Abstract
Purpose
This study investigated patterns of ischemic injury observed in brain images from patients with neonatal group B Streptococcal (GBS) meningitis.
Methods
Clinical findings and brain images from eight term or near-term newborn infants with GBS meningitis were reviewed.
Results
GBS meningitis was confirmed in all 8 infants via cerebrospinal fluid (CSF) analysis, and patients tested positive for GBS in both blood and CSF cultures. Six infants (75.0%) showed early onset manifestation of the disease (<7 days); the remaining 2 (25.0%) showed late onset manifestation. In 6 infants (75%), cranial ultrasonography showed focal or diffuse echogenicity, suggesting hypoxic-ischemic injury in the basal ganglia, cerebral hemispheres, and periventricular or subcortical white matter; these findings are compatible with meningitis. Findings from magnetic resonance imaging (MRI) were compatible with bacterial meningitis, showing prominent leptomeningeal enhancement, a widening echogenic interhemisphere, and ventricular wall thickening in all infants. Restrictive ischemic lesions observed through diffusion-weighted imaging were evident in all eight infants. Patterns of ischemic injury as detected through MRI were subdivided into 3 groups: 3 infants (37.5%) predominantly showed multiple punctuate lesions in the basal ganglia, 2 infants (25.0%) showed focal or diffuse cerebral infarcts, and 3 infants (37.5%) predominantly showed focal subcortical or periventricular white matter lesions. Four infants (50%) showed significant developmental delay or cerebral palsy.
Conclusion
Certain patterns of ischemic injury are commonly recognized in brain images from patients with neonatal GBS meningitis, and this ischemic complication may modify disease processes and contribute to poor neurologic outcomes.
Keywords: Neonate, Streptococcus agalactiae, Meningitis, Brain ischemia, Magnetic resonance imaging
Introduction
Neonatal bacterial meningitis is a serious and potentially life-threatening central nervous system (CNS) infection. It is associated with a variety of neurologic complications including seizure, hydrocephalus, arachnoiditis, subdural empyema, and encephalopathy [1]. Neonatal bacterial meningitis occurs usually with concomitant bacteremia, and group B Streptococcus (GBS) infection is responsible for 40%–50% of cases [2,3]. GBS meningitis can manifest early (before 1 week of age) by vertical transmission, late (1 week to 3 months of age), or very late (after 3 months of age) by interpersonal or nosocomial horizontal transmission [4,5]. In South Korean studies of GBS meningitis, most cases showed late onset manifestation [6,7], with one report of a nosocomial GBS infection outbreak [8].
Meningeal inflammation in neonatal bacterial meningitis extends to the brain parenchyma, causing various complications that include cerebritis, cerebral edema, and impaired cerebrospinal fluid (CSF) circulation, which results in hydrocephalus [9,10]. Also, prothrombotic process in the cerebral vasculature due to regional infection and inflammation can result in arterial and/or venous infarction [11-13]. In a large series of 166 children with perinatal and childhood meningitis, ischemic injury in the brain was identified in 10% of patients [12]. Suggested mechanisms for cerebral ischemic injury in bacterial meningitis include direct invasion of infection/inflammation with prothrombic process resulting in arterial and/or venous infarction, hematological disturbances causing hypercoagulable states, cardiovascular disorders causing distant embolisms, and systemic hypotension [13]. However, the precise mechanism for cerebral ischemic injury in neonatal bacterial meningitis, particularly in GBS infection, is still unclear.
Several recent brain imaging studies reported specific patterns of cerebrovascular injury in neonatal meningitis including arterial stroke and venous thrombosis [5,13,14]. These findings indicate that ischemic injury associated with neonatal meningitis could modify the disease process and contribute to severe neurologic outcomes [11,12,15,16]. In this case series study, we report the specific patterns of ischemic injury identified on the brain images by cranial ultrasonography (US) and brain magnetic resonance imaging (MRI) in cases of neonatal GBS meningitis and the relationship of these patterns with neurodevelopmental outcomes.
Material and methods
1. Subjects
Eight term or near-term newborn infants with GBS meningitis were identified from the database of Dankook University Hospital for the 3 years from 2013 to 2015. The 8 infants fulfilled the following criteria, based on the previous studies [5,17]: (1) gestational age (GA)>35 weeks, (2) <28 days of age at presentation of meningitis, (3) bacterial meningitis identified by clinical findings of CNS infection and confirmed by laboratory findings, usually defined as CSF white blood cell (WBC) count >50/mm3, and (4) GBS-positive CSF or blood culture. All eight infants with GBS meningitis underwent cranial US and brain MRI including diffusion weighted imaging (DWI) during admission.
2. Brain imaging
Cranial US was performed by the bedside in the neonatal intensive care unit using a IU-22 US equipped with a 5–12 MHz linear array (Philips Medical Systems, Bothell, WA, USA). Brain MRI was performed using a 1.5 Tesla medical system (GE, Milwaukee, WI. USA). Brain MRI examination included axial T1-weighted image and T2-weighted image, fluid attenuated inversion recovery, DWI, and apparent diffusion coefficient. All images of cranial US and brain MRI were retrospectively reviewed and analyzed by a pediatric radiologist and a neonatologist. Lesions clearly suspected as arterial stroke or focal cerebral infarct were usually observed.
Infants were sedated with oral chloral hydrate (30–50 mg/kg) under the supervision of an attending physician during the MRI examination. Oxygen saturation was monitored during examination. Earmuffs were used to minimize noise exposure.
3. Clinical findings and laboratory findings
Perinatal and neonatal clinical characteristics of infants and laboratory findings including CSF analysis were retrospectively reviewed.
4. Neurodevelopmental outcomes
Neurodevelopmental outcomes were observed between 1 and 2 years of age, based on physical examination and/or Bayley II neurodevelopmental examination. Cerebral palsy was considered as neurologic disorder characterized by muscular impairment caused by nonprogressive brain damage, and significant developmental delay was defined as MDI (mental developmental index) or PDI (psychomotor developmental index) <70 in the Bayley II examination.
Results
1. Clinical characteristics
Mean age of the eight infants at initial presentation was 7.1±5.9 days (range, 1–17 days). Five infants were male (62.5%). Six infants (75.0%) were in early onset (<7 days) and 2 (25.0%) were in late onset (>6 days). Seven infants were born at term with their GA>36 weeks and one was a late preterm with a GA of 35 weeks+4 days. The mean birth weight was 3,185.0±360.4 g (range, 2,820–3,860 g). One birth (12.5%) was by cesarean section. All eight infants were outborn. All infants had severe CSF pleocytosis, elevated CSF protein, and low CSF glucose level. Mean WBC count, protein and glucose levels of CSF were 6,768.5±7,179.2/mm3 (range, 468– 21,960/mm3), 457.9±286.6 mg/dL (range, 138.3–800.0 mg/dL), and 11.6±12.9 mg/dL (range, 0–39 mg/dL), respectively. GBS was detected in both CSF and blood cultures in all eight infants (Table 1). Maternal screening and antibiotic prophylaxis for GBS during pregnancy were not done in all cases. Other perinatal histories were non-specific and significant perinatal and maternal risk factors for GBS infection, such as premature rupture of membrane >18 hours, fetal distress, and low Apgar score <7 at 5 minutes, were not found. Initial clinical presentations were nonspecific and included fever, lethargy, irritability, respiratory distress, and poor feeding, etc.
Table 1.
Clinical and laboratory findings
| Case No. | Sex | Age of onset (day) | GA (wk) | BW (g) | Type of delivery | CSF cell count |
Initial empirical antibiotics | Antibiotics after culture result | Age at MRI (day) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC (/mm3) | Protein (mg/dL) | Glucose (mg/dL) | |||||||||
| 1 | M | 5 | 35+4 | 3,000 | Vaginal | 2,280 | 558 | 1 | AMP+CTX | PEN+CTX | 14 |
| 2 | M | 4 | 41+2 | 3,100 | Vaginal | 12,120 | 162.8 | 39 | AMP+GEN | PEN+CTX | 9 |
| 3 | F | 15 | 40+0 | 2,860 | Vaginal | 5,760 | 346 | 7 | AMP+CTX | PEN | 28 |
| 4 | M | 1 | 39+6 | 3,560 | Vaginal | 468 | 138.3 | 16 | AMP+CTX | PEN+CTX | 15 |
| 5 | M | 17 | 40+0 | 3,860 | CS | 720 | 700 | 0 | VAN+CTX | PEN+CTX | 34 |
| 6 | F | 8 | 39+4 | 3,020 | Vaginal | 5,800 | 784.8 | 1 | AMP+CTX | PEN+CTX | 17 |
| 7 | M | 1 | 38+2 | 2,820 | Vaginal | 21,960 | 800 | 13 | AMP+CTX | PEN+CTX | 12 |
| 8 | F | 6 | 38+1 | 3,260 | Vaginal | 5,040 | 173.9 | 16 | AMP+CTX | PEN+CTX | 11 |
GA, gestational age; BW, birth weight; CS, cesarean section; CSF, cerebral spinal fluid; WBC, white blood cell; AMP, ampicillin; CTX, cefotaxime; GEN, gentamicin; VAN, vancomycin; PEN, penicillin-G; MRI, magnetic resonance imaging.
2. Complications associated with GBS meningitis
Five infants (62.5%) displayed clinical seizures and were treated with antiepileptic medication, 3 (37.5%) had pneumonia, and 1 (12.5%) was treated with inhaled nitric oxide for persistent pulmonary hypertension of newborn. Four (50.0%) required respiratory support with mechanical ventilation, 2 (25.0%) were treated with inotropic drugs due to hemodynamic instability, and 1 (12.5%) was treated with continuous renal replacement therapy due to acute renal failure and multiple organ dysfunction syndrome (Table 2). All infants were treated with a combination of appropriate antibiotics (Table 1). Mean duration of antibiotics was 40.6±15.6 days (range, 23–66 days).
Table 2.
Complications associated with group B Streptococcal meningitis (n=8)
| Complication | No. (%) |
|---|---|
| Seizure | 5 (62.5) |
| Pneumonia | 3 (37.5) |
| Persistent pulmonary hypertension of newborn | 1 (12.5) |
| Support with mechanical ventilation | 4 (50.0) |
| Use of inotropics due to hemodynamic instability | 2 (25.0) |
| CRRT with ARF and MODS | 1 (12.5) |
CRRT, continuous renal replacement therapy; ARF, acute renal failure; MODS, multiple organ dysfunction syndrome.
3. Brain imaging
Mean age at Initial examination of cranial US was 8.6±7.1 days (range, 1–21 days). Initial US in 5 infants (62.5%) showed findings compatible with meningitis, which included leptomeningeal thickening, increased ventricular wall thickening, widening echogenic interhemispheric space, and fluid collection in subdural or subarachnoid space. In 6 infants (75%), cranial US showed focal or diffuse increased echogenicity that was suggestive of hypoxic- ischemic injury with findings compatible with meningitis. Particularly, increased echogenicity in the basal ganglia was shown on cranial US in 2 infants (cases 1 and 7), and diffuse parenchymal echogenicity in 4 infants (cases 1, 3, 4, and 6) (Figs. 1, 2; Table 3).
Fig. 1.
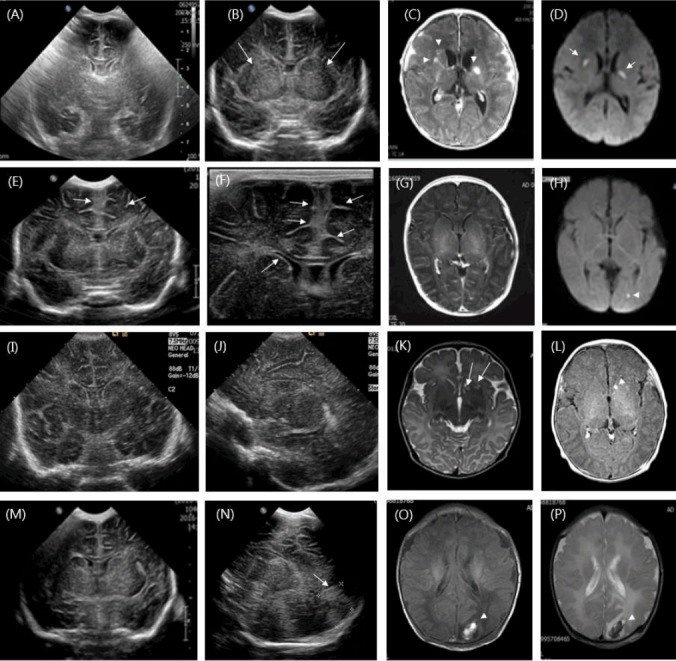
(A-D) Initial coronal ultrasonography of a 6-day-old boy shows diffuse increased echogenicity of the cerebral hemispheres. Ultrasonography conducted 3 days later in the same boy shows increased echogenicity in the both basal ganglia (long arrows). Magnetic resonance imaging (MRI) shows enhanced leptomeningeal thickening and linear enhancement along the ventricular wall with multiple enhanced lesions in both basal ganglia and focal rounded lesions in the head of the right caudate nucleus on an axial T1-weighted image (arrowheads); further, MRI shows multiple restricted lesions in the caudate nucleus and thalamus via diffusion-weighted imaging (DWI) (short arrows). (E-H) Cranial ultrasonography of a 4-day-old boy shows echogenic widening of the anterior interhemispheric fissure with leptomeningeal thickening and ventricular ependymal echogenicity (long arrows). MRI findings demonstrate prominent leptomeningeal enhancement on an axial T1-weighted image and a tiny restrictive lesion in the left occipital region on a DWI (arrowhead). (I–L) Cranial ultrasonography of a 16-day-old girl shows obliteration of ventricles with diffuse increased parenchymal echogenicity. MRI shows multiple lesions with high signal intensities in the head of the right caudate nucleus on a T2-weighted image (long arrows); one of these lesions is enhanced on a T1-weighted image (arrowhead). (M–P) Cranial ultrasonography of a 1-day-old boy shows diffuse increased parenchymal echogenicity, as seen with hypoxic ischemic encephalopathy, and ultrasonography from 3 days later shows a newly developed rounded echogenic lesion in the left occipital lobe (long arrow). MRI shows a subcortical heterogenous signal intensity mass lesion in the left occipital lobe on T1- and T2-weighted images without significant enhancement, suggesting hemorrhagic infarct (arrowheads).
Fig. 2.
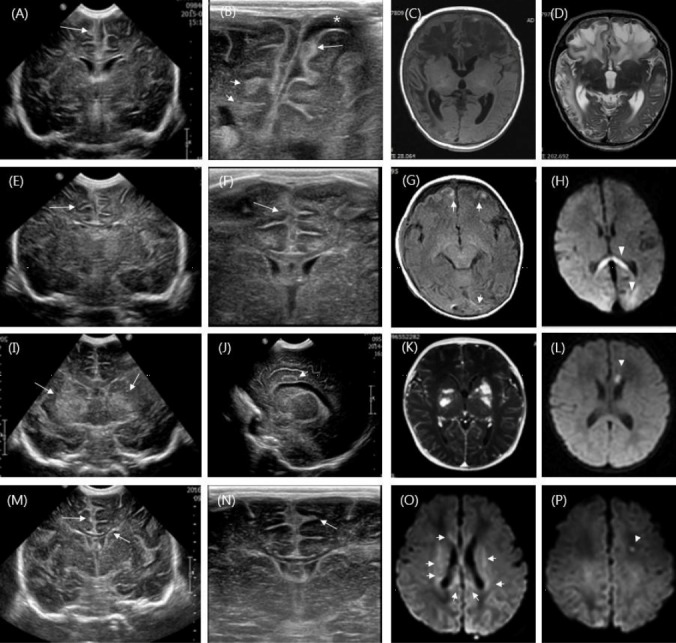
(A–D) Cranial ultrasonography in a 20-day-old boy shows prominent frontal leptomeningeal thickening and a widening interhemispheric fissure. Subarachnoid spaces with dirty echogenic materials (long arrow) on linear high frequency ultrasound are shown with subdural fluid collection (*). Focal echogenic lesions are also shown in the right periventricular white matter and frontal subcortical area (short arrows). Magnetic resonance imaging (MRI) shows diffuse patchy bilateral encephalomalacia in both fronto-temporo-parietal lobes. (E–H) Coronal ultrasonography in an 8-day-old girl shows diffuse parenchymal echogenicity with obliterated ventricles. Leptomeningeal thickening and a widening interhemispheric fissure are shown using linear high frequency ultrasonography (long arrows). MRI shows multiple focal high signal lesions in the frontal and occipital subcortical areas on a T1-weighted image (short arrows). High signal intensity lesions are evident via diffusion-weighted imaging (DWI) in the splenium of the corpus callosum and in both occipital subcortical areas (arrowheads). (I–L) Coronal ultrasonography in a 1-day-old boy shows increased echogenicity in both basal ganglia (long arrows) and oval-shaped lesions in the left cingulate gyrus (short arrow). MRI demonstrates multiple enhanced high signal T1-weighted echogenic lesions in both basal ganglia; further, MRI shows a focal restricted lesion in the genu of the corpus callosum via DWI (arrowhead). (M–P) Cranial sonography in a 6-day-old girl shows echogenic widening of the anterior interhemispheric fissure with leptomeningeal enhancement and ventricular ependymal echogenicity (long arrows). Areas of high signal intensity in the corpus callosum and periventricular area (short arrows) and focal restrictive lesions in the left frontal horn of the lateral ventricle are also shown via DWI (arrowhead).
Table 3.
Brain imaging findings and neurodevelopmental outcomes in group B Streptococcal meningitis
| Case No. | Ultrasonography | MRI | DD or CP |
|---|---|---|---|
| 1 | Diffuse increased echogenicity of the cerebral hemispheres. | Enhanced leptomeningeal thickenings and linear enhancement along the ventricular wall | Normal |
| Bilateral increased echogenicity in both BG | Enhanced multiple lesions in both BG and multiple restricted lesions in the caudate nucleus and thalamus on DWI | ||
| Leptomeningeal thickening and ventricular ependymal, increased echogenicity in interhemispheric fissure | |||
| 2 | Widening of anterior interhemispheric fissure | Prominent leptomeningeal enhancement | DD |
| Leptomeningeal thickening and ventricular ependymal echogenicity | A tiny restrictive lesion in the left occipital region on DWI | ||
| Diffuse echogenic fluid collection in subdural space of both cerebral convexity | |||
| 3 | Diffuse increased parenchymal echogenicity with obliteration of ventricles | Multiple high signal intensities in the head of the right caudate nucleus | Normal |
| Leptomeningeal enhancement | |||
| 4 | Diffuse increased parenchymal echogenicity | Hemorrhagic infarct in the left occipital lobe | DD, CP |
| 5 | Prominent leptomeningeal thickening and ventricular ependymal echogenicity | Diffuse bilateral patch encephalomalacia on both fronto-temporo-parietal lobe. | DD, CP |
| Widening interhemispheric fissure with dense dirty subarachnoidal echogenic materials and subdural fluid collection. Focal echogenic lesions on the periventricular white matter and frontal subcortical area | |||
| 6 | Diffuse parenchymal echogenicity with obliterated ventricles and sulci | Multiple focal high signal lesions in frontal and occipital areas | Normal |
| Leptomeningeal thickening and widening of interhemispheric fissure due to fluid collection | High signal intensity lesion on DWI in the splenium of corpus callosum and both occipital subcortical areas | ||
| 7 | Increased echogenicity in both BG | Multiple enhanced high signal T1-weighted echogenic lesions in both BG | DD |
| Focal restricted lesion in the genu of the corpus callosum on DWI. | |||
| 8 | Echogenic widening of anterior interhemispheric fissure | High signal intensities in the corpus callosum and periventricular area, and focal restrictive lesion in the left frontal horn of lateral ventricle on DWI. | Normal |
| Leptomeningeal enhancement and ventricular ependymal enhancement |
BG, basal ganglia; MRI, magnetic resonance imaging; DWI, diffusion weighted images; DD, developmental delay; CP, cerebral palsy.
Mean age at initial examination of brain MRI was 17.5±8.8 days (range, 9–34 days) (Table 1). The initial findings of brain MRI usually showed the prominent leptomeningeal enhancement and ventricular wall thickening compatible with bacterial meningitis. Also, restrictive lesions suggesting ischemic injury on the DWI were observed in all 8 infants. Patterns of ischemic injury on the brain MRI were subdivided into 3 groups: predominant multiple punctuate lesions in the basal ganglia in 3 cases (37.5%) (cases 1, 3, and 7), focal or diffuse cerebral infarcts in 2 cases (25.0%) (cases 4 and 5), and predominant focal subcortical or periventricular white matter lesions in 3 cases (37.5%) (cases 2, 6, and 8) (Figs. 1, 2; Table 3).
4. Neurodevelopmental outcomes
Four infants (50%) showed significant developmental delay or cerebral palsy between 1 and 2 years of age (Table 3). Neurodevelopmental outcomes according to patterns of ischemic injury on the brain MRI were investigated: basal ganglia lesions in 1 of 4 infants (case 7), predominant subcortical or periventricular white matter lesion in 2 of 4 infants (cases 2 and 6), and diffuse bilateral cerebral infarcts in 1 of 4 infants (case 5). No infant displayed seizure or recurrence of stroke since discharge.
Discussion
In this small case-series study, patterns of cerebral ischemic injury were identified on the brain images of neonatal GBS meningitis. This ischemic injury could greatly affect or aggravate brain damage caused by inflammation in bacterial meningitis. It is presumed that this may be closely related to poor neurological outcomes.
Neonatal bacterial meningitis often occurs concomitantly with the onset of bacteremia, especially those caused by GBS or Gram-negative pathogens like Escherichia coli [1,18,19]. GBS is associated with various neurologic complications including cerebritis and ventriculitis by the spread of inflammation to adjacent brain parenchyma [6,10]. In addition, cerebral ischemic injury is commonly associated with neonatal bacterial meningitis and may affect later neurodevelopmental outcomes [11,12,15,16]. In 2 small case-series studies, patterns of cerebral ischemic injury were commonly observed on the brain MRI in infants with GBS meningitis [5,17], similar to the results of this study.
Local inflammation of the meninges in cases of meningoencephalitis can extend to the adjacent blood vessels, resulting in arteriritis [13,20], and the regional inflammation may promote platelet aggregation, regional thrombosis, and subsequent arterial occlusion, resulting in stroke [5,11,12,21]. However, the precise mechanism for cerebral ischemic injury in neonatal bacterial meningitis is not well understood.
Brain imaging is important in evaluating acute brain injury and predicting prognosis in neonatal bacterial meningitis [11,14,22]. Cranial US is a noninvasive and inexpensive bedside tool, and examinations can be repeated as often as necessary. It is an important tool for initial evaluation of suspected acute brain injury in infants with CNS infection. Possible ultrasonographic findings of bacterial meningitis in neonates are wide echogenic sulci, enhanced leptomeningeal thickening, ventricular wall thickening with ventriculomegaly, hydrocephalus, and change in brain parenchymal echogenicity [9,23]. Such abnormal findings were observed in 61%–73% of cases in previous studies in acute phase of bacterial meningitis [24-28]. In addition, increased diffuse or local brain parenchymal echogenicity is commonly found on the cranial US in bacterial meningitis [23]. These lesions on the cranial US are thought to result from the penetration of organisms from blood vessels into the surrounding brain parenchyma subsequently inducing inflammation, and may be also associated with hypoxia-ischemia [23,29]. The results of this study, similar to the results of previous studies, suggest that increased parenchymal echogenicity on cranial US might be associated with hypoxic-ischemic injury, which has been more clearly demonstrated by brain MRI [5,17]. Presently, focal or diffuse increased echogenicity suggesting hypoxia-ischemia were observed in 6 of the infants using cranial US, along with evidence of meningitis, such as leptomeningeal thickening, increased ventricular wall thickening, wide echogenic interhemispheric space, and fluid collection in the subdural or subarachnoid space. However, cranial US is not an appropriate tool to detect cortical or subcortical lesions, and lesions in the posterior fossa, and is not the best imaging technique to detect subtle white matter lesions [22,30].
On the other hand, brain MRI is a more appropriate tool to detect the pattern of brain structural injury. The initial findings of the brain MRI in this study were usually compatible with those in bacterial meningitis, such as prominent leptomeningeal enhancement and ventricular wall thickening. In addition, in all 8 infants, the patterns of ischemic injury were detected on the brain MRI including DWI. Patterns of ischemic injury on the brain MRI were subdivided into 3 groups: predominant multiple punctuate lesions in the basal ganglia in 3 infants (37.5%), focal or diffuse cerebral infarcts in 2 infants (25.0%), and predominant focal subcortical or periventricular white matter lesions in 3 infants (37.5%). However, we could not identify the association between specific patterns of ischemic injury on the brain MRI and neurodevelopmental outcomes, since the number of infants enrolled was too small and the duration of follow-up for neurodevelopment was short.
Usually, it is presumed that major cerebral arteries entering through the subarachnoid space are involved in the deep injury pattern in basal ganglia, and consequently gray matter and white matter are thought to be damaged [4,21]. As mentioned before, the suggested mechanism of ischemic injury accompanying the inflammation in bacterial meningitis is that regional inflammation may promote platelet aggregation, regional thrombosis, and subsequent arterial occlusion [5,11,12 21]. The precise mechanism remains unclear. A previous study suggested that subarachnoid inflammation directly invades the major vessels of the circle of Willis and the smaller perforating lenticulostriate and thalamostriate arteries, and affects both pial arteries and the veins [4]. Further studies including detailed angiographic imaging are necessary.
Among the infants enrolled in this study, 6 (75%) were early onset (before 1 week of age) and all 8 had invasive GBS disease. However, in South Korean studies of GBS meningitis, most cases showed late onset manifestation, especially after 3rd week of age [6,7]. This difference may suggest the possibility of recent changes in the prevalence and epidemiology of invasive GBS infection in Korea. The previous studies for prevalence of maternal GBS colonization in Korea have reported that the colonization rate of GBS is very low compared to other countries (1.96%–8% vs. 20%–30%) [31-33]. Therefore, the results of this study may suggest that the rate of GBS colonization has been likely to increase in Korea, and this tendency may be reflected in the results of this study. Further study for GBS prevalence in pregnancy is needed and routine maternal screening for GBS infection with antibiotic prophylaxis is also necessary in South Korea.
All infants in this study had proven GBS infection in both CSF and blood culture, but no infant underwent serotype identification. Therefore, we could not identify the correlation associated with serotype difference. Other limitations of this study are the retrospective observational nature of the study on the basis of the brain images and the small number of subjects. However, despite the above limitations, the results of this study clearly demonstrate that ischemic injury is commonly associated with GBS meningitis in neonates, and specific injury patterns could be identified on the brain images of GBS meningitis. So, we can suggest that management for neonatal GBS meningitis in acute phase should be considered to include anticoagulants in addition to rapid appropriate antibiotics to prevent further brain damage by ischemia and for better neurodevelopmental outcomes.
In conclusion, we suggest that cerebral ischemic injury is a common complication of neonatal GBS meningitis, which is identified on the brain images. This ischemic complication may modify the disease process and contribute to later poor neurologic outcomes. In neonatal bacterial meningitis, brain image studies including angiography have to be performed during the acute phase and the appropriate management including anticoagulant should be considered if there is evidence of arterial ischemic stroke or venous thrombosis.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Chu SM, Hsu JF, Lee CW, Lien R, Huang HR, Chiang MC, et al. Neurological complications after neonatal bacteremia: the clinical characteristics, risk factors, and outcomes. PLoS One. 2014;9:e105294. doi: 10.1371/journal.pone.0105294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heath PT, Nik Yusoff NK, Baker CJ. Neonatal meningitis. Arch Dis Child Fetal Neonatal Ed. 2003;88:F173–8. doi: 10.1136/fn.88.3.F173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ku LC, Boggess KA, Cohen-Wolkowiez M. Bacterial meningitis in infants. Clin Perinatol. 2015;42:29–45. doi: 10.1016/j.clp.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berardi A, Rossi C, Lugli L, Creti R, Bacchi Reggiani ML, Lanari M, et al. Group B streptococcus late-onset disease: 2003-2010. Pediatrics. 2013;131:e361–8. doi: 10.1542/peds.2012-1231. [DOI] [PubMed] [Google Scholar]
- 5.Hernández MI, Sandoval CC, Tapia JL, Mesa T, Escobar R, Huete I, et al. Stroke patterns in neonatal group B streptococcal meningitis. Pediatr Neurol. 2011;44:282–8. doi: 10.1016/j.pediatrneurol.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Cheong J, Byun SY, Kim MJ. Group B streptococcal meningitis in neonate: 2001-2011. Korean J Perinatol. 2013;24:142–7. [Google Scholar]
- 7.Lee SY, You SJ, Kim DS, Ko TS. Clinical study of group B beta-Hemolytic streptococcal meningitis. J Korean Pediatr Soc. 2003;46:1224–9. [Google Scholar]
- 8.Kim HJ, Kim SY, Seo WH, Choi BM, Yoo Y, Lee KH, et al. Outbreak of late-onset group B streptococcal infections in healthy newborn infants after discharge from a maternity hospital: a case report. J Korean Med Sci. 2006;21:347–50. doi: 10.3346/jkms.2006.21.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yikilmaz A, Taylor GA. Sonographic findings in bacterial meningitis in neonates and young infants. Pediatr Radiol. 2008;38:129–37. doi: 10.1007/s00247-007-0538-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briand C, Levy C, Baumie F, Joao L, Béchet S, Carbonnelle E, et al. Outcomes of bacterial meningitis in children. Med Mal Infect. 2016;46:177–87. doi: 10.1016/j.medmal.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Bodilsen J, Dalager-Pedersen M, Schønheyder HC, Nielsen H. Stroke in community-acquired bacterial meningitis: a Danish population-based study. Int J Infect Dis. 2014;20:18–22. doi: 10.1016/j.ijid.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Chang CJ, Chang WN, Huang LT, Chang YC, Huang SC, Hung PL, et al. Cerebral infarction in perinatal and childhood bacterial meningitis. QJM. 2003;96:755–62. doi: 10.1093/qjmed/hcg128. [DOI] [PubMed] [Google Scholar]
- 13.Takeoka M, Takahashi T. Infectious and inflammatory disorders of the circulatory system and stroke in childhood. Curr Opin Neurol. 2002;15:159–64. doi: 10.1097/00019052-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Chatue Kamga HB. Neuroimaging complication of neonatal meningitis in full-term and near-term newborns: a retrospective study of one center. Glob Pediatr Health. 2016;3:2333794X16681673. doi: 10.1177/2333794X16681673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katchanov J, Heuschmann PU, Endres M, Weber JR. Cerebral infarction in bacterial meningitis: predictive factors and outcome. J Neurol. 2010;257:716–20. doi: 10.1007/s00415-009-5395-9. [DOI] [PubMed] [Google Scholar]
- 16.Snyder RD, Stovring J, Cushing AH, Davis LE, Hardy TL. Cerebral infarction in childhood bacterial meningitis. J Neurol Neurosurg Psychiatry. 1981;44:581–5. doi: 10.1136/jnnp.44.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tibussek D, Sinclair A, Yau I, Teatero S, Fittipaldi N, Richardson SE, et al. Late-onset group B streptococcal meningitis has cerebrovascular complications. J Pediatr. 2015;166:1187–92. doi: 10.1016/j.jpeds.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 18.May M, Daley AJ, Donath S, Isaacs D; Australasian Study group for neonatal infections. Early onset neonatal meningitis in Australia and New Zealand, 1992-2002. Arch Dis Child Fetal Neonatal Ed. 2005;90:F324–7. doi: 10.1136/adc.2004.066134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Synnott MB, Morse DL, Hall SM. Neonatal meningitis in England and Wales: a review of routine national data. Arch Dis Child. 1994;71:F75–80. doi: 10.1136/fn.71.2.f75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mineyko A, Kirton A. Mechanisms of pediatric cerebral arteriopathy: an inflammatory debate. Pediatr Neurol. 2013;48:14–23. doi: 10.1016/j.pediatrneurol.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 21.Amayo EO, Kayima JK, Joshi MD. Platelet functions in patients with meningococcal meningitis at the Kenyatta National Hospital, Nairobi. East Afr Med J. 2002;79:405–7. doi: 10.4314/eamj.v79i8.8825. [DOI] [PubMed] [Google Scholar]
- 22.Rademaker KJ, Uiterwaal CS, Beek FJ, van Haastert IC, Lieftink AF, Groenendaal F, et al. Neonatal cranial ultrasound versus MRI and neurodevelopmental outcome at school age in children born preterm. Arch Dis Child Fetal Neonatal Ed. 2005;90:F489–93. doi: 10.1136/adc.2005.073908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta N, Grover H, Bansal I, Hooda K, Sapire JM, Anand R, et al. Neonatal cranial sonography: ultrasound findings in neonatal meningitis-a pictorial review. Quant Imaging Med Surg. 2017;7:123–31. doi: 10.21037/qims.2017.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han BK, Babcock DS, McAdams L. Bacterial meningitis in infants: sonographic findings. Radiology. 1985;154:645–50. doi: 10.1148/radiology.154.3.3881791. [DOI] [PubMed] [Google Scholar]
- 25.Mahajan R, Lodha A, Anand R, Patwari AK, Anand VK, Garg DP. Cranial sonography in bacterial meningitis. Indian Pediatr. 1995;32:989–93. [PubMed] [Google Scholar]
- 26.Raju VS, Rao MN, Rao VS. Cranial sonography in pyogenic meningitis in neonates and infants. J Trop Pediatr. 1995;41:68–73. doi: 10.1093/tropej/41.2.68. [DOI] [PubMed] [Google Scholar]
- 27.Soni JP, Gupta BD, Soni M, Gupta M, Dabi DR, Nemal KR. Cranial ultrasonic assessment of infants with acute bacterial meningitis. Indian Pediatr. 1994;31:1337–43. [PubMed] [Google Scholar]
- 28.Arrumugham R, Katariya S, Singhi P, Singhi S, Suri S, Walia BN. Sonography in pyogenic meningitis. Indian Pediatr. 1994;31:1329–36. [PubMed] [Google Scholar]
- 29.Abels L, Lequin M, Govaert P. Sonographic templates of newborn perforator stroke. Pediatr Radiol. 2006;36:663–9. doi: 10.1007/s00247-006-0125-2. [DOI] [PubMed] [Google Scholar]
- 30.Roelants-van Rijn AM, Groenendaal F, Beek FJ, Eken P, van Haastert IC, de Vries LS. Parenchymal brain injury in the preterm infant: comparison of cranial ultrasound, MRI and neurodevelopmental outcome. Neuropediatrics. 2001;32:80–9. doi: 10.1055/s-2001-13875. [DOI] [PubMed] [Google Scholar]
- 31.Lee BK, Song YR, Kim MY, Yang JH, Shin JH, Seo YS, et al. Epidemiology of group B streptococcus in Korean pregnant women. Epidemiol Infect. 2010;138:292–8. doi: 10.1017/S0950268809990859. [DOI] [PubMed] [Google Scholar]
- 32.Choi KU, Koh SK, Lee JY, Park JH, Hwang SO, Lee BI, et al. Clinical significance of group B streptococcal infection in pregnant women. Korean J Obstet Gynecol. 2002;45:811–5. [Google Scholar]
- 33.Madhi SA, Dangor Z, Heath PT, Schrag S, Izu A, Sobanjo-Ter Meulen A, et al. Considerations for a phase-III trial to evaluate a group B Streptococcus polysaccharide-protein conjugate vaccine in pregnant women for the prevention of early- and late-onset invasive disease in young-infants. Vaccine. 2013;31 Suppl 4:D52–7. doi: 10.1016/j.vaccine.2013.02.029. [DOI] [PubMed] [Google Scholar]


