Abstract
An in situ enzymatic screening (ISES) approach to rapid catalyst evaluation recently pointed to Ni(0) as a new candidate transition metal for intramolecular allylic amination. This led to further exploration of chiral bidentate phosphine ligands for such transformations. Herein, a variety of P,N-ligands are examined for this Ni(0)-chemistry, using a model reaction leading into the vinylglycinol scaffold. On the one hand, an N,N-bis(2-diphenylphosphinoethyl)alkylamine (‘PNP’) ligand proved to be the fastest ligand yet seen for this Ni(0)-transformation. On the other, phosphinooxazoline (PHOX) ligands of the Pfaltz–Helmchen–Williams variety gave the highest enantioselectivities (up to 51% ee) among P,N-ligands examined.
1. Introduction
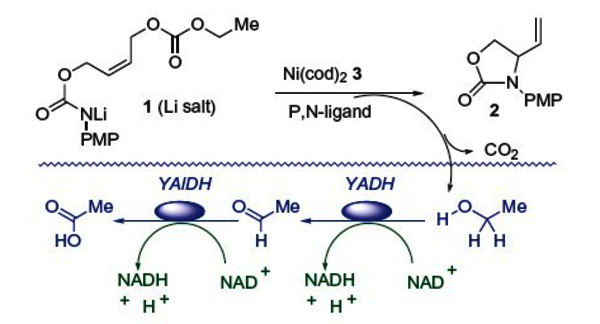
The surge of activity in combinatorial catalysis has led to a keen interest in catalyst screening methods.1 We have found that enzymes can be used to assist organic chemists in this regard, using an approach that we term in situ enzymatic screening (ISES).[2] & [3] To demonstrate proof of principle for ISES, we chose to study transition metal (TM)-mediated intramolecular allylic amination.4 Specifically, the transformation of 1→2 was chosen, as it yields a protected vinylglycinol product, commensurate with our interest in vinylic amino acids as PLP enzyme inhibitors.[5] & [6]
Clearly, the most well studied TM for allylic amination, and particularly for asymmetric variants, is palladium.7 By contrast, there is remarkably little literature on the use of other TM’s for asymmetric allylic amination. Notable exceptions are recent reports on the use of Ru(II) from Takahashi et al.,8 and Ir(I) from Hartwig et al.,9 and Helmchen et al.,10 in which impressive levels of stereoinduction are achieved. Interestingly, Evans has shown that, with the appropriate ligand sphere, Rh(I)-complexes can be employed for allylic amination with preservation of stereochemistry at a pre-existing stereocenter, presumably via a strict double inversion (σ-allyl metal) mechanism.11 Similar observations have been made by Martin et al. recently, for unligated Rh(I) in allylic alkylation chemistry.12
An initial screen of late TM’s for the transformation of 1→2 turned up Ni(0) as a good candidate for further development.2a Those studies also identified Ni(cod)2 as useful catalyst precursor and relatively electron rich and bidentate phosphines (i.e., dppb or dppf) as excellent supporting ligands for this chemistry. The internal carbamate nitrogen nucleophile was found to perform best when outfitted with a PMP (4′-methoxyphenyl) or TMP (3′,4′,5′-trimethoxyphenyl) protecting group and when deprotonated with one equivalent of LiHMDS.
These findings raised the interesting prospect that one might be able to develop the first asymmetric allylic amination chemistry supported by Ni(0).13 Indeed, this turns out to be the case, with members of the Josiphos (Solvias) and BIPHEP (Roche) ligand families providing ee’s at the 75−82% level. This led to an enantioselective synthesis of L-vinylglycine, based on this new Ni chemistry.2b
Given these developments, it seemed a reasonable next step to screen bidentate ligands more broadly, for support of this chemistry. Herein, then, we report our findings on ISES screening across a range of P,N-ligands, followed by closer examination of the most promising hits under typical RB-fiask conditions.
2. Results and discussion
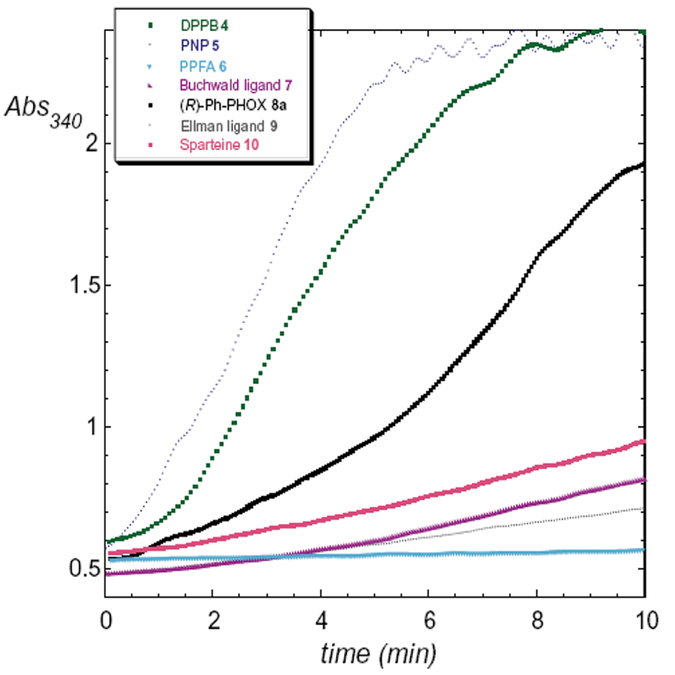
The set of P,N-ligands chosen for the initial ISES survey is illustrated in Figure 1 and Figure 2. This set is bracketed by two ‘homonuclear’ bidentate reference ligands. The fastest P,P-ligand previously seen, DPPB 4, was included as a bis-phosphine reference ligand. For the other ‘bookend,’ we chose sparteine. Sparteine was seen as a reasonable choice for a ‘representative’ N,N-ligand as it represents one of the earliest chiral ligands ever examined for asymmetric allylic alkylation with palladium in pioneering work by Trost and Dietsch.14 Later, Togni et al. showed that sparteine indeed displays bidentate coordination in a π-allyl-Pd complex.15 Finally, the recent successes with sparteine as a chiral element in the Pd(II)-mediated oxidative kinetic resolution of secondary alcohols that have been registered by the groups of Sigman et al.16 and Stoltz et al.17 suggest that renewed attention should be paid to this chiral ligand for late transition metal chemistry.
Figure 1.
ISES data from the initial ligand screen.
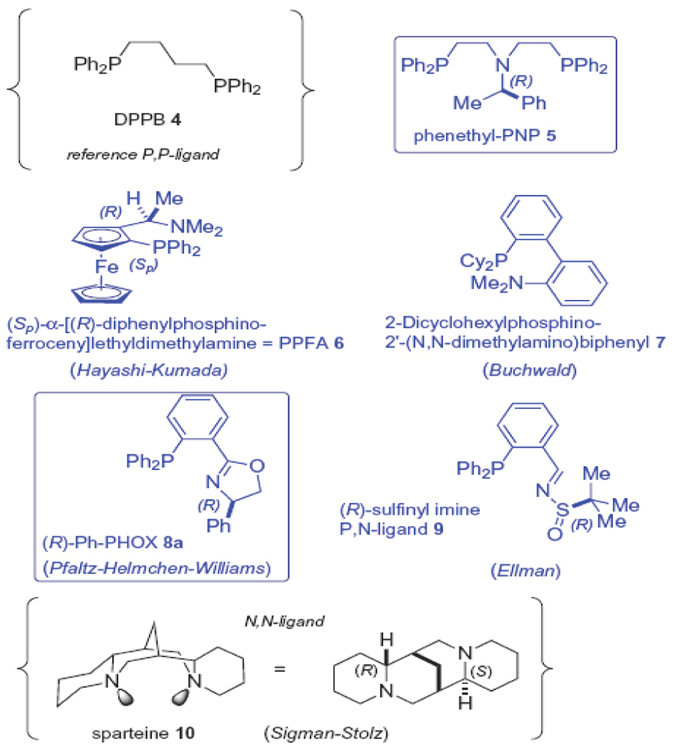
Figure 2.
Structures of the ligands in the initial screen.
The selected P,N-ligands themselves span a range of hybridization states on nitrogen, from sp3 (amine nitrogen; ligands 5 and 6), to intermediate between sp2 and sp3 (aniline nitrogen, ligand 7), to sp2 (oxazoline/imine nitrogen, ligands 8 and 9). All, in principle, offer the possibility for five- or six-ring bidentate chelation to nickel. Whereas, the PNP-ligand 5 has been relatively little studied heretofore,18 the other amine-based ligand, PPFA 6, was developed by Hayashi and Kumada in the 1970’s, and represents the first planar chiral P,N-ligand developed.19 It has been widely studied and has found early application in asymmetric Grignard cross-couplings with vinylic halides, mediated by nickel.19b It also served as the direct precursor to the Josiphos ligands20 with which we have found some success in early asymmetric versions of this nickel chemistry.2b
The biphenyl ligand 7, developed in the Buchwald group, has proved to be one of the most successful ligands for Pd-mediated Suzuki couplings and aminations (Buchwald–Hartwig reaction) of aryl chlorides and bromides.21 Ligand class 8 represents the most well-studied phosphinooxazoline family, wherein the chirality usually resides in the oxazoline moiety, and often is derived from an amino acid. These PHOX ligands22 were developed concurrently in the laboratories of Pfaltz,23a Helmchen,23b and Williams,23c about a decade ago, and have found quite widespread application in late transition metal chemistry, including allylic substitution chemistry. Finally, Schenkel and Ellman have reported that substitution of the chiral oxazoline moiety with a chiral tert-butylsulfinamide-based imine, leads to a P,N-ligand 9 that also supports Pd(0)-based allylic substitution with malonate upon 1,3-diphenylpropenyl acetate.24
In the ISES assay (see Table 1 figure), turnover of substrate 1 implies loss of an ethyl carbonate leaving group, that following decarboxylation and protonation (perhaps at the organic/aqueous interface), leads to release of ethanol. The ethanol signal is diffusible and can be detected by the tandem action of yeast alcohol dehydrogenase and yeast aldehyde dehydrogenase in the reporting aqueous layer. This results in the formation of two molecules of NADH per EtOH detected. Catalysts that turn over the carbonate substrate more rapidly should lead to a greater rate of NADH formation in the aqueous layer. Several catalysts can be screened in parallel, using a UV/vis-spectrophotometer with a multicell changer. The method is sensitive, since even 0.1 μmol of NADH in approximately a 1 mL volume gives rise to a significant absorbance (~0.6) at 340 nm, the λmax for the 1,4-dihydronicotinamide chromophore of reduced pyridine nucleotide co-factors. This then allows for an approximate catalyst ranking, in terms of relative turnover rates.
Table 1.
Surveying new ligand classes for intramolecular Ni(0)-mediated allylic amination using in situ enzymatic screening (ISES)
|
| Noa | Ligand | ΔO.D.340/timeb | %Conv.c |
|---|---|---|---|
| 1 | DPPB 4 | 180±40d | 70 |
| 2 | (R)-Phencthyl-PNP 5 | 360e | 90 |
| 3 | PPFA 6 | 4± 1 | f |
| 4 | Buchwald ligand 7 | 20 ± 15 | f |
| 5 | (R)-Ph-PHOX 8a | 137 ±22 | 38 |
| 6 | Ellman ligand 9 | 18 ± 6 | f |
| 7 | (–)-Sparteine 10 | 21 ± 10 | f |
Conditions for the biphasic ISES screen (YADH= yeast alcohol dehydrogenase and YA1DH = yeast aldehyde dehydrogenase) as described in Note 25.
Obs’d rates (10 min) of NADH formation in units of ΔO.D.340min−1 (Fig. 1). ISES slopes are reported as mean ± SD (duplicate runs) unless otherwise indicated.
Reaction conditions: 67mM 1, 10mol% Ni(cod)2, 10mol% ligand, LiHMDS (1 equiv), THF, rt, 10 min. Product:educt ratio estimated by NMR following work-up.
Average of four runs.
This value is derived from extrapolation as the absorbance rises above the detection limit over the course of the screen (Fig. 1).
Crude NMR shows ≤5% conversion to product.
For reactions to which it applies, the ISES method has the advantage of providing a rapid readout, as no aliquots need be drawn and no work-up is necessary, and the readout is semi-continuous. Another important advantage is that one need not modify the substrate by installing a chromophore, for example. This avoids the synthetic manipulation entailed in such approaches and, more importantly, does not raise the spectre of potentially altered reactivity associated with structural modifications.
The actual UV/vis data obtained for P,N-ligands of classes 4–9 are shown in Figure 1 and the reporting rates are tabulated in Table 1. As noted, the DPPB ligand was the most effective ligand previously found to promote this Ni(0)-transformation (1→2), and so provides a useful calibration point. One notices immediately that two of the new P,N-ligand classes screened, namely the chiral-PNP ligand 5, and the PHOX ligand 8a, give much more significant ISES signals than the others.
Unfortunately, for this substrate, relatively slow rates were seen by ISES with the other P,N-ligand classes screened, including the ligands of Hayashi and Kumada 6, Buchwald 7 and Ellman 9, as well as sparteine. As can be seen from Table 1, a good correlation was seen between ISES rankings (10 min window, biphasic conditions) of the ligands screened and NMR conversions for the same ligands under RB fiask conditions (10 min window, THF solvent).
The results for PNP-ligand 5 are striking, in terms of both the dramatic ISES rate seen, and the nearly complete conversion of 1 to 2 that is seen within 10 min of performing the reaction under standard conditions in THF (Table 1). This ligand accelerates this Ni(0) chemistry more effectively than any other ligand yet studied. Unfortunately, that catalytic power does not translate into any significant enantiodiscrimination, as 2 is obtained in essentially racemic form (chiral HPLC).
Ligand 5 has been previously shown to support the Pd(II)-mediated intramolecular hydroamination of 6-aminohexyne to 2-methyl-1,2-dehydropiperidine.18a Tridentate coordination to palladium was proposed in that work, though a monomer–dimer equilibrium was also postulated to rationalize the NMR data seen. Perhaps more striking, Bianchini et al.[18b] & [18c] have found that ligand 5 promotes the Ir(I)-mediated enantioselective transfer hydrogenation of α,β-unsaturated ketones, in up to 54% ee. Interestingly, these workers succeeded in crystallizing both the (cod)Ir(I)-hydride-5 complex and an Ir(III)-5-dihydride complex. The former exhibits bidentate P,P-coordination to the iridium(I) center, whereas the latter clearly shows P,N,P-tridentate coordination to the Ir(III) center (Fig. 3).
Figure 3.
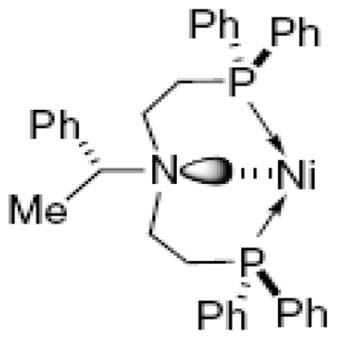
Potential tridentate Ni-coordination for ligand 5.
This latter observation raises the interesting possibility that 5 may exhibit tridentate coordination to the nickel center, either at the Ni(0) or Ni(II) oxidation state along the reaction coordinate, for the conversion of 1 to 2. Given the impressive rate seen here, future experiments are warranted to assess whether rate acceleration correlates well with this ‘tridentate’ ligand motif, and to establish whether alterations in the chiral scaffold within this PNP class can lead to appreciable asymmetric induction in this Ni(0) chemistry.
Given the modular nature of the PHOX ligands, and the potential for readily introducing a range of chiral directing groups into these ligands, we were particularly intrigued that the ISES screen identified 8a as one of the better promoters of this Ni(0)-mediated intramolecular amination chemistry. It was decided to expand upon this lead result. A family of PHOX ligands 8a–g was assembled for a more focused screen, in a second round of ISES.
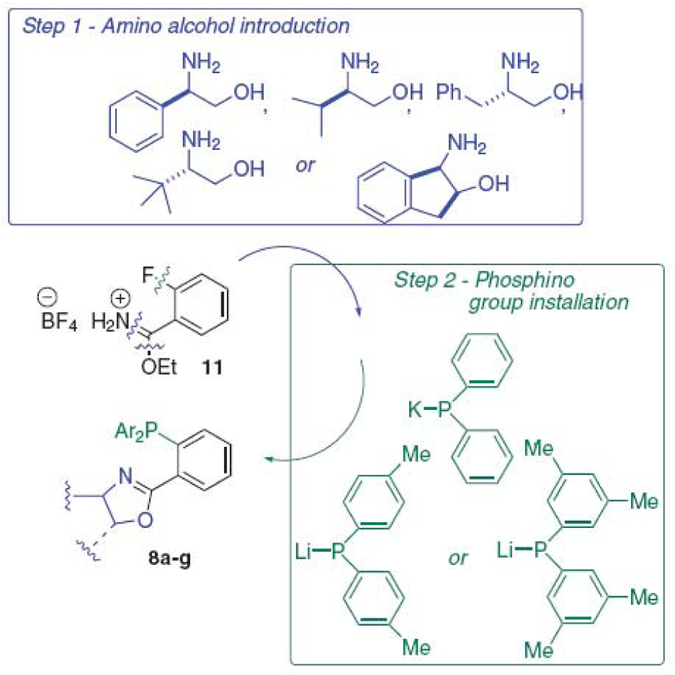
It was found that O-ethyl 2-fiuorobenzimidate tetrafiuorobo-rate salt 11, a reagent introduced recently by Busacca et al. for the synthesis of phosphinoimidazoline ligands,26 provides an excellent nucleus for the assembly of a focused array of PHOX ligands. The approach taken is illustrated in Scheme 1, and involves initial condensation of a chiral amino alcohol with 11, followed by introduction of the desired diarylphosphino group by nucleophilic aromatic substitution upon the resulting (2-fiuoro)aryloxazoline.27
Scheme 1.
Synthesis of the PHOX ligand array.
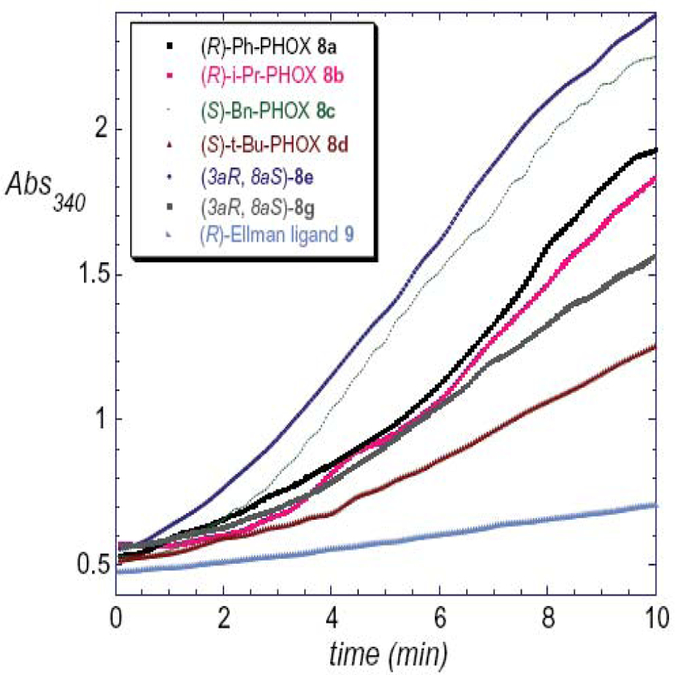
Five different chiral amino alcohols were chosen, derived from D-phenylglycine (a), D-valine (b), L-phenylalanine (c), L-tert-leucine (d) and (1R,2S)-1-amino-2-indanol (e), respectively. The corresponding diphenylphosphinooxazolines, 8a–e, all known ligands, were examined for promotion of the title transformation by ISES. The average reporting rates observed and actual UV traces are presented in Table 2 and Figure 4, respectively.
Table 2.
An ISES examination of chiral PHOX ligands

|
| Noa | L | Ar | R1(R2) | ΔO.D.340/tb | Conv.c (%) |
|---|---|---|---|---|---|
| 1 | (R)-8a | Ph | Ph | 137 ± 22d | 38 |
| 2 | (R)-8b | Ph | i-Pr | 166 ± 29 | 36 |
| 3 | (S)-8c | Ph | Bn | 198 ± 15d | 41 |
| 4 | (S)8d | Ph | t-Bu | 77 | 16 |
| 5 | (3aR,8aS)-8e | Ph | Lnde | 224 ± 18 | 53 |
| 6 | (3aR,8aS)-8g | 3,5-Xyl | lnde | 100 ± 11 | 33 |
| 7 | (R)-9 | Ph | f | 18 ± 6 | <5 |
Conditions for the biphasic ISES screen (YADH = yeast alcohol dehydrogenase and YA1DH = yeast aldehyde dehydrogenase) as described in Note 25.
Obs’d rates (10 min) of NADH formation in units of ΔO.D.340min−1 (Fig. 4). ISES slopes are reported as mean ± SD (duplicate runs) unless otherwise indicated.
Reaction conditions: 67mM 1, 10mol% Ni(cod)2, 10mol% ligand, LiHMDS (1 equiv), THF, rt, 10 min. Product:editct ratio estimated by NMR following work-up.
This slope is the average of four runs.
The chiral element here is the oxazoline derived from (1 R,2S)-l-amino-2-indanol.
This is miman’s ligand, bearing the (R)-t-butylsulfinyl imine chiral element.
Figure 4.
ISES data from the PHOX ligand screen.
Given the impressive rate displayed by ligand 8e, bearing the aminoindanol chiral scaffold, it was selected for further modification. Thus, alteration of the phosphide nucleophile employed in the second module of the synthesis (Scheme 1), permitted for the facile introduction of either a bis(p-toluyl)phosphino group or a bis(3,5-xylyl)phosphino group, to give the previously undescribed ligands 8f and 8g, respectively.27
While 8e and 8f showed comparable rates, 8g showed somewhat attenuated reactivity for the model reaction. Nonetheless, all of the PHOX ligands screened showed respectable ISES rates and clearly supported this Ni(0) chemistry better than even the closely related ligand 9 (Fig. 4). Given these observations, it was decided to examine this ligand set further, under standard reaction conditions, over more extended periods of time, with purification of the product 2 and evaluation of its enantiomeric purity by chiral HPLC. The results are collected in Table 3.
Table 3.
RB flask results: Ni(0)-PHOX-mediated cyclizations of 1 to 2
| Noa | Ligand | Base | Yieldb (%) | Eec | Configd |
|---|---|---|---|---|---|
| 1 | (R)-8a | LiHMDS | 49 | 0 | |
| 2 | (R)-8b | LiHMDS | 41(74)e | 28 | (R) |
| 3 | (S)-8c | LiHMDS | 49 | 24 | (S) |
| 4 | (S)-8d | LiHMDS | 27(46)e | 36 | (S) |
| 5 | (3aR,8aS)-8e | LiHMDS | 57 | 30 | (R) |
| 6 | (3aR,8aS)-8f | LiHMDS | 66–82f | 38 | (R) |
| 7 | (3aR,8aS)-8f | NaHMDS | 61 | 34 | (R) |
| 8 | (3aR,8aS)-8f | KHMDS | 60 | 4 | (R) |
| 9 | (3aR,8aS)-8g | LiHMDS | 37 | 45 | (R) |
| 10 | (R)-9 | LiHMDS | 14 | 31 | (R) |
| 11 | (3aR,8aS)-8f | NaH | 48 | 46 | (R) |
| 12 | (3aR,8aS)-8f | Na2CO3g | 23 | 50 | (R) |
| 13 | (3aR,8aS)-8f | KO-t-Bu | 43 | 37 | (R) |
| 14 | (3aR,8aS)-8f | K2CO3g | 38 | 45 | (R) |
| 15h | (R)-8a | None | 13 | 24 | (R) |
| 16h | (R)-8b | None | 35 | 48 | (R) |
| 17h | (S)-8c | None | <5 | ND | |
| 18h | (S)-8d | None | 37 | 48 | (S) |
| 19h | (3aR,8aS)-8e | None | 40(69)e | 50 | (R) |
| 20 | (3aR,8aS)-8f | None | 38 | 51 | (R) |
Reaction conditions: 67 mM 1, 10mol% Ni(cod)2, 10mol% ligand, base (1 equiv), THF, rt, overnight.
Yields reflect isolated pure product, following chromatography.
HPLC with a chiral stationary phase was used to determine ee [Chiralcel OD; hexane-i-PrOH (80/20)]. ND = Not determined.
Absolute configuration established by correlation of the second-eluting peak of 2 with L-vinylglycine (Ref. 2b).
The yields in parentheses reflect runs in which a second portion of Ni(cod)2 and ligand were added at t= 2h.
Range for two runs.
Nominally, 3 equiv base were employed here, though the base was not completely soluble.
These runs employed a Ni:L ratio of 1:2.
Several trends are apparent. With few exceptions, the inclusion of base improves both rate and yield. In some cases (i.e., entries 5–8), yields in the 60–80% range are attained. However, base generally leads to a lower ee in the product than that observed in the, albeit incomplete, reactions carried out in the absence of base. In the best cases, ee’s in the 48–51% range are seen for the i-Pr, t-Bu, and aminoindanol-based directing groups (entries 16 and 18–20). One can drive these base-free reactions to higher conversions, and maintain these ee’s, by adding a second portion of Ni(0) and ligand (i.e., entry 19), if desired.
3. Conclusions
Recently, the ISES approach to catalyst screened uncovered conditions (model substrate 1, N-PMP protecting group, LiHMDS base, Ni(cod)2 catalyst precursor) that were particularly conducive to Ni(0)-mediated allylic amination chemistry.2 The pattern of ligand performance initially found2a set the stage for the identification of the first asymmetric such transformation with chiral bidentate phosphine ligands (1→2 in 88% yield and 75% ee with MeO-BIPHEP).2b This prompted us to screen other classes of bidentate ligands, such as the P,N-array examined here. This has led to the discovery of the ‘fastest’ ligand yet uncovered for the title transformation; namely PNP-ligand 5. We also find that PHOX ligands 8b and 8d–g promote this chemistry with ee’s up to 51%, though conversion remains an issue here.
Finally, we note that imidate salt 1126 provides a very convenient and modular vehicle into the PHOX ligand class. This approach allowed for the efficient synthesis of the parent 1-amino-2-indanol-based PHOX ligand, 8e, as well as two new congeners thereof, 8f and 8g.27 Whereas ligand 8e remains incompletely studied,28 though Wiese and Helmchen have examined allylic substitutions with Pd here,28c ligands 8f and 8g are new. All three ligands appear to have promise when compared to the other PHOX ligands surveyed. Future studies will exploit this modular ligand synthesis, as substrate and catalyst structure are further varied.
In this light, it is perhaps useful to survey the limited but emerging landscape of catalytic, asymmetric Ni(0)-mediated C–C bond forming reactions, with an eye toward PHOX ligand performance. Interestingly, PHOX ligands (i) perform poorly in Mori’s R2Zn-initiated carboxylative bis-diene cyclizations,29 (ii) provide modest ee’s in Uemura’s allylic substitutions involving hard RMgX or arylboronate-ate nucleophiles,30 and (iii) perform either brilliantly (high conversions and ee’s with dinaphthothiophenes) or not at all (with dibenzothiophenes) in Hayashi’s Grignard-based fused arylthiophene ring openings, depending on subtle nuances of substrate structure.31 This suggests that further exploration of PHOX-based Ni(0)-allylic amination chemistry across a greater expanse of substrate space may reap dividends.
Acknowledgements
The authors thank the NSF (CHE-0317083) for support. D.B.B. acknowledges the Alfred P. Sloan Foundation for a fellowship. This research was facilitated by shared instrumentation grants for NMR (NIH SIG-1–510-RR-06301, NSF CHE-0091975, NSF MRI-0079750) and GC/MS (NSF CHE-9300831), respectively.
References
- 1.(a) Reviews MT Reetz, Compr. Coord. Chem. II 9 (2004), pp. 509–548; [Google Scholar]; (b) Stambuli JP and Hartwig JF, Curr. Opin. Chem. Biol 7 (2003), pp. 420–426. [DOI] [PubMed] [Google Scholar]; (c) Traverse JF and Snapper ML, Drug Discovery Today 7 (2002), pp. 1002–1012; [DOI] [PubMed] [Google Scholar]; (d) Finn MG, Chirality 14 (2002), pp. 534–540; [DOI] [PubMed] [Google Scholar]; (e) Reetz MT, Angew. Chem., Int. Ed 41 (2002), pp. 1335–1338; [DOI] [PubMed] [Google Scholar]; (f) Loch JA and Crabtree RH, Pure Appl. Chem 73 (2001), pp. 119–128. [Google Scholar]
- 2.(a) Berkowitz DB, Bose M and Choi S, Angew. Chem., Int. Ed 41 (2002), pp. 1603–1607; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Berkowitz DB and Maiti G, Org. Lett 6 (2004), pp. 2661–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) For examples, of the use of enzymes to analyze chiral reaction products, post work-up, see:Abato P and Seto CT, J. Am. Chem. Soc 123 (2001), pp. 9206–9207; [DOI] [PubMed] [Google Scholar]; (b) Onaran MB and Seto CT, J. Org. Chem 68 (2003), pp. 8136–8141; [DOI] [PubMed] [Google Scholar]; (c) Li Z, Buetikofer L and Witholt B, Angew. Chem., Int. Ed 43 (2004), pp. 1698–1702; [DOI] [PubMed] [Google Scholar]; (d) For related approaches, using antibodies, see:Matsushita M, Yoshida K, Yamamoto N, Wirsching P, Lerner RA and Janda KD, Angew. Chem., Int. Ed 42 (2003), pp. 5984–5987; [DOI] [PubMed] [Google Scholar]; (e) Taran F, Gauchet C, Mohar B, Meunier S, Valleix A, Renard P-Y, Créminon C, Grassi J, Wagner A and Mioskowski C, Angew. Chem., Int. Ed 41 (2002), pp. 124–127. [DOI] [PubMed] [Google Scholar]
- 4.(a) Reviews concerning allylic amination:Johannsen M and Jorgensen KA, Chem. Rev 98 (1998), pp. 1708–1869; [Google Scholar]; (b) Trost BM, Pure Appl. Chem 68 (1996), pp. 779–784. [Google Scholar]
- 5.For references on PLP–enzyme inhibition with α-vinylic amino acids, see:Berkowitz DB, Jahng W-J and Pedersen ML, Bioorg. Med. Chem. Lett 6 (1996), pp. 2151–2156 and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) For leading references on the stereocontrolled synthesis of α-vinylic amino acids, see:Ma D and Zhu W, J. Org. Chem 66 (2001), pp. 348–350; [DOI] [PubMed] [Google Scholar]; (b) Berkowitz DB, McFadden JM, Chisowa E and Semerad CL, J. Am. Chem. Soc 122 (2000), pp. 11031–11032; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Berkowitz DB, McFadden JM and Sloss MK, J. Org. Chem 65 (2000), pp. 2907–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) For examples, of related asymmetric allylic aminations with Pd, see:Overman LO and Remarchuk TP, J. Am. Chem. Soc 124 (2002), pp. 12–13 (A Pd(II) cycle is proposed here); [DOI] [PubMed] [Google Scholar]; (b) Trost BM, Bunt RC, Lemoine RC and Calkins TL, J. Am. Chem. Soc 122 (2000), pp. 5968–5976; [Google Scholar]; (c) Larksarp C and Alper H, J. Am. Chem. Soc 119 (1997), pp. 3709–3715; [Google Scholar]; (d) Hayashi T, Yamamoto A and Ito Y, Tetrahedron Lett 29 (1988), pp. 99–102. [Google Scholar]
- 8.Matsushima Y, Onitsuka K, Kondo T, Mitsudo T and Takahashi S, J. Am. Chem. Soc 123 (2001), pp. 10406–10495. [DOI] [PubMed] [Google Scholar]
- 9.(a) Leitner A, Shu C and Hartwig JF, Proc. Natl. Acad. Sci. U.S.A 101 (2004), pp. 5830–5833; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kiener CA, Shu C, Incarvito C and Hartwig JF, J. Am. Chem. Soc 125 (2003), pp. 14272–14273; [DOI] [PubMed] [Google Scholar]; (c) Ohmura T and Hartwig JF, J. Am. Chem. Soc 124 (2002), pp. 15164–15165. [DOI] [PubMed] [Google Scholar]
- 10.(a) Welter C, Koch O, Lipowsky G and Helmchen G, Chem. Commun (2004), pp. 896–897; [DOI] [PubMed] [Google Scholar]; (b) Lipowsky G and Helmchen G, Chem. Commun (2004), pp. 116–117; [DOI] [PubMed] [Google Scholar]; (c) Bartels B, Garcia-Yebra C, Rominger F and Helmchen G, Eur. J. Inorg. Chem (2002), pp. 2569–2586. [Google Scholar]
- 11.(a) Evans PA, Robinson JE and Moffett KK, Org. Lett 3 (2001), pp. 3269–3271; [DOI] [PubMed] [Google Scholar]; (b) Evans PA and Robinson JE, Org. Lett 1 (1999), pp. 1929–1931; [DOI] [PubMed] [Google Scholar]; (c) Evans PA, Robinson JE and Nelson JD, J. Am. Chem. Soc 121 (1999), pp. 6761–6762. [Google Scholar]
- 12.Ashfeld BL, Miller KA and Martin SF, Org. Lett 6 (2004), pp. 1321–1324. [DOI] [PubMed] [Google Scholar]
- 13.(a) To our knowledge, there had not been a previous report of asymmetric Ni(0)-mediated allylic amination, prior to our recent findings (Ref. b). However, there were several studies of nonstereocontrolled allylic aminations using Ni(0):Bricout H, Carpentier J-F and Mortreux A, Tetrahedron 54 (1998), pp. 1073–1084; [Google Scholar]; (b) Bricout H, Carpentier J-F and Mortreux A, J. Chem. Soc., Chem. Commun (1995), pp. 1863–1864; [Google Scholar]; (c) For evidence of π-allyl-nickel intermediates, see:Moberg C, Tetrahedron Lett 21 (1980), pp. 4539–4542; [Google Scholar]; (d) Yamamoto T, Ishizu J and Yamamoto A, J. Am. Chem. Soc 103 (1981), pp. 6863–6869; [Google Scholar]; (e) Tolman CA, J. Am. Chem. Soc 92 (1970), pp. 6785–6790; [Google Scholar]; (f) For examples, of substrate-directed, regio- and stereoselective Ni(0)-mediated substitutions of allylic ethers with Grignard reagents, see:Didiuk MT, Morken JP and Hoveyda AH, Tetrahedron 54 (1998), pp. 1117–1130; [Google Scholar]; (g) Farthing CN and Kocovsky P, J. Am. Chem. Soc 120 (1998), pp. 6661–6672. [Google Scholar]
- 14.Trost BM and Dietsch TJ, J. Am. Chem. Soc 95 (1973), pp. 8200–8201. [Google Scholar]
- 15.Togni A, Rihs G, Pregosin PS and Ammann C, Helv. Chim. Acta 73 (1990), pp. 723–732. [Google Scholar]
- 16.(a) Mandal SK and Sigman MS, J. Org. Chem 68 (2003), pp. 7535–7537; [DOI] [PubMed] [Google Scholar]; (b) Mueller JA and Sigman MS, J. Am. Chem. Soc 125 (2003), pp. 7005–7013; [DOI] [PubMed] [Google Scholar]; (c) Mueller JA, Jensen DR and Sigman MS, J. Am. Chem. Soc 124 (2002), pp. 8202–8203; [DOI] [PubMed] [Google Scholar]; (d) Jensen DR, Pugsley JS and Sigman MS, J. Am. Chem. Soc 123 (2001), pp. 7475–7476. [DOI] [PubMed] [Google Scholar]
- 17.(a) Bagdanoff JT, Ferreira EM and Stoltz BM, Org. Lett 5 (2003), pp. 835–837; [DOI] [PubMed] [Google Scholar]; (b) Ferreira EM and Stoltz BM, J. Am. Chem. Soc 123 (2001), pp. 7725–7726. [DOI] [PubMed] [Google Scholar]
- 18.(a) Mueller TE, Berger M, Grosche M, Herdtweck E and Schmidtchen F, Organometallics 20 (2001), pp. 4384–4393; [Google Scholar]; (b) Bianchini C, Glendenning L, Zanobini F, Farnetti E, Graziani M and Nagy E, J. Mol. Catal. A: Chem 132 (1998), pp. 13–19; [Google Scholar]; (c) Bianchini C, Farnetti E, Glendenning L, Graziani M, Nardin G, Peruzzini M, Rocchini E and Zanobini F, Organometallics 14 (1995), pp. 1489–1502. [Google Scholar]
- 19.(a) Hayashi T, Yamamoto K and Kumada M, Tetrahedron Lett (1974), pp. 4405–4408; [Google Scholar]; (b) Hayashi T, Tajika M, Tamao K and Kumada M, J. Am. Chem. Soc 98 (1976), pp. 3718–3719; [Google Scholar]; (c) Fernandez-Galan R, Jalon FA, Manzano BR, Rodriguez-de la Fuente J, Vrahami M, Jedlicka B, Weissensteiner W and Jogl G, Organometallics 16 (1997), pp. 3758–3768; [Google Scholar]; (d) Cammidge AN and Crepy KVL, J. Chem. Soc., Chem. Commun (2000), pp. 1723–1724; [Google Scholar]; (e) Bringmann G, Hamm A and Schraut M, Org. Lett 5 (2003), pp. 2805–2808. [DOI] [PubMed] [Google Scholar]
- 20.Togni A, Breutel C, Schnyder A, Spindler F, Landert H and Tijanit A, J. Am. Chem. Soc 116 (1994), pp. 4062–4066. [Google Scholar]
- 21.(a) Harris MC, Huang X and Buchwald SL, Org. Lett 4 (2002), pp. 2885–2888; [DOI] [PubMed] [Google Scholar]; (b) Tomori H, Fox JM and Buchwald SL, J. Org. Chem 65 (2000), pp. 5334–5341; [DOI] [PubMed] [Google Scholar]; (c) Old DW, Wolfe JP and Buchwald SL, J. Am. Chem. Soc 120 (1998), pp. 9722–9723. [Google Scholar]
- 22.(a) For reviews, see:Helmchen G and Pfaltz A, Acc. Chem. Res 33 (2000), pp. 336–345; [DOI] [PubMed] [Google Scholar]; (b) Helmchen G, Kudis S, Sennhenn P and Steinhagen H, Pure. Appl. Chem 69 (1997), pp. 513–518; [Google Scholar]; (c) Pfaltz A, Acta Chem. Scand. B 50 (1996), pp. 189–194; [Google Scholar]; (d) Williams JMJ, Synlett (1996), pp. 705–710. [Google Scholar]
- 23.(a) von Matt P and Pfaltz A, Angew. Chem., Int. Ed 32 (1993), pp. 566–568; [Google Scholar]; (b) Sprinz J and Helmchen G, Tetrahedron Lett 34 (1993), pp. 1769–1772; [Google Scholar]; (c) Dawson GJ, Frost CG, Williams JMJ and Coote SJ, Tetrahedron Lett 34 (1993), pp. 3149–3150. [Google Scholar]
- 24.Schenkel LB and Ellman JA, Org. Lett 5 (2003), pp. 545–548. [DOI] [PubMed] [Google Scholar]
- 25.Typical ISES conditions: Assays were run in Ar purged, septum-sealed 1 cm-path length quartz cuvets that are nominally 1 mL in volume. The organic layer (500 μL) was layered upon a lower aqueous enzymatic ‘reporting’ layer (900 μL) such that the light beam of the UV/vis spectrometer would pass cleanly through the aqueous layer. This permits for the ready monitoring of NADH formation in the aqueous layer (see Figure 1 and Figure 3 for representative UV traces). Aqueous layer composition: 7.2 mM NAD+, 1.3 U of YADH, 0.12 U of YAlDH, 10 mM KCl in 50 mM sodium pyrophosphate buffer pH 8.6. Organic layer composition/preparation: To a solution of Ni(cod)2 and ligand (10 mol % each) in THF (300 μL), under Ar was added substrate 1 (100 μmol) dissolved in toluene (100 μL). LiHMDS (1 equiv) in hexane (100 μL) was added, and the vial briefiy vortexed, followed by layering onto the aqueous solution
- 26.Busacca C, Grossbach D, So RC, O’Brien EM and Spinelli EM, Org. Lett 5 (2003), pp. 595–598. [DOI] [PubMed] [Google Scholar]
- 27.Representative PHOX ligand synthesis. Step 1: Oxazoline installation via 11. A mixture of (1R,2S)-1-amino-2-indanol (200 mg, 1.34 mmol) and O-ethyl-2-fiuoro-benzimidate, tetrafiuoroborate salt (11, 350 mg, 1.37 mmol) in dry ethanol (10 mL), under Ar, was stirred at rt for 1 h, and then heated at refiux for 2 h. Following removal of the solvent, SiO2 chromatography (10% EtOAc–hexanes), provided the desired oxazoline (220 mg, 65%): 1H NMR (400 MHz, CDCl3) δ 3.35 (dd, J = 2, 18 Hz, 1H), 3.48 (dd, J = 11, 18 Hz, 1H), 5.45 (dt, J = 2, 7 Hz, 1H), 5.76 (d, J = 8 Hz, 1H), 7.07 (dd, J = 9, 11 Hz, 1H), 7.10 (t, J = 8 Hz, 1H), 7.24–7.28 (m, 3H), 7.34–7.40 (m, 1H), 7.57–7.59 (m, 1H), 7.83 (dt, J = 2, 8 Hz, 1H); HRMS calcd for C16H13NOF (M+H)+ 254.0981, found 254.0978. Step 2: Phosphine installation/synthesis of (3aR,8aS)-8f. To a solution of lithium di-(p-tolyl)-phosphide (1.1 mmol) in THF (3 mL) was added the oxazoline from Step 1 (200 mg, 0.8 mmol) in THF (1.5 mL) at rt. The reaction was complete within 10 min and the reaction mixture was cannulated into a separatory funnel and partitioned between H2O and CH2Cl2. The organic layer was dried (Na2SO4), filtered and evaporated. Flash column chromatography under an Ar stream (10% EtOAc–hexanes; degassed and satd with Ar before use) gave 8f (340 mg, 96%): (c 1.22, CHCl3); 1H NMR (500 MHz, CDCl3) δ 2.29 (s, 3H), 2.30 (s, 3 H), 3.05 (d, J = 18 Hz, 1H), 3.26 (dd, J = 7.18 Hz, 1H), 5.19 (app dt, J = 1.4, 18 Hz, 1H), 6.80–6.84 (m, 1H), 7.0–7.2 (m, 11H), 7.82–7.88 (m, 1H); 31P NMR (162 MHz, CDCl3) δ 6.47; HRMS calcd for C30H26NOP (M+H)+ 448.1830, found 448.1839
- 28.(a) Bernardi L, Gothelf AS, Hazell RG and Jorgensen KA, J. Org. Chem 68 (2003), pp. 2583–2591; [DOI] [PubMed] [Google Scholar]; (b) Carmona D, Lahoz FJ, Elipe S, Oro LA, Lamata MP, Viguri F, Sánchez F, Martínez S, Catviela C and López-Ram de Víu MP, Organometallics 21 (2002), pp. 5100–5114; [Google Scholar]; (c) Wiese B and Helmchen G, Tetrahedron Lett 39 (1998), pp. 5727–5730; [Google Scholar]; (d) Carmona D, Catviela C, Elipe S, Lahoz FJ, Lamata MP, López-Ram de Víu MP, Oro LA, Vega C and Viguri F, J. Chem. Soc., Chem. Commun 21 (1997), pp. 2351–2352. [Google Scholar]
- 29.(a) For a review on Ni(0)-mediated cyclizations leading to C–C bond installation, see:Montgomery J, Acc. Chem. Res 33 (2000), pp. 467–473; [DOI] [PubMed] [Google Scholar]; (b) Takimoto M, Nakamura Y, Kimura K and Mori M, J. Am. Chem. Soc 126 (2004), pp. 5956–5957. [DOI] [PubMed] [Google Scholar]
- 30.(a) Chung K-G, Miyake Y and Uemura S, J. Chem. Soc., Perkin Trans 1 (2000), pp. 2725–2729; [Google Scholar]; (b) Chung K-G, Miyake Y and Uemura S, J. Chem. Soc. Perkin Trans 1 (2000), pp. 15–18. [Google Scholar]
- 31.(a) Cho Y-H, Kina A, Shimada T and Hayashi T, J. Org. Chem 69 (2004), pp. 3811–3823; [DOI] [PubMed] [Google Scholar]; (b) Shimada T, Cho Y-H and Hayashi T, J. Am. Chem. Soc 124 (2002), pp. 13396–13397. [DOI] [PubMed] [Google Scholar]