Abstract
A prominent target of the basal ganglia is the superior colliculus (SC) which controls gaze orientation (saccadic eye movement in primates) to an important object. This ‘object choice’ is crucial for choosing an action on the object. SC is innervated by the substantia nigra pars reticulata (SNr) which is controlled mainly by the caudate nucleus (CD). This CD-SNr-SC circuit is sensitive to the values of individual objects and facilitates saccades to good objects. The object values are processed differently in two parallel circuits: flexibly by the caudate head (CDh) and stably by the caudate tail (CDt). To choose good objects, we need to reject bad objects. In fact, these contrasting functions are accomplished by the circuit originating from CDt: the direct pathway focuses on good objects and facilitates saccades to them; the indirect pathway focuses on bad objects and suppresses saccades to them. Inactivation of CDt deteriorated the object choice, because saccades to bad objects was no longer suppressed. This suggests that the indirect pathway is important for object choice. However, the direct and indirect pathways for ‘object choice’, which aim at the same action (i.e., saccade), may not work for ‘action choice’. One possibility is that circuits controlling different actions are connected through the indirect pathway. Additional connections of the indirect pathway with brain areas outside the basal ganglia may also provide a wider range of behavioral choice. In conclusion, basal ganglia circuits are composed of the basic direct/indirect pathways and additional connections, and thus have acquired multiple functions.
Keywords: caudate tail, substantia nigra, saccade, reward value, monkey
Graphical Abstract
Parallel circuits from the caudate nucleus to the superior colliculus choose objects by their values, but selectively: anterior circuit using flexible values, posterior circuit using stable values.In the posterior circuit, the object choice is done by saccading to good objects (by direct pathway) and saccading a way from bad objects (by indirect pathway). In contrast, action choice may require interactions among multiple basal ganglia circuits, especially through indirect pathways, according to our hypothesis.
Introduction
The basal ganglia contribute to decision making (Hikosaka et al., 2017). A typical process of decision making is to choose an object (object choice) and then choose an action (action choice) to manipulate the object, which leads to a rewarding outcome (Kim & Hikosaka, 2015). Object choice is largely controlled by the caudate nucleus and its downstream circuits (Hikosaka et al., 2000), while action choice is largely controlled by the putamen and its downstream circuits (Samejima et al., 2005).
Neural mechanisms in the basal ganglia have been revealed especially for object choice. To choose an object, most animals first look at the object with a saccade (i.e., quick rotation of eyes and/or head) which is controlled mainly by the superior colliculus (SC). SC receives excitatory inputs from many other brain areas, including the frontal eye field (FEF), supplementary eye field (SEF), lateral intraparietal cortex (LIP), and cerebellum (May, 2006). In addition, SC receives GABAergic inhibitory inputs from the basal ganglia, specifically the substantia nigra pars reticulata (SNr) (Hikosaka & Wurtz, 1983c), which plays a unique role in controlling saccades (Hikosaka & Wurtz, 1983b). Since SNr neurons keep firing rapidly, SC neurons, especially pre-saccadic neurons, are tonically inhibited by SNr neurons. Reversible inactivation of SNr neurons (with muscimol injection) causes irrepressible saccades to the contralateral side in the monkey (Hikosaka & Wurtz, 1985b) and rat (Sakamoto & Hikosaka, 1989). This suggests that the basal ganglia are crucial for suppressing body movements. If this suppression does not work, many body parts would move persistently, which often occurs in patients with various kinds of basal ganglia disorders, including Huntington’s disease, dystonia, and hemiballismus (Denny-Brown, 1960).
In the normal condition, SC-projecting SNr neurons stop firing, which causes a disinhibition of SC neurons and facilitates saccades to the contralateral side (Hikosaka & Wurtz, 1983c). The inhibition of SNr neurons is caused by the GABAergic inhibitory input from the caudate nucleus (CD) (Yoshida & Precht, 1971; Hikosaka et al., 1993). Therefore, the CD-SNr-SC circuit is capable of facilitating a desired saccade (Hikosaka et al., 2000). Such a mechanism based on the sequential inhibitory connections is suitable for choice-based decision making. To reach the goal, CD neurons must be able to judge the value of each saccade or its target object. This question evolved to be multiple questions which are related to many aspects of brain functions, including short-term vs. long-term memory, voluntary vs. automatic behavior (Figure 1), direct vs. indirect pathway (Figure 2), and parallel circuits and their integration (Figure 4). We will discuss them below.
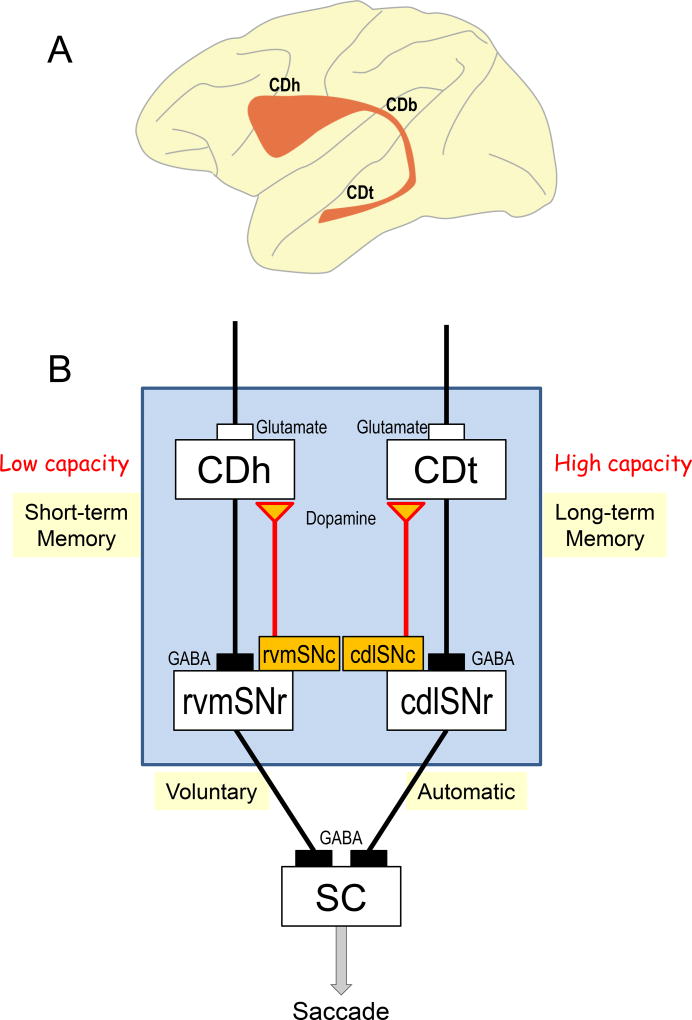
Figure 1.
Parallel circuits in the basal ganglia. A: Sagittal view of the caudate nucleus in the macaque monkey. It is roughly divided to caudate head (CDh), caudate body (CDb), and caudate tail (CDt). They are actually located beneath the cerebral cortex. B: Parallel circuits originating from CDh and CDt, both of which control saccadic eye movement by sending signals to the superior colliculus (SC) through the substantia nigra pars reticulata (SNr). These circuits are separate until they reach SC: CDh to rostral-ventral-medial part of SNr (rvmSNr), CDt to caudal-dorsal-lateral part of SNr (cdlSNr). Both circuits process reward values of visual objects, but in completely different ways: 1) CDh circuit uses short-term memories of object values and chooses objects flexibly, 2) CDt circuit uses long-term memories of object values and chooses objects stably. CDh and CDt are innervated by different groups of dopamine neurons in the substantia nigra pars compacta (rvmSNc and cdlSNc).
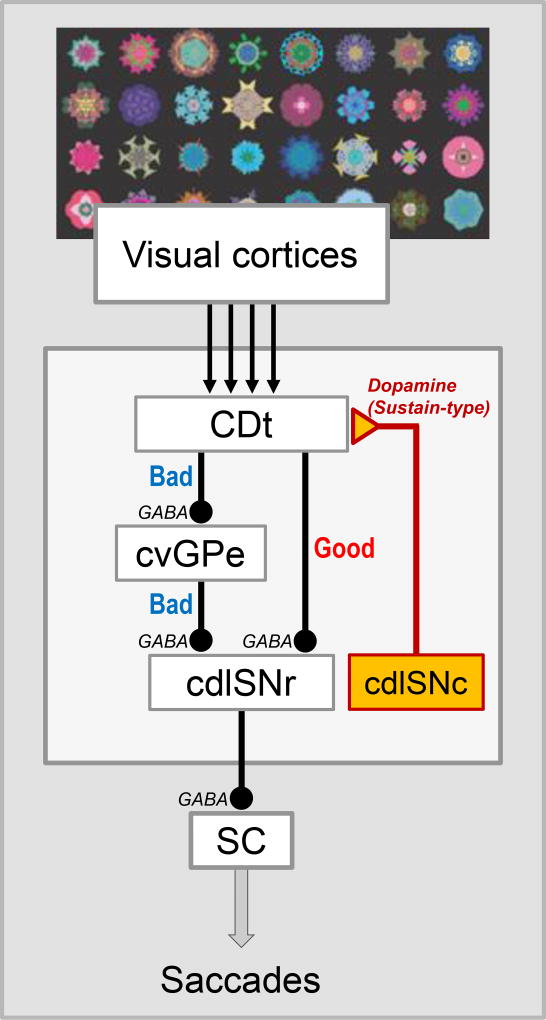
Figure 2.
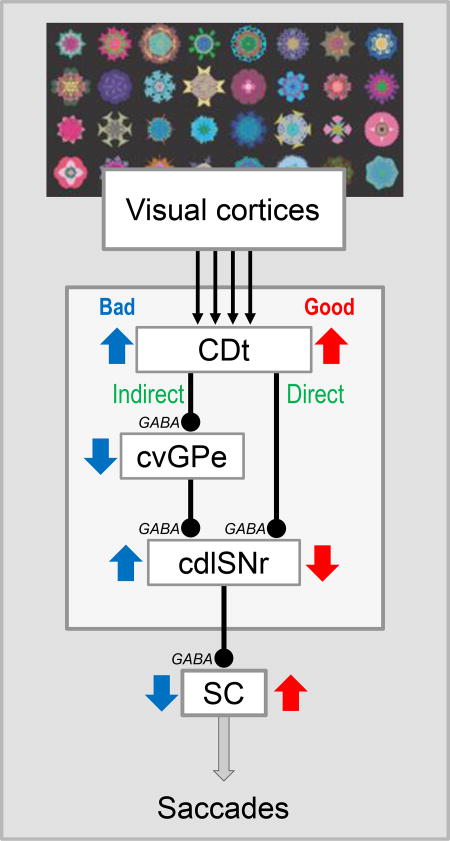
Direct and indirect pathways of CDt circuit. CDt processes visual objects, mainly based on inputs from visual cortical areas. The direct pathway is composed of two serial inhibitory connections: CDt to cdlSNr, cdlSNr-SC. Its function is to choose good objects. The indirect pathway is composed of three serial inhibitory connections: CDt-cvGPe, cvGPe-cdlSNr, cdlSNr-SC. Its function is to reject bad objects. cvGPe: caudal-ventral part of the globus pallidus externus.
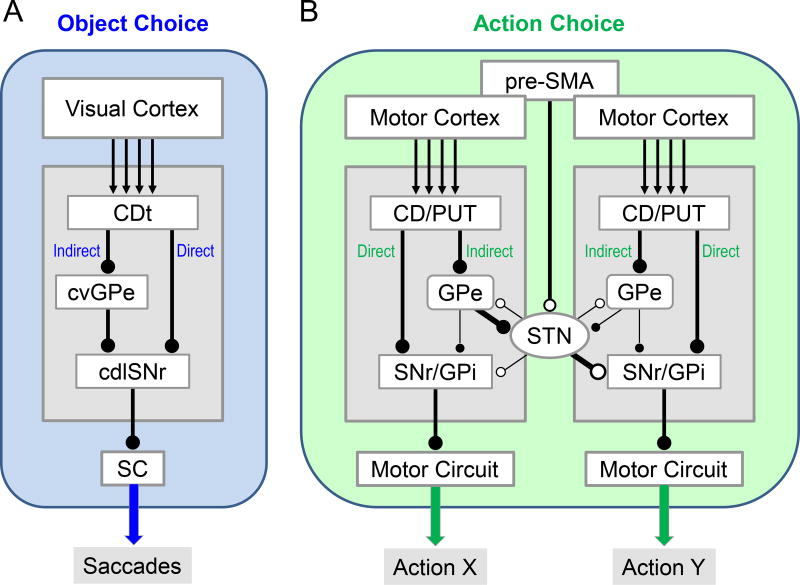
Figure 4.
Basal ganglia circuits for object choice (A) and action choice (B). A: CDt circuit (same as Figure 2). B: Hypothetical circuits for choosing action X while suppressing action Y. pre-SMA: pre-supplementary motor area. STN: subthalamic nucleus. GPi: globus pallidus internus. Black and white circles at line end (i.e., axon terminal) indicate inhibitory and excitatory connections, respectively.
Caudate head circuit
According to classical circuit models, the inputs to the basal ganglia are sent from the cerebral cortex to the striatum (including CD) and the outputs are sent from SNr or the globus pallidus internus (GPi) to the thalamus or midbrain motor circuits (Alexander & Crutcher, 1990). The striatum is thought to be involved in multiple functions, because different areas in the striatum receive inputs from different regions of the cerebral cortex and other areas (Kemp & Powell, 1970; Selemon & Goldman-Rakic, 1985). Differential functions may be maintained by parallel and separate circuits originating from different striatal areas (Alexander et al., 1986). Such parallel mechanisms work clearly in the CD-SNr-SC circuit (Figure 1).
A majority of neurons in the striatum are GABAergic projection neurons (medium spiny neurons) and become active only in limited conditions, otherwise quiet. The head of the caudate nucleus (CDh) contains several groups of quiet neurons which respond to (Hikosaka et al., 1989b) or predict sensory events (Hikosaka et al., 1989c), many of which can judge values. The predictive value-coding was revealed by 1DR (one-direction-rewarded) task (Kawagoe et al., 1998), which turned out to be very effective also for non-primate animals (Stephenson-Jones et al., 2016). On each trial of 1DR saccade task, the visual target is presented at a random position (among 2 or 4 positions), but the saccade to only 1 position is followed by a reward. The reward-associated position is fixed in a block of 20–30 trials, but changed in the next block.
Typically, the visual response of CDh neurons is enhanced if the target (by its position) predicts a reward; if no reward is predicted, the response is suppressed (Kawagoe et al., 1998). Some neurons show the opposite pattern. When the position-reward association is changed (in the next block of trials), their responses changed very quickly (< 5 trials). Most visual neurons in CDh (> 80%) encode such object-values. The value-coding of CDh neurons would change the generation of saccade through the CD-SNr-SC circuit: When a reward is expected, stronger excitation in most CDh neurons, stronger inhibition of SNr neurons, and stronger excitation (i.e., disinhibition) of SC neuron. This should lead to an earlier and faster saccade, which actually occurs consistently (Lauwereyns et al., 2002; Takikawa et al., 2002).
The expected value is based on the recent experience in 1DR task: If the saccade to the left (not right) object was followed by a reward recently, the subject would hope that the object appear on the left side. If the object-reward association has changed (which occurs in 1DR task), the expected value must be changed quickly. To perform this task, the subject needs to use short-term (or working) memory so that the object choice can change flexibly (Figure 1). Such a behavior is likely to be controlled consciously (Baddeley, 2003).
Since CDh projects to SNr (rather than GPi) (Parent et al., 1984), SNr neurons are likely to encode short-term memory. Notably, some of the SC-projecting SNr neurons decrease their activity selectively before memory-guided saccades (Hikosaka & Wurtz, 1983b). CDh also includes pre-saccadic neurons (i.e., increase activity before saccades), some of which are selective to memory-guided saccades (Hikosaka et al., 1989a). These data on 1DR task and memory-guided saccade suggest that CDh simply transfers predictive signals (based on short-term memory) to SNr, while reversing their polarity (i.e., excitation to inhibition).
However, this circuit model was incomplete. First, many SC-projecting SNr neurons showed simple visual responses unrelated to memory-guided saccades (Hikosaka & Wurtz, 1983a). Second, electrical stimulation of CDh often changed (mostly inhibited) the activity of memory-related SNr neurons, but rarely changed the activity of simple visual neurons (Hikosaka et al., 1993). Third, simple visual neurons were located in the caudal-dorsal-lateral part of SNr (cdlSNr) (Hikosaka et al., 1993). Anatomically, it was known that CDh projects to the rostral part of SNr, except for cdlSNr (Smith & Parent, 1986). Instead, cdlSNr receives inputs from the tail of the caudate nucleus (CDt) (Saint-Cyr et al., 1990; Kim et al., 2017). These results suggested that CD has at least two separate circuits (Figure 1B).
Caudate tail circuit
The caudate nucleus is divided into three parts (i.e., head, body, tail) (Figure 1A), especially in primates (humans and monkeys), although there are no clear borders. Yet, CDh and CDt receive inputs from mostly different areas (e.g., frontal cortex to CDh, temporal cortex to CDt) (Yeterian & Van Hoesen, 1978). Even when one area projects to both CDh and CDt, each neuron projects to either CDh or CDt (Griggs et al., 2017).
GABAergic projection neurons (medium spiny neurons) in CDt are also very quiet, and most of them show visual responses with two prominent features: 1) object-selective, 2) spatially selective (Yeterian & Van Hoesen, 1978). When many fractal objects are presented one at a time, CDt neurons respond to only a few of them with different magnitudes. This is the case even when the objects are completely new. These results suggest that each CDt neuron receives inputs randomly from some visual neurons in the inferotemporal cortex (ITC) and other areas. As a population, however, CDt neurons respond to all objects.
Their visual responses are also spatially selective, with receptive fields mostly somewhere in the contralateral hemifield (Yamamoto et al., 2012). They often do not respond to objects positioned at the center. This suggests that CDt neurons receive input from a wide part of ITC where visual topographic map is represented (Boussaoud et al., 1991; Yasuda et al., 2010). The spatial selectivity turned out to be critical for the function of CDt-circuit, as explained below.
It was found that the CDt-circuit controls saccades strongly and selectively. First, weak electrical stimulation (often 20 uA) in CDt evokes saccades to the position which is close to the receptive fields of adjacent CDt neurons (Yamamoto et al., 2012). Such weak stimulation causes a strong inhibition in cdlSNr neurons, and a majority of cdlSNr neurons are antidromically activated by stimulation of SC (Yasuda & Hikosaka, 2015).
These results suggest that CDt controls saccades selectively using CDt-cdlSNr-SC circuit (Figure 1). Importantly, this circuit is basically separate from CDh-circuit, because CDt stimulation and CDh stimulation inhibit separate groups of SNr neurons. Moreover, these groups are separated spatially: CDt-inhibited neurons in cdlSNr, CDh-inhibited neurons in the rostral-ventral-medial part of SNr (rvmSNr) (Yasuda & Hikosaka, 2015). Therefore, there are, at least, two parallel circuits in the basal ganglia that control saccades (Figure 1B). Whether these circuits connect to the same or different neurons in SC remains a question.
However, it is still unclear whether the parallel circuits are completely separated. CDt or CDh stimulation sometimes causes an excitation in either cdlSNr or rvmSNr neurons, not consistently following CDt-cdlSNr or CDh-rvmSNr circuit. Such an excitation may be induced by the indirect pathway which is mediated by the globus pallidus externus (GPe) and/or subthalamic nucleus (STN) (Yasuda & Hikosaka, 2015). This is an important question which will be discussed later.
Stable value-coding in CDt circuit
We previously found that cdlSNr neurons are characterized by their simple visual responses (see above). Therefore, we predicted that CDt-cdlSNr-SC circuit simply transfers the visual information from ITC. To test this hypothesis, we used a flexible object–value task (Yasuda et al., 2012): In one block of trials, object A (not B) is associated with a reward; in the other block, object B (not A) is associated with a reward. This is equivalent to 1DR task. When two objects (A and B) are presented at the same time, the monkey almost always chooses whichever object is currently associated with a reward. Yet, the response of a CDt neuron (Kim & Hikosaka, 2013; Yamamoto et al., 2013) or a cdlSNr neuron (Yasuda et al., 2012) to a fractal object is invariable across trials, even when the predicted reward outcome has been reversed. Thus, neither CDt nor cdlSNr is sensitive to the predictive reward value. These results appear to confirm the hypothesis described above: CDt-circuit is insensitive to value memory. This turned out to be wrong.
In real life, there are so many objects and individuals (e.g., food, friends, family …) and their values do not change often or quickly. But to learn their values, we may need to experience these objects many times. Based on this thought, we tried a new procedure: stable value learning (Yasuda et al., 2012). The subject (monkey) viewed many fractal objects, half associated with a large reward (to be called ‘good objects’) and the other half with a small reward (‘bad objects’). This is done repeatedly across days. We then presented some of the fractal objects and let the monkey view (or not view) them. Even though no reward was delivered by this free viewing, the monkey tended to look at good objects and avoid bad objects. Notably, the free viewing bias developed slowly and took about 5 days of object-reward association before reaching the maximum bias.
We then discovered that the visual responses of CDt neurons (Kim & Hikosaka, 2013; Yamamoto et al., 2013) and cdlSNr neurons (Yasuda et al., 2012) developed biases in response to good vs. bad objects. Most CDt neurons are more excited by good objects than bad objects; some developed the opposite bias (Kim & Hikosaka, 2013). However, the bias is often unclear for individual neurons, because the object-selectivity remained robust. In contrast, cdlSNr neurons discriminated good objects and bad objects completely: inhibition by good objects vs. excitation by bad objects (Yasuda et al., 2012). The inhibition of cdlSNr neurons would cause a disinhibition of SC neurons, thus facilitating saccades to good objects; the excitation of cdlSNr neurons would cause an enhanced inhibition of SC neurons, thus suppressing saccades to bad objects. The value-coding also developed slowly in about 5 days. These features are exactly how monkeys behave during free viewing task. This slow learning explains the lack of flexible value coding in both CDt and cdlSNr neurons, described above.
In addition, CDt and cdlSNr neurons have three surprising features: high-capacity memory, long-term memory, automatic peripheral vision (Figure 1B). Monkeys learned so many fractal objects (so far up to 500 objects) using the stable value learning task, and became able to classify them into good and bad objects during the free viewing task (Yasuda et al., 2012; Kim & Hikosaka, 2013; Yamamoto et al., 2013). An equivalent classification occurs in individual neurons as well, especially in cdlSNr neurons: inhibition by all good objects and excitation by all bad objects (Yasuda et al., 2012).
Moreover, the behavioral and neuronal value coding is very stable. We sometimes stopped showing a group of good-bad objects for a long time (e.g., >100 days) and then presented them suddenly to the monkey. cdlSNr neurons were still inhibited by good objects and the monkey’s gaze was attracted by them; cdlSNr neurons were excited by bad objects and monkey’s gaze avoided them (Yasuda et al., 2012). This occurs even when the monkey viewed many other objects during the long delay period. These results indicate that the value coding is based on long-term memories of object values.
Automatic peripheral vision is also critical. The visual responses of CDt and cdlSNr neurons as well the monkey’s gaze during free viewing are biased even when the reward outcome is absent or incongruent (Yasuda et al., 2012; Kim & Hikosaka, 2013; Yamamoto et al., 2013). These processes occur even when the objects are located in periphery. This is surprising because it is thought that peripheral vision is too poor to discriminate objects (Strasburger et al., 2011). In any case, CDt-cdlSNr-SC circuit discriminate objects automatically by their stable value, even when they are located in periphery. Notably, the intermediate part of the caudate nucleus (caudate body, CDb) (Figure 1A) shares the features of CDh and CDt: it contains neurons with flexible values and neurons with stable values (Kim & Hikosaka, 2013).
Object skill
The importance of CDt-cdlSNr-SC circuit was shown by a visual search task (Ghazizadeh et al., 2016b). One good object and several bad objects are chosen randomly from >100 good/bad objects and presented simultaneously. A large reward is delivered if the monkey looks at the good object; a small reward if one of the bad object is chosen. After the repeated object-reward association, the monkey became able to make a saccade, often directly, to the good object presented in periphery. The saccade reaction time is sometimes less than 150 ms.
This behavior is crucial in real life. There are many objects around us, many of which are unexpectedly present and their locations are unexpected. Without automaticity, we would need to explore all of these objects and evaluate them. CDt-cdlSNr-SC circuit would thus enable us (and animals) to obtain a larger amount of reward per time, which is called ‘object skill’ (Hikosaka et al., 2013).
Notably, the uniqueness of CDt was demonstrated previously by Mort Mishkin and colleagues (Mishkin et al., 1984), which is related to the multiplicity of memory. They invented a memory task in which multiple pairs of objects are presented sequentially and the subject chooses the good object for each pair. In the monkey, the learning of this task was impaired by local lesions of CDt (Fernandez-Ruiz et al., 2001). Humans with lesions in the hippocampal area can learn this task, although their episodic memories are devastated (Bayley et al., 2005). These results confirmed the theory that memory is classified into, at least, two types: conscious memory (which may be called declarative or explicit memory) and subconscious memory (which may be called non-declarative or implicit memory).
Roles of DA neurons in reward value memory
How then do CDh-circuit and CDt-circuit acquire reward value memory in different manners? It is known that dopamine (DA) controls synaptic plasticity in the basal ganglia, especially cortico-striatal synapses (Reynolds & Wickens, 2002; Surmeier et al., 2007). In an experiment using 1DR task, dopamine D1 antagonist injected into the visual region of CDh delayed the saccade to the reward-associated object, whereas dopamine D2 antagonist in CDh delayed the saccade to the reward-unassociated object (Nakamura & Hikosaka, 2006). These results suggest that dopamine (DA) neurons contribute to the flexibility of CDh neurons, according to a computational model (Hong & Hikosaka, 2011). This model is based on the discovery that DA neurons encode reward prediction error (RPE): excitation (or inhibition) if reward or its prediction is higher (or lower) than expected based on the recent experience (Schultz, 1998).
We then found that different groups of DA neurons innervate CDh and CDt: rostral-ventral-medial part of substantia nigra pars compacta (rvmSNc) to CDh, caudal-dorsal-lateral part of substantia nigra pars compacta (cdlSNc) to CDt (Kim et al., 2014). Importantly, CDt-projecting DA neurons do not encode RPE, but instead encode stable values, similarly to CDt and cdlSNr neurons (Kim et al., 2015). The stable value coding develops earlier in CDt-projecting DA neurons than CDt/cdlSNr neurons, suggesting that the DA neurons contribute to the long-term memory of object value in CDt-cdlSNr circuit. On the other hand, CDt-cdlSNr circuit is likely to contribute to the discrimination of visual objects in the DA neurons, since the electrical stimulation of CDt activates CDt-projecting DA neurons orthodromically (as well as antidromically), which may be mediated by the axon collaterals of cdlSNr neurons (Fig. 5 in Kim & Hikosaka, 2015).
Notably, cdlSNc include DA neurons that are excited by both rewarding and dangerous objects (salience type), while DA neurons in rvmSNc are inhibited by dangerous objects (value type) (Matsumoto & Hikosaka, 2009). In fact, gaze is attracted automatically to dangerous object as well as rewarding objects (Ghazizadeh et al., 2016a). However, it is still unclear whether CDt/cdlSNr neurons are sensitive to dangerous objects.
Direct & Indirect pathways
The direct and indirect pathways are thought to control motor behavior in opposite manners: facilitation by the direct pathway and inhibition by the indirect pathway (Hikosaka et al., 2000). This idea is originally based on the neuronal connections (Smith et al., 1998). The output of the direct pathway would be disinhibition induced by two serial inhibitory connections (e.g., CD-SNr and SNr-SC). The output of the indirect pathway would be an enhanced inhibition induced by three serial inhibitory connections (e.g., CD-GPe, GPe-SNr, SNr-SC). This scheme is confirmed by recent studies in which the direct and indirect pathways were activated selectively (Kravitz et al., 2010).
These data raise a further question: Do the direct and indirect pathways aim at the same behavior (e.g., hand movement) or different behaviors (e.g., hand and leg movements)? No clear answer has been provided so far, except for the CDt-circuit (Figure 2). Anatomical studies showed that CDt projects to the cdlSNr and the caudal-ventral GPe (cvGPe), and does so locally and densely (Saint-Cyr et al., 1990; Kim et al., 2017). cvGPe then projects to cdlSNr locally and densely (Kim et al., 2017). Electrophysiological studies confirmed that these connections are inhibitory: direct pathway (CDt-cdlSNr) (Yasuda & Hikosaka, 2015) and indirect pathway (CDt-cvGPe, cvGPe-cdlSNr) (Kim et al., 2017) (Figure 2). Interestingly, a very similar set of the direct and indirect pathways was already shown in the rat (Smith & Bolam, 1991). The role of STN in the indirect pathway is less clear: some STN neurons project to cdlSNr, but there is no clear data showing the connection between STN and cvGPe (Amita et al., 2016). These data suggest that the direct and indirect pathways of CDt, both, aim at saccadic eye movement by acting on SC.
Another important question is about the transformation of information to behavior. Do the direct and indirect pathways transform the same or different types of information to behavior? This was shown clearly for the CDt-circuit. First, a majority of neurons in each region (CDt, cdlSNr, cvGPe) are visually sensitive, responding to many visual (fractal) objects selectively (selectivity higher in CDt than cdlSNr, cvGPe) (Yamamoto et al., 2012; Yasuda et al., 2012; Kim & Hikosaka, 2013; Kim et al., 2017). Furthermore, a majority of these neurons encode stable values based on long-term object value memories (Kim & Hikosaka, 2013; Yasuda & Hikosaka, 2015) (Yamamoto et al., 2013; Kim et al., 2017). These data details the above statement: The direct and indirect pathways of CDt aim at saccadic eye movement based on the stable values of visual objects.
The final question is: What are the behavioral goal of the direct and indirect pathways? Overall, bad objects are encoded by the indirect pathway, whereas good objects are encoded by the direct pathway (Kim et al., 2017). Typically, bad objects inhibit cvGPe neurons, disinhibit cdlSNr neurons, and then inhibit SC neurons, thus suppressing saccades to the bad objects. In contrast, good objects inhibit cdlSNr neurons and then disinhibit SC neurons, thus facilitating saccades to the good objects. Then, the final conclusion is: CDt-circuit suppresses saccades to bad objects using the indirect pathway and facilitates saccades to good objects using the direct pathway.
These data suggest that the projection neurons in CDt are divided into two groups (Figure 2): 1) cvGPe-projecting neurons (which prefer bad object), and 2) cdlSNr-projecting neurons (which prefer good objects). This is supported anatomically by injecting different tracers in cvGPe and cdlSNr: Many neurons in CDt were retrogradely labeled, but mostly from either cvGPe or cdlSNr (Amita et al., 2016). Physiologically, CDt contains good-preferring neurons (i.e., excited more by good than bad objects) and bad-preferring neurons (Kim & Hikosaka, 2013). However, good-preferring neurons are more common than bad-preferring neurons, although cvGPe-projecting neurons are as common as cdlSNr-projecting neurons, anatomically.
Rejection of Bad objects by CDt-circuit
We then asked whether CDt-circuit is necessary for choosing objects automatically based on their stable values. To address this question, we reversely inactivated CDt neurons by locally injecting a GABA agonist (muscimol) (Kim & Hikosaka, 2013) (Figure 3B). This method is useful because functions of different brain areas can be examined repeatedly in the same animal (Hikosaka & Wurtz, 1985a).
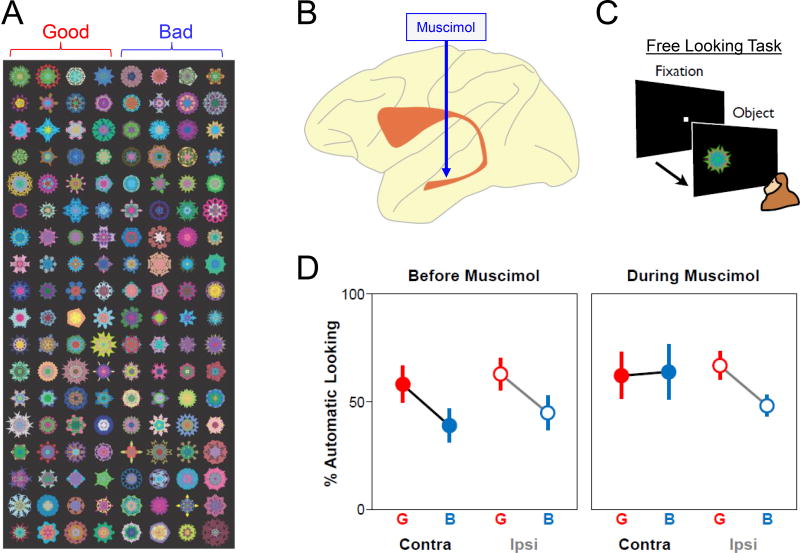
Figure 3.
Inactivation of CDt eliminates the automatic choice of good objects. A: Two groups of fractal objects, one associated with a large reward (good) and the other associated with a small reward (bad). After viewing many of such objects (n>100) repeatedly across several days, the gaze of the subject (macaque monkey) continues to be attracted by good objects automatically. B: Inactivation of CDt neurons by a local injection of muscimol (GABA agonist). C: Free looking task. After gaze fixation at the central spot, on some trials, a randomly chosen fractal object is presented on the left or right side. The subject may or may not make a saccade to it. This is automatic because no reward is delivered. D: The percentage of automatic saccade before (left) and during (right) muscimol injection, shown separately for good (G) and bad (B) objects, and contralateral (Contra) and ipsilateral (Ipsi) saccades.
To test the effect of stable values, we used ‘free looking task’ (Figure 3C). The subject (monkey) performed a simple fixation task to obtain reward. In some trials, a fractal object appeared early on the left or right side. The monkey often looked at the object spontaneously, but no reward was delivered after the saccade. The object was chosen randomly from many fractals (>100) (Figure 3A) which the monkey had viewed previously in association with a large or small reward (i.e., good or bad object). Normally, the frequency of the free looking was higher for good objects than bad objects (Kim & Hikosaka, 2013) (Figure 3D, left), as shown in the free viewing task (Yasuda et al., 2012; Yamamoto et al., 2013).
After muscimol injection in CDt, this gaze bias disappeared (Figure 3D, right), but this happened only when the object was presented on the side contralateral to the inactivated CDt (Figure 3D, right) (Kim & Hikosaka, 2013). Bad object is critical for this change: The frequency of saccades to bad objects increased, whereas the frequency of saccades to good objects showed no change. This result suggests that saccades to bad objects are suppressed only by the indirect pathway of CDt-circuit, but saccades to good objects are facilitated by the direct pathway as well as other brain areas. The dysfunction is contralateral-selective because cvGPe neurons (as well as CDt neurons) respond selectively to contralateral objects (Kim & Hikosaka, 2013). This conclusion raises an important concept, as shown below.
In real life, we (and animals) are surrounded by so many objects (including other animals), but we need to focus on a small number of the objects at each time point (Duncan, 1980). This is done by attention and/or gaze (Hikosaka et al., 2013). To understand the underlying neural mechanism, most studies aim at the neural and behavioral (e.g., perceptual) responses to the focused (or attended) objects. Then, how should the brain deal with other (unfocused) objects? There have been few studies that addressed this question (Bichot & Schall, 1999). Theoretically, the brain needs to activate two contrasting mechanisms: 1) aim attention/gaze to the focused objects, 2) avert attention/gaze from the unfocused objects. This is exactly what the direct and indirect pathways of CDt-circuit are doing. Moreover, the second mechanism (avert attention/gaze from the unfocused objects) is essential for CDt-circuit, and probably also for other circuits (e.g., attention circuit).
There is another important factor: position. For example, to facilitate a saccade to the left side, it is important to suppress saccades to the right side. This is actually what a previous study on cats indicated (Jiang et al., 2003): some SNr neurons (e.g., left side) are excited by contralateral visual stimuli (right side) and project to the contralateral SC (right side), thus suppressing saccades to the left side. This would act as the rejection of non-targeting saccades based on their positions, rather than their values. If the excitation of such SNr neurons is mediated by the indirect pathway (i.e., disinhibition), this part of the indirect pathway would be activated by good objects, rather than bad objects. This is an interesting possibility and should be investigated in near future.
General mechanisms of indirect pathway – hypothesis
Discussion so far suggests that a fundamental role of the direct-indirect pathways is to choose objects based on the same action (saccade) (Figure 4A). However, this does not indicate that the direct and indirect pathways work only within a local circuit (i.e., CDt-circuit) (Joel & Weiner, 1997). In fact, CDt-circuit and CDh-circuit seem to interact with each other by extending the indirect pathway to the other (Yasuda & Hikosaka, 2015).
Moreover, once an important object is chosen, an appropriate action needs to be chosen. For example, if a fruit is found while walking, we need to reach our hand to the fruit. It is likely that different circuits in the basal ganglia contribute to different body movements (Alexander & Crutcher, 1990), including walking and reaching (Figure 4B). In order to control such a behavioral switching (‘walking’ then ‘reaching’), these two circuits must interact with each other. The indirect pathway would be suitable for this goal, according to our circuit model (below).
We hypothesize that the two motor circuits (for action X and Y) are connected through the indirect pathway which is mediated by GPe and STN (Figure 4B). It is known that STN receives inputs from GPe and send outputs to SNr, GPi, or GPe, which was shown anatomically (Parent & Hazrati, 1995; Smith et al., 1998; Bolam et al., 2000) and electrophysiologicaly (Nambu et al., 2000; Tachibana et al., 2008). However, the connections of STN as well as GPe are often not restricted to a particular basal ganglia circuit (Joel & Weiner, 1997; Parent et al., 2000). If STN receives inputs mainly from GPe in X-circuit and sends output mainly to SNr/GPi in Y-circuit (Figure 4B), action Y would be suppressed when action X is facilitated. This mechanism would be important to choose action X, because action Y needs to be suppressed. It is also critical for sequential behavior: action Y (e.g., walking), then action X (e.g., reaching). According to Joel & Weiner (1997), this is called ‘open indirect pathway’ (Figure 4B), instead of ‘closed indirect pathway’ (Figure 4A). This ‘open indirect pathway’ mechanism suggests that two groups of striatal neurons, which control the direct and indirect pathways respectively, may be active simultaneously. This actually occurs in natural behavior (Cui et al., 2013; Tecuapetla et al., 2016).
However, the across-circuit interaction must occur in different combinations depending on the goal of natural behavior. To this end, the efficacy of individual STN connections needs to be changed depending on the context. Mechanistically, this requires connections with many brain areas, with which various kinds of contextual information can be shared. In fact, STN has such connections, for example, with the striatum (Nakano et al., 1990; Smith et al., 1990), PPTg (Mena-Segovia et al., 2004; Kita & Kita, 2011), and the frontal cortex (Monakow et al., 1978; Nambu et al., 1996; Inase et al., 1999; Haynes & Haber, 2013). STN is even connected to the cerebellum (Bostan et al., 2010). Such capability may not be restricted to STN and may be shared by GPe which also has connections with many areas (Bolam et al., 2000), including the striatum (Kita et al., 1999; Sato et al., 2000), and the thalamic reticular nucleus (Hazrati & Parent, 1991; Gandia et al., 1993).
By the way, a main action of STN is the suppression of motor behavior, since its lesion causes involuntary movements (hemiballismus) (Crossman et al., 1984). A mechanism to activate STN could then stop ongoing movements. For example, while we are doing something in a usual way (e.g., eating), something unexpected may happen suddenly (e.g., door open sound) and we typically stop the ongoing movement (e.g., eating). Then, we can switch to another behavior (e.g., gaze orientation) and/or initiate a new thought. This is caused, at least partially, by the direct excitatory inputs from the pre-supplementary motor area (pre-SMA) to STN (Isoda & Hikosaka, 2007; 2008) (Figure 4B), which is called ‘hyperdirect’ pathway (Nambu et al., 2002).
The role of STN in switching to the thought process (mentioned above) is generally important. For example, when a fox tries to catch a rat, the fox often stops moving until the rat becomes reachable. During the waiting period, most movement circuits should be suppressed while ‘thought’ circuits should be activated. An equivalent process actually works before memory-guided saccades (Yasuda & Hikosaka, 2017). After a visual cue stimulus appears to indicate the future target location, the subject must keep suppressing a saccade while remembering the position until the saccade is allowed. This may be caused by the interaction between a ‘thought’ circuit and a motor (saccade) circuit. The indirect pathway of the ‘thought’ circuit is connected, through STN, to SNr neurons in CD-SNr-SC circuit. In fact, most SNr neurons related to memory-guided saccades are excited in response to the visual cue (due to the input from STN) and later inhibited before the saccade. This reflects the process: first, wait and think, and then make the targeting saccade.
Conclusion
The basal ganglia are a large subcortical area and have multiple functions, which are thought to be controlled by multiple circuits. Notably, each circuit, which controls a particular action, is composed of two parallel pathways: the direct pathway that facilitates the action and the indirect pathway that suppresses the action. Our studies on CD-SNr-SC circuit confirmed these schemes. Depending on the origin within the caudate nucleus, CD-SNr-SC circuit is divided into two parallel circuits: CDh-circuit and CDt-circuit. Both of these circuits choose a good object and guide the same action – saccadic eye movement – to the good object. The object choice by CD-SNr-SC circuit is based on the previous experiences of objects (learning) which is supported by DA neurons.
Why then are the two circuits (CDh-circuit and CDt-circuit) necessary for choosing good objects? Learning of CDh-circuit is based on recent experiences (short-term memory) and its output can be changed flexibly. In contrast, learning of CDt-circuit is based on old-historical experiences (long-term memory) and its output is maintained stably. Their outputs are often the same, but can be different. This is important because the correct choice varies in the real world. Accordingly, CDh- and CDt-circuits are supported by spatially-functionally different groups of DA neurons.
The main function of these parallel CD-SNr-SC circuits is object choice. For this purpose, both the direct and indirect pathways discriminate visual objects by their values and thereby control saccades. While the direct pathway focuses on good objects and facilitates saccade to them, the indirect pathway focuses on bad objects and suppresses saccade to them. These two functions together, especially the saccade suppression by the indirect pathway, are critical for choosing good objects among many bad objects. This mechanism was shown clearly for CDt-circuit.
The object choice by saccade (or gaze) needs to be followed by other actions (e.g., reach, manipulate, eat) in order to obtain a rewarding outcome. It is likely that the basal ganglia contain multiple circuits, each of which controls a particular action. If so, action choice requires a mechanism that is different from object choice. To choose an action, other actions need to be suppressed. This may be accomplished by across-circuit interactions, especially through the indirect pathway. During evolution, the indirect pathway may have acquired multiple connections, which may have enabled the brain to use various kinds of behavioral choice. This is a hypothesis similar to the one presented before (Stephenson-Jones et al., 2011), and needs to be studied in future research.
Acknowledgments
This research was supported by the Intramural Research Program at the National Institutes of Health, National Eye Institute.
Abbreviations
- cdlSNc
caudal-dorsal-lateral part of substantia nigra pars compacta
- cdlSNr
caudal-dorsal-lateral part of substantia nigra pars reticulata
- cvGPe
caudal-ventral
- CD
caudate nucleus
- CDh
caudate head
- CDt
caudate tail
- DA
dopamine
- FEF
frontal eye field
- GPe
globus pallidus externus
- GPi
globus pallidus internus
- LIP
lateral intraparietal cortex
- pre-SMA
pre-supplementary motor area
- rvmSNc
rostral-ventral-medial part of substantia nigra pars compacta
- rvmSNr
rostral-ventral-medial part of substantia nigra pars reticulata
- RPE
reward prediction error
- SNr
substantia nigra pars reticulata
- SC
superior colliculus
- SEF
supplementary eye field
- STN
subthalamic nucleus
Footnotes
Authors’ contributions
Okihide Hikosaka Wrote the review article.
Hyoung F. Kim Revised the review article.
Hidetoshi Amita Revised the review article.
Masaharu Yasuda Revised the review article.
Masaki Isoda Revised the review article.
Yoshihisa Tachibana Revised the review article.
Atsushi Yoshida Revised the review article.
References
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends in neurosciences. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Amita H, Kim HF, Griggs W, A G, Gopal A, Hikosaka O. Direct and indirect pathways in basal ganglia signaling opposite reward values: anatomical study. Society for Neuroscience Meeting abstract 2016 [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Bayley PJ, Frascino JC, Squire LR. Robust habit learning in the absence of awareness and independent of the medial temporal lobe. Nature. 2005;436:550–553. doi: 10.1038/nature03857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichot NP, Schall JD. Effects of similarity and history on neural mechanisms of visual selection. Nat Neurosci. 1999;2:549–554. doi: 10.1038/9205. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Hanley JJ, Booth PA, Bevan MD. Synaptic organisation of the basal ganglia. Journal of anatomy. 2000;196(Pt 4):527–542. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8452–8456. doi: 10.1073/pnas.1000496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussaoud D, Desimone R, Ungerleider LG. Visual topography of area TEO in the macaque. J Comp Neurol. 1991;306:554–575. doi: 10.1002/cne.903060403. [DOI] [PubMed] [Google Scholar]
- Crossman AR, Sambrook MA, Jackson A. Experimental hemichorea/hemiballismus in the monkey. Brain. 1984;107:579–596. doi: 10.1093/brain/107.2.579. [DOI] [PubMed] [Google Scholar]
- Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494:238–242. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny-Brown D. Diseases of the basal ganglia. Their relation to disorders of movement. Lancet. 1960;2:1155–1162. doi: 10.1016/s0140-6736(60)92353-9. concl. [DOI] [PubMed] [Google Scholar]
- Duncan J. The locus of interference in the perception of simultaneous stimuli. Psychol Rev. 1980;87:272–300. [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Wang J, Aigner TG, Mishkin M. Visual habit formation in monkeys with neurotoxic lesions of the ventrocaudal neostriatum. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4196–4201. doi: 10.1073/pnas.061022098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandia JA, De Las Heras S, Garcia M, Gimenez-Amaya JM. Afferent projections to the reticular thalamic nucleus from the globus pallidus and the substantia nigra in the rat. Brain Res Bull. 1993;32:351–358. doi: 10.1016/0361-9230(93)90199-l. [DOI] [PubMed] [Google Scholar]
- Ghazizadeh A, Griggs W, Hikosaka O. Ecological Origins of Object Salience: Reward, Uncertainty, Aversiveness, and Novelty. Front Neurosci. 2016a;10:378. doi: 10.3389/fnins.2016.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazizadeh A, Griggs W, Hikosaka O. Object-finding skill created by repeated reward experience. J Vis. 2016b;16:17. doi: 10.1167/16.10.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs WS, Kim HF, Ghazizadeh A, Costello MG, Wall KM, Hikosaka O. Flexible and stable value coding areas in caudate head and tail receive anatomically distinct cortical and subcortical inputs. Front Neuroanat. 2017;11:106. doi: 10.3389/fnana.2017.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes WI, Haber SN. The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for Basal Ganglia models and deep brain stimulation. The Journal of neuroscience. 2013;33:4804–4814. doi: 10.1523/JNEUROSCI.4674-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazrati LN, Parent A. Projection from the external pallidum to the reticular thalamic nucleus in the squirrel monkey. Brain research. 1991;550:142–146. doi: 10.1016/0006-8993(91)90418-u. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Ghazizadeh A, Griggs W, Amita H. Parallel basal ganglia circuits for decision making. J Neural Transm. 2017 doi: 10.1007/s00702-017-1691-1. (In Press) [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakamoto M, Miyashita N. Effects of caudate nucleus stimulation on substantia nigra cell activity in monkey. Experimental brain research. 1993;95:457–472. doi: 10.1007/BF00227139. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakamoto M, Usui S. Functional properties of monkey caudate neurons. I. Activities related to saccadic eye movements. J Neurophysiol. 1989a;61:780–798. doi: 10.1152/jn.1989.61.4.780. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakamoto M, Usui S. Functional properties of monkey caudate neurons. II. Visual and auditory responses. J Neurophysiol. 1989b;61:799–813. doi: 10.1152/jn.1989.61.4.799. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakamoto M, Usui S. Functional properties of monkey caudate neurons. III. Activities related to expectation of target and reward. J Neurophysiol. 1989c;61:814–832. doi: 10.1152/jn.1989.61.4.814. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiological reviews. 2000;80:953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. I. Relation of visual and auditory responses to saccades. J Neurophysiol. 1983a;49:1230–1253. doi: 10.1152/jn.1983.49.5.1230. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. III. Memory-contingent visual and saccade responses. J Neurophysiol. 1983b;49:1268–1284. doi: 10.1152/jn.1983.49.5.1268. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. IV. Relation of substantia nigra to superior colliculus. J Neurophysiol. 1983c;49:1285–1301. doi: 10.1152/jn.1983.49.5.1285. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in the monkey superior colliculus. J Neurophysiol. 1985a;53:266–291. doi: 10.1152/jn.1985.53.1.266. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. II. Effects of muscimol in monkey substantia nigra pars reticulata. J Neurophysiol. 1985b;53:292–308. doi: 10.1152/jn.1985.53.1.292. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Yamamoto S, Yasuda M, Kim HF. Why skill matters. Trends Cogn Sci. 2013;17:434–441. doi: 10.1016/j.tics.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Hikosaka O. Dopamine-mediated learning and switching in cortico-striatal circuit explain behavioral changes in reinforcement learning. Frontiers in behavioral neuroscience. 2011;5:15. doi: 10.3389/fnbeh.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inase M, Tokuno H, Nambu A, Akazawa T, Takada M. Corticostriatal and corticosubthalamic input zones from the presupplementary motor area in the macaque monkey: comparison with the input zones from the supplementary motor area. Brain research. 1999;833:191–201. doi: 10.1016/s0006-8993(99)01531-0. [DOI] [PubMed] [Google Scholar]
- Isoda M, Hikosaka O. Switching from automatic to controlled action by monkey medial frontal cortex. Nat Neurosci. 2007;10:240–248. doi: 10.1038/nn1830. [DOI] [PubMed] [Google Scholar]
- Isoda M, Hikosaka O. Role for subthalamic nucleus neurons in switching from automatic to controlled eye movement. J Neurosci. 2008;28:7209–7218. doi: 10.1523/JNEUROSCI.0487-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Stein BE, McHaffie JG. Opposing basal ganglia processes shape midbrain visuomotor activity bilaterally. Nature. 2003;424:982–986. doi: 10.1038/nature01698. [DOI] [PubMed] [Google Scholar]
- Joel D, Weiner I. The connections of the primate subthalamic nucleus: indirect pathways and the open-interconnected scheme of basal ganglia-thalamocortical circuitry. Brain Res Brain Res Rev. 1997;23:62–78. doi: 10.1016/s0165-0173(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Kawagoe R, Takikawa Y, Hikosaka O. Expectation of reward modulates cognitive signals in the basal ganglia. Nature Neuroscience. 1998;1:411–416. doi: 10.1038/1625. [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TP. The cortico-striate projection in the monkey. Brain. 1970;93:525–546. doi: 10.1093/brain/93.3.525. [DOI] [PubMed] [Google Scholar]
- Kim HF, Amita H, Hikosaka O. Indirect Pathway of Caudal Basal Ganglia for Rejection of Valueless Visual Objects. Neuron. 2017;94:920–930. e923. doi: 10.1016/j.neuron.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HF, Ghazizadeh A, Hikosaka O. Separate groups of dopamine neurons innervate caudate head and tail encoding flexible and stable value memories. Frontiers in neuroanatomy. 2014;8:120. doi: 10.3389/fnana.2014.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HF, Ghazizadeh A, Hikosaka O. Dopamine Neurons Encoding Long-Term Memory of Object Value for Habitual Behavior. Cell. 2015;163:1165–1175. doi: 10.1016/j.cell.2015.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HF, Hikosaka O. Distinct Basal Ganglia circuits controlling behaviors guided by flexible and stable values. Neuron. 2013;79:1001–1010. doi: 10.1016/j.neuron.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HF, Hikosaka O. Parallel basal ganglia circuits for voluntary and automatic behaviour to reach rewards. Brain. 2015;138:1776–1800. doi: 10.1093/brain/awv134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Tokuno H, Nambu A. Monkey globus pallidus external segment neurons projecting to the neostriatum. NeuroReport. 1999;10:1467–1472. doi: 10.1097/00001756-199905140-00014. [DOI] [PubMed] [Google Scholar]
- Kita T, Kita H. Cholinergic and non-cholinergic mesopontine tegmental neurons projecting to the subthalamic nucleus in the rat. The European journal of neuroscience. 2011;33:433–443. doi: 10.1111/j.1460-9568.2010.07537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauwereyns J, Watanabe K, Coe B, Hikosaka O. A neural correlate of response bias in monkey caudate nucleus. Nature. 2002;418:413–417. doi: 10.1038/nature00892. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PJ. The mammalian superior colliculus: laminar structure and connections. Prog Brain Res. 2006;151:321–378. doi: 10.1016/S0079-6123(05)51011-2. [DOI] [PubMed] [Google Scholar]
- Mena-Segovia J, Bolam JP, Magill PJ. Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci. 2004;27:585–588. doi: 10.1016/j.tins.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Malamut B, Bachevalier J. Memories and habits: Two neural systems. In: Lynch G, McGaugh JL, Weinberger NM, editors. Neurobiology of Human Learning and Memory. The Guilford Press; New York: 1984. pp. 65–77. [Google Scholar]
- Monakow KH, Akert K, Kunzle H. Projections of the precentral motor cortex and other cortical areas of the frontal lobe to the subthalamic nucleus in the monkey. Experimental brain research. 1978;33:395–403. doi: 10.1007/BF00235561. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Hikosaka O. Role of dopamine in the primate caudate nucleus in reward modulation of saccades. J Neurosci. 2006;26:5360–5369. doi: 10.1523/JNEUROSCI.4853-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Hasegawa Y, Tokushige A, Nakagawa S, Kayahara T, Mizuno N. Topographical projections from the thalamus, subthalamic nucleus and pedunculopontine tegmental nucleus to the striatum in the japanese monkey, Macaca fuscata. Brain research. 1990;537:54–68. doi: 10.1016/0006-8993(90)90339-d. [DOI] [PubMed] [Google Scholar]
- Nambu A, Takada M, Inase M, Tokuno H. Dual somatotopical representations in the primate subthalamic nucleus: evidence for ordered but reversed body-map transformations from the primary motor cortex and the supplementary motor area. The Journal of neuroscience. 1996;16:2671–2683. doi: 10.1523/JNEUROSCI.16-08-02671.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Hamada I, Kita H, Imanishi M, Akazawa T, Ikeuchi Y, Hasegawa N. Excitatory cortical inputs to pallidal neurons via the subthalamic nucleus in the monkey. J Neurophysiol. 2000;84:289–300. doi: 10.1152/jn.2000.84.1.289. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal 'hyperdirect' pathway. Neurosci Res. 2002;43:111–117. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- Parent A, Bouchard C, Smith Y. The striatopallidal and striatonigral projections: two distinct fiber systems in primate. Brain research. 1984;303:385–390. doi: 10.1016/0006-8993(84)91224-1. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati L-N. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Research Reviews. 1995;20:128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- Parent A, Sato F, Wu Y, Gauthier J, Levesque M, Parent M. Organization of the basal ganglia: the importance of axonal collateralization. Trends Neurosci. 2000;23:S20–27. doi: 10.1016/s1471-1931(00)00022-7. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Wickens JR. Dopamine-dependent plasticity of corticostriatal synapses. Neural Netw. 2002;15:507–521. doi: 10.1016/s0893-6080(02)00045-x. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr JA, Ungerleider LG, Desimone R. Organization of visual cortical inputs to the striatum and subsequent outputs to the pallido-nigral complex in the monkey. J Comp Neurol. 1990;298:129–156. doi: 10.1002/cne.902980202. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Hikosaka O. Eye movements induced by microinjection of GABA agonist in the rat substantia nigra pars reticulata. Neuroscience Research. 1989;6:216–233. doi: 10.1016/0168-0102(89)90061-8. [DOI] [PubMed] [Google Scholar]
- Samejima K, Ueda Y, Doya K, Kimura M. Representation of action-specific reward values in the striatum. Science. 2005;310:1337–1340. doi: 10.1126/science.1115270. [DOI] [PubMed] [Google Scholar]
- Sato F, Lavallee P, Levesque M, Parent A. Single-axon tracing study of neurons of the external segment of the globus pallidus in primate. J Comp Neurol. 2000;417:17–31. [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. Journal of Neuroscience. 1985;5:776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86:353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bolam JP. Convergence of synaptic inputs from the striatum and the globus pallidus onto identified nigrocollicular cells in the rat: a double anterograde labelling study. Neuroscience. 1991;44:45–73. doi: 10.1016/0306-4522(91)90250-r. [DOI] [PubMed] [Google Scholar]
- Smith Y, Hazrati L-N, Parent A. Efferent projections of the subthalamic nucleus in the squirrel monkey as studied by the PHA-L anterograde tracing method. J Comp Neurol. 1990;294:306–323. doi: 10.1002/cne.902940213. [DOI] [PubMed] [Google Scholar]
- Smith Y, Parent A. Differential connections of caudate nucleus and putamen in the squirrel monkey (Saimiri sciureus) Neuroscience. 1986;18:347–371. doi: 10.1016/0306-4522(86)90159-4. [DOI] [PubMed] [Google Scholar]
- Stephenson-Jones M, Samuelsson E, Ericsson J, Robertson B, Grillner S. Evolutionary conservation of the basal ganglia as a common vertebrate mechanism for action selection. Curr Biol. 2011;21:1081–1091. doi: 10.1016/j.cub.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Stephenson-Jones M, Yu K, Ahrens S, Tucciarone JM, van Huijstee AN, Mejia LA, Penzo MA, Tai LH, Wilbrecht L, Li B. A basal ganglia circuit for evaluating action outcomes. Nature. 2016;539:289–293. doi: 10.1038/nature19845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasburger H, Rentschler I, Juttner M. Peripheral vision and pattern recognition: a review. J Vis. 2011;11:13. doi: 10.1167/11.5.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Tachibana Y, Kita H, Chiken S, Takada M, Nambu A. Motor cortical control of internal pallidal activity through glutamatergic and GABAergic inputs in awake monkeys. The European journal of neuroscience. 2008;27:238–253. doi: 10.1111/j.1460-9568.2007.05990.x. [DOI] [PubMed] [Google Scholar]
- Takikawa Y, Kawagoe R, Itoh H, Nakahara H, Hikosaka O. Modulation of saccadic eye movements by predicted reward outcome. Experimental brain research. 2002;142:284–291. doi: 10.1007/s00221-001-0928-1. [DOI] [PubMed] [Google Scholar]
- Tecuapetla F, Jin X, Lima SQ, Costa RM. Complementary Contributions of Striatal Projection Pathways to Action Initiation and Execution. Cell. 2016;166:703–715. doi: 10.1016/j.cell.2016.06.032. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Kim HF, Hikosaka O. Reward value-contingent changes of visual responses in the primate caudate tail associated with a visuomotor skill. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:11227–11238. doi: 10.1523/JNEUROSCI.0318-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Monosov IE, Yasuda M, Hikosaka O. What and where information in the caudate tail guides saccades to visual objects. The Journal of neuroscience. 2012;32:11005–11016. doi: 10.1523/JNEUROSCI.0828-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda M, Banno T, Komatsu H. Color selectivity of neurons in the posterior inferior temporal cortex of the macaque monkey. Cereb Cortex. 2010;20:1630–1646. doi: 10.1093/cercor/bhp227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda M, Hikosaka O. Functional territories in primate substantia nigra pars reticulata separately signaling stable and flexible values. J Neurophysiol. 2015;113:1681–1696. doi: 10.1152/jn.00674.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda M, Hikosaka O. To Wait or Not to Wait-Separate Mechanisms in the Oculomotor Circuit of Basal Ganglia. Front Neuroanat. 2017;11:35. doi: 10.3389/fnana.2017.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda M, Yamamoto S, Hikosaka O. Robust representation of stable object values in the oculomotor Basal Ganglia. The Journal of neuroscience. 2012;32:16917–16932. doi: 10.1523/JNEUROSCI.3438-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeterian EH, Van Hoesen GW. Cortico-striate projections in the rhesus monkey: the organization of certain cortico-caudate connections. Brain research. 1978;139:43–63. doi: 10.1016/0006-8993(78)90059-8. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Precht W. Monosynaptic inhibition of neurons of the substantia nigra by caudato-nigral fibers. Brain research. 1971;32:225–228. doi: 10.1016/0006-8993(71)90170-3. [DOI] [PubMed] [Google Scholar]