Abstract
Purpose
To introduce a new pH- and oxygen-sensitive MR imaging technique using amine proton chemical exchange saturation transfer echo spin-and-gradient echo echoplanar imaging (CEST-SAGE-EPI).
Methods
pH-weighting was obtained using CEST estimations of magnetization transfer ratio asymmetry (MTRasym) at 3ppm and oxygen-weighting was obtained using R2′ measurements. Glutamine concentration, pH, and relaxation rates were varied in phantoms to validate simulations and estimate relaxation rates. MTRasym and R2′ in normal appearing white matter (NAWM), T2 hyperintensity, contrast enhancement (CE), and macroscopic necrosis were measured in 47 gliomas.
Results
Simulation and phantom results confirmed an increase in MTRasym with decreasing pH. CEST-SAGE-EPI estimates of R2, R2*, and R2′ varied linearly with Gd-DTPA concentration (r2=6.2 mM−1·sec−1, and r2*=6.9 mM−1·sec−1). CEST-SAGE-EPI and CPMG estimates of R2 (R2=0.9943) and multi-echo GRE estimates of R2*(R2=0.9727) were highly correlated. T2 lesions had lower R2′ and higher MTRasym compared with NAWM, suggesting lower hypoxia and high acidity, whereas CE tumor regions had elevated R2′ and MTRasym indicating high hypoxia and acidity.
Conclusion
CEST-SAGE-EPI provides simultaneous pH- and oxygen-sensitive image contrasts for evaluation of the brain tumor microenvironment. Advantages include fast whole brain acquisition, in-line B0 correction, and simultaneous estimation of CEST effects, R2, R2*, and R2′ at 3T.
Keywords: CEST, SAGE, pH, pH-weighted MRI, EPI, amino acid, glioma, hypoxia
INTRODUCTION
Abnormal metabolism is a hallmark of cancer (1,2). Notably, glycolysis is often enhanced in cancers, even in the presence of abundant oxygen (i.e. the Warburg effect (2), Fig 1). This form of aerobic glycolysis results in a dramatic decrease in extracellular pH due to increased concentration of lactic acid (1). This increase in extracellular acidity comes with dramatic consequences, as it can be directly linked to the degree of tumor aggressiveness (3,4) and increases tumor invasion (5,6). Interestingly, histological evidence suggests regions containing pseudopalisades, a pathological trademark of glioblastoma, are also hypoxic, express extracellular matrix proteases, and are the result of active tumor migration (7). Increased acidity within the tumor also has been shown to lead to decreased immune function (8). The acidic microenvironment in tumors is also conducive to elevated VEGF expression (9) and expression of platelet-derived endothelial cell growth factor (10), which has been shown to result in increased angiogenesis. This in turn leads to a positive feedback process leading to further tumor growth, decreasing oxygen tension, increased hypoxia, increasing glycolysis resulting in increased lactic acid, decreasing extracellular tissue pH, and more mutations and/or adaptations of the tumor genome. Thus, extensive in vitro, preclinical, and clinical evidence appears to support the hypothesis that tumor acidity and oxygen metabolism both play a critical role in gliomagenesis. There remains, however, a critical need in furthering our understanding of the role of extracellular acidosis and oxygen metabolism in human gliomas and its clinical relevance due to the lack of a robust non-invasive tool for simultaneously estimating and localizing regions of low pH and oxygen consumption. Thus, the purpose of the study was to develop a new, non-invasive MR imaging technique that can provide high-resolution images with sensitivity to tumor acidity and oxygen metabolism that can be performed on clinical MRI systems.
Fig. 1. The Warburg Effect.
A) Within normal tissues in the presence of oxygen, glucose is converted to pyruvate and then used for oxydative phosphorylation within the mitochondria. B) In normal tissues within a hypoxic environment, glucose is converted to pyruvate then to lactate or lactic acid, decreasing extracellular pH. C) In cancer cells, glucose is converted to pyruvate then to lactate and lactic acid (80–85%) and a small portion of pyruvate enters the citric acid cycle (5–15%), regardless of whether oxygen is present. This altered metabolism, also known as the Warburg Effect or aerobic glycolysis, results in increased extracellular acidity (lower pH) even when tumor tissue is well perfused.
pH-Weighted Metabolic MRI using CEST Contrast from Fast Exchangeable Amine Protons on Glutamine
In addition to glucose, glutamine is also a major source of fuel for malignant tumors (11). It is essential for cellular proliferation, tumor growth, and tumor cell survival. Glutamine is the most abundant amino acid in the body, circulating at concentrations of 0.6–0.9 mM and as high as 20 mM in tissue (11). Tumor cells often consume a significant amount of glutamine (12), acting at times like a “glutamine trap”. Glutamine demand is so high that transport systems are amplified to increase glutamine consumption (13). Glutamine has two nitrogen functional groups, an amine and an amide group, having hydrogen NMR resonance frequencies of 3.0 ppm and 3.5 ppm, respectively, compared to water protons. The chemical exchange between amine and amide protons in bulk water has been shown to be pH dependent using a new imaging method called chemical exchange saturation transfer (CEST) imaging (14). The inherently elevated concentration of glutamine within tumors further increases the available proton exchange, resulting in a higher CEST signal at 3.0ppm (15,16). The combination of increased protons (low pH) and increased glutamine makes pH-weighted MRI using CEST contrast from amine protons on glutamine particularly attractive as a non-invasive tool for assessment of microenvironment acidity within malignant brain tumors.
R2′-Based Blood Oxygenation Level Dependent (BOLD) Imaging
The level of glycolysis depends on both the accumulation of lactic acid as well as the inefficient use of oxygen. Thus, image maps sensitive to tumor oxygen metabolism are critically important for understanding the relative level of aerobic (pathologic) versus anaerobic (normal) glycolysis. Blood oxygen level dependent (BOLD) imaging, based on the contrast mechanisms routinely used for functional imaging (fMRI), allow for non-invasive estimation of blood or tissue oxygenation. Simply stated, oxygenated blood containing oxyhemoglobin is diamagnetic, as the iron at the core is magnetically shielded from blood water, resulting in coherent MR spins and a high MR signal. Early studies from Ogawa et al.(17) confirmed this in mice breathing high concentrations of oxygen. In deoxygenated blood, the iron is exposed to blood water resulting in magnetic interference with the proton magnetic moment on water molecules. This interference results in incoherent MR spins and signal dropout in the areas of high deoxyhemoglobin concentration. The paramagnetic nature of deoxyhemoglobin enhances the effective transverse relaxation rate R2*. Changes in tissue R2* therefore reflect relative changes in concentration of deoxyhemoglobin, and thus an indirect measure of oxygen extraction fraction (OEF). Use of the reversible transverse relaxation rate, R2′ = R2* − R2, both isolates the local susceptibility effect while compensating for R2 changes from factors such as water content variation (18). The sensitivity of R2′ to concentration of deoxyhemoglobin has been reported by several studies (19,20) and has been shown to correlate with the hypoxic state of tissue (21). While R2′ does not allow for a direct measurement of OEF, previous studies have demonstrated that OEF is proportional to R2′, after normalization to relative blood volume fraction and static dephasing expected for protons in blood at the specific magnetic field strength (22–25). Additionally, this approach has been previously used to explore oxygen metabolism in brain tumors (24,26) as well as stroke (27). It is important to note, however, that while higher measures of R2′ may suggest higher concentration of deoxyhemoglobin, OEF, and/or local hypoxia, many other biological and/or technical influences (e.g. B1 homogeneity, etc.) may influence this measurement.
In the current study, we introduce a new technique for obtaining fast, whole brain, non-invasive, high-resolution pH- and oxygen-sensitive MR imaging contrast using multi-echo amine proton chemical exchange saturation transfer echo spin-and-gradient echo echoplanar imaging (CEST-SAGE-EPI) on a clinical 3T MRI system. pH-weighted image sensitivity was obtained through quantification of the z-spectral asymmetry in the magnetic transfer ratio after selective saturation of the longitudinal magnetization of amine protons on glutamine at 3.0ppm (MTRasym @ 3.0ppm), while oxygen sensitivity was obtained through quantification of R2′ using a multi-echo spin-and-gradient-echo (SAGE) EPI readout to quantify relaxation rates R2 and R2*. Sensitivity and accuracy of this approach were confirmed using custom phantoms containing both gadolinium DTPA and glutamine and different concentrations and pH. Lastly, both MTRasym @ 3.0ppm and R2′ for tumor and normal tissue were characterized in patients harboring diffuse or malignant gliomas.
THEORY
pH- and oxygen-sensitive MR contrast using multi-echo amine proton chemical exchange saturation transfer spin-and-gradient echo echoplanar imaging (CEST-SAGE-EPI) (Fig 2)
Fig. 2. Pulse sequence diagram for a multi-echo amine chemical exchange saturation transfer spin-and-gradient echo echoplanar imaging (CEST-SAGE-EPI) sequence.
After non-selective off-resonance CEST excitation consisting of three 100-ms Gaussian pulses, a spectral-spatial water only excitation pulse is invoked. Following excitation two gradient echo EPI trains are acquired (Echoes #1 and #2), followed by a 180-degree refocusing pulse. Lastly, an asymmetric spin echo EPI train (Echo #3) and a standard spin-echo EPI train (Echo #4) are acquired. Partial Fourier k-space acquisition strategies were used to further reduce echo times (e.g. TE1/TE2/TE3/TE4 = 14.0/34.1/58.0/92.4 ms for the current study).
The magnetization of bulk water protons undergoing chemical exchange with amine protons can be described by the Bloch-McConnell equations (28) in the form of:
| [Eq. 1] |
where
| [Eq. 2] |
pool a and pool b are the bulk water protons and amine protons, respectively; Maz0 and Mbz0 are the equilibrium magnetizations of pool a and b, respectively; kb is the exchange rate of protons from pool b to pool a; ka is the exchange rate of protons from pool a to pool b as given by (Mb0/Ma0)·kb; ω1 is the RF pulse amplitude as given by ω1 = γB1(t), where γ is the gyromagnetic ratio and B1(t) is given in μT; δa = ω−ωa and δb = ω −ωb, where ω is the applied RF irradiation frequency, ωa is the bulk water resonance frequency, and ωb is the amine proton resonance frequency; T1a and T1b are the longitudinal relaxation times of pool a and b, respectively; and C1a = (1/T1a)+ka, C2a = (1/T2a)+ka, C1b = (1/T1b)+kb, C2b = (1/T2b)+kb represent the sum of exchange and relaxation rates. Eq. 1 can be solved analytically to yield
| [Eq. 3] |
where Maz(t) represents the longitudinal magnetization of bulk water available for subsequent readout after CEST effects. Assuming the spoiler duration and water excitation pulse duration are negligible, Maz(t) immediately following excitation reflects the available longitudinal magnetization for subsequent readout. The chemical exchange between amine protons and bulk water protons can be characterized as a base-catalyzed process, governed by pH:
| [Eq. 4] |
where k0 is the default exchange rate, kbase is the base-catalyzed rate constant, and kb is the exchange rate of protons from the metabolite proton pool to the water pool (29). In glutamine phantoms with concentration of 50 mM, k0 and kbase have been estimated to be 76 Hz and 5.6 Hz, respectively (16).
During the CEST imaging experiment, the RF saturation frequency ω is swept over a range of values to obtain a z-spectral dataset (30). To reduce the effects of T1 and T2 weighting, along with other confounding effects, the attenuation of bulk water magnetization following a saturation pulse is described by the magnetization transfer ratio (MTR), given by:
| [Eq. 5] |
where S(ω) is the amount of bulk water signal available after the saturation pulse with frequency ω and S0 is the signal available without application of RF saturation. Since MTR can be affected by symmetric effects of direct water saturation and conventional magnetization transfer effects, CEST contrast is often described by the asymmetry in the magnetization transfer ratio (MTRasym) with respect to water proton resonance:
| [Eq. 6] |
For amine proton CEST imaging, MTRasym is evaluated at 3.0ppm with respect to the bulk water resonance frequency.
Estimates of R2′ were achieved through use of a SAGE-EPI readout consisting of two gradient echoes (TE1 and TE2), an asymmetric spin echo (TE3), and a spin echo (TE4) EPI acquisition during a single excitation event (Fig 2). The solutions for R2* and R2 using the SAGE EPI were eloquently described by Schmiedeskamp et al. (31) as:
| [Eq. 7] |
where
| [Eq. 8] |
where Sn is signal magnitude for the nth echo and δ is the differences in residual signal differences caused by imperfectly matched slice profiles between echo trains before and after the refocusing pulse (32). Lastly, the reversible transverse relaxation rate, R2′, is calculated as:
| [Eq. 9] |
METHODS
Amine Proton Chemical Exchange Saturation Transfer Spin-and-Gradient Echo Echoplanar Imaging (CEST-SAGE-EPI)
Simultaneous acquisition of pH-sensitive information and relaxometry measures of R2′ were performed through modification of a previously described CEST EPI sequence (16) to include a SAGE-EPI readout (Fig 2). The SAGE-EPI readout consisted of two gradient echoes (TE1=14.0ms;TE2=34.1ms), an asymmetric spin echo (TE3=58.0ms), and a spin echo (TE4=92.4ms). All phantom and human CEST-SAGE-EPI data were acquired with a CEST saturation pulse train consisting of three (3×) 100-ms Gaussian pulses with amplitude B1=6μT, TR>10,000ms, FOV=240×217, matrix size=128×104, partial Fourier encoding =6/8, GRAPPA=3, bandwidth=1630 Hz/pixel, and 25 contiguous slices with a 4mm slice thickness. A total of 29 z-spectral points was acquired with data around +/− 3.0ppm and 0.0ppm with respect to water (from −3.5 to −2.5 in intervals of 0.1; from −0.3 to +0.3 in intervals of 0.1; and from +2.5 to +3.5 in intervals of 0.1). An additional reference S0 scan with identical parameters and no saturation pulse was acquired with NEX=4. The total acquisition time for CEST-SAGE-EPI was 7 minutes and 30 seconds benchmarked on a 3T Siemens Prisma MR scanner (Software Versions VE11A-C; Siemens Healthcare; Erlangen, Germany).
Phantom Testing
To demonstrate the ability to simultaneously acquire pH-sensitive information along with relaxometry measures of R2, R2*, and R2′, we performed CEST-SAGE-EPI, multi-echo gradient echo (ME-GRE), and multi-echo spin echo (Carr-Purcell-Meiboom-Gill, CPMG) MR acquisition in a series of 36 glutamine phantoms (100mM) with combinations of varying pH (5.0, 5.5, 6.0, 6.5, 7.0, 7.5) and gadopentetic acid (Gd-DTPA; Magnevist®) concentration (0, 0.25, 0.5, 1.0, 1.5, and 2.0mM) in 50mL falcon tubes. The 100mM glutamine solution was prepared first using distilled water. The pH in each phantom was titrated using dilute acid (0.1N HCl) and base (0.1N NaOH) solution. The desired Gd-DTPA was then added to the phantom solution and vortexed. All samples were vortexed and pH was re-evaluated prior to MRI acquisition. The ME-GRE sequence used for R2* quantitation was collected with TE = 20/40/60/80ms, TR=10000ms, flip angle=90°, FOV=217×240mm, matrix size=116×128, slice thickness=4mm, and pixel bandwidth=260Hz. The CPMG sequence used for R2 quantitation was performed with TE=49/98/147/196/245/294/343/392ms, TR=10000ms, flip angle=90°, FOV=217×240mm, matrix size=116×128, slice thickness=4mm, and pixel bandwidth=260Hz. Both the ME-GRE and CPMG sequences were repeated to improve signal-to-noise ratio (SNR). All phantom experiments were physically repeated twice to ensure repeatability and compared to theoretical values using Bloch-McConnell simulations. Contrast-to-noise ratio (CNR) of MTRasym at 3ppm between any two samples of differing pH a and b was calculated as
| [Eq. 10] |
Patients
A total of 47 histologically proven glioma patients (WHO IV, N=20; WHO III, N=14; WHO II, N=13) were enrolled in the current study prior to initial surgical resection or at first recurrence. All patients provided informed written consent to have advanced imaging and this information included in our IRB-approved research database. Patient characteristics are outlined in Table 1. In addition to CEST-SAGE-EPI prior to contrast administration, all patients received the anatomic images according to the standardized brain tumor imaging protocol (BTIP)(33) including T2-weighted fluid-attenuated inversion recovery (FLAIR) images, T2-weighted turbo spin echo images, and diffusion weighted images with 3mm slice thickness and no interslice gap, along with parameter matched, 1mm isotropic 3D T1-weighted magnetization-prepared rapid acquisition gradient echo (MPRAGE) scans pre- and post-injection of 0.01 mg/kg Gd-DTPA.
Table 1.
Patient Demographics.
| Patient ID | Age | Sex | 2016 WHO Grade | IDH Status | 2016 WHO Diagnosis | New or Recurrent |
|---|---|---|---|---|---|---|
| 1 | 60 | M | 4 | WT | GBM | New |
| 2 | 62 | M | 4 | WT | GBM | New |
| 3 | 53 | F | 4 | WT | GBM | New |
| 4 | 49 | M | 4 | WT | GBM | New |
| 5 | 60 | M | 4 | WT | GBM | New |
| 6 | 46 | F | 4 | MUT | GBM | Recurrent |
| 7 | 53 | F | 4 | WT | GBM | Recurrent |
| 8 | 76 | F | 4 | WT | GBM | Recurrent |
| 9 | 55 | F | 4 | WT | GBM | Recurrent |
| 10 | 59 | M | 4 | WT | GBM | Recurrent |
| 11 | 60 | M | 4 | WT | GBM | Recurrent |
| 12 | 45 | M | 4 | WT | GBM | Recurrent |
| 13 | 48 | F | 4 | WT | GBM | Recurrent |
| 14 | 66 | M | 4 | WT | GBM | Recurrent |
| 15 | 53 | F | 4 | WT | GBM | Recurrent |
| 16 | 75 | M | 4 | WT | GBM | Recurrent |
| 17 | 56 | M | 4 | WT | GBM | Recurrent |
| 18 | 62 | M | 4 | WT | GBM | Recurrent |
| 19 | 75 | M | 4 | WT | GBM | Recurrent |
| 20 | 60 | M | 4 | WT | GBM | Recurrent |
| 21 | 29 | F | 3 | MUT | AA | New |
| 22 | 51 | M | 3 | MUT | AA | New |
| 23 | 64 | F | 3 | WT | AA | New |
| 24 | 56 | M | 3 | WT | AA | New |
| 25 | 62 | M | 3 | WT | AA | New |
| 26 | 69 | M | 3 | WT | AA | New |
| 27 | 36 | F | 3 | MUT | AO | New |
| 28 | 29 | M | 3 | MUT | AO | New |
| 29 | 82 | M | 3 | WT | AA | Recurrent |
| 30 | 71 | F | 3 | WT | AA | Recurrent |
| 31 | 47 | F | 3 | WT | AA | Recurrent |
| 32 | 76 | F | 3 | WT | AA | Recurrent |
| 33 | 66 | M | 3 | MUT | AO | Recurrent |
| 34 | 38 | F | 3 | MUT | AO | Recurrent |
| 35 | 38 | M | 2 | MUT | A | New |
| 36 | 41 | M | 2 | MUT | A | New |
| 37 | 29 | F | 2 | MUT | A | New |
| 38 | 30 | F | 2 | MUT | A | New |
| 39 | 22 | M | 2 | MUT | A | New |
| 40 | 48 | M | 2 | WT | A | New |
| 41 | 87 | F | 2 | WT | A | New |
| 42 | 41 | M | 2 | MUT | O | New |
| 43 | 42 | F | 2 | MUT | A | Recurrent |
| 44 | 36 | F | 2 | MUT | A | Recurrent |
| 45 | 73 | M | 2 | WT | A | Recurrent |
| 46 | 41 | F | 2 | MUT | O | Recurrent |
| 47 | 91 | M | 2 | WT | O | Recurrent |
M=Male; F=Female; IDH=isocitrate dehydrogenase; MUT=Mutant; WT=Wild Type; WHO=World Health Organization; A=Astrocytoma; O=Oligodendroglioma; AA=Anaplastic Astrocytoma; AO=Anaplastic Oligodendroglioma; GBM=Glioblastoma.
Post-Processing
Clinical post-processing of CEST-SAGE-EPI consisted of affine motion correction (mcflirt; FSL, FMRIB, Oxford, UK) and B0 correction via the WASSR method (34) and/or creating B0 maps using phase information from the two acquired gradient echoes (Supporting Figure S1). An integral of width 0.4 ppm was then taken around both the −3.0 and +3.0 ppm (−3.2 to −2.8 and +2.8 to +3.2, respectively) spectral points of the inhomogeneity-corrected data. These data points were combined with the S0 image to calculate MTRasym at 3.0 ppm as defined by Eq. 5. Estimates of T2*, T2, T2′, R2′, R2, and R2′ from ME-GRE or CPMG were obtained by performing a monoexponential fit to the gradient and spin echoes, respectively, whereas estimates of the same parameters were obtained using Eq. 8. Maps of R2′ were then calculated as
| [Eq. 11] |
with a higher value of R2′ suggesting relatively higher concentration of hemoglobin, oxygen extraction fraction, and/or hypoxia (22–26).
Data Analysis
For all data, average MTRasym at 3.0 ppm calculated from the first (TE=14.0 ms) and second (TE=34.1 ms) gradient echoes were averaged to decrease increase signal-to-noise ratio (SNR) of the resulting MTRasym images. The two gradient echoes were chosen instead of all echoes due to slightly higher variability in MTRasym measurements from asymmetric spin-echo and spin-echo measurements due to the longer TE and additional signal loss from transverse relaxation. In phantom samples, regions of interest (ROIs) were drawn within each sample and the mean (μ) and standard deviation (σ) of MTRasym at 3ppm for voxels within the sample were calculated.
When evaluating glioma patients, volumes of interest (VOIs) were drawn within normal-appearing white matter (NAWM) contralateral to the hemisphere containing evidence of tumor on T2-weighted FLAIR images. Lesions exhibiting abnormal T2 hyperintensity on FLAIR images (“T2 lesions” VOIs) were manually contoured on all patients. To reduce the influence of outliers, median and median absolute deviation (MAD) of MTRasym and R2′ within these regions were calculated and MAD was used to define variability for all measurements. A Wilcoxon signed-rank test was used to determine whether averaging MTRasym from two gradient echoes resulted in a decrease in healthy tissue MTRasym variability compared with a single gradient echo. For glioblastoma patients, VOIs of gadolinium contrast enhancement (CE) were segmented using T1 subtraction maps and a semi-automated thresholding method outlined previously(35,36). Regions of central necrosis were also delineated and examined. A paired t-test was used to determine whether R2′ and MTRasym differed between T2-hyperintense lesions and NAWM. Within glioblastoma patients (WHO grade IV), a one-way repeated-measures ANOVA and Tukey’s test for multiple comparisons was used to determine whether R2′ or MTRasym at 3ppm differed between regions of NAWM, T2 hyperintense lesions, regions of CE, and areas of central necrosis. An additional one-way ANOVA and Tukey’s test for multiple comparisons was used to compare R2′ or MTRasym at 3ppm for T2 lesions across glioma grades II, III, and IV.
RESULTS
MTRasym at 3.0ppm within each glutamine phantom, varying by pH, was similar across all four echoes, showing a characteristic increase in MTRasym at 3.0ppm with decreasing pH (Fig 3A; ANOVA, P = 0.999; Comparison across echoes, P>0.99). These experimental results closely matched simulation results using measured and theoretical relaxation and exchange rates (Fig 3A). CNR was higher when averaging measurements from the two gradient echoes (TE=14.1ms & 34.1ms) compared to a single gradient echo (TE=14.1ms) in phantom samples containing the same concentration of glutamine, but varying pH. In particular, CNR was approximately 13% higher when comparing pH=7.5 to 7.0 (CNR1&2=2.68; CNR1=2.37), 7% higher when comparing pH=6.5 and 7.0 (CNR1&2=5.07; CNR1=4.74), 15% higher when comparing pH=6.0 vs 7.0 (CNR1&2=17.2 CNR1=15.0), and 6.5% higher when comparing pH=6.0 vs. 6.5 (CNR1&2=6.72; CNR1=6.31). Consistent with phantom results, median MTRasym at 3.0ppm in NAWM across all patients was not significantly different when using a single echo and the average of two gradient echoes (P=0.31); however, the median absolute deviation (MAD), a measure of variance in the measurements, was significantly lower when averaging the two gradient echoes (P=0.003).
Fig. 3. Simulation and phantom testing.
A) CEST contrast increases with decreasing pH within a physiological pH range similarly for all four echoes in an amino acid phantom and simulation estimates. These measurements closely match simulation estimates with measured or estimated relaxation and exchange rates (dashed lines). B) R2 and R2* as measured by the multi-echo sequence show a linear increase with increasing Gd-DTPA concentration. Estimates of r2 = 6.24 ± 0.04 mM−1·sec−1, r2* = 6.86 ± 0.10 mM−1·sec−1, and r2′ = 0.61 ± 0.08 mM−1·sec−1. C) Comparison between measured R2 using CEST-SAGE-EPI and CPMG show strong correlation (R2=0.9943, P<0.0001), independent of pH (P=0.9915). D) Comparison between measured R2* using CEST-SAGE-EPI and ME-GRE showing a strong correlation (R2=0.9727, P<0.0001) and no dependence on pH (P=0.2184). E) Estimates of R2′ obtained through subtraction of sequential CPMG and ME-GRE measurements were not significantly different from those obtained using multi-echo SAGE EPI (slope = 1.26±0.1301, P=0.0555) and did not differ by pH (P=0.0533). F) Amine CEST has decreased sensitivity in the presence of contrast agents (Gd-DTPA) that shorten T1 and T2. Thus, edematous tumor tissue with longer T1 and T2 have higher CEST contrast in acidic environments. G) Coefficient of variation (COV) measured from multiple test-retest experiments is also higher in the presence of contrast agents or in environments with shorter T1 and T2 characteristics.
In phantom samples containing varying concentration of Gd-DTPA, CEST-SAGE-EPI estimates of R2, R2*, and R2′ varied linearly with concentration (Fig 3B), with estimates of transverse relaxivities of r2 = 6.24 ± 0.04 mM−1·sec−1 (P<0.0001), r2* = 6.86 ± 0.10 mM−1·sec−1 (P<0.0001), and r2′ = 0.61 ± 0.08 mM−1·sec−1 (P=0.0007). Phantom results identified a strong, significant linear correlation between R2 measurements using CEST-SAGE-EPI and CPMG (Fig 3C; R2=0.9943, P<0.0001) and did not differ by pH (P=0.9915). Estimates of R2 using CEST-SAGE-EPI, however, were lower than estimates of R2 using standard CPMG measurements (slope = 0.8845± 0.006, P<0.0001 compared with slope = 1), with CEST-SAGE-EPI estimates approximately 760us lower on average than CPMG measurements (~7.1%). A strong linear correlation was also observed between R2* measurements obtained using CEST-SAGE-EPI and ME-GRE measurements (Fig 3D; R2=0.9727, P<0.0001) and these measurements also did not differ by pH (P=0.2184). Estimates of R2* were not significantly different from measurements obtained using standard ME-GRE measurements (slope = 0.9862±0.0155, P=0.3819 showing no difference between slope=1). Consistent with these results, calculated estimates of R2′ obtained through subtraction of sequential CPMG and ME-GRE measurements were congruent with SAGE-EPI measurements (Fig 3E; slope = 1.26±0.1301, P=0.0555 showing no difference between slope=1) and did not differ by pH (P=0.0533).
Although measurements of transverse relaxation rate were not affected by pH, the relationship between MTRasym at 3.0ppm and pH was affected by transverse relaxation rate (Fig 3F). Specifically, as transverse relaxation rates R2 or R2* increased as a result of increased concentration of Gd-DTPA, the sensitivity of MTRasym at 3.0ppm to acidic pH decreased, particularly when concentrations were higher than 1mM, corresponding to T2 measurements of 150–170ms and T2* = 130–170ms. Repeated experiments showed higher coefficient of variation (COV) with higher concentrations of Gd-DTPA, or lower T1, as well as higher COV with higher pH (Fig 3G).
Qualitatively, the T2 hyperintense lesions in all patients exhibited heterogeneous areas of both elevated MTRasym at 3.0ppm (acidity) and R2′ (hypoxia). Many areas of non-enhancing tumor in all patients exhibited uncharacteristically low measures of R2′ despite evidence of elevated acidity, suggesting non-enhancing tumor regions may be adequately oxygenated and undergoing aerobic glycolysis (e.g. Fig 1C). For example, Fig 4A illustrates a WHO grade II astrocytoma with an area of moderately elevated acidity in the medial frontal lobe; however, this region exhibited R2′ ~50% lower than surrounding NAWM. Fig 4B illustrates a large non-enhancing, IDH mutant WHO grade III astrocytoma with sizeable regions of macroscopic necrosis, as illustrated by T1 hypointensity. These areas of necrotic tissue exhibited both high levels of acidity and hypoxia, while surrounding non-enhancing components with largely intact blood-brain barrier had elevated acidity, as suggested by higher MTRasym at 3.0ppm, but lower levels of hypoxia, or R2′, compared with regions of NAWM. Figs 4C–D show patients with recurrent and newly diagnosed glioblastoma (WHO IV), respectively. Compared to the WHO grade II and III tumors with largely non-enhancing tumor with intact blood-brain barrier, patients with glioblastoma exhibiting extensive areas of contrast enhancement displayed both high acidity (MTRasym at 3.0ppm) and hypoxia (R2′) as well as regions of moderate acidity and oxygenated tumor. (Additional information including T2 and T2* maps are illustrated in Supporting Figure S2).
Fig. 4. pH- and oxygen-sensitive MR images of human gliomas.
A) A 42-year-old female with a nonenhancing recurrent WHO II IDH mutant astrocytoma (Patient #43) exhibiting a region of focal acidity (high MTRasym at 3ppm) and low oxygen consumption (low R2′). B) A 51-year-old male patient with a newly diagnosed nonenhancing IDH mutant WHO III anaplastic astrocytoma (Patient #22). This lesion showed large, heterogeneous regions of abnormally high and low hypoxia and acidity. C) A 53-year-old female patient with a recurrent IDH wild type WHO IV glioblastoma exhibiting a region of focal acidity and large regions of low oxygen consumption (Patient #15). D) A 53-year-old female with a newly diagnosed IDH wild type WHO IV glioblastoma (Patient #3) displaying ring enhancement, central necrosis, elevated acidity and oxygen extraction (hypoxia) within the enhancing region as well as low oxygen consumption in surrounding nonenhancing tumor.
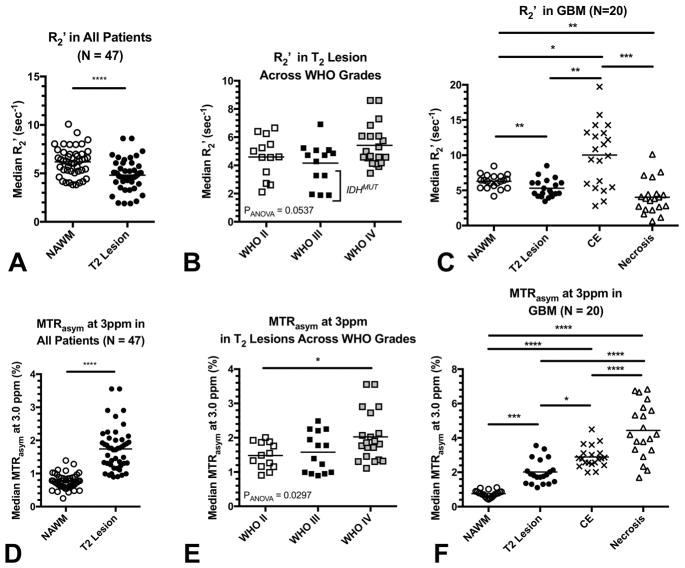
Quantitative evaluation of various regions within these tumors confirmed these observations (Figs 5–6). Regions of T2 hyperintense lesions exhibited a significantly lower median R2′ (4.8 ± 0.2 sec−1) compared with NAWM (6.2 ± 0.2 sec−1) when pooling patients across all tumor grades (Fig 5A; Paired t-test, P<0.0001). Within T2 hyperintense lesions, median R2′ did not vary significantly across tumor grade (Fig 5B; ANOVA, P=0.0537; WHO II = 4.6 ± 0.4 sec−1, WHO III = 4.2 ± 0.4 sec−1, WHO II = 5.4 ± 0.3 sec−1). In glioblastoma patients, significant differences in median R2′ across tissue types were observed (Fig 5C; Repeated measures ANOVA, P=0.0001). In particular, median R2′ was significantly lower in T2 hyperintense lesions (5.3 ± 0.3 sec−1) compared to both NAWM (6.3 ± 0.2 sec−1; Tukey’s Test, Adjusted P=0.0078) and CE regions (10.0 ± 1.0 sec−1; Tukey’s Test, Adjusted P=0.0002); whereas no difference was detected between T2 lesions and areas of central necrosis (4.0 ± 0.5 sec−1; Tukey’s Test, Adjusted P=0.1499). Additionally, median R2′ was significantly higher in CE lesions compared with NAWM (Tukey’s Test, Adjusted P=0.0064) and necrosis (Tukey’s Test, Adjusted P<0.0001), but significantly lower in necrosis compared with NAWM (Tukey’s Test, Adjusted P=0.0064). No differences in median R2′ were observed between recurrent or newly diagnosed tumor or normal tissues (P>0.2), nor were significant differences observed between IDH mutant and wild type tumors (P=0.12); however, IDH mutant tumors tended to have lower R2′ compared with IDH wild type tumors.
Fig. 5. pH and oxygen-sensitive MR measurements in human glioma patients.
A) Median R2′ measurements in normal appearing white matter (NAWM) and the T2 hyperintense lesions in all patients, independent of tumor grade. B) Median R2′ in T2 hyperintense regions for different tumor grades. C) Median R2′ in different brain regions for patients with glioblastoma (GBM). D) Median MTRasym at 3ppm (%) in NAWM and T2 hyperintense lesions in all patients pooled across all tumor grades. E) Median MTRasym at 3ppm (%) in T2 hyperintense regions for different tumor grades. F) Median MTRasym at 3ppm (%) in different tissue types in GBM. * = P<0.05, **=P<0.01, ***=P<0.001, ****=P<0.0001.
Fig. 6. Combined pH and oxygen-sensitive MR measurements in human gliomas illustrating additional separation of tissue types.

A) MTRasym at 3ppm (%) versus R2′ for NAWM and T2 hyperintense lesions in all patients pooled across tumor grades. B) MTRasym at 3ppm (%) versus R2′ in T2 hyperintense regions for different tumor grades. C) MTRasym at 3ppm (%) versus R2′ in different tissue types in GBM.
Median MTRasym at 3.0ppm within T2 hyperintense lesions (1.7 ± 0.1%) were significantly higher than NAWM (0.8 ± 0.03%) when pooling all patients across grade (Fig 5D; Paired t-test, P<0.0001). Within T2 hyperintense lesions, median MTRasym at 3.0ppm was significantly different across tumor grade (Fig 5E; ANOVA, P=0.0297), with WHO IV glioblastoma (2.0 ± 0.2%) exhibiting significantly higher acidity compared with WHO II gliomas (1.5 ± 0.1%; Tukey’s Test, Adjusted P=0.0432). No difference was observed between WHO III gliomas (1.6 ± 0.2%) compared with other grades (Adjusted P>0.05). In a separate comparison, IDH mutant gliomas exhibited a slightly higher degree of tumor acidity compared with IDH wild type tumors when correcting for of grade (Adjusted P=0.0434). Within WHO IV glioblastoma, significant differences in median MTRasym at 3.0ppm were observed between various tissue types (Fig 5F; ANOVA, P<0.0001). Areas of macroscopic necrosis exhibited the highest MTRasym at 3.0ppm degree of acidity (MTRasym at 3.0ppm = 4.4 ± 0.4%) compared with all other tissue types, including CE lesions (2.9 ± 0.1%; Tukey’s Test, Adjusted P<0.0001), T2 hyperintense regions (2.0 ± 0.2%, Adjusted P<0.0001), and NAWM (0.8 ± 0.04%, Adjusted P<0.0001). Additionally, CE tumor exhibited significantly higher median MTRasym at 3.0ppm compared with T2 hyperintense lesions (Tukey’s Test, Adjusted P=0.0218) and NAWM (Tukey’s Test, Adjusted P<0.0001), while T2 lesions showed higher median MTRasym at 3.0ppm (Tukey’s Test, Adjusted P<0.0001).
Examination of acidity and hypoxia characteristics within T2 hyperintense lesions pooled across tumor grade were markedly different from NAWM (Fig 6A), as T2 hyperintense lesions tended to be more acidic but slightly less hypoxic compared with NAWM. T2 hyperintense lesions did not show substantial separation across tumor grade (Fig 6B). Glioblastoma exhibited distinct characteristics across various tissue components (Fig 6C), with NAWM and T2 hyperintense lesions having relatively lower acidity and hypoxia combined with CE tumor and macroscopic necrotic tissue. CE tumor exhibited a moderately high level of acidity, along with high degrees of oxygen extraction, while necrotic tissue showed low oxygen extraction yet high acidity.
DISCUSSION
High-resolution pH- and oxygen-sensitive MR imaging contrast in brain tumors can be achieved on clinical 3T MR systems by implementing a multi-echo SAGE EPI readout after off-resonance saturation or CEST preparation of amine protons (3.0ppm). Comparable techniques for CEST imaging that take advantage of multiple echo readouts are relatively limited. Sun et. al. described a sequence that utilizes a CMPG readout following CEST saturation for improving CNR in agarose phantoms by averaging images from a spin echo train with echo times between 30 and 60 ms (37). However, this sequence only measured R2 characteristics and it could not correct for B0 using phase information available via multiple gradient echoes. The fast EPI readout available using CEST-SAGE-EPI vastly improves acquisition times over slower, single slice or volumetric approaches (38–40). Song et. al. (41) and others have described single echo EPI approaches to speed up acquisition time; however, these studies lacked concurrent measurement of subsequent echoes that would allow for estimation of R2, R2*, R2′ and B0. Thus, CEST-SAGE-EPI has many advantages including speed, in-line B0 mapping, whole brain coverage, and simultaneous CEST, R2, R2*, and R2′ quantitation.
Mathematical simulations and phantom measurements confirmed the relationship between MTRasym at 3.0ppm and pH reported previously (16). Measurements of transverse relaxivity for Gd-DTPA were found to be r2 = 6.24 ± 0.04 mM−1·sec−1 and r2* = 6.86 ± 0.10 mM−1·sec−1, which is consistent with the literature (42,43). Measurements of R2 and R2* also did not vary as a function of pH. As compared to CPMG measurements, CEST-SAGE-EPI tended to underestimate R2, consequently resulting in overestimation of R2′; however, the magnitude of this difference averaged less than 1ms (~760us), or less than 10% difference, and increased with relaxation rate R2. This discrepancy may be related to inaccurate modeling of imperfections during RF inversion during SAGE-EPI readout, as has been suggested in previous SAGE-EPI studies (31,32). In addition to OEF, R2′ value may also be influenced by venous blood fraction as reported by several studies (22–25). A more direct quantification of oxygen consumption or comparison may be achieved with high-field 17O MRI by detecting metabolically produced H217O (44) or with 17O MRS (45).
As demonstrated previously (16), edematous tissues and cancer tissues with long T2 tend to amplify pH-dependent CEST contrast, while tissues with shorter T2 exhibit only a few percentage changes in contrast, even in highly acidic and highly concentrated amino acid environments. In particular, with relatively short T2/T2* including normal appearing white and gray matter may not have suitable sensitivity or CNR to provide meaningful pH information. Thus, the current CEST-SAGE-EPI sequence may not be immediately useful to explore functional or metabolic changes in otherwise normal neural tissues without additional considerations (e.g. higher field strengths, more averages, etc.), but it is uniquely suited for detection and assessment of brain tumor or oncologic metabolism. Although MTRasym contrast could be altered by effects from exchangeable proton pools other than amine protons, we have shown with phantoms (Supporting Figure S3) that the low pH results in elevation of MTRasym at 3.0ppm despite the presence of amide protons and MT effect (introduced with 5% egg-white protein) and this is comparable to previous reports (16). The predominant pH dependence of MTRasym at 3.0ppm could be explained by the high irradiation amplitude (B1 = 6μT) preferably saturating fast-exchanging amine protons compared when with other proton pools. Another concern with using amine CEST contrast at 3.0ppm is it could suffer from an insufficient source of exchangeable protons if glutamine concentration is too low. In addition to glutamine, other amino acids and neurotransmitters possessing a similar amine functional group have similar pH dependence of exchange rate and MTRasym at 3.0ppm (16). Large molecules such as bovine serum albumin (BSA) were also reported to show characteristics of fast-exchanging amine protons at 3.0ppm (46). With a total of around 20–25 mM amino acids within normal neural tissues (47) and other contributions of amine proton pools, sufficient amine CEST contrast within tumor tissues is highly probable.
Combined information about both median tissue acidity (MTRasym at 3.0ppm) and oxygen extraction (R2′) helped to further delineate various tissue types and provide additional insights into the tumor microenvironment. Consistent with tumor biology and the Warburg effect, we observed elevated acidity in tumor tissues even when there was adequate oxygen delivery due to an intact BBB, high neovascularity, and high blood flow. In particular, we observed lower levels of hypoxia and high acidity in regions of non-enhancing tumor (T2/FLAIR hyperintensity) (Fig 5A,D; 6A,C), which can have elevated vascularity depending on the degree of malignancy and/or grade. This was similar across tumor grade, although GBM had slightly higher levels of hypoxia compared with lower grades. In the same tumors we observed substantially higher levels of hypoxia in areas of contrast enhancement (Fig 4D; 5C; 6A,C), which is known to be the most aggressive (48) and hypoxic (49–51). These data suggest tumor acidity and oxygen consumption are both complex and spatially heterogenous, consistent with the known genetic, histopathologic, proteomic, and metabolic spatial heterogeneity (52,53). Hypoxic, non-hypoxic, and/or acidic tumors may also have differing therapeutic responses, as tumor hypoxia and acidity are both known to reduce sensitivity to chemoradiation. Thus, a tool that can co-localize areas of simultaneous pH and oxygen imbalance may allow for future therapeutic strategies to include radiation boost to regions containing the most hypoxic and acidic environments. Additionally, this tool may also allow for evaluation of direct inhibition of energetic pathways as a therapeutic target in these tumors (54–56).
In addition to tissue acidity and reduced oxygen consumption, the Warburg effect is also characterized by altered levels of glucose consumption. Dynamic glucose enhanced MRI (DGE-MRI), which selectively saturates hydroxyl protons of glucose and allows for a dynamic detection of CEST contrast during glucose uptake, has shown promising results in mapping in animals (57,58) and in human tumor patients (59–61) and could potentially add another important piece of information about the tumor microenvironment.
Mutations of isocitrate dehydrogenase (IDH1) are common in less aggressive gliomas and cause inhibition of the conversion of isocitrate to alpha-ketoglutarate, resulting in a buildup of 2-hydroxygluterate (2HG), inhibiting oxidative phosphorylation and therefore inhibiting the ability of cells to utilize aerobic glycolysis (62). Therefore, we hypothesized IDH1-mutated tumors may have lower R2′ measurements compared to IDH1 wild-type tumors, consistent with previous work by Stadbauer et al. (63). Although we observed slightly lower R2′ in IDH1 mutated patients compared to wild-type patients, these effects were not statistically significant. Further studies in a larger patient cohort are needed to better understand this relationship and whether pH- and oxygen-sensitive MR signatures can identify IDH1 mutant tumors.
One potential limitation of our approach is using the conventional asymmetric analysis (MTRasym) to interpret CEST effects. Although the MTRasym approach removes the symmetric effects from direct water saturation (DS), the resulting contrast is still influenced by a number of other effects, including asymmetric magnetic transfer (MT) effect of semi-solid tissue components (64), nuclear Overhauser enhancement (NOE) 0–5ppm upfield to water proton resonance frequency (65), and mixing effects from nearby exchangeable proton pools. Several alternative approaches have been developed to mitigate these problems, including chemical exchange rotation transfer (66), Lorentzian difference (LD) analysis (46), multiple-pool Lorentzian fit (67,68), and the three-point method (69). Further investigation of pH- and oxygen-sensitive imaging with CEST-SAGE-EPI sequence could potentially benefit from applying the mentioned methods.
Additionally, proton exchange rates are influenced by temperature, with faster exchange rates of amine protons observed at higher temperatures, due to increased kinetic energy along with increased T2 relaxation times resulting in increased available NMR signal at a given frequency. Thus, phantom results at room temperature (~18°C) in the current studies are likely to underestimate MTRasym when compared to in vivo comparisons (~37°C), as has been previously demonstrated (16). Thus, care should be given when interpreting absolute values of MTRasym observed in the phantom portion of the current study.
It is important to note that in vivo R2′ mapping using SAGE-EPI readout can be influenced by a variety of technical issues, including B1 inhomogeneity or mismatch between excitation and refocusing slice profiles, as well as biological factors including the effects of water diffusion and multiple water compartments within the tissues (32,70). CEST contrast may also be influenced by multiple water compartments within tissues, although the water signal is thought to be predominantly from the extracellular water pool due to the very short relaxation times of bound intracellular water (71).
Technical imperfections including static magnetic field (B0) and transmit radiofrequency field (B1) inhomogeneity may also have led to altered image contrast. In our study, B0 inhomogeneity was corrected with B0 map generated from WASSR method or from multi-echo B0 mapping; however, B1 inhomogeneities were not compensated for. B1 inhomogeneity under 3T is reported to be relatively small (deviation < 10–20%) and have limited effect on T2 quantification (72) and pH-sensitive CEST contrast (16) within the variation range in the current study. Nevertheless, validation of the effect of B1 inhomogeneity on CEST-SAGE-EPI sequence and incorporation of B1 correction in future work may be beneficial.
A final potential limitation of this study was the relative inhomogeneity of the patient population, which contained untreated and recurrent glioma patients on a variety of therapies. A larger study that separates out specific treatments along with tumor grade is necessary to better understand their influence on our measurements.
CONCLUSION
The current study presents a new CEST-SAGE-EPI sequence for obtaining high-speed, full brain pH- and oxygen-sensitive image contrasts for brain tumor evaluation. Advantages of this technique include fast acquisition, in-line B0 correction using phase information, whole brain coverage, and simultaneous accurate estimation of CEST effects, R2, R2*, and R2′. Results in tumors showed a high degree of spatial heterogeneity and measurements were consistent with known cancer biology.
Supplementary Material
Supporting Figure S1. Examples of B0 correction methods available for CEST-SAGE-EPI. A) B0 maps calculated for a single patient using phase differences from the first two gradient echoes acquired using CEST-SAGE-EPI. B) Corresponding B0 maps for the same patient using the WASSR method. C) Resulting maps of MTRasym at 3ppm after B0 correction using multi-echo phase information. D) MTRasym at 3ppm after the WASSR method for B0 correction. E) MTRasym at 3ppm with no B0 correction.
Supporting Figure S2. Anatomic and relaxometry MR images of human gliomas estimated using CEST-SAGE-EPI. Complimentary T2 and T2* relaxometry images estimated from CEST-SAGE-EPI for the four patients illustrated in Fig. 3.
Supporting Figure S3. Phantom results for 40mM glutamine and 5% egg-white protein. Results demonstrate increasing CEST contrast with decreasing pH, similar to those reported previously with pure glutamine phantoms. This suggests fast-exchanging amine protons are the primary proton pool saturated using the current radiofrequency pulse scheme. Phantom preparation: Combined glutamine and protein phantoms with pH ranging from 6 to 7.5 were prepared by first adding glutamine into liquid egg white solution (5% protein) to reach concentrations of 40mM. The pH within the sample solutions was then titrated to 6.0, 6.3, 6.6, 6.9, 7.2, and 7.4, with acid (1N HCl) and base (1N NaOH) solutions. Scan parameters: B1 = 6uT, TR = 5000ms, TE1/TE2/TE3/TE4 = 14.0/34.1/58.0/92.4ms, FOV = 217×240mm, matrix size = 116×128, slice thickness = 4.0mm, slice number = 10, partial Fourier encoding = 6/8, GRAPPA = 3, bandwidth = 1628Hz/pixel with number of excitations or averages (NEX) = 2. A total of 36 offset frequencies were acquired: ± 2.5ppm to ± 4.0ppm with 0.1ppm interval, ± 6.0ppm and ± 20.0ppm with respect to water proton Larmor frequency. Measurements were taken at room temperature (~20°C). Error bars represent standard deviation within a region of interest within the Falcon tubes.
Acknowledgments
Funding: American Cancer Society (ACS) Research Scholar Grant (RSG-15-003-01-CCE) (Ellingson); Art of the Brain (Cloughesy); University of California Research Coordinating Committee (Ellingson); UCLA Jonsson Comprehensive Cancer Center Seed Grant (Ellingson); UCLA SPORE in Brain Cancer (NIH/NCI 1P50CA211015-01A1) (Ellingson, Liau, Nghiemphu, Lai, Pope, Cloughesy); NIH/NCI 1R21CA223757-01 (Ellingson).
Footnotes
Sponsors: N/A
References
- 1.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4(11):891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 2.Warburg O. In: The metabolism of tumours: investigations from the Kaiser Wilhelm Institute for Biology. Berlin-Dahlem, editor. London, UK: Arnold Constable; 1930. [Google Scholar]
- 3.Morita T, Nagaki T, Fukuda I, Okumura K. Clastogenicity of low pH to various cultured mammalian cells. Mutat Res. 1992;268(2):297–305. doi: 10.1016/0027-5107(92)90235-t. [DOI] [PubMed] [Google Scholar]
- 4.Gatenby RA, Vincent TL. An evolutionary model of carcinogenesis. Cancer Res. 2003;63(19):6212–6220. [PubMed] [Google Scholar]
- 5.Martinez-Zaguilan R, Seftor EA, Seftor RE, Chu YW, Gillies RJ, Hendrix MJ. Acidic pH enhances the invasive behavior of human melanoma cells. Clin Exp Metastasis. 1996;14(2):176–186. doi: 10.1007/BF00121214. [DOI] [PubMed] [Google Scholar]
- 6.Turner GA. Increased release of tumour cells by collagenase at acid pH: a possible mechanism for metastasis. Experientia. 1979;35(12):1657–1658. doi: 10.1007/BF01953252. [DOI] [PubMed] [Google Scholar]
- 7.Brat DJ, Castellano-Sanchez AA, Hunter SB, Pecot M, Cohen C, Hammond EH, Devi SN, Kaur B, Van Meir EG. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res. 2004;64(3):920–927. doi: 10.1158/0008-5472.can-03-2073. [DOI] [PubMed] [Google Scholar]
- 8.Lardner A. The effects of extracellular pH on immune function. J Leukoc Biol. 2001;69(4):522–530. [PubMed] [Google Scholar]
- 9.Shi Q, Le X, Wang B, Abbruzzese JL, Xiong Q, He Y, Xie K. Regulation of vascular endothelial growth factor expression by acidosis in human cancer cells. Oncogene. 2001;20(28):3751–3756. doi: 10.1038/sj.onc.1204500. [DOI] [PubMed] [Google Scholar]
- 10.Griffiths L, Dachs GU, Bicknell R, Harris AL, Stratford IJ. The influence of oxygen tension and pH on the expression of platelet-derived endothelial cell growth factor/thymidine phosphorylase in human breast tumor cells grown in vitro and in vivo. Cancer Res. 1997;57(4):570–572. [PubMed] [Google Scholar]
- 11.Souba WW. Glutamine and cancer. Ann Surg. 1993;218(6):715–728. doi: 10.1097/00000658-199312000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovacevic Z, Morris HP. The role of glutamine in the oxidative metabolism of malignant cells. Cancer Res. 1972;32(2):326–333. [PubMed] [Google Scholar]
- 13.Medina MA, Sanchez-Jimenez F, Marquez J, Rodriguez Quesada A, Nunez de Castro I. Relevance of glutamine metabolism to tumor cell growth. Mol Cell Biochem. 1992;113(1):1–15. doi: 10.1007/BF00230880. [DOI] [PubMed] [Google Scholar]
- 14.Sun PZ, Benner T, Copen WA, Sorensen AG. Early experience of translating pH-weighted MRI to image human subjects at 3 Tesla. Stroke. 2010;41(10 Suppl):S147–151. doi: 10.1161/STROKEAHA.110.595777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris RJ, Cloughesy TF, Liau LM, Prins RM, Antonios JP, Li D, Yong WH, Pope WB, Lai A, Nghiemphu PL, Ellingson BM. pH-weighted molecular imaging of gliomas using amine chemical exchange saturation transfer MRI. Neuro Oncol. 2015;17(11):1514–1524. doi: 10.1093/neuonc/nov106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris RJ, Cloughesy TF, Liau LM, Nghiemphu PL, Lai A, Pope WB, Ellingson BM. Simulation, phantom validation, and clinical evaluation of fast pH-weighted molecular imaging using amine chemical exchange saturation transfer echo planar imaging (CEST-EPI) in glioma at 3 T. NMR Biomed. 2016;29(11):1563–1576. doi: 10.1002/nbm.3611. [DOI] [PubMed] [Google Scholar]
- 17.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87(24):9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujita N, Shinohara M, Tanaka H, Yutani K, Nakamura H, Murase K. Quantitative mapping of cerebral deoxyhemoglobin content using MR imaging. Neuroimage. 2003;20(4):2071–2083. doi: 10.1016/j.neuroimage.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Geisler BS, Brandhoff F, Fiehler J, Saager C, Speck O, Rother J, Zeumer H, Kucinski T. Blood oxygen level-dependent MRI allows metabolic description of tissue at risk in acute stroke patients. Stroke. 2006;37(7):1778–1784. doi: 10.1161/01.STR.0000226738.97426.6f. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Chen YM, Zhang YT. Blood-Oxygenation-Level-Dependent-(BOLD-) Based R2 ′ MRI Study in Monkey Model of Reversible Middle Cerebral Artery Occlusion. Journal of Biomedicine and Biotechnology. 2011 doi: 10.1155/2011/318346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen-Kondering U, Manavaki R, Ejaz S, Sawiak SJ, Carpenter TA, Fryer TD, Aigbirhio FI, Williamson DJ, Baron JC. Brain hypoxia mapping in acute stroke: Back-to-back T2′ MR versus (18)F-fluoromisonidazole PET in rodents. Int J Stroke. 2017;12(7):752–760. doi: 10.1177/1747493017706221. [DOI] [PubMed] [Google Scholar]
- 22.He X, Zhu M, Yablonskiy DA. Validation of oxygen extraction fraction measurement by qBOLD technique. Magn Reson Med. 2008;60(4):882–888. doi: 10.1002/mrm.21719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Domsch S, Mie MB, Wenz F, Schad LR. Non-invasive multiparametric qBOLD approach for robust mapping of the oxygen extraction fraction. Z Med Phys. 2014;24(3):231–242. doi: 10.1016/j.zemedi.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Toth V, Forschler A, Hirsch NM, den Hollander J, Kooijman H, Gempt J, Ringel F, Schlegel J, Zimmer C, Preibisch C. MR-based hypoxia measures in human glioma. J Neurooncol. 2013;115(2):197–207. doi: 10.1007/s11060-013-1210-7. [DOI] [PubMed] [Google Scholar]
- 25.Jensen-Kondering U, Baron JC. Oxygen imaging by MRI: can blood oxygen level-dependent imaging depict the ischemic penumbra? Stroke. 2012;43(8):2264–2269. doi: 10.1161/STROKEAHA.111.632455. [DOI] [PubMed] [Google Scholar]
- 26.Hirsch NM, Toth V, Forschler A, Kooijman H, Zimmer C, Preibisch C. Technical considerations on the validity of blood oxygenation level-dependent-based MR assessment of vascular deoxygenation. NMR Biomed. 2014;27(7):853–862. doi: 10.1002/nbm.3131. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Chen YM, Zhang YT. Blood-oxygenation-level-dependent-(BOLD-) based R2′ MRI study in monkey model of reversible middle cerebral artery occlusion. J Biomed Biotechnol. 2011;2011:318346. doi: 10.1155/2011/318346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woessner DE, Zhang S, Merritt ME, Sherry AD. Numerical solution of the Bloch equations provides insights into the optimum design of PARACEST agents for MRI. Magn Reson Med. 2005;53(4):790–799. doi: 10.1002/mrm.20408. [DOI] [PubMed] [Google Scholar]
- 29.Liepinsh E, Otting G. Proton exchange rates from amino acid side chains--implications for image contrast. Magn Reson Med. 1996;35(1):30–42. doi: 10.1002/mrm.1910350106. [DOI] [PubMed] [Google Scholar]
- 30.Bryant RG. The dynamics of water-protein interactions. Annu Rev Biophys Biomol Struct. 1996;25:29–53. doi: 10.1146/annurev.bb.25.060196.000333. [DOI] [PubMed] [Google Scholar]
- 31.Schmiedeskamp H, Straka M, Newbould RD, Zaharchuk G, Andre JB, Olivot JM, Moseley ME, Albers GW, Bammer R. Combined spin- and gradient-echo perfusion-weighted imaging. Magn Reson Med. 2012;68(1):30–40. doi: 10.1002/mrm.23195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmiedeskamp H, Straka M, Bammer R. Compensation of slice profile mismatch in combined spin- and gradient-echo echo-planar imaging pulse sequences. Magn Reson Med. 2012;67(2):378–388. doi: 10.1002/mrm.23012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellingson BM, Bendszus M, Boxerman J, Barboriak D, Erickson BJ, Smits M, Nelson SJ, Gerstner E, Alexander B, Goldmacher G, Wick W, Vogelbaum M, Weller M, Galanis E, Kalpathy-Cramer J, Shankar L, Jacobs P, Pope WB, Yang D, Chung C, Knopp MV, Cha S, van den Bent MJ, Chang S, Yung WK, Cloughesy TF, Wen PY, Gilbert MR Jumpstarting Brain Tumor Drug Development Coalition Imaging Standardization Steering C. Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Neuro Oncol. 2015;17(9):1188–1198. doi: 10.1093/neuonc/nov095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim M, Gillen J, Landman BA, Zhou J, van Zijl PC. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn Reson Med. 2009;61(6):1441–1450. doi: 10.1002/mrm.21873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellingson BM, Harris RJ, Woodworth DC, Leu K, Zaw O, Mason WP, Sahebjam S, Abrey LE, Aftab DT, Schwab GM, Hessel C, Lai A, Nghiemphu PL, Pope WB, Wen PY, Cloughesy TF. Baseline pretreatment contrast enhancing tumor volume including central necrosis is a prognostic factor in recurrent glioblastoma: evidence from single and multicenter trials. Neuro Oncol. 2017;19(1):89–98. doi: 10.1093/neuonc/now187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellingson BM, Kim HJ, Woodworth DC, Pope WB, Cloughesy JN, Harris RJ, Lai A, Nghiemphu PL, Cloughesy TF. Recurrent glioblastoma treated with bevacizumab: contrast-enhanced T1-weighted subtraction maps improve tumor delineation and aid prediction of survival in a multicenter clinical trial. Radiology. 2014;271(1):200–210. doi: 10.1148/radiol.13131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun PZ, Wang Y, Lu J. Sensitivity-enhanced chemical exchange saturation transfer (CEST) MRI with least squares optimization of Carr Purcell Meiboom Gill multi-echo echo planar imaging. Contrast Media Mol Imaging. 2014;9(2):177–181. doi: 10.1002/cmmi.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Togao O, Hiwatashi A, Keupp J, Yamashita K, Kikuchi K, Yoshiura T, Suzuki Y, Kruiskamp MJ, Sagiyama K, Takahashi M, Honda H. Scan-rescan reproducibility of parallel transmission based amide proton transfer imaging of brain tumors. J Magn Reson Imaging. 2015;42(5):1346–1353. doi: 10.1002/jmri.24895. [DOI] [PubMed] [Google Scholar]
- 39.Heo HY, Zhang Y, Lee DH, Jiang S, Zhao X, Zhou J. Accelerating chemical exchange saturation transfer (CEST) MRI by combining compressed sensing and sensitivity encoding techniques. Magn Reson Med. 2017;77(2):779–786. doi: 10.1002/mrm.26141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Togao O, Yoshiura T, Keupp J, Hiwatashi A, Yamashita K, Kikuchi K, Suzuki Y, Suzuki SO, Iwaki T, Hata N, Mizoguchi M, Yoshimoto K, Sagiyama K, Takahashi M, Honda H. Amide proton transfer imaging of adult diffuse gliomas: correlation with histopathological grades. Neuro Oncol. 2014;16(3):441–448. doi: 10.1093/neuonc/not158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song X, Xu J, Xia S, Yadav NN, Lal B, Laterra J, Bulte JW, van Zijl PC, McMahon MT. Multi-echo length and offset VARied saturation (MeLOVARS) method for improved CEST imaging. Magn Reson Med. 2015;73(2):488–496. doi: 10.1002/mrm.25567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005;40(11):715–724. doi: 10.1097/01.rli.0000184756.66360.d3. [DOI] [PubMed] [Google Scholar]
- 43.Noordin S, Winalski CS, Shortkroff S, Mulkern RV. Factors affecting paramagnetic contrast enhancement in synovial fluid: effects of electrolytes, protein concentrations, and temperature on water proton relaxivities from Mn ions and Gd chelated contrast agents. Osteoarthritis Cartilage. 2010;18(7):964–970. doi: 10.1016/j.joca.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 44.Mellon EA, Beesam RS, Baumgardner JE, Borthakur A, Witschey WR, Reddy R. Estimation of the regional cerebral metabolic rate of oxygen consumption with proton detected O-17 MRI during precision O-17(2) inhalation in swine. Journal of Neuroscience Methods. 2009;179(1):29–39. doi: 10.1016/j.jneumeth.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu XH, Zhang NY, Zhang Y, Zhang XL, Ugurbil K, Chen W. In vivo (17)O NMR approaches for brain study at high field. Nmr in Biomedicine. 2005;18(2):83–103. doi: 10.1002/nbm.930. [DOI] [PubMed] [Google Scholar]
- 46.Jones CK, Huang A, Xu J, Edden RA, Schar M, Hua J, Oskolkov N, Zaca D, Zhou J, McMahon MT, Pillai JJ, van Zijl PC. Nuclear Overhauser enhancement (NOE) imaging in the human brain at 7T. Neuroimage. 2013;77:114–124. doi: 10.1016/j.neuroimage.2013.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perry TL, Hansen S, Berry K, Mok C, Lesk D. Free amino acids and related compounds in biopsies of human brain. J Neurochem. 1971;18(3):521–528. doi: 10.1111/j.1471-4159.1971.tb11980.x. [DOI] [PubMed] [Google Scholar]
- 48.Ellingson BM, Wen PY, Cloughesy TF. Evidence and Context of Use for Contrast Enhancement as a Surrogate of Disease Burden and Treatment Response in Malignant Glioma. Neuro Oncol. 2017 doi: 10.1093/neuonc/nox193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.da Ponte KF, Berro DH, Collet S, Constans JM, Emery E, Valable S, Guillamo JS. In Vivo Relationship Between Hypoxia and Angiogenesis in Human Glioblastoma: A Multimodal Imaging Study. J Nucl Med. 2017;58(10):1574–1579. doi: 10.2967/jnumed.116.188557. [DOI] [PubMed] [Google Scholar]
- 50.Flynn JR, Wang L, Gillespie DL, Stoddard GJ, Reid JK, Owens J, Ellsworth GB, Salzman KL, Kinney AY, Jensen RL. Hypoxia-regulated protein expression, patient characteristics, and preoperative imaging as predictors of survival in adults with glioblastoma multiforme. Cancer. 2008;113(5):1032–1042. doi: 10.1002/cncr.23678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9(6):677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 52.Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, Cahill DP, Nahed BV, Curry WT, Martuza RL, Louis DN, Rozenblatt-Rosen O, Suva ML, Regev A, Bernstein BE. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(6190):1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sottoriva A, Spiteri I, Piccirillo SG, Touloumis A, Collins VP, Marioni JC, Curtis C, Watts C, Tavare S. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci U S A. 2013;110(10):4009–4014. doi: 10.1073/pnas.1219747110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maurer GD, Brucker DP, Bahr O, Harter PN, Hattingen E, Walenta S, Mueller-Klieser W, Steinbach JP, Rieger J. Differential utilization of ketone bodies by neurons and glioma cell lines: a rationale for ketogenic diet as experimental glioma therapy. BMC Cancer. 2011;11:315. doi: 10.1186/1471-2407-11-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nie S, Li K, Huang Y, Hu Q, Gao X, Jie S. miR-495 mediates metabolic shift in glioma cells via targeting Glut1. J Craniofac Surg. 2015;26(2):e155–158. doi: 10.1097/SCS.0000000000001385. [DOI] [PubMed] [Google Scholar]
- 56.Wolf A, Agnihotri S, Micallef J, Mukherjee J, Sabha N, Cairns R, Hawkins C, Guha A. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med. 2011;208(2):313–326. doi: 10.1084/jem.20101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nasrallah FA, Pages G, Kuchel PW, Golay X, Chuang KH. Imaging brain deoxyglucose uptake and metabolism by glucoCEST MRI. Journal of Cerebral Blood Flow and Metabolism. 2013;33(8):1270–1278. doi: 10.1038/jcbfm.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jin T, Mehrens H, Hendrich KS, Kim SG. Mapping brain glucose uptake with chemical exchange-sensitive spin-lock magnetic resonance imaging. Journal of Cerebral Blood Flow and Metabolism. 2014;34(8):1402–1410. doi: 10.1038/jcbfm.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu X, Yadav NN, Knutsson L, Hua J, Kalyani R, Hall E, Laterra J, Blakeley J, Strowd R, Pomper M, Barker P, Chan K, Liu G, McMahon MT, Stevens RD, van Zijl PC. Dynamic Glucose-Enhanced (DGE) MRI: Translation to Human Scanning and First Results in Glioma Patients. Tomography. 2015;1(2):105–114. doi: 10.18383/j.tom.2015.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paech D, Schuenke P, Koehler C, Windschuh J, Mundiyanapurath S, Bickelhaupt S, Bonekamp D, Baumer P, Bachert P, Ladd ME, Bendszus M, Wick W, Unterberg A, Schlemmer HP, Zaiss M, Radbruch A. T1rho-weighted Dynamic Glucose-enhanced MR Imaging in the Human Brain. Radiology. 2017;285(3):914–922. doi: 10.1148/radiol.2017162351. [DOI] [PubMed] [Google Scholar]
- 61.Schuenke P, Koehler C, Korzowski A, Windschuh J, Bachert P, Ladd ME, Mundiyanapurath S, Paech D, Bickelhaupt S, Bonekamp D, Schlemmer HP, Radbruch A, Zaiss M. Adiabatically prepared spin-lock approach for T1rho-based dynamic glucose enhanced MRI at ultrahigh fields. Magn Reson Med. 2017;78(1):215–225. doi: 10.1002/mrm.26370. [DOI] [PubMed] [Google Scholar]
- 62.Borodovsky A, Seltzer MJ, Riggins GJ. Altered cancer cell metabolism in gliomas with mutant IDH1 or IDH2. Curr Opin Oncol. 2012;24(1):83–89. doi: 10.1097/CCO.0b013e32834d816a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stadlbauer A, Zimmermann M, Kitzwogerer M, Oberndorfer S, Rossler K, Dorfler A, Buchfelder M, Heinz G. MR Imaging-derived Oxygen Metabolism and Neovascularization Characterization for Grading and IDH Gene Mutation Detection of Gliomas. Radiology. 2016:161422. doi: 10.1148/radiol.2016161422. [DOI] [PubMed] [Google Scholar]
- 64.Hua J, Jones CK, Blakeley J, Smith SA, van Zijl PCM, Zhou JY. Quantitative description of the asymmetry in magnetization transfer effects around the water resonance in the human brain. Magnetic Resonance in Medicine. 2007;58(4):786–793. doi: 10.1002/mrm.21387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee DH, Heo HY, Zhang K, Zhang Y, Jiang SS, Zhao XN, Zhou JY. Quantitative assessment of the effects of water proton concentration and water T-1 changes on amide proton transfer (APT) and nuclear overhauser enhancement (NOE) MRI: The origin of the APT imaging signal in brain tumor. Magnetic Resonance in Medicine. 2017;77(2):855–863. doi: 10.1002/mrm.26131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zu ZL, Janve VA, Xu JZ, Does MD, Gore JC, Gochberg DF. A new method for detecting exchanging amide protons using chemical exchange rotation transfer. Magnetic Resonance in Medicine. 2013;69(3):637–647. doi: 10.1002/mrm.24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zaiss M, Schmitt B, Bachert P. Quantitative separation of CEST effect from magnetization transfer and spillover effects by Lorentzian-line-fit analysis of z-spectra. Journal of Magnetic Resonance. 2011;211(2):149–155. doi: 10.1016/j.jmr.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 68.Zaiss M, Windschuh J, Paech D, Meissner JE, Burth S, Schmitt B, Kickingereder P, Wiestler B, Wick W, Bendszus M, Schlemmer HP, Ladd ME, Bachert P, Radbruch A. Relaxation-compensated CEST-MRI of the human brain at 7 T: Unbiased insight into NOE and amide signal changes in human glioblastoma. Neuroimage. 2015;112:180–188. doi: 10.1016/j.neuroimage.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 69.Jin T, Wang P, Zong X, Kim S-G. MR imaging of the amide-proton transfer effect and the pH-insensitive nuclear Overhauser effect at 9. 4 T. Magnetic resonance in medicine. 2013;69(3):760–770. doi: 10.1002/mrm.24315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ulrich X, Yablonskiy DA. Separation of Cellular and BOLD Contributions to T2* Signal Relaxation. Magnetic Resonance in Medicine. 2016;75(2):606–615. doi: 10.1002/mrm.25610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ababneh Z, Beloeil H, Berde CB, Gambarota G, Maier SE, Mulkern RV. Biexponential parameterization of diffusion and T2 relaxation decay curves in a rat muscle edema model: decay curve components and water compartments. Magn Reson Med. 2005;54(3):524–531. doi: 10.1002/mrm.20610. [DOI] [PubMed] [Google Scholar]
- 72.Guo H, Au WY, Cheung JS. Myocardial T2 quantitation in patients with iron overload at 3 Tesla (vol 30, pg 394, 2009) Journal of Magnetic Resonance Imaging. 2009;30(5):1230–1230. doi: 10.1002/jmri.21851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figure S1. Examples of B0 correction methods available for CEST-SAGE-EPI. A) B0 maps calculated for a single patient using phase differences from the first two gradient echoes acquired using CEST-SAGE-EPI. B) Corresponding B0 maps for the same patient using the WASSR method. C) Resulting maps of MTRasym at 3ppm after B0 correction using multi-echo phase information. D) MTRasym at 3ppm after the WASSR method for B0 correction. E) MTRasym at 3ppm with no B0 correction.
Supporting Figure S2. Anatomic and relaxometry MR images of human gliomas estimated using CEST-SAGE-EPI. Complimentary T2 and T2* relaxometry images estimated from CEST-SAGE-EPI for the four patients illustrated in Fig. 3.
Supporting Figure S3. Phantom results for 40mM glutamine and 5% egg-white protein. Results demonstrate increasing CEST contrast with decreasing pH, similar to those reported previously with pure glutamine phantoms. This suggests fast-exchanging amine protons are the primary proton pool saturated using the current radiofrequency pulse scheme. Phantom preparation: Combined glutamine and protein phantoms with pH ranging from 6 to 7.5 were prepared by first adding glutamine into liquid egg white solution (5% protein) to reach concentrations of 40mM. The pH within the sample solutions was then titrated to 6.0, 6.3, 6.6, 6.9, 7.2, and 7.4, with acid (1N HCl) and base (1N NaOH) solutions. Scan parameters: B1 = 6uT, TR = 5000ms, TE1/TE2/TE3/TE4 = 14.0/34.1/58.0/92.4ms, FOV = 217×240mm, matrix size = 116×128, slice thickness = 4.0mm, slice number = 10, partial Fourier encoding = 6/8, GRAPPA = 3, bandwidth = 1628Hz/pixel with number of excitations or averages (NEX) = 2. A total of 36 offset frequencies were acquired: ± 2.5ppm to ± 4.0ppm with 0.1ppm interval, ± 6.0ppm and ± 20.0ppm with respect to water proton Larmor frequency. Measurements were taken at room temperature (~20°C). Error bars represent standard deviation within a region of interest within the Falcon tubes.