Abstract
The female reproductive hormones progesterone and estrogen regulate network excitability. Fluctuations in the circulating levels of these hormones during the menstrual cycle cause frequent seizures during certain phases of the cycle in women with epilepsy. This seizure exacerbation, called catamenial epilepsy, is a dominant form of drug-refractory epilepsy in women of reproductive age. Progesterone, through its neurosteroid derivative allopregnanolone, increases γ-aminobutyric acid type-A receptor (GABAR)-mediated inhibition in the brain and keeps seizures under control. Catamenial seizures are believed to be a neurosteroid withdrawal symptom, and it was hypothesized that exogenous administration of progesterone to maintain its levels high during luteal phase will treat catamenial seizures. However, in a multicenter, double-blind, phase III clinical trial, progesterone treatment did not suppress catamenial seizures. The expression of GABARs with reduced neurosteroid sensitivity in epileptic animals may explain the failure of the progesterone clinical trial. The expression of neurosteroid-sensitive δ subunit-containing GABARs is reduced, and the expression of α4γ2 subunit-containing GABARs is upregulated, which alters the inhibition of dentate granule cells in epilepsy. These changes reduce the endogenous neurosteroid control of seizures and contribute to catamenial seizures.
Keywords: Catamenial epilepsy, progesterone, neurosteroids, GABAA receptors
Introduction
Epilepsy is a neurological disorder characterized by recurrent unprovoked seizures. The occurrence of seizures is mostly unpredictable; however, cyclicity in seizure incidence is observed in women of reproductive age. The seizure clustering characteristic of catamenial epilepsy occurs due to menstrual cycle-linked hormonal changes. Female reproductive hormones, progesterone and estrogen, modulate network excitability through their neuroactive derivatives called neurosteroids. This review focuses on the role of neurosteroid regulation of seizures via modulation of GABARs.
A temporal pattern of seizure clustering, called catamenial epilepsy, is observed in as many as 30% of women of reproductive age with epilepsy (Frye, 2008;Reddy and Rogawski, 2009;Reddy, 2013;Bazan et al., 2005;Herzog et al., 2004;Duncan et al., 1993;Herzog et al., 1997;Herzog, 2008;Reddy and Rogawski, 2009;Herzog et al., 2015). Catamenial seizures are seen in all types of epilepsy, but, are more prevalent in patients with temporal lobe epilepsy (TLE) (Taubøll et al., 1991;El-Khayat et al., 2008;Duncan et al., 1993;Quigg et al., 2008) and represent a major form of drug refractory epilepsy in women. Based on the time of seizure exacerbation, catamenial seizures can be divided into three patterns; seizure clustering during the perimenstrual phase is classified as type I or C1 and increased seizure incidence during the follicular phase is classified as type II or C2, whereas seizure exacerbation during the luteal phase is classified as type III or C3 (Herzog et al., 1997) (Fig. 1). The fluctuations in progesterone and estrogen levels during the menstrual cycle underlie these patterns of seizure clustering. Estrogen levels rise during the follicular phase and reach a peak at the time of ovulation. On the other hand, progesterone levels rise following ovulation and decline just before the end of the cycle. Progesterone typically exerts anticonvulsant actions, whereas estrogen is a proconvulsant (see below), and the cyclic changes in the levels of progesterone and estrogen are responsible for the different patterns of catamenial seizures. Type I seizure clustering occurs due to withdrawal form high progesterone levels, whereas a high ratio of estrogen to progesterone during the follicular phase is proposed to cause type II seizure clustering (Herzog et al., 1997). In contrast, type III seizures occur due to an inadequate luteal phase in which progesterone levels do not rise to the levels normally seen in healthy women (Herzog et al., 1997). Perimenstrual seizure exacerbation is the most commonly observed pattern of catamenial seizures (Quigg et al., 2008). A clinical diagnosis of catamenial epilepsy is made if the seizure frequency during a particular phase is greater than twice that seen during other phases (Herzog, 2015). The clinical aspects of catamenial epilepsy are discussed elsewhere in this issue. Here, we will focus on the molecular mechanisms regulating catamenial seizures.
Figure 1. Catamenial seizure exacerbation and the menstrual cycle.
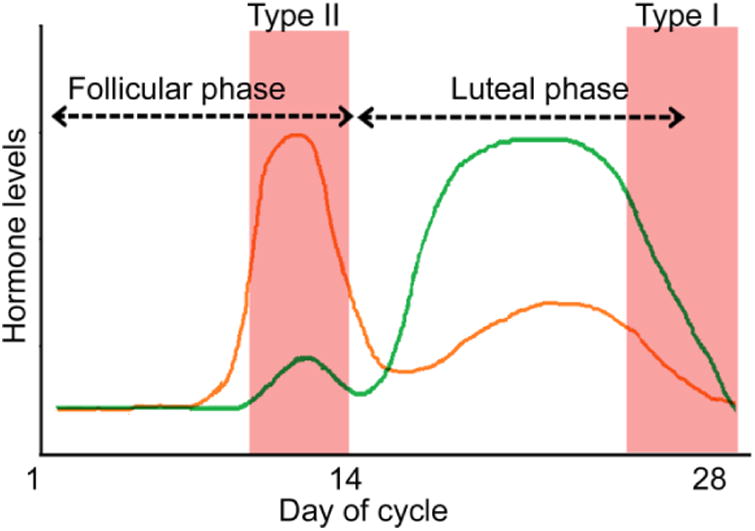
Serum progesterone (green) and estrogen (orange) levels fluctuate during a typical 28-day long menstrual cycle. Estrogen levels rise during the follicular phase, whereas progesterone levels rise during the luteal phase. A mid-cycle high estrogen-to-progesterone ratio underlies type II seizure exacerbation. On the other hand, withdrawal from high progesterone levels at the end of the cycle leads to the increased seizure frequency in type I seizures.
Neurosteroids
Progesterone is metabolized by glia in the brain to compounds called neurosteroids, which can alter inhibitory and excitatory neurotransmission. In addition to progesterone, stress steroids are also a substrate for neurosteroid synthesis. Neurons and glia can also synthesize neurosteroids from cholesterol (Fig. 2), and the neurosteroid levels in the brain can be augmented independent of the levels of circulating steroid hormones (Corpechot et al., 1993;Purdy et al., 1991;Baulieu, 1998). Conversion of cholesterol to allopregnanolone involves multiple steps regulated by an array of enzymes. Cytoplasmic cholesterol is first transported to the inner mitochondrial membrane by the steroidogenic acute regulatory protein (StAR, also called translocator protein (TSPO) or peripheral benzodiazepine receptors), then enzyme P450 side-chain cleavage breaks it down to progesterone, which can enter the allopregnanolone synthetic pathway (Rupprecht et al., 2009;Korneyev et al., 1993;Papadopoulos et al., 2006;Le et al., 1987). The enzymes involved in neurosteroid synthesis are expressed in almost all regions of the brain, as their mRNA and protein can be detected in the principal neurons of the cortex, hippocampus, thalamus, amygdala, and hypothalamus (King et al., 2002;Stoffel-Wagner et al., 2000;Stoffel-Wagner et al., 2003;Stoffel-Wagner, 2003;Petratos et al., 2000;Melcangi et al., 1998;Stoffel-Wagner, 2001;Ibanez et al., 2003;Kimoto et al., 2002). Interestingly GABAergic interneurons are devoid of neurosteroid synthetic enzymes (Agís-Balboa et al., 2006); since neurosteroids synthesized within interneurons could dampen their activity and affect GABA release on principal neurons, the absence of neurosteroid synthetic machinery in interneurons could function to protect the activity of interneurons. Many species of amphibians, birds, and mammals appear to express neurosteroid synthetic enzymes in the brain, suggesting that endogenous neurosteroids may regulate excitability across vertebrates (Do Rego et al., 2009).
Figure 2. Neurosteroid synthetic pathway in the brain.
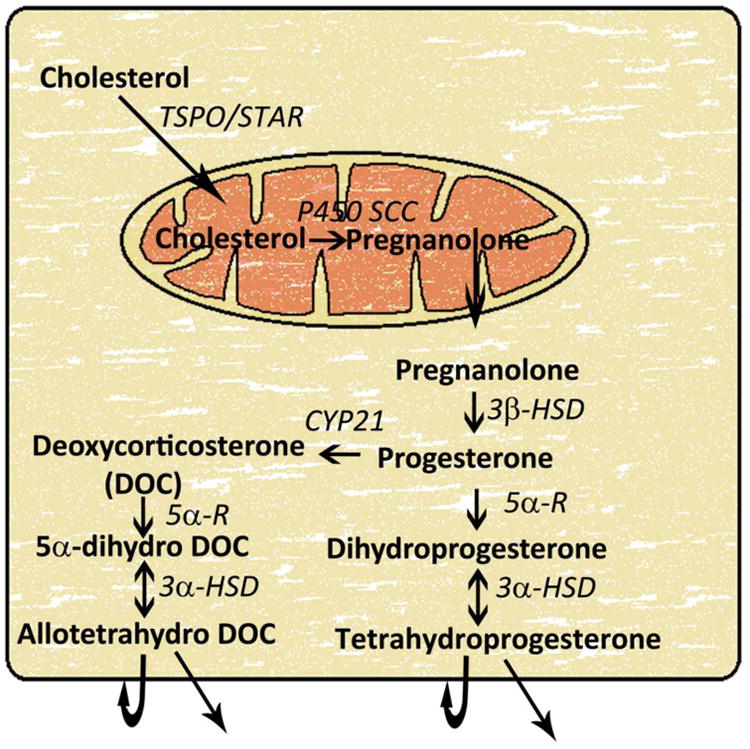
Cytoplasmic cholesterol is transferred to the inner mitochondrial membrane by steroidogenic acute regulatory protein (StAR), also called translocator protein (TSPO), or peripheral benzodiazepine receptors. In the mitochondria, cholesterol is converted to pregnanolone by enzyme cytochrome P450 side-chain cleavage (P450scc) in a rate-limiting step. Pregnanolone is transferred back to the cytoplasm and gets converted to progesterone by enzyme 3β-hydroxysteroid dehydrogenase (3β-HSD). Circulating progesterone can also enter the neurosteroid synthetic pathway at this step. Subsequently, progesterone is converted to dihydroprogesterone by the enzyme 5α-reducatse (5α-R) in the 2nd rate-limiting step in this biosynthetic pathway. Finally, dihydroprogesterone is converted to tetrahydroprogesterone (THP), also called allopregnanolone, by the enzyme 3α-hydroxysteroid dehydrogenase (3α-HSD). Progesterone can also be converted to deoxycorticosterone and then to allotetrahydrodeoxycorticosterone (THDOC). Both allopregnanolone and allotetrahydrodeoxycorticosterone can activate GABAA receptors expressed in the same cell or surrounding cells.
Impaired neurosteroid synthesis in the brain can cause epilepsy. Patients of infantile-onset epileptic encephalopathy protocadherin-19 female-limited epilepsy (PCDH19-FE) have lower serum neurosteroid levels and altered expression of some of the neurosteroid synthetic enzymes (Tan et al., 2015). Whether neurosteroid levels in patients with acquired epilepsy are also lower has not been studied. However, many women with epilepsy experience disorders of reproductive endocrine function (Herzog et al., 1986), which may limit the availability of circulating progesterone for allopregnanolone synthesis. A recent study has found that serum neurosteroid levels are reduced in patients in status epilepticus, which is a disorder characterized by self-sustaining seizures that can last for days to months (Meletti et al, 2017). Serum neurosteroid levels also increase as pregnancy progresses in women (Pennell et al, 2015). A similar measurement of serum neurosteroid levels in epilepsy patients could enhance our understanding of how spontaneous seizures affect endogenous neurosteroid synthesis.
The reproductive cycle of female epileptic animals is also affected (Scharfman et al., 2009;Amado et al., 1987), and the experimental animals may provide a useful system to understand whether endogenous neurosteroid synthetic machinery is impaired by spontaneous seizures. Acquired epilepsy develops following inciting brain insults such as febrile seizures, brain trauma, infection, status epilepticus, and brain tumors. Blockade of endogenous neurosteroid synthesis hastens the onset of spontaneous seizures (Joshi et al., 2017;Biagini et al., 2009;Biagini et al., 2010;Biagini et al., 2006). We found that even a transient blockade of neurosteroid synthesis can accelerate epileptogenesis. Treatment of animals with finasteride, an inhibitor of enzyme 5α-reductase that regulates the rate-limiting step in allopregnanolone synthesis, on a single day following status epilepticus led to an early onset of spontaneous seizures (Joshi et al., 2017). The expression of enzyme cytochrome P450 side-chain cleavage is transiently upregulated in the hippocampus following statue epilepticus (Biagini et al., 2009). Whether this plays a compensatory role is not known. A detailed characterization of the expression of neurosteroidogenic enzymes during epileptogenesis and in epileptic animals will enhance our understanding of epilepsy and provide insights into whether neurosteroid synthetic enzymes can be targeted for therapeutic purposes.
Neurosteroid regulation of seizures
Progesterone is an endogenous anticonvulsant agent, and an inverse correlation exists between progesterone levels and seizure frequency (Bäckström, 1976). Infusion of progesterone suppresses ictal activity in human EEG (Bäckström, 1984). Similarly, acute administration of progesterone also reduces the susceptibility to seizures evoked by chemical or electrical stimulation in experimental animals (Frye et al., 2002;Frye and Scalise, 2000;Kokate et al., 1999a;Reddy et al., 2004;Reddy and Ramanathan, 2012). Multiple lines of evidence show that the anticonvulsant effects of progesterone are mediated through its conversion to allopregnanolone. Progesterone cannot exert anticonvulsant effects in animals treated with finasteride or in mice that lack the expression of enzyme 5α-reductase (Frye et al., 2002;Kokate et al., 1999a;Reddy et al., 2004). Furthermore, administration of allopregnanolone or the stress steroid derivative tetrahydrodeoxycorticosterone can suppress seizures evoked by chemical or electrical stimulation on their own (Frye and Scalise, 2000;Kokate et al., 1994;Kokate et al., 1996;Kokate et al., 1999a;Reddy et al., 2004). Progesterone and neurosteroids also increase the threshold to kindling (Carter et al., 1997;Holmes and Weber, 1984;Reddy et al., 2010;Edwards et al., 2001;Holmes and Weber, 1984;Reddy and Ramanathan, 2012).
All of the above studies were performed in naïve animals; since seizures can affect the expression of neurosteroid synthetic enzymes as well as their cellular targets, it is important to study the anticonvulsant effects of neurosteroids in epileptic animals. Reddy and colleagues (2011) have developed an animal model of catamenial epilepsy in which high progesterone levels are induced by sequential treatment with pregnant mare serum gonadotropin (PMSG) followed by human chorionic gonadotropin (β-HCG); neurosteroid withdrawal, similar to that observed during the perimenstrual period in women, is then triggered by administration of finasteride. Lawrence and colleagues used this model of catamenial seizures in epileptic animals to determine the effects of elevated progesterone levels and subsequent neurosteroid withdrawal on the frequency of spontaneous seizures (Lawrence et al., 2010). They found that PMSG and β-HCG treatment induced a 2-3-fold increase in serum progesterone levels; however, the frequency of spontaneous seizures did not differ between the treated and untreated animals (Lawrence et al., 2010). Furthermore, finasteride treatment not only caused a dramatic increase in seizure frequency in PMSG and β-HCG-treated animals, it also increased seizure frequency in animals with basal levels of progesterone. Thus, in contrast to the findings in non-epileptic female animals, a chronic rise in progesterone levels did not suppress spontaneous seizures in epileptic animals.
The findings of Lawrence and colleagues are similar to the findings of a recent clinical trial of progesterone therapy for catamenial seizures. Since catamenial seizures are believed to be a neurosteroid withdrawal disorder, elevating progesterone levels by twice daily treatment for 14 days was proposed to suppress catamenial seizures (Herzog et al., 2012). However, in this phase III, double-blind, multicenter trial, an equal fraction of women treated with progesterone and placebo treatment reported a reduction in seizure frequency. Thus, chronic progesterone treatment failed to suppress catamenial seizures in patients.
There are reports of a few clinical studies showing suppression of catamenial seizures by progesterone treatment and one anecdotal study, in which a woman on progesterone therapy experienced more seizures when she was treated with finasteride for male pattern baldness (Herzog, 1995;Herzog and Frye, 2003). There may be some protective effect in women with an identified type I seizure exacerbation pattern (Herzog and Frye, 2014). One factor that could contribute to the reduced efficacy of neurosteroids and the failure of the clinical trial is that the expression of GABARs, which are targets of neurosteroids in the brain (see below), is altered. Thus, additional studies are necessary to gain insight into acute versus the chronic effects of progesterone in epileptic and non-epileptic animals.
Neurosteroids and GABARs
The anticonvulsant effects of neurosteroids are mediated via their action on GABARs, which are GABA-gated chloride channels. Activation of GABARs leads to chloride influx in a majority of instances and causes membrane hyperpolarization that dampens excitation. GABARs are assembled from various subunits α, β, γ, δ, and ε, and are expressed throughout the brain and spinal cord (Sieghart, 2006;Whiting, 2003). Some of the subunits have multiple isoforms, whereas others are represented by a single isoform; for example, there are six α subunits (α1 to α6), three β subunits (β1 to β3), and 3 γ subunits (γ1 to γ3), whereas the δ and ε subunits have only one isoform (Whiting et al., 1999). A majority of GABARs are composed of 2 α, 2 β and a γ, δ, or ε subunit (Baumann et al., 2001), and there is region-specific expression of receptors with a specific subunit assembly. For example, α4βxδ subunit-containing receptors are expressed on the extrasynaptic membrane of hippocampal dentate granule cells (DGCs) and thalamic nuclei, whereas α6βxδ subunit-containing receptors are expressed on cerebellar granule cells (Sur et al., 1999;Bencsits et al., 1999;Quirk et al., 1994;Pirker et al., 2000). In contrast, hilar interneurons express α1βxδ subunit-containing receptors (Glykys et al., 2007;Milenkovic et al., 2013;Peng et al., 2004). The γ2 subunits exhibit substantial diversity in partnering with α subunits; receptors containing γ2 subunits that contain α1, α2, α4, or α5 subunits are found under pathophysiological conditions (Glykys et al., 2008;Rajasekaran et al., 2010;Sieghart and Sperk, 2002). Receptors containing only α and β subunits also appear to be expressed on cultured hippocampal pyramidal neurons (Mortensen and Smart, 2006); whether these receptors are expressed in vivo is unclear.
Each subunit is made up of an ‘N’ terminal extracellular domain followed by four transmembrane domains and a short ‘C’ terminal extracellular domain (Fig. 3). The 2nd transmembrane domain lines the ion-channel pore. The intracellular loop joining the transmembrane domains 3 and 4 contains sites for phosphorylation by different kinases such as protein kinase C and protein kinase A (Jacob et al., 2008). This region also interacts with proteins such as GABAR-associated protein (GABARAP) (Jacob et al., 2008). GABAR subunit composition regulates the surface membrane localization and dynamics of trafficking of the receptors. The γ2 subunit-containing receptors are clustered at the synapses through their interaction with gephyrin (Essrich et al., 1998;Sun et al., 2004;Nusser et al., 1998;Zhang et al., 2007;Alldred et al., 2005). On the other hand, receptors lacking γ subunits are restricted to the peri- or extrasynaptic membrane (Nusser et al., 1998;Wei et al., 2003;Sun et al., 2004).
Figure 3. GABAA receptor subunit composition and pharmacology.
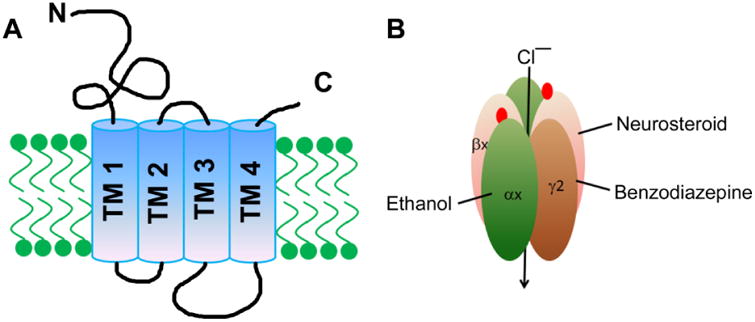
A: A schematic diagram illustrating the structure of GABAR subunits. Each subunit is made up of an “N” terminal extracellular domain, four transmembrane domains (TM1-4), and a short “C” terminal extracellular domain. The intracellular loop between TM3 and TM4 contains residues that play a critical role in the surface membrane trafficking of these receptors. The Cys-loop in the “N” terminal extracellular domain is shown in red. B: Structure of a GABAR showing GABA (red circles) binding sites between the α and β subunits, the benzodiazepine binding site between the α and γ subunits, and the neurosteroid binding site.
Synaptic and extrasynaptic GABARs mediate distinct forms of inhibition, phasic and tonic inhibition, respectively (Nusser and Mody, 2002;Mtchedlishvili and Kapur, 2006;Glykys and Mody, 2007b;Glykys and Mody, 2007a;Glykys et al., 2008). Release of GABA at the synaptic cleft triggers synaptic currents that can be measured as spontaneous or miniature inhibitory post-synaptic currents (Fig. 4). On the other hand activation of extrasynaptic receptors leads to a persistent background current that can be measured in terms of the holding current or the membrane noise (Nusser and Mody, 2002;Mtchedlishvili and Kapur, 2006) (Fig. 4). The δ subunit-containing GABARs desensitize slowly and incompletely (Saxena and Macdonald, 1994); thus, once opened, these receptors remain open for a long time. By virtue of these unique properties, the δ subunit-containing receptors mediate a major fraction of the tonic current, which is substantially attenuated in mice lacking δ subunit expression (Maguire et al., 2005;Stell et al., 2003).
Figure 4. Neurosteroid modulation of synaptic and extrasynaptic receptors of DGCs.
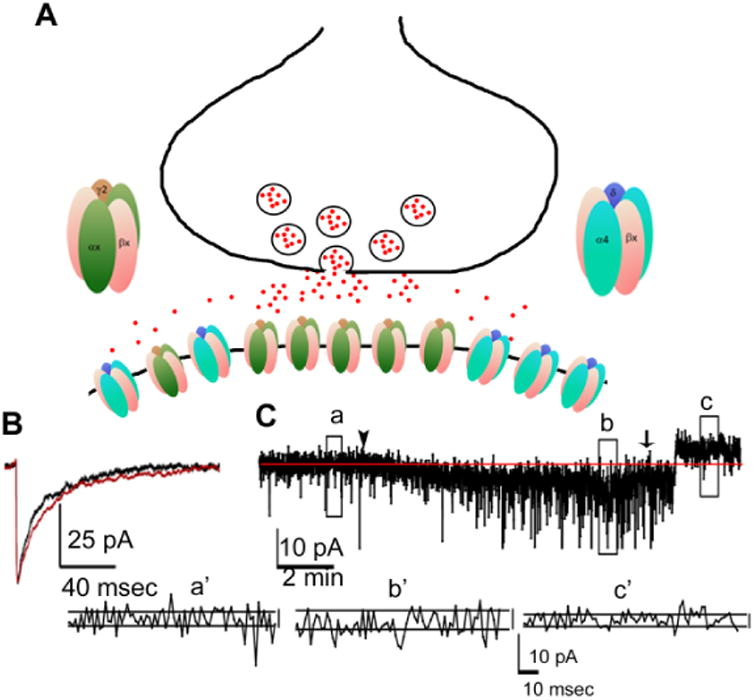
A: A schematic diagram showing the expression of γ2 subunit-containing receptors at the synaptic and extrasynaptic sites on the membrane of DGCs where they are exposed to GABA released from the presynaptic terminal. The excess GABA in the synaptic cleft can spread to extrasynaptic sites to activate α4βxδ subunit-containing receptors. B: A representative averaged miniature inhibitory post-synaptic current showing prolongation of decay after application of allopregnanolone (10 nM, red trace). C: A trace illustrating allopregnanolone modulation of tonic current. Allopregnanolone (10 nM) bath application was started at the arrowhead and it increased the holding current. Once a stable response was obtained, bath application of the GABAR blocker picrotoxin (50 μM) was started (arrow), and it reduced the holding current. Traces below (a′, b′ and c′) show membrane noise, which is another measure of tonic current, at baseline (a), after application of allopregnanolone (b), and after application of picrotoxin (c).
The subunit composition also determines the pharmacological properties of GABARs (Olsen et al., 2007;Olsen and Sieghart, 2009;Sieghart, 2006). The GABA affinity of δ subunit-containing receptors is higher than the γ2 subunit-containing GABARs (Saxena and Macdonald, 1994). Thus, these receptors are activated by the low nanomolar concentrations of GABA that spill over the synaptic cleft and/or are released by glia (Glykys and Mody, 2007b). The δ subunit-containing receptors are also more sensitive to alcohol and neurosteroids than those containing a γ2 subunit (Olsen et al., 2007;Olsen and Sieghart, 2009;Sieghart, 2006). In contrast, the δ subunit-containing GABARs are insensitive to benzodiazepines, which are the widely used anticonvulsant agents that allosterically activate γ2 subunit-containing receptors that do not contain α4 or α6 subunits (Olsen et al., 2007;Olsen and Sieghart, 2009;Sieghart, 2006).
Neurosteroids can activate many GABAR subtypes expressed in the brain (Majewska et al., 1986;Maitra and Reynolds, 1998;Belelli et al., 2002;Puia et al., 1993;Puia et al., 1990). Neurosteroids are lipophilic in nature and can access the binding site from the inside or outside of the cell (Akk et al., 2005;Chisari et al., 2010). The neurosteroid binding site is localized in the interphase between the α and β subunits (Hosie et al., 2006), and there is a stringent structural requirement for the binding of neurosteroids to the receptors (Wittmer et al., 1996). Site-directed mutagenesis studies have helped identify residues that are critical for neurosteroid binding (Hosie et al., 2006). The α1 subunit residue T236 is critical for direct activation of GABARs, whereas the residue Q241 is important for allosteric modulation and activation. Furthermore, residues N407 and Y410 are also involved in binding to neurosteroids. In addition to these residues in the α1 subunit, residue T284 of the β2 subunit is also involved i n the direct activation of GABARs (Hosie et al., 2006). The binding of neurosteroids to residues between TM1 and TM4 of the α1 subunit is proposed to lead to an allosteric modulation of GABARs, whereas neurosteroid binding to a site between α and β subunits leads to direct receptor activation. Recently, another site F301, in the β3 subunits, has also been identified to bind to neurosteroids, although the physiological role of this interaction is currently unclear (Chen et al., 2012). Neurosteroids at nanomolar concentrations act as positive allosteric modulators, whereas at micromolar concentrations, these agents activate the receptors even in the absence of GABA (Callachan et al., 1987;Puia et al., 1990). The residue T236 is involved in the allosteric action, whereas the residue Q241 is involved in the direct agonist-like action (Hosie et al., 2006).
GABARs with known subunit composition expressed in exogenous systems such as HEK293 cells have been valuable in understanding the mechanism of neurosteroid action. One such study found that neurosteroids could enhance the current evoked by low concentrations of GABA but not the current evoked by saturating concentrations of GABA (Bianchi and Macdonald, 2003), indicating that neurosteroids increase the GABA efficacy of these receptors. This is particularly important for δ subunit-containing receptors, as the GABA efficacy of these receptors is low and neurosteroids convert GABA from a partial to full agonist at these receptors. The neurosteroid potentiation of GABARs occurs by increasing the frequency of channel opening and prolonging the duration of the channel open time (Twyman and Macdonald, 1992;Bianchi et al., 2002;Ramakrishnan and Hess, 2010). Furthermore, neurosteroids potentiate the δ subunit-containing receptors more than the γ2 subunit-containing receptors (Wohlfarth et al., 2002). These findings from an exogenous expression system are supported by findings in δ-subunit knockout animals. In these animals, the sedative and anxiolytic effects of neurosteroids were attenuated (Mihalek et al., 1999). Furthermore, neurosteroids also fail to enhance the tonic current of DGCs of δ-subunit knockout animals (Stell et al., 2003).
Neurosteroid regulation of GABARs in epilepsy
Removal of neurosteroid control partially underlies the recurrent spontaneous seizures seen in epileptic animals. Because the hippocampus is primarily involved in seizure generation in temporal lobe epilepsy, changes in GABAR expression and pharmacology have been extensively studied in animal models of TLE. The GABARs expressed on DGCs of epileptic animals have reduced neurosteroid sensitivity, in addition to other changes in the pharmacological properties of these receptors (Rajasekaran et al., 2010;Zhang et al., 2007;Brooks-Kayal et al., 1998;Gonzalez et al., 2013;Mtchedlishvili et al., 2001;Sun et al., 2007;Gibbs, et al., 1997;Joshi et al., 2017). Both the synaptic and extrasynaptic GABARs expressed on DGCs of epileptic animals have reduced neurosteroid sensitivity (Mtchedlishvili et al., 2001). Neurosteroids at physiological concentrations fail to enhance the synaptic and tonic GABAR currents in DGCs of epileptic animals (Rajasekaran et al., 2010;Zhang et al., 2007;Sun et al., 2007). The ongoing neurosteroid synthesis shape GABAR currents, and neurosteroids exert a control on network excitability through GABARs (Keller et al., 2004;Stell et al., 2003;Walker and Semyanov, 2008). Our studies revealed that δ-GABAR expression is reduced as early as 4 days following SE, whereas γ2 subunit upregulation coincided with the onset of spontaneous seizures between 10-14 days following SE (Joshi et al., 2017). Thus, the reduced neurosteroid control during the latent period could contribute to the dentate gating function, which is compromised during epileptogenesis (Heinemann et al., 1992;Lothman et al., 1992;Pathak et al., 2007;Stringer and Lothman, 1989). Furthermore, even though the tonic current appears to be unaltered and the synaptic currents are augmented in epileptic animals, the inhibition of DGCs is likely to be reduced in vivo. The neurosteroid sensitivity of GABARs expressed on cortical neurons of kindled animals is also reduced, and this reduction is associated with dephosphorylation of GABARs (Gavrilovici et al., 2006;Kia et al., 2011). Whether neurosteroid modulation of other principal neurons of the trisynaptic circuit as well as that of thalamic neurons, which also express δ-GABARs, is altered in epilepsy is currently not known.
Altered GABAR expression in epileptic animals underlies the reduced neurosteroid sensitivity of these receptors (Raol et al., 2006;Rajasekaran et al., 2010;Zhang et al., 2007;Sperk et al., 1998;Tsunashima et al., 1997;Brooks-Kayal et al., 1998;Gonzalez et al., 2013;Lund et al., 2008;Peng et al., 2004). Some of these alterations have also been observed in tissue resected from epilepsy patients (Loup et al., 2000;Loup et al., 2006;Palma et al., 2005). The expression of δ and α1 subunit mRNA and protein is reduced in DGCs of epileptic animals, whereas that of α4 and γ2 subunits is increased (Raol et al., 2006;Rajasekaran et al., 2010;Zhang et al., 2007;Sperk et al., 1998;Tsunashima et al., 1997;Peng et al., 2004). The reduction in δ and α1 subunit expression occurs before the onset of spontaneous seizures and could play a role in epileptogenesis (Joshi et al., 2017;Peng et al., 2004). The NMDA receptor activation that occurs during status epilepticus in experimental animals appears to reduce the δ subunit expression, whereas the reduced α1 subunit expression is triggered by activation of the JAK/STAT pathway (Lund et al., 2008;Joshi et al., 2017). Preventing the reduction in α1 subunit expres s ion also suppresses the onset of spontaneous seizures (Raol et al., 2006). Whether blocking the δ subunit expression also prevents epileptogenesis has not been tested. However, mice lacking δ subunit expression have an increased seizure susceptibility, indicating that the δ subunit may play a role in epileptogenesis (Spigelman et al., 2002). In addition, we have shown that activation of ERK1/2 plays a role in the NMDA-triggered reduction in δ subunit expression in cultured hippocampal neurons (Joshi and Kapur, 2013). ERK1/2 are activated during SE and following recurrent spontaneous seizures, and animals with a constitutive activation of ERK1/2 develop epilepsy (Berkeley et al., 2002;Garrido et al., 1998;Kim et al., 1994;Houser et al., 2008;Nateri et al., 2007). These findings suggest that NMDAR activation during SE could increase ERK1/2 signaling, resulting in down-regulation of δ subunit expression and reduced neurosteroid sensitivity of the tonic current.
Potentially compensatory changes in GABARs are seen in epileptic animals; the most prominent alteration includes upregulation of α4γ2 subunit-containing receptors (Rajasekaran et al., 2010;Zhang et al., 2007;Lund et al., 2008). However, the upregulation of these receptors does not occur until the onset of spontaneous seizures (Joshi et al., 2017;Peng et al., 2004). Furthermore, these receptors are not as sensitive to neurosteroids as α4δ or α1γ2 subunit-containing receptors (Belelli et al., 2002;Rajasekaran et al., 2010;Sun et al., 2007;Zhang et al., 2007). The differential time course of changes in GABAR expression is likely to make the brain vulnerable during the epileptogenic period (Pathak et al., 2007). Removal of neurosteroid control of excitability could be one of the mechanisms that contribute to epileptogenesis. Indeed, in one study, daily treatment of animals with finasteride, which inhibits the enzyme 5α-reductase and blocks the ongoing neurosteroid synthesis led to an early onset of epilepsy (Biagini et al., 2009). We found that δ subunit expression is reduced on the 4th day following SE, and a single day of endogenous neurosteroid synthesis blockade on the 4th day following SE accelerated epileptogenesis (Joshi et al., 2017).
Notably, these changes may not be uniform in all models of epilepsy. For example, in the controlled cortical impact model of traumatic brain injury, the tonic current was enhanced, and the expression of the δ subunit increased (Mtchedlishvili et al., 2010;Kharlamov et al., 2011). Furthermore, the frequency of spontaneous seizures may also affect the expression of GABAR subunits (González et al., 2015). The expression of some of the proteins, such as gephyrin and glutamate receptor-interacting protein (GRIP), associated with GABAR trafficking and/or surface membrane anchoring is also reduced following SE (Gonzalez et al., 2013). Whether their expression is also down-regulated in epileptic animals is currently not known.
Neurosteroid regulation of GABAR trafficking and expression
The number of receptors expressed at the surface membrane is regulated by constitutive endocytosis and insertion. We and others have shown that the rate of insertion of synaptic γ2 subunit-containing receptors is rapid, and new receptors can appear at the surface membrane within minutes (Joshi and Kapur, 2009;Joshi et al., 2013b;Bogdanov et al., 2006). In contrast, the insertion of δ subunit-containing receptors is slower than that of γ2 subunit-containing receptors. Interestingly, GABARs containing α4βxδ subunits are inserted at the surface membrane at a faster rate than the receptors containing α1βxδ subunits (Joshi et al., 2013b), which suggests that α subunits influence the trafficking of δ subunit-containing GABARs. In contrast, the rate of insertion of α4βxγ2 or α1βxγ2 subunit-containing r eceptors is similar; thus, the γ2 subunit plays a dominant role in regulating the rate of insertion. The rate of internalization of γ2 subunit-containing receptors is also faster than those containing δ subunits (Joshi and Kapur, 2009). The mechanisms regulating trafficking of synaptic receptors have been extensively studied (Michels and Moss, 2007). In contrast, the mechanisms that regulate trafficking of δ subunit-containing receptors are poorly understood. Neurosteroids appear to enhance cell surface expression of GABARs through receptor phosphorylation (Abramian et al., 2014). However, these studies were performed in α4β3 subunit-containing receptors, and whether neurosteroid regulation of surface expression of δ and γ2 subunit-containing receptors is identical or distinct is not known.
Progesterone, estrogen, and neurosteroids also influence GABAR subunit expression. Estrus cycle-linked fluctuations in progesterone alter the hippocampal expression of δ and γ2 subunits (Maguire et al., 2005;Wu et al., 2013). High progesterone levels correlate with a greater expression of δ subunit-containing GABARs and a lower expression of γ2 subunit-containing GABARs. These estrus cycle-linked fluctuations in GABAR expression persist in animals lacking progesterone receptor expression or when progesterone receptors are blocked but are prevented if allopregnanolone synthesis is inhibited by treatment of animals with finasteride (Maguire and Mody, 2007;Wu et al., 2013). Thus, neurosteroids appear to regulate δ subunit expression (Follesa et al., 2004). In accordance with the role of tonic current and neurosteroids in the regulation of network excitability (Semyanov et al., 2004;Walker and Semyanov, 2008), these changes in the expression of δ subunit expression influence seizure susceptibility. The mice in the diestrus stage that have higher progesterone levels and a greater expression of δ subunit-containing receptors are less susceptible to seizures than mice in the estrus stage of the cycle (Maguire et al., 2005).
Puberty, pregnancy and postpartum are also associated with considerable changes in the hormonal milieu. In accordance with the high progesterone levels during pregnancy, the expression of the δ subunit is also increased during pregnancy, and that of the γ2 subunit is reduced (Sanna et al., 2009;Concas et al., 1998;Concas et al., 1999). The expression of both of these subunits returns to baseline following pregnancy. In contrast, the expression of the α4 subunit is increased following postpartum as a result of withdrawal from high progesterone and neurosteroid levels (Sanna et al., 2009;Concas et al., 1998;Concas et al., 1999;Smith et al., 1998a;Smith et al., 1998b).
Sulfated steroids
In contrast to the anticonvulsant effects of reduced neurosteroids, sulfated neurosteroids exert an excitatory, proconvulsant action. The effects of the sulfated progesterone derivative, pregnanolone sulfate (PS), on seizures and neurotransmission have been studied in vitro and in vivo. Administration of PS increases the convulsant potency of NMDA, reduces the concentration of PTZ necessary to trigger convulsions, and decreases the latency to PTZ-evoked seizures (Kokate et al., 1999b;Reddy and Kulkarni, 1998;Maione et al., 1992;Reddy and Kulkarni, 1998). An intracerebroventricular infusion of PS causes seizures, whereas its infusion into the hippocampus causes prolonged seizures of status epilepticus (Kokate et al., 1999b;Williamson et al., 2004). Sulfated steroids reduce GABA release from presynaptic terminals and affect inhibition (Mtchedlishvili and Kapur, 2003). Other studies have shown that sulfated steroids may also potentiate NMDARs (Majewska and Schwartz, 1987;Majewska et al., 1988;Wu et al., 1991).
Proconvulsant effects of estrogens
Estrogens have a proconvulsant effect (Veliskova and DeSantis, 2013;Veliskova, 2006;Veliskova, 2007;Frye, 2008;Reddy, 2013). A positive relationship has been observed between the levels of estrogens and seizures in women with epilepsy (Backstrom, 1976). Administration of estrogens to experimental animals also increases seizure susceptibility (Woolley and Timiras, 1962;Woolley, 2000;Edwards et al., 1999), whereas blocking estrogen synthesis can even suppress prolonged seizures of status epilepticus (Sato and Woolley, 2016). These excitatory actions of estrogens are mediated through increased glutamatergic transmission (Smith et al., 1987;Smith et al., 1988;Smejkalova and Woolley, 2010;Oberlander and Woolley, 2016). Estrogens enhance the expression of the GluA1 subunit of AMPA receptors via activation of estrogen receptor (ER)-β (Liu et al., 2008;Tada et al., 2015). Estrogens also increase the number of dendritic spines that harbor glutamatergic synapses on CA1 neurons (Woolley and McEwen, 1992;Woolley and McEwen, 1993). In addition, acute application of estrogen to hippocampal slices also suppressed the amplitude of GABAR-mediated IPSCs of CA1 pyramidal neurons by decreasing the probability of GABA release (Huang and Woolley, 2012). Thus, the proconvulsant effects of estrogen involve the reduction of inhibition and activation of excitatory mechanisms.
Conclusions
Menstrual cycle-linked fluctuations in estrogen and progesterone, which exert proconvulsant and anti-convulsant effects respectively, underlie catamenial seizure exacerbation. The progesterone derivative allopregnanolone exerts anticonvulsant actions via potentiation of GABAR-mediated inhibitory transmission in the brain. However, the neurosteroid sensitivity of GABARs is reduced in epilepsy. The mechanisms that trigger the reduction in the expression of neurosteroid-sensitive δ-GABARs in epilepsy are not known. Identifying these mechanisms can provide additional therapeutic targets to alleviate catamenial seizures.
Furthermore, despite catamenial epilepsy being primarily a neurosteroid-withdrawal disorder, prolonged progesterone treatment has failed to exert beneficial effects. Thus, additional studies are warranted to understand the effects of acute and prolonged progesterone treatment in the brain. While progesterone effects mediated by neurosteroids have been extensively studied over the last few decades, the effects mediated via progesterone receptors remain underexplored. Progesterone receptors are ligand-activated nuclear hormone receptors that can regulate gene expression (Conneely et al., 1987;Mani and Oyola, 2012;Singh and Su, 2013). These receptors are widely expressed in the brain (Mitterling et al., 2010), and their signaling may be significant when progesterone treatment is performed over a longer period of time.
Highlights.
Female reproductive cycle-linked seizure exacerbation
Anticonvulsant effects of progesterone and allopregnanolone
Neurosteroid potentiation of GABAA receptor-mediated inhibition
Expression of GABAA receptors with reduced neurosteroid sensitivity in DGCs of epileptic animals
Acknowledgments
This study was supported by NIH grants RO1 NS 040337 and RO1 NS 044370 to JK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramian AM, Comenencia-Ortiz E, Modgil A, Vien TN, Nakamura Y, Moore YE, Maguire JL, Terunuma M, Davies PA, Moss SJ. Neurosteroids promote phosphorylation and membrane insertion of extrasynaptic GABAA receptors. Proc Natl Aca Sci USA. 2014;111:7132–7137. doi: 10.1073/pnas.1403285111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agís-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Aca Sci USA. 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Shu HJ, Wang C, Steinbach JH, Zorumski CF, Covey DF, Mennerick S. Neurosteroid access to the GABAA receptor. J Neurosci. 2005;25:11605–11613. doi: 10.1523/JNEUROSCI.4173-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alldred MJ, Mulder-Rosi J, Lingenfelter SE, Chen G, Luscher B. Distinct γ2 subunit domains mediate clustering and synaptic function of postsynaptic GABAA receptors and gephyrin. J Neurosci. 2005;25:594–603. doi: 10.1523/JNEUROSCI.4011-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amado D, Verreschi IT, Berzaghi MP, Cavalheiro EA. Effects of intrahippocampal injection of kainic acid on estrous cycle in rats. Braz J Med Biol Res. 1987;20:829–832. [PubMed] [Google Scholar]
- Bäckström T, Zetterlund B, Blom S, Romano M. Effects of intravenous progesterone infusions on the epileptic discharge frequency in women with partial epilepsy. Acta Neurol Scand. 1984;69:240–248. doi: 10.1111/j.1600-0404.1984.tb07807.x. [DOI] [PubMed] [Google Scholar]
- Bäckström T. Epileptic seizures in women related to plasma estrogen and progesterone during the menstrual cycle. Acta Neurol Scand. 1976;54:321–347. doi: 10.1111/j.1600-0404.1976.tb04363.x. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Neurosteroids: A novel function of the brain. Psychoneuroendocrinology. 1998;23:963–987. doi: 10.1016/s0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. Subunit arrangement of GABAA receptors. J Biol Chem. 2001;276:36275–36280. doi: 10.1074/jbc.M105240200. [DOI] [PubMed] [Google Scholar]
- Bazan AC, Montenegro MA, Cendes F, Min LL, Guerreiro CA. Menstrual cycle worsening of epileptic seizures in women with symptomatic focal epilepsy. Arq Neuropsiquiatr. 2005;63:751–756. doi: 10.1590/s0004-282x2005000500006. [DOI] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Bencsits E, Ebert V, Tretter V, Sieghart W. A significant part of native GABAA receptors containing α4 subunits do not contain γ or δ subunits. J Biol Chem. 1999;274:19613–19616. doi: 10.1074/jbc.274.28.19613. [DOI] [PubMed] [Google Scholar]
- Berkeley JL, Decker MJ, Levey AI. The role of muscarinic acetylcholine receptor-mediated activation of ERK1/2 in pilocarpine-induced seizures. J Neurochem. 2002;82:192–201. doi: 10.1046/j.1471-4159.2002.00977.x. [DOI] [PubMed] [Google Scholar]
- Biagini G, Longo D, Baldelli E, Zoli M, Rogawski MA, Bertazzoni G, Avoli M. Neu rosteroids and epileptogenesis in the pilocarpine model: evidence for a relationship between P450scc induction and length of the latent period. Epilepsia. 2009;50(s1):53–58. doi: 10.1111/j.1528-1167.2008.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagini G, Panuccio G, Avoli M. Neurosteroids and epilepsy. Curr Opin Neurol. 2010;23:170–176. doi: 10.1097/WCO.0b013e32833735cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagini G, Baldelli E, Longo D, Pradelli L, Zini I, Rogawski MA, Avoli M. Endogenous neurosteroids modulate epileptogenesis in a model of temporal lobe epilepsy. Exp Neurol. 2006;201:519–524. doi: 10.1016/j.expneurol.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Haas KF, Macdonald RL. α1 and α6 subunits specify distinct desensitization, deactivation and neurosteroid modulation of GABAA receptors containing the δ subunit. Neuropharmacology. 2002;43:492–502. doi: 10.1016/s0028-3908(02)00163-6. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABAA receptor channels from low- to high-efficacy gating patterns. J Neurosci. 2003;23:10934–10943. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanov Y, Michels G, Armstrong-Gold C, Haydon PG, Lindstrom J, Pangalos M, Moss SJ. Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts. EMBO J. 2006;25:4381–4389. doi: 10.1038/sj.emboj.7601309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- Callachan H, Cottrell GA, Hather NY, Lambert JJ, Nooney JM, Peters JA. Modulation of the GABAA receptor by progesterone metabolites. Proc R Soc Lond B Biol Sci. 1987;231:359–369. doi: 10.1098/rspb.1987.0049. [DOI] [PubMed] [Google Scholar]
- Carter RB, Wood PL, Wieland S, Hawkinson JE, Belelli D, Lambert JJ, White HS, Wolf HH, Mirsadeghi S, Tahir SH, Bolger MB, Lan NC, Gee KW. Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3α-Hydroxy-3β-methyl-5α-pregnan-20-one), a selective, high-affinity, steroid modulator of the GABAA receptor. J Pharmacol Exp Ther. 1997;280:1284–1295. [PubMed] [Google Scholar]
- Chen ZW, Manion B, Townsend RR, Reichert DE, Covey DF, Steinbach JH, Sieghart W, Fuchs K, Evers AS. Neurosteroid analog photolabeling of a site in the third transmembrane domain of the beta3 subunit of the GABAA receptor. Mol Pharmacol. 2012;82:408–419. doi: 10.1124/mol.112.078410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari M, Eisenman LN, Covey DF, Mennerick S, Zorumski CF. The sticky issue of neurosteroids and GABAA receptors. Trends in Neurosciences. 2010;33:299–306. doi: 10.1016/j.tins.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concas A, Follesa P, Barbaccia ML, Purdy RH, Biggio G. Physiological modulation of GABAA receptor plasticity by progesterone metabolites. Eur J Pharmacol. 1999;375:225–235. doi: 10.1016/s0014-2999(99)00232-0. [DOI] [PubMed] [Google Scholar]
- Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of GABAA receptor in rat brain during pregnancy and after delivery. Proc Natl Aca Sci USA. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conneely OM, Maxwell BL, Toft DO, Schrader WT, O'Malley BW. The A and B forms of the chicken progesterone receptor arise by alternate initiation of translation of a unique mRNA. Biochem Biophys Res Commun. 1987;149:493–501. doi: 10.1016/0006-291x(87)90395-0. [DOI] [PubMed] [Google Scholar]
- Corpechot C, Young J, Calvel M, Wehrey C, Veltz JN, Touyer G, Mouren M, Prasad VV, Banner C, Sjovall J. Neurosteroids: 3α-hydroxy-5α-pregnan-20-one and its precursors in the brain, plasma, and steroidogenic glands of male and female rats. Endocrinology. 1993;133:1003–1009. doi: 10.1210/endo.133.3.8365352. [DOI] [PubMed] [Google Scholar]
- Do Rego JL, Seong JY, Burel D, Leprince J, Luu-The V, Tsutsui K, Tonon MC, Pelletier G, Vaudry H. Neurosteroid biosynthesis: Enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front Neuroendocrinol. 2009;30:259–301. doi: 10.1016/j.yfrne.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Duncan S, Read CL, Brodie MJ. How Common Is Catamenial Epilepsy? Epilepsia. 1993;34:827–831. doi: 10.1111/j.1528-1157.1993.tb02097.x. [DOI] [PubMed] [Google Scholar]
- Edwards HE, Burnham WM, Mendonca A, Bowlby DA, Maclusky NJ. Steroid hormones affect limbic afterdischarge thresholds and kindling rates in adult female rats. Brain Res. 1999;838:136–150. doi: 10.1016/s0006-8993(99)01619-4. [DOI] [PubMed] [Google Scholar]
- Edwards HE, Mo V, Burnham WM, Maclusky NJ. Gonadectomy unmasks an inhibitory effect of progesterone on amygdala kindling in male rats. Brain Research. 2001;889:260–263. doi: 10.1016/s0006-8993(00)03147-4. [DOI] [PubMed] [Google Scholar]
- El-Khayat HA, Soliman NA, Tomoum HY, Omran MA, El-Wakad AS, Shatla RH. Reproductive hormonal changes and catamenial pattern in adolescent females with epilepsy. Epilepsia. 2008;49:1619–1626. doi: 10.1111/j.1528-1167.2008.01622.x. [DOI] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the γ2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Follesa P, Biggio F, Caria S, Gorini G, Biggio G. Modulation of GABAA receptor gene expression by allopregnanolone and ethanol. Eur J Pharmacol. 2004;500:413–425. doi: 10.1016/j.ejphar.2004.07.041. [DOI] [PubMed] [Google Scholar]
- Frye CA. Hormonal influences on seizures: Basic neurobiology. In: Gidal Barry E, Cynthia, editors. International review of neurobiology epilepsy in women the scientific basis for clinical management. chapter 3. Academic Press; 2008. pp. 27–77. [DOI] [PubMed] [Google Scholar]
- Frye CA, Scalise TJ. Anti-seizure effects of progesterone and 3α,5α-THP in kainic acid and perforant pathway models of epilepsy. Psychoneuroendocrinology. 2000;25:407–420. doi: 10.1016/s0306-4530(99)00068-2. [DOI] [PubMed] [Google Scholar]
- Frye C, Rhodes M, Walf A, Harney J. Progesterone reduces pentylenetetrazol-induced ictal activity of wild-type mice but not those deficient in type I 5α-reductase. Epilepsia. 2002;43:14–17. doi: 10.1046/j.1528-1157.43.s.5.19.x. [DOI] [PubMed] [Google Scholar]
- Garrido YCS, Sanabria ERG, Funke MG, Cavalheiro EA, Naffah-Mazzacoratti MG. Mitogen-activated protein kinase is increased in the limbic structures of the rat brain during the early stages of status epilepticus. Brain Res Bull. 1998;47:223–229. doi: 10.1016/s0361-9230(98)00075-6. [DOI] [PubMed] [Google Scholar]
- Gavrilovici C, D'Alfonso S, Dann M, Poulter MO. Kindling-induced alterations in GABAA receptor-mediated inhibition and neurosteroid activity in the rat piriform cortex. Eur J Neurosci. 2006;24:1373–1384. doi: 10.1111/j.1460-9568.2006.05012.x. [DOI] [PubMed] [Google Scholar]
- Gibbs JW, III, Shumate MD, Coulter DA. Differential epilepsy-associated alterations in postsynaptic GABAA receptor function in dentate granule and CA1 neurons. J Neurophysiol. 1997;77:1924–1938. doi: 10.1152/jn.1997.77.4.1924. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mann EO, Mody I. Which GABAA receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mody I. Activation of GABAA reecptors: views from outside the synaptic cleft. Neuron. 2007a;56:763–770. doi: 10.1016/j.neuron.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus. J Physiol. 2007b;582:1163–1178. doi: 10.1113/jphysiol.2007.134460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABAA receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Gonzalez MI, Grabenstatter HL, Cea-Del Rio CA, Cruz Del Angel Y, Carlsen J, Laoprasert RP, White AM, Huntsman MM, Brooks-Kayal A. Seizure-related regulation of GABAA receptors in spontaneously epileptic rats. Neurobiol Disease. 2015;77:246–256. doi: 10.1016/j.nbd.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez MI, Cruz DA, Brooks-Kayal A. Down-regulation of gephyrin and GABAA receptor subunits during epileptogenesis in the CA1 region of hippocampus. Epilepsia. 2013;54:616–624. doi: 10.1111/epi.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann U, Beck H, Dreier JP, Ficker E, Stabel J, Zhang CL. The dentate gyrus as a regulated gate for the propagation of epileptiform activity. Epilepsy Res Suppl. 1992;7:273–280. [PubMed] [Google Scholar]
- Herzog AG. Progesterone therapy in women with complex partial and secondary generalized seizures. Neurology. 1995;45:1660–1662. doi: 10.1212/wnl.45.9.1660. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Catamenial epilepsy: Update on prevalence, pathophysiology and treatment from the findings of the NIH Progesterone Treatment Trial. Seizure. 2015;28:18–25. doi: 10.1016/j.seizure.2015.02.024. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Fowler KM, Smithson SD, Kalayjian LA, Heck CN, Sperling MR, Liporace JD, Harden CL, Dworetzky BA, Pennell PB, Massaro JM. Progesterone vs placebo therapy for women with epilepsy: A randomized clinical trial. Neurology. 2012;78:1959–1966. doi: 10.1212/WNL.0b013e318259e1f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog AG, Frye CA. Seizure exacerbation associated with inhibition of progesterone metabolism. Ann Neurol. 2003;53:390–391. doi: 10.1002/ana.10508. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Frye CA. Allopregnanolone levels and seizure frequency in progesterone-treated women with epilepsy. Neurology. 2014;83:345–348. doi: 10.1212/WNL.0000000000000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog AG, Harden CL, Liporace J, Pennell P, Schomer DL, Sperling M, Fowler K, Nikolov B, Shuman S, Newman M. Frequency of catamenial seizure exacerbation in women with localization-related epilepsy. Ann Neurol. 2004;56:431–434. doi: 10.1002/ana.20214. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia. 1997;38:1082–1088. doi: 10.1111/j.1528-1157.1997.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Seibel MM, Schomer DL, Vaitukaitis JL, Geschwind N. Reproductive endocrine disorders in women with partial seizures of temporal lobe origin. Arch Neurol. 1986;43:341–346. doi: 10.1001/archneur.1986.00520040029014. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Catamenial epilepsy: Definition, prevalence pathophysiology and treatment. Seizure. 2008;17:151–159. doi: 10.1016/j.seizure.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Fowler KM, Sperling MR, Massaro JM Progesterone Trial Study Group. Distribution of seizures across the menstrual cycle in women with epilepsy. Epilepsia. 2015;56:e58–e62. doi: 10.1111/epi.12969. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Weber DA. The effect of progesterone on kindling: a developmental study. Brain Res. 1984;318:45–53. doi: 10.1016/0165-3806(84)90061-0. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HMA, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Houser CR, Huang CS, Peng Z. Dynamic seizure-related changes in extracellular signal-regulated kinase activation in a mouse model of temporal lobe epilepsy. Neuroscience. 2008;156:222–237. doi: 10.1016/j.neuroscience.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Woolley C. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74:801–808. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez C, Guennoun R, Liere P, Eychenne B, Pianos A, El Etr M, Baulieu EE, Schumacher M. Developmental expression of genes involved in neurosteroidogenesis: 3a-Hydroxysteroid Dehydrogenase/Δ5-Δ4 Isomerase in the Rat Brain. Endocrinology. 2003;144:2902–2911. doi: 10.1210/en.2002-0073. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Moss S, Jurd R. GABAA receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Kapur J. Slow intracellular accumulation of GABAA receptor δ subunit is modulated by brain-derived neurotrophic factor. Neuroscience. 2009;164:507–519. doi: 10.1016/j.neuroscience.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Kapur J. NMDA receptor activation down-regulates expression of δ subunit-containing GABAA receptors in cultured hippocampal neurons. Mol Pharmacol. 2013;84:1–11. doi: 10.1124/mol.112.084715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Rajasekaran K, Kapur J. GABAergic transmission in temporal lobe epilepsy: The role of neurosteroids. Exp Neurol. 2013a;244:36–42. doi: 10.1016/j.expneurol.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Keith KJ, Ilyas A, Kapur J. GABAA receptor membrane insertion rates are specified by their subunit composition. Mol Cell Neurosci. 2013b;56:201–211. doi: 10.1016/j.mcn.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Rajasekaran K, Williamson J, Kapur J. Neurosteroid-sensitive δ-GABAA receptors: A role in epileptogenesis? Epilepsia. 2017;58:494–504. doi: 10.1111/epi.13660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller AF, Breton JD, Schlichter R, Poisbeau P. Production of 5α-reduced neurosteroids Is developmentally regulated and shapes GABAA miniature IPSCs in lamina II of the spinal cord. J Neurosci. 2004;24:907–915. doi: 10.1523/JNEUROSCI.4642-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharlamov EA, Lepsveridze E, Meparishvili M, Solomonia RO, Lu B, Miller ER, Kelly KM, Mtchedlishvili Z. Alterations of GABAA and glutamate receptor subunits and heat shock protein in rat hippocampus following traumatic brain injury and in posttraumatic epilepsy. Epilepsy Res. 2011;95:20–34. doi: 10.1016/j.eplepsyres.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Kia A, Ribeiro F, Nelson R, Gavrilovici C, Ferguson SSG, Poulter MO. Kindling alters neurosteroid-induced modulation of phasic and tonic GABAA receptor-mediated currents: role of phosphorylation. J Neurochem. 2011;116:1043–1056. doi: 10.1111/j.1471-4159.2010.07156.x. [DOI] [PubMed] [Google Scholar]
- Kim YS, Hong KS, Seong YS, Park JB, Kuroda S, Kishi K, Kaibuchi K, Takai Y. Phosphorylation and activation of mitogen-activated protein kinase by kainic acid-induced seizure in rat hippocampus. Biochem Biophys Res Commun. 1994;202:1163–1168. doi: 10.1006/bbrc.1994.2050. [DOI] [PubMed] [Google Scholar]
- Kimoto T, Tsurugizawa T, Ohta Y, Makino J, Tamura Ho, Hojo Y, Enami T, Kawato S. Cytochrome P450-dependent neurosteroid synthesis in the rat brain hippocampal neurons. International Congress Series. 2002;1233:127–137. [Google Scholar]
- King SR, Manna PR, Ishii T, Syapin PJ, Ginsberg SD, Wilson K, Walsh LP, Parker KL, Stocco DM, Smith RG, Lamb DJ. An essential component in steroid synthesis, the steroidogenic acute regulatory protein, is expressed in discrete regions of the brain. J Neurosci. 2002;22:10613–10620. doi: 10.1523/JNEUROSCI.22-24-10613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neurosteroids: correlation with gamma-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther. 1994;270:1223–1229. [PubMed] [Google Scholar]
- Kokate TG, Cohen AL, Karp E, Rogawski MA. Neuroactive steroids protect against pilocarpine- and kainic acid-induced limbic seizures and status epilepticus in mice. Neuropharmacology. 1996;35:1049–1056. doi: 10.1016/s0028-3908(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Banks MK, Magee T, Yamaguchi S, Rogawski MA. Finasteride, a 5α-reductase inhibitor, blocks the anticonvulsant activity of progesterone in mice. J Pharmacol Exp Ther. 1999a;288:679–684. [PubMed] [Google Scholar]
- Kokate TG, Juhng KN, Kirkby RD, Llamas J, Yamaguchi Si, Rogawski MA. Convulsant actions of the neurosteroid pregnenolone sulfate in mice. Brain Research. 1999b;831:119–124. doi: 10.1016/s0006-8993(99)01287-1. [DOI] [PubMed] [Google Scholar]
- Korneyev A, Pan BS, Polo A, Romeo E, Guidotti A, Costa E. Stimulation of brain pregnenolone synthesis by mitochondrial diazepam binding inhibitor receptor ligands in vivo. J Neurochem. 1993;61:1515–1524. doi: 10.1111/j.1471-4159.1993.tb13647.x. [DOI] [PubMed] [Google Scholar]
- Lawrence C, Martin BS, Sun C, Williamson J, Kapur J. Endogenous neurosteroid synthesis modulates seizure frequency. Ann Neurol. 2010;67:689–693. doi: 10.1002/ana.21989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le GC, Robel P, Gouezou M, Sananes N, Baulieu EE, Waterman M. Neurosteroids: cytochrome P-450scc in rat brain. Science. 1987;237:1212–1215. doi: 10.1126/science.3306919. [DOI] [PubMed] [Google Scholar]
- Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ. Activation of estrogen receptor-β regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Stringer JL, Bertram EH. The dentate gyrus as a control point for seizures in the hippocampus and beyond. Epilepsy Res Suppl. 1992;7:301–313. [PubMed] [Google Scholar]
- Loup F, Picard F, Andre VM, Kehrli P, Yonekawa Y, Wieser HG, Fritschy JM. Altered expression of α3-containing GABAA receptors in the neocortex of patients with focal epilepsy. Brain. 2006;129:3277–3289. doi: 10.1093/brain/awl287. [DOI] [PubMed] [Google Scholar]
- Loup F, Wieser HG, Yonekawa Y, Aguzzi A, Fritschy JM. Selective alterations in GABAA receptor subtypes in human temporal lobe epilepsy. J Neurosci. 2000;20:5401–5419. doi: 10.1523/JNEUROSCI.20-14-05401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund IV, Hu Y, Raol YH, Benham RS, Faris R, Russek SJ, Brooks-Kayal AR. BDNF selectively regulates GABAA receptor transcription by activation of the JAK/STAT pathway. Sci STKE. 2008;1:ra9. doi: 10.1126/scisignal.1162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABAA receptors: relevance to the ovarian cycle and stress. J Neurosci. 2007;27:2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maione S, Berrino L, Vitagliano S, Leyva J, Rossi F. Pregnenolone sulfate increases the convulsant potency of NMDA in mice. Eur J Pharmacol. 1992;219:477–479. doi: 10.1016/0014-2999(92)90493-n. [DOI] [PubMed] [Google Scholar]
- Maitra R, Reynolds JN. Modulation of GABAA receptor function by neuroactive steroids: evidence for heterogeneity of steroid sensitivity of recombinant GABAA receptor isoforms. Can J Physiol Pharmacol. 1998;76:909–920. doi: 10.1139/cjpp-76-9-909. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Mienville JM, Vicini S. Neurosteroid pregnenolone sulfate antagonizes electrophysiological responses to GABA in neurons. Neurosci Lett. 1988;90:279–284. doi: 10.1016/0304-3940(88)90202-9. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Schwartz RD. Pregnenolone-sulfate: an endogenous antagonist of the gamma-aminobutyric acid receptor complex in brain? Brain Res. 1987;404:355–360. doi: 10.1016/0006-8993(87)91394-1. [DOI] [PubMed] [Google Scholar]
- Mani S, Oyola MG. Progesterone signaling mechanisms in brain and behavior. Frontiers in Endocrinology. 2012;3:1–8. doi: 10.3389/fendo.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcangi RC, Poletti A, Cavarretta I, Celotti F, Colciago A, Magnaghi V, Motta M, Negri-Cesi P, Martini L. The 5alpha-reductase in the central nervous system: expression and modes of control. J Steroid Biochem Mol Biol. 1998;65:295–299. doi: 10.1016/s0960-0760(98)00030-2. [DOI] [PubMed] [Google Scholar]
- Meletti S, Lucchi C, Monti G, Giovannini G, Bedin R, Trenti T, Rustichelli C, Biagini G. Decreased allopregnanolone levels in cerebrospinal fluid obtained during status epilepticus. Epilepsia. 2017;58:e16–e20. doi: 10.1111/epi.13625. [DOI] [PubMed] [Google Scholar]
- Michels G, Moss SJ. GABAA receptors: properties and trafficking. Crit Rev Biochem Mol Biol. 2007;42:3–14. doi: 10.1080/10409230601146219. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in GABAA receptor δ subunit knockout mice. Proc Natl Acad Sci U S A. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milenkovic I, Vasiljevic M, Maurer D, Höger H, Klausberger T, Sieghart W. The parvalbumin-positive interneurons in the mouse dentate gyrus express GABAA receptor subunits α1, β2, and δ along their extrasynaptic cell membrane. Neuroscience. 2013;254:80–96. doi: 10.1016/j.neuroscience.2013.09.019. [DOI] [PubMed] [Google Scholar]
- Mitterling KL, Spencer JL, Dziedzic N, Shenoy S, McCarthy K, Waters EM, McEwen BS, Milner TA. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J Comp Neurol. 2010;518:2729–2743. doi: 10.1002/cne.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen M, Smart TG. Extrasynaptic αβ subunit GABAA receptors on rat hippocampal pyramidal neurons. J Physiol. 2006;577:841–856. doi: 10.1113/jphysiol.2006.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Bertram EH, Kapur J. Diminished allopregnanolone enhancement of GABAA receptor currents in a rat model of chronic temporal lobe epilepsy. J Physiol. 2001;537:453–465. doi: 10.1111/j.1469-7793.2001.00453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Kapur J. A presynaptic action of the neurosteroid pregnenolone sulfate on GABAergic synaptic transmission. Mol Pharmacol. 2003;64:857–864. doi: 10.1124/mol.64.4.857. [DOI] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Kapur J. High-affinity, slowly desensitizing GABAA receptors mediate tonic inhibition in hippocampal dentate granule cells. Mol Pharmacol. 2006;69:564–575. doi: 10.1124/mol.105.016683. [DOI] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Lepsveridze E, Xu H, Kharlamov EA, Lu B, Kelly KM. Increase of GABAA receptor-mediated tonic inhibition in dentate granule cells after traumatic brain injury. Neurobiol Dis. 2010;38:464–475. doi: 10.1016/j.nbd.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Nateri AS, Raivich G, Gebhardt C, Da Costa C, Naumann H, Vreugdenhil M, Makwana M, Brandner S, Adams RH, Jefferys JG, Kann O, Behrens A. ERK activation causes epilepsy by stimulating NMDA receptor activity. EMBO J. 2007;26:4891–4901. doi: 10.1038/sj.emboj.7601911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander JG, Woolley CS. 17β-estradiol acutely potentiates glutamatergic synaptic transmission in the hippocampus through distinct mechanisms in males and females. J Neurosci. 2016;36:2677–2690. doi: 10.1523/JNEUROSCI.4437-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Hanchar HJ, Meera P, Wallner M. GABAA receptor subtypes: the “one glass of wine” receptors. Alcohol. 2007;41:201–209. doi: 10.1016/j.alcohol.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABAA receptors: Subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma E, Spinelli G, Torchia G, Martinez-Torres A, Ragozzino D, Miledi R, Eusebi F. Abnormal GABAA receptors from the human epileptic hippocampal subiculum microtransplanted to Xenopus oocytes. Proc Natl Aca Sci USA. 2005;102:2514–2518. doi: 10.1073/pnas.0409687102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell KD, Woodin MA, Pennell PB. Quantification of neurosteroids during pregnancy using selective ion monitoring mass spectrometry. Steroids. 2015;95:24–31. doi: 10.1016/j.steroids.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V, Lecanu L, Brown RC, Han Z, Yao ZX. Peripheral-type benzodiazepine receptor in neurosteroid biosynthesis, neuropathology and neurological disorders. Neuroscience. 2006;138:749–756. doi: 10.1016/j.neuroscience.2005.05.063. [DOI] [PubMed] [Google Scholar]
- Pathak HR, Weissinger F, Terunuma M, Carlson GC, Hsu FC, Moss SJ, Coulter DA. Disrupted dentate granule cell chloride regulation enhances synaptic excitability during development of temporal lobe epilepsy. J Neurosci. 2007;27:14012–14022. doi: 10.1523/JNEUROSCI.4390-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the δ subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci. 2004;24:8629–8639. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petratos S, Hirst JJ, Mendis S, Anikijenko P, Walker DW. Localization of P450scc and 5α-reductase type-2 in the cerebellum of fetal and newborn sheep. Dev Brain Res. 2000;123:81–86. doi: 10.1016/s0165-3806(00)00076-6. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Puia G, Ducic I, Vicini S, Costa E. Does neurosteroid modulatory efficacy depend on GABAA receptor subunit composition? Receptors Channels. 1993;1:135–142. [PubMed] [Google Scholar]
- Puia G, Santi M, Vicini S, Pritchett DB, Purdy RH, Paul SM, Seeburg PH, Costa E. Neurosteroids act on recombinant human GABAA receptors. Neuron. 1990;4:759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci U S A. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigg M, Fowler KM, Herzog AG the NIH Progesterone Trial Study Group. Circalunar and ultralunar periodicities in women with partial seizures. Epilepsia. 2008;49:1081–1085. doi: 10.1111/j.1528-1167.2008.01537.x. [DOI] [PubMed] [Google Scholar]
- Quirk K, Gillard NP, Ragan CI, Whiting PJ, McKernan RM. Model of subunit composition of GABAA receptor subtypes expressed in rat cerebellum with respect to their alpha and gamma/delta subunits. J Biol Chem. 1994;269:16020–16028. [PubMed] [Google Scholar]
- Rajasekaran K, Joshi S, Sun C, Mtchedlishvilli Z, Kapur J. Receptors with low affinity for neurosteroids and GABA contribute to tonic inhibition of granule cells in epileptic animals. Neurobiol Dis. 2010;40:490–501. doi: 10.1016/j.nbd.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan L, Hess GP. Mechanism of potentiation of a dysfunctional epilepsy-linked mutated GABAA receptor by a neurosteroid (3α, 21-Dihydroxy-5α-pregnan-20-one): transient kinetic investigations. Biochemistry. 2010;49:7892–7901. doi: 10.1021/bi901241g. [DOI] [PubMed] [Google Scholar]
- Raol YH, Lund IV, Bandyopadhyay S, Zhang G, Roberts DS, Wolfe JH, Russek SJ, Brooks-Kayal AR. Enhancing GABAA receptor α1 subunit levels in hippocampal dentate gyrus inhibits epilepsy development in an animal model of temporal lobe epilepsy. J Neurosci. 2006;26:11342–11346. doi: 10.1523/JNEUROSCI.3329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Castaneda DC, O'Malley BW, Rogawski MA. Anticonvulsant activity of progesterone and neurosteroids in progesterone receptor knockout mice. J Pharmacol Exp Ther. 2004;310:230–239. doi: 10.1124/jpet.104.065268. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kim HY, Rogawski MA. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia. 2001;42:328–336. doi: 10.1046/j.1528-1157.2001.10100.x. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. Proconvulsant effects of neurosteroids pregnenolone sulfate and dehydroepiandrosterone sulfate in mice. Eur J Pharmacol. 1998;345:55–59. doi: 10.1016/s0014-2999(98)00034-x. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Neurosteroid replacement therapy for catamenial epilepsy. Neurotherapeutics. 2009;6:392–401. doi: 10.1016/j.nurt.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Neuroendocrine aspects of catamenial epilepsy. Hormones and Behavior. 2013;63:254–266. doi: 10.1016/j.yhbeh.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Gangisetty O, Briyal S. Disease-modifying activity of progesterone in the hippocampus kindling model of epileptogenesis. Neuropharmacology. 2010;59:573–581. doi: 10.1016/j.neuropharm.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Ramanathan G. Finasteride inhibits the disease-modifying activity of progesterone in the hippocampus kindling model of epileptogenesis. Epilepsy Behav. 2012;25:92–97. doi: 10.1016/j.yebeh.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht R, Rammes G, Eser D, Baghai TC, Schüle C, et al. Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science. 2009;325:490–493. doi: 10.1126/science.1175055. [DOI] [PubMed] [Google Scholar]
- Sanna E, Mostallino MC, Murru L, Carta M, Talani G, Zucca S, Mura ML, Maciocco E, Biggio G. Changes in expression and function of extrasynaptic GABAA receptors in the rat hippocampus during pregnancy and after delivery. J Neurosci. 2009;29:1755–1765. doi: 10.1523/JNEUROSCI.3684-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato SM, Woolley CS. Acute inhibition of neurosteroid estrogen synthesis suppresses status epilepticus in an animal model. eLife. 2016;5:e12917. doi: 10.7554/eLife.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Assembly of GABAA receptor subunits: role of the δ subunit. J Neurosci. 1994;14:7077–7086. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Malthankar-Phatak GH, Friedman D, Pearce P, McCloskey DP, Harden CL, Maclusky NJ. A rat model of epilepsy in women: a tool to study physiological interactions between endocrine systems and seizures. Endocrinology. 2009;150:4437–4442. doi: 10.1210/en.2009-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Structure, pharmacology, and function of GABAA receptor subtypes. Adv Pharmacol. 2006;54:231–263. doi: 10.1016/s1054-3589(06)54010-4. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABAA receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Singh M, Su C. Progesterone and neuroprotection. Hormones and Behavior. 2013;63:284–290. doi: 10.1016/j.yhbeh.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smejkalova T, Woolley CS. Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J Neurosci. 2010;30:16137–16148. doi: 10.1523/JNEUROSCI.4161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JM, Li X. GABAA receptor alpha4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998a;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, Hsu FC. Withdrawal from 3a-hydroxy-5α-pregnan-20-One using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor α4 subunit in association with increased anxiety. J Neurosci. 1998b;18:5275–5284. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Waterhouse BD, Woodward DJ. Sex steroid effects on extrahypothalamic CNS. I. Estrogen augments neuronal responsiveness to iontophoretically applied glutamate in the cerebellum. Brain Res. 1987;422:40–51. doi: 10.1016/0006-8993(87)90538-5. [DOI] [PubMed] [Google Scholar]
- Smith SS, Waterhouse BD, Woodward DJ. Locally applied estrogens potentiate glutamate-evoked excitation of cerebellar Purkinje cells. Brain Res. 1988;475:272–282. doi: 10.1016/0006-8993(88)90615-4. [DOI] [PubMed] [Google Scholar]
- Sperk G, Schwarzer C, Tsunashima K, Kandlhofer S. Expression of GABAA receptor subunits in the hippocampus of the rat after kainic acid-induced seizures. Epilepsy Res. 1998;32:129–139. doi: 10.1016/s0920-1211(98)00046-1. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Li Z, Banerjee PK, Mihalek RM, Homanics GE, Olsen RW. Behavior and physiology of mice lacking the GABAA-receptor δ subunit. Epilepsia. 2002;43(s5):3–8. doi: 10.1046/j.1528-1157.43.s.5.8.x. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel-Wagner B. Neurosteroid metabolism in the human brain. Eur J Endocrinol. 2001;145:669–679. doi: 10.1530/eje.0.1450669. [DOI] [PubMed] [Google Scholar]
- Stoffel-Wagner B, Beyenburg S, Watzka M, Blumcke I, Bauer J, Schramm J, Bidlingmaier F, Elger CE. Expression of 5α-reductase and 3α-hydroxisteroid oxidoreductase in the hippocampus of patients with chronic temporal lobe epilepsy. Epilepsia. 2000;41:140–147. doi: 10.1111/j.1528-1157.2000.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Stoffel-Wagner B, Watzka M, Steckelbroeck S, Ludwig M, Clusmann H, Bidlingmaier F, Casarosa E, Luisi S, Elger CE, Beyenburg S. Allopregnanolone serum levels and expression of 5α-reductase and 3α-hydroxysteroid dehydrogenase isoforms in hippocampal and temporal cortex of patients with epilepsy. Epilepsy Res. 2003;54:11–19. doi: 10.1016/s0920-1211(03)00036-6. [DOI] [PubMed] [Google Scholar]
- Stoffel-Wagner B. Neurosteroid biosynthesis in the human brain and its clinical implications. Ann N Y Acad Sci. 2003;1007:64–78. doi: 10.1196/annals.1286.007. [DOI] [PubMed] [Google Scholar]
- Stringer JL, Lothman EW. Maximal dentate gyrus activation: characteristics and alterations after repeated seizures. J Neurophysiol. 1989;62:136–143. doi: 10.1152/jn.1989.62.1.136. [DOI] [PubMed] [Google Scholar]
- Sun C, Mtchedlishvili Z, Erisir A, Kapur J. Diminished neurosteroid sensitivity of synaptic inhibition and altered location of the α4 subunit of GABAA receptors in an animal model of epilepsy. J Neurosci. 2007;27:12641–12650. doi: 10.1523/JNEUROSCI.4141-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Sieghart W, Kapur J. Distribution of α1, α4, γ2, and δ subunits of GABAA receptors in hippocampal granule cells. Brain Res. 2004;1029:207–216. doi: 10.1016/j.brainres.2004.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Farrar SJ, Kerby J, Whiting PJ, Atack JR, McKernan RM. Preferential coassembly of α4 and δ subunits of the GABAA receptor in rat thalamus. Mol Pharmacol. 1999;56:110–115. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- Tada H, Koide M, Ara W, Shibata Y, Funabashi T, Suyama K, Goto T, Takahashi T. Estrous cycle-dependent phasic changes in the stoichiometry of hippocampal synaptic AMPA receptors in rats. PLoS ONE. 2015;10:e0131359. doi: 10.1371/journal.pone.0131359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C, Shard C, Ranieri E, Hynes K, Pham D, et al. Mutations of protocadherin 19 in female epilepsy (PCDH19-FE) lead to allopregnanolone deficiency. Hum Mol Genet. 2015;24:5250–5259. doi: 10.1093/hmg/ddv245. [DOI] [PubMed] [Google Scholar]
- Taubøll E, Lundervold A, Gjerstad L. Temporal distribution of seizures in epilepsy. Epilepsy Research. 1991;8:153–165. doi: 10.1016/0920-1211(91)90084-s. [DOI] [PubMed] [Google Scholar]
- Tsunashima K, Schwarzer C, Kirchmair E, Sieghart W, Sperk G. GABAA receptor subunits in the rat hippocampus III: altered messenger RNA expression in kainic acid-induced epilepsy. Neuroscience. 1997;80:1019–1032. doi: 10.1016/s0306-4522(97)00144-9. [DOI] [PubMed] [Google Scholar]
- Twyman RE, Macdonald RL. Neurosteroid regulation of GABAA receptor single-channel kinetic properties of mouse spinal cord neurons in culture. J Physiol. 1992;456:215–245. doi: 10.1113/jphysiol.1992.sp019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veliskova J, DeSantis KA. Sex and hormonal influences on seizures and epilepsy. Hormones and Behavior. 2013;63:267–277. doi: 10.1016/j.yhbeh.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veliskova J. The role of estrogens in seizures and epilepsy: the bad guys or the good guys? Neuroscience. 2006;138:837–844. doi: 10.1016/j.neuroscience.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Veliskova J. Estrogens and epilepsy: why are we so excited? Neuroscientist. 2007;13:77–88. doi: 10.1177/1073858406295827. [DOI] [PubMed] [Google Scholar]
- Walker MC, Semyanov A. Regulation of excitability by extrasynaptic GABAA receptors. Results Probl Cell Differ. 2008;44:29–48. doi: 10.1007/400_2007_030. [DOI] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of δ subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting P. GABAA receptor subtypes in the brain: a paradigm for CNS drug discovery? Drug Discovery Today. 2003;8:445–450. doi: 10.1016/s1359-6446(03)02703-x. [DOI] [PubMed] [Google Scholar]
- Whiting PJ, Bonnert TP, McKernan RM, Farrar S, le BB, Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJ, Thompson SA, Wafford KA. Molecular and functional diversity of the expanding GABAA receptor gene family. Ann N Y Acad Sci. 1999;868:645–653. doi: 10.1111/j.1749-6632.1999.tb11341.x. [DOI] [PubMed] [Google Scholar]
- Williamson J, Mtchedlishvili Z, Kapur J. Characterization of the convulsant action of pregnenolone sulfate. Neuropharmacology. 2004;46:856–864. doi: 10.1016/j.neuropharm.2003.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmer LL, Hu Y, Kalkbrenner M, Evers AS, Zorumski CF, Covey DF. Enantioselectivity of steroid-induced GABAA receptor modulation and anesthesia. Mol Pharmacol. 1996;50:1581–1586. [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABAA receptors containing the delta subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. Estradiol facilitates kainic acid-induced, but not flurothyl-induced, behavioral seizure activity in adult female rats. Epilepsia. 2000;41:510–515. doi: 10.1111/j.1528-1157.2000.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Woolley DE, Timiras PS. The gonad-brain relationship: effects of female sex hormones on electroshock convulsions in the rat. Endocrinology. 1962;70:196–209. doi: 10.1210/endo-70-2-196. [DOI] [PubMed] [Google Scholar]
- Wu FS, Gibbs TT, Farb DH. Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-D-aspartate receptor. Mol Pharmacol. 1991;40:333–336. [PubMed] [Google Scholar]
- Wu X, Gangisetty O, Carver CM, Reddy DS. Estrous cycle regulation of extrasynaptic δ-containing GABAA receptor-mediated tonic inhibition and limbic epileptogenesis. J Pharmacol Exp Ther. 2013;346:146–160. doi: 10.1124/jpet.113.203653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Wei W, Mody I, Houser CR. Altered localization of GABAA receptor subunits on dentate granule cell dendrites influences tonic and phasic inhibition in a mouse model of epilepsy. J Neurosci. 2007;27:7520–7531. doi: 10.1523/JNEUROSCI.1555-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


