Abstract
Background
The long-term trajectories of lipid and glucose levels in subjects who experience a major cardiovascular (CV) event at a young age has not been well studied. Our objective was to investigate lipid, lipoprotein, apolipoprotein (apo), and glucose levels in individuals experiencing a CV event before 50 years of age.
Methods and findings
A first CV event [non-fatal myocardial infarction (MI), coronary revascularisation, or CV related death] before age 50 was recorded in 2,939 (cumulative incidence 1.2% in males and 0.3% in females) of 361,353 individuals included in the prospective Swedish AMORIS (Apolipoprotein-related MOrtality RISk) study with health examinations 1985–1996 and follow-up through 2011. In a nested case-control analysis, cases with a CV event were matched to randomly selected controls. Population risk factor trajectories were calculated up to 20 years prior to an event. Total cholesterol (TC), triglyceride (TG), and glucose levels were higher in cases than in controls as early as 20 years prior to the event with differences increasing over time. Low density lipoprotein, apoB, and the apoB/apoA-1 ratio were higher and increased over time, while HDL and apoA-1 were lower in cases compared to controls. The odds ratio was 2.5 (95% confidence interval 1.6–3.7) for TC ≥5 mmol/L and TG ≥1.7 mmol/L in cases versus controls. The adjusted population-attributable fractions including lipids, glucose, diabetes, smoking, hypertension, and obesity indicated that about 50% of CV events before age 50 may be associated with elevated lipid and glucose levels.
Conclusions
Elevated TC, TG, LDL, apoB, and glucose levels and high apoB/apo A-1 ratio documented two decades before a CV event in subjects younger than 50 years may account for about half of CV events before age 50, which calls for early recognition and possibly treatment of modifiable CV risk factors in young individuals.
Introduction
Dyslipidaemia, smoking, hypertension, and diabetes are known risk factors for atherosclerosis and its complications, primarily myocardial infarction (MI) and stroke [1–7]. Atherosclerotic changes have been observed at young ages [8, 9],but clinical manifestations are uncommon in individuals younger than 50 years, although risk factors may be present [4, 5, 10–14]. High total cholesterol (TC) and glucose levels have been associated with an increased risk of cardiovascular (CV) disease in young persons [7, 10]. Asymptomatic men with multiple risk factors have an increased risk of death from CV causes [15]. The risk of a future CV event can be estimated from information on sex, age, smoking, TC, and blood pressure [1]. In recent decades, the prevalence of cardiovascular disease, diabetes, and, especially, obesity in young individuals and women has increased worldwide [1, 16]. Therefore early identification of CV risk factors is of importance from a primary prevention perspective. The long-term pattern of CV risk factors in individuals who experience a major CV event at a young age has not been extensively studied.
The goal of this nested case control study was to review and evaluate trajectories of lipid, lipoprotein, apolipoprotein, and glucose levels in the years preceding a CV event in individuals younger than 50 years.
Methods
Design and study population
The study was based on the Apolipoprotein-related MOrtality RISK (AMORIS) Cohort. The AMORIS study was designed to study apolipoprotein (apo) B (atherogenic) and apoA-1 (athero-protective) levels relative to future CV disease [17–19] and includes 812,073 subjects chiefly from the greater Stockholm area who underwent health examinations with laboratory testing through occupational health screening or primary care from 1985–1996.
For the present study, we selected from the AMORIS cohort subjects younger than 50 years who had measurement of total cholesterol (TC), triglycerides (TG), and glucose at the initial health examination (baseline) (n = 361,353 individuals; 46% females). No subject was hospitalized at the time of the baseline health examination or had experienced MI or coronary revascularization prior to this date. Information on low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), and apoB and apoA-1 as well as traditional CV risk factors including tobacco smoking and hypertension was obtained in sub-sets of patients. To analyse long-term trajectories of CV risk factors we conducted a nested case control study within the cohort.
All data were anonymous before any analyses. Informed consent was not required. The study complied with the Declaration of Helsinki and was approved by the Regional Ethical Review Board in Stockholm (2010/1047-31/1, 2011/1406-32].
Case identification and follow-up
For the identification of a first CV event before age 50 years, records of all subjects were reviewed through 2011 in the Swedish National Patient Registry and the National Cause of Death Registry by linkage using a unique personal identification number assigned to all individuals living in Sweden. A first CV event was defined as a non-fatal MI, coronary revascularisation [coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI)], or CV death. The mean period from initial data collection to event was nine years, and the maximum was 27 years. The Swedish National Patient Registry includes information on inpatient care regionally from 1964 and nationally from 1987. The National Cause of Death Registry includes all deaths recorded in Sweden along with information on the cause of death. Information regarding coronary revascularization was obtained from the Swedish Web System for Enhancement and Development of Evidence-based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART) [www.swedeheart.se]. No individual experiencing a previous MI or revascularisation before the index examination was included in the analyses.
Patient characteristics
To obtain information on patient characteristics beyond the laboratory data, the AMORIS cohort was linked to several additional registers and research cohorts described in detail with references in an AMORIS cohort paper [19]. In brief, information on socioeconomic class was obtained from mandatory Swedish national censuses from 1970 to 1990. Information on diabetes, history of smoking, blood pressure, and self-reported hypertension was obtained from several sources including research cohorts at the Karolinska Institutet (the Work, Lipids, and Fibrinogen study, the Cohort of Swedish Men study, the Swedish Mammography Cohort, the cohort of 60-year-old subjects in Stockholm, the Sollentuna Primary Prevention Study and for women undergoing pregnancy also from the national Swedish Medical Birth Register) [19]. Information on smoking and blood pressure was obtained from the SWEDHEART registry for many of the individuals experiencing a CV event, hence providing more data for CV cases than for subjects with no CV diagnosis. Care was taken to, as much as possible, implement similar definitions of patient characteristics derived from different sources in the analyses of this study. Information on dispensed medication was available for all subjects by the National Prescribed Drug Register beginning in July 2005.
Blood sampling and laboratory analyses
Analyses were conducted on fresh blood serum samples (55% fasting) at CALAB Medical Laboratories, Stockholm, Sweden. Total cholesterol and TG were determined by enzyme techniques, and LDL, HDL, and apolipoproteins were measured as previously described [18, 19]. Glucose levels were analysed with the glucose oxidase/peroxidase technique, using automated multichannel analysers [AutoChemist-PRISMA® (New Clinicon, Stockholm, Sweden) and Technicon DAX® TM 96 (Technicon Instruments Corp., Tarrytown, NY, USA)]. Creatinine levels were assessed with the non-kinetic alkaline picrate method (Jaffé), using AutoChemist-PRISMA from 1985 through 1992 and, from 1993 through 1996, a DAX-96 analyser. The coefficient of variation was <3% for all laboratory tests. The glomerular filtration rate was estimated using the Chronic Kidney Disease Epidemiology Collaboration formula.
Definitions
Cardiovascular deaths were classified according to the International Classification of Diseases (ICD)-9 as 390–459 and, from 1997, as ICD-10 codes I00–I99. Acute myocardial infarction was defined as ICD-9 code 410 or ICD-10 code I21. Percutaneous coronary intervention and CABG included all interventions reported to the SWEDEHEART registry. Body mass index was calculated as weight in kg divided by the square of height in meters, with obesity defined as BMI ≥ 30. Hypertension was identified as either >140/90 mm Hg, self-reported hypertension, or a dispensed anti-hypertensive drug recorded in the National Prescribed Drug Registry. Socioeconomic status was classified as manual or non-manual occupation and by level of education (≤9 years, 9–12 years, or >12 years). High serum glucose level was defined as a fasting glucose level of ≥7 mmol/L (126 mg/dL), a random glucose level of ≥11.1 mmol/L (200 mg/dL), or a diagnosis of diabetes registered in the National Diabetes Register [20]. To reflect the relative contribution of TG to TC as a risk factor, combined dyslipidaemia was defined as Fredrickson classification [21]—Type IIa with TC ≥ 5 mmol/L (193 mg/dl) and TG < 1.7 mmol/L (150 mg/dl); Type IIb with TC ≥ 5 mmol/L and TG level ≥ 1.7 mmol/L; or as Type IV with TC < 5 mmol/L and TG ≥ 1.7 mmol/L.
Statistical methods
We used the entire cohort to calculate the cumulative incidence of a first CV event before age 50. We estimated sex- and age-specific incidence rates (number of events per person-years at risk) for subjects exhibiting a combination of total serum cholesterol ≥6.0 mmol/L, triglycerides ≥1.40 mmol/L, and glucose ≥5.6 mmol, as well as incidence rate ratios with 95% confidence intervals.
Population trajectories of risk factors and association of a first CV event with risk factors were analysed using a nested case-control approach. Cases included all subjects experiencing a CV event as defined above (non-fatal MI, coronary intervention, or CV death) before age 50 during the study period. Incidence density sampling was used to randomly select five control subjects per case from the cohort population at risk of a new CV event [22]. Controls were matched to cases according to age (five-year cohorts), sex, and calendar year of the event. We generated trajectories of mean values of risk factors in cases and controls separately, starting 25 years prior to diagnosis of the case or selection as a control subject. These trajectories constitute a comparison between cases and controls at given time points and do not represent repeat measurements in the same individual. The trajectories are based on a single baseline assessment per subject. They do not represent individual development over time, but the mean values of cases and controls each year prior to diagnosis of the case or selection as a control subject. This type of trajectory can be designated a population trajectory.
Population trajectories for TC, TG, glucose, lipoprotein, and apolipoprotein mean values with 95% confidence intervals (CI) were calculated stratified by years preceding the CV event or selection as control. The geometric mean was used for TG because of the skewed distribution. We did not perform statistical tests to compare differences in mean values of cases and controls but calculated 95% confidence intervals for the means of cases and controls. Non-overlapping confidence intervals indicated significant difference between cases and controls. Logistic regression was used to calculate odds ratios (OR) with 95% CI for risk of a CV event. Cut-off levels were set to ≥ 5 mmol/L and ≥1.7 mmol/L for hypercholesterolaemia and hypertriglyceridaemia, respectively [1]. SAS software 9.3 TS Level 1M0 (SAS Institute Inc., Cary, NC, USA) was used for data programming.
Results
Entire study group
We identified 2,939 subjects with a first CV event before age 50. The events occurred four times more often in males (1.2%; 2,422/197,095) than in females (0.3%; 517/177,333). Few events occurred before age 40, with the majority (61%) at 45–49 years.
The estimated incidence rate of a CV event before age 50 years in subjects 40–49 years of age was 47/10,000 person-years in males and 15/10,000 person-years in females with initial measurements of serum cholesterol ≥6.0 mmol/L, triglycerides ≥1.40 mmol/L, and glucose ≥5.6 mmol/L (Table 1). This represented an incidence rate ratio of 2.6 in males and 3.0 in females compared to subjects with levels below these values.
Table 1. Incidence rate and incidence rate ratio with 95% confidence interval for an adverse cardiac event before age 50 among 361,353 men and women stratified to three age groups with total cholesterol ≥6.0 mmol/L, triglycerides ≥1.40 mmol/L, and glucose ≥5.6 mmol/L (Exposed) and those with lower levels (unexposed).
| Exposed (n = 8,467) | Unexposed (n = 352,886) | |||||||
|---|---|---|---|---|---|---|---|---|
| Sex | Age at initial testing (years) |
Number of events | Person years | Incidence rate /10,000 years |
Number of events | Person years | Incidence rate /10,000 years |
Incidence rate ratio (95% CI) |
| Male | 20–29 | 18 | 5,122 | 35 | 461 | 999,480 | 5 | 7.0 (4.4–11.2) |
| Male | 30–39 | 104 | 21,996 | 47 | 986 | 921,445 | 11 | 4.3 (3.5–5.2) |
| Male | 40–49 | 109 | 23,187 | 47 | 726 | 405,435 | 18 | 2.6 (2.1–3.2) |
| Female | 20–29 | 1 | 1,769 | 6 | 119 | 900,186 | 1 | 6.0 (0.8–42.9) |
| Female | 30–39 | 8 | 3,315 | 24 | 207 | 733,296 | 3 | 8.0 (3.9–16.2) |
| Female | 40–49 | 7 | 4,624 | 15 | 163 | 353,052 | 5 | 3.0 (1.4–6.4) |
Nested-case control analyses
The nested case-control study with matching to five controls was performed in 2,925 CV cases (99.5%), with 14 cases matched to fewer controls due to lack of available appropriate referent subjects. A total of 14,660 controls were included. Cardiovascular cases and controls were well balanced by design with respect to age, sex, and calendar year of the event (Table 2). The mean time from the initial blood sampling to a CV event or selection as control was nine years. All subjects exhibited normal renal function. Smoking, diabetes, hypertension, manual work, and fewer than nine years of education were more common, while body mass index, TC, TG, LDL, apoB, and the ratio apoB/apoA-1 and glucose levels were higher, among CV cases than controls. HDL and apoA-1 were lower in cases than in controls.
Table 2. Characteristics of subjects experiencing an adverse cardiovascular event before age 50 and controls.
| Variable | Cases (n = 2,939) |
Controls (n = 14,660) |
P-value |
|---|---|---|---|
| Years since blood sampling (SD) | 9.25 (6.15) | 9.03 (6.18) | |
| Age a (years) (SD) | 45.4 (4.22) | 45.0 (4.51) | |
| Female (%) | 18 | 18 | |
| History of smoking (%) | 62 | 25 | <0.001 |
| Diabetes/High glucose levelb (%) | 13 | 3 | <0.001 |
| Hypertension (%) | 35 | 5 | <0.001 |
| eGFR <60 mL/min/1.73 m2 (%) | 0 | 0 | 0.61 |
| Body mass index (kg/m2) (SD) | 26.7 (4.82) | 24.2 (3.84) | <0.001 |
| Body mass index ≥30 (kg/m2) (%) | 21 | 6 | <0.001 |
| Manual work (%) | 53 | 40 | <0.001 |
| Non-manual work (%) | 41 | 54 | <0.001 |
| Unclassified (%) | 6 | 5 | 0.5910 |
| Education ≤9 years (%) | 27 | 19 | <0.001 |
| Education 9–12 years (%) | 47 | 45 | 0.0950 |
| Education >12 years (%) | 20 | 34 | <0.001 |
| Education Unclassified (%) | 6 | 2 | <0.001 |
| TC (mmol/L) c (SD) | 6.06 (1.36) | 5.30 (1.09) | <0.001 |
| TG (mmol/L) (SD) | 1.91 (1.51) | 1.34 (1.04) | <0. 001 |
| Glucose (mmol/L) (SD) | 5.29 (2.09) | 4.85 (0.94) | <0.001 |
| LDL (mmol/L) (SD) | 4.24 (1.29) | 3.47 (1.01) | <0.001 |
| Non-HDL (mmol/L) (SD) | 5.06 (1.51) | 4.06 (1.31) | <0. 001 |
| HDL (mmol/L) (SD) | 1.23 (0.41) | 1.43 (0.40) | <0.001 |
| Apolipoprotein B (mmol/L) (SD) | 1.49 (0.43) | 1.23 (0.36) | <0. 001 |
| Apolipoprotein A-1 (mmol/L) (SD) | 1.29 (0.22) | 1.37 (0.21) | <0.001 |
| ApoB/ApoA-I ratio (SD) | 1.20 (0.50) | 0.93 (0.31) | <0.001 |
| Fredrickson Classification | |||
| IIa (TC ≥ 5, TG <1.7) (%) | 39 | 41 | 0.10 |
| IIb (TC ≥ 5, TG ≥1.7) (%) | 41 | 19 | <0.001 |
| IV (TC < 5, TG ≥1.7) (%) | 4 | 5 | 0.024 |
| TC < 5, TG <1.7 (%) | 16 | 36 | <0.001 |
TC, total cholesterol; TG, triglycerides; LDL, low density lipoprotein cholesterol; HDL, high density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate
a Age at first event or selection as control subject
b Diabetes mellitus or fasting glucose ≥7 mmol/L or any glucose ≥11 mmol/L
c Geometric means.
Available data (Cases/Controls); Smoking (1,374/1,485), Diabetes (2,766/14,066); Hypertension (972/6,228); Body mass index (1,169/3758); Apolipoprotein (1,076/4,340)
Trajectories
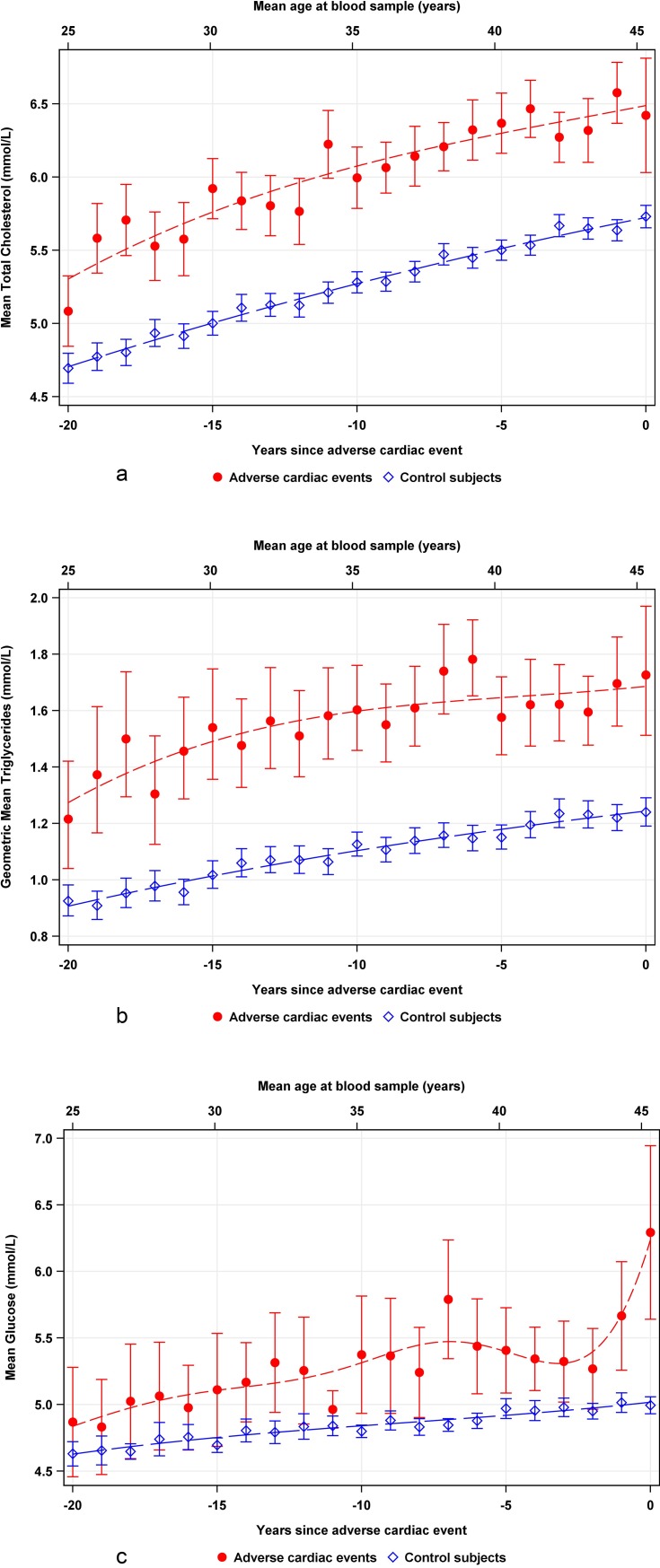
Total cholesterol and TG levels were found significantly higher in CV cases than in controls 20 years prior to a CV event. Total cholesterol, TG, and glucose levels increased continuously and almost in parallel with time closer to the event in both cases and controls (Fig 1A–1C). The slope for glucose was steeper among cases than controls a few years before the CV event.
Fig 1.
Weighted scatterplot smoothed curves of mean values with 95% confidence interval (CI) for (a) total cholesterol, (b) triglycerides, and (c) glucose in 2939 cases (red line) and 14660 controls (blue line) obtained during 20 years preceding a CV event. Clinical reference levels are indicated on the y-axis. Mean age is given at the top of each graph, and years before a cardiac event is indicated on lower x-axis.
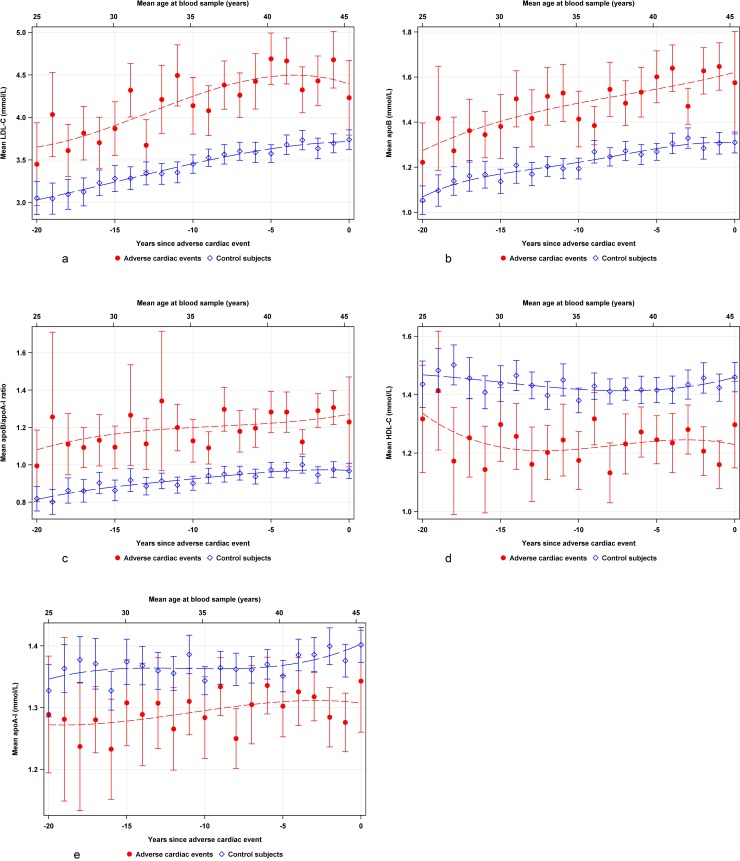
Low density lipoprotein levels were higher in cases than in controls 20 years before the event and increased in parallel with controls but decreased closer to the event (Fig 2A). The ApoB values and the apoB/apoA-1 ratio were higher in cases than in controls and increased during 20 years before the event (Fig 2B and 2C). High density lipoprotein and apoA-1 remained almost stable up to the event in both cases and controls (Fig 2D and 2E).
Fig 2.
Weighted scatterplot smoothed curves of mean values with 95% confidence interval (CI) for (a) low density lipoprotein cholesterol (LDL) in 1164 cases (red line), and 4988 controls (blue line), (b) apolipoproteins B (988 cases, red line; 3927 controls blue line), (c) apolipoproteins B/A-1 (940 cases, redline; 3596 controls, blue line) ratio (d) high density lipoprotein cholesterol (HDL) (1144 cases; red line, 4963 controls, blue line) (e) apolipoproteins A-1 (1076 cases, red line; 4340 controls, blue line) obtained during 20 years preceding a CV event. Clinical reference levels are indicated on the y-axis. Mean age is given at the top of each graph, and years before a cardiac event is indicated on lower x-axis.
Risk of a CV event
Complete information of target variables was available for 628 cases and 859 controls (S1 Table). High TC and TG levels, hyperglycaemia or diabetes mellitus, smoking, hypertension, and obesity were all significantly associated with an increased incidence of a CV event before age 50 (Table 3). The multivariable adjusted population attributable risk (PAR) suggested that ~50% of CV events before age 50 may be associated with elevated TC, TG, and hyperglycaemia. High PAR values were also observed for smoking, hypertension, and obesity (Table 3).
Table 3. Odds ratios (OR) and population attributable risk (PAR) with 95% confidence intervals (CI) for an adverse cardiovascular event before age 50 calculated for cases and controls from available data.
| Variable | Cases(628)a (%) |
Controls(859)b (%) |
OR (95% CI) | Adjusted c OR (95% CI) |
%PAR (95% CI) | Adjusted c %PAR (95%CI) |
|---|---|---|---|---|---|---|
| High TC (≥5 mmol/L) | 72.1 | 42.9 | 2.0 (1.5–2.6) | 1.5 (1.1–2.1) | 36.4 (24.1–46.7) | 24.5 (7.2–38.5) |
| High TG (≥1.7 mmol/L) | 39.9 | 10.9 | 2.9 (2.1–4.0) | 1.7 (1.2–2.4) | 26.1 (17.7–33.6) | 15.9 (4.6–25.8) |
| Diabetes/High glucosed | 15.6 | 2.4 | 6.4 (3.7–11.3) | 3.3 (1.8–5.9) | 13.2 (5.5–20.3) | 10.8 (2.8–18.1) |
| History of smoking | 64.0 | 25.7 | 3.1 (2.4–4.1) | 2.8 (2.1–3.7) | 43.6 (34.8–51.2) | 41.1 (31.5–49.4) |
| Hypertension | 33.3 | 7.6 | 4.0 (2.8–5.7) | 2.9 (2.0–4.2) | 25.0 (17.2–32.0) | 21.6 (13.2–29.2) |
| BMI ≥ 30 (kg/m2) | 24.5 | 3.6 | 6.7 (4.2–10.8) | 4.1 (2.4–6.8) | 20.9 (13.3–27.8) | 18.5 (10.4–25.8) |
BMI, body mass index; TC, total cholesterol; TG, triglycerides.
a Data available in 628/2939 cases (22%)
bData available in 859/14,660 controls (6%)
cAdjusted for variables in Table 2
dDiabetes mellitus or fasting glucose ≥7 mmol/L or any glucose ≥11 mmol/L.
Combined high TC and TG (Type IIb hyperlipidaemia) (adjusted OR 2.5, 95% CI 1.6–3.7) and isolated hypertriglyceridaemia (type IV) were associated with a more than doubled risk of a CV event before age 50 compared to controls. Isolated hypercholesterolaemia (Type IIa) showed lower risk (Table 4).
Table 4. Odds ratios (OR) with 95% confidence intervals (CI) for a cardiovascular event before age 50 years in relation to hyperlipidaemia, calculated for cases and controls with available data.
| Blood lipid levels according to Fredrickson[18] |
Cases (n = 628) (%) |
Controls (n = 859) (%) |
OR (95% CI) | Adjusted a OR (95% CI) |
|---|---|---|---|---|
| TC < 5 and TG <1.7 (mmol/L) | 24.0 | 55.1 | 1.0 | 1.0 |
| Type IIa, TC ≥ 5, TG<1.7 (mmol/L) | 36.1 | 34.0 | 1.7 (1.3–2.3) | 1.6 (1.2–2.3) |
| Type IIb, TC ≥ 5, TG≥1.7 (mmol/L) | 36.0 | 8.9 | 3.8 (2.6–5.5) | 2.5 (1.6–3.7) |
| Type IV, TC <5, TG = >1.7 | 3.8 | 2.1 | 3.9 (1.8–8.4) | 2.6 (1.1–6.3) |
TC, total cholesterol; TG, triglycerides.
a Adjusted for all variables presented in Table 2
In subjects with multiple risk factors Type IIb hyperlipidaemia, history of smoking and hypertension was associated with an adusted risk of a CV event nine-fold that of non-smokers without hypertension and with TC < 5 mmol/L and TG <1.7 mmol/L (OR 9.1, 95% CI 3.9–21.2) (Table 5)
Table 5. Odds ratios (OR) of obesity, high glucose level, and combinations of smoking, hypertension, and type IIb hyperlipidaemia for a cardiovascular event before age 50 years in cases and controls.
| Variable | Cases (n = 628) (%) |
Controls(n = 859) (%) |
OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|---|---|
| BMI = > 30 (kg/m2) | 24.5 | 3.6 | 6.7 (4.1–11.0) | 4.5 (2.7–7.5) |
| High glucose levela | 15.6 | 2.4 | 6.3 (3. 6–11.2) | 3.4 (1.8–6.2) |
| Type IIb hyperlipidaemia | 6.6 | 4.0 | 3.57 (2.0–6.5) | 3.0 (1.6–5.5) |
| Smoking | 26.9 | 19.3 | 3.59 (2.5–5.1) | 3.6 (2.5–5.2) |
| Hypertension | 7.7 | 4.4 | 3.30 (1.9–5.8) | 2.5 (1.4–4.6) |
| Smoking and hypertension | 12.2 | 1.9 | 14.6 (7.6–28.1) | 10.6 (5.4–20.7) |
| Type IIb+Hypertension | 4.5 | 0.4 | 19.7 (5.2–74.6) | 14.8 (3.4–63.9) |
| Type IIb and smoking | 16.0 | 3.6 | 8.1 (4.7–13.8) | 6.8 (3.9–11.7) |
| Type IIb+smoking+hypertension | 9.1 | 0.9 | 16.4 (7.0–38.3) | 9.1 (3.9–21.2) |
BMI, body mass index.
afasting glucose level of ≥7 mmol/L, any glucose ≥11 mmol/L, or diagnosis of diabetes
Fredrickson Type IIb hyperlipidaemia, high glucose level, smoking, obesity, and low socioeconomic status were more common among the 1,315 subjects (45%) who had undergone coronary revascularization than among the 1,624 subjects (55%) experiencing non-fatal MI or CV-associated death (Table 6). Those subjects showed a longer time from the baseline measurement to event than those with MI/CV death (11 versus 6 years)
Table 6. Characteristics of subjects undergoing PCI or CABG and those with MI or death from cardiovascular cause before age 50.
| Variable | All cases n = 2939 |
PCI/CABG n = 1315 (45%) |
MI/Death n = 1624 (55%) |
Difference PCI/CABG and MI/death (p Value) |
|||
|---|---|---|---|---|---|---|---|
| Years to event (median, (range)) |
8 (4–14) | 11 (7–16) | 6 (3–11) | <0.001 | |||
| Age years (mean (SD)) | 45.4 (4.21) | 45.9 (3.4) | 44.9 (4.7) | <0.001 | |||
| Fredrickson Classification | (%) | (%) | (%) | ||||
| IIa (TC ≥ 5, TG <1.7) | 1132/2918 | 38.8 | 491/1304 | 37.7 | 641/1614 | 39.7 | 0.07 |
| IIb (TC ≥ 5, TG ≥1.7) | 1198/2918 | 41.1 | 570/1304 | 43.7 | 628/1614 | 38.9 | <0.001 |
| IV (TC < 5, TG ≥1.7) | 105/2918 | 3.6 | 47/1304 | 3.6 | 58/1614 | 3.6 | nc |
| TC < 5, TG <1.7 | 483/2918 | 16.6 | 196/1304 | 15.0 | 287/1614 | 17.8 | 0.003 |
| Female | 517/2939 | 17.6 | 185/1315 | 14.1 | 332/1315 | 20.4 | <0.001 |
| High glucose levela | 348/2766 | 12.6 | 197/1221 | 16.1 | 151/1545 | 9.8 | <0.001 |
| History of smoking | 863/1374 | 62.8 | 628/967 | 64.9 | 235/407 | 57.7 | <0.001 |
| BMI ≥30 (kg/m2) | 251/1169 | 21.5 | 172/700 | 24.6 | 79/469 | 16.8 | <0.001 |
| SES/Low | 1569/2939 | 53.4 | 731/1315 | 55.6 | 838/1624 | 51.6 | 0.002 |
| Hypertension | 342/972 | 35.2 | 266/759 | 35.0 | 76/213 | 35.7 | 0.35 |
| GFR <60 mL/min/1.73 m2 | 24/2939 | 0.82 | 6/1315 | 0.46 | 18/1624 | 1.1 | <0.001 |
BMI, body mass index; CABG, coronary artery bypass grafting, CVD, cardiovascular disease; MI, myocardial infarction; GFR, glomerular filtration rate; nc, not calculated; PCI, percutaneous coronary intervention; SES, socioeconomic status; TC, total cholesterol; TG, triglycerides.
a Diabetes mellitus or fasting glucose ≥7 mmol/L or any glucose ≥11 mmol/L
Discussion
To the best of our knowledge, this study based on the AMORIS cohort is the largest prospective study evaluating associations of major CV risk factors with a CV event before age 50 years. We found that TC, TG, LDL, apoB, the apoB/apoA-1 ratio, and glucose levels were higher in cases experiencing a CV event than among matched controls as early as 20 years before the event, i.e. at age 30 or younger. Trajectories showed that all potentially atherogenic fractions TC, TG, LDL, apoB, and the apoB/apoA-1 ratio increased and remained higher over time, whereas the protective HDL and apoA-1 remained lower in cases than in controls up to the event. These accumulated differences in lipids, lipoproteins, and apolipoproteins for many years may be strong drivers of atherosclerosis risk resulting in CV events.
At a young age, TC and TG were higher in those experiencing a CV event than in controls, albeit below diagnostic cut-off levels commonly used in adult populations. In agreement with previous studies, we found smoking, obesity, hypertension, and low socioeconomic status to be associated with adverse CV events in young individuals [5, 12, 23]. Information on smoking and hypertension was more frequently available for CV cases than for controls.
Glucose levels were higher in CV cases than in controls, and increased more substantially closer to the CV event. This may indicate early development of hyperglycaemia. Our findings of a significant contribution of high glucose levels, i.e. signs of pre-diabetes (impaired fasting glucose) and manifestations of metabolic syndrome or diabetes confirmed findings of an earlier study [24]. In individuals hospitalized for MI, impaired glucose metabolism is a common finding, with less than 35% showing normal glucose tolerance [25]. In a previous study, we found elevation of lipids, lipoproteins, and the apoB/apoA-1 ratio to be present about 15 years before diagnosis of type 2 diabetes [26].
Our data are in accordance with epidemiological and genetic evidence supporting elevated TG as a major risk factor for CV disease and mortality [27]. We found the combination of smoking, hypertension, and Type IIb hyperlipidaemia according to the Fredrickson classification to be associated with a nine-fold risk of a CV event before age 50 years compared to controls. The increased risk of a CV event associated with Type IIb and Type IV dyslipidaemia reaffirms the need for early diagnosis and management of lipid levels, particularly when combined with other risk factors.
The value of health check-ups and laboratory screening for young individuals is often questioned, as in a Cochrane review of 152,435 asymptomatic participants [28]. However, when high TC and TG levels are recognized early in life, effective treatment can reduce CV risk [29]. Thus, patients with familial hypercholesterolaemia or combined Type IIb and Type III dyslipidaemias are candidates for pharmaceutical intervention at a young age, especially if the case of hereditary dyslipidaemia [29]. Clearly, attention to young individuals presenting multiple conventional risk factors, including metabolic factors, is warranted to detect significant risk and to offer appropriate preventive therapy. This may be particularly important, since lipid and glucose disorders, including type 2 diabetes, contribute to increase in worldwide prevalence of CV disease in young men and women. The estimated population-attributable fractions suggest that approximately 50% of CV events before age 50 could be prevented if elevated TC, TG, and glucose levels were normalized. These results are consistent with the INTERHEART study findings of a 60% population-attributable for myocardial infarction associated with risk high apoB/apoA-1 ratio in combination with diabetes in individuals of mean age 58 years from 52 countries world-wide [19, 30, 31]. Our findings of high LDL, high apoB, and, especially, high apoB/apoA-1 ratio indicate that imbalance between atherogenic (LDL and apoB) and athero-protective (HDL and apoA-1) lipoproteins is a major risk factor for severe CV disease not only in elderly but also in early life. The apoB/apoA-1 ratio has emerged in international studies as a solid and important predictor of risk of CV events [16,18, 31].
The strengths of this study include the investigation of a large cohort of young individuals with records reviewed up to 20 years, enabling assessment of lipids, lipoproteins, apolipoproteins, and glucose levels at least two decades preceding a CV event before age 50. A large proportion of the blood samples were from occupational health screenings in healthy subjects, with analyses of fresh blood samples conducted by a single laboratory. In the interpretation of the trajectories, it is important to note that these represent differences between cases and controls at time of measurement of the biomarkers relative to time of diagnosis. The trajectories do not represent a time series of data for a single individual. Similar population trajectories were previously used in an AMORIS study on development of type 2 diabetes [26].
The timing of the blood sampling relative to the CV event and inclusion of control subjects is of critical importance to interpretation of the results of this study. We had access to the dates of both the examinations and the CV events. The National Patient, National Cause of Death, and SWEDEHEART registries cover dates, CV diagnoses, and interventions with a high degree of completeness. The diagnostic quality in the national registers used to identify cases in this study is high, and any misclassification of disease is unlikely to substantially influence our findings. Our present findings are based on a cohort that has been shown to be representative of the employed population of greater Stockholm County according to the 1990 census with regard to social class, country of birth, and marital status [19]. The AMORIS cohort comprised about 30% of the population of Stockholm County during the inclusion period. Since a large segment of the cohort was included via routine health screenings in the occupational setting, there was a higher proportion of employed subjects in the AMORIS cohort compared to the general population. Consequently, the AMORIS cohort is associated with a healthy worker effect, and the standardized mortality ratio was 0.86 compared to the general population in Stockholm County for the study period [19]. This may have led to an underestimate of the absolute rate of CV events under age 50, but is less likely to have biased the internal validity of the comparison of risk factor levels in CV cases and controls.
An important limitation of this study was that information on apolipoproteins, smoking, hypertension, and obesity were, due to screening procedures, not available for all subjects, which restricts the completeness of multivariable analyses. Notably, smoking habits and hypertension were more commonly reported in individuals who had been in contact with the health care system, especially those who had suffered a CV event.
In addition, we did not have access to several other factors that may affect the likelihood of developing CV disease, including family history, diet, alcohol consumption, abdominal obesity, physical activity, working hours, environmental factors, and level of psychosocial stress. However, since the analyses of population trajectories was basically descriptive, lack of adjustment for other major risk factors in these analyses may not be a major limitation but needs to be kept in mind in the interpretation.
Total cholesterol, TG, LDL, apoB, apoB/apoA-1 ratio and glucose levels were higher in cases compared to controls up to two decades before a CV event occurring before age 50. The differences between levels in cases and controls increased over time up to the event and may account for about half of CV events before age 50. This provides support for early identification and possibly treatment of modifiable CV risk factors in young individuals.
Supporting information
(PDF)
Data Availability
The data underlying the study cannot be made available for ethical and legal reasons. This study is based not only on the AMORIS cohort but also on information from the Swedish National Patient Registry, the National Cause of Death Registry, SWEDEHEART, the Work, Lipids, and Fibrinogen study, the Cohort of Swedish Men study, the Swedish Mammography Cohort, the cohort of 60 year old subjects in Stockholm, the Sollentuna Primary Prevention Study and the National Prescribed Drug Register. The merged database from these sources used for the present analyses contains sensitive information and is therefore anonymized and located in a security server with restricted access at the Institute of Environmental Medicine, Karolinska Institutet in Stockholm. The database is available upon request given that the interested party can obtain approval from the data owners including the National Board of Health and Welfare in Sweden (http://www.socialstyrelsen.se/english) and Statistics Sweden (http://www.scb.se/en_/) as well as from the owners of the research registers at KI, Stockholm, Sweden. The AMORIS cohort data is located at the Institute of Environmental Medicine (IMM), Karolinska Institutet (KI) and governed by a Steering Committee where Professor Göran Walldius is Chair (goran.walldius@ki.se) and Professor Niklas Hammar is vice Chair (niklas.hammar@ki.se). Other members of the Steering Committee include Professor Ulf De Faire, IMM, KI, (ulf.defaire@ki.se) Professor Mats Lambe, Department of Medical Epidemiology and Biostatistics (MEB), KI (mats.lambe@ki.se) and Associate Professor Ingmar Jungner, IMM, KI (ingmar.jungner@ki.se). Requests regarding access to data from the AMORIS cohort can be sent to any member of the Steering Committee.
Funding Statement
The Gunnar and Ingmar Jungner Foundation is a non-commercial foundation with a primary goal to fund laboratory medicine and medical research. This foundation has contributed to the establishment of the AMORIS cohort at Karolinska Institutet and also provided a non-restricted grant to the Institute of Environmental Medicine, Karolinska Institutet for research based on this cohort. The foundation has not made any claims or suggestions as to the contents of this research or had any influence on the work on the current manuscript. The chair of the foundation, Associate Professor Ingmar Jungner, is co-author of this manuscript based on his expertise in laboratory medicine. The foundation was registered in Stockholm, Sweden 1998-03-02 (No. 671-97-14421) (GW).
References
- 1.Authors/Task Force M, Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur J Prev Cardiol. 2016;23(11):NP1–NP96. 10.1177/2047487316653709 [DOI] [PubMed] [Google Scholar]
- 2.Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Boren J, Catapano AL, et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. European heart journal. 2011;32(11):1345–61. 10.1093/eurheartj/ehr112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castelli WP. Epidemiology of coronary heart disease: the Framingham study. The American journal of medicine. 1984;76(2A):4–12. [DOI] [PubMed] [Google Scholar]
- 4.De Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova R, Dallongeville J, et al. European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force of European and other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of eight societies and by invited experts). Atherosclerosis. 2004;173(2):381–91. [PubMed] [Google Scholar]
- 5.Grundy SM, Balady GJ, Criqui MH, Fletcher G, Greenland P, Hiratzka LF, et al. Primary prevention of coronary heart disease: guidance from Framingham: a statement for healthcare professionals from the AHA Task Force on Risk Reduction. American Heart Association. Circulation. 1998;97(18):1876–87. [DOI] [PubMed] [Google Scholar]
- 6.Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabetic medicine: a journal of the British Diabetic Association. 2002;19(9):708–23. [DOI] [PubMed] [Google Scholar]
- 7.Stamler J, Daviglus ML, Garside DB, Dyer AR, Greenland P, Neaton JD. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. Jama. 2000;284(3):311–8. [DOI] [PubMed] [Google Scholar]
- 8.Tracy RE, Newman WP, 3rd, Wattigney WA, Berenson GS. Risk factors and atherosclerosis in youth autopsy findings of the Bogalusa Heart Study. The American journal of the medical sciences. 1995;310 Suppl 1:S37–41. [DOI] [PubMed] [Google Scholar]
- 9.Enos WF, Holmes RH, Beyer J. Coronary disease among United States soldiers killed in action in Korea; preliminary report. Journal of the American Medical Association. 1953;152(12):1090–3. [DOI] [PubMed] [Google Scholar]
- 10.Klag MJ, Ford DE, Mead LA, He J, Whelton PK, Liang KY, et al. Serum cholesterol in young men and subsequent cardiovascular disease. The New England journal of medicine. 1993;328(5):313–8. 10.1056/NEJM199302043280504 [DOI] [PubMed] [Google Scholar]
- 11.Bergstrand R, Vedin A, Wilhelmsson C, Wilhelmsen L. Incidence and prognosis of acute myocardial infarction among men below age 40 in Goteborg, Sweden. European heart journal. 1982;3(2):130–5. [DOI] [PubMed] [Google Scholar]
- 12.Carlsson AC, Li X, Holzmann MJ, Wandell P, Gasevic D, Sundquist J, et al. Neighbourhood socioeconomic status and coronary heart disease in individuals between 40 and 50 years. Heart. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukherjee D, Hsu A, Moliterno DJ, Lincoff AM, Goormastic M, Topol EJ. Risk factors for premature coronary artery disease and determinants of adverse outcomes after revascularization in patients < or = 40 years old. The American journal of cardiology. 2003;92(12):1465–7. [DOI] [PubMed] [Google Scholar]
- 14.Hegele RA, Ginsberg HN, Chapman MJ, Nordestgaard BG, Kuivenhoven JA, Averna M, et al. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. The lancet Diabetes & endocrinology. 2014;2(8):655–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. Jama. 2002;288(21):2709–16. [DOI] [PubMed] [Google Scholar]
- 16.European Association for Cardiovascular P, Rehabilitation, Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). European heart journal. 2011;32(14):1769–818. 10.1093/eurheartj/ehr158 [DOI] [PubMed] [Google Scholar]
- 17.Jungner I, Marcovina SM, Walldius G, Holme I, Kolar W, Steiner E. Apolipoprotein B and A-I values in 147576 Swedish males and females, standardized according to the World Health Organization-International Federation of Clinical Chemistry First International Reference Materials. Clinical chemistry. 1998;44(8 Pt 1):1641–9. [PubMed] [Google Scholar]
- 18.Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. 2001;358(9298):2026–33. 10.1016/S0140-6736(01)07098-2 [DOI] [PubMed] [Google Scholar]
- 19.Walldius G, Malmstrom H, Jungner I, de Faire U, Lambe M, Van Hemelrijck M, et al. Cohort Profile: The AMORIS cohort. Int J Epidemiol. 2017;46(4):1103–i. 10.1093/ije/dyw333 [DOI] [PubMed] [Google Scholar]
- 20.Eeg-Olofsson K, Cederholm J, Nilsson PM, Zethelius B, Svensson AM, Gudbjornsdottir S, et al. Glycemic control and cardiovascular disease in 7,454 patients with type 1 diabetes: an observational study from the Swedish National Diabetes Register (NDR). Diabetes care. 2010;33(7):1640–6. 10.2337/dc10-0398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beaumont JL, Carlson LA, Cooper GR, Fejfar Z, Fredrickson DS, Strasser T. Classification of hyperlipidaemias and hyperlipoproteinaemias. Bulletin of the World Health Organization. 1970;43(6):891–915. [PMC free article] [PubMed] [Google Scholar]
- 22.Rothman KJ, Greenland S. Modern Epidemiology Lippincott Williams & Wilkins; 1988 [Google Scholar]
- 23.Schiele F, Ecarnot F, Chopard R. Coronary artery disease: Risk stratification and patient selection for more aggressive secondary prevention. Eur J Prev Cardiol. 2017;24(3_suppl):88–100. [DOI] [PubMed] [Google Scholar]
- 24.DeFronzo RA, Abdul-Ghani M. Assessment and treatment of cardiovascular risk in prediabetes: impaired glucose tolerance and impaired fasting glucose. The American journal of cardiology. 2011;108(3 Suppl):3B–24B. [DOI] [PubMed] [Google Scholar]
- 25.Norhammar A, Tenerz A, Nilsson G, Hamsten A, Efendic S, Ryden L, et al. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet. 2002;359(9324):2140–4. 10.1016/S0140-6736(02)09089-X [DOI] [PubMed] [Google Scholar]
- 26.Malmström H, Walldius G, Carlsson S, Grill V, Jungner I, Gudbjörnsdottir S, et al. Elevation of metabolic risk factors 20 years or more before diagnosis of type 2 diabetes—experience from the AMORIS study. Diabetes Obesity Metabolism.2018. February 5 10.1111/dom.13241 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384(9943):626–35. 10.1016/S0140-6736(14)61177-6 [DOI] [PubMed] [Google Scholar]
- 28.Krogsboll LT, Jorgensen KJ, Gronhoj Larsen C, Gotzsche PC. General health checks in adults for reducing morbidity and mortality from disease: Cochrane systematic review and meta-analysis. Bmj. 2012;345:e7191 10.1136/bmj.e7191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vallejo-Vaz AJ, Kondapally Seshasai SR, Cole D, Hovingh GK, Kastelein JJ, Mata P, et al. Familial hypercholesterolaemia: A global call to arms. Atherosclerosis. 2015;243(1):257–9. 10.1016/j.atherosclerosis.2015.09.021 [DOI] [PubMed] [Google Scholar]
- 30.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–52. 10.1016/S0140-6736(04)17018-9 [DOI] [PubMed] [Google Scholar]
- 31.Walldius G. The apoB/apoA-1 Ratio is a Strong Predictor of Cardiovascular Risk Lipoproteins in Health and Diseases: Frank S and Kostner G; 2012. p. 95–148. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
The data underlying the study cannot be made available for ethical and legal reasons. This study is based not only on the AMORIS cohort but also on information from the Swedish National Patient Registry, the National Cause of Death Registry, SWEDEHEART, the Work, Lipids, and Fibrinogen study, the Cohort of Swedish Men study, the Swedish Mammography Cohort, the cohort of 60 year old subjects in Stockholm, the Sollentuna Primary Prevention Study and the National Prescribed Drug Register. The merged database from these sources used for the present analyses contains sensitive information and is therefore anonymized and located in a security server with restricted access at the Institute of Environmental Medicine, Karolinska Institutet in Stockholm. The database is available upon request given that the interested party can obtain approval from the data owners including the National Board of Health and Welfare in Sweden (http://www.socialstyrelsen.se/english) and Statistics Sweden (http://www.scb.se/en_/) as well as from the owners of the research registers at KI, Stockholm, Sweden. The AMORIS cohort data is located at the Institute of Environmental Medicine (IMM), Karolinska Institutet (KI) and governed by a Steering Committee where Professor Göran Walldius is Chair (goran.walldius@ki.se) and Professor Niklas Hammar is vice Chair (niklas.hammar@ki.se). Other members of the Steering Committee include Professor Ulf De Faire, IMM, KI, (ulf.defaire@ki.se) Professor Mats Lambe, Department of Medical Epidemiology and Biostatistics (MEB), KI (mats.lambe@ki.se) and Associate Professor Ingmar Jungner, IMM, KI (ingmar.jungner@ki.se). Requests regarding access to data from the AMORIS cohort can be sent to any member of the Steering Committee.