Abstract
The effect of sodium-glucose cotransporter 2 inhibitors on peripheral nerves and kidneys in diabetes mellitus (DM) remains unexplored. Therefore, this study aimed to explore the effect of empagliflozin in diabetic rats. DM in rats was induced by streptozotocin injection, and diabetic rats were treated with empagliflozin 3 or 10 mg/kg. Following 24-week treatment, response thresholds to four different stimuli were tested and found to be lower in diabetic rats than in normal rats. Empagliflozin significantly prevented hypersensitivity (P<0.05) and the loss of skin intraepidermal nerve fibers, and mesangial matrix expansion in diabetic rats. Results of this study demonstrate the potential therapeutic effects of empagliflozin for the treatment of diabetic peripheral neuropathy and nephropathy.
Keywords: Diabetes mellitus, experimental; Diabetic nephropathies; Diabetic neuropathies; Peripheral nerves; Sodium-glucose transporter 2
INTRODUCTION
Sodium-glucose cotransporter 2 (SGLT2) inhibitors as oral hypoglycemic agents have been approved for treating type 2 diabetes mellitus (T2DM) [1]. The insulin-independent action mechanism and extra-metabolic benefits of these agents have encouraged ongoing preclinical and clinical trials for evaluating the efficacy and safety of SGLT2 inhibitors for type 1 diabetes mellitus (T1DM), and favorable results have been reported [2]. In addition to glucose-lowering effects without hypoglycemia, SGLT2 inhibitors retard the development and progression of diabetic complications [3]. However, it is uncertain whether this effect of SGLT2 inhibitors is due to their glucose-lowering effect or not. In addition, unlike diabetic nephropathy (DN), the effects of SGLT2 inhibitors on diabetic peripheral neuropathy (DPN) are unexplored. Therefore, the present study aimed to investigate the effect of empagliflozin, a selective SGLT2 inhibitor, on the kidney and peripheral nerves in streptozotocin (STZ)-induced diabetic rats.
METHODS
All experiments and relevant protocols were approved by the Institutional Animal Care and Use Committee of the Chonbuk National University Medical School (CBU 2011-0055). Male Sprague-Dawley rats (6 to 8 weeks, 160 to 180 g) were housed under optimal conditions on a 12-hour light/dark cycle. The animals had free access to food and water. STZ (Sigma-Aldrich Chemical Corp., St. Louis, MO, USA) was dissolved in 0.1 mol/L sodium citrate buffer (pH 4.5) and intraperitoneally injected into the experimental rats (60 mg/kg) to induce diabetes mellitus (DM). Rats with blood glucose levels ≥20 mmol/L were considered diabetic. Following intraperitoneal injections of STZ and sodium citrate buffer for 2 weeks, diabetic rats and their age-matched controls were randomly assigned to the following four groups (n=6/group): normal plus vehicle (NOR), DM plus vehicle (DM), and DM plus empagliflozin 3 and 10 mg/kg (DM+E3 and DM+E10, respectively). Empagliflozin was administered orally once a day for 24 weeks at doses based on those of a previous study [4]. The body weight and blood glucose levels of rats in each group were measured every 2 weeks. Urine was collected for 24 hours after the administration of empagliflozin or the vehicle using metabolic cages, and urinary concentrations of glucose, creatinine, and albumin were measured at weeks 24. At weeks 12 and 24, we performed behavioral assessments using von Frey filaments (Stoelting Co., Wood Dale, IL, USA), a hot plate (Ugo Basile, Collegeville, PA, USA), an infrared radiometer (Jeungdo B&P, Seoul, Korea), and Randall-Sellito analgesia meter (Ugo Basile, Comerio, Italy). We performed a morphometric comparison of small nerve fibers in the dorsum of the foot, the sciatic nerve, and the renal cortex. Tissue samples were harvested from the dorsum of the hind leg by a skin biopsy at weeks 24. Segments of the right sciatic nerve and renal cortex were also collected from each rat after euthanasia. Protein gene product 9.5 (PGP 9.5)-immunoreactive nerve fibers in the epidermis and renal cortex were counted as described previously [5], and the number of intraepidermal nerve fibers per length (fibers/mm) was used to quantify innervation. Immunohistochemical analysis was performed as described previously [6,7]. In addition, we compared the glomerular histology in each group by staining kidney tissue sections with periodic acid-Schiff. All data are expressed as mean±standard error of the mean. Kruskal-Wallis test was used to compare intergroup differences. A 95% confidence interval was set for determining the significant differences, and P<0.05 was considered statistically significant.
RESULTS
Effects on body weight and blood glucose level
Orally administered empagliflozin (10 mg/kg) significantly reduced plasma glucose levels in diabetic rats; however, this effect was not statistically significant at 3 mg/kg dose compared to the untreated DM group (6.3±0.4, 30.0±2.6, 24.4±4.4, and 21.7±2.7 mmol/L in NOR, DM, DM+E3, and DM+E10, respectively, at weeks 24; P<0.05). After 24 weeks of treatment, the mean body weight of rats in the two empagliflozin-treated groups decreased significantly compared to that of rats in the diabetic control group (645.5±31, 359.2±13, 299.8±8.2, and 244.7±13.3 g in NOR, DM, DM+E3, and DM+E10, respectively; P<0.05).
Effects on peripheral nerves
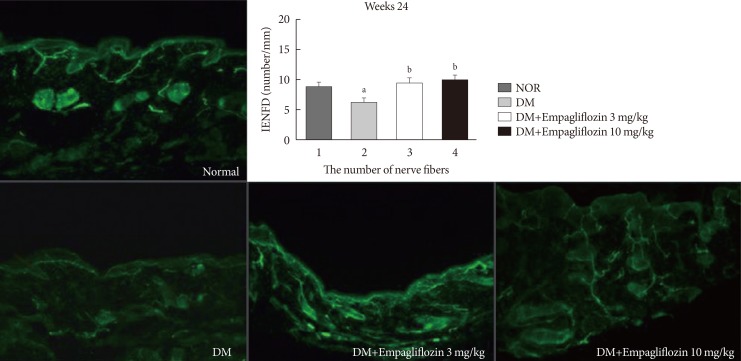
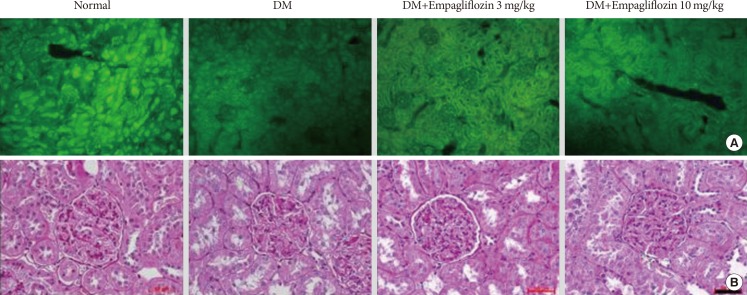
Response threshold to the tactile stimulus in the von Frey test was lower in the DM group than in the NOR group (P<0.05). Empagliflozin significantly inhibited hypersensitivity responses in a dose-dependent manner in diabetic rats (P<0.05). Similarly, response thresholds to the stimuli in the hot plate, infrared radiometer, and analgesia meter tests were lower in the DM group than in the NOR group (P<0.05). These hypersensitivity responses were significantly prevented in both empagliflozin-treated DM groups without a significant difference between the two dose groups (P>0.05). PGP 9.5-immunoreactive small nerve fiber loss in the rat hind foot dorsum was significantly prevented in both empagliflozin-treated diabetic rats compared to that in untreated diabetic rats (Fig. 1). The diameter of the sciatic nerve in the DM group decreased compared to that in the NOR group. Although sciatic nerve diameters of the DM+E3 and DM+E10 groups decreased to a lesser extent than in the DM group, this effect was not statistically significant among the experimental groups (7.96±0.97, 7.35±0.90, 7.73±1.13, and 7.76±0.81 µm in the NOR, DM, DM+E3, and DM+E10 groups, respectively; P>0.05). The number of PGP 9.5-positive small nerve fibers innervating the renal cortex significantly decreased in the DM group compared to that in the NOR group. Despite the lack of a quantitative comparison, considerably more nerve fibers were observed in the empagliflozin-treated groups (Fig. 2A).
Fig. 1. Intraepidermal nerve fiber density and protein gene product (PGP) 9.5-positive small nerve fibers in the dorsum of nondiabetic and diabetic rats treated with or without empagliflozin. PGP-9.5 immunoreactive small nerve fibers were markedly decreased in the diabetes mellitus (DM) group. However, nerve fiber loss was significantly prevented in the empagliflozin-treated DM groups compared to the untreated DM group. Horizontal bar indicates 100 µm. NOR, normal plus vehicle treated group; IENFD, intraepidernal nerve fiber density. aP<0.05 vs. NOR, bP<0.05 vs. DM.
Fig. 2. Protein gene product (PGP) 9.5-positive small nerve fibers in the renal cortex and periodic acid-Schiff staining of kidney tissue sections. (A) Numerous PGP-9.5-stained small nerve fibers were observed in the empagliflozin-treated groups compared to the diabetes mellitus (DM) group. (B) The PAS-positive glomerular mesangial area was expanded in the DM group compared to the normal plus vehicle treated group. These changes were significantly ameliorated in the DM+E10 group; however, the effect was not significant in the DM+E3 group. Horizontal bar indicates 50 µm. DM+E3 and DM+E10, diabetes mellitus plus empagliflozin at 3 and 10 mg/kg, respectively.
Effects on urinary protein excretion and glomerular histopathology
Urine collection over 24 hours revealed more severe albuminuria in the DM group than in the NOR group, and empagliflozin treatment ameliorated albuminuria in a dose-dependent manner (urine albumin/creatinine: 0.24±0.03, 25.6±2.0, 6.67±0.5, and 2.14±0.7 µg/g in the NOR, DM, DM+E3, DM+E10 groups, respectively; P<0.05). Glomerulosclerosis and mesangial matrix expansion were observed in the DM group but not in the NOR group, and these changes were significantly ameliorated in the DM+E10 group; however, the effect was not significant in the DM+E3 group (Fig. 2B).
DISCUSSION
The prevention or delayed progression of chronic DM complications or both is crucial for DM management. However, because both DN and DPN are caused by a complex pathophysiology initiated by chronic hyperglycemia, their effective treatment is also difficult. Although strict glucose control is the most important strategy for primary prevention and delayed progression of these complications, current anti-hypoglycemic treatments have failed to achieve optimal glucose levels and are associated with weight gain and hypoglycemia. Therefore, based on their insulin-independent mechanisms and metabolic benefits of losing weight and lowering blood pressure, SGLT2 inhibitors would be expected to have advantages over current therapies as hypoglycemic agents in DM. Empagliflozin, a selective SGLT2 inhibitor, attenuates both DN and cardiovascular diseases in patients with T2DM [8,9]. SGLT2 inhibitors also exert glucose-dependent and -independent renoprotective effects. Glucose-independent renoprotective effects of SGLT2 inhibitors include the reduction of blood pressure, glomerular hyperfiltration, renin-angiotensin-aldosterone system component activation, renal inflammation, and the expression of antioxidant enzymes [10,11,12]. These effects of SGLT2 inhibitors are considered pleiotropic. Similarly, other antidiabetic drugs, such as dipeptidyl peptidase 4 inhibitors, glucagon-like peptide 1 receptor agonists, and statins have shown renoprotective effects by attenuating inflammation and oxidative stress independent of their glucose- and lipid-lowering effects [13,14]. However, the effect of SGLT2 inhibitors on peripheral nerves remains unexplored. To date, only two studies regarding the effects of SGLT2 inhibitors for DPN in T2DM animal models are reported [3,15]. They evaluated neuronal effects in terms of simple functional parameters, such as motor nerve conduction velocity [3] and tail flick test [15]. Results of these studies verified the beneficial effects of SGLT2 inhibitors for DPN, and these effects were considered to indirect effects of the improvement of hyperglycemia. In our study, four behavioral tests using different stimuli and the histopathological comparison of small nerve fibers in the skin, sciatic nerves, and kidneys were performed, and the results showed preserved neuronal functions and intraepidermal nerve fiber density in both empagliflozin-treated DM groups; however, the anti-hyperglycemic effect was not significant in the DM+E3 group. In other words, present study demonstrating the neuroprotective effects of a SGLT2 inhibitor not only via functional parameters, but also through morphologic comparison in various peripheral nerves with two different doses of the SGLT2 inhibitor. DPN pathogenesis involves chronic inflammation and oxidative stress. Therefore, SGLT2 inhibitors may exert beneficial effects on peripheral nerves through pleiotropic effects mentioned earlier. Further biochemical and histological analyses of various neuronal tissues are needed to confirm these results. This preliminary study showed that empagliflozin ameliorates DN, and DPN in experimental DM. Although the mechanisms underlying its renoprotective and neuroprotective effects remain to be elucidated, SGLT2 inhibitors may be an attractive therapeutic option for DM patients with microvascular complications. This study had some limitations. Our animal model was a late stage T2DM or T1DM model using STZ, but since SGLT2 inhibitors are not currently approved for in T1DM, experiments with animal models of T2DM are needed to confirm the efficacy of the drug. Furthermore, because of SGLT2 is exclusively expressed in the kidney, in vitro and in vivo experiments with positive controls such as insulin treatment groups are necessary to confirm the direct effect of empagliflozin on the peripheral nerves. Lastly, further researches are warranted on the precise signaling pathways and related mediators between SGLT2 inhibition and complication outcomes other than blood glucose.
ACKNOWLEDGMENTS
This work was partly supported by a grant (Kyung Ae Lee, 2013) from the Korean Diabetes Association. The authors also thank the Research Institute of Clinical Medicine of Chonbuk National University-Biomedical Research Institute of Chonbuk National University Hospital for partly supporting this study through grant funding and access to experimental facilities.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Jung CH, Jang JE, Park JY. A novel therapeutic agent for type 2 diabetes mellitus: SGLT2 inhibitor. Diabetes Metab J. 2014;38:261–273. doi: 10.4093/dmj.2014.38.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmadieh H, Ghazal N, Azar ST. Role of sodium glucose cotransporter-2 inhibitors in type I diabetes mellitus. Diabetes Metab Syndr Obes. 2017;10:161–167. doi: 10.2147/DMSO.S122767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takakura S, Toyoshi T, Hayashizaki Y, Takasu T. Effect of ipragliflozin, an SGLT2 inhibitor, on progression of diabetic microvascular complications in spontaneously diabetic Torii fatty rats. Life Sci. 2016;147:125–131. doi: 10.1016/j.lfs.2016.01.042. [DOI] [PubMed] [Google Scholar]
- 4.Neumiller JJ. Empagliflozin: a new sodium-glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. Drugs Context. 2014;3:212262. doi: 10.7573/dic.212262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asensio-Pinilla E, Udina E, Jaramillo J, Navarro X. Electrical stimulation combined with exercise increase axonal regeneration after peripheral nerve injury. Exp Neurol. 2009;219:258–265. doi: 10.1016/j.expneurol.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 6.Jin HY, Kang SM, Liu WJ, Song CH, Lee KA, Baek HS, Park TS. Comparison of peripheral nerve damages according to glucose control timing in experimental diabetes. Exp Clin Endocrinol Diabetes. 2012;120:451–459. doi: 10.1055/s-0032-1306350. [DOI] [PubMed] [Google Scholar]
- 7.Jin HY, Lee KA, Song SK, Liu WJ, Choi JH, Song CH, Baek HS, Park TS. Sulodexide prevents peripheral nerve damage in streptozotocin induced diabetic rats. Eur J Pharmacol. 2012;674:217–226. doi: 10.1016/j.ejphar.2011.05.059. [DOI] [PubMed] [Google Scholar]
- 8.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B EMPA-REG OUTCOME Investigators. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 9.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 10.Kawanami D, Matoba K, Takeda Y, Nagai Y, Akamine T, Yokota T, Sango K, Utsunomiya K. SGLT2 inhibitors as a therapeutic option for diabetic nephropathy. Int J Mol Sci. 2017;18:E1083. doi: 10.3390/ijms18051083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatanaka T, Ogawa D, Tachibana H, Eguchi J, Inoue T, Yamada H, Takei K, Makino H, Wada J. Inhibition of SGLT2 alleviates diabetic nephropathy by suppressing high glucose-induced oxidative stress in type 1 diabetic mice. Pharmacol Res Perspect. 2016;4:e00239. doi: 10.1002/prp2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin SJ, Chung S, Kim SJ, Lee EM, Yoo YH, Kim JW, Ahn YB, Kim ES, Moon SD, Kim MJ, Ko SH. Effect of sodium-glucose co-transporter 2 inhibitor, dapagliflozin, on renal renin-angiotensin system in an animal model of type 2 diabetes. PLoS One. 2016;11:e0165703. doi: 10.1371/journal.pone.0165703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawanami D, Matoba K, Sango K, Utsunomiya K. Incretin-based therapies for diabetic complications: basic mechanisms and clinical evidence. Int J Mol Sci. 2016;17:E1223. doi: 10.3390/ijms17081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawanami D, Matoba K, Utsunomiya K. Signaling pathways in diabetic nephropathy. Histol Histopathol. 2016;31:1059–1067. doi: 10.14670/HH-11-777. [DOI] [PubMed] [Google Scholar]
- 15.Ueta K, Ishihara T, Matsumoto Y, Oku A, Nawano M, Fujita T, Saito A, Arakawa K. Long-term treatment with the Na+-glucose cotransporter inhibitor T-1095 causes sustained improvement in hyperglycemia and prevents diabetic neuropathy in Goto-Kakizaki Rats. Life Sci. 2005;76:2655–2668. doi: 10.1016/j.lfs.2004.09.038. [DOI] [PubMed] [Google Scholar]