Abstract
Separate lines of research have revealed that the rapid development of inhibitory control in the preschool period is closely tied to normative brain development and influenced by early mother-child interactions. One potential theory is that maternal behavior in the context of early interactions influences the neural underpinnings of inhibitory control in development, with implications for child behavior. The purpose of this paper was to examine whether maternal emotional support, measured during a mother-child problem-solving game, predicted child neural responses (frontal-central N2 event-related potential) and behavioral performance (discrimination index, d′) in a go/no-go task of inhibitory control in a large, diverse sample of mother-child dyads (N=276) observed in children’s last year of preschool (Mean age = 56 months). Results of a structural equation model revealed significant direct effects from maternal emotional support to child right hemisphere frontal-central N2 responses to no-go (inhibitory control) trials; greater observed emotional support predicted larger N2 responses. Larger right hemisphere N2 responses to no-go trials were also associated with better overall observed task performance (d′). A test of indirect effects from maternal emotional support to child observed performance via right hemisphere N2 responses was significant, suggesting that underlying neurophysiology is one mechanism through which maternal emotional support is associated with a child’s rapidly developing inhibitory control behavior in the preschool period. This work joins a growing literature demonstrating that caregiver behavior within a “normative” range is an important environmental factor contributing to the development of neural processes supporting child functioning.
Keywords: Inhibitory control, maternal emotional support, preschoolers, event-related potentials, parenting
Introduction
Inhibitory control (IC) is defined as the process of inhibiting a well-learned or prepotent response in favor of a subdominant one and is a core component of Executive Functions (EFs), a set of cognitive processes involved in regulating one’s thoughts and behaviors (Bernier, Carlson, & Whipple, 2010; Durston et al., 2002; Miyake & Friedman, 2012; Willoughby, Blair, Wirth & Greenberg, & the Family Life Project Investigators, 2012). Improvements in IC have been shown to mediate the acquisition of other critically important skills and predict positive outcomes in development such as the ability to delay gratification, better social-emotional competence, and a successful transition to formal schooling and subsequent academic achievement (e.g. Blair & Razza, 2007; Espy et al., 2004; McClelland et al., 2007; Masten et al., 2012; Mischel et al., 2011; Monette, Bigras, & Guay, 2011; Sasser, Bierman, & Heinrichs, 2015). In contrast, deficits in IC are implicated in a range of developmental disorders, including attention deficit and hyperactivity disorder and obsessive-compulsive disorder (Casey, Castellanos et al., 1997; George, 1991; George, Ketter, & Post, 1993; Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005). Poor IC has also been identified as a risk factor for pathways to psychopathology such as anxiety and mood disorders, and alcoholism and substance abuse (see Nigg, 2000 for a review). Thus, identifying factors that promote the development of IC is important for functioning across a variety of domains.
A rich literature has established that IC processes improve rapidly in the preschool and early school-aged years (e.g. Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002; Carver, Livesey, Charles, 2001; Casey, Trainor, Orendi et al., 1997; Davidson, Amso, Anderson, & Diamond, 2006; Diamond, 1990; Durston et al., 2002; Gerstadt, Hong, & Diamond, 1994; Jones, Rothbart, & Posner, 2003) and that this depends heavily on coincident brain development occurring in the frontal cortex (e.g. Casey, Castellanos et al., 1997; Casey, Trainor, Orendi et al., 1997; Diamond, 1990; Durston et al., 2002). Recent work has provided evidence that the child’s social environment, and particularly close relationships like that of the mother-child relationship, may also have an important influence on the development of neurocognitive functions like IC and other dimensions of EF in early childhood (e.g. Bernier, Beauchamps, Carlson, & Lalonde, 2015; Bernier et al., 2010; Matte-Gagné, Bernier, & Lalonde, 2015; Rhoades, Greenberg, Lanza, & Blair, 2011; Valcan, Davis, Pino-Pasternak, 2017). One possibility is that maternal behavior may have a direct influence on neural mechanisms that support the development of IC in early childhood, and that this results in individual differences in behavior. Although theoretical work has suggested that experience with a caregiver may shape a child’s early brain development, and that this would have implications for cognitive processes in development (Bernier et al., 2010; Bernier et al., 2015; Greenough & Black, 1992; Schore, 1996), relatively little work has examined this empirically in the preschool period (but see Bernier, Calkins, & Bell, 2016; Swingler, Perry, Calkins, & Bell, 2017 for work with infants and toddlers).
In addition, previous work that has examined the influence of caregiving experience on neural development has predominately focused on child emotion development in the context of extreme cases of child neglect, abuse, or abnormal social environments (e.g. Curtis & Cicchetti, 2007; De Bellis, 2001; Marshall, Fox, & the BEIP core group, 2004; Parker, Nelson, & the BEIP core group, 2005; Pollak, Klorman, Thatcher, & Cicchetti, 2001; Rutter & O’Connor, 2004; Tottenham, 2012). While this work has provided important evidence that atypical caregiving experiences are related to abnormal structural and functional brain development, a focus on extreme or atypical caregiving experiences of neglect or abuse on offspring emotion or stress regulation has limited the generalizability of these findings. In response, there is a growing emphasis on the importance of examining caregiver behavior within a normative range in the context of typical interactions in relation to child neural development (e.g. Bernier et al., 2016; Schneider-Hassloff et al., 2016; Swingler et al., 2017).
Thus, the goal of the current paper is to examine maternal behavior as one important environmental factor that may impact both neural and behavioral processes associated with IC in a period of rapid developmental change in these abilities. We examined maternal emotional support in a dyadic problem-solving game as a means of capturing normative variation in positive, emotionally supportive maternal behavior in the context of typical parent-child interactions. Emotionally supportive maternal behavior is characterized as highly sensitive, responsive, and positive behavior that is low on intrusiveness and negativity, and thus may support the child’s developing IC skills by creating a well-regulated and supportive environmental context in which IC can be efficiently and effectively practiced and learned. We hypothesized that emotionally supportive maternal behavior may be predictive of neural mechanisms, as well as behavioral performance, in a task that requires IC. To test this, we examined the influence of maternal behavior on one purported neural index of IC, the N2 component of the ERP, as well as behavioral performance on a go/no-go (GNG) task.
Neural Basis of Inhibitory Control
Work examining the development of IC in children has frequently utilized a GNG task as a well-established measure of IC in both adults and children (e.g. Durston et al., 2002; Lahat, Todd, Mahy, Lau, & Zelazo, 2010). This task requires children to make a response to frequently presented stimuli (go trials), but inhibit responding to a subset of infrequently presented stimuli (no-go trials). Importantly, this task lends itself to pairing with event-related potential (ERP) methodology to examine the neural underpinnings of IC in development. Event-related potentials (ERPs) result from postsynaptic potentials occurring in many thousands of similarly oriented cortical neurons responding in synchrony to an event (e.g. stimuli, responses, decisions) such as a go or a no-go trial (Fabiani, Gratton, & Federmeier, 2007; Luck, 2012; Pizzagalli, 2007). This synchronization of activity can be measured at the scalp level with electrodes and can provide information about the extent and timing of cortical activity in response to each trial type. Thus, ERPs are considered neural manifestations of psychological functions or processes (Fabiani et al., 2007). Previous research using ERP methodology has demonstrated that a fronto-centrally distributed negative component, labeled the N2, is observed in a GNG task in both adults and children (e.g. Bokura, Yamaguchi, & Kobayashi, 2001; Cragg, Fox, Nation, Reid, & Anderson, 2009; Davis, Bruce, Snyder, & Nelson, 2003; Eimer, 1993; Falkenstein, Hoormann, & Hohnsbein, 1999; Jonkman, 2006; Jonkman, Lansbergen, & Stauder, 2003; Lamm, Zelazo, & Lewis, 2006; Lahat et al., 2010). The N2 is typically observed between 200 and 500 ms following stimulus presentation over medial frontal and central electrode sites and is generally found to be larger to no-go (response inhibition) trials than to go trials, suggesting increased neural activity when prepotent responses need to be inhibited (e.g. Eimer, 1993; Falkenstein et al., 1999; Jonkman, et al., 2003; Lahat et al., 2010; Lamm et al., 2006; but see Cragg et al., 2009; Davis et al, 2003 for discrepant findings).
Although the N2 has been described by some as indexing response inhibition, or inhibiting the prepotent tendency to respond on all trials or on frequently occurring trials (Bokura et al., 2001; Falkenstein et al., 1999; Jodo & Kayama, 1992); others have defined the N2 as indexing conflict monitoring, or the co-activation and resolution of competing responses in the brain (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Jonkman et al., 2003; Jonkman, 2006; Nieuwenhuis, Yeung, van den Wildenberg, & Ridderinkhof, 2003). Despite these differences in conceptualization, there is general agreement that the N2 is associated with neural processes necessary for the inhibition of a behavioral response (e.g. Lamm et al., 2006). ERP work combined with source analysis methodology has provided additional support for this notion by localizing the cortical generators of the N2 to brain areas and structures previously identified as active during IC processes in fMRI work. In particular, the anterior cingulate cortex and ventromedial, ventrolateral, and dorsomedial prefrontal cortex and orbitofrontal cortex have all been identified as N2 generators in work with adults as well as children and adolescents (Bokura et al., 2001; Lahat et al., 2010; Lamm et al., 2006; Nieuwenhuis et al., 2003).
Indeed, age-related improvement in IC performance has been theorized to parallel development of regions of ventral and dorsal prefrontal cortex, anterior cingulate cortex, and orbital frontal cortex, as well as associated structures in the brain (Carlson, 2005; Durston et al., 2002; Gerstadt et al., 1994; Jones et al., 2003; Luu & Posner, 2003; Zelazo, Carlson, & Kesek, 2008). Neuroscience work with adult and developmental populations has demonstrated that these areas are involved in IC processes and sensitive to manipulation of task demands across ages, although the specific location, extent, and volume of activation change with age, and parallel developmental change in performance (e.g. Casey, Castellanos et al., 1997; Casey, Trainor, Giedd et al., 1997; Casey, Trainor, Orendi et al., 1997; Durston et al., 2002). Thus, one interpretation of this work is that normative brain development supports improvements in IC behavior in development.
Influences of Caregiving Behavior on Neural Development
Although the development of IC across the preschool period is clearly supported by normative brain development, recent theoretical and empirical work has suggested that developing EF skills are also influenced by the child’s caregiving environment (Bernier et al., 2010; Towe-Goodman et al., 2014; Valcan et al., 2017; Zeytinoglu, Calkins, Swingler, & Leerkes, 2017). Given that the development of IC depends heavily on underlying neural development also occurring in the preschool period, one way in which the environment may impact the development of IC abilities is through an influence on neural processes which support them. In effect, the neural mechanisms which support the emergence of IC in development may not be hard-wired processes in the brain that are incapable of being influenced or changed by the environment. Instead, it has been suggested that brain development supporting these processes (i.e. neuronal connections and pathways) continues to occur in the postnatal period, and is capable of being molded by input from the social environment (De Bellis, 2001; Greenough, Black & Wallace, 1987; Gunnar, Fisher, & the Early Experience, Stress and Prevention Network, 2006; Nelson, 2000; Nelson & Bloom, 1997; Propper & Moore, 2006).
In particular, neuroplasticity work has demonstrated that brain areas with longer developmental trajectories, like the frontal lobe, are more malleable in development because of a larger window of susceptibility to environmental input (Stevens & Neville, 2013). This is because early brain development is characterized by a process of synaptogenesis in which an over-production of synapses occurs, followed by a period of gradual pruning (Nelson & Bloom, 1997; Nelson, 2000). At least some neural development occurs because of experience-dependent processes in which synaptic connections are formed or eliminated to optimize a child’s adaptation to features of their environment (Greenough & Black, 1992; Black, Jones, Nelson & Greenough, 1998; Nelson, 2000; Nelson & Bloom, 1997; Nelson, Thomas, & de Haan, 2006; Singer, 1995). The prolonged window of development characteristic of brain systems associated with the development of IC makes it likely that interactions with the caregiver during early childhood may have an impact on the number and type of synaptic connections that are formed in these systems (Kolb et al., 2012).
Importance of Maternal Emotional Support
The caregiver-child relationship undergoes a shift in early development such that during infancy and toddlerhood caregivers serve as more direct external regulators of their child’s behavior, but as children enter the preschool period they rapidly gain and apply new skills like IC, which allow them to begin to regulate and control their own behavior (e.g. Calkins, 2011; Fox & Calkins, 2003; Kopp, 1982; Kopp & Neufeld, 2003). These emerging skills alter the nature of parent-child interactions in important ways as children require less direct intervention and parents take on more of a support role for child independent functioning. Several studies have examined mother-child interaction in the context of joint problem-solving tasks as a way of examining the role of maternal behavior on child cognitive and emotional development and associated outcomes. This work has demonstrated that maternal behavior in these contexts predicts child performance on tasks measuring EF abilities like IC (e.g. Blair et al., 2011; Conway & Stifter, 2012; Cuevas et al., 2014; Leerkes, Blankson, O’Brien, Calkins, & Marcovitch, 2011; Rochette & Bernier, 2014; Rogoff, 1990; Zeytinoglu et al., 2017). In particular, maternal use of emotional support, defined as encouraging autonomy, providing positivity, encouragement, and praise when needed, and helping the child to manage frustration and negativity, has been shown to uniquely relate to these, even when other aspects of parenting behavior like cognitive support and general emotional responsiveness were also measured (Leerkes et al., 2011; Valcan et al., 2017, Zeytinoglu, Calkins, & Leerkes, in press).
The Current Study
We hypothesized that one way in which maternal emotional support may influence IC during a critical period in development is through a direct association with neural processes underlying inhibition of a response. Previous work has demonstrated that maternal education and child variables such as age, gender, and minority status are sometimes associated with observed maternal behavior and child EF performance (e.g. Bernier et al., 2010, 2015, 2016; Mills-Koonce et al., 2007; NICHD Early Child Care Research Network, 2004; Zeytinoglu et al., 2017). Therefore, these were all included as covariates in the current model.
After accounting for these sociodemographic variables, we expected that maternal emotional support would be associated with a pattern of greater neural activity on trials which require the inhibition of a response in an IC task; thus, we hypothesized that more maternal emotional support would be associated with larger N2 amplitudes to no-go trials. In addition, previous developmental ERP studies of the N2 component have revealed a larger right-lateralized response to no-go trials in young children, suggesting a potential right hemisphere specialization for IC (Lahat et al., 2010; Todd, Lewis, Meusel, & Zelazo, 2008; Pérez-Edgar & Fox, 2007). Thus, we examined both left and right hemisphere N2 responses to no-go trials, and expected maternal behavior to be particularly associated with right hemisphere N2 amplitudes, indicating a potential specificity with IC processes. Finally, we expected that greater maternal emotional support would be associated with better behavioral performance on the IC task, both directly, and also through its association with neural mechanisms of IC.
Method
Participants
As part of a longitudinal study examining physiological, cognitive, and emotional predictors of early academic success, 278 children were recruited in a mid-sized city in the Southeastern United States when children were in their final year of preschool. Families were recruited primarily from local daycare centers and public establishments (e.g. libraries, museums, parks), and word of mouth. Of the 278 children, two were reported by parents to have atypical neuropsychological development (1 microcephaly, 1 ADHD) and were excluded from the final sample. Therefore, the current study utilized data from 276 typically developing children (150 girls, 126 boys; Mean Age = 56 Months, SD = 5 months) and their primary caregivers (96% mothers) who participated in a preschool laboratory visit. Parent report of child race indicated that 59% of the children were White, 28% Black, 2% Asian, and 11% multi-racial. For analysis purposes, these reports were recoded into a variable indicating minority status (non-Hispanic white = 0, minority = 1); 46% of children were coded as minority. For mothers who reported educational information (N=275), 2% did not graduate from high school, 8% had a high school diploma or GED only, 29% had some college or a 2-year degree, 32% had a 4-year college degree, and 29% had done post-graduate work.
Procedures
Upon arrival at the research laboratory, participants were greeted by a research assistant who explained the study procedures and obtained written consent from the mother and verbal assent from the child prior to the beginning of data collection. Following the consent process and a brief warm-up period, children were fitted with an EEG net and equipment for physiology data collection before participating in the computerized IC task. Mothers filled out questionnaires in an adjacent room using survey software by Qualtrics (Qualtrics, Provo, UT) and participated in a problem-solving game with the child. The mother-child problem-solving game was recorded for later behavioral coding. Families received monetary compensation for their time and children selected a small toy at the completion of the visit. All procedures were approved by the university institutional review board and complied with the US Federal Policy for the Protection of Human Subjects.
Measures
Maternal emotional support
Mother-child interaction was observed during a 7-minute semi-structured planning and problem-solving task (Leerkes et al., 2011). The treasure game task required mother-child dyads to complete multiple steps in sequence with the goal of getting a bear figurine from the start position on the playing board to a treasure chest located on an island at the other end of the board. Steps along the path were marked by colors that matched the sides of a die rolled to determine where to move the bear. Before getting to the treasure chest, the bear had to be moved to other locations in a correct order (e.g. retrieve a key to unlock a boat, take the boat across a river). A research assistant explained the game to the mother and child before leaving the room. The task ended when mother-child dyads reached the treasure chest, or when 7 minutes had elapsed. Interactions were videotaped and later coded by two trained and reliable coders.
Maternal behavior was rated using a 5-point coding system adapted from Neitzel and Stright (2003) to capture the frequency and quality of behavior on the following three dimensions: (1) Emotional responsiveness was defined as the extent to which the mother was sensitive, appeared to enjoy being with the child, appropriately responded to the child’s requests and emotions, provided positive reinforcement for the child, and anticipated and minimized potential problems or disruptions to the game. (2) Intrusiveness was defined as the extent to which the mother took over during the game and did not let the child participate, thereby undermining the child’s autonomy and participation. Examples of this behavior include giving the child many directions with very little time in between for the child to make decisions or take independent action, playing the game without the child, or physically moving the child’s hand along the board. (3) Negativity was defined as the extent to which the mother displayed negative verbal or non-verbal emotions, including direct criticism, frowning or scowling, irritability, and impatience. Interrater reliability on these rating scales was calculated using intra-class correlation coefficients (ICCs; Winer, Brown, & Michels, 1971) based on 15% (n = 42) of the interactions which were double coded. ICCs ranged from r = .76 to .91, all p < .01. Based on our previous work (Zeytinoglu et al., 2017, Zeytinoglu et al., in press), these three dimensions of maternal behavior were used as indicators to construct a latent factor of maternal emotional support.
Inhibitory Control
A computerized version of an animal GNG task (Lahat et al., 2010) was used to assess children’s inhibitory control. The task was administered using E-Prime Version 2.0 (PST, Pittsburgh, PA) while ongoing electroencephalography (EEG) was acquired. Children were given a brief introduction to the task before beginning. At the beginning of each trial, a fixation point, accompanied by a “ding” sound, appeared in the middle of the screen, and was displayed for 1500 ms. This was followed by the presentation of an animal stimulus that was displayed on the screen for 1500 ms, or until a response was registered (see Figure 1 for schematic of trial structure). Children were instructed to respond via a response button as quickly as they could as soon as an animal appeared on the screen (go), except when it was a dog (no-go). To increase child motivation to participate, feedback was displayed for 500 ms after each trial. A yellow smiley face followed each correct response and a red frowning face followed an incorrect response (i.e. a response on a no-go trial), an omitted response (on a go trial), or a response that occurred after the 1500 ms stimulus window. Before beginning the task, children completed 10 practice trials, consisting of 6 go and 4 no-go trials. The practice block was repeated until children responded correctly on 9 out of 10 trials. The task itself consisted of 144 trials divided into four blocks. Each block contained 27 (75%) go trials and 9 (25%) no-go trials, for a total of 108 go trials and 36 no-go trials. This ratio of go to no-go trials is designed to encourage a prepotent tendency to respond. To avoid predictability, no-go trials were preceded by two, three, or four go trials. Accuracy on each go and no-go trial was recorded. Accuracy on inhibitory (no-go) trials was used as our primary measure of inhibitory control. Successful discrimination between go and no-go targets was measured using a discriminability index (d-prime), computed by subtracting the z-transformed false alarm rate from the z-transformed hit rate: d′ = Z(Correct/Hit) – Z (Incorrect/False Alarm) (e.g. Cohen-Gilbert & Thomas, 2013; McCarthy & Davison, 1979; Tripp & Alsop, 1999; Pizzagalli, Jahn, & O’Shea, 2004).
Figure 1.
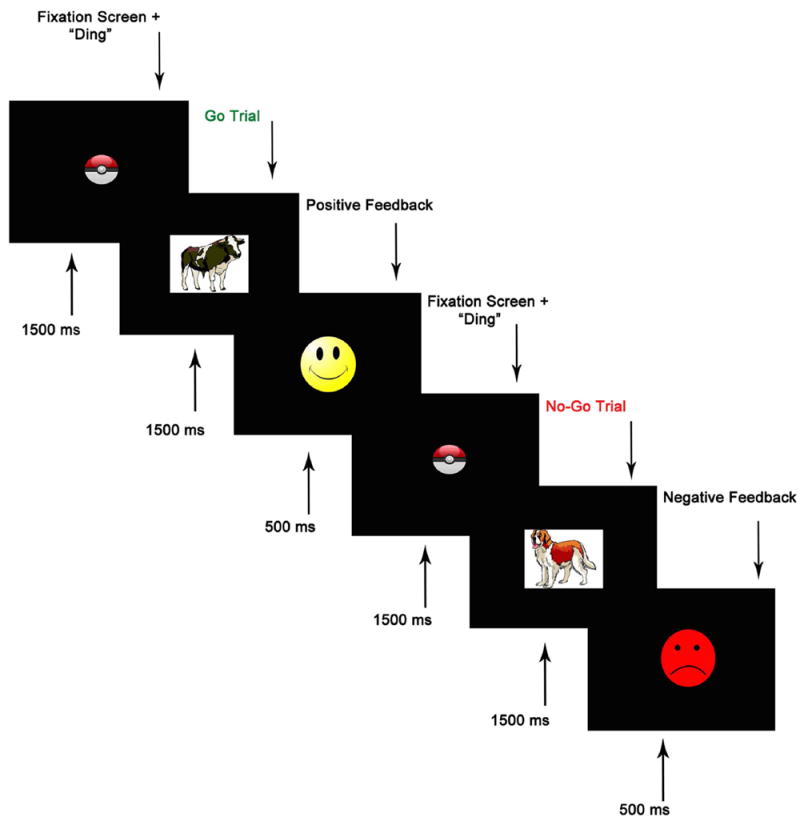
Schematic of Go/No-go (GNG) task structure. Reprinted from Lahat et al., 2010, retrieved from doi: 10.3389/neuro.09.072.2009, © 2010 Lahat, Todd, Mahy, Lau, and Zelazo.
EEG data collection and analysis
At the commencement of the testing session, the circumference of the child’s head was measured and the child was fitted with an appropriate sized 64-channel Geodesic Sensor Net (see Figure 2 for schematic of the electrode configuration) from Electrical Geodesics Inc. (EGI, Eugene, OR). The sensor net was connected to a NetAmps 300 amplifier (Electrical Geodesic Inc., Eugene, OR) and channel impedances were accepted if they were below 80 kΩ. During the go/no-go task, children were seated in front of the computer monitor; distance and alignment to the monitor were controlled and kept consistent across children. The experimenter instructed the children to hold as still as possible during the task and to push the response button with just the fingers of their dominant hand. EEG data were sampled at 250 Hz using EGI software (Netstation 4.5.4; EGI, Eugene, OR) and all channels were referenced to a single vertex electrode (Cz) during recording.
Figure 2.
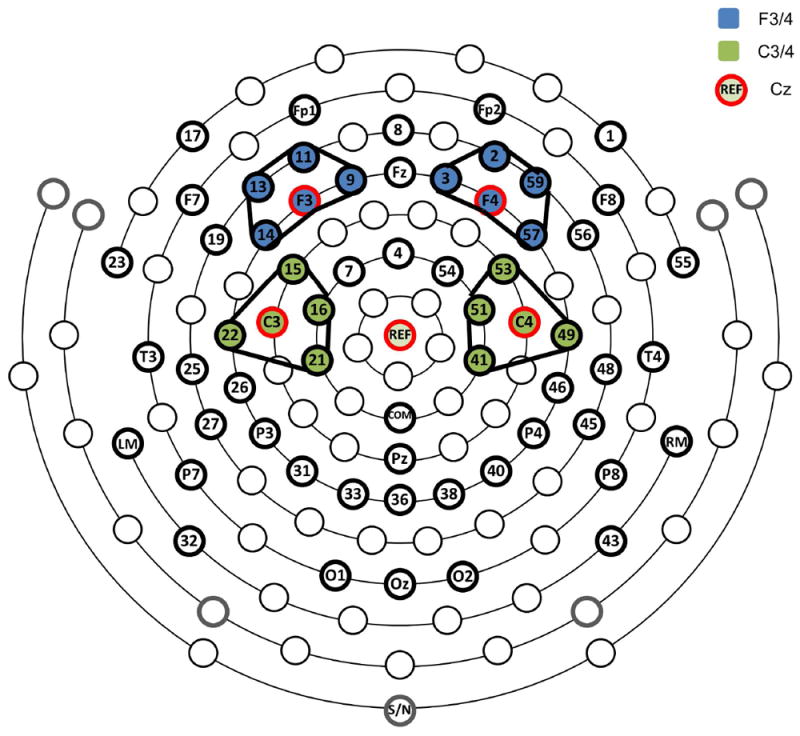
64-channel net with 10-20 channels and frontal and central clusters for each hemisphere identified. Within-hemisphere composites of frontal and central clusters were used in analyses.
ERP analyses were carried out using EEGLAB (Delorme & Makeig, 2004) and ERPLAB (Lopez-Calderon & Luck, 2014). EEG data were band-pass filtered from 0.1 to 30 Hz with a linear finite impulse response (FIR) filter. Following an advisory notice released by EGI on anti-alias filter effects on EEG and timing, event latencies were recoded by adding 8 ms to the original event latencies. Electrodes approximating the international 10-20 locations were renamed and clusters were defined around these electrodes (e.g. Vanderwert, Zeanah, Fox, & Nelson, 2016) as shown in Figure 2 (e.g. F4 cluster: E2, E3, F4, E57, E59). Initial inspection of the data revealed that, electrodes 23, 29, 47, and 55 were artifact-laden in more than half of the participants. Therefore, these electrodes were excluded from further processing. Electrodes 1, FP1, FP2, and 17 were used only for the detection of eye blinks and saccades. For the remaining 52 electrodes, a multi-step procedure was followed to replace bad electrodes. For greater precision, detection of bad electrodes was conducted on single electrodes rather than on clusters. As an initial step, bad electrodes were automatically marked using the pop_rejchan function in EEGLAB when they exceeded a spectrum threshold of 3 SD; additional bad electrodes were identified via visual inspection of the data by a trained research assistant. All electrodes identified as bad were replaced with the average mean amplitude of neighboring within-cluster electrodes (see Figure 2). No more than 5 electrodes (~10%) were replaced for any individual participant. Following this electrode replacement procedure, the EEG data were re-referenced to an average reference configuration (Lehmann, 1987).
The average referenced EEG data were segmented offline into epochs from 200 ms pre- to 600 ms post-stimulus onset; the first 200 ms were identified as the pre-stimulus-onset baseline. For eye blinks and saccades, artifact rejection was executed using a 200 ms moving window in 50 ms increments. A peak-to-peak rejection threshold of 100 or 125 μV was applied to electrodes 1, FP1, FP2, and 17 for the detection of eye-blinks and saccades; if necessary, this threshold was adjusted for individual children following visual inspection of the epochs marked by ERPLAB. For all other electrodes, the peak-to-peak rejection threshold was 200 μV. Children who did not have at least 10 artifact-free go and no-go trials were excluded from the analyses. The mean number of artifact free trials contributing to the final sample was 53 (SD = 19.6) go trials and 19 (SD = 6.3) no-go trials; this is comparable to previous developmental ERP work on inhibitory control (e.g. Lahat et al., 2010; Lamm et al., 2006; Spronk, Jonkman, & Kemner, 2008).
The ERP component of interest was the frontal N2 component, generally considered to be an index of neural processes involved in the inhibition of a response, measured here between 250-500 ms over frontal and central electrodes. This time window is consistent with previous work using this paradigm with a similar age group (Lahat et al., 2010). The appropriateness of this time window and scalp topography was confirmed via visual inspection of individual ERP plots and the grand-average plot (see Figure 3) for frontal and central electrode locations prior to data analysis. For analysis purposes, mean amplitude of the N2 component was extracted for each child at left and right hemisphere frontal-central electrode composites which were created separately for each hemisphere as follows: left hemisphere composite = F3 and C3 clusters; right hemisphere composite = F4 and C4 clusters (see Figure 4). Larger (more negative) values indicate greater neural activity in response to no-go trials.
Figure 3.
Grand-average ERP waveforms at frontal and central electrode locations with time (in milliseconds) depicted on the x-axis, and amplitude (in microvolts) on the y-axis. Note: Midline electrodes (4, 7,8,54, Fz) included for illustrative purposes.
Figure 4.
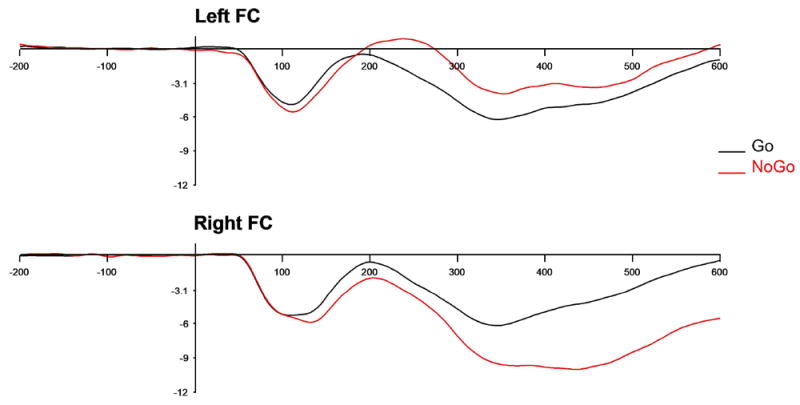
Grand average waveforms for go (black) and no-go (red) conditions over left (top) and right (bottom) frontal-central clusters with time (in milliseconds) depicted on the x-axis, and amplitude (in microvolts) depicted on the y-axis.
Results
As an initial step, the data were screened for missing values, outliers, and normality of distributions. See Table 1 for Ns and descriptive information for all primary study variables. EEG data were unavailable for 25 children for a variety of reasons (15 did not participate in EEG data collection; 3 equipment errors; 7 did not participate in the Go/No-Go task). An additional 51 children did not provide enough useable ERP data to be included. This was most commonly due to excessive EEG artifacts and/or not having a minimum of 10 artifact-free trials in each task condition. Independent samples t-tests revealed that children with (N = 200) and without (N = 76) useable ERP data were not different in terms of age (M = 56.46 vs. 56.12, t (274) = .56, p = .58, d = .07), minority status (M = .48 vs .42, t (274) = .85, p = .39, d = .12), maternal education (M = 4.76 vs 4.56, t (273) = .86, p = .39, d = .12) or gender (M= 1.51 vs. 1.63, t (274) = -1.71, p = .09, d = .24). Children with usable ERP data performed slightly higher on the GNG task than those without useable ERP data (M = 2.30 vs 2.01, t (260) = 1.95, p = .05, d = .28), although the effect size was small (e.g. Cohen, 1992). Bivariate correlations between study variables are presented in Table 2. Given that child age, gender, minority status and maternal education were all significantly correlated with maternal behavior and child behavioral performance, these were included as predictors in the model.
Table 1.
Descriptive Statistics for Model Variables
| N | Mean | SD | Min | Max | Skewness | Kurtosis | |
|---|---|---|---|---|---|---|---|
| Maternal emotional responsivenessa | 274 | 3.85 | 1.03 | 1.00 | 5.00 | -0.51 (.15) | -0.54 (.29) |
| Maternal intrusiveness (r)a,b | 274 | 4.00 | 1.17 | 1.00 | 5.00 | -0.94 (.15) | -0.18 (.29) |
| Maternal negativity (r)a,b | 274 | 4.32 | 0.97 | 1.00 | 5.00 | -1.49 (.15) | 1.75 (.29) |
| Right Frontal-Central N2c | 200 | -8.47 | 4.64 | -21.74 | 7.57 | 0.13 (.17) | 0.39 (.34) |
| Left Frontal-Central N2c | 200 | -2.66 | 4.79 | -20.47 | 10.69 | -0.24 (.17) | 0.73 (.34) |
| GNG Behavioral Performance (d′)d | 263 | 2.23 | 1.04 | -0.08 | 5.39 | 0.19 (.15) | -0.20 (.30) |
Note:
Maternal emotional responsiveness, intrusiveness, and negativity were observed during the problem-solving task and coded on a 1-5 scale.
(r) maternal intrusiveness and negativity are reverse-coded so that higher values equal less of the behavior.
N2 component of the ERP.
discriminability index (d-prime).
Table 2.
Correlations among Model Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Child Gender | - | |||||||||
| 2. | Child Minority status | .02 | - | ||||||||
| 3. | Child Age | -.11 | -.08 | - | |||||||
| 4. | Maternal education | -.01 | -.25** | -.02 | - | ||||||
| 5. | Maternal emotional resp. | .13* | -.21** | .00 | .28** | - | |||||
| 6. | Maternal intrusiveness (r)a | .15* | -.43** | .27** | .31** | .47** | - | ||||
| 7. | Maternal negativity (r)a | .08 | -.36** | .14* | .31** | .52** | .66** | - | |||
| 8. | Right Frontal-Central N2b | .00 | .19** | -.02 | -.14* | -.21** | -.16* | -.18* | - | ||
| 9. | Left Frontal-Central N2b | .00 | .05 | -.01 | -.07 | -.05 | -.03 | .01 | .21** | - | |
| 10. | GNG Beh. Performance (d′)c | .20** | -.24** | .25** | .19** | .19** | .33** | .34** | -.29** | -.08 | - |
p < .05.
p < .01.
Note:
(r) denotes that maternal intrusiveness and negativity are reverse-coded so that higher values equal less of the behavior.
N2 component of the ERP.
discriminability index (d-prime).
We used a structural equation model to examine the associations between maternal emotional support, child left and right hemisphere frontal-central no-go N2 ERPs, and child behavioral performance (d’) on a GNG task in Mplus (Version 8; e.g. Muthén & Muthén, 1998-2014). Full Information Maximum Likelihood (FIML) was used to handle missing data; all data were missing at random. Maternal emotional support was constructed as a latent factor using three indicators: maternal emotional responsiveness, maternal intrusiveness (reversed), and maternal negativity (reversed) (Zeytinoglu et al., 2017). All three indicators loaded significantly on the maternal emotional support construct, as displayed in Figure 5. A bias-corrected bootstrapping procedure was used in evaluating the significance of the indirect pathway from maternal emotional support to child behavioral performance (d’) via N2 ERP responses. This procedure is known to generate accurate confidence intervals (CI) for indirect effects, which are considered significant if the CIs for unstandardized betas do not include zero (Little, 2013).
Figure 5.
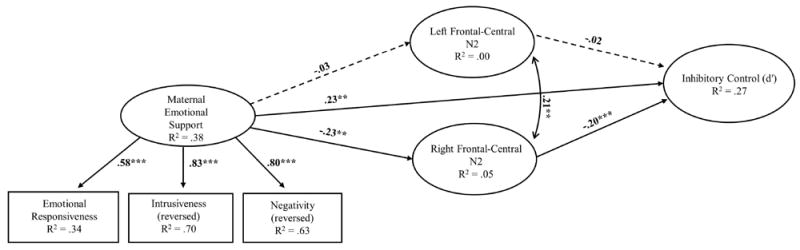
Standardized estimates for the model predicting neural and behavioral performance on the GNG task in preschoolers. Child age, gender, and minority status, and maternal education were included as covariates, but are not depicted here. Note: The N2 is a negative component, thus “larger” values are more negative. *p < .05, **p < .01, ***p<.001
Model fit of the structural model was evaluated using four fit indices: chi-square, the root mean square error of approximation (RMSEA), Bentler comparative fit index (CFI), and standardized root mean square residual (SRMR). The chi-square value tests whether there are differences between the population and model covariance matrices. RMSEA is a parsimony-adjusted index that allows for the identification of lower and upper confidence intervals. Close-fit hypothesis is supported if the RMSEA estimate is lower than. 08, and the test of not acceptable fit can be rejected if the upper confidence interval is lower than .08 (Little, 2013). CFI tests model fit based on a baseline model and values higher than .95 are considered excellent fit. Lastly, SRMR is a measure of the mean absolute correlation residual and estimates lower than .08 are considered indicative of good fit (Hu & Bentler, 1999).
The hypothesized model fit the data well, χ2 (26, N = 276) = 39.45, p = .04, CFI = .97, RMSEA = .04 (CI = .01, .07), SRMR = .03. Unstandardized and standardized coefficients are presented in Table 3 and standardized coefficients are presented in Figure 5. As reported in Figure 5 and Table 3, mothers with higher levels of education and mothers of White non-Hispanic, female, and older children were observed to be more emotionally supportive. Older children and females performed significantly better on the GNG task (d′). Maternal education and child minority status were not significant predictors of child task performance (d’). Below, we walk through specific model pathways that directly reflect our primary research questions.
Table 3.
Unstandardized and Standardized Model Estimates and 95% Confidence Intervals
| Confidence Interval | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Unstand. Est. | Stand. Est. | SE | p | Lower | Upper | ||
| Covariances | |||||||
| Maternal Education | ↔ Minority Status | -0.21 | -0.25 | 0.05 | .00 | -0.31 | -0.11 |
| Left Frontal-Central N2 | ↔ Right Frontal-Central N2 | 4.53 | 0.21 | 1.62 | .01 | 1.35 | 7.72 |
|
| |||||||
| Covariates | |||||||
| Child Gender | → Maternal Emotional Support | 0.22 | 0.19 | 0.07 | .00 | 0.08 | 0.37 |
| → GNG Beh. Performance (d′) | 0.38 | 0.18 | 0.13 | .00 | 0.14 | 0.63 | |
| Child Age | → Maternal Emotional Support | 0.03 | 0.22 | 0.01 | .00 | 0.01 | 0.04 |
| → GNG Beh. Performance (d′) | 0.05 | 0.21 | 0.01 | .00 | 0.02 | 0.07 | |
| Maternal Education | → Maternal Emotional Support | 0.11 | 0.30 | 0.03 | .00 | 0.06 | 0.16 |
| → GNG Beh. Performance (d′) | 0.04 | 0.06 | 0.04 | .33 | -0.04 | 0.12 | |
| Minority Status | → Maternal Emotional Support | -0.47 | -0.39 | 0.08 | .00 | -0.62 | -0.32 |
| → GNG Beh. Performance (d′) | -0.15 | -0.07 | 0.15 | .32 | -0.43 | 0.14 | |
|
| |||||||
| Direct Paths | |||||||
| Maternal Emotional Support | → Left Frontal-Central N2 | -0.25 | -0.03 | 0.60 | .68 | -1.44 | 0.93 |
| → Right Frontal-Central N2 | -1.80 | -0.23 | 0.61 | .00 | -2.99 | -0.59 | |
| → GNG Beh. Performance (d′) | 0.41 | 0.23 | 0.17 | .01 | 0.08 | 0.73 | |
| Left Frontal-Central N2 | → GNG Beh. Performance (d′) | -0.01 | -0.02 | 0.01 | .69 | -0.03 | 0.02 |
| Right Frontal-Central N2 | → GNG Beh. Performance (d′) | -0.05 | -0.20 | 0.01 | .00 | -0.07 | -0.02 |
|
| |||||||
| Indirect Paths | |||||||
| Maternal Emotional Support | → GNG Beh. Performance (d′) | 0.08 | 0.05 | 0.04 | .03 | 0.01 | 0.16 |
| → Left Frontal-Central N2 | 0.00 | 0.00 | 0.01 | .88 | -0.01 | 0.02 | |
| → Right Frontal-Central N2 | 0.08 | 0.05 | 0.04 | .03 | 0.01 | 0.15 | |
The path from maternal emotional support to child N2 no-go ERPs was significant, although this was specific to right frontal-central N2 responses only (see Figure 5). Greater observed maternal emotional support was associated with larger (more negative) right frontal-central N2 no-go ERPs. However, the path from maternal emotional support to left frontal-central N2 no-go ERPs was not significant. As predicted, the direct path from maternal emotional support to child performance on the task (d′) was also significant; higher levels of maternal emotional support were associated with better task performance.
Child frontal-central N2 ERPs to no-go trials were also associated with task performance. However, again, only the direct path from right frontal-central no-go N2s was associated with observed task performance (see Figure 5). This association was negative, such that larger (more negative) N2 amplitudes were associated with better observed performance.
Finally, we examined whether observed maternal emotional support was an indirect predictor of child behavioral performance in the GNG task via right hemisphere no-go N2 amplitudes. Maternal emotional support was not associated with left hemisphere N2 amplitudes; therefore, only the indirect effect from maternal emotional support to task performance via the right frontal-central N2 component was tested. To test this indirect effect, a bias-corrected bootstrapping procedure (10,000 draws) was performed. This approach has been shown to generate the most accurate confidence intervals for indirect effects, reducing Type 1 error rates and increasing power over other similar tests (MacKinnon, Lockwood, & Williams, 2004). The indirect path was significant [unstandardized estimate = .08, S.E. = .037, p = .029, 95% BC Bootstrap (CI .008, .153)], indicating that greater maternal emotional support predicted better child behavioral performance (d′) via larger (more negative) right frontal-central no-go N2 responses. Examination of the standardized regression coefficient of the specific indirect effect (β= .05, see Table 3) indicated that the effect size of the indirect path from maternal emotional support to child GNG performance via right frontal-central no-go N2 amplitudes was small.
Discussion
The goal of this study was to examine the role of maternal emotional support during typical mother-child interactions on children’s developing IC processes in the preschool period. Previous work has suggested that maternal emotional support and related constructs (e.g. quality of the mother-child relationship) play a unique role in behavioral manifestations of child EFs like IC in development (e.g. Blair et al., 2011; Cuevas et al., 2014; Rochette & Bernier, 2014; Valcan et al., 2017; Zeytinoglu et al., 2017). This is presumed to be at least partially a result of an influence of maternal behavior on neural development of the frontal cortex, which has a prolonged window of opportunity for environmental input due to its protracted postnatal development (e.g. Bernier et al., 2015; Kolb et al., 2012). Despite the relative prevalence of this theoretical view, almost no work has demonstrated this relationship empirically in the preschool period, which is a period of rapid developmental change in EF abilities.
To address this gap in the literature, we examined the influence of maternal emotional support during a mother-child problem solving game on one purported neural mechanism of IC, the N2 component of the ERP, as well as observed behavioral performance in an IC task in preschool aged children. Our results indicate that greater observed maternal emotional support during the mother-child problem solving task was associated with larger (more negative) no-go N2 responses in our sample of preschoolers. Interestingly, this result was specific to right hemisphere frontal-central no-go N2 responses; we did not find an association between maternal emotional support and left hemisphere no-go N2 responses. We found a similar pattern of hemispheric results for the association between no-go N2 responses and observed behavioral performance. Specifically, larger (more negative) right frontal-central no-go N2 responses were associated with better observed performance; no such association was evident for left hemisphere no-go N2 responses and observed performance.
Our hemispheric findings are consistent with work in both clinical and developmental populations that has shown a right hemisphere specialization for neural processes that govern the inhibition of a prepotent response. For example, fMRI work has demonstrated that volumes of the right anterior cingulate cortex and other structures of the right frontal cortex correlate with performance on tasks requiring inhibition of a response in middle childhood and early adolescence (Casey, Castellanos et al., 1997; Casey, Trainor, Giedd et al., 1997; Durston et al., 2002). Similarly, ERP work with adults (e.g. Bokura et al., 2001) and children has demonstrated that the N2 response to no-go (IC) trials is largest over the right hemisphere (Lahat et al., 2010; Todd et al., 2008; Peréz-Edgar & Fox, 2007), although there is some indication that this hemispheric specialization increases with age and EF ability (e.g. Bunge et al., 2002; Lamm et al., 2006). Finally, research on EEG alpha power asymmetry has demonstrated a greater role for left prefrontal cortex in approach-related behavior and a greater role for right prefrontal cortex in withdrawal or inhibition (e.g. Davidson, 1992; Davidson & Fox, 1989; Harmon-Jones & Allen, 1997). Thus, there is accumulating evidence for a right hemisphere specialization for IC processes that emerges early in development and is associated with successful IC behavior. In light of this evidence, our finding that maternal emotional support in mother-child interactions in the preschool period is associated with right, but not left, hemisphere no-go N2 responses suggests that maternal behavior has an influence on IC processes specifically in the brain. Further support for this idea comes from the fact that larger right hemisphere no-go N2 responses were associated with better task performance in our sample, while we saw no such association for left hemisphere no-go N2 responses.
Thus, our findings provide some preliminary evidence that maternal emotional support is directly associated with neural mechanisms supporting IC during the preschool period. One potential explanation for this is that more emotionally supportive mothers engage in more mutually reciprocal interactions with their children, particularly in situations which require IC of the child, as this process is still relatively immature and difficult for a child to achieve independently in the preschool period and emotionally supportive mothers may be more sensitive to this. For example, in the mother-child game used in this work, a specific order of events had to be followed in order to achieve the goal of getting the bear to the treasure chest and winning the game. Children were required to inhibit prepotent tendencies to go straight to the treasure chest (the end goal), in order to complete the necessary steps to win the game. Mothers who were more emotionally supportive in this task were more engaged with and sensitive to their child, showed more positive reinforcement and less intrusive or negative behavior, and allowed the child more autonomy to perform the task while providing guidance on how best to achieve the end goal. Thus, these mothers may be more likely to provide the environmental context in which IC can be practiced by the child, thereby strengthening synaptic connections in areas that support IC behavior. This increase in connections and myelination may be reflected in the larger (more negative) right hemisphere N2 no-go amplitudes we found to be associated with greater maternal emotional support. As ERP technology cannot provide information about brain structure, this explanation is purely speculative, however future work testing this hypothesis could utilize imaging methods like Diffusion Tensor Imaging (DTI) to examine white matter tracts in the brain.
Although we, like others, interpret the N2 component of the ERP as reflecting neural processing supporting the inhibition of a response, it is important to acknowledge that multiple processes (e.g. attention and working memory) likely contribute to successful IC and may not be captured by the N2. Thus, although our findings provide evidence for a relationship between maternal emotional support and one neural mechanism for IC indexing inhibition of a response, there are likely other neural and behavioral processes contributing to child behavioral performance that we have not caputred here. In addition, we also found a direct relationship between maternal emotional support and child performance on the task in our sample; that is, more emotionally supportive maternal behavior appears to influence child IC behavior directly, perhaps without altering associated neural underpinnings. One possibility is that mothers who are more emotionally supportive may also exhibit more appropriate IC behavior and strategies themselves that children can model in their own behavior. Support for this idea comes from recent work demonstrating that maternal self-report of effortful control, defined as a process that enables one to voluntarily shift and focus attention and inhibit or activate a response, predicted observed measures of maternal emotional support during mother-child interaction in the preschool period (Zeytinoglu et al., 2017). A second possibility is that mothers with greater IC may pass this capacity to their children biologically, as EFs have been identified as among the most heritable psychological traits (Friedman et al., 2008). Thus, mothers who are more emotionally supportive may also possess greater IC skill themselves, which they pass on to their children through some combination of biology and/or experiential modeling.
There are likely multiple pathways through which maternal emotional support influences child IC development, examination of the standardized path coefficients in Table 3 suggests that in our sample maternal emotional support was associated with child neural responses to the no-go trails. Although this effect was small to moderate in nature, it accounted for a small, but significant, amount of variation in child behavioral performance via neural responses. This is in addition to a significant direct relationship between maternal emotional support behavior and child behavioral IC performance. Taken together, all of our model predictors accounted for approximately 27% of the variance in child IC behavioral performance. Thus, although maternal behavior and child neural functioning (as well as maternal education and child age, gender, and minority status) are clearly factors which contribute to child IC performance in the preschool period, they are by no means the only factors contributing. Nevertheless, our findings implicate maternal emotional support behavior as one predictor of child IC in the preschool period and suggest that even normative variation in maternal behavior during parent-child interactions may influence IC behavior directly, and through neural mechanisms supporting IC in development. We have demonstrated associations for concurrent child behavioral performance here, but there may also be lasting implications for the child IC development as early behaviors and neural development lay the foundation for future functioning across a variety of domains. Although this work is correlational in nature, our findings raise the possibility that interventions and programs aimed at promoting the development of EFs like IC in early childhood could include a focus on teaching mothers and other caregivers to use behavior that encourages child autonomy, and provides positivity, encouragement, and praise to help the child manage frustration and negativity in the course of daily interactions and challenges as a potential means of supporting IC development.
Despite the intriguing nature of these findings with regard to the role of maternal behavior in IC development in the preschool period, this study is not without some limitations. These data were collected from a laboratory visit at a single time point; any conclusions about causal inferences or developmental processes are therefore limited. Future work should include measures of maternal behavior and neural and behavioral measures of child IC at multiple time points to allow for a stronger test of the nature of the relationship between these variables in development. We measured maternal behavior in a laboratory setting in a single parent-child interaction task designed to elicit problem-solving and parent-child cooperation; however, this limited glimpse of maternal behavior and mother-child interaction may not fully capture the range and nuances of maternal behavior in more natural settings (e.g. the home) and across a variety of parent-child interactions.
Despite these limitations, our large, diverse sample is unique, especially with respect to the inclusion of measurement of neural indices of IC; this is a strength of the current work that enhances the generalizability of our findings. We also employed careful observational methods and a detailed, established, and reliable coding scheme in our measurement of maternal behavior and examined child behavioral performance in a well-validated and commonly used computerized version of a GNG task, which minimizes variation in implementation across participants, and facilitates combination with measurement of neural indices during task performance. Our results suggest that children who experience more maternal emotional support during interactions with their mother in the preschool period perform better on an IC task, and that this may be because of an influence of maternal behavior on child behavior, as well as at least one neural mechanism underlying IC. This likely has far reaching consequences for the child in development because of the foundational role IC processes play in healthy cognitive, emotional, and academic functioning throughout the lifespan.
Acknowledgments
This research was supported by Grant HD071957 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health. We are grateful to the families for their participation in our research and to our research team at UNC-Greensboro for their assistance with data collection and coding. We would like to acknowledge the role of Dr. Marion O’Brien, who was instrumental in the design, planning, and implementation of this study prior to her death.
References
- Bernier A, Beauchamp MH, Carlson SM, Lalonde G. A secure base from which to regulate: Attachment security in toddlerhood as a predictor of executive functioning at school entry. Developmental psychology. 2015;51:1177–1189. doi: 10.1037/dev0000032. [DOI] [PubMed] [Google Scholar]
- Bernier A, Calkins SD, Bell MA. Longitudinal associations between the quality of mother-infant interactions and brain development across infancy. Child Development. 2016;87:1159–1174. doi: 10.1111/cdev.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier A, Carlson S, Whipple N. From external regulation to self-regulation: Early parenting precursors of children’s executive functioning. Child Development. 2010;81:326–339. doi: 10.1111/j.1467-8624.2009.01397.x. [DOI] [PubMed] [Google Scholar]
- Black JE, Jones TA, Nelson CA, Greenough WT. Neuronal plasticity and the developing brain. In: Alessi NE, Coyle JT, Harrison SI, Eth S, editors. Handbook of child and adolescent psychiatry, Basic psychiatric science and treatment. Vol. 6. New York: Wiley; 1998. pp. 31–53. [Google Scholar]
- Blair C, Razza RP. Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Development. 2007;78:647–663. doi: 10.1111/j.1467-8624.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- Blair C, Granger DA, Willoughby M, Mills-Koonce R, Cox M, Greenberg MT, Fortunato CK, et al. the FLP Investigators. Salivary cortisol mediates effects of poverty and parenting on executive functions in early childhood. Child Development. 2011;82:1970–1984. doi: 10.1111/j.1467-8624.2011.01643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokura H, Yamaguchi S, Kobayashi S. Electrophysiological correlates for response inhibition in a Go/NoGo task. Clinical Neurophysiology. 2001;112:2224–2232. doi: 10.1016/S1388-2457(01)00691-5. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295X.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JDE. Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/S0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD. Caregiving as coregulation: Psychobiological processes and child functioning. In: Booth A, McHale SM, Landale NS, editors. Biosocial foundations of family processes. New York, NY: Springer; 2011. pp. 49–59. [Google Scholar]
- Carlson SA. Developmentally sensitive measures of executive function in preschool children. Developmental Neuropsychology. 2005;28:595–616. doi: 10.1207/s15326942dn2802_3. [DOI] [PubMed] [Google Scholar]
- Carver AC, Livesey DJ, Charles M. Age related changes in inhibitory control as measured by stop signal task performance. International Journal of Neuroscience. 2001;107:43–61. doi: 10.3109/00207450109149756. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, Rapoport JL, et al. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Giedd JN, Vauss Y, Vaituzis CK, Hamburger S, Rapoport JL, et al. The role of the anterior cingulate in automatic and controlled processes: A developmental neuroanatomical study. Developmental Psychobiology. 1997;30:61–69. doi: 10.1002/(SICI)1098-2302(199701)30:1<61∷AID-DEV6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Geidd JN, Castellanos FX, Rapoport JL, et al. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. Journal of Cognitive Neuroscience. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Cragg L, Fox A, Nation K, Reid C, Anderson M. Neural correlates of successful and partial inhibitions in children: An ERP study. Developmental Psychobiology. 2009;51:533–543. doi: 10.1002/dev.20391. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological bulletin. 1992;112:155. doi: 10.1037/0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cohen-Gilbert JE, Thomas KM. Inhibitory control during emotional distraction across adolescence and early adulthood. Child Development. 2013;84:1954–1966. doi: 10.1111/cdev.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway A, Stifter CA. Longitudinal antecedents of executive function in preschoolers. Child Development. 2012;83:1022–1036. doi: 10.1111/j.1467-8624.2012.01756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas K, Deater-Deckard K, Kim-Spoon J, Watson AJ, Morasch KC, Bell MA. What’s mom got to do with it? Contributions of maternal executive function and caregiving to the development of executive function across early childhood. Developmental Science. 2014;17:224–238. doi: 10.1111/desc.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis W, Cicchetti D. Emotion and resilience: A multilevel investigation of hemispheric electroencephalogram asymmetry and emotion regulation in maltreated and nonmaltreated children. Development and Psychopathology. 2007;19:811–840. doi: 10.1017/S0954579407000405. [DOI] [PubMed] [Google Scholar]
- Davis EP, Bruce J, Snyder K, Nelson CA. The X-trials: Neural correlates of an inhibitory control task in children and adults. Journal of Cognitive Neuroscience. 2003;15:432–443. doi: 10.1162/089892903321593144. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain and Cognition. 1992;20:125–151. doi: 10.1016/0278-2626(92)90065-T. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Fox NA. Frontal brain asymmetry predicts infants’ response to maternal separation. Journal of Abnormal Psychology. 1989;98:127–131. doi: 10.1037/0021-843X.98.2.127. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD. Developmental traumatology: The psychobiological development of maltreated children and its implications for research, treatment, and policy. Development and Psychopathology. 2001;13:539–564. doi: 10.1017/S0954579401003078. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Diamond A. Developmental time course in human infants and infant monkeys, and the neural bases of inhibitory control in reaching. Annals of the New York Academy of Sciences. 1990;608:637–676. doi: 10.1111/j.1749-6632.1990.tb48913.x. [DOI] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Yang Y, Uluğ AM, Zimmerman RD, Casey BJ. A neural basis for the development of inhibitory control. Developmental Science. 2002;5:F9–F16. doi: 10.1111/1467-7687.00235. [DOI] [Google Scholar]
- Eimer M. Effects of attention and stimulus probability on ERPs in a Go/Nogo task. Biological Psychology. 1993;35:123–138. doi: 10.1016/0301-0511(93)90009-W. [DOI] [PubMed] [Google Scholar]
- Espy KA, McDiarmid MM, Cwik MF, Stalets MM, Hamby A, Senn TE. The contribution of executive functions to emergent mathematic skills in preschool children. Developmental Neuropsychology. 2004;26:465–486. doi: 10.1207/s15326942dn2601_6. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Gratton G, Federmeier KD. Event-related brain potentials: Methods, theory, and applications. In: Cacioppo J, Tassinary L, Bernston G, editors. Handbook of Psychophysiology. 3. New York: Cambridge University Press; 2007. pp. 85–119. [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychologica. 1999;101:267–291. doi: 10.1016/S0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Fox NA, Calkins SD. The development of self-control of emotion: Intrinsic and extrinsic influences. Motivation and Emotion. 2003;27:7–26. doi: 10.1023/A:1023622324898. [DOI] [Google Scholar]
- Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology: General. 2008;137:201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstadt C, Hong Y, Diamond A. The relationship between cognition and action: Performance of children 3½–7 years old on a Stroop-like day–night test. Cognition. 1994;53:129–153. doi: 10.1016/0010-0277(94)90068-X. [DOI] [PubMed] [Google Scholar]
- George MS. Obsessive-compulsive disorder. International Clinical Psychopharmacology. 1991;6:57s–68s. [PubMed] [Google Scholar]
- George MS, Ketter TA, Post RM. SPECT and PET imaging in mood disorders. The Journal of Clinical Psychiatry. 1993;54:6–13. [PubMed] [Google Scholar]
- Greenough WT, Black JE. Induction of brain structure by experience: Substrates for cognitive development. In: Gunnar MR, Nelson CA, editors. Developmental behavioral neuroscience. Hillsdale, NJ, England: Lawrence Erlbaum Associates, Inc; 1992. pp. 155–200. [Google Scholar]
- Greenough WT, Black JE, Wallace CS. Experience and brain development. Child Development. 1987;58:539–559. doi: 10.2307/1130197. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Fisher PA, Gunnar MR, Fisher PA the Early Experience, Stress, and Prevention Network. Bringing basic research on early experience and stress neurobiology to bear on preventive interventions for neglected and maltreated children. Development and Psychopathology. 2006;18:651–677. doi: 10.1017/S0954579406060330. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJB. Behavioral activation sensitivity and resting frontal EEG asymmetry: Covariation of putative indicators related to risk for mood disorders. Journal of Abnormal Psychology. 1997;106:159–163. doi: 10.1037/0021-843X.106.1.159. [DOI] [PubMed] [Google Scholar]
- Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6:1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- Jodo E, Kayama Y. Relation of a negative ERP component to response inhibition in a Go/No-go task. Electroencephalography and Clinical Neurophysiology. 1992;82:477–482. doi: 10.1016/0013-4694(92)90054-L. [DOI] [PubMed] [Google Scholar]
- Jones LB, Rothbart MK, Posner MI. Development of executive attention in preschool children. Developmental Science. 2003;6:498–504. doi: 10.1111/1467-7687.00307. [DOI] [Google Scholar]
- Jonkman LM. The development of preparation, conflict monitoring and inhibition from early childhood to young adulthood; a go/nogo ERP study. Brain Research. 2006;1097:181–193. doi: 10.1016/j.brainres.2006.04.064. [DOI] [PubMed] [Google Scholar]
- Jonkman LM, Lansbergen M, Stauder JEA. Developmental differences in behavioral and event-related brain responses associated with response preparation and inhibition in a go/nogo task. Psychophysiology. 2003;40:752–761. doi: 10.1111/1469-8986.00075. [DOI] [PubMed] [Google Scholar]
- Kolb B, Mychasiuk R, Muhammad A, Li Y, Frost DO, Gibb R. Experience and the developing prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:17186–17193. doi: 10.1073/pnas.1121251109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp CB. Antecedents of self-regulation: A developmental perspective. Developmental Psychology. 1982;18:199–241. doi: 10.1037/0012-1649.18.2.199. [DOI] [Google Scholar]
- Kopp CB, Neufeld SJ. Emotional development during infancy. In: Davidson RJ, Scherer KR, Goldsmith H, editors. Handbook of affective sciences. New York, NY, US: Oxford University Press; 2003. pp. 347–374. [Google Scholar]
- Lahat A, Todd RM, Mahy CEV, Lau K, Zelazo PD. Neurophysiological correlates of executive function: A comparison of European-Canadian and Chinese-Canadian 5-year-old children. Frontiers in Human Neuroscience. 2010;3:1–10. doi: 10.3389/neuro.09.072.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Zelazo PD, Lewis MD. Neural correlates of cognitive control in childhood and adolescence: Disentangling the contributions of age and executive function. Neuropsychologia. 2006;44:2139–2148. doi: 10.1016/j.neuropsychologia.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Leerkes EM, Blankson AN, O’Brien M, Calkins SD, Marcovitch S. The relation of maternal emotional and cognitive support during problem solving to pre academic skills in preschoolers. Infant and Child Development. 2011;20:353–370. doi: 10.1002/icd.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann D. Principles of spatial analysis. In: Gevins AS, Remond A, editors. Handbook of electroencephalography and clinical neurophysiology: Methods of analysis of brain electrical and magnetic signals. Vol. 1. Amsterdam: Elsevier; 1987. pp. 309–354. [Google Scholar]
- Little TD. Longitudinal structural equation modeling. New York: Guilford Press; 2013. [Google Scholar]
- Lopez-Calderon J, Luck SJ. ERPLAB: an open-source toolbox for the analysis of event-related potentials. Frontiers in Human Neuroscience. 2014;8:213. doi: 10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ. Event-related potentials. In: Cooper H, Camic PM, Long DL, Panter AT, Rindskopf D, Sher KJ, editors. APA Handbook of Research Methods in Psychology: Foundations, Planning, Measures, and Psychometrics. Vol. 1. Washington, DC: American Psychological Association; 2012. pp. 523–546. [Google Scholar]
- Luu P, Posner MI. Anterior cingulate cortex regulation of sympathetic activity. Brain. 2003;126:2119–2120. doi: 10.1093/brain/awg257. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate Behavioral Research. 2004;39:99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, Fox NA the BEIP core group. A Comparison of the Electroencephalogram between Institutionalized and Community Children in Romania. Journal of Cognitive Neuroscience. 2004;16:1327–1338. doi: 10.1162/0898929042304723. [DOI] [PubMed] [Google Scholar]
- Masten AS, Herbers JE, Desjardins CD, Cutuli JJ, McCormick CM, Sapienza JK, Zelazo PD, et al. Executive function skills and school success in young children experiencing homelessness. Educational Researcher. 2012;41:375–384. doi: 10.3102/0013189X12459883. [DOI] [Google Scholar]
- Matte-Gagné C, Bernier A, Lalonde G. Stability in maternal autonomy support and child executive functioning. Journal of Child and Family Studies. 2015;24:2610–2619. doi: 10.1007/s10826-014-0063-9. [DOI] [Google Scholar]
- McCarthy D, Davison M. Signal probability, reinforcement, and signal detection. Journal of Experimental Analysis of Behavior. 1979;32:373–382. doi: 10.1901/jeab.1979.32-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland MM, Cameron CE, Connor CM, Farris CL, Jewkes AM, Morrison FJ. Links between behavioral regulation and preschoolers’ literacy, vocabulary, and math skills. Developmental Psychology. 2007;43:947–959. doi: 10.1037/0012-1649.43.4.947. [DOI] [PubMed] [Google Scholar]
- Mills-Koonce WR, Propper CB, Gariepy JL, Blair C, Garrett-Peters P, Cox MJ. Bidirectional genetic and environmental influences on mother and child behavior: The family system as the unit of analyses. Development and Psychopathology. 2007;19:1073–1087. doi: 10.1017/S0954579407000545. [DOI] [PubMed] [Google Scholar]
- Mischel W, Ayduk O, Berman MG, Casey BJ, Gotlib IH, Jonides J, Shoda Y, et al. “Willpower” over the life span: decomposing self-regulation. Social Cognitive and Affective Neuroscience. 2011;6:252–6. doi: 10.1093/scan/nsq081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP. The nature and organization of individual differences in executive functions: Four general conclusions. Current Directions in Psychological Science. 2012;21:8–14. doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monette S, Bigras M, Guay MC. The role of the executive functions in school achievement at the end of Grade 1. Journal of Experimental Child Psychology. 2011;109:158–173. doi: 10.1016/j.jecp.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide: Statistical analysis with latent variables. 7. Los Angeles, CA: 1998-2014. [Google Scholar]
- Nieuwenhuis S, Yeung N, Van Den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:17–26. doi: 10.3758/CABN.3.1.17. [DOI] [PubMed] [Google Scholar]
- Nelson CA. The Neurobiological Bases of Early Intervention. In: Shonkoff JP, Meisels SJ, editors. Handbook of Early Childhood Intervention. 2. New York, NY: Cambridge University Press; 2000. pp. 204–227. [Google Scholar]
- Nelson CA, Bloom FE. Child development and neuroscience. Child Development. 1997;68:970–987. doi: 10.1111/j.1467-8624.1997.tb01974.x. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Thomas KM, de Haan M. Neural bases of cognitive development. In: Kuhn D, Siegler RS, Damon W, Lerner RM, editors. Handbook of child psychology: Cognition, perception, and language. 6. Vol. 2. Hoboken, NJ, US: John Wiley & Sons Inc; 2006. pp. 3–57. [Google Scholar]
- NICHD Early Child Care Research Network. Affect dysregulation in the mother-child relationship in the toddler years: Antecedents and consequences. Development and Psychopathology. 2004;16:43–68. doi: 10.1017/S0954579404044402. [DOI] [PubMed] [Google Scholar]
- Neitzel C, Stright AD. Mothers’ scaffolding of children’s problem solving: establishing a foundation of academic self-regulatory competence. Journal of Family Psychology. 2003;17:147–159. doi: 10.1037/0893-3200.17.1.147. [DOI] [PubMed] [Google Scholar]
- Nigg JT. On inhibition/disinhibition in developmental psychopathology: Views from cognitive and personality psychology and a working inhibition taxonomy. Psychological Bulletin. 2000;126:220–246. doi: 10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- Parker SW, Nelson CA the BEIP core group. The impact of early institutional rearing on the ability to discriminate facial expressions of emotion: An event-related potential study. Child Development. 2005;76:54–72. doi: 10.1111/j.1467-8624.2005.00829.x. [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K, Fox NA. Temperamental contributions to children’s performance in an emotion-word processing task: A behavioral and electrophysiological study. Brain and Cognition. 2007;65:22–35. doi: 10.1016/j.bandc.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA. Electroencephalography and high-density electrophysiological source localization. In: Cacioppo J, Tassinary L, Bernston G, editors. Handbook of Psychophysiology. 3. New York: Cambridge University Press; 2007. pp. 56–84. [Google Scholar]
- Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: A signal-detection approach. Biological Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, Klorman R, Thatcher JE, Cicchetti D. P3b reflects maltreated children’s reactions to facial displays of emotion. Psychophysiology. 2001;38:267–274. doi: 10.1017/S0048577201990808. [DOI] [PubMed] [Google Scholar]
- Propper C, Moore GA. The influence of parenting on infant emotionality: A multi level psychobiological perspective. Developmental Review. 2006;26:427–460. doi: 10.1016/j.dr.2006.06.003. [DOI] [Google Scholar]
- Rhoades BL, Greenberg M, Lanza ST, Blair C. Demographic and familial predictors of early executive function development: Contribution of a person-centered perspective. Journal of Experimental Child Psychology. 2011;107:638–662. doi: 10.1016/j.jecp.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochette É, Bernier A. Parenting, family socio-economic status and child executive functioning: A longitudinal study. Merrill-Palmer Quarterly. 2014;60:431–460. doi: 10.13110/merrpalmquar1982.60.4.0431. [DOI] [Google Scholar]
- Rogoff B. Apprenticeship in thinking: Cognitive development in social context. New York, NY: Oxford University Press; 1990. [Google Scholar]
- Rutter M, O’Connor TG. Are there biological programming effects for psychological development? Findings from a study of Romanian adoptees. Developmental Psychology. 2004;40:81–94. doi: 10.1037/0012-1649.40.1.81. [DOI] [PubMed] [Google Scholar]
- Sasser TR, Bierman KL, Heinrichs B. Executive functioning and school adjustment: The mediational role of pre-kindergarten learning-related behaviors. Early Childhood Research Quarterly. 2015;30:70–79. doi: 10.1016/j.ecresq.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Hassloff H, Zwönitzer A, Künster AL, Mayer C, Ziegenhain U, Kiefer M. Emotional availability modulates electrophysiological correlates of executive functions in preschool children. Frontiers in Neuroscience. 2016;10:299–326. doi: 10.3389/fnhum.2016.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schore AN. The experience-dependent maturation of a regulatory system in the orbital prefrontal cortex and the origin of developmental psychopathology. Development and Psychopathology. 1996;8:59–87. doi: 10.1017/S0954579400006970. [DOI] [Google Scholar]
- Singer W. Development and plasticity of cortical processing architectures. Science. 1995;270:758–764. doi: 10.1126/science.270.5237.758. [DOI] [PubMed] [Google Scholar]
- Spronk M, Jonkman LM, Kemner C. Response inhibition and attention processing in 5- to 7-year-old children with and without symptoms of ADHD: An ERP study. Clinical Neuropsychology. 2008;119:2738–2752. doi: 10.1016/j.clinph.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Stevens C, Neville H. Specificity of experiential effects in neurocognitive development. In: Gazzaniga MS, editor. The new cognitive neurosciences. 5. Cambridge, MA: The MIT Press; 2013. pp. 2–21. [Google Scholar]
- Swingler MM, Perry NB, Calkins SD, Bell MA. Maternal behavior predicts infant neurophysiological and behavioral attention processes in the first year. Developmental Psychology. 2017;53:13–27. doi: 10.1037/dev0000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd RM, Lewis MD, Meusel LA, Zelazo PD. The time course of social emotional processing in early childhood: ERP responses to facial affect and personal familiarity in a Go-Nogo task. Neuropsychologia. 2008;46:595–613. doi: 10.1016/j.neuropsychologia.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Tottenham N. Human amygdala development in the absence of species-expected caregiving. Developmental Psychobiology. 2012;54:598–611. doi: 10.1002/dev.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towe-Goodman NR, Willoughby M, Blair C, Gustaffson HC, Mills-Koonce WR, Cox MJ the Family Life Project Key Investigators. Fathers’ sensitive parenting and the development of early executive functioning. Journal of Family Psychology. 2014;28:867–876. doi: 10.1037/t39316-000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp G, Alsop B. Sensitivity to reward frequency in boys with attention deficit hyperactivity disorder. Journal of Clinical Child Psychology. 1999;28:366–375. doi: 10.1207/S15374424280309. [DOI] [PubMed] [Google Scholar]
- Valcan DS, Davis H, Pino-Pasternak D. Parental behaviors predicting early childhood executive functions: A meta-analysis. Educational Psychology Review. 2017:1–43. doi: 10.1007/s10648-017-9411-9. [DOI] [Google Scholar]
- Vanderwert RE, Zeanah CH, Fox NA, Nelson CA. Normalization of EEG activity among previously institutionalized children placed into foster care: A 12-year follow-up of the Bucharest early intervention project. Developmental Cognitive Neuroscience. 2016;17:68–75. doi: 10.1016/j.dcn.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Willoughby MT, Blair C, Wirth RJ, Greenberg M the Family Life Project Investigators. The measurement of executive function at age 5: Psychometric properties and relationship to academic achievement. Psychological Assessment. 2012;24:226–239. doi: 10.1037/a0025361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer BJ, Brown DR, Michels KM. Statistical principles in experimental design. Vol. 2. New York: McGraw-Hill; 1971. [Google Scholar]
- Zelazo PD, Carlson SM, Kesek A. The development of executive function in childhood. In: Nelson CA, Luciana M, editors. Developmental cognitive neuroscience: Handbook of developmental cognitive neuroscience. Cambridge, MA: MIT Press; 2008. pp. 553–574. [Google Scholar]
- Zeytinoglu S, Calkins SD, Leerkes EM. Maternal emotional support but not cognitive support predicts increases in child cognitive flexibility in early childhood. International Journal of Behavioral Development. doi: 10.1177/0165025418757706. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeytinoglu S, Calkins SD, Swingler MM, Leerkes EM. Pathways from maternal effortful control to child self-regulation: The role of maternal emotional support. Journal of Family Psychology. 2017;31:170–180. doi: 10.1037/fam0000271. [DOI] [PMC free article] [PubMed] [Google Scholar]


