Abstract
Introduction
Prognostication in cancer is challenging and inaccurate. C-Reactive Protein (CRP), a cheap and sensitive marker of inflammation may help. This study investigated the relationship between CRP and prognosis in a large cohort of solid tumors with mixed cancer diagnoses and stages.
Methods
Electronic medical records of 4931 adults with solid tumors who attended the Taussig Cancer Institute from 2006–2012 were reviewed. Demographic and clinical characteristics were recorded. Maximum CRP (mCRP) was identified for each individual. CRP was analysed as a time-dependent, continuous and categorical variable for association with survival.
Results
Two thirds of patients had a high mCRP. This was consistently associated with shorter survival, even after correction for time from diagnosis, and when analysed as a continuous or a categorical variable. When mCRP values above 10 mg/L were subcategorized, a higher mCRP was always worse. Even among those with normal values, statistically and clinically significant shorter survival was noted at mCRP levels >5 mg/L.
Conclusions
In a large representative cohort of consecutive solid tumor patients the risk of death was clinically and statistically significantly greater with a high mCRP. This was independent of other variables and regardless of statistical method from both dates of diagnosis and test. CRP appeared to be underutilized. Our results support the routine use of CRP as a universal cost-effective independent prognostic indicator in most solid tumors.
Introduction
Prognostication in cancer is still an inexact science. Physicians are often inaccurate and overly optimistic [1]. Clinical characteristics (lymph node status, male gender, performance status, tumor size) and some biomarkers (alfa-fetoprotein, lactate dehydrogenase) are helpful in certain tumors. Other established biomarkers (e.g. carbohydrate antigen 19–9, carcinoembryonic antigen, prostate specific antigen) are only valuable in specific tumors. Some have been clinically validated [2]. A more widely applicable prognostic biomarker is needed. A few biologic compounds meet the criteria for an ideal tumor marker; C-reactive protein (CRP) is one [3]. It is a non-specific acute phase reactant which reflects tissue damage. Serum concentration depends upon synthesis rate. Serum CRP is a sensitive and stable marker of inflammation. It can be measured by simple inexpensive methods [4].
Hepatocyte CRP secretion is controlled by interleukin 6 (IL-6). Interleukin-1 (IL-1) and tumor necrosis factor (TNF) also stimulate synthesis. A rise in serum levels often reflects the intensity of various pathological processes [5]. High serum CRP is also associated with a greater risk of cardiovascular events. In some chronic inflammatory diseases e.g. rheumatoid arthritis, serial levels correlate with both disease severity and therapeutic response.
There is some evidence to support the role of CRP as a prognostic indicator in specific primary sites [6, 7] and advanced disease [8]. Baseline CRP predicted mortality in operable lung cancer. [9]. In pancreatic cancer CRP, poor functional ability and rate of weight loss all increased near death [10]. CRP may also predict post-surgical tumor recurrence therapeutic response and toxicity [11]. Elevated high sensitivity (hs-CRP) levels have been associated with increased mortality in breast, lung and renal cell carcinomas [12]. Despite these observations CRP seems underused as a biomarker in routine oncology practice. It has been included in some cancer prognostic scoring systems [13]. However, in most reports of CRP and cancer prognosis, survival was not the primary study objective [14]. We decided to investigate the relationship between CRP level and prognosis utilizing the electronic medical records of 4931 persons with solid tumors who had CRP measured subsequent to their cancer diagnosis.
Methods
Study aims
Are high serum CRP levels predictive of prognosis in solid tumors?
Do CRP levels correlate with other known clinical characteristics (comorbidities, metastatic disease, treatment modalities or laboratory values)?
Are specific solid tumors associated with higher CRP levels?
Study design
This retrospective cohort study utilized clinical data from an electronic medical record (EMR) (My Practice/EPIC, 1979–2014 Epic Systems Corporation, WI, USA). The protocol was approved by the Cleveland Clinic Institutional Review Board (IRB). Waiver of informed consent was granted. Adults who attended the Taussig Cancer Institute from 2006–2012 with a solid tumor diagnosis identified by International Classification of Disease Codes Version 9 (ICD-9, World Health Organization 2008) and with at least one serum CRP measurement and at least one total white blood cell count (TWBC) post-diagnosis were included.
People with hematological malignancies or without a TWBC and those whose CRP and/or TWBC measurements preceded the cancer diagnosis were excluded. Those with only one CRP value and a concurrent high TWBC were excluded to avoid co-morbid inflammatory illnesses or intercurrent procedures which may have temporarily elevated CRP. All CRP tests were performed with turbidimetric immunoassay (Roche Kobas, North America) at the Department of Pathology in the Cleveland Clinic.
C-reactive protein
Serum CRP level was the primary measure. Due to the observational nature of the study, CRP had not been measured at consistent time points. A value >10 mg/L is a clinically accepted (though biologically unproven) cutoff-point of CRP into high versus normal groups. Maximum CRP (mCRP) was initially chosen for analysis as it likely reflects peak inflammatory status. In clinical practice it is impossible to identify the mCRP prospectively since this requires all values from diagnosis to death. mCRP years or decades before death may not adequately represent the actual level at death. CRP levels were therefore evaluated in three ways:
mCRP post-diagnosis as a continuous variable
mCRP post-diagnosis categorized as normal (maximum CRP ≤10 mg/L) and high
CRP as a time-dependent variable: this took into account time from diagnosis.
Data
These included: age, race, gender, primary cancer site(s), primary sites (number), metastatic sites (number), liver metastases (yes/no), co-morbidities (heart, liver or inflammatory bowel disease, rheumatoid arthritis), thromboembolic events (superficial or deep vein thrombosis or pulmonary embolism), possible cancer-related symptoms recorded (anorexia, cachexia, delirium, dysphagia, fatigue, malaise, pain, early satiety, weight loss), serum CRP (mg/L), total white blood cell (TWBC) count (k/μL), albumin (g/dL), hemoglobin (g/dL), body mass index (BMI) (Underweight <18.5; Normal 18.5–24.9; Overweight 25–29.9; Obese >30) [15], therapeutic interventions (anti-neoplastic chemotherapy, aspirin, biologic therapies, corticosteroids, hormonal therapy, non-steroidal anti-inflammatory drugs, radiotherapy, statins) and any invasive procedures (biopsies, stents, surgeries) within the four weeks before the mCRP test date. Only (clinical or laboratory) data from the same day or within 4 weeks prior to the mCRP were included. The exception was post-diagnosis mTWBC. TWBC was treated both as a time-dependent and a continuous variable (maximum TWBC). The modified Glasgow Prognostic Score (mGPS) (score 0–2) was also calculated: normal mCRP (≤10 mg/L) = 0; high mCRP (>10 mg/L) plus albumin (≥ 35 g/L) = 1; high mCRP (>10 mg/L) plus hypoalbuminemia (<35 g/L) = 2.
Statistical analyses
Survival time was measured from two index dates; time to death from cancer diagnosis and time to death from date of mCRP. The former is clinically relevant. The latter is biologically relevant as it represents the time from assumed peak inflammatory state: it is less helpful clinically as the mCRP value date is only known retrospectively. Date of death was retrieved either from the Social Security Death Index (United States Social Security Administration) or the electronic medical record (EMR). Those alive at the last visit date were censored. This date was defined as either the last visit to the Cancer Institute (for any purpose) or the final laboratory measurement on record (last CRP; last TWBC).
Data was reported with descriptive statistics: frequencies (percentages) for categorical variables, mean +/- standard deviation or median (interquartile range) for continuous variables. Normal and high mCRP groups were compared with respect to continuous variables by independent t-tests and categorical variables by Fisher’s exact tests. Kaplan-Meier survival curves were generated to compare mCRP groups with log-rank tests. For subgroup analysis of mCRP values, groups were created based solely on frequencies and blinded to survival. Cox proportional hazards regression was performed to obtain hazard ratios (HR). HR quantified associations among clinical factors (e.g. mCRP, TWBC, mGPS) and mortality. Regression models were both adjusted and unadjusted for multiple variables. All percentages were rounded off to the nearest whole number. Analyses were performed with SAS (version 9.3) and JMP Pro (version 9) statistical software (SAS Institute Inc., Cary, NC, USA). P-values ≤ 0.05 (two sided) were considered statistically significant.
Prognosis was analyzed by mCRP group. A significant survival difference was observed between the normal and high groups both from the date of cancer diagnosis and mCRP test date. A similar survival analysis was done from mCRP test date. In both adjusted and unadjusted models, higher levels were associated with shorter survival both from date of diagnosis and test date.
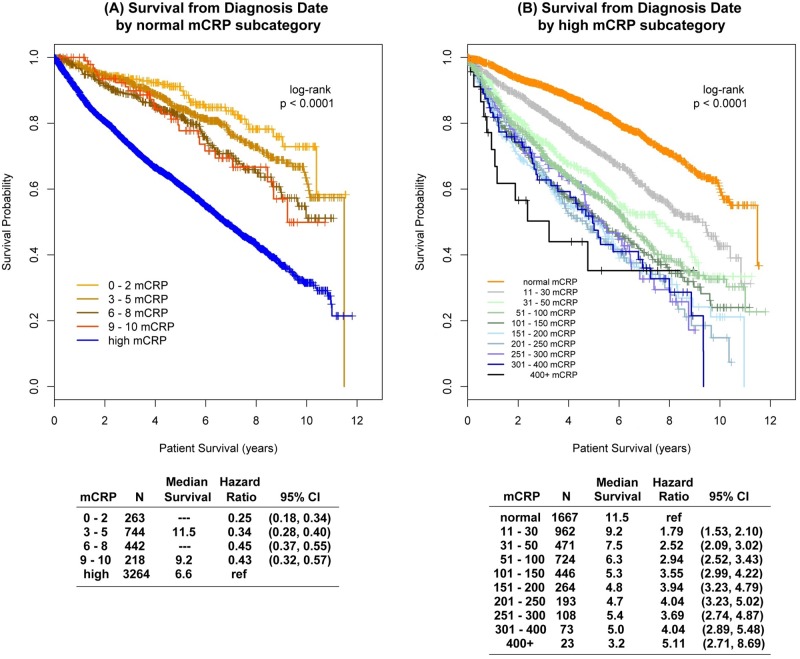
To further elucidate the mCRP association with survival, both the normal and high mCRP groups were subcategorized (Fig 1). In general, survival times were shorter in successive categories with higher mCRP values in both groups. When stratified by primary cancer site, higher mCRP values were associated with increased risk of death, regardless of tumor site. When mCRP was categorized the hazard ratio for high compared to normal mCRP ranged from 1.35 to 7.37.
Fig 1. Prognosis by maximum CRP subgroup.
Results
7716 patients attended the Taussig Cancer Institute from 2006–2012 with a solid tumor diagnosis. The total study population was representative of the US population by gender (49% male) and race (83% Caucasians, 13% African Americans). The study cohort (N = 4931) was also representative of cancer mortality in the United States by tumor site prevalence. Their median length of follow-up was 4.0 years (range 0–11.8). The median number of CRP measurements per patient was 1 (range 1–87) and for TWBC 2 (range 1–96).
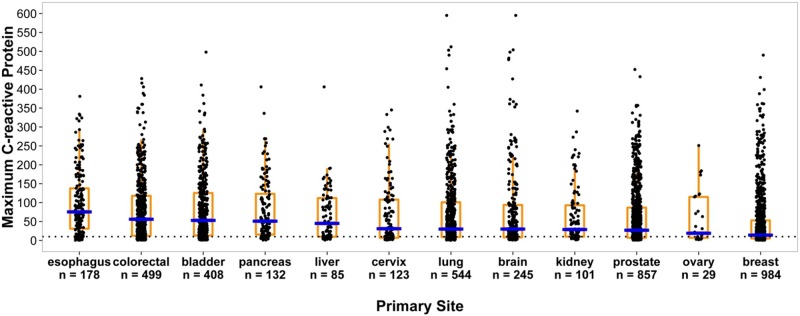
4931 (63%) met all inclusion/exclusion criteria (Table 1). Table 2 compares the normal (34%, n = 1667) and high (66%, n = 3264) mCRP groups. The two groups were similar with respect to age, race, comorbidities and BMI. The normal group median (Q1—Q3) mCRP value was 5 (3–7) mg/L and the high group 62 (26–130) mg/L. More of the high group were male (53% vs. 41%), with a greater number of metastatic sites, and more often had surgery (31% vs. 8%) and stent insertions (2% v 1%) post-cancer diagnosis. Serum albumin and plasma hemoglobin levels were lower and maximum TWBC greater in the high mCRP group. Primary cancer site(s) were identified for each patient. mCRP values were higher in certain solid tumor primary sites (Fig 2). The highest median mCRP values = mg/L in descending order were: esophagus = 76, colorectal = 56, bladder = 53, pancreas = 51, liver = 45, cervix = 31, lung = 30, brain = 30, kidney = 29, prostate = 27, ovary = 19, and breast = 14. The association of mCRP and survival by primary cancer type was statistically significant for all sites.
Table 1. Patient demographic characteristics.
| Variable | Solid Tumor Cancer Patients n = 4931 |
|---|---|
| Age at Diagnosis (years)* | 64 ± 14 |
| Male | 2415 (49%) |
| Primary Cancer Site* | |
| Breast | 984 (20%) |
| Prostate | 857 (17%) |
| Lung | 544 (11%) |
| Colorectal | 499 (10%) |
| Bladder | 408 (8%) |
| Brain | 245 (5%) |
| Esophagus | 178 (4%) |
| Pancreas | 132 (3%) |
| Cervix | 123 (3%) |
| Kidney | 101 (2%) |
| Liver | 85 (2%) |
| Ovary | 29 (0.5%) |
| Metastatic sites | |
| 0 | 3667 (74%) |
| 1 | 727 (15%) |
| 2 | 295 (6%) |
| 3+ | 242 (5%) |
mean ± standard deviation or count (%)
* few patients had multiple primary sites
Table 2. Demographic and clinical variables in solid tumors by maximum CRP status.
| Normal mCRP (≤ 10 mg/L) | High mCRP (> 10 mg/L) | p-value** | |
|---|---|---|---|
| Count | 1667 (34%) | 3264 (66%) | |
| Age at Diagnosis (years) | 64 ± 13 | 65 ± 14 | 0.06 |
| Male | 685 (41%) | 1730 (53%) | < .0001 |
| Body Mass Index, BMI* | 28.0 (24.6–32.4) | 27.0 (23.5–31.6) | 0.0001 |
| Metastatic Sites | < .0001 | ||
| 0 | 1399 (84%) | 2268 (69%) | |
| 1 | 164 (10%) | 563 (17%) | |
| 2 | 52 (3%) | 243 (7%) | |
| 3+ | 52 (3%) | 190 (6%) | |
| Liver metastases | 64 (4%) | 284 (9%) | < .0001 |
| Comorbidities | |||
| Cardiovascular inflammatory disease | 220 (13%) | 628 (19%) | < .0001 |
| Inflammatory bowel disease | 40 (2%) | 84 (3%) | 0.77 |
| Liver disease | 81 (5%) | 265 (8%) | < .0001 |
| Rheumatoid arthritis | 127 (8%) | 230 (7%) | 0.49 |
| Venous thromboembolism | 178 (11%) | 613 (19%) | < .0001 |
| Treatment | |||
| Aspirin | 460 (28%) | 890 (27%) | 0.81 |
| Biologic therapy | 14 (1%) | 55 (2%) | 0.02 |
| Chemotherapy | 367 (22%) | 616 (19%) | 0.01 |
| Corticosteroid | 258 (15%) | 719 (22%) | < .0001 |
| Hormonal therapy | 179 (11%) | 283 (9%) | 0.02 |
| Non-steroidal anti-inflammatory | 397 (24%) | 499 (15%) | < .0001 |
| Statin | 588 (35%) | 1063 (33%) | 0.06 |
| Surgery after Cancer Diagnosis | 132 (8%) | 1008 (31%) | < .0001 |
| Stent after Cancer Diagnosis | 11 (1%) | 57 (2%) | 0.002 |
| Maximum TWBC, k/uL* | 7.1 (5.9–8.4) | 8.8 (7.0–11.2) | < .0001 |
| Albumin, g/dL* | 4.1 (3.8–4.4) | 3.2 (2.6–3.9) | < .0001 |
| Hemoglobin, g/dL* | 13.2 (12.1–14.2) | 11.0 (9.5–12.6) | < .0001 |
| Modified Glasgow Prognostic Score (mGPS) | < .0001 | ||
| 0: CRP ≤ 10 mg/L | 1667 (100%) | 0 (0%) | |
| 1: CRP > 10 mg/L & Albumin ≥ 35 gm/L | 0 (0%) | 1130 (42%) | |
| 2: CRP > 10 mg/L & Albumin < 35 gm/L | 0 (0%) | 1559 (58%) |
*mean ± standard deviation, median (Q1—Q3) or count (%)
** independent t test or Fisher's exact test
Fig 2. Maximum CRP value by primary cancer site.
CRP was analyzed in three ways as a continuous, categorical, and time-dependent variable. Regardless of whether CRP or mCRP were used, higher levels were associated with increased mortality where survival was time from cancer diagnosis to death. Unadjusted and adjusted regression models evaluated this association (Table 3). For CRP analyzed as a time-dependent continuous variable, for every ten unit rise (10 mg/L) the risk of death increased 3% (HR 1.003; 95% CI 1.003–1.004; p < .0001) when controlled for all other model variables.
Table 3. CRP and prognosis.
| Univariable | Multivariable* | |||||
| Survival from Cancer Diagnosis Date | Hazard Ratio | 95% CI | p-value | Hazard Ratio | 95% CI | p-value |
| CRP (time dependent)¥ | 1.005 | (1.005, 1.006) | < .0001 | 1.003 | (1.003, 1.004) | < .0001 |
| Maximum CRP (continuous) | 1.004 | (1.004, 1.005) | < .0001 | 1.001 | (1.000, 1.002) | 0.005 |
| Maximum CRP (categorized: high v normal) | 2.76 | (2.44, 3.11) | < .0001 | 1.57 | (1.34, 1.84) | < .0001 |
| TWBC (time dependent)§ | 1.06 | (1.05, 1.07) | < .0001 | 1.02 | (1.004, 1.04) | 0.01 |
| Maximum TWBC (continuous) | 1.06 | (1.05, 1.07) | < .0001 | 1.01 | (0.998, 1.02) | 0.10 |
| Modified Glasgow Prognostic Score (mGPS)** | ||||||
| Maximum CRP ≤ 10 mg/L | ref | ref | ||||
| Maximum CRP > 10 mg/L & Albumin ≥ 35 g/L | 1.68 | (1.44, 1.95) | < .0001 | 1.43 | (1.22, 1.68) | < .0001 |
| Maximum CRP > 10 mg/L & Albumin < 35 g/L | 4.71 | (4.14, 5.36) | < .0001 | 2.42 | (2.06, 2.85) | < .0001 |
| ¥ 10mg/L u = increase § K/μL increase |
||||||
| Univariable | Multivariable* | |||||
| Survival from Maximum CRP Test Date | Hazard Ratio | 95% CI | p-value | Hazard Ratio | 95% CI | p-value |
| CRP (time dependent)¥ | 1.003 | (1.002, 1.004) | < .0001 | 1.001 | (1.000, 1.003) | 0.03 |
| Maximum CRP (continuous) | 1.004 | (1.004, 1.005) | < .0001 | 1.001 | (1.000, 1.002) | 0.005 |
| Maximum CRP (categorized: high v normal) | 2.65 | (2.35, 3.00) | < .0001 | 1.50 | (1.23, 1.76) | < .0001 |
| TWBC (time dependent)§ | 1.02 | (0.99, 1.05) | 0.08 | 1.00 | (0.97, 1.03) | 0.98 |
| Maximum TWBC (continuous) | 1.05 | (1.04, 1.06) | < .0001 | 1.01 | (0.999, 1.02) | 0.07 |
| modified Glasgow Prognostic Score (mGPS)** | ||||||
| CRP ≤ 10 mg/L | ref | < .0001 | ref | < .0001 | ||
| CRP > 10 mg/L & Albumin ≥ 35 g/L | 1.69 | (1.46, 1.97) | 1.39 | (1.18, 1.63) | ||
| CRP > 10 mg/L & Albumin < 35 g/L | 4.18 | (3.68, 4.76) | 2.31 | (1.96, 2.71) | ||
¥ 10mg/L u = increase
§ K/μL increase
* Each multivariable model adjusted for the following: patient age at diagnosis, patient gender, WBC or CRP, hemoglobin, albumin, BMI, primary cancer site (respiratory, genital, digestive, urinary, breast, brain), liver metastases, number of metastatic sites, comorbidities (liver, cardiac, inflammatory bowel disease, rheumatic arthritis, venous thromboembolism)
** CRP and albumin removed from multivariable model to avoid multicollinearity
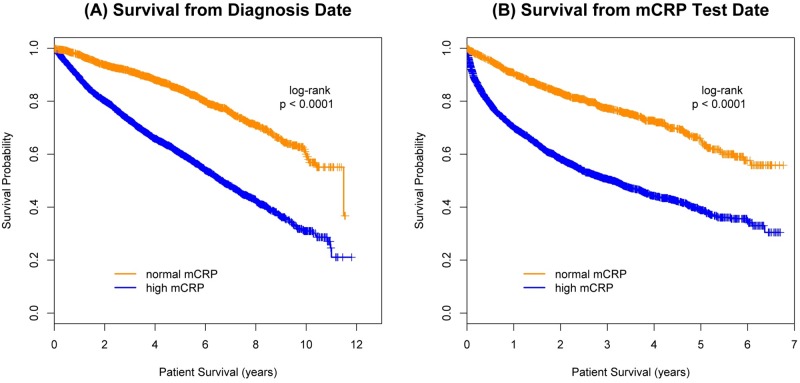
Prognosis was analyzed by mCRP group (Fig 3). A significant survival difference was observed between the normal and high groups both from the date of cancer diagnosis (p < .0001; Fig 3A) and mCRP test date (p < .0001; Fig 3B). The risk of death increased 46% when mCRP was high (> 10 mg/L) compared to normal (≤ 10 mg/L) (HR 1.46; 95% CI 1.25–1.71; p < .0001) after control for all other model variables. A similar survival analysis was done from mCRP test date (Table 3). When the analyses were repeated, adjusting for time from cancer diagnosis, results did not change (Table 3). In both adjusted and unadjusted models, (regardless of how mCRP was analyzed), higher levels were associated with shorter survival, both from date of diagnosis and from date of test.
Fig 3. Prognosis by maximum CRP group.
To further elucidate the mCRP association with survival, both the normal and high mCRP groups were subcategorized (Fig 1). In general, survival times were shorter in successive categories with higher mCRP values in both groups. The same analysis was performed from mCRP test day with similar results. When stratified by primary cancer site, higher mCRP values were associated with increased risk of death, regardless of tumor site. With mCRP as a time dependent variable, the hazard ratios (Δ = 10 mg/L) ranged from 1.05 to 1.13 (all p < .0001). When mCRP was categorized the hazard ratio for high compared to normal mCRP ranged from 1.35 to 7.37. The combination of low albumin and high mCRP in the (modified) Glasgow Prognostic Score supported these observations. Compared to the normal mCRP group, mGPS = 1 was associated with a 35% higher death risk whereas mGPS = 2 had an almost 250% increased risk. While not as strongly predictive, a higher TWBC was also associated with increased mortality. For each unit (k/μL) increase in TWBC, the risk of death rose 2% (HR 1.02; 95% CI 1.004–1.04; p = 0.01).
Discussion
Two thirds of the large cohort of consecutive solid tumor patients who met the inclusion criteria had a high mCRP. This was consistently associated with shorter survival and was a powerful prognostic predictor in most solid tumors, regardless of statistical technique. The differences observed were both statistically and clinically important. When mCRP values above 10 mg/L were subcategorized, a higher mCRP was always worse. This approximate dose-response relationship provided further biological plausibility to the overall findings. Risk of early death also increased with high mCRP irrespective of cancer site. The highest mCRP values were noted among cancers of the esophagus, colon-rectum, bladder, and pancreas. This was the largest study of this issue to date. The total patient population was representative in terms of gender, ethnicity, and cancer primary sites. CRP seemed underused as a prognostic indicator as just two thirds met the inclusion criteria and most of those had few tests.
There were more males in the high mCRP group. Males with advanced cancers are known to lose more weight and have shorter survival than females [16]. High CRP may be positive and low albumin a negative marker of inflammation and loss of lean tissue mass. Lower hemoglobin and albumin levels were also noted amongst the high mCRP group. The hemoglobin and albumin abnormalities might be due to the generalized nutritional and functional decline common in cancer and/or reflect the secondary effects of a high inflammatory load.
Even among those with normal values, statistically and clinically significant shorter survival was noted at mCRP levels >5 mg/L. This suggests the need for a revised CRP reference range of 1–5 mg/L for prognostication in cancer patients. Few studies [17] have investigated this and it needs further research. Our results support the importance of CRP as a cost-effective independent prognostic indicator in most solid tumors. In addition, the scale and magnitude of the relationship suggest CRP should be recommended for routine use from diagnosis.
A relationship between inflammation and the origin of cancer was first hypothesized in 1863 [18]. This is now accepted but incompletely understood. Immune modulators have therapeutic benefit in specific tumors [19]. Some have advocated non-steroidal anti-inflammatory drugs (NSAIDs) in chemo-prevention and treatment and limited epidemiological evidence supports this [20]. NSAIDs (or corticosteroids, other immunomodulators) might be used as an adjuvant therapy to individualize therapy for solid tumor in those identified by high CRP values. The potential effectiveness of NSAIDs to reduce tumor recurrence, improve treatment response and reduce chemotherapeutic treatment-related toxicities needs further investigation.
This was a retrospective study. The original indication for the CRP measurement was not known that include nor was it part of any known research protocol or clinical pathway in that time frame. Some had multiple tests and others few. Currently, cancer prognosis is often (and usually inaccurately) estimated by clinical data like disease stage, performance status and histology. We found the EMR to be surprisingly unreliable regarding these characteristics and therefore were unable to incorporate them into the current analyses. Nonetheless, CRP was a highly significant independent predictor of survival from both diagnosis and test dates after adjustment for multiple other clinical variables (e.g. liver metastases) and exclusion of those potentially at risk for intercurrent infections.
Although adjustment for such additional covariates might have impacted some of our results, the sheer strength of the intimate association between high CRP and poor prognosis across multiple solid tumor primary sites makes this unlikely. Additionally, the strong relationship was observed even after control of multiple other covariates (that were available in the EMR). The available covariates e.g. liver metastases may also be seen as proxies for the information we could not access, like tumor histology. It is also noteworthy that the current use of parameters like performance status has not satisfactorily resolved the everyday prognostication clinical dilemma. Our results support the hypothesis that inflammation has an important role in cancer natural history and prognosis.
We believe our observations and conclusions are robust. We conducted a prior systematic review which found CRP to be prognostic of survival and treatment response specifically in gastrointestinal and renal cell carcinomas [21]. Our findings support these earlier observations [21, 22] but extend them to nearly all solid tumors. Some have proposed that tumoral CRP is superior to serum CRP for estimation of recurrence and prognosis [2, 23]. Others consider the CRP gene a potential target for individual therapy [24]. Polymorphism may increase cancer risk and has been associated with worse survival in colorectal cancer [25]. A relationship has also been proposed between systemic inflammation and various cancer symptoms. It appears that even one elevated CRP in a cancer patient (unexplained by a co-morbid illness or other intercurrent event) suggests a significantly worse disease outcome. Our data also supports the role of the GPS (although it might be enhanced by addition of TWBC).
Serial measurements to establish CRP kinetics may be clinically useful in different solid tumors and perhaps hematological malignancies and predict clinical course, cancer recurrence and survival [18]. CRP levels in both serum and tumor tissue could be evaluated among various solid tumors for prognostic purposes [23]. The relationship of CRP levels to specific histologies should also be investigated. Serum and tumoral CRP may also help target individualized therapy [2]. High sensitivity CRP (hsCRP), tumor CRP and CRP gene polymorphism should be evaluated for better insight into risk of recurrence, treatment response and toxicity [13, 23, 26]. Given the known close relationship between a high CRP and risk of cardiovascular disease, it is possible that accelerated atheromatous disease is part of the metastatic process and accompanies progressive disease. Lastly, it is possible that unexplained high CRP levels in otherwise healthy people may indicate a later risk of developing cancer (and not just cardiovascular disease). A recent systematic review and meta-analysis supports our observations [27]. CRP should be routinely used as a prognostic indicator in solid tumor oncology practice.
Conclusions
Two thirds of the tested solid tumor population had a high mCRP. This was more common in males. In this representative cohort of consecutive solid tumor patients, the risk of death was clinically and statistically significantly greater for those with a high (or high normal) mCRP level independent of all other variables from both cancer diagnosis and test date. This was true regardless of primary cancer site and after exclusion of those with concurrent elevated white cell counts. Particular diseases (esophagus, colon-rectum, bladder, and pancreas) were more often associated with higher values. Risk of death was 46% greater when mCRP was high compared to normal. With each ten unit (mg/dl) increase the risk of increased 3%. There was also an inverse relationship between absolute mCRP value and survival even within the normal reference range. Lower serum albumin and hemoglobin levels were also noted among the high mCRP group. CRP seemed underutilized as a prognostic marker. Further analysis of the data set by the modified Glasgow Prognostic Score supported our observations. CRP should be used routinely in medical oncology practice to improve prognostic accuracy and further research is warranted in this important area.
Supporting information
(XLSX)
Data Availability
The de-identified data set is available as a Supporting Information file.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Glare P. Clinical predictors of survival in advanced cancer. J Support Oncol. 2005;3(5):331–9. Epub 2005/10/13. . [PubMed] [Google Scholar]
- 2.Schilsky RL, Taube SE. Tumor markers as clinical cancer tests—are we there yet? Semin Oncol. 2002;29(3):211–2. Epub 2002/06/14. . [DOI] [PubMed] [Google Scholar]
- 3.Jaye DL, Waites KB. Clinical applications of C-reactive protein in pediatrics. Pediatr Infect Dis J. 1997;16(8):735–46; quiz 46–7. Epub 1997/08/01. . [DOI] [PubMed] [Google Scholar]
- 4.Black S, Kushner I, Samols D. C-reactive protein. J Biol Chem. 2004;279(47):48487–90. 10.1074/jbc.R400025200 [DOI] [PubMed] [Google Scholar]
- 5.Emery P, Gabay C, Kraan M, Gomez-Reino J. Evidence-based review of biologic markers as indicators of disease progression and remission in rheumatoid arthritis. Rheumatol Int. 2007;27(9):793–806. 10.1007/s00296-007-0357-y [DOI] [PubMed] [Google Scholar]
- 6.He X, Li JP, Liu XH, Zhang JP, Zeng QY, Chen H, et al. Prognostic value of C-reactive protein/albumin ratio in predicting overall survival of Chinese cervical cancer patients overall survival: comparison among various inflammation based factors. J Cancer. 2018;9(10):1877–84. Epub 2018/05/29. 10.7150/jca.23320 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shinohara S, Sugaya M, Onitsuka T, Machida K, Matsuo M, Tanaka F. Prognostic Impact of Postoperative C-reactive Protein for Non-small Cell Lung Cancer Following Lobectomy. Anticancer Res. 2018;38(5):3193–8. Epub 2018/05/02. 10.21873/anticanres.12584 . [DOI] [PubMed] [Google Scholar]
- 8.Amano K, Maeda I, Morita T, Miura T, Inoue S, Ikenaga M, et al. Clinical Implications of C-Reactive Protein as a Prognostic Marker in Advanced Cancer Patients in Palliative Care Settings. J Pain Symptom Manage. 2016;51(5):860–7. Epub 2016/01/31. 10.1016/j.jpainsymman.2015.11.025 . [DOI] [PubMed] [Google Scholar]
- 9.Pastorino U, Morelli D, Leuzzi G, Gisabella M, Suatoni P, Taverna F, et al. Baseline and postoperative C-reactive protein levels predict mortality in operable lung cancer. Eur J Cancer. 2017;79:90–7. Epub 2017/05/05. 10.1016/j.ejca.2017.03.020 . [DOI] [PubMed] [Google Scholar]
- 10.Barber MD, Ross JA, Fearon KC. Changes in nutritional, functional, and inflammatory markers in advanced pancreatic cancer. Nutr Cancer. 1999;35(2):106–10. Epub 2000/02/29. 10.1207/S15327914NC352_2 . [DOI] [PubMed] [Google Scholar]
- 11.Ramsey S, Lamb GW, Aitchison M, McMillan DC. Prospective study of the relationship between the systemic inflammatory response, prognostic scoring systems and relapse-free and cancer-specific survival in patients undergoing potentially curative resection for renal cancer. BJU Int. 2008;101(8):959–63. Epub 2008/01/15. 10.1111/j.1464-410X.2007.07363.x . [DOI] [PubMed] [Google Scholar]
- 12.Ko YJ, Kwon YM, Kim KH, Choi HC, Chun SH, Yoon HJ, et al. High-sensitivity C-reactive protein levels and cancer mortality. Cancer Epidemiol Biomarkers Prev. 2012;21(11):2076–86. Epub 2012/11/09. 10.1158/1055-9965.EPI-12-0611 . [DOI] [PubMed] [Google Scholar]
- 13.Walsh D, Mahmoud F, Barna B. Assessment of nutritional status and prognosis in advanced cancer: interleukin-6, C-reactive protein, and the prognostic and inflammatory nutritional index. Support Care Cancer. 2003;11(1):60–2. 10.1007/s00520-002-0390-z [DOI] [PubMed] [Google Scholar]
- 14.Iwasa S, Nakajima TE, Nakamura K, Takashima A, Kato K, Hamaguchi T, et al. Systemic chemotherapy for peritoneal disseminated gastric cancer with inadequate oral intake: a retrospective study. Int J Clin Oncol. 2011;16(1):57–62. Epub 2010/10/16. 10.1007/s10147-010-0135-9 . [DOI] [PubMed] [Google Scholar]
- 15.Obesity: Preventing and Managing The Global Epidemic. Geneva, Swizerland: 2000. [PubMed]
- 16.Sarhill N, Mahmoud F, Walsh D, Nelson KA, Komurcu S, Davis M, et al. Evaluation of nutritional status in advanced metastatic cancer. Support Care Cancer. 2003;11(10):652–9. Epub 2003/08/16. 10.1007/s00520-003-0486-0 . [DOI] [PubMed] [Google Scholar]
- 17.Saito K, Tatokoro M, Fujii Y, Iimura Y, Koga F, Kawakami S, et al. Impact of C-reactive protein kinetics on survival of patients with metastatic renal cell carcinoma. Eur Urol. 2009;55(5):1145–53. Epub 2008/10/22. 10.1016/j.eururo.2008.10.012 . [DOI] [PubMed] [Google Scholar]
- 18.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45. Epub 2001/03/07. 10.1016/S0140-6736(00)04046-0 . [DOI] [PubMed] [Google Scholar]
- 19.Moschos SJ, Edington HD, Land SR, Rao UN, Jukic D, Shipe-Spotloe J, et al. Neoadjuvant treatment of regional stage IIIB melanoma with high-dose interferon alfa-2b induces objective tumor regression in association with modulation of tumor infiltrating host cellular immune responses. J Clin Oncol. 2006;24(19):3164–71. 10.1200/JCO.2005.05.2498 [DOI] [PubMed] [Google Scholar]
- 20.Harris RE, Chlebowski RT, Jackson RD, Frid DJ, Ascenseo JL, Anderson G, et al. Breast Cancer and Nonsteroidal Anti-Inflammatory Drugs Prospective Results from the Women’s Health Initiative. Cancer Res. 2003;63(18):6096–101. [PubMed] [Google Scholar]
- 21.Shrotriya S, Walsh D, Bennani-Baiti N, Thomas S, Lorton C. C-Reactive Protein Is an Important Biomarker for Prognosis Tumor Recurrence and Treatment Response in Adult Solid Tumors: A Systematic Review. PLoS One. 2015;10(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMillan DC, Crozier JE, Canna K, Angerson WJ, McArdle CS. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Colorectal Dis. 2007;22(8):881–6. 10.1007/s00384-006-0259-6 [DOI] [PubMed] [Google Scholar]
- 23.Nakatsu T, Motoyama S, Maruyama K, Usami S, Sato Y, Miura M, et al. Tumoral CRP expression in thoracic esophageal squamous cell cancers is associated with poor outcomes. Surg Today. 2012;42(7):652–8. Epub 2012/02/22. 10.1007/s00595-012-0147-3 . [DOI] [PubMed] [Google Scholar]
- 24.Spentzos D, Levine DA, Kolia S, Otu H, Boyd J, Libermann TA, et al. Unique gene expression profile based on pathologic response in epithelial ovarian cancer. J Clin Oncol. 2005;23(31):7911–8. 10.1200/JCO.2005.02.9363 [DOI] [PubMed] [Google Scholar]
- 25.Yang SH, Huang CJ, Chang SC, Lin JK. Association of C-reactive protein gene polymorphisms and colorectal cancer. Ann Surg Oncol. 2011;18(7):1907–15. Epub 2011/02/05. 10.1245/s10434-011-1575-9 . [DOI] [PubMed] [Google Scholar]
- 26.Slattery ML, Curtin K, Poole EM, Duggan DJ, Samowitz WS, Peters U, et al. Genetic variation in C-reactive protein in relation to colon and rectal cancer risk and survival. Int J Cancer. 2011;128(11):2726–34. Epub 2010/10/16. 10.1002/ijc.25721 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dolan RD, McSorley ST, Horgan PG, Laird B, McMillan DC. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: Systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;116:134–46. Epub 2017/07/12. 10.1016/j.critrevonc.2017.06.002 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
The de-identified data set is available as a Supporting Information file.