Abstract
Background:
Sexual dysfunction is a common symptom of multiple sclerosis (MS) that often goes unreported by both the patient and the clinician. Sexual dysfunction can affect a person's mood, relationships, daily functioning, and quality of life. Gaining a better understanding of the prevalence and nature of sexual dysfunction in individuals with MS would not only help identify patients with this problem but also determine contributing factors, which can inform treatment alternatives available to the patient.
Methods:
Patients with a diagnosis of MS (n = 162) completed the Multiple Sclerosis Intimacy and Sexuality Questionnaire-19 during their neurology appointments at the Mellen Center for Multiple Sclerosis at Cleveland Clinic. These data were merged with Knowledge Program data collected as part of standard practice and included measures of mood, disability, and quality of life.
Results:
Sexual dysfunction was present in 64.2% of the clinic sample. Patients with sexual dysfunction had significantly worse average MS-related disability and depressive symptom scores.
Conclusions:
Sexual dysfunction is highly prevalent in the MS clinic sample. Assessment and treatment of depression may serve as a starting point for intervention in patients with MS who experience sexual dysfunction. Identifying individuals who are at risk for sexual dysfunction concerns may help with clinician and patient burden in terms of routine assessment of this symptom.
Sexual dysfunction is among the myriad symptoms of multiple sclerosis (MS).1 Although sexual dysfunction is a common symptom of MS, it often goes unreported, underassessed, and, thus, undertreated.2 Sexual dysfunction can affect a person's mood, daily functioning, relationships, and quality of life (QOL). Although this literature is continually growing, studies have shown an estimated prevalence of 40% to 80% in women and 50% to 90% in men; this includes several types of sexual dysfunction disorders (ie, disorders of desire, arousal, orgasm, and pain).3 Some of the most common complaints observed in this population include erectile dysfunction, loss of sexual confidence, orgasmic dysfunction, and genital numbness in men and loss of libido, inadequate vaginal lubrication, and genital numbness in women.3
Although the etiology of sexual dysfunction in MS is still not entirely understood, Foley and Iverson4 developed a conceptual model for sexual dysfunction in MS, which describes it as having different levels of contributing factors in terms of primary, secondary, and tertiary causes. For example, primary causes are those generally associated with cortex and spinal cord lesions and can include paresthesia, sensory numbness, loss of libido, decreased lubrication, and erectile dysfunction. Certain medications can have an effect on primary causes as well. Secondary causes are associated with MS symptoms such as fatigue, spasticity, pain, bladder and bowel dysfunction, and cognitive difficulties. Finally, tertiary causes are those stemming from psychosocial factors and can include changes in social roles, mood disorders, demoralization, interpersonal difficulties, body image concerns, fear of rejection, and others. Of note, most patients will likely present with a combination of primary, secondary, and tertiary levels of contributing factors.
Despite the prevalence of sexual dysfunction in MS, it is still a symptom that is not routinely assessed by the clinician.5 The Multiple Sclerosis Intimacy and Sexuality Questionnaire-19 (MSISQ-19) is a 19-item self-report tool that measures sexual dysfunction stemming from the primary, secondary, or tertiary domains and has been validated in the MS population.6 An advantage of the MSISQ-19 is that it is quick, taking approximately 2 minutes to complete, and can be done before any patient visit. Although there are several studies that have incorporated the MSISQ-19 as a primary measure of sexual dysfunction in MS, the research is limited in terms of closely examining each level of contributing factors. Moreover, identifying what specific factors are more highly associated with sexual dysfunction can potentially reduce clinician and patient burden in terms of assessing directly for sexual dysfunction. In addition, gaining a better understanding of sexual dysfunction and contributing factors can also potentially aid in the design of efficacious treatment alternatives and, thus, improve QOL in individuals with MS. The objectives of the present study were 1) to survey the prevalence of sexual dysfunction in patients with MS in a clinic sample and 2) to further examine factors associated with sexual dysfunction, including demographic variables (ie, age, sex, race, median income, and marital status), mood, QOL, and disability.
Methods
Participants
Participants were recruited from a clinic sample at the Mellen Center for Multiple Sclerosis at Cleveland Clinic (Cleveland, OH). Participants who fit the study criteria and verbally expressed interest in participation completed the MSISQ-19 during their established neurology appointment. The survey was introduced by an advanced practice clinician and was completed by the participant in a private consultation room. Individuals aged 18 to 65 years with a diagnosis of MS who could speak, read, and write in English were included in the study. Individuals who were physically unable to complete the form on their own, those living in an assisted living facility, patients with severe cognitive impairment, and non–English-speaking patients were excluded from participation. This study was approved by the institutional review board at the Cleveland Clinic Foundation.
Measures
As part of standard practice, participants also completed other measures, including the MS Performance Scales, the Patient Health Questionnaire-9 (PHQ-9), and the Patient-Reported Outcomes Measurement Information System 10 (PROMIS-10), and these data were available through the Knowledge Program Data Registry at Cleveland Clinic.7 The Knowledge Program data are collected via systematic administration of questionnaires to patients at each appointment, which helps track patients' physical and emotional functioning over time. The MS Performance Scales is an instrument that measures disability in bladder control, cognitive functioning, fatigue, hand function, mobility, sensory symptoms, spasticity, and vision in MS.8 The PHQ-9 has been shown to be a reliable measure of depression severity.9 In addition, a medical record review was conducted to identify clinical covariates, including demographic information, current or past use of disease-modifying therapy, disease duration, present disease course, behavioral medicine consultations, and use of antidepressant medications.
Statistical Analyses
Sexual dysfunction was defined as a score of 4 or 5 on any MSISQ-19 item. Descriptive statistics were calculated for the entire cohort and stratified by the presence of any sexual dysfunction. Health status measure data were collected on the day of MSISQ-19 completion. If the data were not available on the day of completion, the data from the nearest visit within 210 days were substituted. This period was chosen because many patients had 6-month follow-ups with health status measure collection. Association between other measures and MSISQ-19 was assessed using multivariate linear regression models. Each model included the demographic covariates of age at time of completion (years), sex, race (white vs. nonwhite), marital status (married vs. not married), and median income by zip code (US dollars) as well as clinical covariates, including current or past use of disease-modifying therapy, disease duration (years), and present disease course categorized as progressive (clinically isolated syndrome, primary progressive, secondary progressive) or relapsing-remitting. Each subscale model had additional specific predictors of interest: primary included Timed 25-Foot Walk test score and PROMIS-10 item 6 inquiring about daily activities; secondary used PROMIS-10 items 7 and 8 detailing pain and fatigue; and tertiary included PHQ-9 score, PROMIS-10 item 2 asking about QOL, use of antidepressant drugs, and behavioral consultation reported in the previous year. Higher scores on the PROMIS-10 fatigue item represent less fatigue, whereas a higher score on the pain item indicates greater pain. The MS Performance Scales was also considered but ultimately excluded owing to a large proportion of missing data (>40%). All the analyses were computed using R, version 3.3.1 (R Core Team, R Foundation for Statistical Computing, 2016). A P < .05 was considered significant.
Results
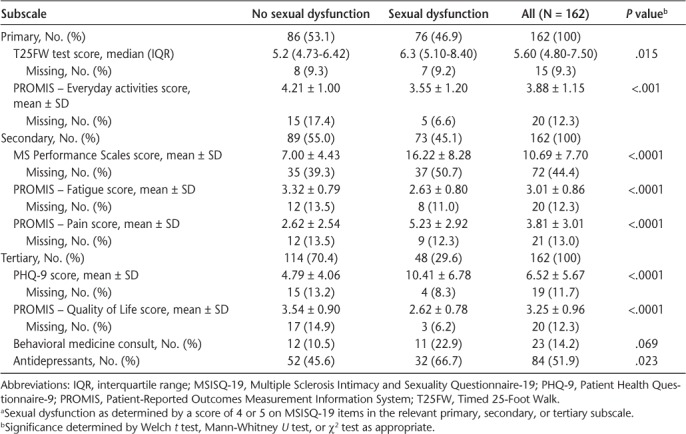
Overall, 162 patients completed the MSISQ-19. Demographic and clinical characteristics are given in Table 1. Sexual dysfunction was present in 104 of the patients (64.2%). The cohort had a mean ± SD age of 45.6 ± 9.5 years and was majority female (n = 126 [77.8%]), white race (n = 132 [81.5%]), and married (n = 111 [68.5%]). Patients with and without sexual dysfunction did not differ in median income, age, sex, race, or marital status. Use of disease-modifying therapy and present disease course also did not differ significantly. The MS Performance Scales, PHQ-9, Timed 25-Foot Walk test, and PROMIS QOL, everyday activities, fatigue, and pain scores were significantly worse in those with sexual dysfunction. Use of antidepressant medications and history of behavioral consultations were significantly higher in those with sexual dysfunction (Table 2). Missing data for the demographic and clinical variables ranged from 0% to 3.1% except for disease duration (22.2%). One individual had a confirmed diagnosis of MS but no clinical course information. Predictors of interest (Table 2) had moderate rates of missingness, ranging from 9.3% to 13%. Overall, 75.3% of patients were not missing any covariates and 87.8% were missing only one.
Table 1.
Demographic and clinical characteristics stratified by sexual dysfunction a
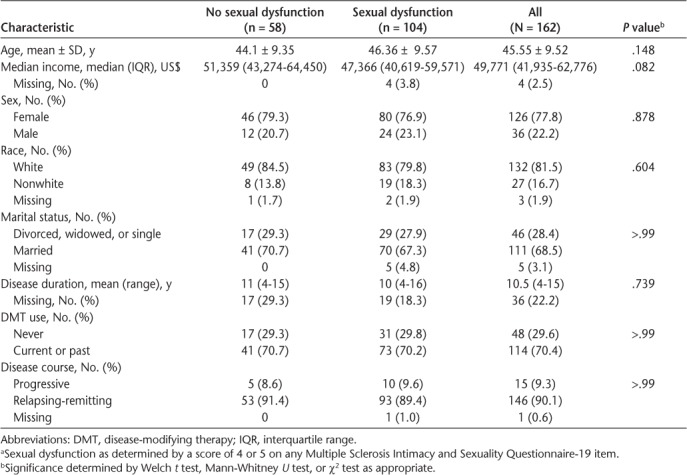
Table 2.
Health status measures stratified by sexual dysfunction in relevant MSISQ-19 subscales a
Multiple imputation was used to create 30 imputed data sets for multivariate analysis.10 The imputation model included MSISQ-19 items, demographic and clinical variables, and predictors of interest. Calculations were performed using the mice: Multivariate Imputation by Chained Equations in R 2.25 package.11 Continuous variables were imputed using predictive mean matching, and binary variables were imputed using logistic regression. Data were assumed missing-at-random based on visual inspection and sensitivity analyses. Linear regression models were computed in each imputed data set separately, with results combined using Rubin's rules.
Results for the primary, secondary, and tertiary models are presented in Table 3. A moderate degree of collinearity was present between the PROMIS item asking about daily activity and the Timed 25-Foot Walk test score in the primary model; the PROMIS item was retained. The PROMIS items, rated from 1 to 5, except for pain (rated 0–10), were strong predictors of associated MSISQ-19 subscales after adjustment for demographic and clinical factors. On average, an increase of 1 on the PROMIS everyday physical activities item decreased the MSISQ-19 primary score by 1.225. An increase of 1 on the PROMIS fatigue item, indicating less fatigue, was associated with a decrease of 3.493 on the secondary MSISQ-19 score, and an increase in the rating of pain increased the MSISQ-19 score by 0.726. Finally, tertiary sexual dysfunction increased by 1.798 per unit decrease in QOL.
Table 3.
Results of multivariate linear regression models for primary, secondary, and tertiary subscales of MSISQ-19
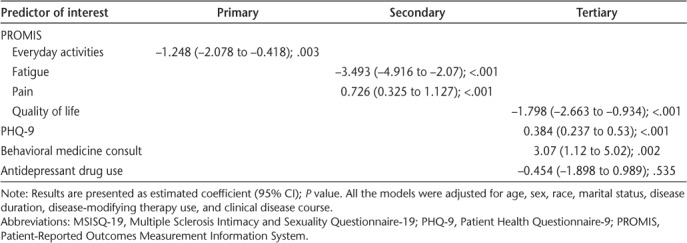
An increase of 5 units in the PHQ-9 score, considered a clinically meaningful change,12 was associated with an increase of 1.92 in the MSISQ-19 tertiary subscale. Patients who had a behavioral medicine consultation in the previous year had a score 3.07 higher than those who did not, on average. Use of antidepressant medications was not significantly associated with change in tertiary sexual dysfunction scores.
Discussion
This study sought to explore the prevalence of sexual dysfunction in an MS clinic population and to identify variables associated with sexual dysfunction in MS. The results of the study demonstrated that sexual dysfunction is highly prevalent in a clinic sample at the Mellen Center for MS, consistent with existing literature. It was thought that older individuals with a progressive disease course would have a higher prevalence of sexual dysfunction. Interestingly, there were no differences between those who endorsed sexual dysfunction and those who did not in terms of age, sex, race, median income, marital status, or disease course. These results support the notion that sexual dysfunction is more prevalent in people with MS than in the general population. Moreover, participants who endorsed sexual dysfunction also had a higher prevalence of pain, fatigue, and depression and were more likely to have been referred for a behavioral medicine consultation in the past year and to have received a prescription for an antidepressant medication. Patients with sexual dysfunction had higher PHQ-9 scores, and there were no significant differences in terms of sexual dysfunction domain.
Other studies have also found a strong association between depression severity and higher rates of sexual dysfunction.13–15 Depressive symptoms in MS can stem from both physiologic and psychological factors, which requires close assessment to determine appropriate treatment options (ie, pharmacologic and behavioral approaches). Many antidepressants can include loss of libido and delayed orgasm as common adverse effects, so it is imperative to focus on individual factors when recommending treatment options.16 The findings of this study suggest that focusing on assessment of depression, pain, and fatigue can help identify individuals who are at risk of experiencing sexual dysfunction symptoms.
This study has several limitations. First, some of the health status measures were performed at different times than when the MSISQ-19 was completed. Second, given that the Knowledge Program data are collected in a clinic setting versus a research setting, it is possible that rigorous methods of data collection were not applied, which generated some complications regarding missing data.
Despite these limitations, the results of this study have important clinical implications, as understanding the individual factors affecting sexual dysfunction will facilitate the development of evidence-based treatments. Given that time and efficiency are valued aspects of managing patient care, the development of treatment algorithms and workflow can help facilitate treatment delivery. Assessment and treatment of depression may serve as a starting point of intervention in patients with MS who experience sexual dysfunction. Assessment and treatment of sexual dysfunction in adults with MS is an important component of comprehensive care. Ideally, this should be part of a clinician's routine assessment; however, many providers feel that they do not have the time or lack the training and comfort regarding treatment of sexual dysfunction. Knowing the impact of sexual dysfunction on QOL and general wellness, it is important to identify and offer treatment to individuals who experience these issues. We recommend narrowing assessment of sexual dysfunction to individuals who can be identified as being at risk, and, thus, help address some of these barriers by facilitating assessment and treatment. Due to the complexities of sexual dysfunction in MS, it will be important to identify appropriate means of intervention from an interdisciplinary perspective.
PRACTICE POINTS
Sexual dysfunction is a highly prevalent symptom in individuals with MS that commonly goes unreported by the patient. Conversely, many clinicians choose not to assess for sexual dysfunction for a variety of reasons (eg, visit time limits, perceived patient discomfort, and perceived lack of competency in the area).
Identifying factors that are commonly associated with sexual dysfunction in MS can help reduce clinician burden by understanding appropriate assessment for it in individuals at risk.
The assessment and treatment of depression can serve as a starting point for intervention in individuals with MS who experience sexual dysfunction.
Further understanding of sexual dysfunction has the potential to assist in the design of efficacious treatment alternatives and, thus, improve quality of life in individuals with MS.
Acknowledgments
The authors acknowledge the Knowledge Program Data Registry of Cleveland Clinic for providing part of the data used in these analyses.
Financial Disclosures
Mr. Thompson has disclosed salary support to his institution from Novartis Pharmaceuticals for research outside of this study. Dr Sullivan has received a consulting fee from and served on a speakers' bureau for Novartis. The other authors declare no conflicts of interest.
Funding/Support
This research was made possible by the Cleveland Clinic Neurological Institute Center for Outcomes Research and Evaluation Statistical Support Grant.
Prior Presentation
Aspects of this study have been presented in abstract form at the Consortium of Multiple Sclerosis Centers (CMSC) Annual Meeting; June 1–4, 2016; National Harbor, MD.
References
- 1.Zwibel HL, Smrtka J. Improving quality of life in multiple sclerosis: an unmet need. Am J Manag Care. 2011;17:S139–S145. [PubMed] [Google Scholar]
- 2.Gromisch ES, Schairer LC, Pasternak E et al. Assessment and treatment of psychiatric distress, sexual dysfunction, sleep disturbances, and pain in multiple sclerosis: a survey of members of the Consortium of Multiple Sclerosis Centers. Int J MS Care. 2016;18:291–297. doi: 10.7224/1537-2073.2016-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schairer LC, Foley FW, Zemon V et al. The impact of sexual dysfunction on health-related quality of life in people with multiple sclerosis. Mult Scler. 2014;20:610–616. doi: 10.1177/1352458513503598. [DOI] [PubMed] [Google Scholar]
- 4.Foley FW, Iverson J. Sexuality and multiple sclerosis. In: Kalb RC, Scheinberg LC, editors. Multiple Sclerosis and Family. New York, NY: Demos; 1992. pp. 63–82. [Google Scholar]
- 5.Griswold GA, Foley FW, Halper J et al. Multiple sclerosis and sexuality: a survey of MS health professionals' comfort, training, and inquiry about sexual dysfunction. Int J MS Care. 2003;5:37–51. [Google Scholar]
- 6.Sanders AS, Foley FW, LaRocca NG et al. The Multiple Sclerosis Intimacy and Sexuality Questionnaire-19 (MSISQ-19) Sex Disabil. 2000;18:3–26. [Google Scholar]
- 7.Katzan I, Speck M, Dopler C et al. The Knowledge Program: an innovative, comprehensive, electronic data capture system and warehouse. AMIA Annu Symp Proc. 2011;2011:683–692. [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz CE, Vollmer T, Lee H. Reliability and validity of two self-report measures of impairment and disability for MS. Neurology. 1999;52:63–70. doi: 10.1212/wnl.52.1.63. [DOI] [PubMed] [Google Scholar]
- 9.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: John Wiley & Sons Inc; 1987. [Google Scholar]
- 11.Van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J Stat Softw. 2011:45. [Google Scholar]
- 12.Löwe B, Kroenke K, Herzog W, Gräfe K. Measuring depression outcome with a brief self-report instrument: sensitivity to change of the Patient Health Questionnaire (PHQ-9) J Affect Disord. 2004;81:61–66. doi: 10.1016/S0165-0327(03)00198-8. [DOI] [PubMed] [Google Scholar]
- 13.Lew-Starowicz M, Rola R. Correlates of sexual function in male and female patients with multiple sclerosis. J Sex Med. 2014;11:2172–2180. doi: 10.1111/jsm.12622. [DOI] [PubMed] [Google Scholar]
- 14.Gumus H, Akpinar Z, Yilmaz H. Effects of multiple sclerosis on female sexuality: a controlled study. J Sex Med. 2014;11:481–486. doi: 10.1111/jsm.12397. [DOI] [PubMed] [Google Scholar]
- 15.Calabrò R, Russo M. Sexual dysfunction and depression in individuals with multiple sclerosis: is there a link? Innov Clin Neurosci. 2015;12:11–12. [PMC free article] [PubMed] [Google Scholar]
- 16.Calabrò R, De Luca R, Conti-Nibali V et al. Sexual dysfunction in male patients with multiple sclerosis: a need for counseling! Int J Neurosci. 2014;124:547–557. doi: 10.3109/00207454.2013.865183. [DOI] [PubMed] [Google Scholar]


