Abstract
The dorsal lateral geniculate nucleus of the thalamus (LGN) receives the main outputs of both eyes and relays those signals to the visual cortex. Each retina projects to separate layers of the LGN so that each LGN neuron is innervated by a single eye. In line with this anatomical separation, visual responses of almost all of LGN neurons are driven by one eye only. Nonetheless, many LGN neurons are sensitive to what is shown to the other eye as their visual responses differ when both eyes are stimulated compared to when the driving eye is stimulated in isolation. This, predominantly suppressive, binocular modulation of LGN responses might suggest that the LGN is the first location in the primary visual pathway where the outputs from the two eyes interact. Indeed, the LGN features several anatomical structures that would allow for LGN neurons responding to one eye to modulate neurons that respond to the other eye. However, it is also possible that binocular response modulation in the LGN arises indirectly as the LGN also receives input from binocular visual structures. Here we review the extant literature on the effects of binocular stimulation on LGN spiking responses, highlighting findings from cats and primates, and evaluate the neural circuits that might mediate binocular response modulation in the LGN.
Keywords: binocular vision, binocular combination, binocular integration, lateral geniculate nucleus, neurophysiology
Graphical Abstract
Our brains combine the signals from the two eyes to create a singular view. Where the separate streams from the two eyes meet in the primate brain is unclear, with debate centering on whether the signals from the two eyes remain separate within the main target of retinal projections, the lateral geniculate nucleus (LGN). Here we review the effects of binocular stimulation on LGN neurons and evaluate neural circuits that might mediate binocular interactions in this structure.
Introduction
Our visual perception of the world is characterized by a singular view despite the fact that each eye’s perspective differs from the other. To create this unified perspective, our brains need to combine the separate outputs of the two eyes into a unified binocular signal. In order to allow for this binocular combination, outputs from one eye must meet and interact with outputs from the other eye. Knowing where the outputs from the two eyes converge is critical for our understanding of binocular vision and promises the discovery of new therapeutic targets for binocular vision disorders such as strabismus and amblyopia. The neuroanatomical framework of binocular convergence has been authoritatively summarized in seminal work by Casagrande and Boyd (Casagrande & Boyd, 1996). Here we provide an extension of their preeminent review, adding recent developments and functional measurements that augment the structural findings.
The primary visual pathway of mammals accommodates two structures where the outputs of the two eyes might first meet and interact (Figure 1): Retinal ganglion cells from each eye project in two isolated streams to the dorsal lateral geniculate nucleus of the thalamus (LGN). The LGN projects to the primary visual cortex, which constitutes a bottleneck for visual input to all other cortical areas (Felleman & Van Essen, 1991; Lennie & Movshon, 2005; Markov et al., 2013; Schmid et al., 2013; Schmiedt et al., 2014). The primary visual cortex is the first structure in the primary visual pathway where almost all neurons are excited by stimulation of either eye (Hubel & Wiesel, 1962; Hubel & Wiesel, 1977; Smith et al., 1997a). This feature suggests that the signals from the two eyes are combined, or merged together, in this structure (Casagrande & Kaas, 1994; Hubel & Wiesel, 1969). Unlike in primary visual cortex, almost neurons at the previous stage of visual processing, the LGN, are excited by stimulation of one eye only. This means that only stimulation from one eye, but not the other, will result in a significant increase or decrease of in the frequency of action potentials, or spikes, in LGN neurons. Nevertheless, the LGN is a candidate structure for the two eyes’ outputs to meet and interact, in that the spike rate of a neuron that responds to the neuron’s driving eye is modulated (enhanced or suppressed) when the other eye is stimulated as well. This type of binocular modulation could serve computations that require the two eyes’ outputs to interact before they are merged together.
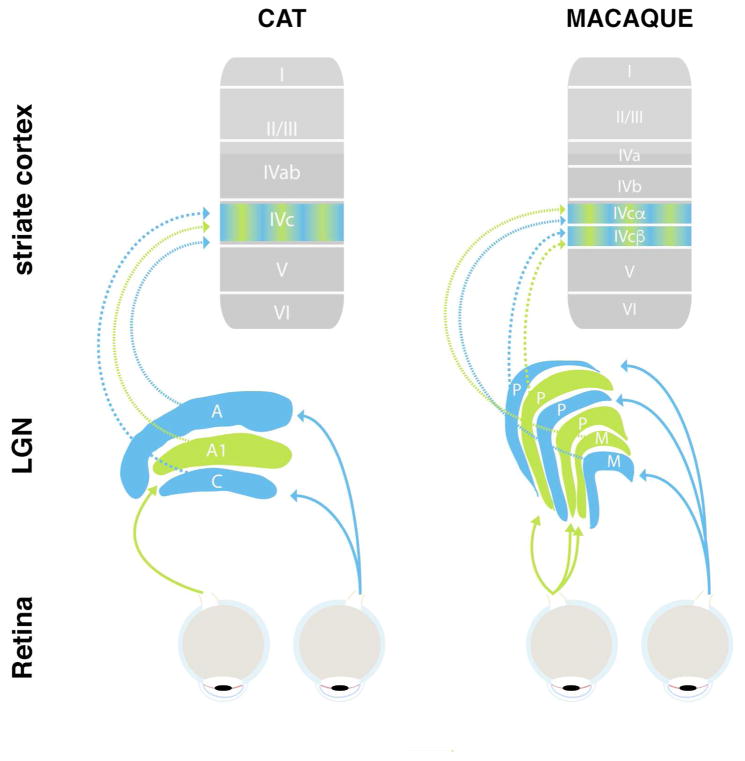
Figure 1.
Simplified schematic of primary visual pathway of cat (left) and macaque monkeys (right). Retinal neurons project visual sensory information to the LGN, which is divided into several, eye-specific layers (blue and green). LGN neurons primarily project to layer 4 (arrows) as well as other sublayers of the primary visual cortex. Note the differences in anatomy and nomenclature between cats and monkeys.
For example, we know that the visual system adjusts the relative strength of each eye’s outputs before merging them into a single binocular signal. This fact is evidenced by the observation that our visual perception hardly changes when we close one eye, despite the fact that this action virtually halves the visual input to the visual system. In order to account for the difference in visual activation between these two viewing conditions, the brain needs to adjust the relative strength, or gain, of the signal from each eye in a way that depends on the activation of the other eye. This computational step likely takes place prior to binocular merging because the relative strength of the outputs from each eye are lost in the merged binocular signal. Indeed, several neurophysiological and psychophysical studies on this subject convergingly determined that the gain of the outputs of the eyes are adjusted while the two signals are still separate (Baker, Meese, & Summers, 2007; Ding & Sperling, 2006; Meese, Georgeson, & Baker, 2006; Moradi & Heeger, 2009; Truchard, Ohzawa, & Freeman, 2000).
Based on the above, we can conclude that the outputs from the two eyes meet and interact somewhere in the brain before binocular combination in primary visual cortex. The underlying neural process termed binocular modulation is most likely carried out by neurons that are excited by one eye only (so-called monocular neurons), in that these neurons modulate their responses depending on what is shown to the other eye. In other words, when monocular neurons are excited by a visual stimulus in their driving, “dominant eye”, this visual response is either enhanced or inhibited when the other, “non-dominant eye” is also stimulated. These two types of binocular modulation have been termed binocular facilitation and binocular suppression, respectively. Based on the anatomy discussed above, binocular modulation could arise in three different ways along the primary visual pathway: 1) Binocular modulation is exclusively generated by LGN neurons. (2) Binocular modulation is exclusively generated by V1 neurons, and LGN neurons inherit binocular modulation following cortical feedback. (3) Binocular modulation is generated by both LGN and V1 neurons (Figure 2). Below, we will discuss these three alternatives, and summarize our current understanding of binocular modulation in the primary visual pathway.
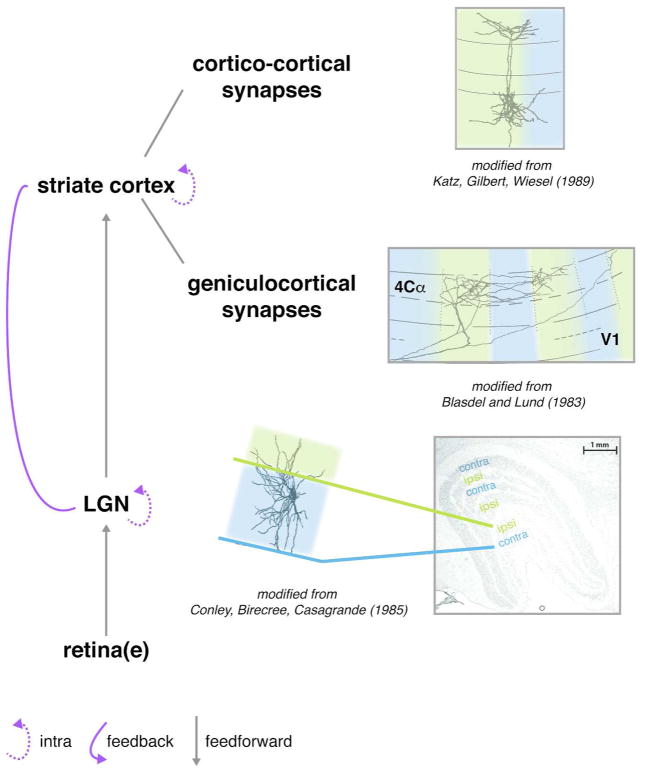
Figure 2.
Possible sites of binocular modulation in the primary visual pathway. 1) The outputs of the two eyes arrive in segregated eye-specific (green/blue) layers in the LGN, but some anatomical connections can bridge between them (top). 2) The projections of LGN neurons to primary visual cortex are also largely segregated by eye along the tangential dimension, terminating in eye-specific ocular dominance columns (green/blue). However, some of these LGN projections appear to form synapses outside their respective ocular dominance columns. 3) Projections from layer 4 neurons to other neurons within primary visual cortex are not bound to the boundaries of the ocular dominance columns. 4) Connections within the LGN or visual cortex as well as corticogeniculate feedback could provide a structural substrate for binocular modulation. Adopted from (Blasdel & Lund, 1983; Conley et al., 1985; Fitzpatrick, Lund, & Blasdel, 1985; Katz, Gilbert, & Wiesel, 1989).
Binocular modulation of LGN spiking responses
Over the past half century, neurophysiological studies across many eminent laboratories have probed whether LGN neurons exhibit binocular modulation. The bulk of this work was based on extracellular recordings in anesthetized cats and monkeys. These model species were chosen because their eyes are positioned on the head in a way that is similar to humans, resulting in similarly sized binocular visual fields (Heesy, 2009). Many other mammalian species feature a more lateralized position of the eyes and consequently deviate in their anatomy of binocular combination (Grieve, 2005; M. Howarth, Walmsley, & Brown, 2014; Jeffery, Cowey, & Kuypers, 1981; Kondo, Takada, Honda, & Mizuno, 1993; Longordo, To, Ikeda, & Stuart, 2013; Niell & Stryker, 2008; Scholl, Burge, & Priebe, 2013a).
Studies in cat LGN
Only a very small number (2% – 11%) of cat LGN neurons can be driven to increase or reduce their spontaneous spiking rate through stimulation of either eye (Bishop, Burke, & Davis, 1962a; Erulkar & Fillenz, 1960; Kinston, Vadas, & Bishop, 1969) (Table 1). However, visual responses of most cat LGN neurons are significantly altered when a second visual stimulus is simultaneously presented to the non-dominant eye, i.e. their spike rate differs between binocular and monocular stimulation (Figure 3) (Guido, Tumosa, & Spear, 1989; Sengpiel, Blakemore, & Harrad, 1995a; Xue, Ramoa, Carney, & Freeman, 1987). The retinal region of the non-dominant eye that, when stimulated, elicits binocular modulation is called the non-dominant eye receptive field. More than three fourths of cat LGN cells (~82%) feature such a non-dominant eye receptive field, and 88% of these neurons are suppressed by stimulation of the non-dominant eye (Sanderson, Bishop, & Darian-Smith, 1971). Non-dominant eye receptive fields are generally larger than dominant-eye receptive fields (Sanderson et al., 1971). And whereas dominant eye receptive fields in the LGN typically follow a center-surround organization, where stimulation of the center results in the opposite effect as stimulation of the surrounding region, non-dominant eye receptive fields feature a spatially homogenous organization (Sanderson et al., 1971), but see (Schmielau & Singer, 1977).
Table 1.
| CAT | MONKEY | |
|---|---|---|
| LGN | LGN | |
| Exclusively Monocular Neurons | 20% | 70–90% |
| Interocular Facilitated Neurons | 10% | 5% |
| Interocular Suppressed Neurons | 70%% | 10–30% |
| Binocular Neurons | 2–10% | 3% |
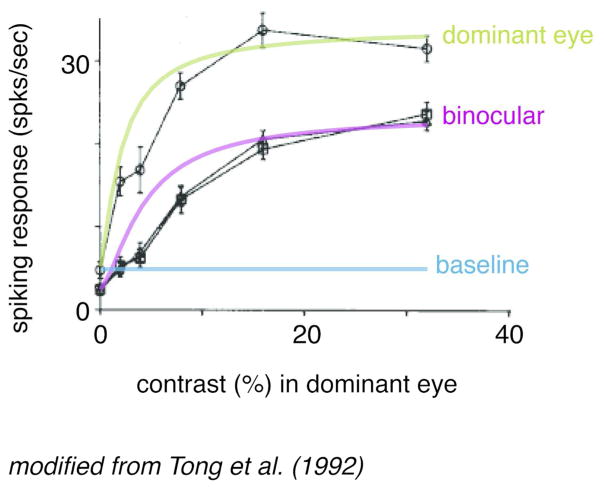
Figure 3.
Binocular modulation of monocular neurons. Data from a previously published example cat LGN neuron. Ordinate represents the magnitude of the neuron’s spiking response to visual stimulation and abscissa plots the contrast of the visual stimulus (shown to the neuron’s dominant eye). Model fits using a Naka-Rushton equation (Naka & Rushton, 1966) for binocular (purple line) and monocular (green line) responses are superimposed on the actual data (black traces). The solid blue line represents the estimated baseline firing rate of the neuron based on the activity plotted for the monocular condition at 0% contrast. Note the overall drop in response gain for the binocular stimulation condition, indicating that even though the LGN neuron can only be activated by one eye (the dominant eye), this neuron is nonetheless sensitive to stimulation of the opposite (non-dominant) eye, resulting in an overall reduced visual response when both eyes are stimulated. No difference was found between a dioptic condition (triangles) and a dichoptic condition (squares) in which the spatial frequency in the two eyes were different. Adapted from (Tong et al., 1992).
Binocular modulation of cat LGN responses is greatest when the stimulus in the non-dominant eye matches the spatial frequency of the stimulus in the dominant eye (Sengpiel, Blakemore, & Harrad, 1995a; Tong, Guido, Tumosa, Spear, & Heidenreich, 1992), though binocular modulation can be evoked across a wide range of spatial frequencies (Moore, Spear, Kim, & Xue, 1992; Sengpiel, Blakemore, & Harrad, 1995a). While most studies used stimuli of relatively low spatial frequencies (< 0.8 cycles per degree), binocular modulation has been observed for stimuli as high as five or more cycles per degree, suggesting that binocular modulation in cat LGN acts at high spatial acuity (Guido et al., 1989) (the peak contrast sensitivity for cats lies between 1 and 5 cycles per degree (Blake, Cool, & Crawford, 1974; Pasternak & Merigan, 1981)).
The spiking responses of most cat LGN neurons increase with increasing stimuli contrast. The resulting contrast response functions can be measured for stimuli presented to one eye alone (monocular stimulation) or for identical stimuli presented at the same position of both eyes’ retina (dioptic stimulation) (Tong et al., 1992). Assuming binocular suppression, which dominates cat LGN, binocular modulation under these conditions could take one of three different forms:
Dioptic stimulation results in decreased responses relative to monocular stimulation at all contrast levels, i.e., stimulating the non-dominant eye has the same suppressive effect for any stimulus contrast;
dioptic stimulation results in decreased responses relative to monocular stimulation for high contrasts only, i.e., binocular suppression is limited to high contrasts;
dioptic stimulation results in a shift of the slope of the contrast response function relative to that for monocular stimulation, i.e., the dynamic range of contrast responses is enhanced.
The majority of cat LGN cells exhibit the first type of binocular modulation (Tong et al., 1992). Therefore, binocular modulation in cat LGN appears to primarily reduce the neurons’ response gain.
Studies in monkey LGN
Similar to the cat, only a small minority (~3%) of neurons in monkey LGN can be driven through either eye (binocular responses have only been shown for ~30% of K neurons, which constitute ~10% of all LGN neurons) (Cheong, Tailby, Solomon, & Martin, 2013; Dacey, 1994; Zeater, Cheong, Solomon, Dreher, & Martin, 2015). As in cats, monkey LGN also contains neurons that modulate their spiking under binocular viewing (Marrocco & McClurkin, 1979; Rodieck & Dreher, 1979). And, similar to the cat, between 70% and 100% of this modulation in monkeys takes the form of binocular suppression. One notable difference between the two species is that the fraction of LGN cells for which binocular modulation has been reported is drastically smaller in monkeys (<10–30%) compared to cats (Marrocco & McClurkin, 1979; Rodieck & Dreher, 1979) (Table 1). This difference in proportions of binocularly modulated LGN neurons might be due to differences in LGN anatomy between carnivores and primates, which we will discuss below. Another possibility is that the magnitude of binocular modulation depends on the type of visual stimulation. Specifically, investigations in cat LGN relied primarily on slowly moving grating stimuli to isolate visual contrast responses. In contrast, almost all studies on binocular modulation in macaque monkey LGN used bars or light flashes that covered the entire visual field to evoke neural responses, which change global luminance in addition to local visual contrast (Marrocco & McClurkin, 1979; Rodieck & Dreher, 1979) but see Schroeder, Tenke, Arezzo, & Vaughan, 1990).
Primate Specializations
The primate LGN is organized in parvocellular (P), magnocellular (M), and koniocellular (K) layers (Brunso-Bechtold & Casagrande, 1982; Norton, Casagrande, Irvin, Sesma, & Petry, 1988; Xu et al., 2001)(Figure 1). P cells, which make up the majority of LGN neurons, form the primary four dorsal layers of the LGN (Dreher, Fukada, & Rodieck, 1976). M cells, which constitute less than 20% of LGN neurons, are located in the two ventral-most primary layers (Hendry & Reid, 2000). K neurons, which constitute less than a tenth of LGN neurons, are almost exclusively located within the intercalated zones that span between the primary LGN layers (Casagrande, Yazar, Jones, & Ding, 2007; Hendry & Reid, 2000). M and P neurons can be reliably distinguished using neurophysiological criteria, which includes systematic differences in the transiency of their responses, selectivity to spatial and temporal frequency, color (cone) opponency, contrast response functions and recording locations within the LGN (Brunso-Bechtold & Casagrande, 1982; Norton et al., 1988; Xu et al., 2001). K neurons tend to differ in their spectral response from M and P neurons, and can be cytochemically distinguished via optogenetic targeting by their expression of CamKII, a protein kinase, which is absent in all other LGN neurons (Hendry & Yoshioka, 1994; Klein et al., 2016).
The distinction between P, M and K neurons is particularly important for binocular vision because the physiology and anatomical connectivity of these cells differs distinctively, which might affect binocular modulation in these pathways. Several psychophysical studies have found that motion information, believed to be carried by the M pathway, is integrated across the eyes differently than other visual properties (Andrews & Blakemore, 2002; Carlson & He, 2000; Sun, Tong, Yang, Tian, & Hung, 2002). This hypothesis is corroborated by neurophysiological data (Tailby, Majaj, & Movshon, 2010), but how this finding relates to the responses of specific LGN subpopulations is unclear. Some researchers suggest that binocular modulation occurs with equal frequency in P and M layers (Marrocco & McClurkin, 1979; Schroeder et al., 1990), while others report that binocular modulation is exclusive to the M layers (Rodieck & Dreher, 1979). Interestingly, anatomical studies in New World monkeys show that M neurons tend to be oriented orthogonally to the laminar boundaries and stay less confined to their home layer than P cells (Conley, Birecree, & Casagrande, 1985). This idiosyncratic morphology might constitute a unique mechanism to provide M cells with inputs from both eyes. No neurophysiological data to date speak to whether K neurons exhibit binocular modulation by altering their responses to the dominant eye when the non-dominant is stimulated as well. Intriguingly, a small fraction (~10–30%) of K neurons respond to both eyes (Cheong et al., 2013; Zeater et al., 2015), suggesting that (some) K neurons might play a special role for binocular interactions. K neurons uniquely receive input from the superior colliculus (Stepniewska, Qi, & Kaas, 1999), which might provide them with exclusive binocular input. The superior colliculus receives inputs from both eyes as well as inputs from V1, and approximately 80% of superior colliculus neurons respond to stimuli shown to either eye in primates (Moors & Vendrik, 1979), with ~30% even responding equally strong to input from either eye (Marrocco & Li, 1977).
Subcortical circuits supporting binocular modulation
Potential role of intrageniculate cells
Neurons in the primary layers of the LGN receive input from one eye only (Guillery, 1970; Hayhow, 1958; Hickey & Guillery, 1974; Kaas, Guillery, & Allman, 1972; Laties & Sprague, 1966; Stone & Hansen, 1966). In order for binocular modulation to be generated locally in the LGN, the processes of any involved LGN neurons must extend across these laminar boundaries. In the cat, several types of intrageniculate cells could fulfill this criterion (Sanderson et al., 1971). First, large multipolar class I cells, located in interlaminar zones, receive binocular input from the optic tract, and also feature dendrites that extend beyond the cells’ home layer (Hayhow, 1958; Laties & Sprague, 1966). Second, geniculocortical class II cells feature dendrites that extend into other layers (Guillery, 1966). Third, one type of LGN interneuron (subtype b) features axons that extend into other layers of the LGN (Tömböl, 1969). Lastly, another type of cell that is present in all major laminae has dendrites that cross into other layers and contacts other dendrites there (Famiglietti, 1970). Binocular modulation in cat LGN could arise through the activity of any of these four cell types, or a combination thereof.
Evidence of similar anatomical connections among LGN layers in primates is much sparser. However, some primate LGN neurons, particularly those close to primary laminar borders, have dendrites that extend across the border of origin into interlaminar spaces (K layers) and sometimes even into the adjacent primary layer (Campos Ortega, Glees, & Neuhoff, 1968; Saini & Garey, 1981). In addition, neurons within interlaminar zones feature dendrites that span into both neighboring laminae, suggesting potential for interactions between monocular neurons across laminar borders in primates (Guillery & Colonnier, 1970).
Potential role of other subcortical structures for binocular modulation in the LGN
Both cats and primates feature a group of cell bodies superior to the LGN, known as the perigeniculate nucleus (PGN) and thalamic reticular nucleus (TRN), respectively, that receive inputs from both the LGN and early visual cortex (Ahlsén & Lindström, 1983; Ahlsén, Lindström, & Sybirska, 1978; Dubin & Cleland, 1977; Updyke, 1975). Some PGN cells respond to visual stimulation in either eye (Xue, Carney, Ramoa, & Freeman, 1988). These binocular responses in PGN are robust to ablation of areas 17, 18, and 19 (Xue et al., 1988), suggesting that PGN cells combine monocular LGN inputs locally. In turn, PGN cells inhibit LGN principal cells (Lindström, 1982). Given that LGN neurons generally reduce their responses under binocular viewing (Guido et al., 1989; Sengpiel, Blakemore, & Harrad, 1995a; Xue et al., 1987), PGN/TRN neurons might play a role in subcortical binocular modulation (Funke & Eysel, 1998). Other possible subcortical routes for LGN binocular modulation involve the superior colliculus, as discussed above, as well as the pretectum or the parabigeminal nucleus (Casagrande & Boyd, 1996; Feig & Harting, 1994; Harting, Hashikawa, & Van Lieshout, 1986; Harting, Van Lieshout, Hashikawa, & Weber, 1991). Future research may discern the role of each of these pathways for binocular modulation.
Binocular modulation in primary visual cortex
An alternative to the hypothesis that the outputs from the two eyes first meet and interact in the LGN is that this meeting first occurs in primary visual cortex. LGN relay cells from M and P laminae primarily project onto granular layer 4 stellate cells in primary visual cortex (Figure 2). Layer 4 cells have been described as predominantly monocular (Hubel & Wiesel, 1968). Lesioning a geniculate layer corresponding to one eye results in patchy degeneration of cortical tissue in macaque V1 layer 4, suggesting spatial segregation of each eye’s input (Hubel & Wiesel, 1972). Proline (dye) injections in one eye reveal alternating bands of ocular dominance in the granular layer, which further demonstrates that eye-specific LGN inputs to macaque V1 layer 4 are spatially distinct (Blasdel & Lund, 1983; Hubel & Wiesel, 1972). However, the spatial segregation of eye-specific inputs in V1 seems far less strict than in the LGN as some LGN afferents terminate in V1 ocular dominance bands corresponding to the other eye (Blasdel & Lund, 1983). Therefore, the signals from the two eyes might interact at the level of geniculocortical synapses in layer 4, i.e. at the input stage to visual cortex. Another possibility is that binocular modulation in primary visual cortex is mediated via local interneurons. For example, inhibitory basket cells in layer 3 of cat visual cortex span ocular dominance bands (Buzás, Eysel, Adorján, & Kisvárday, 2001). Basket cells also reside in layer 4, although it is unknown whether these neurons span ocular dominance as well (Martin, Somogyi, & Whitteridge, 1983). In any case, most neurons outside of layer 4 of primary visual cortex are driven through either eye, which suggests that the signals from the two eyes are merged when visual activation reaches these layers (Hubel & Wiesel, 1962).
Single neuron studies in cat visual cortex
A large body of literature on binocular modulation in cat visual cortex has shown that binocular stimulation generally results in a reduction of activity compared to monocular stimulation, especially if the orientation of the stimuli in each eye are orthogonal (Sengpiel & Blakemore, 1994; Sengpiel & Vorobyov, 2005; Sengpiel, Baddeley, Freeman, Harrad, & Blakemore, 1998; Sengpiel, Blakemore, & Harrad, 1995a; Sengpiel, Freeman, & Blakemore, 1995b).
Several neurophysiological studies have probed the specific origins of binocular modulation in cat area A17 and A18. For example, Ohzawa and Freeman relied on the fact that, unlike LGN neurons (Xue et al., 1987), responses of visual cortical neurons vary with binocular disparity (i.e., a slight positional shift of the same image between the two eyes). In this study, both the relative phase (disparity) and contrast of grating stimuli shown to each eye were varied (Freeman & Ohzawa, 1990). Interestingly, the neurons’ disparity tuning remained constant, even for large interocular contrast differences, such as 2.5% contrast in one eye and 50% contrast in the other eye. This result suggests that binocular modulation occurs before area 17 neurons produce action potentials since their spiking output has already been adjusted to the contrast of the stimulus shown to the other eye.
Truchard, Ohzawa, & Freeman (2000) developed a model for contrast encoding that incorporates monocular and binocular stages of visual processing. They also recorded spiking responses from binocular cells in area 17 in anesthetized cats while a grating of either high or low contrast was presented to one eye, and another grating of varying contrast and spatial phase was simultaneously shown to the opposite eye. They found that increasing the contrast of a grating in one eye results in a large reduction in monocular contrast gain, and that this reduction is largely independent of the contrast gain of the other eye (Truchard, Ohzawa, & Freeman, 2000). In other words, their data suggest that, under binocular viewing, most contrast gain control occurs at the monocular level.
On the other hand, transfer of visual adaptation from one eye to the other (interocular transfer) has been pointed out as evidence that some interocular gain control occurs at the binocular level. Specifically, following several hundred milliseconds to several seconds of exposure to stimuli presented to one eye, responses to stimulation of the other eye are reduced for both binocular neurons (Hammond & Mouat, 1988; Maffei, Berardi, & Bisti, 1986) and monocular neurons alike (Howarth, Vorobyov, & Sengpiel, 2009). This finding suggests that monocular neurons are not only modulated by their counterparts that encode the other eye, but also by neurons that receive inputs from both eyes and therefore encode the binocular signal.
Single neuron studies in macaque V1
There have been numerous studies on the effects of binocular stimulation in monkey primary visual cortex. The vast majority of them were limited to stimuli that did not match between the eyes (see Cumming & DeAngelis, 2001; Freeman, 2017; Henriksen, Tanabe, & Cumming, 2016; Leopold, Maier, Wilke, & Logothetis, 2005; Logothetis, 1998; Macknik & Martinez-Conde, 2007; Parker & Cumming, 2001; Parker, Smith, & Krug, 2016, for review). Similar to the cat, most neurons in monkey V1 are driven through either eye (Hubel & Wiesel, 1968), and the predominant effect of binocular stimulation (outside very low contrast levels) is binocular suppression (Endo, Kaas, Jain, Smith, & Chino, 2000; Kumagami, Zhang, Smith, & Chino, 2000).
One study in monkey V1 specifically addressed the question if signals from the two eyes interact before reaching this area. In this experiment, a grating was presented at one contrast level to one eye, and a target of varying contrast was presented to the other eye. The authors found that when a lower contrast grating is shown to one eye, a higher contrast grating needs to be shown to the other eye to elicit a certain criterion response (Smith, Chino, Ni, & Cheng, 1997b). This finding mirrors the results from the cat outlined above (Truchard et al., 2000): Given that dichoptic gratings of varying contrast can elicit the same neuronal response, binocular modulation seems to occur prior to the spiking of V1 neurons. This conclusion allows for the possibility that the site of initial binocular modulation lies either within the LGN or at the synaptic input level to V1 (such as in layer 4C), prior to spiking output.
Cortical Feedback to the LGN and its Potential Role for Binocular Modulation
In both cats and primates, some cells in primary visual cortex project back to the LGN (Figure 2). In cats, layer 6 neurons in areas 17, 18, and 19 project to the LGN (Gilbert & Kelly, 1975). In monkeys, ~15% of V1 layer 6 neurons project to the LGN (Fitzpatrick, Usrey, Schofield, & Einstein, 1994). To a much smaller extent, neurons in extrastriate areas, such as area V2, project to the LGN as well (Briggs, Kiley, Callaway, & Usrey, 2016). This corticogeniculate projection is retinotopically aligned with the retinogeniculate input (Ichida, Mavity-Hudson, & Casagrande, 2014; Updyke, 1975). But in contrast to retinal inputs that mostly terminate proximally on LGN neurons and activate ionotropic receptors, corticogeniculate inputs to LGN tend to terminate distally and act on metabotropic receptors, effectively serving a modulatory role (see Sherman, 2007). Given the specificity of corticogeniculate projections and their termination pattern, it is conceivable that binocular modulation in the LGN is not computed locally, but fed back from primary visual cortex. As discussed above, corticogeniculate cells also synapse on cells in the PGN/TRN, which in return project to and inhibit cells in the LGN (Ahlsén & Lindström, 1983; Dubin & Cleland, 1977). Therefore, corticogeniculate feedback could also act through the PGN/TRN to mediate binocular modulation in the LGN.
To investigate the potential role of cortical feedback on binocular modulation in the LGN, several researchers studied whether binocular modulation is sensitive to stimulus orientation (Moore et al., 1992; Sengpiel, Freeman, & Blakemore, 1995b; Varela & Singer, 1987). The rationale behind these studies is that neuronal orientation selectivity (orientation tuning) is of cortical origin (Scholl, Tan, Corey, & Priebe, 2013b). Therefore, orientation dependence of binocular modulation in the LGN seems indicative of involvement of corticogeniculate feedback. Only one of these studies found an orientation bias of binocular inhibition of LGN neurons (Sengpiel, Blakemore, & Harrad, 1995a; Varela & Singer, 1987), leaving the question of feedback-mediated mechanisms unresolved. Notably, cortex could also be involved in LGN binocular modulation in an orientation-independent manner if it arises by a population of cortico-geniculate neurons with varying orientation tuning or by cortical neurons without orientation tuning (Moore et al., 1992). Furthermore, a small, but measurable orientation bias has been found among retinal ganglion cells (Kuffler, 1953; Levick & Thibos, 1982) as well as geniculate neurons of both cats (Creutzfeldt & Nothdurft, 1978; Daniels, Norman, & Pettigrew, 1977; Shou, Ruan, & Zhou, 1986; Soodak, Shapley, & Kaplan, 1987; Vidyasagar & Urbas, 1982) and monkeys (Lee, Virsu, & Creutzfeldt, 1977; Smith, Chino, Ridder, Kitagawa, & Langston, 1990; Xu, Ichida, Shostak, Bonds, & Casagrande, 2002). This orientation bias of LGN neurons survives inactivation of cortical feedback (Thompson, Leventhal, Zhou, & Liu, 1994; Vidyasagar & Urbas, 1982). Therefore, (modest) orientation-dependent binocular modulation may not be sufficient to exclusively implicate cortical feedback for binocular modulation (Shou & Leventhal, 1989; Xu et al., 2002).
In cats, the role of the cortical feedback for binocular modulation has also been investigated during reversible or permanent inactivation of visual cortex. One of these studies found that removal of areas 17, 18, and 19 had little-to-no impact on LGN cells that exhibit binocular modulation (Sanderson et al., 1971). In line with this result, two related studies found that LGN binocular modulation remained unchanged after cooling areas 17, 18, and 19 (Pape & Eysel, 1986; Singer, 1970). However, other groups found results that stand in direct contradiction to the above. In one of these studies, areas 17 and 18 were cooled to inactivate neural activity in the deep layers that project to the LGN, and binocular modulation in the LGN was greatly diminished in the LGN under this condition (Schmielau & Singer, 1977). Another study found that removing areas 17, 18 and 19 permanently eliminated binocular modulation in the LGN (Varela & Singer, 1987). Therefore, based on the existing data, it is too early to conclude about the involvement of cortical feedback in LGN binocular modulation.
Binocular modulation and binocular rivalry
When the two eyes are simultaneously stimulated using two very different (non-fusible) stimuli at the same retinal location, perception alternates stochastically between each eye’s view (Alais & Blake, 2005; Blake & Logothetis, 2002; Leopold et al., 2005; Maier, Panagiotaropoulos, Tsuchiya, & Keliris, 2012; Tong, Meng, & Blake, 2006). For example, when one eye views a face while the other eye is looking at a house that is presented at the same retinal location, perception dynamically switches between the face and the house. In other words, although retinal stimulation remains constant, perception fluctuates under these conditions. This dynamic perceptual phenomenon termed binocular rivalry seems related to binocular modulation in that it involves stimulation of the two eyes as well as suppression of one eye’s view at the expense of the other. However, there are several important differences between these two phenomena. First, binocular rivalry only occurs when the stimuli in the two eyes cannot be fused, whereas the kind of binocular suppression discussed here occurs even when the stimuli are perceptually fused. Second, binocular rivalry suppresses only one eye’s view, whereas the kind of binocular modulation discussed here affects the responses of both eyes. Third, binocular rivalry fluctuates over time, whereas the binocular modulation discussed here does not co-vary with these perceptual alternations. The relationship between binocular modulation and binocular rivalry does remain unclear.
One benefit of binocular rivalry is that it can be utilized by experimenters to track if certain groups of visual neurons alter their activity whenever perception changes between the two simultaneously presented stimuli. Two independent studies with human volunteers found that fMRI signals in LGN strongly correlate with perceptual report (Haynes, Deichmann, & Rees, 2005; Wunderlich, Schneider, & Kastner, 2005). In contrast, neurophysiological studies in trained macaques did not show any population firing rate changes in LGN during similar stimulation conditions (Lehky & Maunsell, 1996; Wilke, Mueller, & Leopold, 2009). The involvement of LGN in binocular rivalry therefore remains unclear. An empirically supported explanation for the apparent discrepancies is that the fMRI signal is more sensitive to metabolically costly synaptic modulation than spiking output (Logothetis, Pauls, Augath, Trinath, & Oeltermann, 2001). This difference in measurement might explain why spiking activity and fMRI signals diverge when feedback modulation is too weak to elicit large changes in population spiking (Boynton, 2011; Maier et al., 2008; Schmid & Maier, 2015).
Discussion
The LGN is often conceptualized as a “monocular” structure. This classification is well-supported by anatomy and physiology that show that almost all LGN neurons receive input and are driven through only one eye (Bishop, Kozak, Levick, & Vakkur, 1962b; Cleland, Dubin, & Levick, 1971; Guillery, 1970; Guillery & Kaas, 1971; Hayhow, 1958; Hickey & Guillery, 1974; Hubel & Wiesel, 1961; Kaas et al., 1972; Laties & Sprague, 1966; Stone & Hansen, 1966). Yet, as described in this review, several LGN circuits, both local and extra-geniculate, could provide a substrate for binocular modulation of LGN neurons. Indeed, most single neuron studies in both cats and primates have found evidence for binocular modulation in the LGN.
Convergent observations of binocular modulation in the LGN of cats and primates suggest a homologous mechanism for processing binocular inputs. Could this geniculate processing serve to adjust the gain of responses under binocular viewing? The input to the visual system is greater under binocular than monocular viewing, yet the appearance of the visual world remains largely the same under these viewing conditions. This phenomenon suggests that the brain accounts for the difference in inputs between monocular and binocular viewing, likely through a gain control mechanism. Intriguingly, in both cats and monkeys, most binocular modulation in the LGN is suppressive. Furthermore, where contrast-dependent modulation was measured, the majority of binocular modulation suggested an adjustment in responses at all contrast levels (Tong et al., 1992).
While both cats and monkeys show (mostly suppressive) binocular modulation in the LGN, the data also reveal several differences between these two model species. Most strikingly, while the majority of LGN neurons in the cat modulate under binocular viewing, this binocular modulation is limited to a minority of macaque neurons. Some of these interspecies differences in neuronal response properties might be explained by species-specific anatomical adaptations. Several researchers have drawn homologies between cat X, Y, and W cells and primate P, M, and K cells, respectively, based on similarities of their response properties (Dreher et al., 1976; Irvin, Norton, Sesma, & Casagrande, 1986; Norton & Casagrande, 1982; Schiller & Malpeli, 1978; Sherman, Wilson, Kaas, & Webb, 1976) but see Kaplan & Shapley, 1982). In cats, the superior colliculus projects to the C layers of cat LGN where W cells reside (Torrealba, Partlow, & Guillery, 1981). Similarly, in primates, the superior colliculus projects to K neurons (Stepniewska et al., 1999). Given this homology, it is possible that this tecto-thalamic projection serves similar roles for binocular processing for cat W and primate K neurons. However, several other observations suggest that binocular processing differs significantly between cats and primates. Chief among them is the difference in anatomical layout of the cat LGN and primate LGN. Although homologies have been drawn between X, Y, W and P, M, and K neurons, their distribution in the LGN in each species is different. X cells terminate in layers A and A1 of cat LGN, Y cells terminate in layer A and C, and W cells terminate in C layers only (Stone & Fukuda, 1974; Sur & Sherman, 1982b; 1982a; Wilson, Rowe, & Stone, 1976). In contrast, primate P, M and K neurons are segregated in separate layers. Second, neurons in cat LGN that cross eye-specific layers appear to be more frequent in cats than in monkeys (Guillery, 1966; Levitt, Schumer, Sherman, Spear, & Movshon, 2001; Movshon et al., 1987; Sanderson et al., 1971). Lastly, whereas cat LGN projects to three visual areas (A17, A18 and A19), primate LGN predominantly projects to the primary visual cortex (V1). Cat LGN also receives feedback from three cortical areas (A17–19), whereas primate LGN receives its cortical feedback almost exclusively from V1 (but see Briggs et al., 2016; Hendrickson, Wilson, & Ogren, 1978). Given these differences in organization, the LGN circuitry for binocular combination in cats and primates may differ significantly, which may translate directly into functional differences that have been observed across neurophysiological studies (Conway & Schiller, 1983).
Another consideration regarding the extant literature on binocular modulation is that most of the work from cat and primate LGN were performed using anesthetized preparations that relied on GABAergic action. LGN neurons are highly sensitive to altered states of arousal such as wakefulness, drowsiness, sleep and general anesthesia (Alitto, Moore, Rathbun, & Usrey, 2011). In addition, response normalization, which is one potential mechanism by which the gain of monocular signals might be adjusted under binocular viewing, is significantly altered by such anesthetic preparations (Vaiceliunaite, Erisken, Franzen, Katzner, & Busse, 2013). In line with this concern, direct comparison between anesthetized and awake recordings in cat LGN found that the anesthetized state produced significantly different results than the awake state (Garraghty, Salinger, MacAvoy, Schroeder, & Guido, 1982; Schroeder, Salinger, & Guido, 1988).
All findings reviewed in this paper were collected in animals with normal vision, but, as outlined in the introduction, binocular modulation in the LGN is of great relevance for abnormal vision as well. In particular, strabismic amblyopia, a disorder of binocular vision that affects children with misaligned eyes, and causes lifelong perceptual suppression of one eye if not treated before they reach adolescence, causes “shrinkage” of LGN neurons (primarily of the affected eye) in cats, macaques and humans (Barnes et al., 2010; G. Cheng et al., 2008; Noorden & Crawford, 1992; Noorden & Middleditch, 1975; Tremain & Ikeda, 1982). However, whether and how this structural malformation affects binocular modulation among LGN neurons remains unknown as existing studies in the LGN of strabismic cats were generally limited to testing the acuity of LGN neurons related to the perceptually suppressed eye (Chino et al., 1994; Gillard-Crewther & Crewther, 1988).
An impressive amount of work on the topic of binocular modulation in the LGN has laid a strong foundation to fully elucidate the role of this structure in binocular processing. These advances will be critical to determining the origins of binocular modulation and its possible basis for interocular gain control and binocular contrast normalization (Murphy & Sillito, 1989; Pape & Eysel, 1986; Sanderson et al., 1971; Schmielau & Singer, 1977; Singer, 1970; Varela & Singer, 1987). The apparent paradox that outputs from both eyes are brought together in the LGN in retinotopic register while keeping each eye’s output segregated may explain binocular modulation within this structure through mechanisms yet to be uncovered.
Acknowledgments
This work was supported by a research grant from the National Eye Institute (1R01EY027402-01) as well as research grants from the European Research Council, the Whitehall Foundation and the Knights Templar Eye Foundation. K.D. is supported by a National Eye Institute Training Grant (2T32 EY007135-21).
Footnotes
In Memoriam of Vivien A. Casagrande
Among her many scientific breakthroughs, Vivien made fundamental contributions to the neuronal basis of binocular combination in LGN and V1 across different primate species. Much of her work laid the groundwork for the research we discuss in this review. In addition to her shining legacy as a scientist, Vivien was a deeply admired colleague and an inspiring mentor. She is sorely missed.
References
- Ahlsén G, Lindström S. Corticofugal projection to perigeniculate neurones in the cat. Acta Physiologica Scandinavica. 1983;118(2):181–184. doi: 10.1111/j.1748-1716.1983.tb07259.x. [DOI] [PubMed] [Google Scholar]
- Ahlsén G, Lindström S, Sybirska E. Subcortical axon collaterals of principal cells in the lateral geniculate body of the cat. Brain Research. 1978;156(1):106–109. doi: 10.1016/0006-8993(78)90084-7. [DOI] [PubMed] [Google Scholar]
- Alais D, Blake R. Binocular Rivalry. MIT Press; 2005. [Google Scholar]
- Alitto HJ, Moore BD, Rathbun DL, Usrey WM. A comparison of visual responses in the lateral geniculate nucleus of alert and anaesthetized macaque monkeys. The Journal of Physiology. 2011;589(Pt 1):87–99. doi: 10.1113/jphysiol.2010.190538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews TJ, Blakemore C. Integration of motion information during binocular rivalry. Vision Research. 2002;42(3):301–309. doi: 10.1016/s0042-6989(01)00286-3. [DOI] [PubMed] [Google Scholar]
- Baker DH, Meese TS, Summers RJ. Psychophysical evidence for two routes to suppression before binocular summation of signals in human vision. Neuroscience. 2007;146(1):435–448. doi: 10.1016/j.neuroscience.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Barnes GR, Li X, Thompson B, Singh KD, Dumoulin SO, Hess RF. Decreased gray matter concentration in the lateral geniculate nuclei in human amblyopes. Investigative Ophthalmology & Visual Science. 2010;51(3):1432–1438. doi: 10.1167/iovs.09-3931. [DOI] [PubMed] [Google Scholar]
- Bishop PO, Burke W, Davis R. The interpretation of the extracellular response of single lateral geniculate cells. The Journal of Physiology. 1962a;162(3):451–472. doi: 10.1113/jphysiol.1962.sp006944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop PO, Kozak W, Levick WR, Vakkur GJ. The determination of the projection of the visual field on to the lateral geniculate nucleus in the cat. The Journal of Physiology. 1962b;163(3):503–539. doi: 10.1113/jphysiol.1962.sp006991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake R, Logothetis NK. Visual competition. Nature Reviews Neuroscience. 2002;3(1):13–21. doi: 10.1038/nrn701. [DOI] [PubMed] [Google Scholar]
- Blake R, Cool SJ, Crawford ML. Visual resolution in the cat. Vision Research. 1974;14(11):1211–1217. doi: 10.1016/0042-6989(74)90218-1. [DOI] [PubMed] [Google Scholar]
- Blasdel G, Lund J. Termination of afferent axons in macaque striate cortex. The Journal of Neuroscience. 1983;3(7):1389–1413. doi: 10.1523/JNEUROSCI.03-07-01389.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton GM. Spikes, BOLD, Attention, and Awareness: A comparison of electrophysiological and fMRI signals in V1. Journal of Vision. 2011;11(5):12–12. doi: 10.1167/11.5.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs F, Kiley CW, Callaway EM, Usrey WM. Morphological Substrates for Parallel Streams of Corticogeniculate Feedback Originating in Both V1 and V2 of the Macaque Monkey. Neuron. 2016;90(2):388–399. doi: 10.1016/j.neuron.2016.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunso-Bechtold JK, Casagrande VA. Early postnatal development of laminar characteristics in the dorsal lateral geniculate nucleus of the tree shrew. The Journal of Neuroscience. 1982;2(5):589–597. doi: 10.1523/JNEUROSCI.02-05-00589.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzás P, Eysel UT, Adorján P, Kisvárday ZF. Axonal topography of cortical basket cells in relation to orientation, direction, and ocular dominance maps. The Journal of Comparative Neurology. 2001;437(3):259–285. doi: 10.1002/cne.1282. [DOI] [PubMed] [Google Scholar]
- Campos Ortega JA, Glees P, Neuhoff V. Ultrastructural analysis of individual layers in the lateral geniculate body of the monkey. Zeitschrift Fur Zellforschung Und Mikroskopische Anatomie (Vienna, Austria: 1948) 1968;87(1):82–100. doi: 10.1007/BF00326562. [DOI] [PubMed] [Google Scholar]
- Carlson TA, He S. Visible binocular beats from invisible monocular stimuli during binocular rivalry. Current Biology. 2000;10(17):1055–1058. doi: 10.1016/s0960-9822(00)00672-2. [DOI] [PubMed] [Google Scholar]
- Casagrande VA, Boyd JD. The neural architecture of binocular vision. Eye (London, England) 1996;10(Pt 2)(2):153–160. doi: 10.1038/eye.1996.40. [DOI] [PubMed] [Google Scholar]
- Casagrande VA, Kaas JH. Primary Visual Cortex in Primates. Vol. 10. Boston, MA: Springer US; 1994. The Afferent, Intrinsic, and Efferent Connections of Primary Visual Cortex in Primates; pp. 201–259. [Google Scholar]
- Casagrande VA, Yazar F, Jones KD, Ding Y. The morphology of the koniocellular axon pathway in the macaque monkey. Cerebral Cortex. 2007;17(10):2334–2345. doi: 10.1093/cercor/bhl142. [DOI] [PubMed] [Google Scholar]
- Cheng G, Kaminski HJ, Gong B, Zhou L, Hatala D, Howell SJ, et al. Monocular visual deprivation in macaque monkeys: a profile in the gene expression of lateral geniculate nucleus by laser capture microdissection. Molecular Vision. 2008;14:1401–1413. [PMC free article] [PubMed] [Google Scholar]
- Cheong SK, Tailby C, Solomon SG, Martin PR. Cortical-like receptive fields in the lateral geniculate nucleus of marmoset monkeys. Journal of Neuroscience. 2013;33(16):6864–6876. doi: 10.1523/JNEUROSCI.5208-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chino YM, Cheng H, Smith EL, Garraghty PE, Roe AW, Sur M. Early discordant binocular vision disrupts signal transfer in the lateral geniculate nucleus. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(15):6938–6942. doi: 10.1073/pnas.91.15.6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland BG, Dubin MW, Levick WR. Simultaneous recording of input and output of lateral geniculate neurones. Nature: New Biology. 1971;231(23):191–192. doi: 10.1038/newbio231191a0. [DOI] [PubMed] [Google Scholar]
- Conley M, Birecree E, Casagrande VA. Neuronal classes and their relation to functional and laminar organization of the lateral geniculate nucleus: a Golgi study of the prosimian primate, Galago crassicaudatus. The Journal of Comparative Neurology. 1985;242(4):561–583. doi: 10.1002/cne.902420407. [DOI] [PubMed] [Google Scholar]
- Conway JL, Schiller PH. Laminar organization of tree shrew dorsal lateral geniculate nucleus. Journal of Neurophysiology. 1983;50(6):1330–1342. doi: 10.1152/jn.1983.50.6.1330. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt OD, Nothdurft HC. Representation of complex visual stimuli in the brain. Die Naturwissenschaften. 1978;65(6):307–318. doi: 10.1007/BF00368371. [DOI] [PubMed] [Google Scholar]
- Cumming BG, DeAngelis GC. The physiology of stereopsis. Annual Review of Neuroscience. 2001;24(1):203–238. doi: 10.1146/annurev.neuro.24.1.203. [DOI] [PubMed] [Google Scholar]
- Dacey DM. Physiology, morphology and spatial densities of identified ganglion cell types in primate retina. Ciba Foundation Symposium. 1994;184:12–28. doi: 10.1002/9780470514610.ch2. discussion 28–34– 63–70. [DOI] [PubMed] [Google Scholar]
- Daniels JD, Norman JL, Pettigrew JD. Biases for oriented moving bars in lateral geniculate nucleus neurons of normal and stripe-reared cats. Experimental Brain Research. 1977;29(2):155–172. doi: 10.1007/BF00237039. [DOI] [PubMed] [Google Scholar]
- Ding J, Sperling G. A gain-control theory of binocular combination. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(4):1141–1146. doi: 10.1073/pnas.0509629103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher B, Fukada Y, Rodieck RW. Identification, classification and anatomical segregation of cells with X-like and Y-like properties in the lateral geniculate nucleus of old-world primates. The Journal of Physiology. 1976;258(2):433–452. doi: 10.1113/jphysiol.1976.sp011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin MW, Cleland BG. Organization of visual inputs to interneurons of lateral geniculate nucleus of the cat. Journal of Neurophysiology. 1977;40(2):410–427. doi: 10.1152/jn.1977.40.2.410. [DOI] [PubMed] [Google Scholar]
- Endo M, Kaas JH, Jain N, Smith EL, Chino Y. Binocular cross-orientation suppression in the primary visual cortex (V1) of infant rhesus monkeys. Investigative Ophthalmology & Visual Science. 2000;41(12):4022–4031. [PubMed] [Google Scholar]
- Erulkar SD, Fillenz M. Single-unit activity in the lateral geniculate body of the cat. The Journal of Physiology. 1960;154(1):206–218. doi: 10.1113/jphysiol.1960.sp006574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti EV. Dendro-dendritic synapses in the lateral geniculate nucleus of the cat. Brain Research. 1970;20(2):181–191. doi: 10.1016/0006-8993(70)90287-8. [DOI] [PubMed] [Google Scholar]
- Feig S, Harting JK. Ultrastructural studies of the primate lateral geniculate nucleus: morphology and spatial relationships of axon terminals arising from the retina, visual cortex (area 17), superior colliculus, parabigeminal nucleus, and pretectum of Galago crassicaudatus. The Journal of Comparative Neurology. 1994;343(1):17–34. doi: 10.1002/cne.903430103. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cerebral Cortex. 1991;1(1):1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D, Lund JS, Blasdel GG. Intrinsic connections of macaque striate cortex: afferent and efferent connections of lamina 4C. The Journal of Neuroscience. 1985;5(12):3329–3349. doi: 10.1523/JNEUROSCI.05-12-03329.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick D, Usrey WM, Schofield BR, Einstein G. The sublaminar organization of corticogeniculate neurons in layer 6 of macaque striate cortex. Visual Neuroscience. 1994;11(2):307–315. doi: 10.1017/s0952523800001656. [DOI] [PubMed] [Google Scholar]
- Freeman RD. 2015 Charles F. Prentice Medal Award Lecture: Neural Organization of Binocular Vision. Optometry and Vision Science: Official Publication of the American Academy of Optometry. 2017 Oct; doi: 10.1097/OPX.0000000000001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman RD, Ohzawa I. On the neurophysiological organization of binocular vision. Vision Research. 1990;30(11):1661–1676. doi: 10.1016/0042-6989(90)90151-a. [DOI] [PubMed] [Google Scholar]
- Funke K, Eysel UT. Inverse correlation of firing patterns of single topographically matched perigeniculate neurons and cat dorsal lateral geniculate relay cells. Visual Neuroscience. 1998;15(4):711–729. doi: 10.1017/s0952523898154111. [DOI] [PubMed] [Google Scholar]
- Garraghty PE, Salinger DWL, MacAvoy MG, Schroeder CE, Guido W. The shift in X/Y ratio after chronic monocular paralysis: A binocularly mediated, barbiturate-sensitive effect in the adult lateral geniculate nucleus. Experimental Brain Research. 1982;47(2):301–308. doi: 10.1007/BF00239390. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Kelly JP. The projections of cells in different layers of the cat’s visual cortex. The Journal of Comparative Neurology. 1975;163(1):81–105. doi: 10.1002/cne.901630106. [DOI] [PubMed] [Google Scholar]
- Gillard-Crewther S, Crewther DP. Neural site of strabismic amblyopia in cats: X-cell acuities in the LGN. Experimental Brain Research. 1988;72(3):503–509. doi: 10.1007/BF00250595. [DOI] [PubMed] [Google Scholar]
- Grieve KL. Binocular visual responses in cells of the rat dLGN. The Journal of Physiology. 2005;566(Pt 1):119–124. doi: 10.1113/jphysiol.2005.090878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guido W, Tumosa N, Spear PD. Binocular interactions in the cat’s dorsal lateral geniculate nucleus. I. Spatial-frequency analysis of responses of X, Y, and W cells to nondominant-eye stimulation. Journal of Neurophysiology. 1989;62(2):526–543. doi: 10.1152/jn.1989.62.2.526. [DOI] [PubMed] [Google Scholar]
- Guillery RW. A study of Golgi preparations from the dorsal lateral geniculate nucleus of the adult cat. The Journal of Comparative Neurology. 1966;128(1):21–50. doi: 10.1002/cne.901280104. [DOI] [PubMed] [Google Scholar]
- Guillery RW. The laminar distribution of retinal fibers in the dorsal lateral geniculate nucleus of the cat: A new interpretation. Journal of Comparative Neurology. 1970;138(3):339–367. [Google Scholar]
- Guillery RW, Colonnier M. Synaptic patterns in the dorsal lateral geniculate nucleus of the monkey. Zeitschrift Für Zellforschung Und Mikroskopische Anatomie. 1970;103(1):90–108. doi: 10.1007/BF00335403. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Kaas JH. A study of normal and congenitally abnormal retinogeniculate projections in cats. The Journal of Comparative Neurology. 1971;143(1):73–100. doi: 10.1002/cne.901430106. [DOI] [PubMed] [Google Scholar]
- Hammond P, Mouat GS. Neural correlates of motion after-effects in cat striate cortical neurones: interocular transfer. Experimental Brain Research. 1988;72(1):21–28. doi: 10.1007/BF00248496. [DOI] [PubMed] [Google Scholar]
- Harting JK, Hashikawa T, Van Lieshout D. Laminar distribution of tectal, parabigeminal and pretectal inputs to the primate dorsal lateral geniculate nucleus: connectional studies in Galago crassicaudatus. Brain Research. 1986;366(1–2):358–363. doi: 10.1016/0006-8993(86)91319-3. [DOI] [PubMed] [Google Scholar]
- Harting JK, Van Lieshout DP, Hashikawa T, Weber JT. The parabigeminogeniculate projection: connectional studies in eight mammals. The Journal of Comparative Neurology. 1991;305(4):559–581. doi: 10.1002/cne.903050404. [DOI] [PubMed] [Google Scholar]
- Hayhow WR. The cytoarchitecture of the lateral geniculate body in the cat in relation to the distribution of crossed and uncrossed optic fibers. The Journal of Comparative Neurology. 1958;110(1):1–63. doi: 10.1002/cne.901100102. [DOI] [PubMed] [Google Scholar]
- Haynes JD, Deichmann R, Rees G. Eye-specific effects of binocular rivalry in the human lateral geniculate nucleus. Nature. 2005;438(7067):496–499. doi: 10.1038/nature04169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesy CP. Seeing in stereo: The ecology and evolution of primate binocular vision and stereopsis. Evolutionary Anthropology: Issues, News, and Reviews. 2009;18(1):21–35. [Google Scholar]
- Hendrickson AE, Wilson JR, Ogren MP. The neuroanatomical organization of pathways between the dorsal lateral geniculate nucleus and visual cortex in Old World and New World primates. The Journal of Comparative Neurology. 1978;182(1):123–136. doi: 10.1002/cne.901820108. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Reid RC. The koniocellular pathway in primate vision. Annual Review of Neuroscience. 2000;23:127–153. doi: 10.1146/annurev.neuro.23.1.127. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Yoshioka T. A neurochemically distinct third channel in the macaque dorsal lateral geniculate nucleus. Science. 1994;264(5158):575–577. doi: 10.1126/science.8160015. [DOI] [PubMed] [Google Scholar]
- Henriksen S, Tanabe S, Cumming B. Disparity processing in primary visual cortex. Philosophical Transactions of the Royal Society B: Biological Sciences. 2016;371(1697):20150255. doi: 10.1098/rstb.2015.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey TL, Guillery RW. An autoradiographic study of retinogeniculate pathways in the cat and the fox. The Journal of Comparative Neurology. 1974;156(2):239–253. doi: 10.1002/cne.901560207. [DOI] [PubMed] [Google Scholar]
- Howarth CM, Vorobyov V, Sengpiel F. Interocular transfer of adaptation in the primary visual cortex. Cerebral Cortex. 2009;19(8):1835–1843. doi: 10.1093/cercor/bhn211. [DOI] [PubMed] [Google Scholar]
- Howarth M, Walmsley L, Brown TM. Binocular Integration in the Mouse Lateral Geniculate Nuclei. Current Biology. 2014;24(11):1241–1247. doi: 10.1016/j.cub.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Integrative action in the cat’s lateral geniculate body. The Journal of Physiology. 1961;155(2):385–398. doi: 10.1113/jphysiol.1961.sp006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. The Journal of Physiology. 1962;160(1):106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. 1968;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Anatomical demonstration of columns in the monkey striate cortex. Nature. 1969;221(5182):747–750. doi: 10.1038/221747a0. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Laminar and columnar distribution of geniculo-cortical fibers in the macaque monkey. 1972;146(4):421–450. doi: 10.1002/cne.901460402. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Ferrier lecture. Functional architecture of macaque monkey visual cortex. 1977;198(1130):1–59. doi: 10.1098/rspb.1977.0085. Retrieved from. [DOI] [PubMed] [Google Scholar]
- Ichida JM, Mavity-Hudson JA, Casagrande VA. Distinct patterns of corticogeniculate feedback to different layers of the lateral geniculate nucleus. Eye and Brain. 2014;2014(6 Suppl 1):57–73. doi: 10.2147/EB.S64281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin GE, Norton TT, Sesma MA, Casagrande VA. W-like response properties of interlaminar zone cells in the lateral geniculate nucleus of a primate (Galago crassicaudatus) Brain Research. 1986;362(2):254–270. doi: 10.1016/0006-8993(86)90450-6. [DOI] [PubMed] [Google Scholar]
- Jeffery G, Cowey A, Kuypers HG. Bifurcating retinal ganglion cell axons in the rat, demonstrated by retrograde double labelling. Experimental Brain Research. 1981;44(1):34–40. doi: 10.1007/BF00238747. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Guillery RW, Allman JM. Some principles of organization in the dorsal lateral geniculate nucleus. Brain, Behavior and Evolution. 1972;6(1):253–299. doi: 10.1159/000123713. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Shapley RM. X and Y cells in the lateral geniculate nucleus of macaque monkeys. The Journal of Physiology. 1982;330:125–143. doi: 10.1113/jphysiol.1982.sp014333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Gilbert CD, Wiesel TN. Local circuits and ocular dominance columns in monkey striate cortex. PLOS Biology. 1989 doi: 10.1523/JNEUROSCI.09-04-01389.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinston WJ, Vadas MA, Bishop PO. Multiple projection of the visual field to the medical portion of the dorsal lateral geniculate nucleus and the adjacent nuclei of the thalamus of the cat. The Journal of Comparative Neurology. 1969;136(3):295–315. doi: 10.1002/cne.901360304. [DOI] [PubMed] [Google Scholar]
- Klein C, Evrard HC, Shapcott KA, Haverkamp S, Logothetis NK, Schmid MC. Cell-Targeted Optogenetics and Electrical Microstimulation Reveal the Primate Koniocellular Projection to Supra-granular Visual Cortex. Neuron. 2016;90(1):143–151. doi: 10.1016/j.neuron.2016.02.036. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Takada M, Honda Y, Mizuno N. Bilateral projections of single retinal ganglion cells to the lateral geniculate nuclei and superior colliculi in the albino rat. Brain Research. 1993;608(2):204–215. doi: 10.1016/0006-8993(93)91460-a. [DOI] [PubMed] [Google Scholar]
- Kuffler SW. Discharge patterns and functional organization of mammalian retina. Journal of Neurophysiology. 1953;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Kumagami T, Zhang B, Smith EL, Chino YM. Effect of onset age of strabismus on the binocular responses of neurons in the monkey visual cortex. Investigative Ophthalmology & Visual Science. 2000;41(3):948–954. [PubMed] [Google Scholar]
- Laties AM, Sprague JM. The projection of optic fibers to the visual centers in the cat. The Journal of Comparative Neurology. 1966;127(1):35–70. doi: 10.1002/cne.901270104. [DOI] [PubMed] [Google Scholar]
- Lee BB, Virsu V, Creutzfeldt OD. Responses of cells in the cat lateral geniculate nucleus to moving stimuli at various levels of light and dark adaptation. Experimental Brain Research. 1977;27(1):51–59. doi: 10.1007/BF00234824. [DOI] [PubMed] [Google Scholar]
- Lehky SR, Maunsell JHR. No binocular rivalry in the LGN of alert macaque monkeys. Vision Research. 1996;36(9):1225–1234. doi: 10.1016/0042-6989(95)00232-4. [DOI] [PubMed] [Google Scholar]
- Lennie P, Movshon JA. Coding of color and form in the geniculostriate visual pathway (invited review) Journal of the Optical Society of America. a, Optics, Image Science, and Vision. 2005;22(10):2013–2033. doi: 10.1364/josaa.22.002013. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Maier A, Wilke M, Logothetis NK. In: Binocular Rivalry and the Illusion of Monocular Vision. Alais D, Blake R, editors. MIT Press; 2005. pp. 231–243. [Google Scholar]
- Levick WR, Thibos LN. Analysis of orientation bias in cat retina. The Journal of Physiology. 1982;329:243–261. doi: 10.1113/jphysiol.1982.sp014301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt JB, Schumer RA, Sherman SM, Spear PD, Movshon JA. Visual response properties of neurons in the LGN of normally reared and visually deprived macaque monkeys. Journal of Neurophysiology. 2001;85(5):2111–2129. doi: 10.1152/jn.2001.85.5.2111. [DOI] [PubMed] [Google Scholar]
- Lindström S. Synaptic organization of inhibitory pathways to principal cells in the lateral geniculate nucleus of the cat. Brain Research. 1982;234(2):447–453. doi: 10.1016/0006-8993(82)90885-x. [DOI] [PubMed] [Google Scholar]
- Logothetis NK. Single units and conscious vision. Phil Trans R Soc Lond B. 1998;353(1377):1801–1818. doi: 10.1098/rstb.1998.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412(6843):150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Longordo F, To MS, Ikeda K, Stuart GJ. Sublinear integration underlies binocular processing in primary visual cortex. Nature Publishing Group. 2013;16(6):714–723. doi: 10.1038/nn.3394. [DOI] [PubMed] [Google Scholar]
- Macknik SL, Martinez-Conde S. The role of feedback in visual masking and visual processing. Advances in Cognitive Psychology/University of Finance and Management in Warsaw. 2007;3(1–2):125–152. doi: 10.2478/v10053-008-0020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei L, Berardi N, Bisti S. Interocular transfer of adaptation after effect in neurons of area 17 and 18 of split chiasm cats. Journal of Neurophysiology. 1986;55(5):966–976. doi: 10.1152/jn.1986.55.5.966. [DOI] [PubMed] [Google Scholar]
- Maier A, Panagiotaropoulos TI, Tsuchiya N, Keliris GA. Introduction to research topic - binocular rivalry: a gateway to studying consciousness. Frontiers in Human Neuroscience. 2012;6:263. doi: 10.3389/fnhum.2012.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier A, Wilke M, Aura C, Zhu C, Ye FQ, Leopold DA. Divergence of fMRI and neural signals in V1 during perceptual suppression in the awake monkey. Nature Neuroscience. 2008;11(10):1193–1200. doi: 10.1038/nn.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markov NT, Ercsey-Ravasz M, Van Essen DC, Knoblauch K, Toroczkai Z, Kennedy H. Cortical High-Density Counterstream Architectures. 2013;342(6158):1238406–1238406. doi: 10.1126/science.1238406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrocco RT, Li RH. Monkey superior colliculus: properties of single cells and their afferent inputs. Journal of Neurophysiology. 1977;40(4):844–860. doi: 10.1152/jn.1977.40.4.844. [DOI] [PubMed] [Google Scholar]
- Marrocco RT, McClurkin JW. Binocular interaction in the lateral geniculate nucleus of the monkey. Brain Research. 1979;168(3):633–637. doi: 10.1016/0006-8993(79)90319-6. [DOI] [PubMed] [Google Scholar]
- Martin KA, Somogyi P, Whitteridge D. Physiological and morphological properties of identified basket cells in the cat’s visual cortex. Experimental Brain Research. 1983;50(2–3):193–200. doi: 10.1007/BF00239183. [DOI] [PubMed] [Google Scholar]
- Meese TS, Georgeson MA, Baker DH. Binocular contrast vision at and above threshold. Journal of Vision. 2006;6(11):1224–1243. doi: 10.1167/6.11.7. [DOI] [PubMed] [Google Scholar]
- Moore RJ, Spear PD, Kim CB, Xue JT. Binocular processing in the cat’s dorsal lateral geniculate nucleus. III. Spatial frequency, orientation, and direction sensitivity of nondominant-eye influences. Experimental Brain Research. 1992;89(3):588–598. doi: 10.1007/BF00229884. [DOI] [PubMed] [Google Scholar]
- Moors J, Vendrik AJ. Responses of single units in the monkey superior colliculus to stationary flashing stimuli. Experimental Brain Research. 1979;35(2):333–347. doi: 10.1007/BF00236619. [DOI] [PubMed] [Google Scholar]
- Moradi F, Heeger DJ. Inter-ocular contrast normalization in human visual cortex. Journal of Vision. 2009;9(3):131–22. doi: 10.1167/9.3.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon JA, Eggers HM, Gizzi MS, Hendrickson AE, Kiorpes L, Boothe RG. Effects of early unilateral blur on the macaque’s visual system. III. Physiological observations. The Journal of Neuroscience. 1987;7(5):1340–1351. doi: 10.1523/JNEUROSCI.07-05-01340.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PC, Sillito AM. The binocular input to cells in the feline dorsal lateral geniculate nucleus (dLGN) The Journal of Physiology. 1989;415:393–408. doi: 10.1113/jphysiol.1989.sp017727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka KI, Rushton WA. S-potentials from colour units in the retina of fish (Cyprinidae) The Journal of Physiology. 1966;185(3):536–555. doi: 10.1113/jphysiol.1966.sp008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Highly selective receptive fields in mouse visual cortex. Journal of Neuroscience. 2008;28(30):7520–7536. doi: 10.1523/JNEUROSCI.0623-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Noorden GK, Crawford ML. The lateral geniculate nucleus in human strabismic amblyopia. Investigative Ophthalmology & Visual Science. 1992;33(9):2729–2732. [PubMed] [Google Scholar]
- von Noorden GK, Middleditch PR. Histology of the monkey lateral geniculate nucleus after unilateral lid closure and experimental strabismus: further observations. Investigative Ophthalmology. 1975;14(9):674–683. [PubMed] [Google Scholar]
- Norton TT, Casagrande VA. Laminar organization of receptive-field properties in lateral geniculate nucleus of bush baby (Galago crassicaudatus) Journal of Neurophysiology. 1982;47(4):715–741. doi: 10.1152/jn.1982.47.4.715. [DOI] [PubMed] [Google Scholar]
- Norton TT, Casagrande VA, Irvin GE, Sesma MA, Petry HM. Contrast-sensitivity functions of W-, X-, and Y-like relay cells in the lateral geniculate nucleus of bush baby, Galago crassicaudatus. Journal of Neurophysiology. 1988;59(6):1639–1656. doi: 10.1152/jn.1988.59.6.1639. [DOI] [PubMed] [Google Scholar]
- Pape HC, Eysel UT. Binocular interactions in the lateral geniculate nucleus of the cat: GABAergic inhibition reduced by dominant afferent activity. Experimental Brain Research. 1986;61(2):265–271. doi: 10.1007/BF00239516. [DOI] [PubMed] [Google Scholar]
- Parker AJ, Cumming BG. Cortical mechanisms of binocular stereoscopic vision. Progress in Brain Research. 2001;134:205–216. doi: 10.1016/s0079-6123(01)34015-3. [DOI] [PubMed] [Google Scholar]
- Parker AJ, Smith JET, Krug K. Neural architectures for stereo vision. Philosophical Transactions of the Royal Society B: Biological Sciences. 2016;371(1697):20150261. doi: 10.1098/rstb.2015.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak T, Merigan WH. The luminance dependence of spatial vision in the cat. Vision Research. 1981;21(9):1333–1339. doi: 10.1016/0042-6989(81)90240-6. [DOI] [PubMed] [Google Scholar]
- Rodieck RW, Dreher B. Visual suppression from nondominant eye in the lateral geniculate nucleus: a comparison of cat and monkey. Experimental Brain Research. 1979;35(3):465–477. doi: 10.1007/BF00236765. [DOI] [PubMed] [Google Scholar]
- Saini KD, Garey LJ. Morphology of neurons in the lateral geniculate nucleus of the monkey. A Golgi study. Experimental Brain Research. 1981;42(3–4):235–248. doi: 10.1007/BF00237491. [DOI] [PubMed] [Google Scholar]
- Sanderson KJ, Bishop PO, Darian-Smith I. The properties of the binocular receptive fields of lateral geniculate neurons. Experimental Brain Research. 1971;13(2):178–207. doi: 10.1007/BF00234085. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Malpeli JG. Functional specificity of lateral geniculate nucleus laminae of the rhesus monkey. Journal of Neurophysiology. 1978;41(3):788–797. doi: 10.1152/jn.1978.41.3.788. [DOI] [PubMed] [Google Scholar]
- Schmid MC, Maier A. To see or not to see - Thalamo-cortical networks during blindsight and perceptual suppression. Progress in Neurobiology. 2015;126:36–48. doi: 10.1016/j.pneurobio.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Schmid MC, Schmiedt JT, Peters AJ, Saunders RC, Maier A, Leopold DA. Motion-sensitive responses in visual area v4 in the absence of primary visual cortex. Journal of Neuroscience. 2013;33(48):18740–18745. doi: 10.1523/JNEUROSCI.3923-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt JT, Maier A, Fries P, Saunders RC, Leopold DA, Schmid MC. Beta oscillation dynamics in extrastriate cortex after removal of primary visual cortex. Journal of Neuroscience. 2014;34(35):11857–11864. doi: 10.1523/JNEUROSCI.0509-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmielau F, Singer W. The role of visual cortex for binocular interactions in the cat lateral geniculate nucleus. Brain Research. 1977;120(2):354–361. doi: 10.1016/0006-8993(77)90914-3. [DOI] [PubMed] [Google Scholar]
- Scholl B, Burge J, Priebe NJ. Binocular integration and disparity selectivity in mouse primary visual cortex. Journal of Neurophysiology. 2013a;109(12):3013–3024. doi: 10.1152/jn.01021.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl B, Tan AYY, Corey J, Priebe NJ. Emergence of orientation selectivity in the Mammalian visual pathway. Journal of Neuroscience. 2013b;33(26):10616–10624. doi: 10.1523/JNEUROSCI.0404-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder CE, Salinger WL, Guido W. The influence of anesthesia upon binocular processes controlling the recordability of X- and Y-cells in the lateral geniculate nucleus of the cat. Brain Research. 1988;454(1–2):227–237. doi: 10.1016/0006-8993(88)90822-0. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Tenke CE, Arezzo JC, Vaughan HG. Binocularity in the lateral geniculate nucleus of the alert macaque. Brain Research. 1990;521(1–2):303–310. doi: 10.1016/0006-8993(90)91556-v. [DOI] [PubMed] [Google Scholar]
- Sengpiel F, Blakemore C. Interocular control of neuronal responsiveness in cat visual cortex. 1994;368(6474):847–850. doi: 10.1038/368847a0. [DOI] [PubMed] [Google Scholar]
- Sengpiel F, Vorobyov V. Intracortical origins of interocular suppression in the visual cortex. 2005;25(27):6394–6400. doi: 10.1523/JNEUROSCI.0862-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengpiel F, Baddeley RJ, Freeman TC, Harrad R, Blakemore C. Different mechanisms underlie three inhibitory phenomena in cat area 17. 1998;38(14):2067–2080. doi: 10.1016/s0042-6989(97)00413-6. [DOI] [PubMed] [Google Scholar]
- Sengpiel F, Blakemore C, Harrad R. Interocular suppression in the primary visual cortex: a possible neural basis of binocular rivalry. 1995a;35(2):179–195. doi: 10.1016/0042-6989(94)00125-6. [DOI] [PubMed] [Google Scholar]
- Sengpiel F, Freeman TC, Blakemore C. Interocular suppression in cat striate cortex is not orientation selective. 1995b;6(16):2235–2239. doi: 10.1097/00001756-199511000-00032. [DOI] [PubMed] [Google Scholar]
- Sherman SM. The thalamus is more than just a relay. Current Opinion in Neurobiology. 2007;17(4):417–422. doi: 10.1016/j.conb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Wilson JR, Kaas JH, Webb SV. X- and Y-cells in the dorsal lateral geniculate nucleus of the owl monkey (Aotus trivirgatus) Science. 1976;192(4238):475–477. doi: 10.1126/science.816006. [DOI] [PubMed] [Google Scholar]
- Shou TD, Leventhal AG. Organized arrangement of orientation-sensitive relay cells in the cat’s dorsal lateral geniculate nucleus. The Journal of Neuroscience. 1989;9(12):4287–4302. doi: 10.1523/JNEUROSCI.09-12-04287.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou T, Ruan D, Zhou Y. The orientation bias of LGN neurons shows topographic relation to area centralis in the cat retina. Experimental Brain Research. 1986;64(1):233–236. doi: 10.1007/BF00238218. [DOI] [PubMed] [Google Scholar]
- Singer W. Inhibitory binocular interaction in the lateral geniculate body of the cat. Brain Research. 1970;18(1):165–170. doi: 10.1016/0006-8993(70)90463-4. [DOI] [PubMed] [Google Scholar]
- Smith EL, Chino YM, Ni J, Cheng H, Crawford ML, Harwerth RS. Residual binocular interactions in the striate cortex of monkeys reared with abnormal binocular vision. Journal of Neurophysiology. 1997a;78(3):1353–1362. doi: 10.1152/jn.1997.78.3.1353. [DOI] [PubMed] [Google Scholar]
- Smith EL, Chino YM, Ridder WH, Kitagawa K, Langston A. Orientation bias of neurons in the lateral geniculate nucleus of macaque monkeys. Visual Neuroscience. 1990;5(6):525–545. doi: 10.1017/s0952523800000699. [DOI] [PubMed] [Google Scholar]
- Smith EL, Chino Y, Ni J, Cheng H. Binocular combination of contrast signals by striate cortical neurons in the monkey. Journal of Neurophysiology. 1997b;78(1):366–382. doi: 10.1152/jn.1997.78.1.366. [DOI] [PubMed] [Google Scholar]
- Soodak RE, Shapley RM, Kaplan E. Linear mechanism of orientation tuning in the retina and lateral geniculate nucleus of the cat. Journal of Neurophysiology. 1987;58(2):267–275. doi: 10.1152/jn.1987.58.2.267. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Qi HX, Kaas JH. Do superior colliculus projection zones in the inferior pulvinar project to MT in primates? European Journal of Neuroscience. 1999;11(2):469–480. doi: 10.1046/j.1460-9568.1999.00461.x. [DOI] [PubMed] [Google Scholar]
- Stone J, Fukuda Y. Properties of cat retinal ganglion cells: a comparison of W-cells with X- and Y-cells. Journal of Neurophysiology. 1974;37(4):722–748. doi: 10.1152/jn.1974.37.4.722. [DOI] [PubMed] [Google Scholar]
- Stone J, Hansen SM. The projection of the cat’s retina on the lateral geniculate nucleus. The Journal of Comparative Neurology. 1966;126(4):601–624. [PubMed] [Google Scholar]
- Sun F, Tong J, Yang Q, Tian J, Hung GK. Multi-directional shifts of optokinetic responses to binocular-rivalrous motion stimuli. Brain Research. 2002;944(1–2):56–64. doi: 10.1016/s0006-8993(02)02706-3. [DOI] [PubMed] [Google Scholar]
- Sur M, Sherman SM. Linear and nonlinear W-cells in C-laminae of the cat’s lateral geniculate nucleus. Journal of Neurophysiology. 1982a;47(5):869–884. doi: 10.1152/jn.1982.47.5.869. [DOI] [PubMed] [Google Scholar]
- Sur M, Sherman SM. Retinogeniculate terminations in cats: morphological differences between X and Y cell axons. Science. 1982b;218(4570):389. doi: 10.1126/science.7123239. [DOI] [PubMed] [Google Scholar]
- Tailby C, Majaj NJ, Movshon JA. Binocular integration of pattern motion signals by MT neurons and by human observers. Journal of Neuroscience. 2010;30(21):7344–7349. doi: 10.1523/JNEUROSCI.4552-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KG, Leventhal AG, Zhou Y, Liu D. Stimulus dependence of orientation and direction sensitivity of cat LGNd relay cells without cortical inputs: a comparison with area 17 cells. Visual Neuroscience. 1994;11(5):939–951. doi: 10.1017/s0952523800003898. [DOI] [PubMed] [Google Scholar]
- Tong F, Meng M, Blake R. Neural bases of binocular rivalry. Trends in Cognitive Sciences. 2006;10(11):502–511. doi: 10.1016/j.tics.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Tong L, Guido W, Tumosa N, Spear PD, Heidenreich S. Binocular interactions in the cat’s dorsal lateral geniculate nucleus, II: Effects on dominant-eye spatial-frequency and contrast processing. Visual Neuroscience. 1992;8(6):557–566. doi: 10.1017/s0952523800005654. [DOI] [PubMed] [Google Scholar]
- Torrealba F, Partlow GD, Guillery RW. Organization of the projection from the superior colliculus to the dorsal lateral geniculate nucleus of the cat. Neuroscience. 1981;6(7):1341–1360. doi: 10.1016/0306-4522(81)90192-5. [DOI] [PubMed] [Google Scholar]
- Tömböl T. Role of the Golgi II cell type in the intranuclear synaptic organization of the medial geniculate body. Verhandlungen Der Anatomischen Gesellschaft. 1969;63:661–665. [PubMed] [Google Scholar]
- Tremain KE, Ikeda H. Relationship between amblyopia, LGN cell “shrinkage” and cortical ocular dominance in cats. Experimental Brain Research. 1982;45(1–2):243–252. doi: 10.1007/BF00235784. [DOI] [PubMed] [Google Scholar]
- Truchard AM, Ohzawa I, Freeman RD. Contrast gain control in the visual cortex: monocular versus binocular mechanisms. Journal of Neuroscience. 2000;20(8):3017–3032. doi: 10.1523/JNEUROSCI.20-08-03017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updyke BV. The pattern of projection of cortical areas 17, 18, and 19 onto the laminae of the dorsal lateral geniculate nucleus in the cat. Journal of Comparative Neurology. 1975;163(4):377–395. doi: 10.1002/cne.901630402. [DOI] [PubMed] [Google Scholar]
- Vaiceliunaite A, Erisken S, Franzen F, Katzner S, Busse L. Spatial integration in mouse primary visual cortex. Journal of Neurophysiology. 2013;110(4):964–972. doi: 10.1152/jn.00138.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela FJ, Singer W. Neuronal dynamics in the visual corticothalamic pathway revealed through binocular rivalry. Experimental Brain Research. 1987;66(1):10–20. doi: 10.1007/BF00236196. [DOI] [PubMed] [Google Scholar]
- Vidyasagar TR, Urbas JV. Orientation sensitivity of cat LGN neurones with and without inputs from visual cortical areas 17 and 18. Experimental Brain Research. 1982;46(2):157–169. doi: 10.1007/BF00237172. [DOI] [PubMed] [Google Scholar]
- Wilke M, Mueller KM, Leopold DA. Neural activity in the visual thalamus reflects perceptual suppression. Proceedings of the National Academy of Sciences. 2009;106(23):9465–9470. doi: 10.1073/pnas.0900714106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PD, Rowe MH, Stone J. Properties of relay cells in cat’s lateral geniculate nucleus: a comparison of W-cells with X- and Y-cells. Journal of Neurophysiology. 1976;39(6):1193–1209. doi: 10.1152/jn.1976.39.6.1193. [DOI] [PubMed] [Google Scholar]
- Wunderlich K, Schneider KA, Kastner S. Neural correlates of binocular rivalry in the human lateral geniculate nucleus. Nature Neuroscience. 2005;8(11):1595–1602. doi: 10.1038/nn1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Ichida JM, Allison JD, Boyd JD, Bonds AB, Casagrande VA. A comparison of koniocellular, magnocellular and parvocellular receptive field properties in the lateral geniculate nucleus of the owl monkey (Aotus trivirgatus) The Journal of Physiology. 2001;531(Pt 1):203–218. doi: 10.1111/j.1469-7793.2001.0203j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Ichida J, Shostak Y, Bonds AB, Casagrande VA. Are primate lateral geniculate nucleus (LGN) cells really sensitive to orientation or direction? Visual Neuroscience. 2002;19(1):97–108. doi: 10.1017/s0952523802191097. [DOI] [PubMed] [Google Scholar]
- Xue JT, Carney T, Ramoa AS, Freeman RD. Binocular interaction in the perigeniculate nucleus of the cat. Experimental Brain Research. 1988;69(3):497–508. doi: 10.1007/BF00247304. [DOI] [PubMed] [Google Scholar]
- Xue JT, Ramoa AS, Carney T, Freeman RD. Binocular interaction in the dorsal lateral geniculate nucleus of the cat. Experimental Brain Research. 1987;68(2):305–310. doi: 10.1007/BF00248796. [DOI] [PubMed] [Google Scholar]
- Zeater N, Cheong SK, Solomon SG, Dreher B, Martin PR. Binocular Visual Responses in the Primate Lateral Geniculate Nucleus. Current Biology. 2015;25(24):3190–3195. doi: 10.1016/j.cub.2015.10.033. [DOI] [PubMed] [Google Scholar]