Abstract
Objective
Maternal obesity accounts for the majority of large-for-gestational-age infants and newborn percent fat (NB%fat) correlates strongest with childhood obesity. In addition to maternal glucose, fasting triglycerides may contribute but postprandial triglycerides (PPTG) are unstudied. We hypothesized that fasting and PPTG are higher in obese (OB) compared to normal-weight (NW) women throughout pregnancy, correlate more strongly with NB%fat than glucose, and may relate to dietary chylomicron-TG.
Methods
Fasting and PPTG, FFA, glucose, and insulin were prospectively measured 10 times over 4 hours after a controlled liquid breakfast early (14-16 wks) and later (26-28 wks) in 27 NW and 27 OB mothers. Newborn %fat was measured by DXA.
Results
Fasting and PPTG were already ≥30% higher in OB mothers at 14-16 wks (p<0.001) vs. NW. In OB mothers, a simple 1-hr (r=0.71; p<0.01) or 2-hr (r=0.69; p<0.01) PPTG at 14-16 wks correlated strongest with NB%fat. In NW, the increase in TG from early to later pregnancy correlated strongest with NB%fat(r=0.57; p< 0.01). Maternal glucose did not statistically add to prediction models.
Conclusions
These novel data suggest that 1- or 2-hr PPTG might be a new target for early intervention in obese pregnancies to prevent excess newborn adiposity and attenuate child obesity risk.
Keywords: Maternal Obesity, Pregnancy, Triglycerides, Newborn Fat Mas
INTRODUCTION
Obesity affects nearly 40% of women of child-bearing age(1) and accounts for a greater number of large-for-gestational-age (LGA) infants than pregnancies complicated by gestational diabetes mellitus (GDM)(2). The fetal over-nutrition hypothesis suggests maternal fuels contributing to excess fetal growth and childhood obesity are not limited to glucose and these fuels are in greater abundance in maternal obesity(3). Our group previously demonstrated that despite a controlled diet, women with obesity have higher 24-hour glycemic patterns than normal-weight (NW) both early and later in gestation(4) but a single fasting triglyceride (FTG), measured once in early pregnancy, correlated more strongly than glucose with newborn percent fat (NB%fat). Postprandial lipids were not measured and NB%fat was estimated by skinfolds. Some studies(4) but not others, have shown that FTG and fasting free fatty acids (FFA) may contribute to fetal fat accretion, but well-controlled, prospective studies in women with obesity have not been performed(5). Further, recent evidence suggests that early exposure to excess maternal fuels may increase the risk for excess fetal growth equal to or greater than later exposure(6).
Due to inconsistent data, maternal lipids are not recognized risk factors for excess fetal fat accretion and are not currently targeted for intervention. Whether postprandial TG (PPTG) elevations more strongly contribute to fetal growth requires elucidation(5, 7, 8, 9, 10, 11, 12), especially given that PP glucose predicts excess growth and is standard-of-care in the management of GDM. Most studies have not controlled for maternal diet, which markedly affects both FTG and PPTG for up to 3 days before measurement(13). Moreover, differential lipoprotein contributions to total TG from maternal diet (chylomicrons [CM-TG]) versus liver-synthesized TG (very-low- density lipoproteins [VLDL-TG]) are unreported. Both may be important given TG are hydrolyzed by placental lipases to FFA for fetal fat accretion(14). Our group recently demonstrated that the activity of placental lipoprotein lipase is correlated with newborn adiposity(15). If one type of TG lipoprotein correlates more strongly with fetal fat accretion, targeting FTG (VLDL-TG) versus PPTG (CM-TG) through interventions may be more effective(16). New data also support that the early intrauterine metabolic environment may influence placental nutrient transport and fetal growth more than later pregnancy. This may be, in part, why mid-gestation interventional trials to prevent LGA infants are often unsuccessful(17). Although birth weight (BW) is typically used to quantify growth, NB%fat is a better proxy for intrauterine nutrient exposure and stronger predictor of childhood obesity(18). To account for these important factors, we designed a prospective trial that carefully controls maternal diet to test the hypothesis that fasting and PPTG are higher in obese (OB) versus NW women both early and later in pregnancy, and more strongly predict NB%fat than glucose.
METHODS
Study Population and Design
This was a NIH-funded prospective trial (R56DK078645; R01DK078645) approved by the Colorado Multiple Institutional Review Board (COMIRB 07-0535). Pregnant women (18-35 yrs) were recruited at University of Colorado Hospital from 2008-2015. Fifty-four English-speaking mothers with singleton pregnancies were enrolled at ~12 wks based on pre-pregnancy BMI (n=27 NW, BMI 20-25 kg/m2; n=27 OB, BMI 30-38) who were otherwise healthy. All chronic medical conditions were excluded as was a history of preeclampsia to minimize the risk of placental insufficiency and growth restriction. Mothers with a history of preterm birth (PTB) were excluded due to the progressive increase in fetal fat accretion from 28 to 40 wks given the primary outcome was fat mass in term infants (≥37 weeks) by dual X-ray absorptiometry (DXA). All OB women were screened for glucose intolerance before enrollment(19) and excluded if they failed an early 100-gram oral glucose-tolerance-test (OGTT) by Carpenter and Coustan criteria(19).
All 27 NW and 24/27 OB were studied both early (14-16 wks) and later (27-28 wks) for their fasting/postprandial response to a liquid test meal. Three OB mothers studied early could not be studied later due to gall bladder disease, pregnancy loss, or relocation. At 28 wks, all subjects underwent a 100-gram OGTT to exclude GDM(19), and measure glucose and insulin at baseline/fasting, 1-, 2- and 3-hrs for insulin sensitivity estimates. Given rapid fetal fat accumulation over third trimester, only term (≥37 wks), healthy newborns were analyzed for %fat by DXA. One newborn from a NW mother was excluded due to PTB. Of the 24 OB mothers who completed both early and later studies, 5 newborns could not undergo DXA due to PTB, preterm premature rupture of membranes (PPROM), preeclampsia, subchorionic hemorrhage, and birth trauma, resulting in 26 NW and 19 OB offspring for term NB%fat analysis.
Liquid Meal Studies for Metabolic Measures
Because diet affects glucose and lipids for several days(13), subjects were provided with 3-day standardized lead-in diets prepared by the Colorado Clinical Translational Science Institute (CCTSI) metabolic kitchen preceding the two liquid breakfast visits. The lead-in and liquid breakfast diets were matched for macronutrients; 50% carbohydrate (30% complex/20% simple sugars), 35% fat (12% saturated/12% monounsaturated/11% polyunsaturated), 15% protein. Energy requirements were based on Institute of Medicine guidelines (OB-25 kcal/kg; NW-30 kcal/kg). Studies were completed within tight gestational windows of 14-16 and 26-28 wks given the progressive increase in insulin resistance of pregnnacy.
After 3 days of standardized diet at 14-16 and 26-28 wks, fasted subjects (10 hrs) had baseline labs drawn on the CCTSI. They were then given a liquid breakfast shake (30% of total calories) based on data that the PPTG response can be completely captured within 4 hrs after a liquid breakfast(20). Following breakfast, 9 additional samples were collected over 4 hrs (T=20,40,60,90,120,150,180,210,240 mins) for plasma TG, glucose (GLUC), insulin (INS) and FFA (CCTSI laboratory) using assays previously reported(4, 21). Using the trapezoidal method, the 4-hr area-under-the-curve (AUC) was calculated to characterize the postprandial GLUC, INS, TG, and FFA response to the meal(21). To determine if the 4-hr AUC correlated with simpler 1-hr or 2-hr PP measures used in GDM to capture postprandial glucose responses, the 4-hr TG-AUC and 4-hr GLU-AUC were correlated with the 1-hr and 2-hr PPTG and glucose. Because maternal insulin resistance (IR) increases availability of all nutrients to the fetal-placenal unit, maternal IR was estimated by 3 indices: 1) Fasting IR by HOMA-IR(22); 2) Postprandial IR by 4-hr glucose and insulin AUC product (Meal-IR; GLU-AUC*INS-AUC); and 3) OGTT-IR glucose and insulin AUC product (OGTT; GLU-AUC*INS-AUC)(23) on the 28wk 3-hr 100-gram OGTT.
Total FTG/PPTG were separated to measure TG-rich lipoproteins synthesized from the liver (VLDL-TG) versus dietary chylomicrons from the gut (CM-TG), given both can be hydrolyzed by pLPL(14) into FFA for fetal fat accretion. Triglyceride-rich lipoprotein separations were performed successfully as previously described(24) from samples collected (T=0,60,120,180,240 minutes) in 33 women.
Newborn Fat Measures
Forty-five term newborns underwent DXA at Children’s Hospital Colorado at ~2wks of life (mean 15.6 days, range=12-20 days; QDR Discovery fan beam densitometer, Hologic Delphi-W, Waltham, MA; Apex version 3.2) as described previously(25) for measurement of fat mass and fat free mass. The DXA was performed at 2 wks due to the expected newborn diuresis affecting total body water and the re-equilibration of fat mass by 7-14 days(26), especially in breast-fed infants. Later in the study, air displacement plethysomography (PEA POD) was added (same day, ±2 hrs of DXA) to also estimate NB%fat due to scanning ease(25) and absence of any radiation; however, because it was unavailable for the first third of the cohort, %fat by DXA is reported. In n=2 newborns (1 from NW, 1 from obese), DXA revealed a calibration error. Given our previous data(25) showed a strong correlation between 2-wk DXA and PEA POD (r=0.74), both were used (n=33 paired cases) to determine a regression allowing prediction of NB%fat by DXA “y” using the PEA POD estimate “x”(y=0.77× + 1.35) in the 2 cases of DXA calibration error.
Statistical Analysis
Data are mean±SEM; all approximated a normal distribution. Between-group differences were assessed using t-tests for independent groups; within-group differences by paired t-tests. Correlations were assessed using Pearson’s r. Variables that exhibited univariate significance were included in simple and multiple linear regression models to test for predictive associations (IBM SPSS Statistics v24; Armonk, NY). Power was calculated a-priori (PASS software; Kaysville, UT) to test the hypothesis that obese mothers have higher FTG in early and later gestation. Based on pilot data (R56DK078645), 15 women/group would detect a FTG difference of 58 mg/dL (SD=34.12) for 97% power (α=0.017) using a 2-sided/2-sample t-test. Further, it was calculated a-priori from our pilot data (R2=0.81; 2-sided) that a total of 45 women would allow for >80% power (α=0.05) to detect a significant association between the change in FTG from early to late pregnancy and NB%fat. A Bonferroni correction was applied to minimize risk of a Type 1 error from multiple comparisons; α≤0.01 was considered statistically significant.
RESULTS
Maternal and Newborn Characteristics
Age, gestational weight gain, and %primigravidas were not statistically different between groups; 93% were Caucasian (Table 1). By design, the mean pre-pregnancy BMI in NW was 22.3 versus 31.7 kg/m2 in OB; gestational age at delivery was identical. A slightly higher percentage of males was born to OB compared to NW (68% vs 50%; p>0.05). Birthweight was significantly higher in OB offspring with a trend for increased NB%fat. All except 1 infant in each group were exclusively breastfed.
Table 1.
Maternal and Newborn Characteristics of NW and Obese Subjects
| Maternal Characteristics | ||
|---|---|---|
| Normal Weight (n=27) | Obese (n=27) | |
| Age (yrs) | 30.5 ± 0.63 | 29.8 ± 0.80 |
| Pre-Pregnancy BMI (kg/m2) | 22.3 ± 0.34 | 31.7 ± 0.62* |
| Gestational Weight Gain (kg) | 13.7 ± 0.84 | 14.2 ± 1.6 |
| Primigravida (% total) | 14 (51.9) | 11 (40.7) |
| Caucasian (% total) | 25 (92.6) | 25 (92.6) |
| Newborn Characteristics | ||
| Normal Weight (n=26) | Obese (n=19) | |
| Gestational Age at Delivery (wks) | 39.7 ± 0.2 | 39.7 ± 0.23 |
| Cesarean (% total) | 23% | 37% |
| Birthweight (grams) | 3258.0 ± 73.6 | 3557.6 ± 107.8* |
| Female (% total) | 50% | 32% |
| 2-wk NB %fat | 8.9 ± 0.72 | 11.0 ± 1.2 |
| 2-wk Total Mass (grams) | 3864.8 ± 95.4 | 4122.5 ± 136.9 |
Data are mean ± SEM; NB = newborn
Indicates NW vs. OB, p = 0.004
Metabolic Differences Between Groups
Table 2 and Figure 1 show that FTG were higher in OB, early and later, as were fasting INS, GLUC, and the 1- and 2-hr PP and 4-hr AUC measures. Fasting glucose was <80 mg/dL, early and later in both groups, indicative of relatively healthy OB women and glucoses were overall ~10%higher in the OB mothers. More striking than differences in glucose were the 30-40% differences in fasting, 1-hr, and 2-hr PPTG and the 4-hr TG-AUC (OB vs. NW FTG: 126.2 vs. 89.2 mg/dL, early; 174.9 vs. 135.1, later; both p<0.01). The change from early to late gestation between groups did not differ. Obese women had 50-60% higher fasting and PP INS (Figure 1B) and demonstrated greater IR by all estimates both early and later (p<0.01; Table 2). In fact, insulin and glucose were already higher in OB women early in pregnancy compared to NW later in pregnancy (Figure 1B,C).
Table 2.
Early (12-14 wks) and late (26-28 wks) gestation metabolic measures between NW and obese women.
| Normal(Weight | Obese | |||||
|---|---|---|---|---|---|---|
| Early (n=27) | Later (n=27) | Delta | Early (n=27) | Later (n=24) | Delta | |
| Fasting TG (mg/dL) | 89.2±3.98 | 135.1±7.8 | 45.9±5.3 | 126.2±8.7* | 174.9±12.2# | 50.2±5.9 |
| 1-hr PPTG (mg/dL) | 95.3 ± 4.6 | 153.2 ± 8.0 | 58.0±4.6 | 143.4 ± 10.8* | 201.2 ± 13.3# | 60.1±6.0 |
| 2-hr PPTG (mg/dL) | 86.6 ± 5.2 | 137.9 ± 8.1 | 51.3±5.1 | 135.3 ± 10.7* | 189.1 ± 13.1 * | 55.9±6.4 |
| TG-AUC (mg*min/dL) | 22708.7 ± 1216.4 | 34467.6 ± 1879.9 | 11758.9±1005.7 | 33584.6 ± 2629.2* | 46209.2 ± 3245.5# | 13244.1±481.1 |
| Fasting Glucose (mg/dL) | 73.0 ± 0.88 | 72.7 ± 1.13 | −0.37±0.94 | 76.7 ± 0.99# | 76.9 ± 1.1# | 0.25±1.32 |
| 1-hr PP Glucose (mg/dL) | 83.3 ± 2.5 | 83.6 ± 2.5 | 0.26±2.4 | 90.3 ± 2.5 † | 96.3 ± 2.9# | 5.1±2.8 |
| 2-hr PP Glucose (mg/dL) | 85.6 ± 1.6 | 86.6 ± 1.9 | 1.1±1.7 | 93.2 ± 1.5* | 95.5 ± 1.7* | 2.1±1.9 |
| Glucose-AUC (mg*min/dL) | 19513.9±255.3 | 19472.2±331.5 | −41.7±230.62 | 21280.6±337.8 * | 21629.6±281.9 * | 379.1±413.3 |
| Fasting Insulin (μIu/mL) | 6.6±0.6 n=27 | 9.1±0.73 | 2.5±0.62 | 10.5±1.2# | 13.7±1.4# | 3.0±0.96 |
| 1-hr PP Insulin (μIu/mL) | 37.9 ± 4.2 | 48.6 ± 4.8 | 10.8±4.0 | 62.2 ± 9.4 † | 85.0 ± 11.1# | 22.5±5.5 |
| Insulin-AUC (μIU*min/mL) | 4995.9 ± 408.9 | 6539.4 ± 430.9 | 1543.5±291.9 | 9423.3 ± 132.3 * | 12766.7 ±1673.2 * | 3328.0±596.2 |
| Fasting FFA (μEq/L) | 655.2±30.5 | 502.96±96 | −152.2±34.0 | 628.6±42.7 | 557.42±30.2 | −49.3±33.0 |
| FFA-AUC (μEq*min/L) | 68017.6±2343.4 | 64410.2±2834.8 | −3607.4±2685.9 | 67548.3±4784.9 | 65942.1±3188.5 | −1880.7±2758.3 |
| HOMA-IR | 1.2 ± 0.12 | 1.7 ± 0.15 | 0.47±0.12 | 2.02 ± 0.25# | 2.6 ± 0.29# | 0.59±0.2 |
| Meal-IR (×107) | 9.9 ± 0.89 | 13.0 ± 1.0 | 3.1±0.69 | 20.6 ± 3.3# | 28.2 ± 4.1 * | 7.61±1.54# |
| OGTT-IR (×108) | 2.4 ± 0.33 | 3.96 ± 0.48# | ||||
Data are mean ±SEM;
indicates NW vs. OB at same time point p≤0.001;
indicates NW vs. OB at same time point p<0.01;
indicates NW vs. OB at same time point p<0.05; PP = postprandial; AUC = area-under-the-curve; IR = insulin resistance; OGTT = oral glucose tolerance test.
Figure 1.
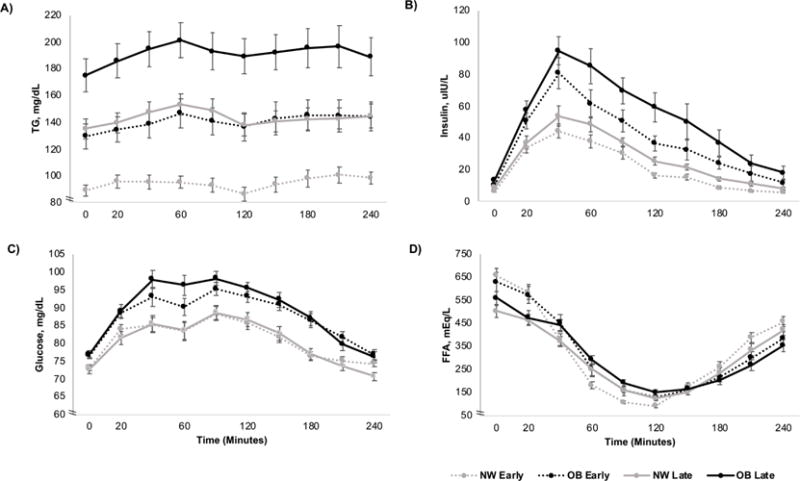
The 4-hr AUC of total TG (A), insulin (B), glucose (C) and FFA (D) in NW vs. obese (OB) women early (12-14 wks) and later (26-28 wks) in pregnancy.
Obese women later in pregnancy had the highest total TG (including CM-TG and VLDL-TG; Figure 1A) in the fasting and PP states. Importantly, early pregnancy TG in OB women were already as high as later TG in NW women. Postprandial TG peaked 1-hr after the meal and the increase in 1-hr PPTG in the total cohort was accounted for by both dietary CM-TG (r=0.91, p<0.001) and VLDL-TG (r=0.78; p<0.01). We further determined that the 4-hr TG-AUC could be entirely captured by a single 1-hr PPTG or 2 hr PPTG (both r=0.98; p<0.001). We emphasize the 1-hr PPTG measure for simplicity. Postprandial FFA were not different between the groups (Figure 1D) and were similarly suppressed by the much higher insulin levels in OB women.
Predictors of Newborn Adiposity
Table 3 and Figures 2–5 show correlations and simple and multiple regressions for TG and glucose with NB%fat, early and later, and by group. In the total cohort (Figure 2), FTG early and later similarly correlated with NB%fat (r=0.47 early, r=0.56 later; both p≤0.001) as did 1-hr and 2-hr PPTG (r=0.57, early; r=0.54-0.58, later; both p<0.001) and predicted ~32% of the variance in NB%fat (R2=0.32; p<0.001). The TG lipoprotein fractionations in 33/45 mothers revealed that in the total cohort, the early 1-hr CM-TG significantly correlated with NB%fat (r=0.61) as did the 1-hr VLDL-TG (r=0.59). In OB mothers (Figure 3), the FTG in early pregnancy (r=0.60; p=0.006) obtained at 14-16 wks correlated more strongly with NB%fat than later FTG(r=0.55; p=0.01) obtained at 26-28 wks. Notably, the early 1 hr PPTG (r=0.71; p=0.001, Figure 3C) most strongly correlated with NB%fat compared to later 1-hr PPTG (r=0.63; p=0.004, Figure 3D). In fact, in OB mothers, the early 1-hr or 2-hr PPTG predicted ~50% of the variance of NB%fat (R2=0.50; p<0.01). In contrast to OB, among NW mothers (Figure 4), the increase (delta) in FTG from early to later pregnancy (r=0.59; p=0.001) as well as later FTG (r=0.48; p≤0.01) correlated with NB%fat. Similarly, the increase in total TG-AUC from early to later (r=0.57; p=0.002) as well as later TG-AUC (r=0.44; p=0.02) was correlated with NB%fat. The early to later changes in FTG/PPTG predicted similar variance in NB%fat (R2=0.32-0.35; p≤0.01) in NW. Unlike OB mothers, there were no significant predictions in NW mothers between early fasting or early PPTG and NB%fat (Table 3).
Table 3.
Pearson correlations, simple and multiple regression models for the total cohort and by group, early and later in gestation. Dependent variable for regression is NB%fat
| Entire Cohort | Normal-Weight | Obese | |||||
|---|---|---|---|---|---|---|---|
| Early (n=54) |
Later (n=51) |
Early (n=27) |
Later (n=27) |
Early (n=27) |
Later (n=24) |
||
| Pre-Pregnancy BMI (kg/m2) | 0.12 | ||||||
| Total GWG (kg) | 0.07 | ||||||
| Fasting TG (mg/dL) | 0.47* | 0.56* | 0.04 | 0.48# | 0.60# | 0.55# | |
| 1-hr PPTG (mg/dL) | 0.57* | 0.54* | 0.14 | 0.27 | 0.71* | 0.63# | |
| 2-hr PPTG (mg/dL) | 0.57* | 0.58* | 0.21 | 0.47† | 0.69# | 0.61# | |
| TG-AUC (mg*min/dL) | 0.53* | 0.57* | 0.12 | 0.44† | 0.66# | 0.60# | |
| Fasting Glucose (mg/dL) | 0.08 | 0.25 | −0.10 | 0.07 | 0.05 | 0.29 | |
| 1-hr PP Glucose (mg/dL) | 0.25 | 0.26 | 0.05 | 0.29 | 0.36 | 0.11 | |
| 2-hr PP Glucose (mg/dL) | 0.40# | 0.43# | 0.26 | 0.35 | 0.39 | 0.50† | |
| Glucose-AUC (mg*min/dL) | 0.43# | 0.42# | 0.10 | 0.26 | 0.54† | 0.47† | |
| Total Cohort Early | Obese Early | ||||||
|---|---|---|---|---|---|---|---|
| β | p | R2 | β | p | R2 | ||
| Simple Regression | 0.22 | Simple Regression | 0.36 | ||||
| Fasting TG | 0.07 | ≤ 0.001 | Fasting TG | 0.08 | ≤ 0.01 | ||
| Multiple Regression | 0.32 | Multiple Regression | 0.58 | ||||
| Fasting TG | 0.06 | ≤ 0.01 | Fasting TG | 0.07 | ≤ 0.01 | ||
| GLUC-AUC | 0.001 | 0.1 | GLUC-AUC | 0.001 | 0.1 | ||
| Simple Regression | 0.32 | Simple Regression | 0.50 | ||||
| 1-hr PPTG | 0.06 | ≤ 0.001 | 1-hr PPTG | 0.08 | ≤ 0.001 | ||
| Multiple Regression | 0.35 | Multiple Regression | 0.57 | ||||
| 1-hr PPTG | 0.05 | ≤ 0.01 | 1-hr PPTG | 0.06 | ≤ 0.01 | ||
| GLUC-AUC | 0.0005 | 0.2 | GLUC-AUC | 0.001 | 0.1 | ||
| Total Obese Later: | Cohort Later: | ||||||
| β | p | R2 | β | p | R2 | ||
| Simple Regression | 0.31 | Simple Regression | 0.30 | ||||
| Fasting TG | 0.05 | ≤ 0.001 | Fasting TG | 0.05 | ≤ 0.01 | ||
| Multiple Regression | 0.33 | Multiple Regression | 0.36 | ||||
| Fasting TG | 0.04 | ≤ 0.01 | Fasting TG | 0.04 | 0.1 | ||
| GLUC-AUC | 0.0004 | 0.3 | GLUC-AUC | 0.001 | 0.3 | ||
| Simple Regression | 0.29 | Simple Regression | 0.40 | ||||
| 1-hr PPTG | 0.05 | ≤ 0.001 | 1-hr PPTG | 0.05 | ≤ 0.01 | ||
| Multiple Regression | 0.32 | Multiple Regression | 0.44 | ||||
| 1-hr PPTG | 0.04 | ≤ 0.01 | 1-hr PPTG | 0.05 | < 0.05 | ||
| GLUC-AUC | 0.0004 | 0.2 | GLUC-AUC | 0.001 | 0.3 | ||
Data are Pearson correlation (r-values) with NB%Fat;
indicates p≤0.001,
indicates p≤0.01,
indicates p<0.05; GWG= Gestational Weight Gain; PP = postprandial; AUC = area-under-the-curve; IR = insulin resistance; OGTT = oral glucose tolerance test. Fasting FFA (r=0.22); Fasting Insulin (r=0.07); HOMA-IR (r=0.11) for total cohort (all p>0.05) so not added to table.
Figure 2.
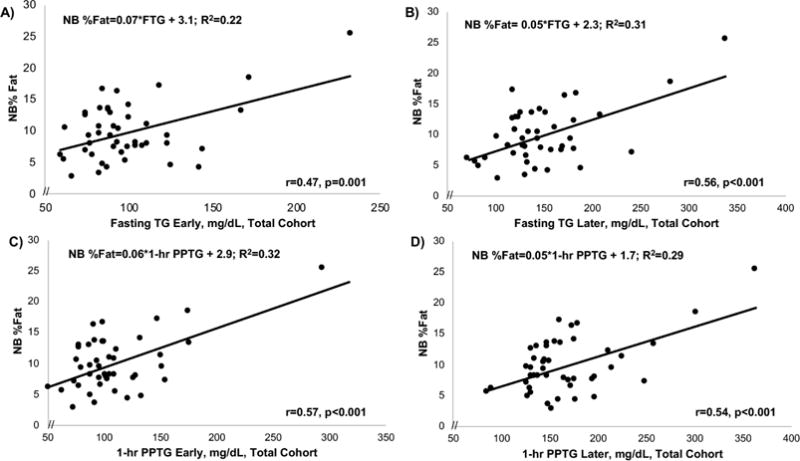
In the total cohort fasting TG early (A), fasting TG later (B), 1-hr PPTG early (C) and 1-hr PP TG later (D) were similarly predictive of newborn (NB) %fat. The 2-hr PPTG showed a similar association.
Figure 5.
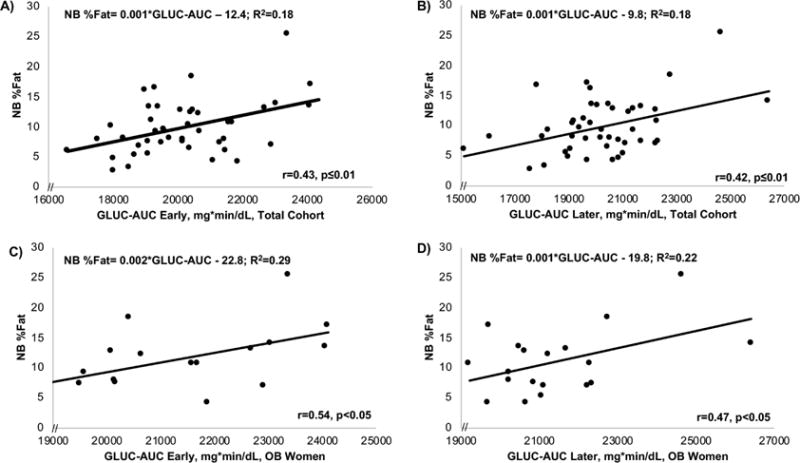
Across the total cohort, the early 4-hr glucose AUC (A) was similarly predictive of NB%fat compared to the later 4-hr glucose AUC (B). In the OB (but not NW) women, the 4-hr glucose AUC was also modestly predictive of NB%fat both early (C) and later (D) in gestation.
Figure 3.
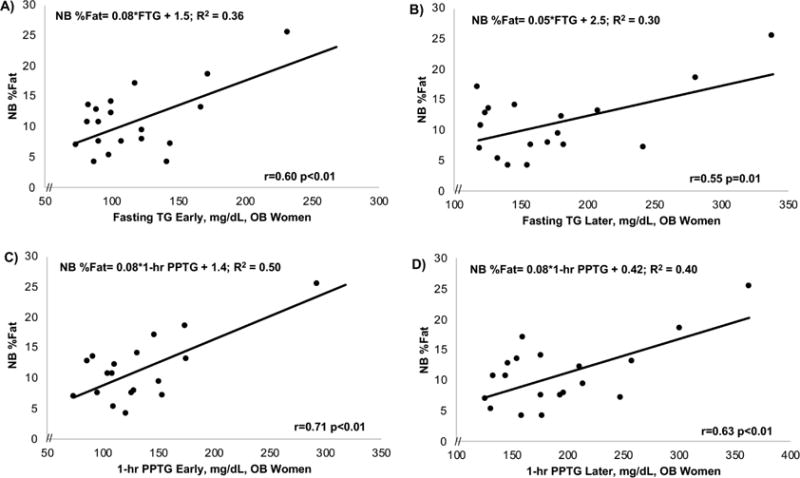
In obese women, fasting TG early (A) were more predictive of NB%fat than fasting TG later (B), and the early 1-hr PPTG (C) was also more strongly predictive of NB%fat than the later 1-hour PPTG (D). The 2-hr PPTG showed a similar association.
Figure 4.
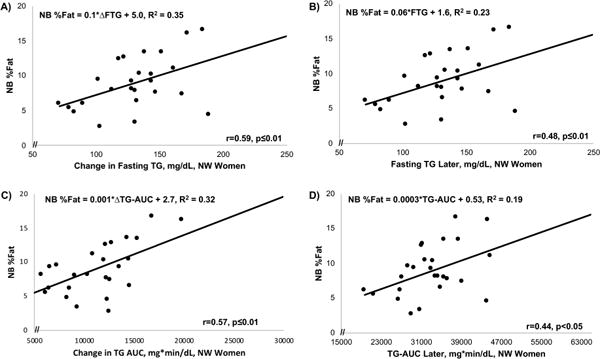
In NW women, the change (delta) in fasting TG (A) and in total TG AUC (C) from 12-14 to 26-28 wks gestation were strongly predictive of NB%fat, as was the later gestation fasting TG (B) and TG AUC (D). (Δ = change in [delta])
In comparison to FTG, fasting glucose was not correlated with NB%fat in the total cohort or by group (Table 3). However, in the total cohort, both the early and later 4-hr GLUC-AUC were correlated with NB%fat (r=0.43, early; r=0.42, later; both p≤0.01; Figure 5A,B) as was the 2-hr PP GLUC (r=0.40, early; r=0.43, late; both p≤0.01). These glucose correlations were driven by OB mothers given that the early and later 4-hr GLUC-AUC significantly correlated with NB%fat in OB (r=0.54 and r=0.47, respectively, p<0.05; Figure 5C,D), but not in NW mothers.
As specified in our sample size calculation, we used linear regression to determine the influence of TG or other measures on the prediction of NB%fat. Whereas the early 1-hr PPTG was most predictive of NB%fat in OB mothers (R2=0.50; Table 3), GLUC-AUC did not significantly add to predictions (all p>0.05), nor did maternal BMI. Insulin, IR estimates, or FFA did not correlate with NB%fat in the total cohort or by group. Although there were strong correlations between TG measures and NB%fat, there were no significant correlations with birthweight.
DISCUSSION
This study, to our knowledge, is the first to demonstrate that women with obesity have substantially higher FTG and PPTG compared to NW both early and later in pregnancy, despite a controlled diet. The PPTG response was explained by both dietary CM-TG and VLDL-TG. Highly relevant to clinical practice, a single 1-hr or 2-hr PPTG in pregnancy captured the entire 4-hr TG-AUC meal response. Surprisingly, in OB mothers, the early 14-16 wk 1-hr PPTG correlated most strongly with NB%fat (r=0.71; p=0.001). Newborn %fat was not correlated with fasting glucose in any groups and only the 4-hr Glucose-AUC or 2-hr PP GLUC correlated modestly in OB mothers (Table 3). Together these data suggest that FTG and, more importantly, PPTG may be under-recognized important contributors to fetal fat accretion, particularly in OB glucose tolerant mothers. Most striking in OB women, the prediction between maternal TG and NB%fat was strongest early in pregnancy, related to both CM-TG and VLDL-TG, and captured by a 1- or 2-hr PPTG.
The American College of Obstetricians and Gynecologists identified maternal obesity as the greatest public health risk in pregnancy and the largest number of LGA infants are born to mothers with obesity(2). The intrauterine environment in obesity is characterized by nutrient excess and associated with increased risk for childhood obesity (20% of preschoolers)(27), glucose intolerance, and non-alcoholic fatty liver disease (NAFLD); the latter occuring in ~40% of children with obesity and the leading cause of liver transplantation(28). Offspring from women with obesity or GDM are not only born with increased subcutaneous fat(29). We have shown by MRI spectroscopy that these 2-wk newborns compared to NW mothers already had 68% more intrahepatic fat(30), potentially a risk factor for the subsequent development of NAFLD. Offspring obesity risk was shown to be best predicted by maternal obesity more so than GDM in a cohort of offspring who had fat mass measured by DXA at 9 years of age(18). Further, fat mass at birth, not birthweight, predicted fat mass at 9 yrs, supporting that NB%fat is a better predictor of childhood obesity(18).
Investigators have sought to determine whether glucose, lipids, or excess gestational weight gain may be driving the risk of excess fetal growth in maternal obesity(31). We show that despite similar weight gain (Table 1), both fasting and PPTG were ~30-40% higher over gestation in OB mothers and an early 1-hr PPTG (similar to a 2-hr PPTG) most strongly predicted NB%fat (R2=0.50; p<0.01), accounting for 50% of the variance. Studies have inconsistently shown a correlation between FFA or fasting TG and fetal growth (4, 5, 7, 8, 9) but PPTG are rarely measured. Placental lipid metabolism and transport play a central role in determining fetal FFA availability which is complex. Our group has shown that the activity of placental lipoprotein lipase, which hydrolyzes maternal TG to FFA for fetal-placental availability, is correlated with NB%fat estimated by skinfolds at birth(15). The inconsistency in trials exploring maternal TG and fetal growth may not only be due to absent newborn fat mass measurements, but also due to the influence of maternal diet on TG. Diet influences fasting TG by ~20% for up to 3 days before sampling(13). We therefore provided a eucaloric diet with identical macronutrient composition to isolate the effect of maternal obesity from diet on maternal lipid and glucose metabolism. We demonstrate that fasting and PP insulin concentrations are already impressively ~50% higher in OB versus NW mothers in early pregnancy (Table 2). Mothers with obesity also have higher IR entering gestation, previously shown by pre-conception insulin clamp data(32). Maternal IR in obesity increases maternal lipolysis, elevating fasting TG and FFA(31, 33). Because the fetus has limited capacity for de novo lipogenesis and fatty acid oxidation, excess maternal TG can be hydrolyzed to FFA by placental lipases for fetal fat accretion(14, 33, 34, 35). Although the high insulin levels generated by OB women suppressed postprandial FFA to levels similar to NW, the fasting and PP TG early in OB women were already as high as in NW mothers later in pregnancy (Figure 1A), providing early fetoplacental exposure to excess lipid(36).
The 50-60% higher fasting insulin concentrations in OB mothers suppressed fasting glucose to only slightly higher than NW (Table 2) and this may be why, in part, fasting glucose did not correlate with NB%fat. However, the 4-hr GLUC-AUC, which better captures the entire glucose exposure, was ~10% higher in obese mothers early and later compared to NW (Figure 1C) and the 4-hr GLUC-AUC early and later were modestly correlated with NB%fat in OB mothers, but not in NW (Table 3). This is consistent with our previous study(4), in which OB mothers had ~8% higher 24-hr glucose profiles by CGM early and later but a single early fasting TG was more strongly correlated with NB%fat.
There is growing recognition of the importance of early excess nutrient transport shifting the focus to earlier or pre-pregnancy interventions(6, 17, 31, 33) to decrease LGA. This earlier focus is further suggested by the disappointing outcomes with later pregnancy interventions and support that early fetal hyperinsulinemia drives fetal growth(6). Our data strongly support this focus, especially in obese pregnancies. Although in NW mothers, the increase in PPTG from early to later pregnancy most strongly predicted NB%fat, in OB women the strongest predictor was the early 1-hr PPTG. This suggests that over the course of pregnancy, TG increases in NW women contribute to fetal fat accretion. However, in OB women, TG are already high in early pregnancy and strongly relate to infant adiposity, suggesting interventions to reduce them must occur early or, perhaps, even pre-pregnancy.
Limitations include that this was primarily a Caucasian cohort and findings may be different in other ethnicities. Furthermore, risk factors for preeclampsia or PTD were excluded because they could confound NB%fat measures given fetal fat accretion continues at ~0.1%/day until 40 wks gestation(37, 38). Except for higher IR and TG, this obese cohort was relatively healthy as demonstrated by the low glucoses. Generalizability to to less healthy obese populations is limited. Despite enrolling relatively healthy OB women, 19/27 newborns from the OB group were evaluable by DXA because of obstetric complications or delivery <37 wks. This underscores the increased risk of adverse outcomes even in OB women without risk factors for preterm delivery or preeclampsia. With a larger sample size, glucose may be found to independently contribute to a prediction model in mothers with obesity. Analysis of newborn sex differences between groups were limited by power. Although the 1-hr PPTG correlation with NB%fat in the pooled cohort was strongest for males (r=0.68, early; r=0.59, later) versus females (r=0.43, early; r=0.49, later), we are unable to draw conclusions of significance given we were not powered to detect sex differences. Lastly, female newborns have slightly more %fat than males(37). Although more male offspring were delivered from OB mothers, adjustment for sex showed that the effect between sex and groups was of borderline significance (p=0.1) suggesting that the lack of a statistically significant difference in NB%fat between groups was also likely due to the limited sample size.
CONCLUSIONS
Going forward, the clinical implication of these findings is that measuring a 1- or 2-hr PPTG after a meal, similar to 1- or 2-hr PP glucose typically measured in GDM, may identify an important metabolic contributor to newborn adiposity previously unrecognized. Given that the 1- and 2-hr PPTG were correlated strongest with NB%fat, these data suggest that the maternal TG response to diet might be targeted and lowered by a dietary intervention and/or omega-3 fatty acid supplements, especially in those with higher TG levels. In OB women, interventions should be targeted <14 wks or preconception and if proven successful in a future RCT, could represent a paradigm shift in focus and management. Our mothers ingested a healthy diet prior to the studies. How a fast-food diet, higher in saturated fat and simple carbohydrate, is likely to further increase PPTG and impact newborn adiposity remains to be studied in a real world environment. Discerning whether this PPTG association occurs in GDM, for which the focus of treatment has primarily been glucose-lowering, deserves further study given the persistently high LGA rate. With portable point-of-care lipid testing using meters similar to the size of glucometers becoming more affordable and precise(39, 40), obtaining repeated fasting and PPTG measures in a larger population of women at risk for fetal overgrowth is feasible and should be done to confirm these associations. Current glucose-centered strategies are only partially effective. With an ever-growing maternal obesity epidemic accompanied by LGA infants, novel approaches which target excess fetal growth earlier in pregnancy to attenuate risk for childhood metabolic disease are merited.
Study Importance.
What Is Already Known About This Subject?
-
-
Obesity occurs in ~40% of women of childbearing age in the U.S. and accounts for a greater number of large-for-gestational-age (LGA) infants than pregnancies complicated by gestational diabetes mellitus.
-
-
Maternal BMI and fat mass at birth, but not birth weight, are the strongest risk factors for childhood obesity.
-
-
Mothers with obesity have higher fasting and postprandial glucose than normal-weight mothers which increase fetal growth but there are few data on the role of fasting or postprandial triglycerides as contributors to fetal fat accretion in early and later pregnancy, after controlling for maternal diet.
What Does This Study Add?
-
-
We prospectively demonstrate that women with obesity in early pregnancy (14-16 weeks) already have higher fasting and postprandial glucose and insulin, and 30-40% higher triglycerides than normal-weight women later (26-28 weeks) despite a controlled diet.
-
-
In mothers with obesity, a 1-hr and 2-hr postprandial triglyceride (PPTG) early in pregnancy (14-16 weeks) was correlated with newborn %fat (1-hr PPTG r=0.71; 2-hr PPTG r=0.69; both p<0.01) by DXA more strongly than glucose and was related to both dietary chylomicron-TG and VLDL-TG.
-
-
Fifty percent of the variance in newborn fat mass at birth was predicted by an early 1-hr PPTG in women with obesity, which was the strongest predictor in a simple regression model. No other metabolic biomarkers including glucose, insulin resistance indices, or free fatty acids added significantly to this prediction model, suggesting that a 1-hr or 2-hr PPTG might be a new therapeutic target in early pregnancy to prevent excess fetal growth and future child obesity risk.
Acknowledgments
We thank Catherine Chartier-Logan for assistance in study execution.
Data from the R56-DK078645 pilot were competitively selected for an oral abstract at the American Diabetes Association Scientific Sessions, 2009 and most recently data from the completed trial (R01 DK078645) were competitively selected for an oral abstract presented at the American Diabetes Association Scientific Sessions, June 2017.
Funding Source: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: R56 DK078645 and R01 DK078645; National Institutes of Health, National Center for Advancing Translational Sciences: UL1 TR001082.
Footnotes
Clinical Trial ID: NCT00826904, https://clinicaltrials.gov
Conflict of Interest: The authors have no conflicts of interest.
Author Contributions: L.A.B wrote the manuscript and researched data; S.S.F. edited the manuscript and researched data; J.E.F edited the manuscript; N.M.H. researched data and reviewed the manuscript; M.S.R. researched data and reviewed the manuscript; R.E. VP. researched data and edited the manuscript; T.L.H. researched data and edited the manuscript
References
- 1.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ACOG Practice Bulletin No 156: Obesity in Pregnancy. Obstet Gynecol. 2015;126:e112–126. doi: 10.1097/AOG.0000000000001211. [DOI] [PubMed] [Google Scholar]
- 3.Barbour LA. Changing perspectives in pre-existing diabetes and obesity in pregnancy: maternal and infant short- and long-term outcomes. Curr Opin Endocrinol Diabetes Obes. 2014;21:257–263. doi: 10.1097/MED.0000000000000079. [DOI] [PubMed] [Google Scholar]
- 4.Harmon KA, Gerard L, Jensen DR, Kealey EH, Hernandez TL, Reece MS, et al. Continuous Glucose Profiles in Obese and Normal-Weight Pregnant Women on a Controlled Diet: Metabolic determinants of fetal growth. Diabetes Care. 2011;34:2198–2204. doi: 10.2337/dc11-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett HL, Dekker NM, McIntyre HD, Callaway LK. Normalizing metabolism in diabetic pregnancy: is it time to target lipids? Diabetes Care. 2014;37:1484–1493. doi: 10.2337/dc13-1934. [DOI] [PubMed] [Google Scholar]
- 6.Desoye G, Nolan CJ. The fetal glucose steal: an underappreciated phenomenon in diabetic pregnancy. Diabetologia. 2016;59:1089–1094. doi: 10.1007/s00125-016-3931-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olmos PR, Rigotti A, Busso D, Berkowitz L, Santos JL, Borzone GR, et al. Maternal hypertriglyceridemia: A link between maternal overweight-obesity and macrosomia in gestational diabetes. Obesity (Silver Spring) 2014 doi: 10.1002/oby.20816. [DOI] [PubMed] [Google Scholar]
- 8.Schaefer-Graf UM, Graf K, Kulbacka I, Kjos SL, Dudenhausen J, Vetter K, et al. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care. 2008;31:1858–1863. doi: 10.2337/dc08-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vrijkotte TG, Algera SJ, Brouwer IA, van Eijsden M, Twickler MB. Maternal triglyceride levels during early pregnancy are associated with birth weight and postnatal growth. J Pediatr. 2011;159:736–742.e731. doi: 10.1016/j.jpeds.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Di Cianni G, Miccoli R, Volpe L, Lencioni C, Ghio A, Giovannitti MG, et al. Maternal triglyceride levels and newborn weight in pregnant women with normal glucose tolerance. Diabet Med. 2005;22:21–25. doi: 10.1111/j.1464-5491.2004.01336.x. [DOI] [PubMed] [Google Scholar]
- 11.Whyte K, Kelly H, O’Dwyer V, Gibbs M, O’Higgins A, Turner MJ. Offspring birth weight and maternal fasting lipids in women screened for gestational diabetes mellitus (GDM) Eur J Obstet Gynecol Reprod Biol. 2013;170:67–70. doi: 10.1016/j.ejogrb.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Geraghty AA, Alberdi G, O’Sullivan EJ, O’Brien EC, Crosbie B, Twomey PJ, et al. Maternal Blood Lipid Profile during Pregnancy and Associations with Child Adiposity: Findings from the ROLO Study. PLoS One. 2016;11:e0161206. doi: 10.1371/journal.pone.0161206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaskolowski J, Ritz C, Sjodin A, Astrup A, Szecsi PB, Stender S, et al. Weekday variation in triglyceride concentrations in 1. 8 million blood samples J Lipid Res. 2017;58:1204–1213. doi: 10.1194/jlr.M074062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magnusson AL, Waterman IJ, Wennergren M, Jansson T, Powell TL. Triglyceride hydrolase activities and expression of fatty acid binding proteins in the human placenta in pregnancies complicated by intrauterine growth restriction and diabetes. J Clin Endocrinol Metab. 2004;89:4607–4614. doi: 10.1210/jc.2003-032234. [DOI] [PubMed] [Google Scholar]
- 15.Heerwagen MJR, Gumina DL, Hernandez TL, Van Pelt RE, Kramer AW, Janssen RC, et al. Placental lipoprotein lipase activity is positively associated with newborn adiposity. Placenta. 2018;64:53–60. doi: 10.1016/j.placenta.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Fisher RM, Coppack SW, Humphreys SM, Gibbons GF, Frayn KN. Human triacylglycerol-rich lipoprotein subfractions as substrates for lipoprotein lipase. Clin Chim Acta. 1995;236:7–17. doi: 10.1016/0009-8981(95)06032-3. [DOI] [PubMed] [Google Scholar]
- 17.Simmons D. Prevention of gestational diabetes mellitus: Where are we now? Diabetes Obes Metab. 2015;17:824–834. doi: 10.1111/dom.12495. [DOI] [PubMed] [Google Scholar]
- 18.Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, de Mouzon SH, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90:1303–1313. doi: 10.3945/ajcn.2008.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ACOG Practice Bulletin No. 180: Gestational Diabetes Mellitus. Obstet Gynecol. 2017;130:e17–e37. doi: 10.1097/AOG.0000000000002159. [DOI] [PubMed] [Google Scholar]
- 20.Horton TJ, Commerford SR, Pagliassotti MJ, Bessesen DH. Postprandial leg uptake of triglyceride is greater in women than in men. Am J Physiol Endocrinol Metab. 2002;283:E1192–1202. doi: 10.1152/ajpendo.00164.2002. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez TL, van Pelt RE, Anderson MA, Daniels LJ, West NA, Donahoo WT, et al. A higher-complex carbohydrate diet in gestational diabetes mellitus achieves glucose targets and lowers postprandial lipids: a randomized crossover study. Diabetes Care. 2014;37:1254–1262. doi: 10.2337/dc13-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallace TM, Matthews DR. The assessment of insulin resistance in man. Diabet Med. 2002;19:527–534. doi: 10.1046/j.1464-5491.2002.00745.x. [DOI] [PubMed] [Google Scholar]
- 23.Van Pelt RE, Gozansky WS, Kohrt WM. A novel index of whole body antilipolytic insulin action. Obesity (Silver Spring) 2013;21:E162–165. doi: 10.1038/oby.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernandez TL, Bessesen DH, Cox-York KA, Erickson CB, Law CK, Anderson MK, et al. Femoral lipectomy increases postprandial lipemia in women. Am J Physiol Endocrinol Metab. 2015;309:E63–71. doi: 10.1152/ajpendo.00080.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbour LA, Hernandez TL, Reynolds RM, Reece MS, Chartier-Logan C, Anderson MK, et al. Striking differences in estimates of infant adiposity by new and old DXA software, PEAPOD and skin-folds at 2 weeks and 1 year of life. Pediatr Obes. 2016;11:264–271. doi: 10.1111/ijpo.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fields DA, Gilchrist JM, Catalano PM, Gianni ML, Roggero PM, Mosca F. Longitudinal body composition data in exclusively breast-fed infants: a multicenter study. Obesity (Silver Spring) 2011;19:1887–1891. doi: 10.1038/oby.2011.11. [DOI] [PubMed] [Google Scholar]
- 27.Cunningham SA, Kramer MR, Narayan KM. Incidence of childhood obesity in the United States. N Engl J Med. 2014;370:1660–1661. doi: 10.1056/NEJMc1402397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brumbaugh DE, Friedman JE. Developmental origins of nonalcoholic fatty liver disease. Pediatr Res. 2014;75:140–147. doi: 10.1038/pr.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sewell MF, Huston-Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol. 2006;195:1100–1103. doi: 10.1016/j.ajog.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 30.Brumbaugh DE, Tearse P, Cree-Green M, Fenton LZ, Brown M, Scherzinger A, et al. Intrahepatic Fat Is Increased in the Neonatal Offspring of Obese Women with Gestational Diabetes. J Pediatr. 2012 doi: 10.1016/j.jpeds.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ. 2017;356:j1. doi: 10.1136/bmj.j1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol. 1999;180:903–916. doi: 10.1016/s0002-9378(99)70662-9. [DOI] [PubMed] [Google Scholar]
- 33.Herrera E, Desoye G. Maternal and fetal lipid metabolism under normal and gestational diabetic conditions. Horm Mol Biol Clin Investig. 2016;26:109–127. doi: 10.1515/hmbci-2015-0025. [DOI] [PubMed] [Google Scholar]
- 34.Perazzolo S, Hirschmugl B, Wadsack C, Desoye G, Lewis RM, Sengers BG. The influence of placental metabolism on fatty acid transfer to the fetus. J Lipid Res. 2017;58:443–454. doi: 10.1194/jlr.P072355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrett HL, Kubala MH, Scholz Romero K, Denny KJ, Woodruff TM, McIntyre HD, et al. Placental lipases in pregnancies complicated by gestational diabetes mellitus (GDM) PLoS One. 2014;9:e104826. doi: 10.1371/journal.pone.0104826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis RM, Desoye G. Placental Lipid and Fatty Acid Transfer in Maternal Overnutrition. Ann Nutr Metab. 2017;70:228–231. doi: 10.1159/000463397. [DOI] [PubMed] [Google Scholar]
- 37.Hawkes CP, Hourihane JO, Kenny LC, Irvine AD, Kiely M, Murray DM. Gender- and gestational age-specific body fat percentage at birth. Pediatrics. 2011;128:e645–e651. doi: 10.1542/peds.2010-3856. [DOI] [PubMed] [Google Scholar]
- 38.Moore GS, Allshouse AA, Fisher BM, Kahn BF, Hernandez TL, Reece MS, et al. Can Fetal Limb Soft Tissue Measurements in the Third Trimester Predict Neonatal Adiposity? J Ultrasound Med. 2016;35:1915–1924. doi: 10.7863/ultra.15.06028. [DOI] [PubMed] [Google Scholar]
- 39.Barrett HL, Dekker Nitert M, D’Emden M, McIntyre HD, Callaway LK. Validation of a triglyceride meter for use in pregnancy. BMC Res Notes. 2014;7:679. doi: 10.1186/1756-0500-7-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barrett HL, McIntyre HD, D’Emden M, Dekker Nitert M, Callaway LK. Home Monitoring of Fasting and Postprandial Triglycerides in Late Pregnancy: A Pilot Study. Diabetes Care. 2017;40:e1–e2. doi: 10.2337/dc16-2181. [DOI] [PubMed] [Google Scholar]


