Abstract
Malignancies are one of the leading causes of mortality in women during their reproductive life. Treatment of gynecological malignant tumors during pregnancy is possible but not simple, since it creates a conflict between care of the mother and the fetus. BC is the most prevalent malignancy diagnosed in pregnancy, ranking up to 21% of all pregnancy-related malignancies. Due to its stets increasing prevalence, aggressive cancer subtype, and severe ethical and psychological aspects linked to the disease, experts raise an alarm for an acute necessity to improve the overall management of the PABC—the issue which has strongly motivated our current paper. Comprehensive research data and clinical experience accumulated in recent years have advanced our understanding of the disease complexity. PABC treatment must be individualized with an emphasis on optimal care of the mother, while observing standard treatment protocols with regard to safety of the fetus. Treatment protocols should be elaborated based on the individualized patient profile, bearing in mind the acute danger to the mother, maximizing the therapy efficacy and minimizing harmful effects to the fetus. Complex consulting on treatment options, their impacts on pregnancy and potential teratogenic effects requires tight “doctor-patient” collaboration. Complications that may arise due to the treatment of breast cancer in pregnancy require a multiprofessional expertise including oncologists, neonatologists, perinatologists, obstetricians, teratologists, and toxicologists, and an extensive psychological support throughout the pregnancy and after giving birth. Thereby, specifically psychological aspects of PABC diagnosis and follow-up are frequently neglected, being not yet adequately explored in the entire disease management approach. Herewith, we update the status quo regarding the currently available diagnostic modalities, complex treatment algorithms, and novel clinical approaches which altogether argue for an urgent necessity of a paradigm shift moving away from reactive to predictive, preventive, and personalized medical approach in the overall management of PABC meeting the needs of young populations, persons at high risk, affected patients, and families as the society at large.
Keywords: Pregnancy, Breast cancer, Diagnostics, Treatment, Chemotherapy, Radiotherapy, Psychological aspects, Preventive predictive personalized medicine
Introduction
Currently, breast cancer (BC) epidemic is characterized by around two million of new cases and a half of million disease-related deaths noticed annually in the world [1]. Furthermore, BC is the most prevalent malignancy diagnosed in pregnancy, ranking up to 21% of all pregnancy-related malignancies [2, 3]. Depending on the applied definition, geographic region, specific including and excluding criteria of corresponding statistical analysis, the incidence of PABC represents 15–35 cases/100,000 births, 1/3000 pregnancies, or between 1 and 21% of PABC prevalence, as it has been recently overviewed [4]. The Pregnancy Associated Breast Cancer (PABC) is most commonly defined as BC diagnosed during pregnancy or within 2 years post-delivery [5]. Due to its stets increasing prevalence, aggressive cancer subtype, and severe ethical and psychological aspects linked to the disease, experts raise an alarm for an acute necessity of the PABC dedicated studies (consolidated definitions, population studies, international database, disease etiology, molecular mechanisms) and innovative approaches for more adequate PABC management based on predictive and preventive medical services such as innovative screening programs adapted to the needs of young populations, predictive diagnostics, and targeted prevention [4]. There are multifaceted problems linked with the PABC predisposition and increasing prevalence. On the one hand, there is an enhancing tendency of BC prevalence in pregnant women towards the trend of their increasing age at planned pregnancies, specifically in Western countries [6]. Further, an induced abortion is a well-acknowledged causal factor for BC predisposition, and according to the currently available statistics, about a half of all unintended pregnancies ends in abortion. Consequently, some cohort studies consider the pregnancy-related BC portion representing ≥ 20% of all BC cases [4].
Pregnancy-associated breast cancers are typically found at an advanced stage, are more aggressive, and have a poorer prognosis. Delays in diagnosis of PABC are frequently associated with increased morbidity and mortality. In this regard, our article considers currently available approaches for PABC diagnosis and management with all their pros and cons, suggesting potential improvements to be considered for advanced medical services in order to satisfy the needs of PABC patients, including psychosocial aspects.
Characteristics of patients and the disease
PABC patients are most often in their 30s at diagnosis, with a median age of 33 years, and at the end of the second trimester, with a median gestation age of 21 weeks. Up to 80% of cases are found in the second and third trimesters. Histologically, 75–90% involve invasive ductal carcinoma, often in locally advanced stages with nodal positivity (T3, N+), Her2 positivity, and a higher grading (G3). Despite hormonal overload in pregnancy, the prevalence of hormone receptor negative cases (60–70% pregnant versus 25–39% not pregnant) is observed. The phenomenon is explained by downregulation of ER and PR receptors with high levels of free steroidal hormones [7–9] and the higher proportional occurrence of TNBC as luminal types, as TNBC is typical for the younger age group of patients [10].
For PABC, there is no proof that a therapeutic abortion improves prognosis [11]. It may become justified if the pregnancy interferes with therapy. Worse results of PABC treatment can be attributed to delayed diagnosis: determining a diagnosis from the first symptoms lasts in pregnancy approximately a month, in comparison with weeks in non-pregnant patients and suboptimal, insufficiently intensive therapy in the fear of exposing the fetus to the toxicity of the treatment. Moreover, the processes of a breast tissue healing and inflammation during involution and remodeling of tissues of the mammary glands after birth and post-lactation obviously share a role in cancer biology (origin or progression). The problems with the late diagnosis of any breast tumor in the pregnancy was the reason for translational research, focusing on modern multiomic approach with the aim to identify specific biomarkers not only for early detection but also for prediction of tumor biology, treatment response, and risk of metastatic spread. Growing evidence demonstrates that it could be the microRNA (miRNA) profile playing the central role in the regulation of the signaling pathways and tumor biology in PABC. Here, the aberrant miRNA expression could initiate cancer development, cell proliferation, invasion, migration, metastatic spread, or drug resistance. Reflecting this, Muñoz-Rodríguez et al. [12] conducted miRNA profiling study on 56 tumors from a series of multiparous Hispanic women and assessed the pattern of expression according to the time since the last full-term pregnancy. The authors recognized 15 miRNAs with significant differential expression between the early and late postpartum groups, while 60% of them were X-chromosome encoded miRNAs. Moreover, two-fold or higher differences in expression in overexpressed miR-17, miR-31, miR-135b, miR-138, miR-454, miR-660, and miR-934 and underexpressed miR-199a 5p, miR-542-5p, and miR-892a, which were found in the early group compared to the late postpartum group. Moreover, the DNA methylation status of three miRNAs (miR-31, miR-135b, and miR-138) was decreased in the early versus the late postpartum group. This concluded that differentially expressed and methylated miRNAs in tumors of the early and late postpartum groups suggest potential differences in epigenetic dysfunction, which may be operative in postpartum BC [12].
Predictive, preventive, and personalized medicine (PPPM) implemented in the overall management of PABC includes individualized medicine approach which recognizes a multidimensional interaction of internal and external risk factors, genetic background, age, gender, environmental risk factors, lifestyle, culture, and beliefs as well as social status in the overall predisposition of individuals to cancer diseases, the disease development, the natural course of disease, and the response to therapy [13]. BC is extremely complex clinical problem; therefore, the including of new paradigm representing a spectrum of complementary components that constitute the innovative concept of PPPM is needed to solve this medical puzzle [14].
Currently available diagnostic modalities
Clinical examination
The basis of determining a diagnosis is the case history and a physical examination. Although 80% of the time, a palpable resistance in the breast during pregnancy is benign (adenoma, fibroadenoma, hyperplasia, lipoma, hamartoma, galactocele, abscess, etc.), each de novo arising lesion which persists for more than 2 weeks should be investigated. During pregnancy, the classic symptoms of disease are easily hidden as a consequence of changes caused by pregnancy—hypertrophy of the breasts, colostrum secretion, and others. The trivialization of a palpable lump in the breast not only has a negative impact on the patient but also can lead to serious negative consequences for the examining physician as well. A tumor can be masked under the image of mastitis not responding to antibiotic treatment. To make differential diagnosis of a suspicious find easier, it is important to have a record on the palpation find on the breasts optimally before pregnancy and then to palpate the breast at the beginning of each trimester. Examination of the breasts during pregnancy is, with respect to the ongoing physiological changes in the mammary glands, demanding for interpretation and burdened by a higher false positivity and lower sensitivity [15].
Radiologic examination
Screening display examinations in tumor diseases during pregnancy are very important, since an appropriate treatment approach is based on them. Ultrasound examination (USG) and magnetic resonance imaging (MRI) are the predominant forms of examination, with USG as the preferred method, as it is completely safe during pregnancy. A USG examination can differentiate a cystic lesion from a solid one, which at the same time can be biopsied. The sensitivity and specificity are not influenced by the ongoing changes caused by pregnancy [16]. Mammographic examination with abdominal shielding is not contraindicated but has limited application in young women. With sufficient shading of the uterus, it is safe in the first and second trimesters. The dose of radiation on the fetus is 4 mGy, which is well under the estimated deterministic threshold, which depending on the source gives from 50 to 100 mGy [17, 18]. It is irreplaceable in depicting microcalcifications, focal asymmetry, or multifocal lesions. It has sensitivity of approximately 86% in pregnancy [16]. With unclear results on the USG examination, MRI is indicated. According to the American College of Radiology exposure to MRI, based on the current knowledge, it is without side effects on a developing fetus in any trimester of pregnancy [19]. Gadolinium (a category C medicament according to the US Food and Drug Administration) should be used only in an absolutely essential case, even though thus far, no undesired effects of gadolinium on the child in all three trimesters of pregnancy have been found [20]. MRI without the use of gadolinium may also provide sufficient information on the invasiveness of a tumor. Although exposure to ionization radiation in distant parts of the mother’s body exposes a fetus to low doses of radiation, when accumulated, it may damage the fetus. Radiography of the chest exposes a fetus to a negligible dosage of approximately 0.1 mSv, and 1 mSv is the dose absorbed by the mother [20]. CT of the chest with shielding the belly can be performed in the case of suspicion of the presence of a mediastinal, pleural, and/or lung metastasis.
C/histological examination (tumor biopsy)
Histological examination of a suspicious lesion in breast during the pregnancy has very high importance, even though it includes a certain level of risk hazard, where, e.g., fine-needle biopsy bears the risk of false negative results. Fine-needle aspiration (FNA) of breast masses in pregnant or lactating women is a common procedure, but the cytological interpretation is considered problematic due to the atypia inherent to secretory changes in glandular epithelia. FNA cases from breast masses in pregnant or lactating women can be processed as ThinPrep (TP) and/or direct smears (DS). Heymann et al. [21] by comparing DS to TP found out that TP samples of lactating adenomas (the most common diagnosis for breast masses in pregnant and lactating women/up to 78.5%) demonstrated “lacy” fragments, a tissue-paper-like texture and globular clumps of “milky” background material, with embedded singly dispersed “bare” epithelial cell nuclei containing cherry-red macronucleoli. Moreover, the architecture in lactating adenomas appeared to be disrupted with isolated cells, smaller cell clusters, and lobules. Despite the cellular morphology better preserved in TP biopsies compared to DC, the cytological features of carcinoma on TP were similar to DS.
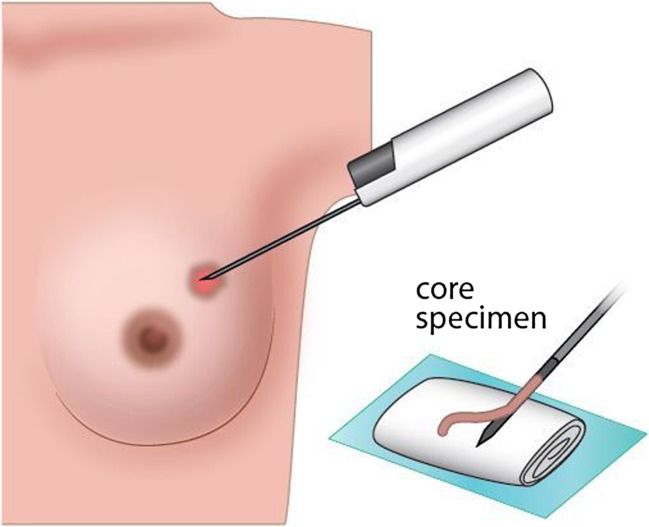
As the open excision of the breast during pregnancy increases the risk for developing the milk fistula during lactation, the gold standard in the histopathological diagnosis of PABC become a core-cut (needle) biopsy (Fig. 1) yielding sensitivity over 97–98% and low variability range, overweighting the sensitivity of FNA, which varies from 77 to 97% [22]. While FNA offers several advantages, such as the rapidity, low cost, and minimal invasion with good sensitivity and specificity, core biopsy provides higher specificity than FNA with the additional advantage of ancillary tests and comment on the histopathological prognostic markers, which can be influenced by pregnancy hormonal status on the other hand. Thus, it is very important to inform the pathologist that biopsy was taken during pregnancy. Up to 80% of biopsies in pregnancy are of benign dignity; the rest is linked to premalignant or malignant features, when each month of delayed treatment increases the risk of the lymph node involvement by 0.9–1.8% [23].
Fig. 1.
The histopathological diagnosis of pregnancy-associated breast cancer by core-needle biopsy. Core-needle biopsy is the gold standard method for breast cancer histological examination. This method is outpatient procedure, well tolerated, and quick. If compared to other biopsy techniques, this method provides significantly better interpretation and tissue characterization than fine-needle aspiration and allows specifying the definitive histopathological diagnosis of PABC. As it is less invasive than vacuum-assisted biopsy or surgical/core biopsy, also the risks associated with this procedure is lower
D/staging examinations
Staging examinations for the exclusion of distant metastases are not indicated in asymptomatic patients in the early stage of disease (T1–2N0); in others, it is recommended to undergo them [22]. Process includes:
RTG X-ray image of the chest with abdominal shielding,
USG examination of the liver,
MRI without Gd contrast (whereby it is necessary), and
Low-dose scintigraphy of the body (is also considered allowable).
While indicating X-ray imaging examinations, it is necessary to consider the ratio of benefit for the patient versus risk for the fetus associated with radiation exposure.
E/blood tests (tumor markers)
Tumor markers in PABC scope are used especially for monitoring the effectiveness of treatment. The number of studies which have dealt with examination of serum tumor markers during pregnancy is limited. Tumor marker profile may also be increased during a normal pregnancy, including, e.g., hCG, alpha-fetoprotein, CA 15–3, SCCA, and CA 125 [24].
Levels of inhibin B, AMH, HE4, and LDH are not raised in sera of mothers during a normal pregnancy, and therefore, they could be used in differential diagnostics [25, 26].
Nowadays, there occurs other potential markers due to the comprehensive multiomic research, such as genomics, proteomics, metabolomics, and epigenomics, which has been focused on complex understanding of biological functions and dynamics of specific diseases, including breast cancer. In this regard, e.g., miRNA signatures seem to be very promising markers. Apart from tumor microenvironment, miRNAs can be found and isolated from various body fluids including serum, plasma, but also saliva, urine, or breast milk [27], making them an ideal source for tumor marker study in PABC.
Clinical management of high-risk PABC individuals: general approaches and necessity for more predictive, preventive, and personalized medical services
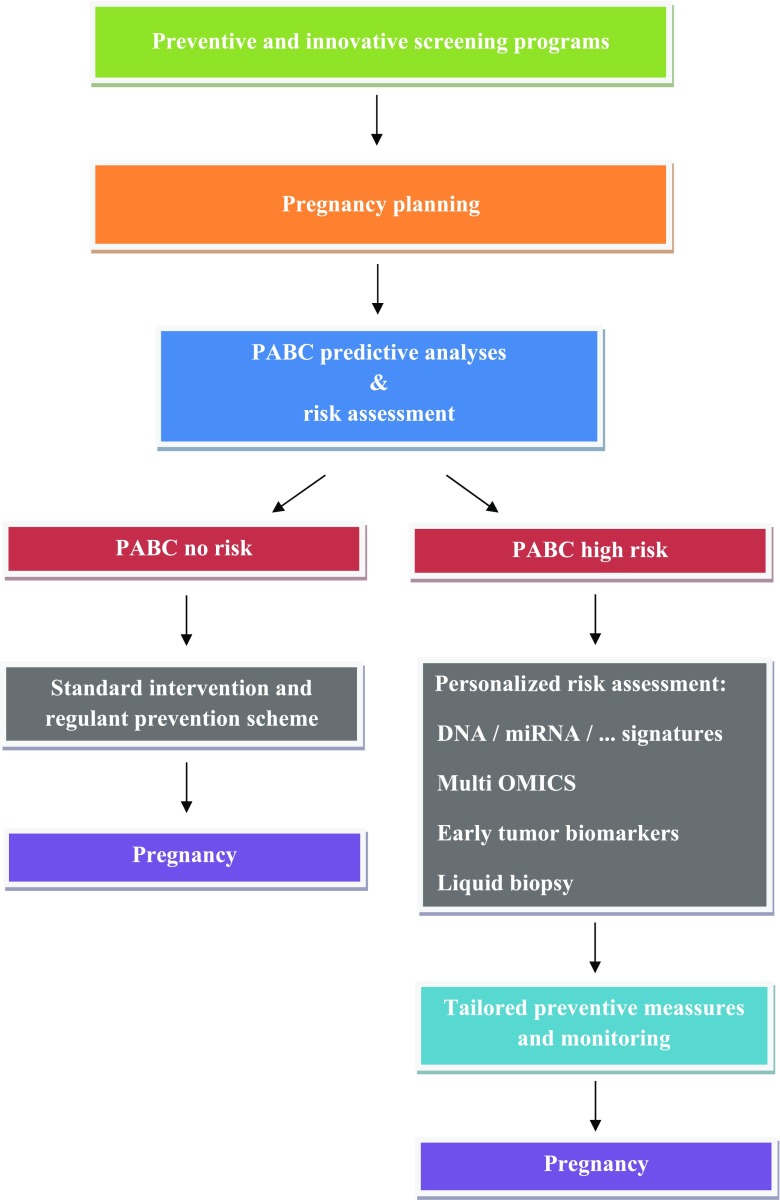
The important question of modern oncology is the prediction and effective protection against clinical manifestation of the cancer disease by distinguishing between “silent” carriers of tumor lesions and individuals who are predisposed to a disease development and progression. Implementing the PPPM, Polivka et al. [4] described the concept for advancing medical devices in the overall management of high-risk PABC women. It comprises innovative screening programs adapted to the needs of young populations relevant to PABC, phenotyping of predisposed individuals, multilevel diagnostics, and preventive measures tailored to the patient. Based on these data, we propose innovative preventive, predictive, and personalized algorithm summarized in Fig. 2.
Fig. 2.
Innovative preventive, predictive, and personalized algorithm for planned pregnancies. The algorithm strongly focuses on the prediction and personalized characteristics by innovative approaches of multilevel diagnostics
Young populations (teenagers, youth) potentially relevant to PABC include several breast cancer risk factors, such as reproductive factors (early menarche), physical inactivity and sedentary lifestyle, previous cancer and chest radiotherapy, and family history of breast cancer or genetic mutation, especially carriers with BRCA1/2 mutation. Exogenous risk factors increasing the risk of BC are environmental risk factors (industrial air pollution or toxic environmental contamination with heavy metals and polychlorinated biphenyls), smoking cigarettes before the first full-term pregnancy, alcohol consumption, flight attendants, type 2 diabetes mellitus, or anorexia nervosa [4]. Currently, genetic testing is not recommended in adolescents under 18 years, as medical surveillance is not usually recommended until around 25 years [28]. Imaging modalities (ultrasound, mammography, and MRI diagnostics) are effective for early BC diagnosis. However, the most high-risk breast surveillance programs now offer annual MRI to eligible high-risk women from age 25 to 30, usually supplemented by regular mammography. Regarding younger women, careful screening measures including thorough clinical breast exam, breast self-awareness, and breast self-examinations are crucial for the detection of preneoplastic and neoplastic lesions. Compared to MRI plus mammography, the inclusion of clinical breast exam (CBE) and/or ultrasound used in high-risk young women has lower impact on the cancer detection. There is now considerable evidence that MRI is by far the most sensitive imaging modality in young high-risk women [28]. The reduction of advanced stage interval cancers (especially in BRCA1/2 mutation carriers) could include screening programs offering additional semi-annual CBE and/or ultrasound or alternate MRI and mammography every 6 months. In this regard, the important question is rising—how long the regular MRI should be continued in young high-risk women? Clinical data on long-term survival and mortality in women undergoing high-risk surveillance with MRI is still rare [29].
Breast cancer risk assessment that includes the identification of most important markers, phenotyping, and genotyping in young female populations might be extremely useful for innovative screening programs specifically focused on the selection of high-risk population for the PABC development. In this regard, lifestyle factors, reproductive and endocrine factors, family history, phenotype, and genotype are crucial parameters defining the BC risk [30]. The risk of early premenopausal BC development increases with tobacco and alcohol consumption, obesity, early menarche, absence or low number of children, number of miscarriages or voluntary termination of pregnancy, length of breastfeeding, and hormonal contraceptive use. When differentiating sporadic BC from familial cases, the history of breast and/or ovarian cancer in first- or second-degree relatives should be taken into consideration. Moreover, within the family history of BC, the parameters like histological type, grade, tumor size, lymph node involvement, metastatic spread, molecular classification, and genotype of BRCA1 and BRCA2 genes presence should be assessed [31].
In addition to previously mentioned risk factors, young women affected by Flammer syndrome are at the increased risk of the developing fast progressing BC [4, 32–34]. This knowledge points out to the FS relevance for particularly aggressive BC subtypes such as the triple-negative breast cancer, which is typical for PABC patient cohort. Based on abovementioned data, it seems reasonable to implement valuable screening programs for individuals predisposed for PABC. This clinical approach might be of great importance; it could be applied to teenagers by introducing dedicated questionnaires at the level of primary care (general practitioners).
Predictive clinical approaches applied to the individuals at high risk for premenopausal BC requires multilevel diagnostics including family history, questionnaires, imaging tools, and multiomic approach evaluating predisposition and disease-specific molecular patterns. Currently, the deficits in PABC management include suboptimal screening programs demonstrating many cases with both false-positive and false-negative diagnosis [35], the deficiency of screening programs directed specifically on BC predisposition and introduction in young population with individualized risk profiling for more precise diagnostics and treatment options. An apparent lack of studies providing biomarker panels for multilevel diagnostic approaches in the detection of early premenopausal BC warrants the development and successful clinical application of innovative approaches such as using of integrative bioinformatics. The multilevel diagnostic panels consist of genomics, proteomics, epigenetics, miRNA, metabolomics, circulating tumor cells, and cancer stem cells [36]. Predictive biomarkers used for early BC diagnostics detectable by genomics include BRCA1 mutations, MTHFR C677T polymorphism, SNP s28366003 in MT2A, or SNP rs17849079 in PIK3CA. Predictive biomarker patterns detected at epigenetic level in BC include overall DNA hypomethylation. Dysregulations of miR-181a and circulating 9-miR signatures are predictive markers for early BC stages. Moreover, another significant biomarkers of early BC include increased expression of annexin A6 and MMP-9 and dysregulated metabolites, e.g., low levels of histidine, higher levels of glucose and lipids, increased PGE2, enhanced glutamine metabolism (ER and TNBC phenotype), or glycine/serine metabolism (HER2 phenotype). Evaluation of circulating tumor cells is promising clinical diagnostic tool in the detection and associated pathology development of premenopausal BC [37]. However, it is important to mention that despite recent advances in personalized medicine and the identification of novel molecular biomarkers that facilitate diagnostics of early premenopausal BC, the molecular mechanisms of how they exert their effects is still not fully understood [38]. Additional clinical studies aimed on the association of these biomarkers and diagnostics and symptom severity among high-risk BC population are warranted.
The fast advancing era of genomic and molecular medicine provides new possibilities for the most effective accomplishment of PPPM in high-risk population for PABC. It includes innovative approaches of multilevel diagnostics, application of individualized patient characteristics, and creation of optimized screening programs focused on young female populations. This clinical approach is strongly suggested notably for planned pregnancies, with the aim to eliminate the risk of advanced PABC by targeted therapies [4]. New diagnostic strategies including the preventive measures tailored to the risk premenopausal BC individuals, if based on the personalized diagnostic approach, will decrease mortality and improve the cost-effectiveness of medical services in the society.
Treatment management of PABC patients
A personalized therapeutic procedure is important for effective individual patient care. Firstly, it is necessary to develop a better diagnostic method, which is able to predict patients’ responses to chemotherapy, radiation, and targeted therapy. The deeper understanding of the molecular background of carcinogenesis has to progress further to accelerate the development of therapeutically effective drugs and more specific diagnostic techniques for personalized treatment. The purpose of personalized treatment is the precise prescriptions to optimize the right drug to the right patient at the right dose at the right time for the right duration [39]. The goals of treating a pregnant patient with BC is to cure, support the pregnancy, and not damage the fetus. A principle aspect, if we try to preserve pregnancy, is the necessity to respect fetal development, especially its vulnerable period of the first trimester. In this period, there is a 17% risk of malformation occurring; therefore, vital indication of cytostatic treatment in the first trimester can be an indication for a therapeutic abortion [40]. The involved patient or a parent of the patient must be fully involved in such a decision. Good results of treatment of pregnant patients with minimum risk for the fetus depend on the erudition of a multidisciplinary team, including a gynecology specialist on risky pregnancy, a neonatologist, and a psychologist.
During pregnancy, it is possible to use all basic modalities of treatment—surgical treatment, chemotherapy (CTh), and radiotherapy (RT). Their timing and incorporation into treatment have to maximally consider the presence of the fetus and the specific period of its development. On the other side, the biological treatment is contraindicated in PABC [41].
Treatment modalities in PABC are affected a lot of interfering factors:
With a diagnosis of BC in the first trimester of pregnancy, the options are limited. In the case that the patient wants to preserve the pregnancy, surgical treatments are considered, if there has been timely diagnosis of the carcinoma; in the majority of cases, it is necessary to put off treatment to the second trimester.
In the second trimester, CTH can be safely commenced from the 12th week of pregnancy, when organogenesis ends. In the case of advanced findings, neoadjuvant chemotherapy (NACT) is indicated. Routinely, a surgery can be done following adjuvant chemotherapy. Premature birth in the second trimester is burdened by significant fetal morbidity and mortality.
In the third trimester, we can indicate a surgical solution, NACT; induce pulmonary maturation of the fetus; proceed to premature birth; or consider putting off treatment until after birth.
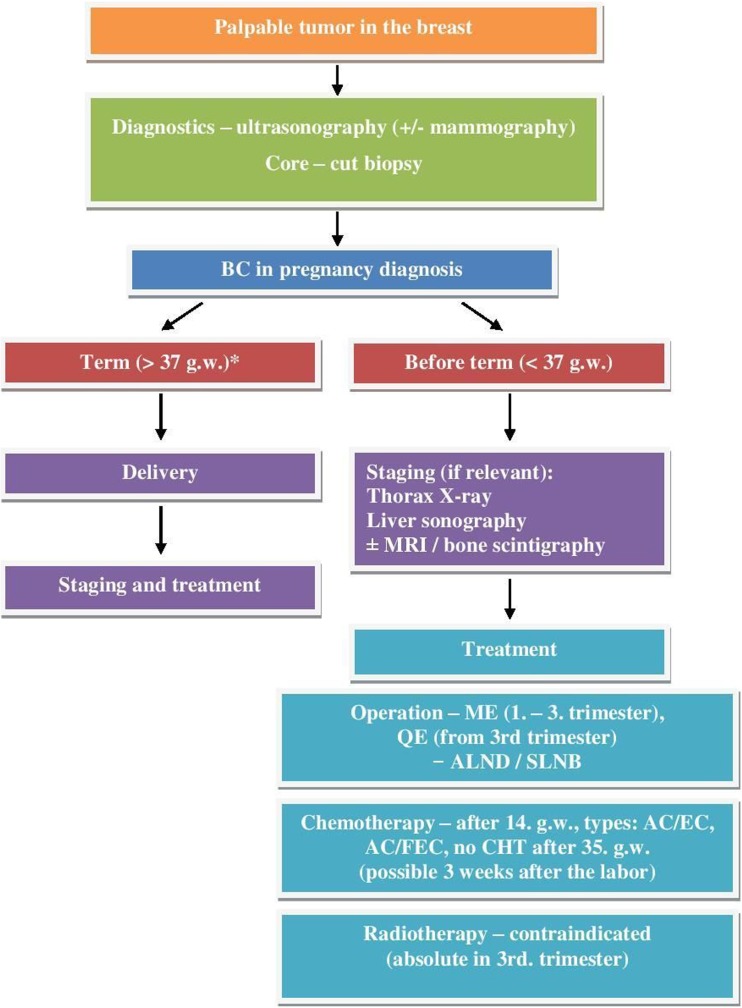
Once the termination of a pregnancy is adopted, it is possible either on the request of the patient to the end of the first trimester or on the basis of oncological indication to the end of the 24th week of pregnancy [42]. The treatment algorithm of pregnancy-associated breast cancer is summarized in Fig. 3.
Fig. 3.
Treatment algorithm of pregnancy-associated breast cancer. CHT chemotherapy, USG ultrasonography, MRI magnetic resonance imagining, ME mastectomy, ALND exenteration of the axilla, SLNB sentinel lymph node biopsy. Asterisk indicates that it is acceptable to wait 2 to 4 weeks for maturation
Surgical treatment
Surgical treatment of PABC (if indicated) is safe throughout the entire period of pregnancy. Ideal, however, is to postpone an operation until after the first trimester due to the higher risk of miscarriage [43]. It is recommended to operate in a position of the patient moderately on the left side, to adequately use analgesics, antibiotics, and mini-heparinization. Mastectomy (ME) may be indicated during the whole pregnancy, and in the first trimester, it is the method of choice, with respect to contraindication of radiotherapy. It needs to be remembered that in the case of breast-preservation surgery, adjuvant RT must follow. By the end of the second and in the third trimester, breast-preservation surgery comes into consideration, with planning of RT after birth.
Gentilini et al. compared breast-preservation surgery with radical modified ME in a set of 21 patients, and during the 24-month follow-up, no signs of local or regional recurrence were found [44]. Kuerer et al. published similar results of survival among patients treated with ME and breast-preservation surgery [45]. It was shown that conservative surgery in the course of pregnancy is not burdened by significantly higher risk of complications [46].
Reconstruction operations should be postponed until the period after birth, with respect to pregnancy-related changes in the other breast and the subsequent undesired cosmetic effects [42]. In early BC with clinically negative lymph nodes in the axilla, standard treatment is currently sentinel lymph node biopsy (SLNB). Several works have dealt with the safety of SLNB during pregnancy. It was found that the radiation dose for the fetus does not exceed 0.45 mGy with the use of a 1-day protocol [45, 47]; furthermore, technetium does not pass through the placental barrier. On the basis of existing experience, the use of technetium was recommended in European international guidelines [48]. Contrary to that, the use of blue dye (isosulfan blue or methylene blue) due to the risk of an anaphylactic reaction for the detection of SLU is not allowed [47]. Regarding the more often occurrence of advanced carcinoma with palpable lymph nodes, complete dissection of the axilla remains the standard procedure.
Radiotherapy
Radiation has dose- and gestational-week-dependent effects on the fetus [6]. Radiotherapy during pregnancy is generally contraindicated. However, it is done in several world-renowned clinics with the experience and equipment allowing radiotherapy. Radiotherapy can be safely applied after a careful dose adjustment (exposures of 0.01 mGy are below the threshold dose) and if not targeting the pelvis or abdomen. It can be carried out in the first and at the beginning of the second trimester of pregnancy using the appropriate abdominal shielding. Radiotherapy becomes riskier as the fetus grows and uterus reaches the radiotherapy area [49]. Children exposed to radiation therapy are under the increased risk of intrauterine growth restriction, mental retardation, and have increased risk of developing cancer during their childhood [50]. Treatment of progressed stages of BC should include surgery, chemotherapy after the first trimester, but wait with radiotherapy after delivery [51]. Women who undergo radiotherapy can keep on breastfeeding even though due to the increased risk of post-radiation mastitis, they should only feed the infant from the healthy breast [52].
Delaying radiotherapy in the management of PABC is likely to lead to an increased rate of local recurrence. Several studies have dealt with the question of deferring radiotherapy in PABC. A retrospective analysis of 13,907 patients in stages I and II determined a worsening prognosis for patients with delayed RT for longer than 3 months [53]. Chenet al. conducted a meta-analysis of 20 publications dealing with the deferment of radiotherapy and its impact on patient prognosis. This study suggested that recurrence rate increases by 1% by each month of deferment of radiotherapy [54]. At the same time, in patients who are diagnosed during the course of second half of pregnancy, the start of radiotherapy could be postponed until after delivery without worsening maternal outcome [51].
Chemotherapy and supportive treatment
Physiological changes in pregnancy can have an impact on the effect of the cytostatic treatment. Therefore, the lack of information on pharmacokinetics and pharmacodynamics of cytotoxic medicines in pregnant women is not surprising [55]. CTh may be indicated to patients with locally advanced BC either as a preoperative (neoadjuvant) therapy or as an adjuvant therapy with negative prognostic factors. The use of CTh during pregnancy has been the subject of many studies, including preclinical ones with experimental therapy. Data on the transplacental transfer of cytostatics and their metabolites are predominately available only from animal models, which show that thanks to a placental barrier equipped with protein pumps (P-glycoprotein, multidrug resistance protein, breast cancer resistance protein) the levels of anthracyclines, docetaxel, paclitaxel, vinblastine, and cyclophosphamide in fetal blood are low [15].
In the first trimester, cytostatics work as a teratogen on the fetus, in general [40]. Treatment after the 14th week of pregnancy is generally considered to be relatively safe. Exposure to CTh in this period does not increase the risk of malformations. There is higher risk of IUGR, ascribed more to hyponutrition and anemia of the mother during CTh, and the risk of myelosuppression, especially with therapies shortly before birth. The following cytostatics are considered safe: anthracyclines (adriamycin A, epirubicin E), 5-fluorouracil (F), and cyclophosphamide (C). These schemes are preferred and verified: AC, EC, FAC, and FEC. At present, the most experience in BC has been with the FAC regimen, whose use has been published in prospective studies with minimal occurrence of complications [56]. Taxanes (paclitaxel) and vinorelbine are probably safe, as well. Therapy with bisphosphonates is due to its potential teratogenic effect in pregnancy contraindicated, although no changes were observed in the body of newborns exposed to their use [57]. The dose on the surface of the body is calculated according to the actual weight, not the weight of the patient before pregnancy. In the course of supporting therapies, it is possible to use setron antiemetics, metoclopramide, and corticoids (hydrocortisone and methylprednisolone are given preference over hydrocortisone and methylprednisolone dexamethasone). Chemotherapy during PABC is advised to be discontinued 3 weeks before delivery or after 35 weeks of gravidity, with the aim of minimizing the risk of sepsis and hemorrhage in the mother or newborn [15]. This provides time for fetal drug excretion via the placenta, mainly for preterm babies with a limited ability to metabolize drugs through immature liver and kidneys. Chemotherapy can continue after adequate recovery from delivery. Breastfeeding is contraindicated during chemotherapy and can proceed 3 to 4 weeks after the last administered dose [58].
Most recently, a 20-year international cohort study of 1170 oncological patients receiving treatment during pregnancy showed 955 (88%) of 1089 singleton pregnancies ended in a live birth, of which 430 (48%) of 887 pregnancies ended preterm [59]. BC was the most frequent oncological disease (39%) in pregnant women. Each 5 years, the authors found more live births (RR 1.04, 95% CI 1.01–1.06) and fewer iatrogenic preterm deliveries (0.91, 0.84–0.98). Moreover, data suggested a relationship between platinum-based chemotherapy and small size for gestational age (odds ratio [OR] 3.12, 95% CI 1.45–6.70), and between taxane chemotherapy and the neonatal intensive care unit admission (OR 2.37, 95% CI 1.31–4.28). Anthracyclines (e.g., epirubicin and doxorubicin) are the most widely used chemotherapeutic agents, as they have a favorable safety profile when administered during pregnancy [60]. However, BC treatment during pregnancy using anthracyclines is sometimes associated with early and late toxicity for the fetus, characterized by cardiac and neurodevelopmental toxicity and low birth weight and birth defects. The risk of congenital malformations is not increased [61].
It is crucial to regularly observe the detailed fetal heart anatomy during the curse of the anthracycline-based chemotherapy treatment. If fetal heart shows signs of damage, it is inevitable to choose alternative therapeutic options. Future research of specific pharmacokinetics and pharmacodynamics of chemotherapeutic drugs in most vulnerable fetuses will improve the choosing of suitable treatment [62].
Alternative treatment choice is found in taxanes [63]. Even though fewer studies have evaluated taxanes compared to anthracyclines and they were done on smaller number of patients, a good tolerability in fetus was observed in vast majority of pregnancies. It might be associated with P-glycoprotein, which is highly expressed in maternal part of the placenta and protects the fetus against xenobiotics and might reduce transfer of taxanes through placenta [64].
Biological and hormonal treatment
Biological treatment is based on using the body’s immune system to treat a disease and protect the body from some of the undesirable effects of certain standard treatments. Biological and molecular targeted therapies have been introduced into standard clinical practice in the last 10 years and are continuously in the development within clinical trials. Biological targeted therapy applied in cancer treatment includes several categories of molecular targets (i.e., cytokines, monoclonal antibodies, cellular therapies, vaccines, or angiogenesis inhibitors). However, regarding the PABC, biological treatment is contraindicated [41, 65]. There is limited experience with anti-HER-2 biological treatment (trastuzumab, lapatinib). HER-2 plays a pivotal role in embryonic cardiac development and acts as an important recovery pathway when the heart is exposed to stress [66]. Moreover, HER-2 is linked to the function of fetal kidney. Clinical practice demonstrated the use of monoclonal antibodies without undesirable consequences in the first trimester of pregnancy. However, trastuzumab crosses the placenta at increased levels starting in the second trimester. Several cases of patients with PABC elected to receive trastuzumab following the first trimester [67, 68]. Several other women unintentionally became pregnant during the treatment with trastuzumab. Despite the fact that the majority of patients were exposed to this monoclonal antibody during the first trimester, no cardiac events or anomalies were found. On the other hand, it is important to mention that very few cases reported long-term follow-ups for the newborns. Regarding the fetal kidney, the treatment in the second and third trimesters leads to oligohydramnios to anhydramnios (incidence up to 61.1%), with the incidence increasing with duration of treatment [61, 68–71]. These results have been imputed mainly to the inhibitory effect of trastuzumab on HER-2, which is highly expressed on the fetal kidney responsible for the production of amniotic fluid. However, oligohydramnios seems to be reversible when discontinuing trastuzumab, with good clinical outcome found in these pregnancies. An international collaborative study evaluated the prognosis of women with primary breast cancer diagnosed during pregnancy [72]. Investigators assessed the disease-free survival and overall survival on exposure (pregnant or not), adjusting for the age, stage, grade, hormone receptor status, human epidermal growth factor 2 status, histology, type of chemotherapy, use of trastuzumab, and radiotherapy. The results showed similar overall survival for women diagnosed with PABC compared to nonpregnant women. These data support the option to start treatment of BC with the continuation of pregnancy.
Another monoclonal antibody bevacizumab targets VEGF, which is a key regulator of angiogenesis. Angiogenesis is a very complex process, therefore is crucial during embryogenesis. VEGF receptor 2 has been described as very important for the vitality of corpus luteum, which is an endocrinal organ supporting the pregnancy development [73]. Moreover, VEGF is a regulator of the amount of the amniotic fluid produced by the fetal kidney. The administration of bevacizumab in pregnant mice resulted in disruption of survival of the preexisting blood vessels in the corpus luteum and terminated the embryonic development [74]. The administration of bevacizumab to BC women is associated with an increased risk of hypertension and proteinuria [75]. These side effects are major problem if encountered during pregnancy, because it would increase the risk of developing preeclampsia, which could endanger the pregnancy with the adverse consequences for maternal and fetal health [76]. To date, there are no data describing the administration of bevacizumab in PABC.
Hormonal therapy is also contraindicated during pregnancy [77]. Gonadoliberins (LHRH analogues) interfere with gestation, and tamoxifen is known serious teratogen, with 20% risk of malformation of the cranio-facial area (Goldenhar syndrome) and genitalia [51]. Moreover, tamoxifen is also transmitted into milk; therefore, it is contraindicated during the breastfeeding, as well [78].
Novel clinical approaches
Preventive multiomic approach within PPPM plays a crucial role in the determination of specific genetic disorders through population screening for carriers of rare, fully penetrant alleles that cause monogenic diseases, and in the genotyping of susceptibility genes within families at high risk of BC development, and thus provides the background of public health genetics [13]. The current management of the PABC requires creating new diagnostic strategies better adapted to the needs of clinical care. Predictive diagnostic approaches (specifically in premenopausal women), innovative screening programs focused on young female populations, targeted prevention in high-risk groups, and optimized treatment concepts are necessary for better controlling of PABC [4]. Recently, miRNAs have emerged as molecular regulators that may play key roles in the pathogenesis, progression, or drug resistance of different malignancies, including BC. These miRNAs can be found circulating body fluid, suggesting their role as non-invasive marker with the use of liquid biopsy. Among circulating miRNAs with well-documented aberrant activation in BC patients are, e.g., miR-16, miR-18a, miR-21, miR-145, let-151a, and miR-155. Well-described tissue-specific miRNAs are miR-7, miR-21, miR-145, miR-155/154, miR-182, miR-203, and miR-213 [79]. MicroRNA profiling studies in different solid tumors have demonstrated cancer type-specific deregulations of tumor suppressor genes [80]. In particular, miR-21 has been observed to be commonly overexpressed in different solid tumors, including breast cancer. Walter et al. [81] evaluated the expression levels of miR-21 in PABC cases to assess its role in the transcriptional regulation of target genes, i.e., PTEN, PDCD4, and Bcl-2, and consequently the impact on tumor onset and progression. The results supported the concept that miR-21 may play a potentially important oncogenic role in breast cancer not only in non-pregnant but also in pregnant women. Overexpressed miR-21 may be a possible indicator of poor prognosis in PABC. This clinical study demonstrated that circulating or tissue-specific miRNAs could potentially serve as promising biomarkers for the detection of early disease stages, prediction of prognosis, and treatment monitoring of the patients with PABC. Additional very perspective and important biomarkers of BC disease in non-pregnant women, such as circulating tumor cells or cell-free DNA, have not yet been described in PABC.
Psychological aspects related to PABC
Once BC is diagnosed during the pregnancy, consequences are frequently dramatic such as psychological aspects of the decision by choosing between following choices:
Interruption of the planned pregnancy which might be the first and the last one in individual cases without a perspective to give a birth to the child in the future.
To treat the pregnant women with a potential to damage the fetus.
Not to treat the mother until the delivery with the potential consequence of very poor outcome of the delayed treatment.
Patient and the medical team face complex ethical concerns and several dilemmas when dealing with PABC. No specific medical evidence exists which would favor termination of the pregnancy over keeping it or postpartum delayed treatment, in means of improving maternal prognosis [58]. That is why there is no medical protocol for PABC. It is completely up to the mother’s personal decision whether to end or proceed with the pregnancy during PABS. She should be offered complex and extensive discussion of the topic with the medical team [82, 83]. The impact of the diagnosis of PABC on maternal health, fetal outcome, and risks to the fetus should be considered on an individual basis [58].
The analysis of existing research and literature on PABC has shown that the psychological aspects of this disease, as well as the psychological state of the patients, are rather neglected topics. Furthermore, the possibilities for a personalized approach to the treatment of these patients with psychotherapy are a topic that is a real challenge. The most important and current issues pertaining to psychological aspects and conditions are mainly focused on the maternal prognosis followed by the effects of the therapy on the fetus and the risk of continuing with the pregnancy. Emotional states as an integral part of the psychological state of these patients are not less important. Apart from anger, grief, anxiety, sadness, and fear [84], the psychological problems include concerns regarding fetal survival and pregnancy outcome. After dealing with problems associated with pregnancy, concerns arise about body and sexuality, loss of fertility, premature ovarian failure, and future childbearing [85]. Important psychological aspects are, of course, uncertainty and insecurity about a relapse of the disease, as well as socioeconomic consequences that can lead to stress related to losing a career or dismissal from workplace.
Furthermore, recent study conducted by Rodsten et al. raised awareness about the importance of experience of patients with PABC. This study focused on major themes such as (1) experiencing a clash of priority between baby’s life and mother’s life, versus mother feeling in alliance with baby; (2) having perceptions of being physically and emotionally saved by the pregnancy and baby; and (3) experiencing a loss of being a mother [86]. Patients with PABC experience psychological challenges concerning identity and new role as “mother-patient,” maternal-infant attachment, coping and support, and impact on family life and relationships.
Psychosocial support and treatment
Counseling and supportive psychotherapy, social support from the patient’s family, and health professionals may improve the patient’s emotional status. In conditions where besides counseling and supportive psychotherapy, there are no empirical data on the effects of the influence of a particular type of psychotherapy in patients with PABC, there is room for debate and questions about which psychotherapy would be most appropriate and with the greatest effect in these patients. Rodsten et al. claimed that cognitive behavioral therapies (CBT), including individual and family systemic approaches, are appropriate within a flexible structural format to accommodate PABC and treatment effects, and especially parenting behavior, wishes, and responsibilities [86]. Having in mind the latest systematic review papers and meta-analyses related to the efficiency of CBT in cancer patients, we consider CBT psychotherapy to be a solid foundation for creating and implementing interventional, personalized programs in patients with CBT.
Recently, CBT has introduced a more trans-diagnostic/process-based and personalized approach. Its primary goal is to link the therapeutic technique to the process and the individual client [87]. CBT is nowadays considered as the gold standard of psychological treatment as it is the most researched form of psychotherapy; there is no data showing superiority of any other form of psychotherapy, and in addition, the CBT theoretical models/mechanisms of change have been the most researched and are in line with the current mainstream paradigms of human mind and behavior (e.g., information processing) [88–90]. Even though support from psychotherapists or counselors are essential for successful therapy, the role of family and friends should not be neglected.
Conclusions and expert recommendations
Malignancies are one of the leading causes of mortality in women during their reproductive life. The effects of treatment of gynecological malignant tumors, including breast cancer, during pregnancy is possible, although it creates a conflict between care of the mother and the fetus. Oncological treatment must be individualized with an emphasis on optimal care of the mother, while observing standard treatment protocols with regard to safety of the fetus. A treatment protocol should be elaborated on an individual basis, bearing in mind the morbidity of the mother, the effect of treatment, and the maximum probability of survival. Complex consulting on treatment options, their impacts on pregnancy, and potential teratogenic effects should be explained in detail to each pregnant patient before commencing treatment. Complications that arise during treatment of breast cancer in pregnancy require a supportive multidisciplinary team, which included neonatologists, perinatologists, oncologists, obstetricians, teratologists, and toxicologists, and provide psychological support throughout the pregnancy and after giving birth. Cumulative research and clinical experience in recent years has improved our understanding of the management of breast cancer during pregnancy. Psychological aspects of PABC and psychological states and issues are important, but neglected and not well-explored topics. We further argue for an integrated scientific psychotherapy, with CBT serving as the foundational platform for integration. This knowledge helps physician and families with complex decision regarding treatment of the mother and safety of the unborn child. Specific psychological states and mental health consequences associated with PABC are strong arguments in favor of predictive diagnostics and targeted preventive measures for planned pregnancies in order to avoid PABC with all negative consequences to the mother, baby, family, health care system, and society at large.
Importantly, value-guided medical approach to economically valid screening programs is necessary regarding the consistent management of PABC. However, no any population (screening) programs, no generally accepted risk factors, and no standardized preventive diagnostic methods specifically focused on PABC are currently available. The establishment of this new concept using appropriate clinical evaluations and measures (mentioned above) is crucial due to the emergency of this medical problem. In this regard, we must emphasize the key role of PPPM in the management of cancer disease which is aimed to substantially decrease the number of deaths, improve the early detection, develop state-of-the-art diagnosis and treatment, improve the quality of life of patients, and develop easy-to-use kits for diagnosis [39]. It is evident that a paradigm shift is needed to move from reactive to predictive, preventive, and personalized medicine as a new philosophy, promoting an integrated approach combining advantages of individual bio/medical fields and consolidating a multiprofessional collaboration [13].
Acknowledgements
This work has been supported by the following organizations: European Association for Predictive, Preventive, and Personalized Medicine, EPMA, Brussels, Belgium; Slovak Research and Development Agency (under contract no. APVV-16-0021), the Scientific Grant Agency, Ministry of Education, Science, and Research, Slovak Republic; and the VEGA Grant Agency (1/0124/17), Ministry of Education, Science and Research, Slovak Republic
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of informed consent
Patients have not been involved in the study.
Statement of human and animal rights
No experiments have been performed including patients and/or animals.
Footnotes
Pavol Zubor and Peter Kubatka co-first/equal authorship
References
- 1.Golubnitschaja O, Debald M, Yeghiazaryan K, Kuhn W, Pešta M, Costigliola V, Grech G. Breast cancer epidemic in the early 21st century: evaluation of risk factors, cumulative questionnaires and recommendations for preventive measures. Tumor Biol. 2016;37(10):12941–12957. doi: 10.1007/s13277-016-5168-x. [DOI] [PubMed] [Google Scholar]
- 2.Triunfo S, Scambia G. Cancer in pregnancy: diagnosis, treatment and neonatal outcome. Minerva Ginecol. 2014;66(3):325–334. [PubMed] [Google Scholar]
- 3.Lee YY, Roberts CL, Dobbins T, Stavrou E, Black K, Morris J, Young J. Incidence and outcomes of pregnancy-associated cancer in Australia, 1994-2008: a population-based linkage study. BJOG. 2012;119(13):1572–1582. doi: 10.1111/j.1471-0528.2012.03475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polivka J, Altun I, Golubnitschaja O. Pregnancy-associated breast cancer: the risky status quo and new concepts of predictive medicine. EPMA J. 2018;9(1):1–13. doi: 10.1007/s13167-018-0129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansson AL, Andersson TM, Hsieh CC, Cnattingius S, Dickman PW, Lambe M. Family history and risk of pregnancy-associated breast cancer (PABC) Breast Cancer Res Treat. 2015;151(1):209–217. doi: 10.1007/s10549-015-3369-4. [DOI] [PubMed] [Google Scholar]
- 6.Keyser EA, Staat BC, Fausett MB, Shields AD. Pregnancy-associated breast Cancer. Rev Obstet Gynec. 2012;5(2):94–99. [PMC free article] [PubMed] [Google Scholar]
- 7.McGrath SE, Ring A. Chemotherapy for breast cancer in pregnancy: evidence and guidance for oncologists. Ther Adv Med Oncol. 2011;3(2):73–83. doi: 10.1177/1758834010392445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vinatier E, Merlot B, Poncelet E, Collinet P, Vinatier D. Breast Cancer during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2009;147(1):9–14. doi: 10.1016/j.ejogrb.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 9.Gupta PB, Kuperwasser CJ. Contributions of estrogen to ER-negative breast tumor growth. Steroid Biochem Mol Biol. 2006;102(1–5):71–78. doi: 10.1016/j.jsbmb.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 10.John EM, Hines LM, Phipps AI, Koo J, Longacre TA, Ingles SA, Baumgartner KB, Slattery ML, Wu AH. Reproductive history, breast-feeding and risk of triple negative breast cancer: the breast Cancer etiology in minorities (BEM) study. Int J Cancer. 2018;142(11):2273–2285. doi: 10.1002/ijc.31258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azim HA, Jr, Pavlidis N, Peccatori FA. Treatment of the pregnant mother with cancer: a systematic review on the use of cytotoxic, endocrine, targeted agents and immunotherapy during pregnancy. Part II: hematological tumors. Cancer Treat Rev. 2010;36(2):110–121. doi: 10.1016/j.ctrv.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Muñoz-Rodríguez JL, Vrba L, Futscher BW, Hu C, Komenaka IK, Meza-Montenegro MM, Gutierrez-Millan LE, Daneri-Navarro A, Thompson PA, Martinez ME. Differentially expressed microRNAs in postpartum breast cancer in Hispanic women. PLoS One. 2015;10(4):e0124340. doi: 10.1371/journal.pone.0124340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, Krapfenbauer K, Mozaffari M, Costigliola V. Medicine in the early twenty-first century: paradigm and anticipation - EPMA position paper 2016. EPMA J. 2016;7:23. doi: 10.1186/s13167-016-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golubnitschaja O, Costigliola V, Grech G. EPMA World Congress: traditional forum in predictive, preventive and personalised medicine for multi-professional consideration and consolidation. EPMA J. 2017;8(Suppl):1–54. [Google Scholar]
- 15.Yu HH, Cheung PS, Leung RC, Leung TN, Kwan WH. Current management of pregnancy-associated breast cancer. Hong Kong Med J. 2017;23(4):387–394. doi: 10.12809/hkmj166049. [DOI] [PubMed] [Google Scholar]
- 16.Ahn BY, Kim HH, Moon WK, Pisano ED, Kim HS, Cha ES, Kim JS, Oh KK, Park SH. Pregnancy- and-lactation-associated breast cancer: mammographic and sonographic findings. J Ultrasound Med. 2003;22(5):491–497. doi: 10.7863/jum.2003.22.5.491. [DOI] [PubMed] [Google Scholar]
- 17.Theriault RL, Litton JK. Pregnancy during or after breast cancer diagnosis: what do we know and what do we need to know? J Clin Oncol. 2013;31(20):2521. doi: 10.1200/JCO.2013.49.7347. [DOI] [PubMed] [Google Scholar]
- 18.Litton JK, Theriault RL, Gonzalez-Angulo AM. Breast cancer diagnosis during pregnancy. Women's Health (Lond Engl) 2009;5(3):243–249. doi: 10.2217/WHE.09.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanal E, Barkovich AJ, Bell C, Borgstede JP, Bradley WG, Jr, Froelich JW, Gilk T, Gimbel JR, Gosbee J, Kuhni-Kaminski E, Lester JW, Jr, Nyenhuis J, Parag Y, Schaefer DJ, Sebek-Scoumis EA, Weinreb J, Zaremba LA, Wilcox P, Lucey L, Sass N. ACR blue ribbon panel on MR safety. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188(6):1447–1474. doi: 10.2214/AJR.06.1616. [DOI] [PubMed] [Google Scholar]
- 20.Sundgren PC, Leander P. Is administration of gadolinium-based contrast media to pregnant women and small children justified? J Magn Reson Imaging. 2011;34(4):750–757. doi: 10.1002/jmri.22413. [DOI] [PubMed] [Google Scholar]
- 21.Heymann JJ, Halligan AM, Hoda SA, Facey KE, Hoda RS. Fine needle aspiration of breast masses in pregnant and lactating women: experience with 28 cases emphasizing Thinprep findings. Diagn Cytopathol. 2015;43(3):188–194. doi: 10.1002/dc.23197. [DOI] [PubMed] [Google Scholar]
- 22.Mitra S, Dey P. Fine-needle aspiration and core biopsy in the diagnosis of breast lesions: a comparison and review of the literature. Cytojournal. 2016;13:18. doi: 10.4103/1742-6413.189637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nettleton J, Long J, Kuban D, Wu R, Shaefffer J, El-Mahdi A. Breast cancer during pregnancy: quantifying the risk of treatment delay. Obstet Gynecol. 1996;87(3):414–418. doi: 10.1016/0029-7844(95)00470-X. [DOI] [PubMed] [Google Scholar]
- 24.Szecsi PB, Andersen MR, Bjørngaard B, Hedengran KK, Stender S. Cancer antigen 125 after delivery in women with normal pregnancy: a prospective cohort study. Acta Obstet Gynecol Scand. 2014;93(12):1295–1301. doi: 10.1111/aogs.12492. [DOI] [PubMed] [Google Scholar]
- 25.Han SN, Lotgerink A, Gziri MM, Van Calsteren K, Hanssens M, Amant F. Physiologic variations of serum tumor markers in gynecological malignancies during pregnancy: a systematic review. BMC Med. 2012;8(10):86. doi: 10.1186/1741-7015-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore RG, Miller MC, Eklund EE, Lu KH, Bast RC, Jr, Lambert-Messerlian G. Serum levels of the ovarian cancer biomarker HE4 are decreased in pregnancy and increase with age. Am J Obstet Gynecol. 2012;206(4):349.e1–349.e7. doi: 10.1016/j.ajog.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, Galas DJ, Wang K. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56(11):1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jayasinghe Y. Preventive care and evaluation of the adolescent with a breast mass. Semin Plast Surg. 2013;27(1):13–18. doi: 10.1055/s-0033-1343990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bick U. Intensified surveillance for early detection of breast cancer in high-risk patients. Breast Care (Basel) 2015;10(1):13–20. doi: 10.1159/000375390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amir E, Freedman OC, Seruga B, Evans DG. Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst. 2010;102(10):680–691. doi: 10.1093/jnci/djq088. [DOI] [PubMed] [Google Scholar]
- 31.Corsini C, Henouda S, Nejima DB, Bertet H, Toledano A, Boussen H, Habib F, Mouhout A, Gaballah A, Ghazaly HE, Bourgier C, Coupier I, Galibert V, Baudry K, Vilquin P, Biquard L, Rey JM, Belkacemi Y, Ihout P, Khayat D, Picot MC, Bensalem A, Pujol P. Early onset breast cancer: differences in risk factors, tumor phenotype, and genotype between north African and south European women. Breast Cancer Res Treat. 2017;166(2):631–639. doi: 10.1007/s10549-017-4434-y. [DOI] [PubMed] [Google Scholar]
- 32.Zubor P, Gondova A, Polivka J, Kasajova P, Konieczka K, Danko J, Golubnitschaja O. Breast cancer and Flammer syndrome: any symptoms in common for prediction, prevention and personalised medical approach? EPMA J. 2017;8(2):129–140. doi: 10.1007/s13167-017-0089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bubnov R, Polivka J, Zubor P, Konieczka K, Golubnitschaja O. “Pre-metastatic niches” in breast cancer: are they created by or prior to the tumour onset? “Flammer syndrome” relevance to address the question. EPMA J. 2017;8(2):141–157. doi: 10.1007/s13167-017-0092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smokovski I, Risteski M, Polivka J, Zubor P, Konieczka K, Costigliola V, Golubnitschaja O. Postmenopausal breast cancer: European challenge and innovative concepts. EPMA J. 2017;8(2):159–169. doi: 10.1007/s13167-017-0094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson SG, Wilcox M. The benefits and harms of breast cancer screening: an independent review. Br J Cancer. 2013;108(11):2205–2240. doi: 10.1038/bjc.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Girotra S, Yeghiazaryan K, Golubnitschaja O. Potential biomarker panels in overall breast cancer management: advancements by multilevel diagnostics. Per Med. 2016;13:469–484. doi: 10.2217/pme-2016-0020. [DOI] [PubMed] [Google Scholar]
- 37.Maltoni R, Fici P, Amadori D, Gallerani G, Cocchi C, Zoli M, Rocca A, Cecconetto L, Folli S, Scarpi E, Serra P, Fabbri F. Circulating tumor cells in early breast cancer: a connection with vascular invasion. Cancer Lett. 2015;367(1):43–48. doi: 10.1016/j.canlet.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 38.Chan CWH, Law BMH, So WKW, Chow KM, Waye MMY. Novel strategies on personalized medicine for breast Cancer treatment: an update. Int J Mol Sci. 2017;18(11):E2423. doi: 10.3390/ijms18112423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grech G, Zhan X, Yoo BC, Bubnov R, Hagan S, Danesi R, Vittadini G. Desiderio DM. EPMA position paper in cancer: current overview and future perspectives. EPMA J. 2015;6(1):9. doi: 10.1186/s13167-015-0030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cardonick E, Iacobucci A. Use of chemotherapy during human pregnancy. Lancet Oncol. 2004;5(5):283–291. doi: 10.1016/S1470-2045(04)01466-4. [DOI] [PubMed] [Google Scholar]
- 41.Azim HA, Jr, Azim H, Peccatori FA. Treatment of cancer during pregnancy with monoclonal antibodies: a real challenge. Expert Rev Clin Immunol. 2010;6(6):821–826. doi: 10.1586/eci.10.77. [DOI] [PubMed] [Google Scholar]
- 42.Ji YI, Kim KT. Gynecologic malignancy in pregnancy. Obstet Gynecol Sci. 2013;56(5):289–300. doi: 10.5468/ogs.2013.56.5.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moran BJ, Yano H, Al Zahir N, Farquharson M. Conflicting priorities in surgical intervention for cancer in pregnancy. Lancet Oncol. 2007;8(6):536–544. doi: 10.1016/S1470-2045(07)70171-7. [DOI] [PubMed] [Google Scholar]
- 44.Gentilini O, Cremonesi M, Toesca A, Colombo N, Peccatori F, Sironi R, Sangalli C, Rotmensz N, Pedroli G, Viale G, Veronesi P, Galimberti V, Goldhirsch A, Veronesi U, Paganelli G. Sentinel lymph node biopsy in pregnant patients with breast cancer. Eur J Nucl Med Mol Imaging. 2010;37(1):78–83. doi: 10.1007/s00259-009-1217-7. [DOI] [PubMed] [Google Scholar]
- 45.Kuerer HM, Gwyn K, Ames FC, Theriault RL. Conservative surgery and chemotherapy for breast carcinoma during pregnancy. Surgery. 2002;131(1):108–110. doi: 10.1067/msy.2002.115357. [DOI] [PubMed] [Google Scholar]
- 46.Dominici LS, Kuerer HM, Babiera G, Hahn KM, Perkins G, Middleton L, Ramirez MM, Yang W, Hortobagyi GN, Theriault RL, Litton JK. Wound complications from surgery in pregnancy- associated breast cancer (PABC) Breast Dis. 2010;31(1):1–5. doi: 10.3233/BD-2009-0289. [DOI] [PubMed] [Google Scholar]
- 47.Khera SY, Kiluk JV, Hasson DM, Meade TL, Meyers MP, Dupont EL, Berman CG, Cox CE. Pregnancy-associated breast cancer patients can safely undergo lymphatic mapping. Breast J. 2008;14(3):250–254. doi: 10.1111/j.1524-4741.2008.00570.x. [DOI] [PubMed] [Google Scholar]
- 48.Amant F, Halaska MJ, Fumagalli M, Dahl Steffensen K, Lok C, Van Calsteren K, Han SN, Mir O, Fruscio R, Uzan C, Maxwell C, Dekrem J, Strauven G, Mhallem Gziri M, Kesic V, Berveiller P, van den Heuvel F, Ottevanger PB, Vergote I, Lishner M, Morice P, Nulman I. ESGO task force ‘Cancer in pregnancy’. Gynecologic cancers in pregnancy: guidelines of a second international consensus meeting. Int J Gynecol Cancer. 2014;24(3):394–403. doi: 10.1097/IGC.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 49.Greskovich JF, Jr, Macklis RM. Radiation therapy in pregnancy: risk calculation and risk minimization. Semin Oncol. 2000;27(6):633–645. [PubMed] [Google Scholar]
- 50.Rovera F, Chiappa C, Coglitore A, Baratelli GM, Fachinetti A, Marelli M, Frattini F, Lavazza M, Bascialla L, Rausei S, Boni L, Corben AD, Dionigi G, Dionigi R. Management of breast cancer during pregnancy. Int J Surg. 2013;11(Suppl 1):S64–S68. doi: 10.1016/S1743-9191(13)60020-5. [DOI] [PubMed] [Google Scholar]
- 51.Amant F, Deckers S, Van Calsteren K, Loibl S, Halaska M, Brepoels L, Beijnen J, Cardoso F, Gentilini O, Lagae L, Mir O, Neven P, Ottevanger N, Pans S, Peccatori F, Rouzier R, Senn HJ, Struikmans H, Christiaens MR, Cameron D, Du Bois A. Breast cancer in pregnancy: recommendations of an international consensus meeting. Eur J Cancer. 2010;46(18):3158–3168. doi: 10.1016/j.ejca.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 52.Cardonick E. Pregnancy-associated breast cancer: optimal treatment options. Int J Womens Health. 2014;6:935–943. doi: 10.2147/IJWH.S52381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hershman DL, Wang X, McBride R, Jacobson JS, Grann VR, Neugut AI. Delay in initiating adjuvant radiotherapy following breast conservation surgery and its impact on survival. Int J Radiat Oncol Biol Phys. 2006;65(5):1353–1360. doi: 10.1016/j.ijrobp.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 54.Chen Z, King W, Pearcey R, Kerba M, Mackillop WJ. The relationship between waiting time for radiotherapy and clinical outcomes: a systematic review of the literature. Radiother Oncol. 2008;87(1):3–16. doi: 10.1016/j.radonc.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 55.Espie M, Cuvier C. Treating breast cancer during pregnancy – what can be taken safely. Drug Saf. 1998;2(18):135–142. doi: 10.2165/00002018-199818020-00005. [DOI] [PubMed] [Google Scholar]
- 56.Han SN, Van Calsteren K, Heyns L, Mhallem Gziri M, Amant F. Breast cancer during pregnancy: a literature review. Minerva Ginecol. 2010;62(6):585–597. [PubMed] [Google Scholar]
- 57.Levy S, Fayez I, Taguchi N, Han JY, Aiello J, Matsui D, Moretti M, Koren G, Ito S. Pregnancy outcome following in utero exposure to bisphosphonates. Bone. 2009;44(3):428–430. doi: 10.1016/j.bone.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 58.Padmagirison R, Gajjar K, Spencer C. Management of breast cancer during pregnancy. Obstet Gynaecol. 2010;12:186–192. [Google Scholar]
- 59.de Haan J, Verheecke M, Van Calsteren K, Van Calster B, Shmakov RG, Mhallem Gziri M, Halaska MJ, Fruscio R, Lok CAR, Boere IA, Zola P, Ottevanger PB, de Groot CJM, Peccatori FA, Dahl Steffensen K, Cardonick EH, Polushkina E, Rob L, Ceppi L, Sukhikh GT, Han SN, Amant F, International Network on Cancer and Infertility Pregnancy (INCIP) Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: a 20-year international cohort study of 1170 patients. Lancet Oncol. 2018;19(3):337–346. doi: 10.1016/S1470-2045(18)30059-7. [DOI] [PubMed] [Google Scholar]
- 60.Azim HA, Jr, Del Mastro L, Scargone G, Peccatori FA. Treatment of breast cancer during pregnancy: regimen selection, pregnancy monitoring and more…. Breast. 2011;20(1):1–6. doi: 10.1016/j.breast.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 61.Krishna I, Lindsay M. Breast cancer in pregnancy. Obstet Gynecol Clin North Am. 2013;40(3):559–571. doi: 10.1016/j.ogc.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 62.Framarino-Dei-Malatesta M, Sammartino P, Napoli A. Does anthracycline-based chemotherapy in pregnant women with cancer offer safe cardiac and neurodevelopmental outcomes for the developing fetus? BMC Cancer. 2017;17(1):777. doi: 10.1186/s12885-017-3772-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mir O, Berveiller P. Increased evidence for use of chemotherapy in pregnancy. Lancet Oncol. 2012;13(9):852–854. doi: 10.1016/S1470-2045(12)70331-5. [DOI] [PubMed] [Google Scholar]
- 64.Zagouri F, Sergentanis TN, Chrysikos D, Dimitrakakis C, Tsigginou A, Zografos CG, Dimopoulos MA, Papadimitriou CA. Taxanes for breast cancer during pregnancy: a systematic review. Clin Breast Cancer. 2013;13(1):16–23. doi: 10.1016/j.clbc.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 65.Zagouri F, Dimitrakakis C, Marinopoulos S, Tsigginou A, Dimopoulos MA. Cancer in pregnancy: disentangling treatment modalities. ESMO Open. 2016;1(3):e000016. doi: 10.1136/esmoopen-2015-000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Negro A, Brar BK, Gu Y, Peterson KL, Vale W, Lee KF. ErbB2 is required for G protein-coupled receptor signaling in the heart. Proc Natl Acad Sci U S A. 2006;103(43):15889–15893. doi: 10.1073/pnas.0607499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goodyer MJ, Ismail JR, O’Reilly SP, Moylan EJ, Ryan CA, Hughes PA, O'Connor A. Safety of trastuzumab (Herceptin) during pregnancy: two case reports. Cases J. 2009;2:9329. doi: 10.1186/1757-1626-2-9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pant S, Landon MB, Blumenfeld M, Farrar W, Shapiro CL. Treatment of breast cancer with trastuzumab during pregnancy. J Clin Oncol. 2008;26(9):1567–1569. doi: 10.1200/JCO.2008.16.0309. [DOI] [PubMed] [Google Scholar]
- 69.Pirvulescu C, Mau C, Schultz H, Sperfeld A, Isbruch A, Renner-Lützkendorf H, Loibl S, Freitag U, Klühs G, Fleige B, Untch M. Breast Cancer during pregnancy: an interdisciplinary approach in our institution. Breast Care (Basel) 2012;7(4):311–314. doi: 10.1159/000341383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.El-Safadi S, Wuesten O, Muenstedt K. Primary diagnosis of metastatic breast cancer in the third trimester of pregnancy: a case report and review of the literature. J Obstet Gynaecol Res. 2012;38(3):589–592. doi: 10.1111/j.1447-0756.2011.01745.x. [DOI] [PubMed] [Google Scholar]
- 71.Mandrawa CL, Stewart J, Fabinyi GC, Walker SP. A case study of trastuzumab treatment for metastatic breast cancer in pregnancy: fetal risks and management of cerebral metastases. Aust N Z J Obstet Gynaecol. 2011;51(4):372–376. doi: 10.1111/j.1479-828X.2011.01314.x. [DOI] [PubMed] [Google Scholar]
- 72.Amant F, von Minckwitz G, Han SN, Bontenbal M, Ring AE, Giermek J, Wildiers H, Fehm T, Linn SC, Schlehe B, Neven P, Westenend PJ, Müller V, Van Calsteren K, Rack B, Nekljudova V, Harbeck N, Untch M, Witteveen PO, Schwedler K, Thomssen C, Van Calster B, Loibl S. Prognosis of women with primary breast cancer diagnosed during pregnancy: results from an international collaborative study. J Clin Oncol. 2013;31(20):2532–2539. doi: 10.1200/JCO.2012.45.6335. [DOI] [PubMed] [Google Scholar]
- 73.Pauli SA, Tang H, Wang J, Bohlen P, Posser R, Hartman T, Sauer MV, Kitajewski J, Zimmermann RC. The vascular endothelial growth factor (VEGF)/VEGF receptor 2 pathway is critical for blood vessel survival in corpora lutea of pregnancy in the rodent. Endocrinology. 2005;146(3):1301–1311. doi: 10.1210/en.2004-0765. [DOI] [PubMed] [Google Scholar]
- 74.Demir R, Kayisli UA, Seval Y, Celik-Ozenci C, Korgun ET, Demir-Weusten AY, Huppertz B. Sequential expression of VEGF and its receptors in human placental villi during very early pregnancy: differences between placental vasculogenesis and angiogenesis. Placenta. 2004;25(6):560–572. doi: 10.1016/j.placenta.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 75.Chen HX, Cleck JN. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev. 2009;6(8):465–477. doi: 10.1038/nrclinonc.2009.94. [DOI] [PubMed] [Google Scholar]
- 76.Berkane N. Gestational hypertensions: definitions and consequences in outcome of pregnancy. Ann Fr Anesth Reanim. 2010;29(3):e1–e6. doi: 10.1016/j.annfar.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 77.Ishizuka S, Satou S. A case of delivery of healthy infant in breast cancer patient incidentally treated with goserelin acetate and tamoxifen during pregnancy. Breast Cancer. 2016;23(1):164–166. doi: 10.1007/s12282-013-0469-z. [DOI] [PubMed] [Google Scholar]
- 78.RCOG Green-top guideline No. 12. Pregnancy and breast cancer. UK royal college of obstetricians and Gynaecologists; 2011.
- 79.Bahrami A, Aledavood A, Anvari K, Hassanian SM, Maftouh M, Yaghobzade A, Salarzaee O, ShahidSales S, Avan A. The prognostic and therapeutic application of microRNAs in breast cancer: tissue and circulating microRNAs. J Cell Physiol. 2018;233(2):774–786. doi: 10.1002/jcp.25813. [DOI] [PubMed] [Google Scholar]
- 80.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6(4):259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 81.Walter BA, Gómez-Macias G, Valera VA, Sobel M, Merino MJ. miR-21 expression in pregnancy-associated breast Cancer: a possible marker of poor prognosis. J Cancer. 2011;2:67–75. doi: 10.7150/jca.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eedarapalli P, Jain S. Breast cancer in pregnancy. J Obstet Gynaecol. 2009;26(1):1–4. doi: 10.1080/01443610500363808. [DOI] [PubMed] [Google Scholar]
- 83.Ha JF, Longnecker N. Doctor-patient communication: a review. Ochsner J. 2010;10(1):38–43. [PMC free article] [PubMed] [Google Scholar]
- 84.Perry S, Kowalski TL, Chang CH. Quality of life assessment in women with breast cancer, benefits, acceptability and utilization. Health Qual Life Outcomes. 2007;5:24. doi: 10.1186/1477-7525-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Avis NE, Crawford S, Manuel J. Quality of life among younger women with breast cancer. J Clin Oncol. 2005;23(15):3322–3330. doi: 10.1200/JCO.2005.05.130. [DOI] [PubMed] [Google Scholar]
- 86.Rodsten JM Understanding the experience of pregnancy-associated breast Cancer - an interpretative phenomenological analysis. In: DCounsPsych, University of the West of England. 2017. http://eprints.uwe.ac.uk/30561. Accessed 26 Jul 2017.
- 87.Hayes SC, Hofmann SG. The third wave of CBT and the rise of process-based care. World Psychiatry. 2017;16(3):245–246. doi: 10.1002/wps.20442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.David D, Cristea I, Hofmann SG. Why cognitive behavioral therapy is the current gold standard of psychotherapy. Front Psychiatry. 2018;9:4. doi: 10.3389/fpsyt.2018.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hofmann SG, Asnaani A, Vonk IJJ, Sawyer AT, Fang A. The efficacy of cognitive behavioral therapy: a review of meta-analyses. Cogn Ther Res. 2012;36(5):427–440. doi: 10.1007/s10608-012-9476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hofmann SG, Asmundson GJ, Beck AT. The science of cognitive therapy. Behav Ther. 2013;44(2):199–212. doi: 10.1016/j.beth.2009.01.007. [DOI] [PubMed] [Google Scholar]