Abstract
Current healthcare is evolving to emphasize cost-effective care by leveraging results and outcomes of genomic and other advanced research efforts in clinical care and preventive health planning. Through a collaborative effort between the University of Tennessee Health Science Center (UTHSC) and Le Bonheur Children’s Hospital (LBCH), the Biorepository and Integrative Genomics (BIG) Initiative was established to set up a pediatric-based DNA biorepository that can serve as a foundation for successful development of delivery platforms for predictive, preventive, and personalized medical services in Memphis, Tennessee, a historically disadvantaged community in the USA. In this paper, we describe the steps that were followed to establish the biorepository. We focused on domains that are essential for implementation of a biorepository for genomic research as an initial goal and identified patient consent, DNA extraction, storage and dissemination, and governance as essential components. Specific needs in each of these domains were addressed by respective solutions developed by multidisciplinary teams under the guidance of a governance model that involved experts from multiple hospital arenas and community members. The end result was the successful launch of a large-scale DNA biorepository, with patient consent greater than 75% in the first year. Our experience highlights the importance of performing pre-design research, needs assessment, and designing an ethically vetted plan that is cost-effective, easy to implement, and inclusive of the community that is served. We believe this biorepository model, with appropriate tailoring according to organizational needs and available resources, can be adopted and successfully applied by other small- to mid-sized healthcare organizations.
Keywords: Biobank, Integrative genomics initiative, Predictive preventive personalized medicine, Pediatrics, Community
Objectives
With interest in predictive, preventive, and personalized medicine (PPPM) gaining momentum in recent years, health systems have started developing PPPM-centric healthcare delivery platforms and population health management strategies. The design of these platforms and strategies are informed by the unique socio-demographic, clinical, genetic, and environmental characteristics of their patient base. Genomic data is a rich and useful source of information for the development of PPPM-based healthcare, and the improvement and dramatically falling costs of sequencing technologies make genomics an apt starting point for PPPM efforts [1]. It is currently estimated that multiple biobanks across the USA store millions of bio-specimens, including DNA, and these numbers are growing exponentially [2]. While a number of institutions have implemented initiatives that collect DNA samples for research and to inform clinical practice, large and inclusive pediatric-focused repositories are rare.
Memphis is one of the poorest cities in the USA [3], with an overall poverty rate of 26.9%, a poverty rate of 32.3% for non-Hispanic Blacks, and a child poverty rate of 44.7%, and ranks among the worst in the nation for many key health indicators, including infant mortality and childhood obesity [4]. The Memphis Metropolitan Statistical Area is the largest in the USA where a majority of residents are African Americans [3], and thus the health disparities inherent in this racial distribution and other extant conditions are further compounded by the large role of genomics in current PPPM-based care. African American populations have been historically underrepresented in genomic-based studies [5–8]. Moreover, it is becoming clear that the diagnostic utility of NGS (Next Generation Sequencing)-based testing is significantly reduced for minorities relative to Caucasian populations in North America [9].
To address this issue, we established the Biorepository and Integrative Genomics (BIG) Initiative, a DNA biorepository effort designed to ultimately generate genomic data on all consenting patients at Le Bonheur Children’s Hospital (Memphis, Tennessee), and to serve as a foundation for pediatric-based genomic research and, ultimately, PPPM-based care in our general hospital setting. While it is clear that a multiomics approach is preferable for some clinical areas, such as cancer [10], we focused on genomics because the prevalent clinical issues treated at Le Bonheur are not cancer-related. Instead, they span a wide range of clinical areas in which current genomics-based testing and analytics can inform clinical decisions, including obesity, diabetes, asthma, autism, epilepsy and seizure disorders, hypertension and cardiovascular disease, metabolic/biochemical/developmental disorders, viral (influenza and RSV) infection, and pain management [11–15]. Our focus on genomics also leverages resident expertise in genomics and the greater potential to recover some costs through insurance reimbursement, when BIG eventually develops CLIA and CAP-certified clinical genomic testing capabilities in conjunction with the Le Bonheur Children’s Hospital (LBCH) clinical laboratories.
Many challenges confronting the development of a pediatric-based biorepository require coordination between multiple departments and units within a healthcare system. Although the design and implementation of processes for data generation, analysis, and implementation of PPPM-based healthcare protocols (for example, the application of pharmacogenomics-based clinical decision-making) have been more newsworthy and discussed at greater length, we found that front-end efforts to develop organizational infrastructure, governance, and pipelines for obtaining informed consent and the collection and management of samples are just as critical, and have focused on them in this report. Challenges we encountered in these areas include the development of a consent process that facilitates participation in an environment where consent or assent is required from multiple parties with varying degrees of health literacy (including parents, children, and legal guardians), balancing of current policies on informed consent and subjects protection with the broadest possible use of collected materials, and the creation of automated systems that manage sample collection and processing (i.e., identification of blood samples available for collection, sample de-identification, sample tracking through DNA extraction, quality control testing, archiving, and distribution) and facilitate cohort selection based on subject and sample characteristics (e.g., subject-associated diagnoses, demographic information, DNA quality control metrics) for distribution and analysis while maintaining subject anonymity. The foremost challenge was establishment of a governance model that proactively develops solutions to anticipated technical, operational, and ethical challenges; facilitates community involvement; and addresses issues in a consistent, timely, and transparent manner.
Here, we describe how the BIG Initiative approached and successfully tackled these challenges to establish a pediatric patient-centered DNA biorepository that is focused on opt-in enrollment with informed broad consent; utilizes a pipeline that scavenges leftover blood from standard lab test samples for DNA extraction and archiving; built a HIPAA-compliant Laboratory Information Management System (LIMS) for efficient tracking, management, and de-identification of collected DNA samples; and distributes DNA samples under a standardized set of rules governing the usage of samples and acquired genomic data for research purposes. We believe our experience in the development of this system and how community and ethical issues were approached may be useful to other pediatric-centered institutions considering similar initiatives.
Methods and results
Planning
The framework for the BIG Initiative was developed after conducting extensive background research in the field of DNA biobanking. We identified academic medical institutions in the USA that had successfully implemented biorepository initiatives, some with a focus on pediatric patients, reviewed their implementation strategies and experiences, and pooled information on the development, design, and implementation of pediatric DNA biorepositories in order to identify common elements and knowledge gaps. We also communicated with management teams at institutions whose established DNA biorepositories we viewed most closely aligned with our goals, and gathered information from those institutions through email, phone conversations, and site visits to understand their processes and procedures. When we began, there existed considerable variation in approaches toward consent, targeted populations, and research objectives, as well as overall goals of each respective biorepository [16]. However, many in the USA have now more closely aligned their policies and protocols with recent revisions to US federal policies and guidelines [17, 18]. International efforts at standardization of PPPM and biobanking policies and procedures have also contributed to this convergence [19–21].
The concept we developed for our own initiative envisions supporting research projects that will generate new genomic information that is clinically relevant to the patient population at LBCH and the surrounding community, which is majority African American and thus historically underrepresented in most genomic studies [5–8]. Ultimately, our vision is to utilize this data in clinical decision support in the future (Fig. 1). We identified initial steps that were critical to successful implementation of the initiative, including the development of (1) a governance model for the biorepository initiative that encompassed all operational aspects with active engagement of ethics and healthcare research experts and the community, (2) a patient-centered consent process with novel ways of obtaining informed consent from patients and families, (3) an efficient pipeline for DNA extraction and storage post-consent that is coordinated through a dedicated LIMS, and (4) an efficient means for researchers to identify samples of interest and for BIG to accept and evaluate requests for those samples.
Fig. 1.
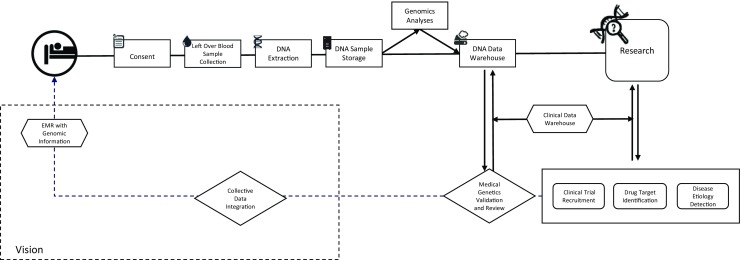
Overall concept for the Biorepository and Integrative Genomics (BIG) Initiative
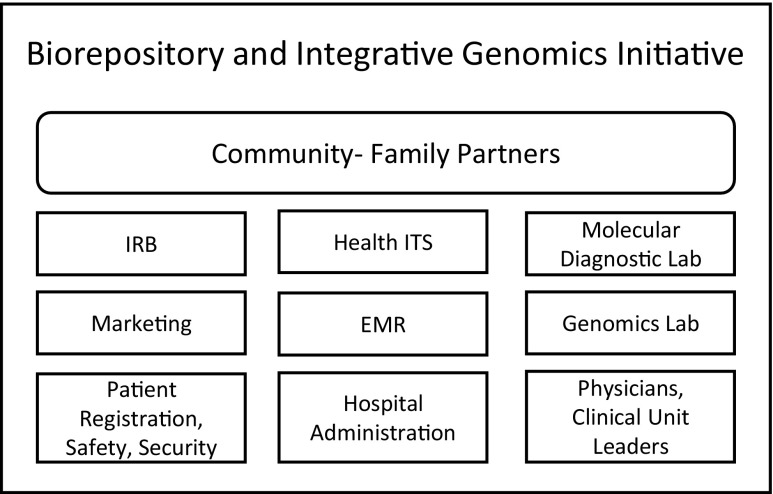
Implementation of these steps was achieved through multidisciplinary collaborations between different units and departments at LBCH and University of Tennessee Health Science Center (UTHSC) that span Information Technology, Clinical Laboratories (Molecular Diagnostics), Patient Registration, Marketing, and Community Partners (Fig. 2), holding regular meetings with representatives from all of these disciplines. By following these steps, the BIG Initiative went operational 12 months after Institutional Review Board (IRB) approval. We recognized that institutional dynamics play a major role in determining the success or failure of such collaborations. It was therefore essential that all contributing units and departments were fully supportive of this effort. This required the establishment and maintenance of positive relationships between all interacting parties, clear delineation of responsibilities, and transparent engagement by the BIG leadership team. To do so, in addition to the full and clearly expressed support by UTHSC and LBCH leadership, we found it crucial that the Initiative’s project leader had familiarity with hospital operations and personnel, strong leadership skills, and exceptional drive in order to successfully champion the effort.
Fig. 2.
Collaborations essential for implementation of the BIG Initiative
Governance
As shown in Fig. 3 and detailed below, the governance model we developed to implement the BIG Initiative, and to ensure uninterrupted operations and long-term growth, consists of four cores that encompass necessary operational arenas of the biorepository: advisory, research oversight, ethics, and community engagement. The boards responsible for these cores are as follows:
-
(i)
The Advisory Board is chaired by the project leader and is responsible for the development and implementation of the biorepository and functions. We found that the effectiveness of this board and indeed the entire initiative required active participation by senior university and hospital faculty and officials. Thus, the BIG Advisory Board includes the LBCH Pediatrician-in-Chief and chair of the UTHSC Department of Pediatrics; the director of the LBCH Children’s Foundation Research Institute; division chiefs within the Department of Pediatrics (Clinical Genetics, Infectious Diseases, Cardiology); the chair of the UTHSC Department of Genetics, Genomics, and Informatics; the director of the UTHSC Center for Biomedical Informatics; the director of the LBCH Biomedical Informatics Core; the head of LBCH patient registration (hospital admissions); and the technical director of LBCH Clinical Laboratories (Molecular Diagnostics).
Fig. 3.
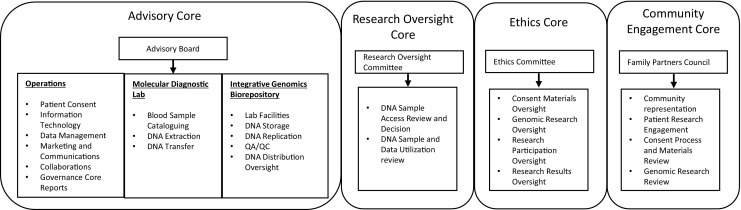
Biorepository and Integrative Genomics Initiative Governance
During initial stages of development of the initiative, the Advisory Board played a key role in developing and approving plans and strategy, in identifying institutional resources available for the initiative as well as those that were missing, and in recruitment of key personnel. As the initiative matured and operational staff were put in place, the Advisory Board became a reporting and advising channel to suggest improvements to ongoing processes. The board receives monthly reports from the project leader and BIG operational staff, including the operations manager, IT manager, and the biorepository director, that cover all operations of the BIG Initiative, i.e., patient consent, enrollment and research records, marketing, patient registration teams, DNA extraction and transfer to the biorepository, DNA storage, DNA dissemination for research, and opt-out pipelines. The project leader and operations manager also report comments, recommendations, and/or decisions from the Ethics Committee and Family Partners Council (see below).
-
(ii)
The Research Oversight Committee (ROC) is a multidisciplinary body comprised of 15 rotating members from different clinical and research departments within UTHSC and LBCH and is administered by the BIG operations manager. Solicitation of ROC members was done in conjunction with recommendations by multiple department chairs to provide a widest possible representation of academic departments and interests. ROC members are grouped into five trios based on similar academic interests and backgrounds, and each month five members (one from each trio) review, prioritize, and approve or deny submitted sample/data request applications. ROC approval letters are provided to investigators for use in grant applications and must be incorporated into IRB applications by the requesting investigators. DNA samples are distributed to investigators upon IRB approval of the project and subsequent execution of a research materials use agreement with BIG. Criteria used for application review and approval decisions, and critical aspects of the research materials use agreement, are discussed below (Sample Acquisition and Use).
-
(iii)
The Ethics Committee oversees the project’s interaction with patients and families, the provision of DNA samples and data to researchers, ethical issues related to specific DNA-related research and participants’ rights, and compliance with applicable state and federal laws and regulations related to human subjects protection, private health information, and the use of genomic data. This committee has representation from the legal, ethical, regulatory, and clinical arenas, including the chair of the UTHSC Institutional Review Board, a UTHSC associate professor who is a certified fellow of the American College of Medical Genetics, a contracts attorney and professor from the University of Memphis Cecil C. Humphreys School of Law, and a research fellow from the Hastings Center Bioethics Research Institute (Hudson, NY). This committee meets quarterly to review all patient materials and documentation, to receive reports on all research-related decisions made by the ROC, and to discuss the impact of proposed or actual changes in public policies on relevant biorepository activities. To avoid conflicts of interest, participating members of the Ethics Committee are independent to all other aspects of the BIG Initiative. As such, the BIG project leader and operations manager attend meetings as non-participating members solely to provide information and administrative support.
The Ethics Committee has been integral to shaping the BIG Initiative, at both strategic and operational levels, by providing guidance to ensure the initiative’s policies and procedures related to patient recruitment, informed consent, patient contact, and sample and data use are consistent with current applicable laws and regulations; specifically, these include HHS and FDA Policies for the Protection of Human Subjects (45 CFR Part 46 and 21 CFR parts 50 and 56, respectively) [22, 23], the Health Insurance Portability and Accountability Act (HIPAA) of 1996 and HHS Standards for Privacy of Individually Identifiable Health (HIPAA Privacy Rules) (45 CFR Part 160 and Subparts A and E of Part 164) [24–26], the Genetic Information Nondiscrimination Act (GINA) of 2008 [27], and the NIH Genomic Data Sharing Policy (NIH GDS Policy issued August 27, 2014) [17, 27], and in anticipation of changes in the NIH Policy, such as the Use of a Single Institutional Review Board of Record for Multi-Site Research (NIH sIRB Policy, effective May 25, 2017 [28]) and revisions to the Common Rule (Subpart A of 45 CFR Part 46, effective January 1, 2018 [18, 29, 30]). All content, language, and images used in consenting and reporting materials (documents, scripts, videos, newsletters, website), patient surveys, the research materials request application and review criteria, and the research materials use agreement are reviewed and finalized by the Ethics Committee. In addition, the Ethics Committee discusses and provides recommendations on potential ethical issues in research materials request applications that are raised by ROC reviewers or the submitting investigators.
-
(iv)
Community Engagement is achieved through the combined efforts of BIG Operations marketing and communications activities, and the LBCH Family Partners Council, which is an existing body within LBCH comprised of over 40 families who serve as a link to the local community for discussion and advocacy of health and medical issues and community awareness. Many hospitals maintain some type of community advisory board that serves a similar purpose. The Family Partners Council was engaged early during development of the BIG Initiative and has provided valuable feedback on the scope and strategy of the project; on the content, tone, and language in consenting materials; on the consent process strategy; and on updates and semi-annual reports to participants and marketing activities toward the community at large. BIG operations create, oversee, and/or coordinate the BIG website, all print and electronic articles on the BIG Initiative that are produced by the LBCH Marketing and Communications department, the production of print and video consent materials, and the content and production of BIG semi-annual newsletters to participating patients and their families. The Family Partners Council receives quarterly reports from the BIG project leader on BIG marketing and reporting activities, summaries of the initiative’s progress (operational activities and research projects), and future propositions.
Consent
The outside institutions we studied had different forms of consent, ranging from opt-out to opt-in and from overly broad to very specific in scope, and were shaped by their specific institutional research policies, goals, and objectives; by the communities they serve; and in part by the prevailing legal and ethics regulations at the time their programs were formulated. Based on these examples, we developed consent materials tailored to our own vision, with input and advice from the Ethics Committee and the Family Partners Council to ensure the content in the consent form and other materials was patient-centric, clearly showed/stated the purpose of the program in language and images that were comprehensible to both parents and children, promoted the active engagement of community partners (which is of particular importance for pediatric biorepositories [31]), and was legally and ethically sound.
Our informed consent materials provide information on the critical elements of the initiative, including operational processes, DNA sample de-identification, DNA sample linkage to health information, the security of bio-specimens and related data, and risks associated with participation. Additionally, the consent form includes a discussion of the laws and policies cited above that protect patients’ and families’ rights to privacy with regard to genetic data. Consent documents and materials were developed to emphasize the voluntary nature of consent, the ability to opt out in the future, and offer patients the choice to be contacted for consideration to participate in future research studies. Consent requirements were split into two tiers due to the fact that children of all ages are included in the initiative; consent for children below 8 years of age is to be obtained solely from parents or legal guardians whereas parental/guardian consent and explicit child assent are to be obtained for cognitively able children 8 years or older.
The LBCH marketing team, the LBCH Family Partners Council, and the LBCH patient registration team heavily assisted in crafting the consent language, shifting the initial emphasis of the consent form from a predominantly institutional focus to more of a patient- or family-centered emphasis (for example, using the term “your child” instead of “subject” or “participant”), limiting the consent form to a one-page document, and developing easily interpretable FAQs. In addition, recommendations were made for the time at which consent should be obtained—specifically emphasizing approaching patients the day following admission to avoid the stress many families experience with a child’s hospitalization. The patient registration team helped map consent processes for patients and families based on their prior experiences with other hospital projects (e.g., patient portal registration). The consent form, coupled with the supporting materials (described below), formed an integral part of the consent process.
Supporting consent materials
We developed complementary media (a brochure and supporting video) to facilitate our ability to communicate information in the consent form. Consenting for biobanks with the support of multimedia tools is rare, particularly with pediatric populations [31]. The consent video content included a narration of the consent by a physician. The video is 2 minutes and 33 seconds in length. Families can also review it on our website after the encounter. The video is accompanied by a brochure with a list of answers to questions that families might have. Contact information and the website address are provided for patients to obtain further information or to opt out if desired (http://www.lebonheur.org/research-and-education/research/biorepository-and-integrative-genomics-initiative/).
Consent process
The consent process (Fig. 4) occurs in two parts and includes dedicated staff, i.e., a person obtaining consent (POC), who obtains consent typically the day following admission. POCs are research associates who are trained by physicians advising the BIG Initiative in approaching and communicating effectively with patients and families in hospital settings. In the first part of the consenting process, the hospital admissions department generates a daily list of newly admitted patients and their hospital room locations from the hospital’s electronic medical record, which is provided to the POCs. A POC approaches each listed room and identifies parents or legal guardians to verify ability to provide consent. The POC shows the parent(s) and patient the consent video on a tablet. If parents express an interest in joining the initiative, the parent(s) and patient are provided with a paper consent form to be signed. Once consent and assent (if required) are obtained, the patient and parent(s) or legal guardian are provided with a copy of the signed consent form and a brochure that contains comprehensive information on the initiative. In the second part of the consent process, the POC updates the consent field on the electronic medical record. LBCH uses Cerner EMR and a BIG consent field was created in Cerner by our hospital’s Information Technology department specifically for this initiative. Updating this field initiates a process by which the BIG LIMS (described below) flags the EMR to provide the clinical lab with information regarding consented patients and their ordered tests. Furthermore, this field is used for future encounters so patients’ families are not re-approached at each subsequent visit.
Fig. 4.
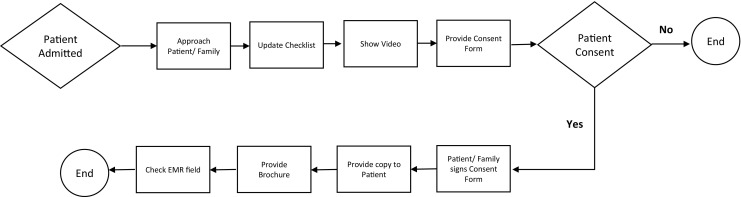
Biorepository and Integrative Genomics Initiative Consent Process
The consent form and an accompanying checklist from the hospital’s Health Information Management (HIM) department are printed with barcodes that specify specific folders in the EMR in which scanned versions of these documents will be stored for each patient. POCs enter the approach and consent result information and a scanned copy of each signed consent form into a secure database (i.e., REDCap [32, 33]), and then transport the paper consent forms and the HIM checklist to HIM for scanning of the consent form into the patients’ EMR and subsequent destruction of the paper form.
DNA extraction, storage, and management
After surveying biorepositories at other institutions, we chose to obtain DNA from pediatric patients by scavenging surplus blood from clinical assays. This strategy reduces the risks and parental concerns associated with an additional dedicated blood draw from their child, and in so doing we believe elevates consent rates. This strategy also allowed us to easily incorporate existing high-throughput DNA extraction equipment in the LBCH Molecular Diagnostics Laboratory (MDL) into the BIG operational pipeline. A second critical component of the BIG is a multi-function LIMS that acquires patient consent and bloodwork order information from the EMR (Cerner) and manages the flow of samples through the clinical lab to the biorepository to facilitate accurate and timely extraction and archiving of DNA samples. Planning and implementation of the LIMS by the BIG Information Technology Management team required the assistance of the LBCH Biomedical Informatics Core and the Information Technology department of the Methodist/Le Bonheur Healthcare (MLH) system. Engaging the cooperation of the Director of the LBCH Clinical Laboratory, the LBCH Chief Biomedical Research Informatics Officer, and the MLH Chief Technology Officer was essential to the development of this operational pipeline.
In practice, after the POC updates the consent flag, the EMR system communicates bloodwork order information, i.e., blood tube barcode (accession number), patient medical record number (MRN), etc., to the LIMS through an HL7 interface. The LIMS provides the clinical labs with a daily picklist of appropriate blood tubes from all consenting patients, filtering out patients for whom more than one aliquot of DNA is present in the DNA repository and blood samples that have not passed the state-mandated 7-day hold period or are older than 14 days post-draw. MDL technologists identify and segregate blood samples meeting these criteria, scan blood tube barcodes (i.e., accession numbers) into the LIMS for verification, and then proceed to automated DNA extraction. Data exported from the DNA extraction system, including blood tube barcodes, deep-well elution plate barcodes, and sample positions in the elution plate, are imported into the LIMS. Sealed elution plates containing the DNA samples are transferred to the Biorepository for quality control testing, aliquoting, and storage. At this point, sample identifiers in the LIMS have been converted from the blood tube accession numbers to a primary identifier that contains the elution plate barcode and sample position coordinates.
The Biorepository uses automated and semi-automated equipment and standardized protocols for DNA aliquoting (five replicates each), quality control (QC) testing of the DNA, indexed sample storage, inventory auditing, and sample retrieval for distribution (or destruction upon receipt of opt-out requests). Samples are replicated in batches of 96 in screw-capped cryotubes manufactured with unique 2D and human-readable 1D barcodes, and stored in barcoded tube racks with lockable lids that are compatible with whole rack barcode readers. Replication tube barcodes, which are linked to the primary identifier in the LIMS, are the only identifier given out to researchers. Automated high-throughput equipment performs all liquid handling steps with DNA samples and is used to measure DNA QC metrics for all archived samples, including DNA concentration and electrophoretic size information (median smear size), all of which uploads into the LIMS and is linked to each sample replicate through the primary identifier. DNA samples are stored in standard − 20 °C freezers containing custom fit drawer-style freezer racks that provide a maximum number of specific locations for storage of the sample tube racks, which are defined in the LIMS prior to each unit’s use. With this freezer configuration, a standard − 20 °C laboratory freezer can hold up to 53,000 tubes. We found the use of individual freezer units to be the most cost-effective storage solution during the startup of our initiative, but will assess the future need for a larger automated cold storage system when our consent pipeline matures and enrollment numbers become more predictable.
After surveying multiple LIMS options and associated costs, we ultimately decided to design and develop our own LIMS in-house. The LIMS contains all necessary components needed to manage and streamline the flow of information between various processes and groups involved in the BIG Initiative, including the LBCH Cerner EMR, the clinical laboratory, and the biorepository. As mentioned above, the LIMS securely imports consent and blood test order data from the EMR using HL7 protocols for integration with sample data. For added security, different data types are segregated into separate tables and databases and applications are housed on different servers. LIMS functionalities include grid-based authentication, audit logging, system QA/QC, equipment tracking and management, sample management (e.g., tracking, validation, quality control metrics), and custom dashboards for display of various statuses. The LIMS was designed to implement a large variety of widgets, applications, and statistical tools (e.g., for sample filtering or subject randomization), and enables single or batch processing of samples with customizable format templates, integration of data from sample processing, and quality control testing equipment with user-defined templates and multiple file formats, and performs sample distribution management and project tracking for distributed samples. A low-cost generalized installation package of our LIMS (BLIMS), with user guide, support documentation and additional technical support, is commercially available for other institutions.
Sample acquisition and use
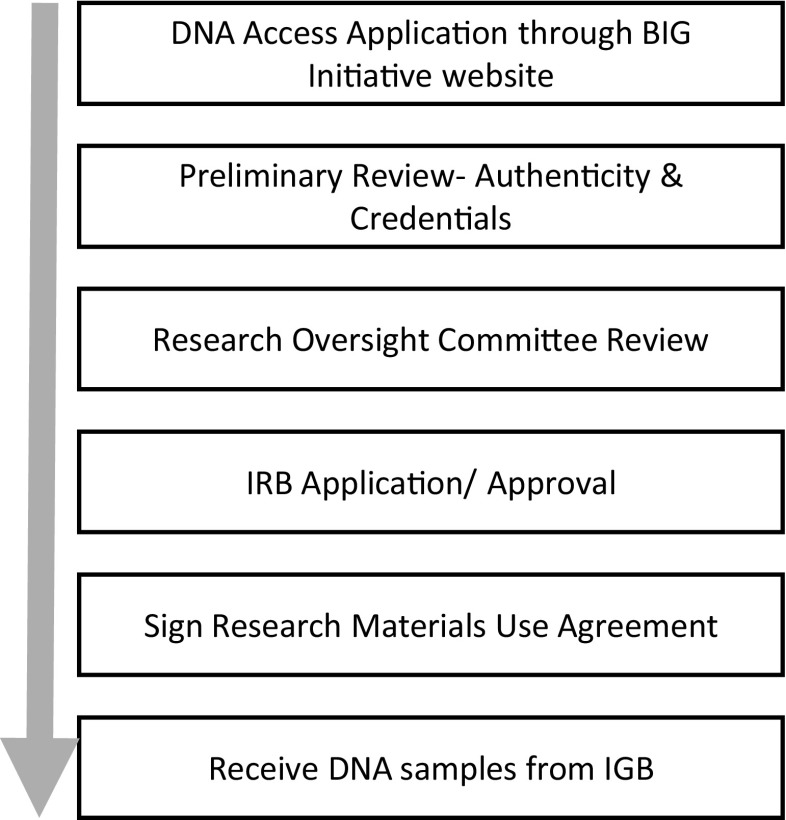
Our framework for efficient utilization of DNA samples operates under the assumption that each sample is a non-renewable resource, and therefore the application process for sample acquisition prioritizes well-designed research projects that propose genome-wide data generation. To do so, we established an efficient pipeline for researchers to identify and request a study cohort of samples, and for BIG to review and approve their judicial use (Fig. 5). This pipeline takes advantage of an existing cohort discovery tool called the Pediatric Research Database (PRD), a quarterly updated i2b2-based data mart that was created by the LBCH Biomedical Informatics Core and contains de-identified clinical data from the LBCH Cerner EMR dating back to 2009. The PRD enables researchers to query EMR clinical data prior to IRB approval and is very similar to research enterprise data warehouses (rEDW) found at a number of academic medical institutions (e.g., [34–36]). PRD can provide de-identified summary information on DNA availability in the biorepository for cohorts selected using clinical or disease-specific data. A crosswalk is used by an honest broker to map BIG DNA samples to PRD data, which enables anonymized information to be used for proposed access to samples in PRD-defined cohorts.
Fig. 5.
Research sample acquisition workflow
The BIG Initiative website (http://www.lebonheur.org/research-and-education/research/biorepository-and-integrative-genomics-initiative/) contains links to an online application that UTHSC researchers can use to place requests for DNA samples and to descriptions of the application process, requirements, and review criteria. The online application (BIG Materials Distribution Request application) is managed using REDCap (UTHSC has been a member of the REDCap consortium since 2013) [33]. Once applications are reviewed by the project manager for authenticity of credentials, they are sent to the Research Oversight Committee (ROC) for review. The ROC meets monthly to review DNA sample use requests based on validity of research, widest utility, and ethical and multiple other considerations. Criteria for unbiased selection by the ROC have been developed. Following review and approval from the ROC, the BIG program coordinator sends the requesting investigator the BIG Research Materials Use Agreement for signature; this binding agreement is meant to ensure that the investigator uses the distributed samples and associated information in a manner that is compliant with all applicable regulations on clinical and genetic/genomic research, in accordance with the scope and methods described in their request application, returns copies of the raw data back to the BIG within 1 year of receipt of the samples, and also stipulates that the returned data will not be made available by BIG to other investigators until 1 year after data receipt by BIG. Samples and/or data are distributed to the requesting investigator only after electronic copies of the ROC approval letter, IRB approval, and the signed research material use agreement are provided to the biorepository director.
Summary and discussion
The Biorepository and Integrative Genomics Initiative represents the first successful step by UTHSC and LBCH to develop a PPPM-based pediatric healthcare platform in a historically disadvantaged US metropolitan area. As mentioned above, the Memphis Metropolitan Statistical Area is the largest in the USA where a majority of residents are African Americans, as well as one of the poorest, with a child poverty rate of 44.7%, and ranks among the worst in the USA for many key pediatric health indicators, including infant mortality and childhood obesity [3, 4]. The health disparities inherent with these factors provide an urgent challenge for the local healthcare community (HRSA score 10; IMV score 51–58.1; last updated July 31, 2014). To help address these issues, we established in a relatively short period of time a resource that will facilitate scientific research on genetic factors that impact health issues prevalent in our local community, including proposed projects on the pharmacogenomics of pediatric pain management and identification of genetic factors associated with neurological disorders (epilepsy and seizure disorders, Tourette’s syndrome), obesity, diabetes, hypertension, and other cardiovascular diseases, and various idiopathic metabolic/biochemical/developmental disorders. We believe information gained from such studies and, just as importantly, the infrastructure developed for the BIG program will ultimately improve local patient care delivery in the coming years.
We recognize that the use of additional high-throughput omics technologies would enhance the clinical and research capabilities of our initiative [10]. However, the BIG Initiative was started as a self-funded program by the UTHSC and LBCH without any additional support by state or US federal agencies. As such, the breadth of technology platforms and informatics infrastructure had to be balanced against the funds available for capital and personnel costs. Our choice was to establish efficient infrastructure and initially focus on genomics platforms, in order to leverage the expertise in genomics already present at UTHSC and the prevalence of genomics-based research in clinical areas of interest at UTHSC and LBCH. As the BIG Initiative and its associated infrastructure mature, we expect to develop additional omics-based platforms as well as other advanced analytical capabilities.
While the initial development of BIG required considerable preliminary research, we identified strengths from other institutions that successfully established similar platforms and leveraged this knowledge to help develop the BIG Initiative. Our overall approach was customized to LBCH operations with approval by various committees in our governance model. In less than 12 months, we established laboratory and archive facilities, trained staff, and established a group of POCs able to consent patients and families. Our marketing team developed a welcoming consent video, brochures, and other informed advertisement materials. Our family partners were engaged at every step of the development of the initiative and were instrumental in making our processes more patient-centered.
The BIG Initiative operationally launched in November 2015 and received positive responses and support from patients and their families. Over the first 12 months of operations, the consent team approached 3571 patient families and over 2780 patients (approximately 78%) were enrolled from inpatient units, whereas 581 patient families declined to participate (approximately 16%) and 205 patient families (6%) were undecided. We found early on that the most significant challenges to the consent and enrollment process included room restrictions (for example, gown/glove requirements for patients’ rooms with infectious diseases), the unavailability of patients due to medical procedures, the absence of parents or legal guardians, language barriers, and early discharge prior to POC revisits. Additional POC training on how to approach patients and family members, increasing consent activity hours, and increased frequency of revisiting rooms improved consent rates from 50 to 60 per week to over 100 per week. Currently, the weekly enrollment averages approximately 110 individual patients in the inpatient units. Plans are underway to extend enrollment activity to the Le Bonheur pediatric outpatient clinic, which experiences more than 250,000 patient encounters each year, as well as increasing capacity to approach more families in the hospital inpatient setting.
As we expand and collect DNA samples, we have begun considering the best utility of these samples and the ethical implications of analyzed DNA data. During the first year of implementation of the initiative, the discussions and decisions made by our Ethics committee focused mainly on four areas: (1) consenting processes and language, (2) responses to questions or concerns raised by parents and families that were conveyed to the consenters and/or project manager, (3) acquisition of a Certificate of Confidentiality from the US National Institutes of Health [37, 38] to provide greater security to participant anonymity, and (4) regulatory aspects of biorepository operations and activities: specifically, supervisory review, personnel training, inventory auditing, risk mitigation, and compliance with State of Tennessee and US Federal regulations for clinical laboratories with regard to performance via standard operating protocols (SOPs) and documentation. Going forward, it is clear that our attention will have to turn toward the utilization of research outcomes to effect actionable changes at a care delivery level, the responsible return of results to participants/patients, handling consent after patients reach adulthood, and how these issues will be impacted by evolving national and international guidelines.
Conclusions and expert recommendations
With the BIG Initiative, we have established a valuable resource that can facilitate research efforts and clinical platform development in PPPM-based healthcare delivery and will ultimately enhance personalized treatment for individual patients in an effective, efficient, and patient-centered manner. We believe the governance model, organizational structure, and operational pipelines developed here can be readily replicated by many other institutions.
Based on our experience from implementing this initiative, we believe the following recommendations are critical for establishment of an institutional PPPM-based biorepository. First, institutional leaders and administrators must enthusiastically support the mission and provide financial backing for the project. Without such institutional engagement, the effort will fail. Second, the project leader must have familiarity with hospital operations and personnel, strong leadership skills, and exceptional drive to successfully champion the effort. Third, multidisciplinary collaboration and partnerships are essential in successful planning, integration, and implementation of diverse areas such as informatics systems, patient consent, biological material processing and storage, sample usage for research, and applicability of research results in clinical practice. This means that resident expertise in these areas must be identified and successfully engaged in the project. Fourth, develop a governance structure that is functionally agile and accurately reflects the institutional participation and operational aspects of your program. Fifth, participant enrollment documentation and other materials must include all required information about the biorepository, should clearly facilitate informed consent with a minimum of paperwork, and should be legally and ethically appropriate and acceptable to the community, especially when pediatric populations are involved. In addition, all current national and regional policies and protocols on biobanking and research should be identified to ensure that the design/framework and governance model of the project are developed within those guidelines, e.g., as set by NIH and NHGRI (National Human Genome Research Institute), as well as aligning with current ethical considerations in the field. To meet these ends, an independent and knowledgeable ethics committee is essential. Lastly, if they do not already exist, devise concrete mechanisms for community engagement and marketing. Enthusiastic community support will facilitate the development of effective enrollment operations and high participation rates.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
The authors provide consent for publication.
Ethical approval
All the patient investigations conformed to the principles outlined in the Declaration of Helsinki and have been performed with the permission (IRB Approval Number—15-03639-XP) released by the responsible Ethics Committee/Institutional Regulatory Board of the University of Tennessee Health Science Center. All the patients were informed about the purposes of the study and have signed their “consent of the patient.” This article does not contain any studies with animals performed by any of the authors.
References
- 1.Christensen KD, Dukhovny D, Siebert U, Green RC. Assessing the costs and cost-effectiveness of genomic sequencing. J Pers Med. 2015;5(4):470–486. doi: 10.3390/jpm5040470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker M. Biorepositories: building better biobanks. Nature. 2012;486(7401):141–146. doi: 10.1038/486141a. [DOI] [PubMed] [Google Scholar]
- 3.Delavarga E. Memphis poverty fact sheet. 2017. http://www.memphis.edu/socialwork/research/2017povertyfactsheetwebversion.pdf.
- 4.Centers for Disease Control. Behavioral risk factor surveillance system 2016 system data and documentation. 2018. https://www.cdc.gov/brfss/annual_data/annual_2016.html.
- 5.Knerr S, Wayman D, Bonham VL. Inclusion of racial and ethnic minorities in genetic research: advance the spirit by changing the rules? J Law Med Ethics. 2011;39(3):502–512. doi: 10.1111/j.1748-720X.2011.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright ML, Housman D, Taylor JY. A perspective for sequencing familial hypercholesterolaemia in African Americans. NPJ Genom Med. 2016;1:16012. doi: 10.1038/npjgenmed.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Popejoy AB, Fullerton SM. Genomics is failing on diversity. Nature. 2016;538:161–164. doi: 10.1038/538161a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garber KB, Vincent LM, Alexander JJ, Bean LJ, Bale S, Hegde M. Reassessment of genomic sequence variation to harmonize interpretation for personalized medicine. Am J Hum Gen. 2016;99(5):1140–1149. doi: 10.1016/j.ajhg.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landry LG, Rehm HL. Association of racial/ethnic categories with the ability of genetic tests to detect a cause of cardiomyopathy. JAMA Cardiol. 2018;3(4):341–345. doi: 10.1001/jamacardio.2017.5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu M, Zhan X. The crucial role of multiomic approach in cancer research and clinically relevant outcomes. EPMA J. 2018;9(1):77–102. doi: 10.1007/s13167-018-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozgüç M. Genetic testing: predictive value of genotyping for diagnosis and management of disease. EPMA J. 2011;2(2):173–179. doi: 10.1007/s13167-011-0077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wysocki K. Lung disease and genomics. AACN Adv Crit Care. 2018;29(1):74–83. doi: 10.4037/aacnacc2018378. [DOI] [PubMed] [Google Scholar]
- 13.Amare AT, Schubert KO, Baune BT. Pharmacogenomics in the treatment of mood disorders: strategies and opportunities for personalized psychiatry. EPMA J. 2017;8(3):211–227. doi: 10.1007/s13167-017-0112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Relling MV, Evans WE. Pharmacogenomics in the clinic. Nature. 2015;526(7573):343–350. doi: 10.1038/nature15817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berg AT, Coryell J, Saneto RP, Grinspan ZM, Alexander JJ, Kekis M, Sullivan JE, Wirrell EC, Shellhaas RA, Mytinger JR, Gaillard WD, Kossoff EH, Valencia I, Knupp KG, Wusthoff C, Keator C, Dobyns WB, Ryan N, Loddenkemper T, Chu CJ, Novotny EJ, Jr, Koh S. Early-life epilepsies and the emerging role of genetic testing. JAMA Pediatr. 2017;171(9):863–871. doi: 10.1001/jamapediatrics.2017.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGregor TL, Van Driest SL, Brothers KB, Bowton EA, Muglia LJ, Roden DM. Inclusion of pediatric samples in an opt-out biorepository linking DNA to de-identified medical records: pediatric BioVU. Clin Pharmacol Ther. 2013;93(2):204–211. doi: 10.1038/clpt.2012.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Human Research Institute. NHGRI implementation of the NIH Genomic Data Sharing (GDS) Policy. https://www.genome.gov/27562511#al-2.
- 18.U.S. Department of Health & Human Services. Federal policy for the protection of human subjects (‘Common Rule’). https://www.hhs.gov/ohrp/regulations-and-policy/regulations/common-rule/index.html.
- 19.Golubnitschaja O, Costigliola V, EPMA General report & recommendations in predictive, preventive and personalised medicine 2012: white paper of the European association for predictive, preventive and personalised medicine. EPMA J. 2012;3(1):14. doi: 10.1186/1878-5085-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, Krapfenbauer K, Mozaffari MS, Costigliola V. Medicine in the early twenty-first century: paradigm and anticipation - EPMA position paper 2016. EPMA J. 2016;7:23. doi: 10.1186/s13167-016-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell LD, Astrin JJ, DeSouza Y, Giri J, Patel AA, Rawley-Payne M, et al. The 2018 revision of the ISBER best practices: summary of changes and the editorial Team's development process. Biopreserv Biobank. 16(1):3–6.21. [DOI] [PMC free article] [PubMed]
- 22.CFR Parts 50 and 56: Additional Safeguards for Children in Clinical Investigations of Food and Drug Administration-Regulated Products https://www.gpo.gov/fdsys/pkg/FR-2013-02-26/pdf/2013-04387.pdf. [PubMed]
- 23.Title 45: Public Welfare, Part 46, Protection of Human Subjects. https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/index.html. [PubMed]
- 24.45 CFR Part 160 and Subparts A and E: https://www.gpo.gov/fdsys/pkg/CFR-2007-title45-vol1/pdf/CFR-2007-title45-vol1.pdf.
- 25.45 CFR Part 160 and Subparts A and E Simplification: https://www.hhs.gov/sites/default/files/ocr/privacy/hipaa/administrative/combined/hipaa-simplification-201303.pdf.
- 26.45 CFR Part 160 and Subparts A and E for Professionals: https://www.hhs.gov/hipaa/for-professionals/privacy/index.html.
- 27.The Genetic Information Nondiscrimination Act of 2008, Information for Researchers and Health Care Professionals: https://www.genome.gov/pages/policyethics/geneticdiscrimination/ginainfodoc.pdf.
- 28.Final NIH Policy on the Use of a Single Institutional Review Board for Multi-Site Research: https://grants.nih.gov/grants/guide/notice-files/NOT-OD-16-094.html.
- 29.Federal Policy for the Protection of Human Subjects; Final Rule: https://www.gpo.gov/fdsys/pkg/FR-2017-01-19/html/2017-01058.htm.
- 30.Federal Policy for the Protection of Human Subjects; Rules and Regulations: https://www.gpo.gov/fdsys/pkg/FR-2018-01-22/pdf/2018-00997.pdf.
- 31.Brothers KB, Lynch JA, Aufox SA, Connolly JJ, Gelb BD, Holm IA, Sanderson SC, McCormack JB, Williams JL, Wolf WA, Antommaria AHM, Clayton E. Practical guidance on informed consent for pediatric participants in a biorepository. Mayo Clin Proc. 2014;89:1471–1480. doi: 10.1016/j.mayocp.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Redcap (Research Electronic Data Capture) https://www.project-redcap.org.
- 34.Evans RS, Lloyd JF, Pierce LA. Clinical use of an enterprise data warehouse. AMIA Ann Symp Proc. 2012;2012:189–198. [PMC free article] [PubMed] [Google Scholar]
- 35.Northwestern Medicine Enterprise Data Warehouse (NMEDW): http://nucats.northwestern.edu/resources/data-science-and-informatics/nmedw/index.html.
- 36.VCU and VCU Health System, single Enterprise Data Warehouse (EDW) https://cctr.vcu.edu/informatics/edw/index.html.
- 37.NIH’s Certificates of Confidentiality Policy Enhances Confidentiality of Participants Enrolled in Clinical Research Studies: https://nexus.od.nih.gov/all/2017/09/07/nih-new-certificates-of-confidentiality-policy.
- 38.NIH Certificates of Confidentiality (CoC): https://humansubjects.nih.gov/coc/index.