Abstract
Background
Depression is common in asthma and is associated with poor outcomes. However, antidepressant therapy in depressed asthma patients has been the topic of little research.
Objective
This study examined the impact of antidepressant treatment with escitalopram vs. placebo on the Hamilton Rating Scale for Depression (HRSD), Inventory of Depressive Symptomatology-Self Report (IDS-SR), Asthma Control Questionnaire (ACQ), and oral corticosteroid use in asthma patients with major depressive disorder (MDD).
Methods
Single site 12-week, randomized, double-blind, placebo-controlled, parallel-group trial of escitalopram (10 mg/d) was conducted in 139 outpatients with asthma and MDD. Randomization was stratified by oral corticosteroid use (≥ 3 bursts in past 12 months, yes or no) and baseline depressive symptom severity (HRSD ≥ 20) (higher severity, n=42) vs. < 3 bursts, HRSD < 20 or both (lower severity, n=97). The primary data analysis was conducted using HLM Version 7.01 on the higher and lower severity samples and post hoc was conducted on the combined sample.
Results
Among the higher severity completers (n=21), a significant reduction in the ACQ (p=0.04) and oral corticosteroid use (p=0.04) was observed with escitalopram. In the combined sample, no significant differences were observed, but a trend toward greater reduction in the IDS-SR was observed with escitalopram (p=0.07). Side effects were comparable across groups.
Conclusion
The findings suggest that patients with more severe asthma and depression symptomatology may have a positive response, in terms of both asthma and depressive symptom reduction, to antidepressant treatment.
Keywords: major depressive disorder, asthma, escitalopram, selective serotonin reuptake inhibitor
Introduction
Asthma is a common, severe respiratory condition characterized by chronic inflammation, airflow obstruction, symptoms of frequent cough, wheezing, chest tightness, and shortness of breath (1). The prevalence of asthma increased from 7.3% in 2001 to 8.4% in 2010 in the United States, with global prevalence estimated at 235 million people (2), (3). The Center for Disease Control (CDC) and Environmental Protection Agency (EPA) estimate approximately 500,000 hospitalizations, 2 million emergency room visits (4), and over 14 million asthma-related doctor visits, resulting in nearly $56 billion in direct and indirect public health costs (5).
Depression is another chronic condition with significant personal and public health burden in the U.S and worldwide (6). Prior research suggests a link between depression and asthma (7), with lifetime MDD prevalence of up to 47% (8), (9) and greater depressive symptom severity in asthma patients compared to healthy controls or patients with other chronic medical conditions (e.g. rheumatoid arthritis, ulcerative colitis, hypertension) (10). Depression may worsen asthma outcomes, such as unscheduled asthma-related doctor and emergency room visits (11), (12), work productivity (13), (14), and may be associated with asthma-related fatalities (15), (16). Furthermore, Strunk suggested depression as a risk factor for asthma-related deaths. (17). More recently, Hannaway (2000) surveyed 400 asthma physicians to find that among fatal to near-fatal asthma cases, 44% of patients had predisposing psychosocial factors, suggesting that more severe asthma cases may be particularly vulnerable to psychiatric comorbidities and may require specialized treatment (18).
Prior literature suggests that these chronic conditions are closely linked and may share common physiological mechanisms. However, the majority of existing research has primarily focused on environmental and psychosocial factors as the main factors underlying the comorbidity of asthma and depression. A recent study by Tedner et al. (2016) suggests that the relationship between these diseases may go beyond shared environmental and genetic factors (19). The researchers conducted a large scale national study that showed an association between depression and asthma (OR 1.20) in adult twins. The association remained significant after controlling for genetic and environmental factors (control co-twin analysis), and was not present in the adults with depression and the asthma diagnosis in their children, further strengthening the hypothesis that asthma and depression share mechanisms beyond genetic and shared environmental factors.
Despite the prevalence and morbidity associated with depression in asthma patients, as well as a possible physiological connection between the diseases, to our knowledge, very few controlled studies examined the impact of antidepressant treatment in depressed asthma patients. Sanger (1969) compared amitriptyline and doxepin in a double-blind, placebo-controlled study investigating the treatment of anxiety and depression in patients with multiple allergies, including dermatological conditions, hay fever, and bronchial asthma (20). Doxepin was significantly more effective than amitriptyline (p = 0.0006) at reducing the 17-item Hamilton Rating Scale for Depression (HRSD17, (21), (22)) total scores in this population. A randomized 12-week clinical trial of citalopram in 82 adults with both asthma and MDD showed a reduction in oral corticosteroid use and depression symptom scores (measured by HRSD17) in patients receiving citalopram vs. placebo (23). Additionally, trends towards reduction in depression scores, as well as a correlation between changes in asthma control, pulmonary function, and depressive symptoms, have been observed in a small proof-of-concept randomized trial of escitalopram in outpatients with asthma and MDD (24). These studies may have meaningful clinical implications for the management of both asthma and MDD with antidepressant medications; however, physiological mechanisms that drive the relationship between both conditions and the medication response remain unclear.
The current report presents data from a randomized, controlled trial of escitalopram in outpatients with asthma and current major depressive disorder. Changes in depression, asthma control and corticosteroid use were assessed. Our hypothesis was that escitalopram therapy would decrease depressive symptom severity, improve asthma control and decrease the need for rescue oral corticosteroids in people with asthma who had higher levels of depressive symptom severity and frequent oral corticosteroid use.
Methods
A 12-week, randomized, double-blind, parallel-group, placebo-controlled trial of escitalopram in 139 outpatient adults (18–70 years old, male and female, English and Spanish speakers) with both asthma and MDD was conducted at UT Southwestern Medical Center, Dallas, TX between 7/21/2010 and 2/18/2015.
All participants were recruited from asthma clinics on campus, as well as through flyers and other advertising in the community (Table 1). The included participants must have had a diagnosis of asthma, were currently receiving asthma treatment (see Table 1 for asthma medication breakdown), and had at least one asthma-related physician visit in the past 6 months, as well as current diagnosis of MDD, baseline HRSD17 score ≥ 15 and 7-item Asthma Control Questionnaire (ACQ total score ≥ 1). ACQ scores are reported as the mean of the items (i.e., total divided by the number of items)). Participants with MDD with current psychotic features (e.g. hallucinations, delusions), other severe psychiatric illness (bipolar disorder, schizophrenia, schizoaffective), age > 70 years old, current tobacco use, cognitive impairment, current use of antidepressants, other psychiatric medications or psychotherapy, high risk of suicide, pregnancy or nursing, or recent (within past 2 weeks) changes in asthma medications, oral corticosteroid treatment, or respiratory tract infections were excluded from the study.
Table 1.
Asthma medication and study referral breakdown by treatment group
| Medication Class | Medication Type | Escitalopram | Placebo | ||
|---|---|---|---|---|---|
| Severity | High (n=13) | Low (n=33) | High (n=16) | Low (n=37) | |
| n (% on each medication) | |||||
| Short-acting beta2-agonist | Albuterol sulfate | 9 (69%) | 31 (94%) | 14 (88%) | 33 (89%) |
| Levalbuterol tartrate | 1 (8%) | 1 (3%) | 2 (13%) | 2 (5%) | |
| Both (Albuterol + Levalbuterol) | 3 (23%) | 0 | 0 | 2 (5%) | |
| Long-acting beta2-agonist | Salmeterol xinafoate | 0 | 1 (3%) | 0 | 1 (3%) |
| Combination medication | Fluticasone propionate/salmeterol xinafoate | 8 (62%) | 13 (39%) | 8 (50%) | 20 (54%) |
| Mometasone furoate/formoterol fumarate | 0 | 5 (15%) | 2 (13%) | 0 | |
| Budesonide/formoterol fumarate | 1 (8%) | 0 | 0 | 2 (5%) | |
| Inhaled corticosteroids | Beclomethasone dipropionate | 1 (8%) | 7 (21%) | 4 (25%) | 8 (22%) |
| Fluticasone propionate | 1 (8%) | 4 (12%) | 1 (6%) | 4 (11%) | |
| Leukotriene receptor antagonist | Montelukast | 4 (31%) | 7 (21%) | 4 (25%) | 6 (16%) |
| Zafirlukast | 0 | 0 | 0 | 1 (3%) | |
| Muscarinic receptor antagonist | Ipratropium bromide | 1 (8%) | 2 (6%) | 6 (38%) | 3 (8%) |
| Tiotropium bromide | 0 | 1 (3%) | 2 (13%) | 0 | |
| Referral Information | Escitalopram (n=46) | Placebo (n=53) | |||
| Community-Oriented Primary Care Clinics (COPC) Flyers | 19 (41%) | 19 (36%) | |||
| Hospital-affiliated Asthma Clinic | 17 (37%) | 14 (26%) | |||
| Free/Paid Media | 6 (13%) | 5 (9%) | |||
| Other Research Studies | 2 (4%) | 12 (23%) | |||
| Word-of-Mouth | 2 (4%) | 3 (6%) | |||
Note: 32% (n=99) of participants were on multiple medications in addition to their rescue inhaler.
At the baseline assessment, the Structured Clinical Interview for DSM 4 (SCID-IV) (26) was administered to establish a current diagnosis of MDD, as well as rule out exclusionary psychiatric illness. The diagnosis was confirmed by a clinical assessment by a psychiatrist. Depression symptomatology was measured using a clinician-rated HRSD17 and a self-report Inventory of Depressive Symptomatology (IDS-SR) (27). Asthma measures included ACQ, forced expiratory volume in 1-second percentage of normal (FEV1%) using a portable spirometer, as well as participant report on oral corticosteroid use. Cumulative Illness Rating Scale (CIRS) was used to evaluate and rule out general medical conditions and the PRD-III Somatic Symptom Scale (PRD III) (28) was used to assess side effects. A blood draw for routine laboratory analyses was conducted to ensure participant health and safety prior to initiating the study medication. All participants were compensated for their time and effort in the study. The study protocol was reviewed and approved by the UT Southwestern Institutional Review Board (IRB), and all participants signed an informed consent form prior to undergoing any study procedures. This trial was registered with clinicaltrials.gov under NCT01324700.
Computer-based randomization (1:1) was performed by an unblinded statistician to allocate participants to receive either escitalopram (10 mg/day) or an identical placebo for 12 weeks, with reassessment visits conducted every two weeks. Separate randomizations were conducted based on the baseline asthma and depression characteristics of the participants. Those with a baseline HRSD17 ≥ 20 and ≥ 3 course of oral corticosteroids (a marker of significant asthma exacerbations (29)) in the past 12 months (“higher severity group”) were randomized separately from those otherwise eligible but with either HRSD17 < 20 or < 3 course of oral corticosteroids in the past 12 months (“lower severity group”). Participants who had not shown evidence of adequate response (<30% decrease in HRSD17) to the antidepressant at week 4, and those without side effects, received a dose increase to 20 mg/day of escitalopram or an equivalent number of placebo capsules. At each follow-up visit, participants met with a research coordinator and a psychiatrist (both blinded to the treatment assignment) and were reevaluated on HRSD17, IDS-SR, ACQ, and spirometry, as well as answered questions about oral corticosteroid use (used = 1, not used = 0 since the last visit). Additionally, a psychiatrist reassessed patients at each visit to ensure safety and protocol adherence.
Statistical Analysis
The sample size was based on sample with post-baseline, but not necessarily compete data, using data from a pilot study subgroup analysis of high severity asthma (≥3 oral corticosteroid bursts/past 12 months) and depression (Hamilton Rating Scale for Depression 17-item, HRSD17) participants (23), using the formula of Rochon (25) to compute the power for a given sample size. Based on these data, the proposed sample size was n=80 in the higher severity group and n=142 in the lower severity group.
Separate analyses of higher and lower severity groups were preplanned. A secondary analysis of the combined groups was also conducted. Hierarchical linear modeling (HLM) was used to analyze the longitudinal data of the combined sample with time included as a within-subjects variable, treatment group included as a between-subjects variable, and baseline levels included as covariates. At level-1, time points were nested under each participant (weeks 2, 4, 6, 8, and 12). At level-2, treatment and baseline (ACQ, corticosteroid use, IDS-SR) scores were included in the model as between-participant variables. Time and treatment were added to the model uncentered because they were coded with a zero-value (0 = placebo, 0 = week 2) and baseline scores included were grand mean centered. Variance and covariance components in HLM are estimated through maximum likelihood procedures (30). Because baseline was used as a covariate, participants needed to have at least two post baseline time points (e.g., weeks 2 and 4) to be included in the analyses. In the “higher severity” completer analysis, SPSS Version 24 was used to run independent t-tests to examine differences between those who were given escitalopram and placebo in those who had high corticosteroid (≥ 3 bursts in the past year) AND depression severity (HRSD17 ≥ 20). If participants did not meet both criteria they were considered “lower severity”, and were not used in this analyses. Change scores (exit – baseline) on mood and asthma symptoms were used as the primary outcome to compare treatment groups, as well as the sum of the times corticosteroids were used throughout the study (i.e., reported at each visit). The number of times participants reported using systemic corticosteroids, of any duration, (coded as 1) for weeks 2 through 12 were added together to equal total number of times corticosteroids were used during the study. If a participant used corticosteroids twice in one week, use was still only coded as 1. For example, if a participant reported using corticosteroids during weeks 2, 6, and 8, their corticosteroid use for the study would be 3. Effect sizes use Cohen’s d.
Results
Demographic characteristics of participants randomized to escitalopram and placebo in the “higher severity group” and “lower severity group” are provided in Table 2, while Table 3 provides baseline data on study completers and dropouts. The groups were similar with the exception of a higher mean years of education in the placebo group. Participants were predominantly female, African-American, and middle-aged which reflects the demographic population of the asthma clinics from which most participants were recruited.
Table 2.
Demographic characteristics of the sample.
| Treatment Group | |||
|---|---|---|---|
| Characteristics | Placebo | Escitalopram | Significance |
| Mean(SD) | Mean(SD) | ||
| Age | 46.69(10.78) | 44.58(12.14) | t(75) = 0.81, p = 0.42 |
| Low | 45.79(11.68) | 45.37(11.29 | t(54) = 0.14, p = 0.89 |
| High | 49.30(7.48) | 42.64(14.40) | t(19) = 1.31, p = 0.21 |
| N | n | ||
| Sex | χ2(1) = 0.21, p = 0.65 | ||
| Low | χ2(1) = 0.18, p = 0.67 | ||
| High | χ2(1) = 0.01, p = 0.82 | ||
| Male | 11 | 9 | |
| Low | 9 | 7 | |
| High | 2 | 2 | |
| Female | 28 | 29 | |
| Low | 20 | 20 | |
| High | 8 | 9 | |
| Ethnicity | χ2(3) = 1.10, p = 0.90 | ||
| Low | χ2(3) = 0.88, p = 0.83 | ||
| High | χ2(3) = 3.36, p = 0.34 | ||
| Caucasian | 7 | 7 | |
| Low | 5 | 7 | |
| High | 2 | 1 | |
| African American | 25 | 24 | |
| Low | 18 | 16 | |
| High | 7 | 8 | |
| Hispanic | 6 | 5 | |
| Low | 5 | 3 | |
| High | 1 | 2 | |
| Asian/Pacific Islander | 1 | 1 | |
| Low | 1 | 1 | |
| High | 0 | 0 | |
| Mean(SD) | Mean(SD) | ||
| Education (years) | 13.45(2.82) | 11.83(3.54) | t(75) = 2.22, p = 0.03 |
| Low | 13.28(2.94) | 12.17(3.92) | t(54) = 1.20, p = 0.23 |
| High | 13.95(2.54) | 11.00(2.28) | t(19) = 2.80, p = 0.01 |
| ACQ Baseline | 2.04(1.07) | 2.15(1.12) | t(75) = 0.43, p = 0.67 |
| Low | 2.04(1.13) | 1.89(1.01) | t(54) = 0.51, p = 0.62 |
| High | 2.04(0.92) | 2.77(1.18) | t(19) = 1.55, p = 0.14 |
| IDS-SR | 37.62(12.25) | 36.84(11.70) | t(75) = 0.28, p = 0.78 |
| Low | 36.45(11.96) | 36.00(11.54) | t(54) = 0.14, p = 0.89 |
| High | 41.00(13.10) | 38.91(12.37) | t(19) = 0.38, p = 0.71 |
| HRSD17 Baseline | 24.85(5.09) | 25.03(5.23) | t(75) = 0.15, p = 0.88 |
| Low | 23.69(4.11) | 24.56(5.45) | t(54) = 0.67, p = 0.50 |
| High | 28.20(6.32) | 26.18(4.69) | t(19) = 0.84, p = 0.41 |
| Body Mass Index (BMI) | 37.29(10.54) | 37.54(10.54) | t(75) = −0.10, p = .92 |
| Low | 36.41(11.35) | 36.47(10.96) | t(54) = −0.02, p = .98 |
| High | 39.85(7.63) | 40.15(9.39) | t(19) = −0.08, p = .94 |
| FEV1% Predicted | 96.55(9.87) | 92.28(11.09) | t(75) = 1.78, p = 0.08 |
| Low | 97.04(9.27) | 95.14(11.00) | t(54) = 0.71, p = 0.49 |
| High | 95.11(11.87) | 85.27(8.00) | t(19) = 2.25, p = 0.04 |
Note: ACQ – Asthma Control Questionnaire; IDS-SR – Inventory of Depressive Symptomatology Self-Report; HRSD17 – Hamilton Rating Scale for Depression.
Table 3.
Baseline characteristics by study completion and treatment group.
| Treatment Group | |||
|---|---|---|---|
| Characteristics | Study Completion | Placebo | Escitalopram |
| Mean(SD) | Mean(SD) | ||
| Age | Completer (n = 77) | 46.69(10.78) | 44.58(12.14) |
| Drop out (n = 62) | 41.52(11.78) | 43.09(12.22) | |
| n | n | ||
| Sex | |||
| Male | Completer (n = 20) | 11 | 9 |
| Drop out (n = 19) | 13 | 6 | |
| Female | Completer (n = 52) | 28 | 29 |
| Drop out (n = 43) | 16 | 27 | |
| Ethnicity | |||
| Caucasian | Completer (n = 14) | 7 | 7 |
| Drop out (n = 8) | 5 | 3 | |
| African American | Completer (n = 49) | 25 | 24 |
| Drop out (n = 34) | 14 | 20 | |
| Hispanic | Completer (n = 11) | 6 | 5 |
| Drop out (n = 20) | 10 | 10 | |
| Asian/Pacific Islander | Completer (n = 2) | 1 | 1 |
| Drop out (n = 0) | 0 | 0 | |
| Asthma/Depression Severity | |||
| High | Completer (n = 21) | 10 | 11 |
| Drop out (n = 21) | 10 | 11 | |
| Low | Completer (n = 56) | 29 | 27 |
| Drop out (n = 41) | 19 | 22 | |
| Mean(SD) | Mean(SD) | ||
| HRSD17 Baseline | Completer (n = 77) | 24.85(5.09) | 25.03(5.23) |
| Drop out (n = 62) | 26.97(4.86) | 26.12(4.99) | |
| IDS-SR Baseline | Completer (n = 77) | 37.61(12.25) | 36.84(11.69) |
| Drop out (n = 62) | 38.28(10.97) | 40.06(12.48) | |
Note: In drop outs there were significantly more women (81.8%) in the escitalopram group compared to men (18.2%), χ2 (1) = 5.16, p = .02. There were no other statistically significant differences between groups on baseline characteristics. HRSD17 – Hamilton Rating Scale for Depression; IDS-SR – Inventory of Depressive Symptomatology Self-Report.
Longitudinal Results for High and Low Severity Groups
Of the 139 participants, 99 had at least two post-baseline visits and were included in the analyses (Table 4). Among those in the higher severity group, there was not a significant time by group interaction (indicating that the two groups responded similarly over time in the study) in HRSD [b = −0.27 SE = 0.65, t(18) = .45, p = .65, d = .21], IDS-SR [b = 0.83 SE = 1.04, t(18) = .80, p = .43, d = .38], or ACQ scores [b = −0.01, SE = 0.09, t(18) = 0.12, p = .91, d = .06]; however, the group by time interaction was trending for corticosteroid use [b = −0.04, SE = 0.02, t(19) = 1.79, p = .09, d = .82]. In the lower severity group, no significant group by time interactions on HRSD [b = 0.11, SE = 0.36, t(53) = 0.33, p = 0.74, d = .09], ACQ [b = −0.02, SE = 0.05, t(53) = 0.31, p = .76, d = .08], or corticosteroid use [b = −0.001, SE = 0.009, t(54) = 0.19, p = .85, d = .05] was observed; however, there was a trending interaction between group and time on IDS-SR scores [b = 1.02, SE = 0.61, t(53) = 1.67, p = .10, d = .46].
Table IV.
Asthma medication and study referral breakdown by treatment group
| Medication Class | Specific Medication | Escitalopram | Placebo | ||
|---|---|---|---|---|---|
| Severity | Severity | ||||
| High (n=13) | Low (n=33) | High (n=16) | Low (n=37) | ||
| n (% on each medication) | |||||
| Short-acting beta2-agonist | Albuterol sulfate | 9 (69%) | 31 (94%) | 14 (88%) | 33 (89%) |
| Levalbuterol tartrate | 1 (8%) | 1 (3%) | 2 (13%) | 2 (5%) | |
| Both (Albuterol + Levalbuterol) | 3 (23%) | 0 | 0 | 2 (5%) | |
| Long-acting beta2-agonist | Salmeterol xinafoate | 0 | 1 (3%) | 0 | 1 (3%) |
| Combination medication | Fluticasone propionate/salmeterol xinafoate | 8 (62%) | 13 (39%) | 8 (50%) | 20 (54%) |
| Mometasone furoate/formoterol fumarate | 0 | 5 (15%) | 2 (13%) | 0 | |
| Budesonide/formoterol fumarate | 1 (8%) | 0 | 0 | 2 (5%) | |
| Inhaled corticosteroids | Beclomethasone dipropionate | 1 (8%) | 7 (21%) | 4 (25%) | 8 (22%) |
| Fluticasone propionate | 1 (8%) | 4 (12%) | 1 (6%) | 4 (11%) | |
| Leukotriene receptor antagonist | Montelukast | 4 (31%) | 7 (21%) | 4 (25%) | 6 (16%) |
| Zafirlukast | 0 | 0 | 0 | 1 (3%) | |
| Muscarinic receptor antagonist | Ipratropium bromide | 1 (8%) | 2 (6%) | 6 (38%) | 3 (8%) |
| Tiotropium bromide | 0 | 1 (3%) | 2 (13%) | 0 | |
| Referral Information | Escitalopram (n=46) | Placebo (n=53) | |||
| Community-Oriented Primary Care Clinics (COPC) Flyers | 19 (41%) | 19 (36%) | |||
| Hospital-affiliated Asthma Clinic | 17 (37%) | 14 (26%) | |||
| Free/Paid Media | 6 (13%) | 5 (9%) | |||
| Other Research Studies | 2 (4%) | 12 (23%) | |||
| Word-of-Mouth | 2 (4%) | 3 (6%) | |||
Note: 32% (n=99) of participants were on multiple medications in addition to their rescue inhaler.
Analysis of Study Completers (data available through week 12)
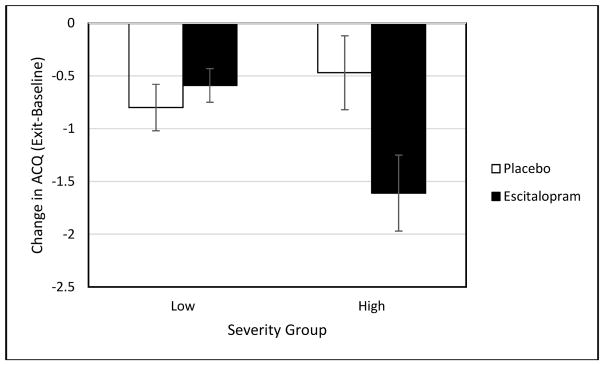
A post hoc completer (those with week 12 data) analysis was also conducted to examine those who had a full 12-week exposure to escitalopram or placebo (i.e., study completers). Completers and non-completers were demographically similar except for a greater proportion of women receiving escitalopram among the non-completers (see Table 3). Among completers in the higher severity group, there was a significant difference between treatment groups on both ACQ scores [t(19) = 2.27, p = .04, d = 1.04] and corticosteroid use [t(19) = 2.18, p = .04, d = 1.00; see Figure 1] but not on the HRSD [t(19) = .63, p = .54, d = .29], or IDS-SR [t(19) = .46, p = .65, d = .21]. Both reported asthma symptoms (ACQ scores), and need for rescue oral corticosteroids was lower in those who received escitalopram compared to those who received placebo. No significant between-group differences in asthma [ACQ t(153) = .75, p = .46, d = .21; corticosteroid use t(54) = .27, p = .79, d = .07] or depression [HRSD t(54) = .26, p = .79, d = .07, IDS-SR t(54) = .27, p = .79, d = .07] outcomes were observed in the lower severity completer sample (see Table 4).
Figure 1.
Change in ACQ in Low and High Severity groups.
Note: ACQ – Asthma Control Questionnaire. Standard error bars (+/−) are shown for each treatment group.
Longitudinal Results for Combined Severity Groups
A post hoc analysis of the combined higher and lower severity groups was also conducted in all participants (escitalopram and placebo groups) who had at least two post-baseline measures. Although there was no significant difference in those who received escitalopram or placebo in asthma symptoms (ACQ) [b = −0.02 (SE = 0.03), t = .44, p = .66, d = .10] or corticosteroid use [b = 0.01 (SE = 0.01), t = 1.18 p = .24, d = .27], IDS-SR scores demonstrated a trending group difference while controlling for baseline IDS-SR scores [b = 0.97(SE = .53), t = 1.83, p = .07, d = .43] where those who received escitalopram had a greater decrease in IDS-SR scores compared to those who received placebo.
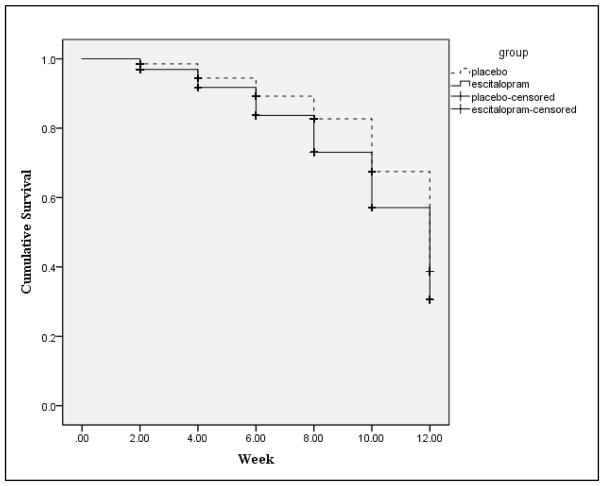
Safety and Tolerability
Escitalopram was well tolerated. There were no group differences in the side effect profile between the escitalopram and the placebo group [t(97) = −.02, p = .98, d = .01]. There was a difference in length of continuation in the study (i.e., survival) between the two groups. Those who were given escitalopram were, on average, continued in the study for a slightly shorter period of time [M = 10.05 (SE = .16)] compared to those in the placebo group [M = 10.65 (SE = .14)] (Figure 2).
Figure 2.
Cumulative survival between groups across 12 weeks.
Discussion
Our hypothesis was that the “higher severity” group (defined as a HRSD17 ≥20 and ≥3 oral corticosteroid courses in past 12 months) would demonstrate a better response to escitalopram, as compared to placebo, than the lower severity group. The results in part support this hypothesis. None of the outcomes reached statistical significance. However, the higher severity group demonstrated a trend on use of rescue oral corticosteroids with a large effect size. The lower severity group, however, showed a trend on the IDS-SR. High severity completers showed statistically significant, as well as clinical significant mean reductions based on the established ACQ change of at least 0.5 (31), reduction on the ACQ and significantly less oral corticosteroid use with escitalopram than placebo while none of the outcomes were significant in the lower severity group and effect sizes were small. The results largely replicate the findings from a very small proof-of-concept study of the effectiveness of escitalopram in asthma and MDD (24) as well as an earlier study with citalopram (23), and suggest that SSRIs may be associated with improvement in asthma outcomes in patients with more severe asthma and depression. The changes observed in the higher severity group were stronger than similar findings using traditional asthma treatments. A meta-analysis examining the effectiveness of omalizumab as an add-on therapy to inhaled corticosteroids for 4 to 6 months in patients with severe asthma found a medium effect on asthma symptom control (d = .77) and small effect size on reduction of corticosteroid use (d = .38) (32). The present work demonstrated a large effect on both ACQ and corticosteroid use in a shorter period of time (12 weeks). A caveat is that the current study was in depressed people with asthma who might respond especially favorably to an antidepressant. Thus, a direct comparison of the findings from this study to the omalizumab study is not possible. Furthermore, the statistically significant effects in the current study were only observed in the completer sample. This might suggest that a relatively long observation period is needed to see the effects of the intervention on asthma outcomes. Although there is a concern that a completer sample might not be representative of all participants, the completers and dropouts were demographically similar with exception of sex differences.
The modest effect of the antidepressant on depressive symptoms is not entirely surprising. A meta-analysis of antidepressant studies in depression reported a mean effect size of d=0.11 (small) for mild-to-moderate depression severity (baseline HRSD ≤ 18), d=0.17 (small) for severe (baseline HRSD 19–23) and d=0.47 (medium) for very severe (baseline HRSD ≥ 23) (33). Thus, the study may not have had a sufficiently large sample size to observe statistically significant improvement in depressive symptoms.
Escitalopram is a well-tolerated selective serotonin reuptake inhibitor (SSRI) that has been shown effective at reducing depressive symptoms (34). The available evidence on the physiological mechanisms involved in both depression and asthma also suggests a possible role of serotonin (5-HT) in modulating the immune system response to both of these conditions. A meta-analysis by Arreola et al. (2015) reviewed the mechanisms of serotonin-induced immunomodulation, focusing on therapeutic implications of the serotonergic system and the immune function in certain diseases, including MDD and asthma (35). MDD patients show decreased expression of serotonin transporter (SERT) in platelets (36) and lymphocytes (37), (38), as well as decreased serum level of serotonin (39). Limited in vitro testing suggests the importance of serotonin in regulating immune system response to chronic inflammatory conditions, such as asthma (40), while Lechin et al. (1998) showed that an antidepressant tianeptine reduced serotonin plasma level and increased FEV1 in patients with asthma, further strengthening the link between serotonergic activity and the inflammatory response (41).
Lechin et al. (1996) also found that both clinical asthma symptoms and the free serotonin level were significantly higher in symptomatic compared to asymptomatic patients, and that the level of serotonin was positively correlated with asthma severity and negatively with FEV1 in symptomatic (FEV1 < 70%), but not asymptomatic patients (FEV1 > 80%) (42). These results may also lend support to our findings, which indicated that escitalopram was particularly effective at reducing asthma symptoms and oral corticosteroid use in the “high severity” group (high depression and high asthma scores), suggesting that SSRIs may be particularly beneficial for depressed asthma patients with frequent asthma exacerbations. However, the findings may not be generalizable to asthma patients with frequent exacerbations who are not depressed, and, at this point, should not be interpreted as suggesting that escitalopram was more effective than standard therapies for asthma.
Escitalopram might also alter asthma medication response through several potential mechanisms. Depression is strongly associated with treatment non-adherence in medically ill populations (43). Thus, improvement in adherence or other aspects of asthma self-management is a potential mechanism through which an antidepressant might improve asthma outcomes. Depression is associated with changes in cognition (44), while cognition is related to asthma adherence (45). Thus, an antidepressant might improve asthma outcomes through an indirect mechanism of cognition, which could then lead to improved asthma medication adherence. Antidepressants appear to potentiate the effects of corticosteroids (46). A subset of depressed patients (47) and asthma patients (48) demonstrate resistance to glucocorticoids. Although highly speculative, antidepressants may decrease resistance to the effects of this type of medication. Finally, depressed asthma patients reportedly demonstrate a decreased bronchodilator response (49), which might be ameliorated with effective depression treatment.
There were several limitations associated with this trial, such as a high attrition rate and modest sample size, which possibly led to an underpowered study and could introduce bias in the findings. Clinical asthma diagnosis was used. Documentation of a positive asthma test (reversibility test or airway hyperresponsiveness) was not required for entry. However, the participants were all receiving asthma treatment, and people over age 70 or current smokers (both potential risk factors for COPD) were excluded. Asthma patients were not specifically phenotyped and thus it is unclear if a specific asthma phenotype may be more responsive to SSRI therapy. However, exacerbation-prone asthma subjects appear to have the most benefit with escitalopram. A longer observation period would have allowed more time to explore the full impact of the SSRI treatment on rescue oral corticosteroid use. Asthma medication changes were allowed up to two weeks prior to entry. Some asthma medications take more than a few weeks to demonstrate full response. Asthma medication adherence was not assessed, preventing an exploration of changes in adherence as a mechanism by which escitalopram exerted positive effects in a subset of asthma patients. The higher severity participants assigned to escitalopram had significantly lower baseline FEV1% predicted scores than those who received placebo. This could suggest that the active treatment group had more severe symptoms and, thus, greater ability to demonstrate change with treatment. However, the ACQ, which was the asthma measure used as an outcome and that includes FEV1% predicted in the score, was not significantly different at baseline in the two groups. Additionally, the mean FEV1% predicted value in the higher severity group was, perhaps, higher than might be expected in people with such frequent need for rescue oral corticosteroid therapy. The relatively high FEV1% predicted may be because participants were generally stable at the time of study entry and were excluded if they had a very recent exacerbation requiring oral corticosteroid therapy. Furthermore, as pointed out in a review, exacerbation-prone asthma appears to be influenced by both intrinsic and extrinsic factors and even people with a history of near-fatal asthma may appear similar to those with mild-to-moderate asthma on many clinical and inflammatory measures (50).
Our current findings provide additional clinically meaningful evidence for the possible role of serotonin in the pathogenesis of both asthma and depression, as well as potential role of SSRIs, including escitalopram, in reducing symptoms of both diseases. Future research, particularly larger-scale clinical trials, are still needed to fully understand the role of serotonin and serotonergic medications in modulating both asthma and depression response. In particular, these trials may benefit from a larger sample size in order to investigate the different components of the serotonin system (e.g., SERT) in subsets of patients with varying severity of both depression and asthma, and underscore new therapeutic approaches to the concurrent management of these two severe chronic conditions.
Highlights Box.
What is already known about this topic?
Major depression a major contributor to poor asthma outcomes; however, there is limited evidence regarding the treatment options for patients with both conditions.
What does this article add to our knowledge?
This study demonstrates improvement in asthma outcomes in patients with severe asthma and depression who received antidepressant therapy.
How does this study impact current management guideline?
Patients with both high severity asthma and depression symptomatology may benefit from a course of selective serotonin reuptake inhibitor (SSRI) antidepressant treatment.
Acknowledgments
Funding: This work was supported by the National Heart, Lung, and Blood Institute (R18 HL092862).
Abbreviations
- ACQ
Asthma Control Questionnaire
- BMI
Body Mass Index
- CDC
Center for Disease Control
- CIRS
Cumulative Illness Rating Scale
- COPD
Chronic Obstructive Pulmonary Disease
- DSM 4
Diagnostic and Statistical Manual of Mental Disorders, 4th. Edition
- EPA
Environmental Protection Agency
- FEV1%
Forced Expiratory Volume for 1 Second (%)
- HLM
Hierarchical Linear Modeling
- HRSD17
Hamilton Rating Scale for Depression
- IDS-SR
Inventory of Depressive Symptomatology Self-Report
- MDD
Major Depressive Disorder
- PRD III
The Psychobiology of Recovery in Depression III – Somatic Symptom Scale
- SCID-IV
Structured Clinical Interview for DSM 4
- SERT
Serotonin Transporter
- SPSS
Statistical Package for the Social Sciences
- SSRI
Selective Serotonin Reuptake Inhibitor
Footnotes
Financial Disclosure
Dr. Brown has research grants from NIH, The Stanley Medical Research Institute, Otsuka, and an honorarium from Genentech. Dr. Ivleva has research grants from NIH. Dr. Khan reports research funding from NIH, a speaker honorarium from Genentech, and service on a Data and Safety Monitoring Board at Aimmune. All other authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McCracken JL, Veeranki SP, Ameredes BT, Calhoun WJ. Diagnosis and Management of Asthma in Adults: A Review. JAMA. 2017;318(3):279–90. doi: 10.1001/jama.2017.8372. [DOI] [PubMed] [Google Scholar]
- 2.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief. 2012;(94):1–8. [PubMed] [Google Scholar]
- 3.(GINA) GIfA. Global Strategy for Asthma Management and Prevention. 2013 Available from: http://www.ginasthma.org/
- 4.Agency USEP. Asthma Facts. 2013. [Google Scholar]
- 5.Control CfD, Prevention. Asthma facts—CDC’s national asthma control program grantees. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2013. [Google Scholar]
- 6.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851–8. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 7.Zielinski TA, Brown ES. Depression in patients with asthma. Adv Psychosom Med. 2003;24:42–50. doi: 10.1159/000073779. [DOI] [PubMed] [Google Scholar]
- 8.Nejtek VA, Brown ES, Khan DA, Moore JJ, Van Wagner J, Perantie DC. Prevalence of mood disorders and relationship to asthma severity in patients at an inner-city asthma clinic. Ann Allergy Asthma Immunol. 2001;87(2):129–33. doi: 10.1016/s1081-1206(10)62206-5. [DOI] [PubMed] [Google Scholar]
- 9.Brown ES, Khan DA, Mahadi S. Psychiatric diagnoses in inner city outpatients with moderate to severe asthma. Int J Psychiatry Med. 2000;30(4):319–27. doi: 10.2190/7U7P-EJYL-5BKG-6106. [DOI] [PubMed] [Google Scholar]
- 10.Lyketsos CG, Lyketsos GC, Richardson SC, Beis A. Dysthymic states and depressive syndromes in physical conditions of presumably psychogenic origin. Acta Psychiatr Scand. 1987;76(5):529–34. doi: 10.1111/j.1600-0447.1987.tb02914.x. [DOI] [PubMed] [Google Scholar]
- 11.Ahmedani BK, Peterson EL, Wells KE, Williams LK. Examining the relationship between depression and asthma exacerbations in a prospective follow-up study. Psychosom Med. 2013;75(3):305–10. doi: 10.1097/PSY.0b013e3182864ee3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang G, Zhou T, Wang L, Wang L, Fu JJ, Zhang HP, et al. Relationship between current psychological symptoms and future risk of asthma outcomes: a 12-month prospective cohort study. J Asthma. 2011;48(10):1041–50. doi: 10.3109/02770903.2011.631238. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Zhang X, Zheng J, Wang L, Zhang HP, Wang L, et al. Co-morbid psychological dysfunction is associated with a higher risk of asthma exacerbations: a systematic review and meta-analysis. J Thorac Dis. 2016;8(6):1257–68. doi: 10.21037/jtd.2016.04.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hakola R, Kauppi P, Leino T, Ojajarvi A, Pentti J, Oksanen T, et al. Persistent asthma, comorbid conditions and the risk of work disability: a prospective cohort study. Allergy. 2011;66(12):1598–603. doi: 10.1111/j.1398-9995.2011.02729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Picado C, Montserrat JM, de Pablo J, Plaza V, Agusti-Vidal A. Predisposing factors to death after recovery from a life-threatening asthmatic attack. J Asthma. 1989;26(4):231–6. doi: 10.3109/02770908909073254. [DOI] [PubMed] [Google Scholar]
- 16.Kuo CJ, Chen VC, Lee WC, Chen WJ, Ferri CP, Stewart R, et al. Asthma and suicide mortality in young people: a 12-year follow-up study. Am J Psychiatry. 2010;167(9):1092–9. doi: 10.1176/appi.ajp.2010.09101455. [DOI] [PubMed] [Google Scholar]
- 17.Strunk RC. Identification of the fatality-prone subject with asthma. J Allergy Clin Immunol. 1989;83(2 Pt 1):477–85. doi: 10.1016/0091-6749(89)90137-1. [DOI] [PubMed] [Google Scholar]
- 18.Hannaway PJ. Demographic characteristics of patients experiencing near-fatal and fatal asthma: results of a regional survey of 400 asthma specialists [see comment] Ann Allergy Asthma Immunol. 2000;84(6):587–93. doi: 10.1016/s1081-1206(10)62408-8. [DOI] [PubMed] [Google Scholar]
- 19.Tedner SG, Lundholm C, Olsson H, Almqvist C. Depression or anxiety in adult twins is associated with asthma diagnosis but not with offspring asthma. Clin Exp Allergy. 2016;46(6):803–12. doi: 10.1111/cea.12714. [DOI] [PubMed] [Google Scholar]
- 20.Sanger MD. The treatment of anxiety and depression in the allergic patient. Ann Allergy. 1969;27(10):506–10. [PubMed] [Google Scholar]
- 21.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamilton M. DEVELOPMENT OF A RATING SCALE FOR PRIMARY DEPRESSIVE ILLNESS. Br J Soc Clin Psychol. 1967;6:278. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 23.Brown ES, Vigil L, Khan DA, Liggin JD, Carmody TJ, Rush AJ. A randomized trial of citalopram versus placebo in outpatients with asthma and major depressive disorder: a proof of concept study. Biol Psychiatry. 2005;58(11):865–70. doi: 10.1016/j.biopsych.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 24.Brown ES, Howard C, Khan DA, Carmody TJ. Escitalopram for severe asthma and major depressive disorder: a randomized, double-blind, placebo-controlled proof-of-concept study. Psychosomatics. 2012;53(1):75–80. doi: 10.1016/j.psym.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Rochon J. Sample Size Calculations for Two-Group Repeated-Measures Experiments. Biometrics. 1991;47(4):1383–98. [Google Scholar]
- 26.First MB. Structured clinical interview for the DSM (SCID) Wiley Online Library; 1995. [Google Scholar]
- 27.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26(3):477–86. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 28.Thase ME, Fava M, Halbreich U, Kocsis JH, Koran L, Davidson J, et al. A placebo-controlled, randomized clinical trial comparing sertraline and imipramine for the treatment of dysthymia. Arch Gen Psychiatry. 1996;53(9):777–84. doi: 10.1001/archpsyc.1996.01830090023004. [DOI] [PubMed] [Google Scholar]
- 29.Fuhlbrigge A, Peden D, Apter AJ, Boushey HA, Camargo CA, Jr, Gern J, et al. Asthma outcomes: exacerbations. J Allergy Clin Immunol. 2012;129(3 Suppl):S34–48. doi: 10.1016/j.jaci.2011.12.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. Sage; 2002. [Google Scholar]
- 31.Juniper EF, Svensson K, Mork AC, Stahl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 2005;99(5):553–8. doi: 10.1016/j.rmed.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Alhossan A, Lee CS, MacDonald K, Abraham I. “Real-life” Effectiveness Studies of Omalizumab in Adult Patients with Severe Allergic Asthma: Meta-analysis. The journal of allergy and clinical immunology In practice. 2017 doi: 10.1016/j.jaip.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Gibbons RD, Hur K, Brown CH, Davis JM, Mann JJ. Benefits from antidepressants: synthesis of 6- week patient-level outcomes from double-blind placebo-controlled randomized trials of fluoxetine and venlafaxine. Arch Gen Psychiatry. 2012;69(6):572–9. doi: 10.1001/archgenpsychiatry.2011.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garnock-Jones KP, McCormack PL. Escitalopram: a review of its use in the management of major depressive disorder in adults. CNS drugs. 2010;24(9):769–96. doi: 10.2165/11204760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 35.Arreola R, Becerril-Villanueva E, Cruz-Fuentes C, Velasco-Velazquez MA, Garces-Alvarez ME, Hurtado-Alvarado G, et al. Immunomodulatory effects mediated by serotonin. Journal of immunology research. 2015;2015:354957. doi: 10.1155/2015/354957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mossner R, Mikova O, Koutsilieri E, Saoud M, Ehlis AC, Muller N, et al. Consensus paper of the WFSBP Task Force on Biological Markers: biological markers in depression. World J Biol Psychiatry. 2007;8(3):141–74. doi: 10.1080/15622970701263303. [DOI] [PubMed] [Google Scholar]
- 37.Pena S, Baccichet E, Urbina M, Carreira I, Lima L. Effect of mirtazapine treatment on serotonin transporter in blood peripheral lymphocytes of major depression patients. Int Immunopharmacol. 2005;5(6):1069–76. doi: 10.1016/j.intimp.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Lima L, Urbina M. Serotonin transporter modulation in blood lymphocytes from patients with major depression. Cell Mol Neurobiol. 2002;22(5–6):797–804. doi: 10.1023/A:1021869310702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fazzino F, Montes C, Urbina M, Carreira I, Lima L. Serotonin transporter is differentially localized in subpopulations of lymphocytes of major depression patients. Effect of fluoxetine on proliferation. J Neuroimmunol. 2008;196(1–2):173–80. doi: 10.1016/j.jneuroim.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 40.Menard G, Turmel V, Bissonnette EY. Serotonin modulates the cytokine network in the lung: involvement of prostaglandin E2. Clin Exp Immunol. 2007;150(2):340–8. doi: 10.1111/j.1365-2249.2007.03492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lechin F, van der Dijs B, Orozco B, Jara H, Rada I, Lechin ME, et al. Neuropharmacologic treatment of bronchial asthma with the antidepressant tianeptine: a double-blind, crossover placebo-controlled study. Clin Pharmacol Ther. 1998;64(2):223–32. doi: 10.1016/S0009-9236(98)90156-4. [DOI] [PubMed] [Google Scholar]
- 42.Lechin F, van der Dijs B, Orozco B, Lechin M, Lechin AE. Increased levels of free serotonin in plasma of symptomatic asthmatic patients. Ann Allergy Asthma Immunol. 1996;77(3):245–53. doi: 10.1016/S1081-1206(10)63263-2. [DOI] [PubMed] [Google Scholar]
- 43.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment - Meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101–7. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 44.McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord. 2009;119(1–3):1–8. doi: 10.1016/j.jad.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 45.Soones TN, Lin JL, Wolf MS, O’Conor R, Martynenko M, Wisnivesky JP, et al. Pathways linking health literacy, health beliefs, and cognition to medication adherence in older adults with asthma. J Allergy Clin Immunol. 2017;139(3):804–9. doi: 10.1016/j.jaci.2016.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pariante CM, Thomas SA, Lovestone S, Makoff A, Kerwin RW. Do antidepressants regulate how cortisol affects the brain? Psychoneuroendocrinology. 2004;29(4):423–47. doi: 10.1016/j.psyneuen.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 47.Lowy MT, Reder AT, Antel JP, Meltzer HY. Glucocorticoid resistance in depression: the dexamethasone suppression test and lymphocyte sensitivity to dexamethasone. Am J Psychiatry. 1984;141(11):1365–70. doi: 10.1176/ajp.141.11.1365. [DOI] [PubMed] [Google Scholar]
- 48.Reddy D, Little FF. Glucocorticoid-resistant asthma: more than meets the eye. J Asthma. 2013;50(10):1036–44. doi: 10.3109/02770903.2013.831870. [DOI] [PubMed] [Google Scholar]
- 49.Han YY, Forno E, Marsland AL, Miller GE, Celedon JC. Depression, Asthma, and Bronchodilator Response in a Nationwide Study of US Adults. The journal of allergy and clinical immunology In practice. 2016;4(1):68–73e1. doi: 10.1016/j.jaip.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dougherty RH, Fahy JV. Acute exacerbations of asthma: epidemiology, biology and the exacerbation-prone phenotype. Clin Exp Allergy. 2009;39(2):193–202. doi: 10.1111/j.1365-2222.2008.03157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]