Abstract
Background:
Exercise training can improve skeletal muscle metabolism in persons with multiple sclerosis (MS). However, quantification of exercise-mediated improvements in muscle metabolism has been limited, particularly in people with high levels of disability. We evaluated the effect of 9 weeks of antigravity treadmill training on muscle oxidative capacity and muscle endurance and assessed the relationship to walking function in a person with MS.
Methods:
One person with MS (Expanded Disability Status Scale score, 6.5) performed treadmill training for 24 minutes approximately twice weekly for 9 weeks (16 sessions) using an antigravity treadmill system. Before and after the intervention phase, we measured muscle oxidative capacity in the medial gastrocnemius using near-infrared spectroscopy after 15 to 20 seconds of electrical stimulation; muscle endurance in the medial gastrocnemius using accelerometer-based mechanomyography during 9 minutes of twitch electrical stimulation in three stages (3 minutes per stage) of increasing frequency (2, 4, and 6 Hz); muscle strength (plantarflexion) using a maximal voluntary contraction; and walking function using the Timed 25-Foot Walk test and the 2-Minute Walk Test.
Results:
Muscle oxidative capacity increased from 0.73 min−1 to 1.08 min−1 (48%). Muscle endurance increased from 75.9% to 84.0% at 2 Hz, from 67.8% to 76.2% at 4 Hz, and from 13.5% to 44.7% at 6 Hz. Maximal voluntary contraction decreased by 0.68 kg (15%), Timed 25-Foot Walk test speed decreased by 0.19 ft/s (20%), and 2-Minute Walk Test distance increased by 65 m (212%).
Conclusions:
Muscle oxidative capacity and muscle endurance, as well as walking function, improved in a person with MS after training on an antigravity treadmill.
Multiple sclerosis (MS) is an autoimmune disease characterized by the demyelination of axons in the central nervous system. Persons with MS commonly have decreased mobility related to declines in walking function.1–3 Impairments in mobility can promote deconditioning by decreasing participation in physical activity,1,2,4 and several studies have shown that reductions in skeletal muscle endurance, strength, and metabolism in persons with MS are related to walking dysfunction.5–9
Various lines of evidence suggest that aerobic and resistance exercise training can improve muscle strength, exercise capacity, and walking function in persons with MS.10–13 One recent study using muscle-specific training (functional electrical stimulation cycling) reported increases in muscle oxidative metabolism.14 However, quantifying changes in muscle metabolism with training as changes in muscle oxidative capacity in persons with MS would be helpful in characterizing the physiologic response to exercise training in this population. Furthermore, the link between muscle function and walking capacity is not yet established, and whether either or both can be modified with exercise in people with severe walking impairment remains unclear.
Body weight–supported treadmill training can facilitate exercise training in persons with limited mobility.15–18 Recently, one such device has been developed that uses lower-body positive pressure to provide body weight support (BWS), which may give a person with severe walking dysfunction the opportunity to exercise. The purpose of the present study was to evaluate the effects of exercise using antigravity treadmill training on muscle oxidative capacity and muscle endurance in a person with MS who has severe walking dysfunction. In addition, walking function and muscle strength were evaluated for comparison.
Methods
Participant
The participant was a 56-year-old woman diagnosed as having relapsing-remitting MS 22 years before enrollment (Expanded Disability Status Scale [EDSS] score of 6.5). The participant had no history of orthopedic injury or cardiovascular disease that would make exercise unsafe. She was enrolled in a larger pilot study evaluating the safety, feasibility, and effectiveness of antigravity treadmill training using an AlterG treadmill system (AlterG Inc, Fremont, CA) for people with MS. This study was approved by the research review committee at Shepherd Center (Atlanta, GA), and the participant gave written informed consent before participation in the study.
Exercise Training Intervention
The antigravity treadmill training intervention consisted of 16 sessions over 9 weeks. Sessions were held on nonconsecutive days. The participant attended two sessions a week for 9 weeks except in weeks 5 and 9, when only one training session occurred. Each session included 20 minutes of walking exercise during which BWS and speed were altered and progressed based on the participant's walking performance. This participant's BWS and speed ranged from 35% to 70% BWS at 0.4 to 0.8 mph during training. In addition, the training session included 2-minute warm-up and 2-minute cool-down periods before and after the exercise bout, and a mandatory 2- to 5-minute break was provided at minute 10 of the exercise bout to collect vital signs. All the sessions were completed without an adverse event.
Experimental Protocols
Muscle strength, muscle oxidative capacity, muscle endurance, walking function, and perceived walking function were measured before the start of exercise training and after completion of the 16th exercise training session.
Muscle Endurance
Muscle endurance was defined as the preservation of twitch contraction acceleration as measured by accelerometer-based mechanomyography during repeated electrically stimulated twitch contractions as previously described elsewhere.19 In brief, an accelerometer was placed on the skin over the gastrocnemius muscle using double-sided tape, and muscle contractions were measured during a 9-minute electrical stimulation protocol. The electrical stimulation protocol consisted of three low-stimulation (twitch) frequencies (2, 4, and 6 Hz) for 3 minutes each. An endurance index was calculated as the percentage of acceleration measured at the end of each stage of frequency relative to the peak acceleration.19
Muscle Oxidative Capacity
Muscle oxidative capacity was measured using near-infrared spectroscopy (NIRS) on the medial gastrocnemius as previously described elsewhere.20 To summarize, NIRS signal changes during periods of ischemia can be used to measure the metabolism of oxygen in skeletal muscle.20,21 After exercise, increases in the rate of oxygen metabolism as measured by NIRS reflect increases in oxidative metabolism required to restore intramuscular phosphocreatine stores.22,23 Therefore, the recovery of muscle metabolic rate after exercise can be measured using a series of ischemic periods after exercise.20 The oxygen metabolism rates during a series of postexercise ischemic periods can be fitted to the exponential function y(t) = End − Δ × e−kt. In this equation, the rate constant, k, is used as an index of muscle mitochondrial capacity.20 The NIRS recovery tests were performed with the participant positioned on a padded table in a supine position, and a blood pressure cuff was placed just proximal to the knee joint. Electrical stimulation was applied for 10 to 20 seconds to the gastrocnemius muscle. Immediately after electrical stimulation, the blood pressure cuff was inflated to approximately 100 mm Hg above systolic blood pressure for 5 seconds to measure oxygen consumption. Then, 18 to 22 blood pressure cuff inflations were performed (5–20 seconds for each inflation) to measure the recovery of oxygen consumption.
Muscle Strength
Muscle strength was measured using handheld dynamometry (Baseline; Fabrication Enterprises Inc, White Plains, NY) during maximal voluntary contraction of the ankle plantar flexors.24 The average of two trials was recorded.
Walking Function
Walking endurance was measured using the 2-Minute Walk Test (2MWT), and walking speed was measured using the Timed 25-Foot Walk (T25FW) test as previously described elsewhere.25 The 2MWT measures the distance walked during a 2-minute period. The T25FW test measures walking speed as calculated from the time elapsed during a 25-foot walk. The participant used a rolling walker during the 2MWT and T25FW test. Perceived walking function was measured using the 12-item Multiple Sclerosis Walking Scale (MSWS-12),3 a self-report questionnaire about the participant's perception of the impact of MS on walking function.
Interpretation
Statistical analysis was not performed because the present study evaluated only one participant. Changes in outcome measures were considered significant if larger than the SD previously reported in studies of persons with MS.26–31
Results
Muscle oxidative capacity increased by 48% (from 0.73 min−1 to 1.08 min−1) (Figure 1A). Muscle endurance increased 8.1% (from 75.9% to 84.0%) at 2 Hz, 8.4% (from 67.8% to 76.2%) at 4 Hz, and 31.2% (from 13.5% to 44.7%) at 6 Hz of stimulation (Figure 1B). The 2MWT distance increased by 65 m (212%) (Figure 1C). Walking speed as measured by the T25FW test decreased from 0.94 ft/s to 0.75 ft/s (20%), and maximal voluntary contraction decreased from 4.5 kg to 3.9 kg (15%). The MSWS-12 score improved from 46 to 45 (2.2%).
Figure 1.
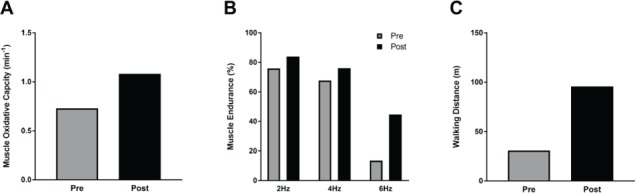
Changes in muscle function and walking endurance before (Pre) and after (Post) antigravity treadmill training in a participant with multiple sclerosis
A, Muscle oxidative capacity as measured by near-infrared spectroscopy in medial gastrocnemius. Oxidative capacity is reported as the time constant of recovery of oxygen consumption after exercise. B, Muscle endurance as measured by the endurance index in medial gastrocnemius at 2, 4, and 6 Hz of electrical stimulation. C, Walking endurance as measure by 2-Minute Walk Test.
Discussion
The primary finding of this study was that muscle oxidative capacity and muscle endurance, as well as walking endurance, improved in one person with MS after antigravity treadmill training. The observed magnitudes of improvements in muscle oxidative capacity and 2MWT distance were greater than the variability previously reported for these measures in persons with MS26,28 and were generally consistent with previous training studies demonstrating improved muscle oxidative metabolism with training.32 To our knowledge, this is the first study to evaluate exercise-mediated changes in muscle-specific oxidative capacity using NIRS in persons with MS, and our participant improved muscle oxidative capacity of the gastrocnemius by 48% with antigravity treadmill training. Moreover, few studies have addressed muscle plasticity in people with MS who have the extent of impairment in walking ability and EDSS score seen in this participant (EDSS score of 6.5).17,33 The participant in this study had severe walking dysfunction as evident by the use of assistive devices and measures of walking function. The participant's walking speed and walking endurance were lower than measures reported in studies of individuals with moderate-to-severe disability (EDSS score of 4.5–6.5).3,25 In addition, muscle oxidative capacity in the medial gastrocnemius muscle of the participant in the present study before exercise training was approximately 50% that of controls and 35% lower than values previously reported in persons with MS.26 Comparably, Kent-Braun et al.6 reported 40% lower oxidative enzyme activity in the lower extremity muscles of persons with MS matched to controls. Muscle strength as measured during maximal voluntary contraction (plantarflexion) was also considerably lower than normative values for controls, which is consistent with studies of muscle strength in persons with MS.24,34 Thus, these findings support the potential for people with MS and substantial mobility impairments to improve muscle and walking function given the opportunity to exercise.
Muscle endurance in the medial gastrocnemius showed substantial improvements with antigravity treadmill training. Similar to previous studies, we quantified muscle endurance as the ability to sustain repeated contractions.5 However, the muscle endurance measurement used in the present study was specific to the peripheral skeletal muscle and was not influenced by the central nervous system. Previous studies evaluating muscle plasticity in neurologic populations have reported that increases in oxidative capacity are associated with improvements in muscle-specific endurance, but few studies have evaluated relationships between changes in muscle endurance or walking endurance and metabolism in people with MS.32 The improvements in muscle endurance observed in the present study provide a physiologic link between the increase in muscle oxidative capacity and improvement in the desired functional improvement (walking endurance). Indeed, understanding how exercise interventions affect skeletal muscle plasticity in persons with MS can help elucidate the mechanisms behind improvements in walking function, and the present findings lend support to the inclusion of muscle endurance and muscle oxidative capacity measurements in the rehabilitation of walking function in persons with MS.
The improvements in walking endurance and perception of walking function after antigravity treadmill training are consistent with improvements in walking function reported in people with MS who can perform unassisted training.12,35 However, walking speed as measured by the T25W test declined approximately 20% after training, which is both consistent18 and in disagreement36 with previous studies evaluating the effects of exercise walking speed and may be considered a clinically meaningful difference.27 The decrease in strength observed in this participant is difficult to interpret in that magnitude of change is lower than the minimal detectable change established for this method and less than the variability of strength measures previously reported in persons with MS.24,31 The lack of improvement in strength could be due to an insufficient stimulus for muscle strength improvements with the body weight–supported treadmill training used in the present study. Whether the decrease in walking speed is related to muscle strength remains unclear but is in keeping with the findings of previous studies showing a relationship between muscle weakness, as well as somatosensory loss, and walking speed over short distances.5,37 On the other hand, the present results indicate that improvements in muscle oxidative capacity and muscle endurance may be associated with an increased capacity to sustain walking speed over longer distances, supporting previous reports that walking endurance is more closely related to muscle oxidative capacity than strength in persons with MS.9 Notwithstanding, the exact mechanisms underlying exercise-mediated improvements in walking function in persons with MS are unknown, and future studies with larger sample sizes are needed to establish the role of muscle plasticity in improving walking function in persons with MS.
In conclusion, muscle oxidative capacity and muscle endurance, as well as walking endurance, improved after antigravity treadmill training in a person with MS who had severe walking dysfunction. These changes occurred without changes in muscle strength and walking speed. Further investigation is warranted to establish the effects of voluntary exercise on muscle plasticity and the role of muscle oxidative capacity in the rehabilitation of persons with MS.
PRACTICE POINTS
The benefits of treadmill training have been demonstrated in persons with MS, yet those with severe walking dysfunction often have difficulty exercising on a treadmill.
An antigravity treadmill, by providing body weight support, may give a person with severe walking dysfunction the opportunity to exercise.
People with MS who have severe walking dysfunction may exhibit improved muscle metabolism, muscle endurance, and walking endurance after antigravity treadmill training.
Financial Disclosures
Dr. McCully is the president of Infrared Rx Inc. The other authors declare no conflicts of interest.
Funding/Support
None.
References
- 1.Higginson IJ, Hart S, Silber E, Burman R, Edmonds P. Symptom prevalence and severity in people severely affected by multiple sclerosis. J Palliat Care. 2006;22:158–165. [PubMed] [Google Scholar]
- 2.Newland PK, Fearing A, Riley M, Neath A. Symptom clusters in women with relapsing-remitting multiple sclerosis. J Neurosci Nurs. 2012;44:66–71. doi: 10.1097/JNN.0b013e3182478cba. [DOI] [PubMed] [Google Scholar]
- 3.Goldman MD, Marrie RA, Cohen JA. Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Mult Scler. 2008;14:383–390. doi: 10.1177/1352458507082607. [DOI] [PubMed] [Google Scholar]
- 4.Sandroff BM, Dlugonski D, Weikert M, Suh Y, Balantrapu S, Motl RW. Physical activity and multiple sclerosis: new insights regarding inactivity. Acta Neurol Scand. 2012;126:256–262. doi: 10.1111/j.1600-0404.2011.01634.x. [DOI] [PubMed] [Google Scholar]
- 5.Broekmans T, Gijbels D, Eijnde BO et al. The relationship between upper leg muscle strength and walking capacity in persons with multiple sclerosis. Mult Scler. 2013;19:112–119. doi: 10.1177/1352458512444497. [DOI] [PubMed] [Google Scholar]
- 6.Kent-Braun JA, Ng AV, Castro M et al. Strength, skeletal muscle composition, and enzyme activity in multiple sclerosis. J Appl Physiol (1985) 1997;83:1998–2004. doi: 10.1152/jappl.1997.83.6.1998. [DOI] [PubMed] [Google Scholar]
- 7.Kent-Braun JA, Sharma KR, Miller RG, Weiner MW. Postexercise phosphocreatine resynthesis is slowed in multiple sclerosis. Muscle Nerve. 1994;17:835–841. doi: 10.1002/mus.880170802. [DOI] [PubMed] [Google Scholar]
- 8.Wetzel JL, Fry DK, Pfalzer LA. Six-minute walk test for persons with mild or moderate disability from multiple sclerosis: performance and explanatory factors. Physiother Can. 2011;63:166–180. doi: 10.3138/ptc.2009-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen D, Feys P, Wens I, Eijnde BO. Is walking capacity in subjects with multiple sclerosis primarily related to muscle oxidative capacity or maximal muscle strength? a pilot study. Mult Scler Int. 2014;2014 doi: 10.1155/2014/759030. 759030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cakt BD, Nacir B, Genc H et al. Cycling progressive resistance training for people with multiple sclerosis: a randomized controlled study. Am J Phys Med Rehabil. 2010;89:446–457. doi: 10.1097/PHM.0b013e3181d3e71f. [DOI] [PubMed] [Google Scholar]
- 11.Kerling A, Keweloh K, Tegtbur U et al. Effects of a short physical exercise intervention on patients with multiple sclerosis (MS) Int J Mol Sci. 2015;16:15761–15775. doi: 10.3390/ijms160715761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmadi A, Arastoo AA, Nikbakht M, Zahednejad S, Rajabpour M. Comparison of the effect of 8 weeks aerobic and yoga training on ambulatory function, fatigue and mood status in MS patients. Iran Red Crescent Med J. 2013;15:449–454. doi: 10.5812/ircmj.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalgas U, Stenager E, Jakobsen J et al. Fatigue, mood and quality of life improve in MS patients after progressive resistance training. Mult Scler. 2010;16:480–490. doi: 10.1177/1352458509360040. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds MA, McCully K, Burdett B, Manella C, Hawkins L, Backus D. Pilot study: evaluation of the effect of functional electrical stimulation cycling on muscle metabolism in nonambulatory people with multiple sclerosis. Arch Phys Med Rehabil. 2015;96:627–632. doi: 10.1016/j.apmr.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Pilutti LA, Lelli DA, Paulseth JE et al. Effects of 12 weeks of supported treadmill training on functional ability and quality of life in progressive multiple sclerosis: a pilot study. Arch Phys Med Rehabil. 2011;92:31–36. doi: 10.1016/j.apmr.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 16.Pilutti LA, Paulseth JE, Dove C, Jiang S, Rathbone MP, Hicks AL. Exercise training in progressive multiple sclerosis: a comparison of recumbent stepping and body weight-supported treadmill training. Int J MS Care. 2016;18:221–229. doi: 10.7224/1537-2073.2015-067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beer S, Aschbacher B, Manoglou D, Gamper E, Kool J, Kesselring J. Robot-assisted gait training in multiple sclerosis: a pilot randomized trial. Mult Scler. 2008;14:231–236. doi: 10.1177/1352458507082358. [DOI] [PubMed] [Google Scholar]
- 18.Lo AC, Triche EW. Improving gait in multiple sclerosis using robot-assisted, body weight supported treadmill training. Neurorehabil Neural Repair. 2008;22:661–671. doi: 10.1177/1545968308318473. [DOI] [PubMed] [Google Scholar]
- 19.Bossie HM, Willingham TB, Van Schoick RA, O'Connor PJ, McCully KK. Mitochondrial capacity, muscle endurance and low energy in Friedreich ataxia. Muscle Nerve. 2017;56:773–779. doi: 10.1002/mus.25524. [DOI] [PubMed] [Google Scholar]
- 20.Ryan TE, Erickson ML, Brizendine JT, Young HJ, McCully KK. Noninvasive evaluation of skeletal muscle mitochondrial capacity with near-infrared spectroscopy: correcting for blood volume changes. J Appl Physiol (1985) 2012;113:175–183. doi: 10.1152/japplphysiol.00319.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamaoka T, Iwane H, Shimomitsu T et al. Noninvasive measures of oxidative metabolism on working human muscles by near-infrared spectroscopy. J Appl Physiol (1985) 1996;81:1410–1417. doi: 10.1152/jappl.1996.81.3.1410. [DOI] [PubMed] [Google Scholar]
- 22.Paganini AT, Foley JM, Meyer RA. Linear dependence of muscle phosphocreatine kinetics on oxidative capacity. Am J Physiol. 1997;272(pt 1):C501–C510. doi: 10.1152/ajpcell.1997.272.2.C501. [DOI] [PubMed] [Google Scholar]
- 23.Ryan TE, Southern WM, Reynolds MA, McCully KK. A cross-validation of near-infrared spectroscopy measurements of skeletal muscle oxidative capacity with phosphorus magnetic resonance spectroscopy. J Appl Physiol (1985) 2013;115:1757–1766. doi: 10.1152/japplphysiol.00835.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mentiplay BF, Perraton LG, Bower KJ et al. Assessment of lower limb muscle strength and power using hand-held and fixed dynamometry: a reliability and validity study. PLoS One. 2015;10:e0140822. doi: 10.1371/journal.pone.0140822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gijbels D, Alders G, Van Hoof E et al. Predicting habitual walking performance in multiple sclerosis: relevance of capacity and self-report measures. Mult Scler. 2010;16:618–626. doi: 10.1177/1352458510361357. [DOI] [PubMed] [Google Scholar]
- 26.Harp MA, McCully KK, Moldavskiy M, Backus D. Skeletal muscle mitochondrial capacity in people with multiple sclerosis. Mult Scler. 2016;2:1–7. doi: 10.1177/2055217316678020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen JA, Krishnan AV, Goodman AD et al. The clinical meaning of walking speed as measured by the timed 25-foot walk in patients with multiple sclerosis. JAMA Neurol. 2014;71:1386–1393. doi: 10.1001/jamaneurol.2014.1895. [DOI] [PubMed] [Google Scholar]
- 28.Gijbels D, Eijnde BO, Feys P. Comparison of the 2- and 6-minute walk test in multiple sclerosis. Mult Scler. 2011;17:1269–1272. doi: 10.1177/1352458511408475. [DOI] [PubMed] [Google Scholar]
- 29.Motl RW, Snook EM. Confirmation and extension of the validity of the Multiple Sclerosis Walking Scale-12 (MSWS-12) J Neurol Sci. 2008;268:69–73. doi: 10.1016/j.jns.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Hobart J, Blight AR, Goodman A, Lynn F, Putzki N. Timed 25-foot walk: direct evidence that improving 20% or greater is clinically meaningful in MS. Neurology. 2013;80:1509–1517. doi: 10.1212/WNL.0b013e31828cf7f3. [DOI] [PubMed] [Google Scholar]
- 31.Guclu-Gunduz A, Citaker S, Irkec C, Nazliel B, Batur-Caglayan HZ. The effects of pilates on balance, mobility and strength in patients with multiple sclerosis. NeuroRehabilitation. 2014;34:337–342. doi: 10.3233/NRE-130957. [DOI] [PubMed] [Google Scholar]
- 32.Ryan TE, Erickson ML, Young HJ, McCully KK. Case report: endurance electrical stimulation training improves skeletal muscle oxidative capacity in chronic spinal cord injury. Arch Phys Med Rehabil. 2013;94:2559–2561. doi: 10.1016/j.apmr.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 33.Skjerbaek AG, Naesby M, Lutzen K et al. Endurance training is feasible in severely disabled patients with progressive multiple sclerosis. Mult Scler. 2014;20:627–630. doi: 10.1177/1352458513505351. [DOI] [PubMed] [Google Scholar]
- 34.Lee Y, Chen K, Ren Y et al. Robot-guided ankle sensorimotor rehabilitation of patients with multiple sclerosis. Mult Scler Relat Disord. 2017;11:65–70. doi: 10.1016/j.msard.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Samaei A, Bakhtiary AH, Hajihasani A, Fatemi E, Motaharinezhad F. Uphill and downhill walking in multiple sclerosis: a randomized controlled trial. Int J MS Care. 2016;18:34–41. doi: 10.7224/1537-2073.2014-072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rampello A, Franceschini M, Piepoli M et al. Effect of aerobic training on walking capacity and maximal exercise tolerance in patients with multiple sclerosis: a randomized crossover controlled study. Phys Ther. 2007;87:545–555. doi: 10.2522/ptj.20060085. [DOI] [PubMed] [Google Scholar]
- 37.Thoumie P, Mevellec E. Relation between walking speed and muscle strength is affected by somatosensory loss in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2002;73:313–315. doi: 10.1136/jnnp.73.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]


