Abstract
Background
For decades, economists and sociologists have documented intergenerational transmission of socioeconomic disadvantage, demonstrating that economic, political, and social factors contribute to ‘inherited hardship’. Drawing on biological factors, the ‘Developmental Origins of Adult Health and Disease model posits that fetal exposure to maternal prenatal distress associated with socioeconomic disadvantage compromises offspring’s neurodevelopment, affecting short and long-term physical and mental health, and thereby psychosocial standing and resources. Increasing evidence suggests that mother-to-child influence does occur prenatally, in part via maternal and offspring atypical HPA axis regulation, with negative effects on the maturation of prefrontal and subcortical neural circuits in the offspring. However, even this in utero timeframe may be insufficient to understand biological aspects of the transmission of factors contributing to disadvantage across generations.
Methods
We review animal studies and emerging human research indicating that parents’ childhood experiences may transfer epigenetic marks that could impact the development of their offspring independently of and in interaction with their offspring’s perinatal and early childhood direct exposures to stress stemming from socioeconomic disadvantage and adversity.
Results
Animal models point to epigenetic mechanisms by which traits that could contribute to disadvantage may be transmitted across generations. However epigenetic pathways of parental childhood experiences influencing child outcomes in the next generation is only beginning to be studied in humans. With a focus on translational research, we point to design features and methodological considerations for human cohort studies to be able to test the intergenerational transmission hypothesis, and we illustrate this with existing longitudinal studies.
Conclusions
Epigenetic intergenerational transmission, if at play in human populations, could have policy implications in terms of reducing the continuation of disadvantage across generations. Further research is needed to address this gap in the understanding of the perpetuation of compromised lives across generations.
Keywords: Development, early life experience, endocrinology, gene-environment interaction, epigenetics, adversity, stress
I. Introduction
Studies over the past several decades have shown that poverty tends to be reproduced across generations (Rodgers, 1995). A number of theories have been proposed over time to explain the intergenerational transmission of socioeconomic disadvantage, generally reflecting the prevailing political-cultural attitudes and state of scientific knowledge of the times. Tackling intergenerational transmission requires a greater knowledge of the mechanisms, including biological, behavioral, and psychological, in the context of socio-economic factors and access to resources, to know when and how to intervene most effectively. In this review we describe a new hypothesized factor in this intergenerational transmission process: epigenetics— molecular processes that change the expression of genes independent of changing DNA sequence. Epigenetic marks have been observed in genes thought to impact stress response (de Rooij et al., 2012, Edelman et al., 2012), emotion regulation (Puglia et al., 2015), disease susceptibility (Dong et al., 2017), and mental disorders (Melas et al., 2013, Dammann et al., 2011, Na et al., 2014, Wang et al., 2017a), all factors that can lead to compromised life courses. While more research is needed to clarify the role of epigenetics in phenotypic expression in these areas, epigenetic intergenerational transmission could potentially be a biological component of the reproduction of poverty across generations.1
Culture was the dominant explanation for the perpetuation of disadvantage across generations during the 1980s and 1990s. Coined in 1961 by the American anthropologist Oscar Lewis in his ethnographic work with Mexican-American communities, the term “culture of poverty” attempted to explain the cyclical quality of poverty by suggesting that parental attitudes and behaviors are transmitted to their children, perpetuating a cycle of poverty (Lewis, 1971). This theory has been highly contentious, perhaps because early scholarship tended to suggest that the culture of poverty would not shift even if socio-political factors did, e.g., access to better schools, and it was up to individuals to change their attitudes and behaviors to lift themselves out of poverty (Small, 2010). This hypothesis began to be challenged around the turn of the 21st century with more emphasis being placed on structural factors constraining the agency of poor individuals (Ludwig and Mayer, 2006). This new focus emphasized a need to ameliorate the effects of parents’ limited financial resources such as access to health care, good nutrition, and quality schools, and it was supported by research showing that incidence, depth, duration and timing of poverty, as well as the neighborhood where a child was raised, all influence a child’s educational attainment, a proxy for socioeconomic status and income (Ferguson et al., 2007). Research also showed that household and neighborhood poverty are related to stress (Schulz et al., 2012), and the cumulative effect of stressors such as housing or food insecurity, child abuse or neglect, parental substance abuse, and violence—factors associated with poverty (Evans and English, 2002)— can induce a toxic stress response in young children, which can lead to long-term changes in brain structure and function in infancy and early childhood (Shonkoff et al., 2012, Shonkoff et al., 2009, McEwen et al., 2015). Neighborhood poverty also was shown to be correlated with poor nutrition and obesity (Morland et al., 2006), which combined with risk factors such as community violence and interpersonal and environmental trauma prevalent in areas of poverty, contribute to chronic physical and mental health problems across the life course (Curry et al., 2008, Leventhal and Brooks-Gunn, 2000, Seligman et al., 2010, Chung et al., 2016). The structural perspective also emphasizes that the lack of access to adequate health care, particularly preventative health care, can exacerbate these problems. Furthermore, smoking and drug use, and lack of physical exercise worsen health problems, creating a negative feedback cycle of poor health and economic problems that confounds behaviors, “culture,” and structural factors. In addition, chronic stress associated with social disadvantage can compromise parents’ ability to provide sensitive caregiving and to stimulate early cognitive and language development (Finegood et al., 2017, Gershoff et al., 2007, Repetti et al., 2002)(Snow, 2006)(Conger, Conger, & Martin, 2010).
In the early 2000’s, biology began to play a more central role in theories on the intergenerational transmission of disadvantage with a focus on low birth weight as a predictor of disease across generations. Specific biological mechanisms were not clearly defined early on, though, and as such, scientists weren’t certain why low birth weight was perpetuated across generations in contexts of poverty and was a predictor of future disease risk, making intervention strategies speculative (Conley and Bennett, 2000, Evans et al., 1994). ‘Fetal Programming’, or the ‘Developmental Origins of Health and Disease (DOHaD) model, demonstrated that fetal exposure to a variety of maternal life experiences (e.g., poor nutrition, pollutants, stress) could alter offspring’s neurodevelopment, with implications for future health and well-being (Barker, 1990, Barker and Martyn, 1992, Barker, 1995). As biological research advanced further over the next two decades, transmission pathways began to come into focus. Strong evidence now shows that mother-to-child influence indeed occurs prenatally, in part via maternal and offspring atypical hypothalamic–pituitary–adrenal (HPA) axis regulation, with effects on the maturation of prefrontal and subcortical neural circuits (Popoli et al., 2012, Hunter et al., 2012). Importantly, this model also suggests that developmental risk can be curtailed by intervention during sensitive periods (Leshem and Schulkin, 2012, McCreary et al., 2016a).
However, even this in utero timeframe may be inadequate to understand biological components of the transmission of factors related to disadvantage across generations. Recent animal work and some human studies now suggest epigenetics as a biological pathway by which parents’ childhood experiences may affect the development of their offspring independently of and in interaction with their offspring’s prenatal and early childhood exposures to adverse experiences. Yet this pathway—parental childhood experiences influencing epigenetics in the next generation—is only beginning to be studied in humans.
In this review, we describe recent research on the epigenetic intergenerational transmission of disadvantage, discussing in detail exemplar studies that both support and challenge this model. First, we define the intergenerational epigenetic transmission hypothesis and briefly describe the potential role of epigenetics in the transmission of disadvantage across generations. Next, we highlight recent animal research on epigenetic transmission in the paternal and maternal germline and point to critiques and limitations with the current state of knowledge on epigenetics and intergenerational transmission in animal models and in humans. We concentrate the review on early life stress and epigenetic changes related to HPA axis functioning, though other factors such as diet should also be included in a model of epigenetic intergenerational transmission of disadvantage. With a focus on translational research, we then highlight research priorities for further testing this hypothesis and point to current longitudinal cohort studies that seek to address gaps in the understanding of the perpetuation of disadvantage across generations. Finally, we discuss the need for additional research in humans within the context of socio-economic and other contextual factors, to ultimately lead toward implications for interventions and public policy to interrupt the cycle of inherited disadvantage.
II. Reviving Lamarck: Epigenetics and Intergenerational Transmission
Fetal programming and DOHaD shifted the equation on intergenerational transmission of adversity from the side of “nurture” more toward the side of “nature”, while re-defining the biological aspect by demonstrating that poor genes do not cause poverty but rather adversity gets “under the skin” to affect growth and development, curtailing individuals’ productive potential. The “Nature” in “Nature versus Nurture,” however, continues to unfold in complexity. Most research on DOHaD and Fetal Programming view conception or the period shortly before conception as the starting point from which adversity can affect the biology of a new life. But advances in epigenetic research point to an expanded timeframe for intergenerational impact. The term “epigenetics,” coined over a half a century ago (Waddington, 1959), has been used in a broad sense to refer to the long-term or stable regulation of gene expression and function induced by environmental factors without a change in DNA sequence (Wang et al., 2017b). There is considerable interest in the possibility that phenotypic traits, disease susceptibility, and even behaviors that are acquired through environmental interactions in one generation can be transmitted through epigenetic processes to future generations, however empirical evidence remains inconclusive and particularly scarce in human populations. (Yehuda, 2011, Dietz et al., 2011, Daxinger and Whitelaw, 2012, Siklenka et al., 2015, Szyf, 2015, Champagne, 2010, Klengel et al., 2015, van Otterdijk and Michels, 2016). If epigenetic inheritance through generations indeed takes place in humans, then environmental risk factors prevalent in contexts of adversity may epigenetically impact not only the individual and the individual’s children but also their children’s children.
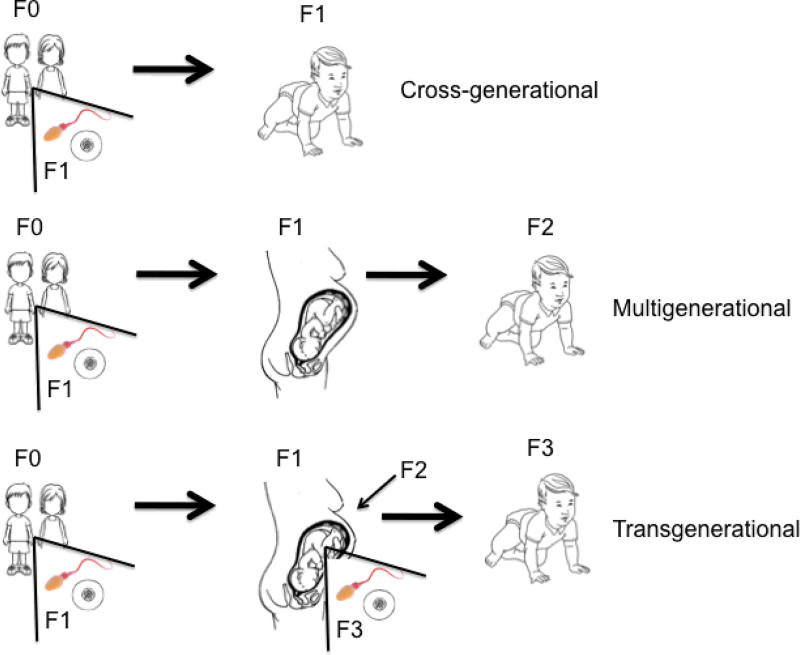
The terms Intergenerational (or cross-generational), multigenerational, and transgenerational currently are used to describe epigenetic transmission across generations, though the use of these terms varies slightly across studies. In a recent review, Wang et al (Wang et al., 2017b) defined these three terms as follows: Using parents’ childhood as the first exposure, Intergenerational or cross-generational describes transmission across one generation, such as the impact of childhood trauma on the child’s germline and thus future children (Carone et al., 2010, Heard and Martienssen, 2014, Szyf, 2015). The children (future parents) would be the initial generation (F0) and their affected offspring would be the F1 generation. Multigenerational effects are then those that originate in the F0 generation and persist from the F1 generation to their children, the F2 generation (Dias & Ressler, 2014), and transgenerational effects are those that persist in more than three generations (F3 or beyond) (Anway et al., 2005; Greer et al., 2011; Rechavi et al., 2011). We use the term “intergenerational” broadly in this review to describe transmission across two or more generations. We depict the three different variants— “cross-generational,” “multigenerational,” and “transgenerational” in Figure 1 with parents’ childhood as the first exposure.
Figure 1.
Model of Intergenerational Transmission of Disadvantage: Parents’ Childhood as the First Exposure
*Epigenetic marks in egg/sperm in children affected by environmental stress are transmitted to their children (cross-generational effects), to their grandchildren (multigenerational effects), and to their great-grandchildren (transgenerational effects).
Given the potential implications for developmental research as well as society, epigenetic intergenerational transmission warrants continued human research. Assimilating findings from a strong and growing body of animal research can lead to efficient translational studies in human populations. Such studies can move toward understanding if and how epigenetic intergenerational transmission occurs in human populations and how epigenetics affect factors associated with disadvantage, including stress response and emotion regulation, cognitive development, disease susceptibility, and mental disorders.
Preconception Stress and Epigenetic Transmission
The epigenetic intergenerational transmission hypothesis states that environmental insults before conception, even early in childhood, (called preconception stress) can cause epigenetic changes to gamete (egg or sperm) cells that can manifest in the offspring on the behavioral and neuroendocrine level, and even at the level of neuronal differentiation and synaptic development (Bock et al., 2016, Zaidan et al., 2013). The results of a number of studies in animals lend support to this theory (Bock et al., 2016, Yao et al., 2014, Harker et al., 2015, Rompala et al., 2016, Zaidan and Gaisler-Salomon, 2015, Pisu et al., 2013, Modir et al., 2014). Yet studies suggesting intergenerational transmission in rodent models have garnered intense attention, including some that is strongly skeptical. Critics claim that most studies on epigenetic intergenerational transmission have not demonstrated causation, and doubt remains regarding the genetic mechanisms of this transmission (Callaway, 2013, Bestor et al., 2015).
Much animal research on epigenetic intergenerational transmission centers on males, in part because male rodents do not engage in rearing, precluding a behavioral explanation for the resulting transmission of traits. A growing body of evidence in animal models suggests that epigenetic changes in both the maternal and paternal germlines induced by environmental stress can be passed to subsequent generations (Bock et al., 2016, Yao et al., 2014, Harker et al., 2015, Rompala et al., 2016, Zaidan and Gaisler-Salomon, 2015, Pisu et al., 2013, Modir et al., 2014). It is important to note that many of the epigenetic changes that have been shown in animal models are sex-specific. The potential mechanisms mediating such sex-specific intergenerational effects have not yet been thoroughly investigated and remain largely unknown. It has been suggested that chromosomal differences between the sexes, including both sex steroid-dependent and sex steroid-independent effects, as well as differences in epigenetic plasticity during gamete maturation, might potentially contribute to the observed sex-specific epigenetic inheritance (Champagne, 2013). Moreover, differential epigenetic processes in the placenta related to the X-linked genes involved in placenta development and early unequal gene expression by the sex chromosomes between males and females, might exert an early impact (Gabory et al., 2013). The different epigenetic effects in the paternal and maternal germlines may also result from differences in the epigenetic reprogramming process between the male and female germlines and from differences between the maternal and paternal genomes post fertilization (Kobayashi et al., 2013, Champagne, 2013, Saavedra-Rodríguez and Feig, 2013), although each of these hypotheses require further experimental evidence (Wang et al., 2017b).
Focus on Mechanisms of Epigenetic Intergenerational Transmission
Critiques of epigenetic intergenerational transmission point out that observations of transmission across generations of phenotypes or disease risk could be caused by intrauterine exposures, mutations in DNA repair mechanisms, shared environment, and the re-production in subsequent generations of the environment/behaviors that influenced gene expression in the first generation (van Otterdijk and Michels, 2016). Buss et al note that epigenetic inheritance (i.e. transmission of epigenetic marks) is often confused with de novo production of stable epigenetic alterations in the offspring (Buss et al., 2017). While not technically intergenerational epigenetic inheritance, such prenatal de novo epigenetic changes in offspring of parents who experienced early life adversity could constitute an epigenetic mechanism by which the effects of adversity might be propagated in the subsequent generation if the epigenetic re-programming in utero is dependent on biological factors in the mother caused by early life adversity. We will return to this possible mechanism in the section on evidence of intergenerational transmission in humans.
Given these critiques on the evidence of intergenerational epigenetic inheritance on animal studies, recent research has focused on elucidating the specific mechanisms of transmission. DNA methylation is an often-cited candidate for transmissible epigenetic modification, given that it is stable, known to be heritable through cell division, and is retained during sperm maturation, when other epigenetic marks are largely removed (Hur et al., 2017). However, the biological significance of DNA methylation can be difficult to interpret, as changes are often small and widespread throughout the genome and affect a variety of genes (Soubry, 2015, Carone et al., 2010). Evidence also suggests that DNA methylation is driven by stable genetics and intrauterine exposure rather than intergenerational epigenetic inheritance (van Otterdijk and Michels, 2016, Gertz et al., 2011). Given that most functional genomic elements are extensively demethylated after fertilization (Wang et al. 2014), there remain significant challenges to support the idea that inherited DNA methylation patterns are responsible for the transmission of programming from parent to child (Hur et al., 2017).
The modification of core histones at their amino-terminal tails by acetylation, phosphorylation, methylation and ubiquitylation has a fundamental role in determining gene activity (Jenuwein and Allis, 2001, Zhang and Reinberg, 2001, Strahl and Allis, 2000). However, whether and how these epigenetic marks could be replicated during embryonic development to affect the offspring had not been demonstrated until very recently (Wang et al., 2017b). Teperek et al showed for the first time that histone modifications in sperm do change gene expression in embryos and are required for healthy offspring development (Teperek et al., 2016). They compared the development of frog embryos by experimentally removing epigenetic marks at fertilization in one group and found that removing these epigenetic changes led to abnormal gene expression in the embryo, followed by less efficient development to the swimming tadpole stage. (Thomson, 2016, Teperek et al., 2016). Ciabrelli et al likewise showed that in Drosophila fruit flies, epigenetic alterations in histone H3 Lys27 are transmitted to future generations, expressing as red eye color phenotype (Ciabrelli et al., 2017), and Klosin et al showed that histone modifications cuased by environmental conditions (temperature change) persisted for 14 generations of nematode worms and were inherited through both eggs and sperm (Klosin et al., 2017). However, studies in other vertebrate and mammal systems are still lacking, and the potential involvement of heritable chromatin alterations in epigenetic programming in mammals remains an open question.
Another prominent theory for epigenetic intergenerational inheritance involves the actions of small non-coding RNA (snRNA), functional RNA moledules that are transcribed from DNA but are not translated into proteins. (Hur et al., 2017, Franklin et al., 2010, Szyf, 2013, Gapp et al., 2014). Only around 2% of genomic transcripts are translated into protein (Peschansky and Wahlestedt, 2014), and the remaining transcripts — which do not code for protein — were first regarded as “junk”. But mounting evidence demonstrates that these snRNA molecules can indeed be functional and play a role in epigenetic transmission (Peschansky and Wahlestedt, 2014). Rogers et al found that offspring of male mice exposed to six weeks of chronic stress prior to breeding, either throughout puberty or in adulthood, displayed significantly reduced HPA axis stress responsivity, regardless of the time period of paternal stress (Rodgers et al., 2013). Gene set enrichment analyses of stress-regulating brain regions revealed global pattern changes in transcription suggestive of epigenetic reprogramming and consistent with altered offspring stress responsivity, including increased expression of glucocorticoid-responsive genes in the paraventricular nuclei (Rodgers et al., 2013). An increase in nine micro RNAs (miRNAs), which are one type of snRNA, was identified in the sperm of stressed sires and associated with reduced HPA stress axis reactivity in offspring (Rodgers et al., 2013), and in a subsequent study these miRNAs in mouse sperm were micro-injected into a single-cell zygote, which was then implanted into surrogate dams, reared normally, and examined for HPA stress axis sensitivity in adulthood (Rodgers et al., 2015). Notably, these animals demonstrated a remarkable reproduction of the stress-induced dysregulation phenotype previously seen in offspring, indicating a clear mechanistic role for sperm miRNAs in the transmission of paternal lifetime experience (Rodgers et al., 2015). Gapp et al also found that traumatic stress in early life altered male mouse miRNA expression in sperm and behavioral and metabolic responses in the progeny: reduced avoidance and fear, depressive-like behaviors, insulin hypersensitivity and hypermetabolism (Gapp et al., 2014). Injection of sperm RNAs from traumatized male rodents into fertilized wild-type oocytes reproduced the behavioral and metabolic alterations in the resulting offspring (Gapp et al., 2014).
One way that information from snRNAs could potentially be transmitted to the sperm cells is through extracellular vesicles— tiny lipid-enclosed vesicles in all body fluids (Eaton et al., 2015). Again, once considered inert substances, they are hypothesized to function as intercellular messengers; if this is the case, extracellular vesicles might be able to transmit information from parental body cells affected by stressors (including stressed brain cells), directly to the germ cells, altering DNA expression in those cells that will become a fetus (Eaton et al., 2015). Advances in research on extracellular vesicles have been supported by the findings that these vesicles can deliver proteins to sperm and RNAs (Koch et al., 2015, Sharma et al., 2016) and by the discovery of extracellular mobile RNAs that can be found outside extracellular vesicles (Sarkies and Miska, 2014).
No robust evidence exists for how epigenetic changes in germ cells can escape the reprogramming that occurs after fertilization and as the embryo and fetus is developing; as indicated, these are major challenges to the epigenetic transmission hypothesis. Additionally, most studies investigating epigenetic intergenerational transmission are based on animal models, leaving it unclear whether intergenerational epigenetic inheritance exists in humans (van Otterdijk and Michels, 2016). Differences in the epigenome between humans and mice do not permit direct inference to humans (Casas and Vavouri, 2014, Chavez et al., 2014), and in addition to biological differences, animal studies cannot replicate complex social and community structure. Human cohort studies are therefore needed to assess whether epigenetic effects may be at play in cycles of human social and economic disadvantage. To date there is no clear evidence of intergenerational epigenetic inheritance in humans (van Otterdijk and Michels, 2016).
III. Intergenerational Epigenetic Transmission in Humans
Though no strong evidence yet exists for epigenetic intergenerational transmission in humans, several observational studies suggest that epigenetic changes in mothers and fathers who experienced adversity long before pregnancy could be passed on or otherwise reproduced in future generations. A cross-sectional study of holocaust survivors and their (adult) children found that holocaust exposure had an effect on Cytosine methylation within the gene encoding for FK506 binding protein 5, an important regulator of glucocorticoid receptor sensitivity (Yehuda et al., 2016). This cross-sectional study in two generations was not able to differentiate between epigenetic inheritance and social transmission, although the study did control for offspring trauma exposure and psychopathology, as well as other socio-demographic factors. Given the small sample size (n=22 adult offspring of holocaust survivors), the results should also be interpreted with caution. The same body of work examining offspring of holocaust survivors found that offspring with both maternal and paternal PTSD showed lower methylation in a glucocorticoid receptor promoter, which was associated with greater cortisol suppression (Yehuda et al., 2014). In the absence of maternal PTSD, offspring with paternal PTSD showed higher methylation in this same location, suggesting different mechanisms for intergenerational transmission by sex (Yehuda et al., 2014). These results have not yet been replicated in the published literature.
A study of children and grandchildren of individuals who survived the Dutch famine of 1944–45 investigated the methylation of 15 loci implicated in growth and metabolic disease in individuals who were and were not exposed prenatally to the famine and found persistent changes in DNA methylation in those with prenatal famine exposure (Tobi et al., 2009). Several of these changes depended on the sex of the exposed children (higher methylation in men for IGF2R and lower in men for LEP, IL10, and APOC1) and the gestational timing of the exposure (lower methylation ain at the GNASAS locus was observed for those exposed to the famine late in gestation rather then in the periconception period). A study of Rwandan genocide survivors also found epigenetic modifications of the glucocorticoid receptor NR3C1 gene in women exposed to genocide during pregnancy as well as in their children exposed in utero (Perroud et al., 2014).
While the inheritance of epigenetic marks as a mechanism for the intergenerational transmission of disadvantage remains equivocal in humans, increasing evidence in humans suggests that an in utero timeframe is too narrow, and mothers’ own childhood experiences are likely to have a lasting biological effect on their offspring. Recent studies examining the impact of early childhood trauma or adverse experiences on pregnancy and birth outcomes have found that maternal early life trauma moderated the impact of pregnancy stressors and even had a more pronounced effect than the stressors measured during pregnancy. For example, in a pregnancy cohort in Michigan, abuse in childhood (measured using retrospective report during pregnancy) significantly increased the odds of pre-term delivery whereas pregnancy stressors did not (Margerison-Zilko et al., 2016). In this study of over 2,500 pregnant women (80% white, 60% with 12+ years of education), abuse/witnessing violence, loss, economic stress, and substance use were measured for three stages in the life course— childhood (via retrospective recall), adulthood (retrospective recall), and in the first six months of pregnancy. Among all women, abuse in childhood increased odds of late pre-term delivery (Margerison-Zilko et al., 2016). In another study, trauma history magnified the effects of maternal prenatal affective symptoms on birthweight, and this moderating effect was limited to those who first experienced a trauma prior to 18 years of age (Blackmore et al., 2016). This study was carried out in a hospital serving a predominantly low-income inner-city population, and nearly 40% of the sample reported experiencing at least one traumatic event. The interaction of trauma exposure and anxiety during pregnancy significantly strengthened the model predicting low birthweight (Blackmore et al., 2016). Examining outcomes such as birthweight and in utero growth, rather than infant, early childhood, or adult outcomes, helps to isolate the effects of mother’s pre-conception and early life trauma on child and infant development. However, such studies cannot rule out the possibility that prior trauma affects a mother’s health practices in pregnancy, unless such prenatal health practices are well controlled for in the studies.
Early life stress in the mother appears to alter the placental environment regardless of a women’s health and experiences during pregnancy, affecting fetal development or even causing de novo epigenetic changes in the developing embryo that could mimic the epigenetic changes identified in the mother. One way this could happen is that maternal early life adversity might produce alterations in her egg cell cytoplasm (such as mitochondria, proteins, and RNA molecules) that, after conception, exerts an influence on the developing embryo or fetus (Buss et al., 2017). Another possibility is that increased placental concentrations of endocrine and immune stress mediators (caused by early life adversity in the mother) could cause alterations in the expression of miRNA and DNA methylation in the fetal brain, which could result in changes in fetal cell proliferation, neuronal differentiation, gliogenesis, availability of neurotrophic growth factors, cell survival, synaptogenesis, neurotransmitter levels, myelination, and adult neurogenesis (Buss et al., 2012, Babenko et al., 2015).
Supporting this latter hypothesis is evidence that adversity in early childhood produces long-term alterations in endocrine and immune-inflammatory physiology, including greater hypothalamic-pituitary-adrenal axis reactivity (Heim et al., 2003) and a greater pro-inflammatory state (Danese et al., 2007). In a study of 295 pregnant women, 34% of whom were Latina, maternal exposure to childhood trauma was significantly associated with placental corticotrophin-releasing hormone production, controlling for sociodemographic, biophysical, obstetric, behavioral, and psychological factors in pregnancy (Moog et al., 2016). Maternal preconception stress, but not current pregnancy stress, was positively associated with concentration of cortisol in maternal hair during pregnancy, exposure to emotional neglect during childhood showing the strongest effect. (Buss et al., 2016). Furthermore, higher maternal hair cortisol concentrations during pregnancy were associated with reduced neonatal brain white matter volume in that same study (Buss et al., 2016).
Cumulative Effects
The idea of intergenerational transmission suggests that insults to pregnant women or stress even earlier in life may have independent negative effects on future generations regardless of the experiences of those later generations. However, it is usually the case that situations of socio-economic deprivation, poverty, and violence are present across generations — perhaps even as a consequence of trans-generational transmission of characteristics that impede overcoming adversity. As such, the effects may be cumulative. Animal studies suggest that this is in fact, the case. In pregnant female rats of three successive generations exposed to prenatal stress, each additional generation of prenatal stress incrementally elevated HPA axis activation and increased anxiety-like and aversive behaviors in adult female offspring (McCreary et al., 2016b). Changes in brain signaling reflecting a simplification of network processing in multi-generationally prenatally stressed rats support the hypothesis that recurrent ancestral stress leads to adaptations in the brain that are different from those caused by single-generation prenatal stress (Skelin et al., 2015). Stress across generations in rats also gradually reduced gestational length, maternal weight gain and behavioral activity, and increased blood glucose levels, with each successive generation (Yao et al., 2014). Human studies of cumulative intergenerational processes are needed, particularly ones examining biological mechanisms of transmission.
It also is important to note that DNA epigenetic changes are not immutable. In fact, initial studies in animals show that stress-elicited epigenetic changes can be reversed when an intervention provides a health-promoting environment. One study, for example, examined rats from three lineages; one with prenatal stress only in the F1 generation, one with compounding effects of multigenerational prenatal stress, and a non-stress control lineage. Reduced axonal density was observed in both stressed lineages and was associated with abnormal corticospinal tract morphology and fine motor movement. However, environmental enrichment for the F3 rats — in the form of increased social interactions and an enriched living space filled with multiple shelters and toys, providing social, physical and sensory stimulation — reduced these consequences (McCreary et al., 2016a). In another study, pre-conception stress altered offspring behavior, but this was ameliorated both by pre-reproductive enrichment to the dam (including larger cages with running wheels, tunnels, climbing objects that were frequently alternated) and by post-weaning enrichment to the offspring (enriched in similar ways to the pre-reproductive period) (Leshem and Schulkin, 2012). The possibility for reversing epigenetic changes contributing to poor health outcomes, or even inducing health-promoting changes through intervention, has important implications for policy, particularly when the consequences of not intervening are health-threatening genetic and physiologic changes that accumulate across generations, as the evidence from animal studies suggests. While there is no direct evidence that epigenetic effects actually lead to the continuation of poverty in families, the findings in animal studies warrant further research in human cohorts to determine whether epigenetic changes may be a biological component of intergenerational transmission processes within a more complex model in humans including socio-economic and other contextual factors.
IV. What is Missing from Existing Human Studies on the Effects of Intergenerational Adversity?
Existing studies have been limited in their ability to test whether parents’ early childhood experiences independently affect the health and development of future offspring. To date, most studies and developmental theories involving biological processes have assumed that a child's own experiences of adversity (including in utero) are primarily influencing their future outcomes. Our review of epigenetic intergenerational transmission suggests that this lens is too narrow. In fact, intergenerational transmission of disadvantage through biological processes may extend to both previous generations and subsequent generations beyond the exposed child. Studies restricted to one generation cannot parse intergenerational cycling of disadvantage from that of a child's own experience of adversity. Studies that retrospectively collect childhood experiences from pregnant women or new parents inherently contain recall bias, an un-quantified, though potentially significant, limitation in previous studies (Reuben et al., 2016). In addition to multigenerational cohort studies with prospectively collected data on biological and psychological factors, there are at least three important data elements that need to be included in multi-generational epigenetic studies to more rigorously test the intergenerational transmission hypothesis in humans.
Data on the offspring during the perinatal period
Data on factors in the perinatal environment are needed to differentiate the effects of the early childhood experiences of the mother from the gestational environment she provides to her child. To fully understand intergenerational epigenetic inheritance, the influence of intrauterine exposures and a shared postnatal environment on epigenetic effects has to be studied to enable a clear distinction between these exposures and the potential for intergenerational epigenetic inheritance.
Studies in minority and disadvantaged groups
Existing multi-generation cohort studies tend not to address disadvantaged and minority youth. This is a critical gap because minority populations are at greater risk for experiencing adversity. It remains to be determined whether the association between parental childhood adversity and negative offspring outcomes, identified primarily within non-disadvantaged populations, is perpetuated in a similar way among disadvantaged children. Associations between stress, biomarkers, and detrimental fetal/infant outcomes may be stronger when the risk factors like poverty and violence are more severe. For example, a meta-analysis found that the association between prenatal depression and low offspring birth weight was stronger in low- and middle-income countries than in high-income ones (Grote et al., 2010). Another study found that most (60%) of IQ variance among 7-y.o. twins living in poverty was accounted for by environmental effects with genes having virtually no influence. However, among affluent families, the influence of genes was the most important with almost no environment effect (Turkheimer et al., 2003). Given these findings, cohort studies examining epigenetic intergenerational transmission should include a substantial sample of disadvantaged families, and future human research on intergenerational epigenetic transmission should examine variations in the level and timing (e.g. childhood vs. adulthood) of risk exposure.
Data on early brain development
The earliest stages of neural development are the foundation of future growth and development that will impact a wide range of outcomes from cognitive abilities and educational success to self-regulation, social competence, and mental and physical health outcomes later in life. New, intergenerational cohort studies that include imaging of newborn functional and structural connectivity in the brain may help to understand whether, and how, parental epigenetic marks influence health and disease susceptibility in offspring (van Otterdijk and Michels, 2016).
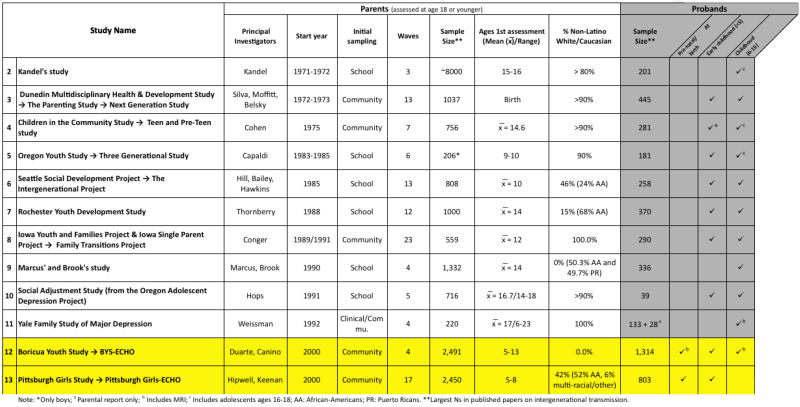
We conducted a PubMed search to identify existing cohorts that have tested or have the potential to test the intergenerational transmission hypothesis. The inclusion criteria were as follows: the original child generation assessed before the age of 18; offspring assessed at least once before the age of 18; parental childhood and offspring assessments include a developmental, cognitive, emotional or behavioral measure; a community or school sample (not solely a clinic-based sample); not a register-based sample; sample size for original generation >200, and first assessment conducted after the 1970s. Combinations of the following terms were used: cohort, intergeneration, transmission, mental health, mood, cognition, emotion, and development. Eleven cohorts were identified. These studies are listed in Figure 2.
Figure 2.
Studies on Intergenerational Transmission with Prospective Information on Parents when 18 or Younger and Offspring’s Childhood
*Studies identified since 1970 that assessed parents as children and later assessed their offspring in childhood using psychosocial/cognitive/emotional measures, a community or school sample of at least 200.
These studies include a predominance of White race/ethnicity samples (in 7 out of the 10 cohorts parents were > 80% whites). Existing studies’ school-based designs also reflect a selective sampling process that often excludes the most disadvantaged individuals who may not attend school. Additionally, none of the identified studies have collected information on early brain development, with the exception of the two discussed below. While the studies listed in Table 2 are valuable for expanding our understanding of intergenerational transmission of disadvantage in humans, research designs that incorporate the design elements described above are needed to more precisely study epigenetic intergenerational transmission in humans.
V. Intergenerational Transmission in Humans: The ECHO Opportunity
As the science of epigenetics in animal models is rapidly progressing, the time is now right to translate findings on intergenerational transmission into human studies with the potential to lead toward intervention models and public policy initiatives. The Environmental Influences on Children’s Health Outcomes (ECHO) study is a 7-year research initiative by the US National Institutes of Health aiming to increase understanding of the effects of environmental exposures on child health and development. ECHO leverages 84 existing cohorts, approximately 50,000 children, to prospectively investigate the role of early life exposures and underlying biological mechanisms in childhood health and disease. We highlight two cohort studies within ECHO (Figure 2, ccohorts 12 and 13) that contain the design study design features described above, providing a unique opportunity to test the intergenerational transmission of disadvantage hypothesis in humans, including on a molecular level using epigenetic analysis. These study designs can inform the development other research studies that aim to contribute to this line of research.
ECHO-Boricua Youth Study (ECHO-BYS)
A well-established population-based study of Puerto Rican youth, ECHO-BYS seeks to understand the lived experiences of Puerto Ricans in the US and Puerto Rico. The study first launched in 2001 and originally enrolled about 2,500 children between the ages of 5 and 13 in two sites: the South Bronx, NY, and San Juan, PR. Now in its fourth wave, the study is currently collecting data on G2, the children of the original child participants. Of the sample, 66% of families live below the Federal Poverty Level, and their experiences of neglect, physical and emotional abuse are four times higher than a nationally representative sample of adolescents. Latinos are one of the largest and most rapidly growing disadvantaged minority group in the United States (Davidson and Smith, 2015, Bureau, 2013). Among all Latino subgroups, Puerto Ricans are at higher risk for a range of negative mental and physical health outcomes (Alegria et al., 2006, Baca-Garcia et al., 2011, Daviglus et al., 2012), as well as for key psychosocial adjustment indicators (Treschan, 2010). ECHO-BYS includes prospective collection of childhood data in G1 parents from early in life, avoiding recall bias. In addition, prenatal risk factors are captured, controlling for gestational exposure to adversity. Data are available from three generations with rich indicators of multiple stressors and factors of disadvantage. Finally, ECHO-BYS includes the collection of fetal/infant behavioral assessments, blood and saliva samples for epigenetic analysis, and MRI on newborns to examine functional and structural connectivity (Posner et al., 2016).
ECHO- Pittsburgh Girls Study (PGS)
Another component study within ECHO, the PGS is a longitudinal, urban, population-based study of 2,450 girls over-sampled from disadvantaged neighborhoods, with 33% of sample households living in poverty (Hipwell et al., 2002, Keenan et al., 2010). Similar to BYS, the PGS precludes recall bias by prospective collection of childhood data in F1 parents from early childhood onwards. Several PGS sub-studies have now collected peri/postpartum data on F1, which include measures of the gestational environment and prenatal health behaviors, as well as early childhood data on F2, including MRI assessments to investigate early brain development. Particular strengths of the PGS include: a) multiple, annual assessments allowing detailed examination of the timing and duration of environmental exposures and patterns of developmental change (Seventeen consecutive annual assessments have been completed to date spanning G1 ages 5–8 years through 21–24 years). b) high sample retention (mean retention over 16 years is 89.5%, and 86.1% of the original sample (N=2,109) was interviewed in the last wave) and therefore good representation of the original population; c) approximately equal representation of African American and Caucasian participants; and d) measurement of broad domains of stress and disadvantage within family, peer, school and neighborhood contexts that can contribute to knowledge on the specificity of exposure effects.
The study designs of these two cohorts, within the sample of approximately 50,000 children in the ECHO initiative, the other cohorts of which have at least some of the same study design features, can be leveraged to understand specific epigenetic mechanisms. The sample size will allow for epigenome-wide analyses with the potential for more exploratory mechanistic analyses, the examination of specific CpG sites, and targeted gene and mechanism-specific investigations. While these two cohorts of the ECHO initiative illustrate the methodological elements that we highlight in this review as important for testing the epigenetic intergenerational transmission of disadvantage, other cohort studies also include a number of these study design features and can also contribute significantly. Studies that have repeated measures of DNA methylation and brain imaging, if extended to future generations, will also be an important advance in understanding epigenetic processes in the intergenerational transmission of factors related to disadvantage.
V. Conclusion
Current research from animal studies provides plausible epigenetic mechanisms by which stress associated with socioeconomic disadvantage could affect future generations, independent of and in interaction with those future generations’ life experiences. Longitudinal studies are now needed to further explore these mechanisms in humans. Such studies should nest analysis of epigenetic processes within a model that considers the complexity of human social conditions. Studies that isolate epigenetic mechanisms without considering social disadvantage run the risk of arriving at reductionist conclusions that overstate the causal role of epigenetics in the reproduction of poverty.
By describing epigenetics as a potential mechanism in the intergenerational transmission of poverty, we do not mean to imply that epigenetic changes alone, or even primarily, cause the intergenerational continuity of disadvantage. Rather, being able to identify a biological component of the complex, interwoven set of mechanisms may be another step toward an integrated understanding of these processes, allowing interventions to target and be measured at multiple levels— biological, psychological, behavioral.
Even though this review focuses on the possible role of epigenetic mechanisms in human cycles of socioeconomic disadvantage, we recognize that these mechanisms act in the context of socio-economic factors that cause families to remain in poverty, e.g. discrimination, economic conditions, educational and occupational opportunities, regional conditions, family and community resources. As such, epigenetic mechanisms must be considered not separately but rather in concert with this complex set of causal factors. Research in humans will need to determine the relative contributions and interaction/mediation of epigenetic effects and social, economic, and cultural factors, and that such considerations should be included in the design of human cohort studies and analyses of data from such studies.
Public health implications of intergenerational transmission of social disadvantage are enormous for the individual child and for society at large. Scientific findings from animal studies suggest that epigenetic intergenerational transmission could be a biological component of the complex set of social and biological processes involved in the reproduction of disadvantage across generations. Yet evidence in humans is lacking. Intergenerational cohort studies in disadvantaged groups that include key design features illustrated by ECHO-BYS and ECHO-PGS are crucial for elucidating epigenetic processes and leading toward integrated targets and measures for interventions meant to break the cycle of disadvantage for generations to come.
Key Points.
Socioeconomic disadvantage is reproduced across generations. Biological factors in utero, including maternal and offspring atypical HPA axis regulation, contribute to intergenerational transmission of socioeconomic disadvantage.
Research from animal models and some human studies suggests epigenetic mechanisms by which stress associated with socioeconomic disadvantage could affect future generations, independent of and in interaction with those future generations’ life experiences.
Evidence in humans is lacking. Current longitudinal studies in humans illustrate key research design features to test the intergenerational biological transmission of disadvantage.
Research in humans should examine epigenetics within context of socio-economic factors that cause families to remain in poverty, e.g. discrimination, economic conditions, educational and occupational opportunities, regional conditions, family and community resources.
Acknowledgments
This research has been supported by the National Institutes of Health, [T32 MH096724 (Wainberg, Arbuckle), UG3OD023328-01 (Duarte, Canino, Monk, Posner) MH56401 (Bird), DA033172 (Duarte), AA020191 (Duarte), MH098374 (Alegria, Canino, Duarte), HD060072 (Martins, Duarte, Canino), HL125761 (Suglia)]. The Pittsburgh Girls Study has been supported by the National Institute of Health [MH056630 (Loeber), DA012237 (Chung), and UG3 OD023244 (Hipwell, Keenan), the US Department of Justice Office of Juvenile Justice and Delinquency Prevention [2013-JF-FX0058 (Hipwell, Stepp)], FISA Foundation and the Falk Foundation. We are indebted to our ECHO colleagues and program staff for their support and feedback in the preparation of this manuscript. The authors have declared no competing or potential conflicts of interest.
Abbreviations
- DOHaD
Developmental Origins of Health and Disease
- HPA
hypothalamic–pituitary–adrenal
- miRNA
micro RNA
- snRNA
small non-coding RNA
- ECHO
Environmental Influences on Children’s Health Outcomes
- BYS
Boricua Youth Study
- PGS
Pittsburgh Girls Study
Footnotes
Conflict of Interest Statement:
On behalf of all authors, the corresponding author states that there is no conflict of interest.
The authors have declared no competing or potential conflicts of interest.
To note, we use the terms “socioeconomic disadvantage,” “disadvantage,” “poverty,” and “adversity” interchangeably throughout this review, as they reflect highly overlapping and interrelated concepts.
Contributor Information
Dr. Pamela Scorza, Department of Psychiatry, Columbia University Medical Center; New York State Psychiatric Institute, New York, NY
Dr. Cristiane S. Duarte, Department of Psychiatry, Columbia University Medical Center; New York State Psychiatric Institute, New York, NY
Dr. Alison E. Hipwell, Department of Psychiatry and Psychology, University of Pittsburgh, Pittsburgh, PA
Dr. Jonathan Posner, Department of Psychiatry, Columbia University Medical Center; New York State Psychiatric Institute, New York, NY
Dr. Ana Ortin, Department of Psychology, Hunter College, City University of New York, New York, NY
Dr. Glorisa Canino, School of Medicine, University of Puerto Rico, San Juan, Puerto Rico
Dr. Catherine Monk, Departments of Psychiatry and Obstetrics and Gynecology, Columbia University Medical Center; New York State Psychiatric Institute, New York, NY
References
- Alegria M, Canino G, Stinson FS, Grant BF. Nativity and DSM-IV psychiatric disorders among Puerto Ricans, Cuban Americans, and non-Latino whites in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry. 2006;67:56–65. doi: 10.4088/jcp.v67n0109. [DOI] [PubMed] [Google Scholar]
- Babenko O, Kovalchuk I, Metz GA. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neuroscience & Biobehavioral Reviews. 2015;48:70–91. doi: 10.1016/j.neubiorev.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Baca-Garcia E, Perez-Rodriguez MM, Keyes KM, Oquendo MA, Hasin DS, Grant BF, Blanco C. Suicidal ideation and suicide attempts among Hispanic subgroups in the United States, 1991–1992 and 2001–2002. Journal of psychiatric research. 2011;45:512–518. doi: 10.1016/j.jpsychires.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. The Wellcome Foundation Lecture, 1994. The fetal origins of adult disease. Proceedings of the Royal Society of London B: Biological Sciences. 1995;262:37–43. doi: 10.1098/rspb.1995.0173. [DOI] [PubMed] [Google Scholar]
- Barker DJ. The fetal and infant origins of adult disease. BMJ: British Medical Journal. 1990;301:1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Martyn CN. The maternal and fetal origins of cardiovascular disease. Journal of epidemiology and community health. 1992;46:8. doi: 10.1136/jech.46.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestor TH, Edwards JR, Boulard M. Notes on the role of dynamic DNA methylation in mammalian development. Proceedings of the National Academy of Sciences. 2015;112:6796–6799. doi: 10.1073/pnas.1415301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore ER, Putnam FW, Pressman EK, Rubinow DR, Putnam KT, Matthieu MM, Gilchrist MA, Jones I, O'connor TG. The Effects of Trauma History and Prenatal Affective Symptoms on Obstetric Outcomes. Journal of Traumatic Stress. 2016;29:245–252. doi: 10.1002/jts.22095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock J, Poeschel J, Schindler J, Börner F, Shachar-Dadon A, Ferdman N, Gaisler-Salomon I, Leshem M, Braun K, Poeggel G. Transgenerational sex-specific impact of preconception stress on the development of dendritic spines and dendritic length in the medial prefrontal cortex. Brain Structure and Function. 2016;221:855–863. doi: 10.1007/s00429-014-0940-4. [DOI] [PubMed] [Google Scholar]
- Bureau, U. S. C. Asians fastest-growing race or ethnic group in 2012, Census Bureau Reports 2013 [Google Scholar]
- Buss C, Entringer S, Moog NK, Toepfer P, Fair DA, Simhan HN, Heim CM, Wadhwa PD. Intergenerational transmission of maternal childhood maltreatment exposure: implications for fetal brain development. Journal of the American Academy of Child & Adolescent Psychiatry. 2017 doi: 10.1016/j.jaac.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Entringer S, Swanson JM, Wadhwa PD. The role of stress in brain development: The gestational environment’s long-term effects on the brain. Cerebrum: the Dana forum on brain science. 2012 Dana Foundation. [PMC free article] [PubMed] [Google Scholar]
- Buss C, Stalder T, Entringer S, Moog N, Kirschbaum C, Heim C, Wadhwa P. Maternal preconceptual and gestational stress, hair cortisol concentrations during pregnancy and newborn brain integrity. Psychoneuroendocrinology. 2016;71:72–73. [Google Scholar]
- Callaway E. Fearful memories haunt mouse descendants. Nature. 2013;1 [Google Scholar]
- Carone BR, Fauquier L, Habib N, Shea JM, Hart CE, Li R, Bock C, Li C, Gu H, Zamore PD. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 2010;143:1084–1096. doi: 10.1016/j.cell.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas E, Vavouri T. Sperm epigenomics: challenges and opportunities. Frontiers in genetics. 2014;5:330. doi: 10.3389/fgene.2014.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA. Epigenetic influence of social experiences across the lifespan. Developmental psychobiology. 2010;52:299–311. doi: 10.1002/dev.20436. [DOI] [PubMed] [Google Scholar]
- Champagne FA. Effects of stress across generations: why sex matters. Biol Psychiatry. 2013;73:2–4. doi: 10.1016/j.biopsych.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Chavez SL, Mcelroy SL, Bossert NL, De Jonge CJ, Rodriguez MV, Leong DE, Behr B, Westphal LM, Pera RAR. Comparison of epigenetic mediator expression and function in mouse and human embryonic blastomeres. Human molecular genetics. 2014 doi: 10.1093/hmg/ddu212. ddu212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung EK, Siegel BS, Garg A, Conroy K, Gross RS, Long DA, Lewis G, Osman CJ, Messito MJ, Wade R. Screening for social determinants of health among children and families living in poverty: a guide for clinicians. Current problems in pediatric and adolescent health care. 2016;46:135–153. doi: 10.1016/j.cppeds.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciabrelli F, Comoglio F, Fellous S, Bonev B, Ninova M, Szabo Q, Xuéreb A, Klopp C, Aravin A, Paro R. Stable Polycomb-dependent transgenerational inheritance of chromatin states in Drosophila. Nature genetics. 2017;49:876–886. doi: 10.1038/ng.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley D, Bennett NG. Is biology destiny? Birth weight and life chances. American Sociological Review. 2000:458–467. [Google Scholar]
- Curry A, Latkin C, Davey-Rothwell M. Pathways to depression: The impact of neighborhood violent crime on inner-city residents in Baltimore, Maryland, USA. Social science & medicine. 2008;67:23–30. doi: 10.1016/j.socscimed.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammann G, Teschler S, Haag T, Altmüller F, Tuczek F, Dammann RH. Increased DNA methylation of neuropsychiatric genes occurs in borderline personality disorder. Epigenetics. 2011;6:1454–1462. doi: 10.4161/epi.6.12.18363. [DOI] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson J, Smith RA. Asian Americans and Latinos: Similarities, differences and the population that intersects both groups 2015 [Google Scholar]
- Daviglus ML, Talavera GA, Avilés-Santa ML, Allison M, Cai J, Criqui MH, Gellman M, Giachello AL, Gouskova N, Kaplan RC. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA. 2012;308:1775–1784. doi: 10.1001/jama.2012.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daxinger L, Whitelaw E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nature Reviews Genetics. 2012;13:153–162. doi: 10.1038/nrg3188. [DOI] [PubMed] [Google Scholar]
- De Rooij SR, Costello PM, Veenendaal MV, Lillycrop KA, Gluckman PD, Hanson MA, Painter RC, Roseboom TJ. Associations between DNA methylation of a glucocorticoid receptor promoter and acute stress responses in a large healthy adult population are largely explained by lifestyle and educational differences. Psychoneuroendocrinology. 2012;37:782–788. doi: 10.1016/j.psyneuen.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Dietz DM, Laplant Q, Watts EL, Hodes GE, Russo SJ, Feng J, Oosting RS, Vialou V, Nestler EJ. Paternal transmission of stress-induced pathologies. Biological psychiatry. 2011;70:408–414. doi: 10.1016/j.biopsych.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Huang Y, Gutin B, Dong Y, Zhu H. Inflammation, Global DNA Methylation and Telomeres in Healthy Adolescents. The FASEB Journal. 2017;31:592.510–592.510. [Google Scholar]
- Eaton SA, Jayasooriah N, Buckland ME, Martin DI, Cropley JE, Suter CM. Roll over Weismann: extracellular vesicles in the transgenerational transmission of environmental effects. Epigenomics. 2015;7:1165–1171. doi: 10.2217/epi.15.58. [DOI] [PubMed] [Google Scholar]
- Edelman S, Shalev I, Uzefovsky F, Israel S, Knafo A, Kremer I, Mankuta D, Kaitz M, Ebstein RP. Epigenetic and genetic factors predict women's salivary cortisol following a threat to the social self. PloS one. 2012;7:e48597. doi: 10.1371/journal.pone.0048597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, English K. The environment of poverty: Multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child development. 2002;73:1238–1248. doi: 10.1111/1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- Evans RG, Barer ML, Marmor TR. Why are some people healthy and others not?: The determinants of the health of populations. Transaction Publishers; 1994. [Google Scholar]
- Ferguson H, Bovaird S, Mueller M. The impact of poverty on educational outcomes for children. Paediatrics & Child Health. 2007;12:701. doi: 10.1093/pch/12.8.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegood ED, Raver CC, Dejoseph ML, Blair C. Parenting in poverty: Attention bias and anxiety interact to predict parents’ perceptions of daily parenting hassles. Journal of family psychology. 2017;31:51. doi: 10.1037/fam0000291. [DOI] [PubMed] [Google Scholar]
- Franklin TB, Russig H, Weiss IC, Gräff J, Linder N, Michalon A, Vizi S, Mansuy IM. Epigenetic transmission of the impact of early stress across generations. Biological psychiatry. 2010;68:408–415. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- Gabory A, Roseboom TJ, Moore T, Moore LG, Junien C. Placental contribution to the origins of sexual dimorphism in health and diseases: sex chromosomes and epigenetics. Biology of sex differences. 2013;4:5. doi: 10.1186/2042-6410-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, Farinelli L, Miska E, Mansuy IM. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nature neuroscience. 2014;17:667–669. doi: 10.1038/nn.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershoff ET, Aber JL, Raver CC, Lennon MC. Income is not enough: Incorporating material hardship into models of income associations with parenting and child development. Child development. 2007;78:70–95. doi: 10.1111/j.1467-8624.2007.00986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertz J, Varley KE, Reddy TE, Bowling KM, Pauli F, Parker SL, Kucera KS, Willard HF, Myers RM. Analysis of DNA methylation in a three-generation family reveals widespread genetic influence on epigenetic regulation. PLoS Genet. 2011;7:e1002228. doi: 10.1371/journal.pgen.1002228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Archives of general psychiatry. 2010;67:1012–1024. doi: 10.1001/archgenpsychiatry.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker A, Raza S, Williamson K, Kolb B, Gibb R. Preconception paternal stress in rats alters dendritic morphology and connectivity in the brain of developing male and female offspring. Neuroscience. 2015;303:200–210. doi: 10.1016/j.neuroscience.2015.06.058. [DOI] [PubMed] [Google Scholar]
- Heard E, Martienssen RA. Transgenerational epigenetic inheritance: myths and mechanisms. Cell. 2014;157:95–109. doi: 10.1016/j.cell.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Focus. 2003;1:282–289. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Hipwell AE, Loeber R, Stouthamer-Loeber M, Keenan K, White HR, Kroneman L. Characteristics of girls with early onset disruptive and antisocial behaviour. Criminal Behaviour and Mental Health. 2002;12:99–118. doi: 10.1002/cbm.489. [DOI] [PubMed] [Google Scholar]
- Hunter RG, Murakami G, Dewell S, Seligsohn MA, Baker ME, Datson NA, Mcewen BS, Pfaff DW. Acute stress and hippocampal histone H3 lysine 9 trimethylation, a retrotransposon silencing response. Proceedings of the National Academy of Sciences. 2012;109:17657–17662. doi: 10.1073/pnas.1215810109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur SS, Cropley JE, Suter CM. Paternal epigenetic programming: evolving metabolic disease risk. Journal of Molecular Endocrinology. 2017;58:R159–R168. doi: 10.1530/JME-16-0236. [DOI] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Keenan K, Hipwell A, Chung T, Stepp S, Stouthamer-Loeber M, Loeber R, Mctigue K. The Pittsburgh Girls Study: overview and initial findings. Journal of Clinical Child & Adolescent Psychology. 2010;39:506–521. doi: 10.1080/15374416.2010.486320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T, Dias BG, Ressler KJ. Models of intergenerational and transgenerational transmission of risk for psychopathology in mice. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosin A, Casas E, Hidalgo-Carcedo C, Vavouri T, Lehner B. Transgenerational transmission of environmental information in C. elegans. Science. 2017;356:320–323. doi: 10.1126/science.aah6412. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Sakurai T, Miura F, Imai M, Mochiduki K, Yanagisawa E, Sakashita A, Wakai T, Suzuki Y, Ito T. High-resolution DNA methylome analysis of primordial germ cells identifies gender-specific reprogramming in mice. Genome research. 2013;23:616–627. doi: 10.1101/gr.148023.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, Acebron SP, Herbst J, Hatiboglu G, Niehrs C. Post-transcriptional Wnt signaling governs epididymal sperm maturation. Cell. 2015;163:1225–1236. doi: 10.1016/j.cell.2015.10.029. [DOI] [PubMed] [Google Scholar]
- Leshem M, Schulkin J. Transgenerational effects of infantile adversity and enrichment in male and female rats. Developmental psychobiology. 2012;54:169–186. doi: 10.1002/dev.20592. [DOI] [PubMed] [Google Scholar]
- Leventhal T, Brooks-Gunn J. The neighborhoods they live in: the effects of neighborhood residence on child and adolescent outcomes. Psychological bulletin. 2000;126:309. doi: 10.1037/0033-2909.126.2.309. [DOI] [PubMed] [Google Scholar]
- Lewis O. The culture of poverty. Poor Americans: How the white poor live. 1971:20–26. [Google Scholar]
- Ludwig J, Mayer S. "Culture" and the Intergenerational Transmission of Poverty: The Prevention Paradox. The Future of Children. 2006:175–196. doi: 10.1353/foc.2006.0017. [DOI] [PubMed] [Google Scholar]
- Margerison-Zilko CE, Strutz KL, Li Y, Holzman C. Stressors Across the Life-Course and Preterm Delivery: Evidence From a Pregnancy Cohort. Maternal and Child Health Journal. 2016:1–11. doi: 10.1007/s10995-016-2151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccreary JK, Erickson ZT, Metz GA. Environmental enrichment mitigates the impact of ancestral stress on motor skill and corticospinal tract plasticity. Neuroscience Letters. 2016a;632:181–186. doi: 10.1016/j.neulet.2016.08.059. [DOI] [PubMed] [Google Scholar]
- Mccreary JK, Truica LS, Friesen B, Yao Y, Olson DM, Kovalchuk I, Cross AR, Metz GA. Altered brain morphology and functional connectivity reflect a vulnerable affective state after cumulative multigenerational stress in rats. Neuroscience. 2016b doi: 10.1016/j.neuroscience.2016.05.046. [DOI] [PubMed] [Google Scholar]
- Mcewen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, Nasca C. Mechanisms of stress in the brain. Nature neuroscience. 2015;18:1353–1363. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melas PA, Wei Y, Wong CC, Sjöholm LK, Åberg E, Mill J, Schalling M, Forsell Y, Lavebratt C. Genetic and epigenetic associations of MAOA and NR3C1 with depression and childhood adversities. International Journal of Neuropsychopharmacology. 2013;16:1513–1528. doi: 10.1017/S1461145713000102. [DOI] [PubMed] [Google Scholar]
- Modir F, Salmani ME, Goudarzi I, Lashkarboluki T, Abrari K. Prenatal stress decreases spatial learning and memory retrieval of the adult male offspring of rats. Physiology & behavior. 2014;129:104–109. doi: 10.1016/j.physbeh.2014.02.040. [DOI] [PubMed] [Google Scholar]
- Moog NK, Buss C, Entringer S, Shahbaba B, Gillen DL, Hobel CJ, Wadhwa PD. Maternal Exposure to Childhood Trauma Is Associated During Pregnancy With Placental-Fetal Stress Physiology. Biological psychiatry. 2016;79:831–839. doi: 10.1016/j.biopsych.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morland K, Roux AVD, Wing S. Supermarkets, other food stores, and obesity: the atherosclerosis risk in communities study. American journal of preventive medicine. 2006;30:333–339. doi: 10.1016/j.amepre.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Na K-S, Chang HS, Won E, Han K-M, Choi S, Tae WS, Yoon H-K, Kim Y-K, Joe S-H, Jung I-K. Association between glucocorticoid receptor methylation and hippocampal subfields in major depressive disorder. PloS one. 2014;9:e85425. doi: 10.1371/journal.pone.0085425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroud N, Rutembesa E, Paoloni-Giacobino A, Mutabaruka J, Mutesa L, Stenz L, Malafosse A, Karege F. The Tutsi genocide and transgenerational transmission of maternal stress: epigenetics and biology of the HPA axis. The World Journal of Biological Psychiatry. 2014;15:334–345. doi: 10.3109/15622975.2013.866693. [DOI] [PubMed] [Google Scholar]
- Peschansky VJ, Wahlestedt C. Non-coding RNAs as direct and indirect modulators of epigenetic regulation. Epigenetics. 2014;9:3–12. doi: 10.4161/epi.27473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisu MG, Garau A, Olla P, Biggio F, Utzeri C, Dore R, Serra M. Altered stress responsiveness and hypothalamic-pituitary-adrenal axis function in male rat offspring of socially isolated parents. Journal of neurochemistry. 2013;126:493–502. doi: 10.1111/jnc.12273. [DOI] [PubMed] [Google Scholar]
- Popoli M, Yan Z, Mcewen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nature Reviews Neuroscience. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Cha J, Roy A, Peterson B, Bansal R, Gustafsson H, Raffanello E, Gingrich J, Monk C. Alterations in amygdala–prefrontal circuits in infants exposed to prenatal maternal depression. Translational Psychiatry. 2016;6:e935. doi: 10.1038/tp.2016.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglia MH, Lillard TS, Morris JP, Connelly JJ. Epigenetic modification of the oxytocin receptor gene influences the perception of anger and fear in the human brain. Proceedings of the National Academy of Sciences. 2015;112:3308–3313. doi: 10.1073/pnas.1422096112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: family social environments and the mental and physical health of offspring. Psychological bulletin. 2002;128:330. [PubMed] [Google Scholar]
- Reuben A, Moffitt TE, Caspi A, Belsky DW, Harrington H, Schroeder F, Hogan S, Ramrakha S, Poulton R, Danese A. Lest we forget: comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. Journal of Child Psychology and Psychiatry. 2016;57:1103–1112. doi: 10.1111/jcpp.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. Journal of Neuroscience. 2013;33:9003–9012. doi: 10.1523/JNEUROSCI.0914-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers AB, Morgan CP, Leu NA, Bale TL. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proceedings of the National Academy of Sciences. 2015;112:13699–13704. doi: 10.1073/pnas.1508347112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JR. An empirical study of intergenerational transmission of poverty in the United States. Social Science Quarterly. 1995;76:178–194. [Google Scholar]
- Rompala GR, Finegersh A, Homanics GE. Paternal preconception ethanol exposure blunts hypothalamic-pituitary-adrenal axis responsivity and stress-induced excessive fluid intake in male mice. Alcohol. 2016;53:19–25. doi: 10.1016/j.alcohol.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra-Rodríguez L, Feig LA. Chronic social instability induces anxiety and defective social interactions across generations. Biological psychiatry. 2013;73:44–53. doi: 10.1016/j.biopsych.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkies P, Miska EA. Small RNAs break out: the molecular cell biology of mobile small RNAs. Nature Reviews Molecular Cell Biology. 2014;15:525–535. doi: 10.1038/nrm3840. [DOI] [PubMed] [Google Scholar]
- Schulz AJ, Mentz G, Lachance L, Johnson J, Gaines C, Israel BA. Associations between socioeconomic status and allostatic load: effects of neighborhood poverty and tests of mediating pathways. American journal of public health. 2012;102:1706–1714. doi: 10.2105/AJPH.2011.300412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. The Journal of nutrition. 2010;140:304–310. doi: 10.3945/jn.109.112573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma U, Conine CC, Shea JM, Boskovic A, Derr AG, Bing XY, Belleannee C, Kucukural A, Serra RW, Sun F. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science. 2016;351:391–396. doi: 10.1126/science.aad6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff J, Boyce W, Cameron J, Duncan G, Fox N, Gunnar M, Thompson R. Excessive stress disrupts the architecture of the developing brain. Working Paper 3. 2009 Retrieved from www.developingchild.havard.edu.
- Shonkoff JP, Garner AS, Siegel BS, Dobbins MI, Earls MF, Mcguinn L, Pascoe J, Wood DL, Child COPAO, Health F, Committee on early childhood, A. Care D. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129:e232–e246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- Siklenka K, Erkek S, Godmann M, Lambrot R, Mcgraw S, Lafleur C, Cohen T, Xia J, Suderman M, Hallett M. Disruption of histone methylation in developing sperm impairs offspring health transgenerationally. Science. 2015;350:aab2006. doi: 10.1126/science.aab2006. [DOI] [PubMed] [Google Scholar]
- Skelin I, Needham M, Molina L, Metz G, Gruber A. Multigenerational prenatal stress increases the coherence of brain signaling among cortico–striatal–limbic circuits in adult rats. Neuroscience. 2015;289:270–278. doi: 10.1016/j.neuroscience.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Small M, Jarding DJ, Lamont M. Reconsidering Culture and Poverty. Annals of the American Academy of Political & Social Science. 2010;29:6–27. [Google Scholar]
- Soubry A. Epigenetic inheritance and evolution: a paternal perspective on dietary influences. Progress in biophysics and molecular biology. 2015;118:79–85. doi: 10.1016/j.pbiomolbio.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- Szyf M. How do environments talk to genes? Nature neuroscience. 2013;16:2–4. doi: 10.1038/nn.3286. [DOI] [PubMed] [Google Scholar]
- Szyf M. Nongenetic inheritance and transgenerational epigenetics. Trends in molecular medicine. 2015;21:134–144. doi: 10.1016/j.molmed.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Teperek M, Simeone A, Gaggioli V, Miyamoto K, Allen GE, Erkek S, Kwon T, Marcotte EM, Zegerman P, Bradshaw CR. Sperm is epigenetically programmed to regulate gene transcription in embryos. Genome research. 2016;26:1034–1046. doi: 10.1101/gr.201541.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson H. Health Depends on Dad's Sperm. New Scientist. 2016;230 [Google Scholar]
- Tobi EW, Lumey L, Talens RP, Kremer D, Putter H, Stein AD, Slagboom PE, Heijmans BT. DNA methylation differences after exposure to prenatal famine are common and timing-and sex-specific. Human molecular genetics. 2009;18:4046–4053. doi: 10.1093/hmg/ddp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treschan L. Latino youth in New York City: School, work, and income trends for New York's largest group of young people. Community Service Society of New York 2010 [Google Scholar]
- Turkheimer E, Haley A, Waldron M, D'onofrio B, Gottesman II. Socioeconomic status modifies heritability of IQ in young children. Psychol Sci. 2003;14:623–628. doi: 10.1046/j.0956-7976.2003.psci_1475.x. [DOI] [PubMed] [Google Scholar]
- Van Otterdijk SD, Michels KB. Transgenerational epigenetic inheritance in mammals: how good is the evidence? The FASEB Journal. 2016;30:2457–2465. doi: 10.1096/fj.201500083. [DOI] [PubMed] [Google Scholar]
- Waddington CH. Canalization of development and genetic assimilation of acquired characters. Nature. 1959;183:1654–1655. doi: 10.1038/1831654a0. [DOI] [PubMed] [Google Scholar]
- Wang W, Feng J, Ji C, Mu X, Ma Q, Fan Y, Chen C, Gao C, Ma X-C, ZHU F. Increased methylation of glucocorticoid receptor gene promoter 1 F in peripheral blood of patients with generalized anxiety disorder. Journal of Psychiatric Research. 2017a;91:18–25. doi: 10.1016/j.jpsychires.2017.01.019. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu H, Sun Z. Lamarck rises from his grave: parental environment-induced epigenetic inheritance in model organisms and humans. Biological Reviews. 2017b doi: 10.1111/brv.12322. [DOI] [PubMed] [Google Scholar]
- Yao Y, Robinson AM, Zucchi FC, Robbins JC, Babenko O, Kovalchuk O, Kovalchuk I, Olson DM, Metz GA. Ancestral exposure to stress epigenetically programs preterm birth risk and adverse maternal and newborn outcomes. BMC medicine. 2014;12:1. doi: 10.1186/s12916-014-0121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. Are different biological mechanisms involved in the transmission of maternal versus paternal stress-induced vulnerability to offspring? Biological psychiatry. 2011;70:402–403. doi: 10.1016/j.biopsych.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Daskalakis NP, Bierer LM, Bader HN, Klengel T, Holsboer F, Binder EB. Holocaust exposure induced intergenerational effects on FKBP5 methylation. Biological psychiatry. 2016;80:372–380. doi: 10.1016/j.biopsych.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Daskalakis NP, Lehrner A, Desarnaud F, Bader HN, Makotkine I, Flory JD, Bierer LM, Meaney MJ. Influences of maternal and paternal PTSD on epigenetic regulation of the glucocorticoid receptor gene in Holocaust survivor offspring. American Journal of Psychiatry. 2014;171:872–880. doi: 10.1176/appi.ajp.2014.13121571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidan H, Gaisler-Salomon I. Prereproductive stress in adolescent female rats affects behavior and corticosterone levels in second-generation offspring. Psychoneuroendocrinology. 2015;58:120–129. doi: 10.1016/j.psyneuen.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Zaidan H, Leshem M, Gaisler-Salomon I. Prereproductive stress to female rats alters corticotropin releasing factor type 1 expression in ova and behavior and brain corticotropin releasing factor type 1 expression in offspring. Biological psychiatry. 2013;74:680–687. doi: 10.1016/j.biopsych.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Reinberg D. Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes & development. 2001;15:2343–2360. doi: 10.1101/gad.927301. [DOI] [PubMed] [Google Scholar]