Abstract
Background:
Up to 70% of people with multiple sclerosis (MS) experience cognitive impairment. Some remain cognitively intact despite advanced disease. Cognitive reserve (CR) theory postulates that individuals with higher levels of intellectual enrichment can tolerate more pathology than others before exhibiting cognitive impairment.
Methods:
Thirty-two individuals with early-phase relapsing-remitting MS with mild physical disability and disease duration less than 10 years and 32 controls were recruited. At baseline and after 3 years, participants completed neuropsychological tests evaluating several cognitive domains. The CR was assessed via a cognitive reserve index (CRI) using educational levels and North American Adult Reading Test scores. Change in cognition was assessed using a reliable change index.
Results:
At baseline, people with MS performed worse than controls on visual memory. There were no significant group differences on information processing speed, learning, language, and executive functions. Most cognitive domains showed no change over time, and CRI was not a significant predictor in the regression model.
Conclusions:
People with MS performed worse on memory tasks at baseline compared with controls. Cognitive change differed between people with MS and controls in executive functions. Although people with MS and controls improved over time, beyond practice effects, people with MS improved less than controls. Overall, no cognitive deterioration was noted over time, and CR did not predict change in cognition. Sample homogeneity in terms of disease stage and CR may explain these findings.
Cognitive impairment is a prevalent concern in multiple sclerosis (MS), with approximately 40% to 70% of people with MS being affected.1,2 The frequency of cognitive impairment in relapsing-remitting MS (RRMS) is lower than that in the MS group as a whole, averaging approximately 30%.3–5 Frequently affected cognitive domains include memory (verbal and working) and information processing speed (IPS), executive functions, attention, abstract/conceptual reasoning, and visuospatial skills.6–9 Intuitively, cognitive impairment is expected to be related to the extent of pathology and/or atrophy. For example, a recent review of 39 studies reports a moderate-to-strong correlation between IPS decline and magnetic resonance imaging measures (T2-weighted lesion volume and atrophy).10 However, there seems to be a discrepancy between cognitive impairment and disease burden, with only one-third to one-half of the variance being explained.11
Emerging research has focused on understanding why two people with MS with the same degree of pathology demonstrate different degrees of cognitive impact. One possible explanation proposed to account for this discrepancy between cognitive impairment and imaging parameters is cognitive reserve (CR) theory. The concept of CR was initially postulated in reference to Alzheimer disease, where autopsy revealed advanced brain pathology despite intact cognition in elders.12,13 The CR theory posits that individuals with higher levels of intelligence are able to process tasks more efficiently than their counterparts with lower CR; thus, they can sustain greater brain damage before demonstrating cognitive decline.12 Similar findings were reported in stroke,14 traumatic brain injury,15 Parkinson disease,16 white matter lesions,17 and recently MS.18–29 Theoretically then, CR is one factor that may help explain why such a discrepancy between cognitive functioning and disease burden exists.
Cognitive reserve is quantified indirectly using estimates of premorbid intellectual and social enrichment, with years of education, verbal intellectual abilities (as measured by the North American Adult Reading Test [NAART]), as well as involvement in leisure activities.18–30 Despite the apparent relevance of the CR theory, longitudinal studies investigating the effects of reserve on cognitive outcomes are scarce. To our knowledge, there have been only four longitudinal studies investigating CR in MS. A 3-year study and a 4.5-year longitudinal study showed that higher CR (estimated by education and vocabulary) protected against cognitive decline.25,28 It may be relevant to note that the sample at baseline consisted of all types of MS (clinically isolated syndrome, RRMS, secondary progressive MS, and primary progressive MS), with 30% of the sample being primary progressive MS. In contrast, a 1.6-year longitudinal study showed that while at baseline an interaction between a cognitive reserve index (CRI; educational level, premorbid IQ, and leisure activities) and cortical atrophy predicted cognitive performance, at follow-up, the CRI was not a predictor of longitudinal change in cognition.30 Another study showed that higher CR (intellectual enrichment quantified as years of education and performance on the NAART) protected against cognitive decline in cognitive efficiency and memory over approximately 5 years.29
Further longitudinal research is needed to conclusively determine whether CR moderates cognitive decline in individuals with MS as the disease progresses. The primary aim of this study was to longitudinally evaluate cognition over a 3-year period. When analyses revealed no change in cognition, we decided post hoc to investigate whether CR may explain the longitudinal stability in cognition observed in the present sample.
Methods
Study Design and Patients
Thirty-two individuals with early-phase RRMS31 were recruited from the MS Clinic of The Ottawa Hospital. During regular clinic visits, individuals with MS were asked to participate in a research study. Those who indicated an interest were later contacted by a research assistant. Only patients with a disease duration of 10 years or less and a level of physical disability less than or equal to 5.5 as indicated by the Expanded Disability Status Scale32 were enrolled. All the participants (including controls) were aged 18 to 65 years. Moreover, to be included in the study, any signs and symptoms attributable to an MS exacerbation had to begin improving at least 28 days before testing. Thirty-two age-, education-, and IQ-matched controls were recruited by word of mouth from the community and via newspaper and website advertisements. All the individuals were fluent in English. Participants had not participated in any cognitive study within 6 months of beginning the present study. All the participants were free of previous neurologic, medical, or psychiatric illnesses (besides MS and depression) that may have impaired cognition. All the participants provided written informed consent. This study was approved by the Ottawa Health Science Network Research Ethics Board and was performed in accordance with the Declaration of Helsinki and the International Conference for Harmonisation (ICH) Harmonised Tripartite Guideline, Guideline for Good Clinical Practice.
Estimates of CR
In the present study, a CRI was calculated similar to in a previous study.30 It included two commonly used surrogate measures of CR: the NAART and years of education. The two variables were combined as follows: scores on each variable were normalized through a z score, using the controls' sample mean and SD, and then the mean value of the two z scores was computed for each person with MS and used as a CRI.
Assessment of Cognitive Functioning
Overview
Enrolled participants completed a comprehensive battery of neuropsychological tests assessing cognitive functioning at two visits, approximately 3 years apart. All 64 individuals (32 people with MS and 32 controls) completed the full neuropsychological battery at both baseline and follow-up. Four different cognitive domains were evaluated: IPS, language, learning and memory, and executive functions. The testing was conducted by a research assistant trained by two neuropsychologists (L.A.S.W and a nonauthor).
Information Processing Speed
The IPS was assessed using the Symbol Digit Modalities Test33 and the Paced Auditory Serial Addition Test.34,35
Language
Language was assessed using measures of phonemic (FAS test) and semantic (animal naming) verbal fluency.36
Learning and Memory
Learning and memory were assessed using the Brief Visuospatial Memory Test–Revised (BVMT-R)37 and the List Learning task from the Learning and Memory Battery (LAMB).38 The variables of interest for the BVMT-R and the LAMB were the total recall raw score (BVMT-R), free + cued recall (LAMB) (ie, learning variables), the delayed recall scores (BVMT-R), and free + cued recall retention score (LAMB) (ie, memory).
Executive Functions
Two executive tasks were administered: the Delis-Kaplan Executive Function System (D-KEFS)39 Sorting Test and the D-KEFS Tower Test. The variable of interest for the Sorting Test was the total number of confirmed correct sorts. The variable of interest for the Tower Test was the total achievement score.
Assessment of Depression and Fatigue
Fatigue was assessed using the Fatigue Impact Scale, a self-report scale that measures the effects that fatigue has on behavior.40 Depression was assessed using the Beck Depression Inventory–Fast Screen for Medical Patients, a brief self-report questionnaire that measures affective state but does not include questions related to vegetative signs.41
Evolution in Cognitive Change
Changes in cognition from baseline to follow-up were assessed on each test using a reliable change index (RCI), which took into account measurement error and practice effects.30 In accordance with previous studies, RCI was defined as ([X2 − X1] − [M2 − M1])/SED, where X1 and X2 are the observed test scores at baseline and follow-up, respectively; M1 and M2 are the controls' mean test scores at baseline and follow-up, respectively; and SED is the SD of the controls' mean observed difference score.30
Statistical Analyses
A statistical software package (IBM SPSS Statistics for Windows, version 24.0; IBM Corp, Armonk, NY) was used for all data analyses. A significance level of α ≤ .05 was used throughout. Group differences at baseline and group differences in RCI were investigated using one-way analysis of variance. Linear regression was used to investigate whether the CRI predicts RCI.
Results
Demographic and Clinical Data
Most of the individuals in the present study (68.8%) were taking a disease-modifying medication and were, therefore, receiving early treatment intervention. Demographics, disease characteristics (for people with MS), and group differences are shown in Table 1.
Table 1.
Demographic and disease characteristics and group differences
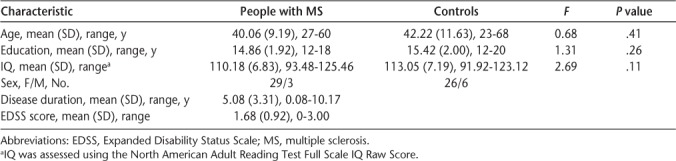
There was no significant difference between people with MS and controls in age, education, and IQ scores, and a χ2 analysis revealed no significant difference in sex distribution between the two groups (χ2 = 1.16, P = .28) (Table 1).
Cognitive Performance
Table 2 illustrates performance data at baseline for people with MS and controls, and any significant group differences. At baseline, people with MS performed worse than controls on visual memory, and no significant group differences were observed in other tests. Covarying fatigue and depression did not significantly change the results.
Table 2.
Group differences on cognitive functions at baseline
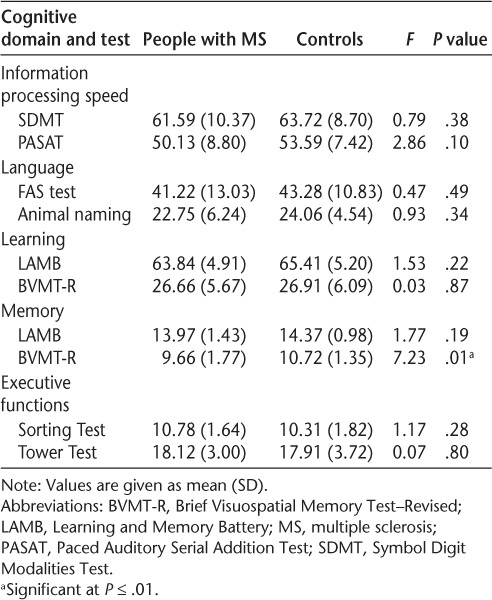
Table 3 illustrates changes in performance (calculated as the RCI between follow-up and baseline scores) for people with MS and controls and any significant group differences. The results are presented for each cognitive test administered from the following domains: IPS, language, learning, memory, and executive functions. Note that when between-group analyses were re-run using simple change scores (ie, not RCI) the results did not differ from those of the original analyses (ie, using RCI scores).
Table 3.
Group differences on reliable change index
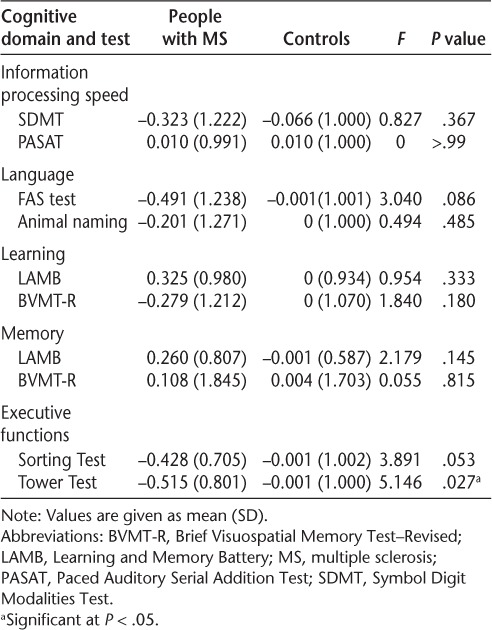
Over time, the groups differed significantly regarding the degree of change on the Tower Test: RCIMS = −0.515 (0.801); RCIcontrol = −0.001 (1.000). Despite the change in cognition being significantly different between groups, a t test showed no significant difference between baseline and follow-up performance in people with MS (t = 1.81, P = .08). An RCI of 0 indicates no change. Results herein indicate that although the MS and control groups improved over time, the MS group improved less than the control group. No other significant group differences were observed.
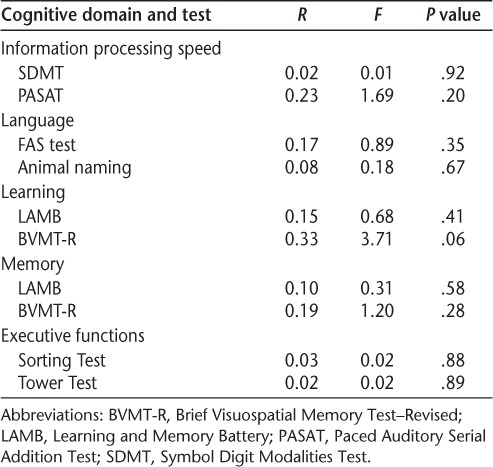
Table 4 shows the regression analysis investigating whether CRI predicts change in cognition in the present sample. The CRI did not predict the (nonsignificant) change in cognition in people with MS in any of the cognitive tests investigated.
Table 4.
Regression analysis of cognitive reserve index as a predictor of change in cognition in patients with multiple sclerosis
Discussion
We report on a longitudinal study evaluating cognition over time in an early-phase RRMS population. Overall, we found very little cognitive impairment, with people with MS performing worse than controls on only a task evaluating visual memory at baseline, which is consistent with past literature suggesting that this domain is among the first to show decline in MS.7 In contrast, tests evaluating IPS, language, learning, and executive functions showed no difference in performance between people with MS and controls. Similarly, there was little evidence of change in cognition over time. Specifically, there was no evidence of deterioration during the 3-year study interval. In fact, the only group difference in cognitive change identified was in the domain of executive functions (specifically, the D-KEFS Tower Test). Specifically, although both the MS and control groups improved, the improvement in the MS group was less than that in the control group. Although the longitudinal study was not originally designed to investigate CR, this was evaluated in a post hoc analysis to determine whether CR was a factor in the lack of change. Given the evidence of cognitive stability, it is not surprising that the CRI did not have a mediating role in the subtle nonsignificant change in cognition.
There is a scarcity of longitudinal studies examining CR in MS. With the present study, there are just five longitudinal studies in all. Three studies show that CR protected against decline in cognitive processing speed,25,28,29 with one of these same studies also showing a similar effect on memory.28 In contrast, the present study and an additional 1.6-year longitudinal study also reported that CR did not predict longitudinal cognitive performance.30
The present study suggests that other factors beyond CR, such as characteristics of the present sample, likely contributed to the stability of cognition in this sample. Specifically, the present sample was restricted to patients in the early phase of RRMS (mean [SD] disease duration, 5.08 [3.31] years) and with minimal disability (mean [SD] Expanded Disability Status Scale score = 1.68 [0.92]). Although cognitive impairment can present in individuals with RRMS who are early in their disease, impairment is typically more severe in individuals with a progressive subtype of MS.3
Oftentimes studies enrolled individuals with advanced types of MS or a mixture of varying subtypes of MS,18,21,23,25,26 suggesting a greater disease burden overall in these samples. According to CR theories, those with higher levels of CR are better able to compensate in the face of accumulated pathology. Although we did not complete neuroimaging with the present sample, we can presume a lower level of disease burden compared with studies with all subtypes because disease burden is often less in RRMS samples.42 As such, with less pathology there is less need for compensation. In other words, the present sample does not need to rely on their CR given that they do not have sufficient pathology to warrant high levels of compensation. Moreover, 68.8% of people with MS in the present study were taking a disease-modifying medication, receiving early treatment intervention, which might further explain the longitudinal stability of cognition in this sample.43
Aside from being in the early phase of the disease, the present MS sample had a high CR as assessed by years of education, with a minimum of 12 years of education (mean [SD]: 14.86 [1.92] years), and relatively high NAART scores (mean [SD]: 110.18 [6.83]). Thus, this study lacked the variability in levels of CR observed in other studies. For example, a study investigating whether individual differences in CR (estimated by NAART score and years of education) protect against the progression of cognitive dysfunction in MS showed that people with MS with high CR (>14 years of education) showed no significant change in IPS between baseline and 1.6 years' follow-up, whereas people with MS with low CR (<14 years of education) had significant decline.29 The limited variability in CR in the present sample prevented us from dividing the sample into high versus low CR; in other words, this sample generally corresponds to the high CR sample of other studies. The fact that individuals in the present study had high levels of CR may have accounted for why they demonstrated relative preservation of cognitive functioning over time.
Finally, in the present sample, people with MS were recruited on a volunteer basis. This may have introduced a selection bias favoring highly educated individuals who value the potential scientific contribution of their participation in research studies. Those who have concerns about their cognition may be less likely to volunteer to avoid being confronted with expected deficits. This contrasts with other studies in which individuals with MS undergo cognitive testing as part of routine medical care. For example, in one previous longitudinal study evaluating CR in MS, patients were undergoing testing as part of standard of care,30 and in another, patients entered the study for one of three reasons: participation in research (57%), routine monitoring of cognitive function (11%), or referral for evaluation of a specified management problem related to suspected cognitive impairment (32%).29 These recruitment methods would yield a more representative sample of the true spectrum of cognitive involvement than those used herein.
There are several limitations in the present study. It is not known whether patients were exposed to additional neuropsychological testing outside the study protocol. As such, individuals may have been exposed to some of these tests in the past. Nonetheless, previous exposure is unlikely because these tests were not a part of the standard protocol at our clinic at the time the study was conducted (beginning in 2011). Regarding data analysis, the problem of multiple comparisons was not directly addressed. Given the nonsignificance, running multiple comparisons would have yielded similar results. We acknowledge that this is a limitation of the study and that the one significant result (BVMT-R) may, in fact, be a result of capitalizing on chance. The present sample does not represent the general MS population in previous studies regarding the variability typically observed in levels of CR. In other words, longitudinal stability of cognition may be due to the homogeneity of the sample, because most participants had high CR as assessed by years of education and NAART scores. Second, the sample size for the study was small (32 with RRMS and 32 controls), which may result in the study being underpowered to detect subtle change.
Overall, the study suggests that in the early phase of MS there are cognitive differences between people with MS and controls in visual memory. Considering that the present sample was limited to individuals with early-phase RRMS, it seems that some cognitive domains are affected by MS sooner than other domains. Cognitive reserve (as measured in the present study) did not predict longitudinal stability of cognition over time. This may be due to the characteristics of the present sample. Indeed, the fact that all the individuals in this study met previously defined average-to-high reserve suggests that this sample was perhaps protected from cognitive decline as a result. Future prospective studies should replicate this study in a sample with a higher variability in years of education and should include all subtypes of MS.
PRACTICE POINTS
Up to 70% of people with MS experience cognitive impairment; however, there seems to be a discrepancy between burden of disease in the brain and cognitive impairment. Some researchers have suggested that this discrepancy can be partially explained by the cognitive reserve (CR) theory, which posits that persons with higher levels of intellectual enrichment are better able cope with cerebral pathology than are individuals with a lower CR.
The present study saw little change in cognition in a 3-year period, with no predictive value of CR. Characteristics of the sample (ie, early-phase relapsing-remitting MS, almost uniformly high CR levels) may account for why CR did not influence outcome.
Findings may suggest that people with MS who are early in the disease course, who are taking disease-modifying therapies, and who have high CR may be less likely to demonstrate early cognitive deterioration. Further study is warranted in patients with early-phase MS using samples with more variable CR levels.
Acknowledgments
The authors thank the research participants for their time and effort; their contributions are invaluable. The authors also thank Dr. Kasia Muldner for support with statistical analyses.
Financial Disclosures
The authors declare no conflicts of interest.
Funding/Support
This study was funded by the Multiple Sclerosis Society of Canada.
Prior Presentation
Aspects of this study have been presented in abstract form at the Consortium of Multiple Sclerosis Centers (CMSC) Annual Meeting; June 1–4, 2016; National Harbor, MD.
References
- 1.Deluca GC, Yates RL, Beale H, Morrow SA. Cognitive impairment in multiple sclerosis: clinical, radiologic and pathologic insights. Brain Pathol. 2014;25:79–98. doi: 10.1111/bpa.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romero K, Shammi P, Feinstein A. Neurologists' accuracy in predicting cognitive impairment in multiple sclerosis. Mult Scler Relat Disord. 2015;4:291–295. doi: 10.1016/j.msard.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Potagas C, Giogkaraki E, Koutsis G et al. Cognitive impairment in different MS subtypes and clinically isolated syndromes. J Neurol Sci. 2008;267:100–106. doi: 10.1016/j.jns.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Patti F, Amato MP, Trojano M et al. Cognitive impairment and its relation with disease measures in mildly disabled patients with relapsing-remitting multiple sclerosis: baseline results from the Cognitive Impairment in Multiple Sclerosis (COGIMUS) study. Mult Scler. 2009;15:779–788. doi: 10.1177/1352458509105544. [DOI] [PubMed] [Google Scholar]
- 5.Sundgren M, Maurex L, Wahlin Å, Piehl F, Brismar T. Cognitive impairment has a strong relation to nonsomatic symptoms of depression in relapsing-remitting multiple sclerosis. Arch Clin Neuropsychol. 2013;4 doi: 10.1093/arclin/acs113. acs113. [DOI] [PubMed] [Google Scholar]
- 6.Martin MYP, Rio PED, Platas MG, Sosa AJ. Cognitive status in patients with multiple sclerosis in Lanzarote. Neuropsychiatr Dis Treat. 2016;12:1553–1559. doi: 10.2147/NDT.S105805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Planche V, Gibelin M, Cregut D et al. Cognitive impairment in a population-based study of patients with multiple sclerosis: differences between late relapsing-remitting, secondary progressive and primary progressive multiple sclerosis. Eur J Neurol. 2015;23:282–289. doi: 10.1111/ene.12715. [DOI] [PubMed] [Google Scholar]
- 8.Sacco R, Bisecco A, Corbo D et al. Cognitive impairment and memory disorders in relapsing-remitting multiple sclerosis: the role of white matter, gray matter and hippocampus. J Neurol. 2015;262:1691–1697. doi: 10.1007/s00415-015-7763-y. [DOI] [PubMed] [Google Scholar]
- 9.Rao SM. Cognitive function in patients with multiple sclerosis: impairment and treatment. Int J MS Care. 2004;6:9–22. [Google Scholar]
- 10.Rao SM, Martin AL, Huelin R et al. Correlations between MRI and information processing speed in MS: a meta-analysis. Mult Scler Int. 2014;2014:1–9. doi: 10.1155/2014/975803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benedict RHB, Zivadinov R. Risk factors for and management of cognitive dysfunction in multiple sclerosis. Nat Rev Neurol. 2011;7:332–342. doi: 10.1038/nrneurol.2011.61. [DOI] [PubMed] [Google Scholar]
- 12.Stern Y. What is cognitive reserve? theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- 13.Katzman R, Aronson M, Fuld P et al. Development of dementing illnesses in an 80-year-old volunteer cohort. Ann Neurol. 1989;25:317–324. doi: 10.1002/ana.410250402. [DOI] [PubMed] [Google Scholar]
- 14.Elkins JS, Longstreth WT, Manolio TA et al. Education and the cognitive decline associated with MRI-defined brain infarct. Neurology. 2006;67:435–440. doi: 10.1212/01.wnl.0000228246.89109.98. [DOI] [PubMed] [Google Scholar]
- 15.Kesler SR, Adams HF, Blasey CM, Bigler ED. Premorbid intellectual functioning, education, and brain size in traumatic brain injury: an investigation of the cognitive reserve hypothesis. Appl Neuropsychol. 2003;10:153–162. doi: 10.1207/S15324826AN1003_04. [DOI] [PubMed] [Google Scholar]
- 16.Poletti M, Emre M, Bonuccelli U. Mild cognitive impairment and cognitive reserve in Parkinson's disease. Parkinsonism Relat Disord. 2011;17:579–586. doi: 10.1016/j.parkreldis.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Dufouil C, Alperovitch A, Tzourio C. Influence of education on the relationship between white matter lesions and cognition. Neurology. 2003;60:831–836. doi: 10.1212/01.wnl.0000049456.33231.96. [DOI] [PubMed] [Google Scholar]
- 18.Sumowski JF, Chiaravalloti N, Leavitt VM, Deluca J. Cognitive reserve in secondary progressive multiple sclerosis. Mult Scler. 2012;18:1454–1458. doi: 10.1177/1352458512440205. [DOI] [PubMed] [Google Scholar]
- 19.Booth AJ, Rodgers JD, Schwartz CE et al. Active cognitive reserve influences the regional atrophy to cognition link in multiple sclerosis. J Int Neuropsychol Soc. 2013;19:1128–1133. doi: 10.1017/S1355617713001082. [DOI] [PubMed] [Google Scholar]
- 20.Feinstein A, Lapshin H, O'Connor P, Lanctôt KL. Sub-threshold cognitive impairment in multiple sclerosis: the association with cognitive reserve. J Neurol. 2013;260:2256–2261. doi: 10.1007/s00415-013-6952-9. [DOI] [PubMed] [Google Scholar]
- 21.Sumowski JF, Rocca MA, Leavitt VM et al. Brain reserve and cognitive reserve in multiple sclerosis: what you've got and how you use it. Neurology. 2013;80:2186–2193. doi: 10.1212/WNL.0b013e318296e98b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinter D, Sumowski J, Deluca J et al. Higher education moderates the effect of T2 lesion load and third ventricle width on cognition in multiple sclerosis. PLoS One. 2014;9:e87567. doi: 10.1371/journal.pone.0087567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silva AMD, Cavaco S, Moreira I et al. Cognitive reserve in multiple sclerosis: protective effects of education. Mult Scler. 2015;21:1312–1321. doi: 10.1177/1352458515581874. [DOI] [PubMed] [Google Scholar]
- 24.Luerding R, Gebel S, Gebel E-M et al. Influence of formal education on cognitive reserve in patients with multiple sclerosis. Front Neurol. 2016;7:46. doi: 10.3389/fneur.2016.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Modica CM, Bergsland N, Dwyer MG et al. Cognitive reserve moderates the impact of subcortical gray matter atrophy on neuropsychological status in multiple sclerosis. Mult Scler. 2015;22:36–42. doi: 10.1177/1352458515579443. [DOI] [PubMed] [Google Scholar]
- 26.Sumowski JF, Leavitt VM. Cognitive reserve in multiple sclerosis. Mult Scler. 2013;19:1122–1127. doi: 10.1177/1352458513498834. [DOI] [PubMed] [Google Scholar]
- 27.Sumowski JF, Chiaravalloti N, DeLuca J. Cognitive reserve protects against cognitive dysfunction in multiple sclerosis. J Clin Exp Neuropsychol. 2009;31:913–926. doi: 10.1080/13803390902740643. [DOI] [PubMed] [Google Scholar]
- 28.Sumowski JF, Rocca MA, Leavitt VM et al. Brain reserve and cognitive reserve protect against cognitive decline over 4.5 years in MS. Neurology. 2014;82:1776–1783. doi: 10.1212/WNL.0000000000000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benedict RH, Morrow SA, Guttman BW et al. Cognitive reserve moderates decline in information processing speed in multiple sclerosis patients. J Int Neuropsychol Soc. 2010;16:829–835. doi: 10.1017/S1355617710000688. [DOI] [PubMed] [Google Scholar]
- 30.Amato MP, Razzolini L, Goretti B et al. Cognitive reserve and cortical atrophy in multiple sclerosis: a longitudinal study. Neurology. 2013;80:1728–1733. doi: 10.1212/WNL.0b013e3182918c6f. [DOI] [PubMed] [Google Scholar]
- 31.Mcdonald WI, Compston A, Edan G et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 32.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 33.Smith A. Symbol Digit Modalities Test. Los Angeles, CA: Western Psychological Services; 1991. [Google Scholar]
- 34.Gronwall D. Paced auditory serial-addition task: a measure of recovery from concussion. Percept Mot Skills. 1977;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- 35.Rao SM, Leo GJ, Haughton VM, St Aubin-Faubert P, Bernardin L. Correlation of magnetic resonance imaging with neuropsychological testing in multiple sclerosis. Neurology. 1989;39(2 pt 1):161–166. doi: 10.1212/wnl.39.2.161. [DOI] [PubMed] [Google Scholar]
- 36.Tombaugh T. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. 1999;14:167–177. [PubMed] [Google Scholar]
- 37.Benedict RH. The Brief Visuospatial Memory Test–Revised. Lutz, FL: Psychological Assessment Resources; 1997. [Google Scholar]
- 38.Tombaugh T, Schmidt J. The Learning and Memory Battery (LAMB): development and standardization. Psychol Assess. 1992;4:193–206. [Google Scholar]
- 39.Delis DC, Kaplan E, Dramer JA. Delis-Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 40.Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis. 1994;18(suppl 1):S79–S83. doi: 10.1093/clinids/18.supplement_1.s79. [DOI] [PubMed] [Google Scholar]
- 41.Brown GK, Beck AT, Steer RA. BDI—Fast Screen for Medical Patients Manual. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 42.Benedict RH, Bruce JM, Dwyer MG et al. Neocortical atrophy, third ventricular width, and cognitive dysfunction in multiple sclerosis. Arch Neurol. 2006;63:1301–1306. doi: 10.1001/archneur.63.9.1301. [DOI] [PubMed] [Google Scholar]
- 43.Amato MP, Langdon D, Montalban X et al. Treatment of cognitive impairment in multiple sclerosis: position paper. J Neurol. 2013;260:1452–1468. doi: 10.1007/s00415-012-6678-0. [DOI] [PubMed] [Google Scholar]


