Abstract
Background
Chronic stress is associated with suboptimal health status (SHS) which is a new public health challenge in China and worldwide. Plasma stress hormones may act as potential objective biomarkers for SHS measure. This study was aimed to evaluate the diagnostic performance of plasma cortisol, catecholamine adrenaline/noradrenaline, and SHS questionnaires (SHSQ) for SHS using latent class analysis (LCA) in the absence of a gold standard.
Methods
A cross-sectional study was conducted among 868 employees in Beijing. The SHS questionnaires-25 (SHSQ-25) was distributed, and plasma cortisol, adrenaline, and noradrenaline were measured in the survey. LCA was used to assess the performance of both subjective and objective measures for SHS recognition.
Results
Akaike information criterion (AIC) and consistent AIC (CAIC) was 14.11 and 54.48 respectively, indicating that the model was well fitted. The sensitivity and specificity of plasma cortisol were 0.836 (95% CI 0.811–0.861) and 0.840 (95% CI 0.816–0.864), respectively. The area under curve (AUC) of receiver operating characteristic (ROC) of SHSQ-25 was 0.743 (95% CI 0.709–777), while the AUC of plasma adrenaline was 0.688 (95% CI 0.651–0.725). The prevalence of SHS in the investigated population was 34.78%.
Conclusion
Plasma cortisol is a valuable biomarker for SHS detection, whereas SHSQ-25 is more suitable for SHS screening in the population-based health survey. The accuracy and applicability of plasma adrenaline are inferior to cortisol and SHSQ-25, respectively. LCA has merit to evaluate performance of plasma cortisol, catecholamines, and SHSQ-25 for recognition of SHS in the absence of a gold standard test.
Keywords: Suboptimal health status, Cortisol, Catecholamine, Latent class analysis, Early recognition, Prediction
Introduction
In contemporary societies, there has been an increase in the number of people who suffer from suboptimal health status (SHS) which is characterized by functional somatic syndromes and absence of a definitive diagnosis [1]. Accumulating evidences indicated associations between psychosocial stress and a wide range of diseases including mental disorders [2, 3], diabetes [4, 5], and cardiovascular disease [6, 7]. Based on the perceived health complaints affected by chronic stress, we have established a questionnaire for measuring SHS (suboptimal health status questionnaire-25, SHSQ-25) [8]. The SHSQ-25 includes 25 items covering five dimensions: fatigue, the cardiovascular system, the digestive tract, the immune system, and mental status [8]. A preliminary diagnostic criterion for SHS (SHSQ-25 score ≥ 35) was also advanced [9, 10]. As a subjective health measure, SHSQ-25 has been recognized internationally and applied to the health survey among African, Chinese, and Caucasian ethnic groups [11–14]. However, in order to better understand the etiology and progress of SHS, it is necessary that an objective criterion for SHS should be identified.
Our previous study confirmed the association between chronic psychosocial stress and SHS [12]. Persistent psychological stress can affect health through neuroendocrine pathways including hypothalamus-pituitary-adrenal (HPA) axis and sympathetic nervous system (SNS). The catecholamine (adrenaline, noradrenalin, and dopamine), produced by the SNS, and cortisol, produced by the HPA axis, are the major stress hormones and important mediators between psychological stress and several chronic diseases including impaired glucose and lipid metabolism and hypertension [15, 16]. Cortisol, adrenaline, and noradrenaline are important allostatic load biomarkers of chronic stress [17]. Salivary cortisol has also been increasingly utilized in the field of stress hormone research as a measure of HPA axis activation, particularly in the setting of psychological stress [18, 19].
Although the stress hormones appear to be the potential biomarkers for SHS, there yet is no generally accepted “gold standard” test to definitively categorize individuals as true SHS (SHS positive) or true health status (SHS negative). Latent class analysis (LCA) is a statistical technique commonly used to compare the performance of diagnostic tests in the absence of a gold standard test [20]. LCA uses available information to estimate potential variables that are difficult to observe directly and accurately, and to analyze potential structural relationships among potential variables [20]. LCA estimates the accuracy (sensitivity and specificity) of each test and the proportion of individuals truly positive in the population based on the observed frequency of the possible combinations of test results. In this study, we applied LCA to assess the performances of objective measurement of plasma adrenaline, noradrenalin, and cortisol, along with the subjective evaluation by SHSQ-25 for diagnosis of SHS, and to estimate the prevalence of SHS.
Materials and methods
Participants
A total of 868 participants (55.3% women) who took routine health medical examination were recruited from Health Management Center, Xuanwu Hospital, Capital Medical University. All participants were employees from companies in Xuanwu district and aged from 18 to 60 years old. The exclusion criteria were as follows: (1) suffering from major pathologies such as cardiovascular and cerebrovascular diseases; liver, kidney, and metabolic diseases; and tumors; (2) medicine consumption in the past 2 weeks; (3) trauma or acute diseases; and (4) psychiatric disorders.
This study was approved by the Ethical Committee of Capital Medical University (2015SY27), Beijing, and each participant signed an informed consent form.
Data collection
Information about demographic data, medical history, and current medication were collected by a structured questionnaire [21]. SHS was measured by the SHSQ-25, a self-reported questionnaire validated in various populations [8, 11–14, 22]. Each participant was asked to rate a specific statement on a five-point Likert-type scale, based on how often they suffered various specific complaints in the preceding 3 months: (1) never or almost never, (2) occasionally, (3) often, (4) very often, and (5) always. The score of each statement was recoded as 0 to 4. SHS scores were calculated by summing the ratings of the 25 items. SHS score ≥ 35 represents SHS and < 35 represented an ideal health status [9].
Plasma collection
Following a 10-h overnight fast, peripheral blood (5 ml) was collected using EDTA-containing tubes between 7:30 and 8:30 a.m. after a 0.5- to 1-h period in health examination area to allow acclimatization to the environment and staff. All blood samples were drawn from an antecubital vein with each participant seated. Samples were immediately centrifuged at 3000 r/s for 15 min at 4 °C, and plasma was preserved at − 80 °C until further analysis. Plasma cortisol, adrenaline, and noradrenaline were measured by commercial radioimmunoassays (RIA) (Beijing Sino-uk Institute of Biological Technology, China) using with a γ counter (XH-6020, North Institute of Bio-Tech, China) [5, 23]. The coefficient of variation (CV) of these assays were < 6.0% for the intra-assay and < 10.0% for the inter-assay, respectively. We measured the plasma stress hormones because it is convenient to obtain blood samples during routine physical examination. The RIA method is simple in operation and cheaper than high-pressure liquid chromatography (HPLC) method and is more suitable for large-scale investigation. The reference range of cortisol, adrenaline, and noradrenaline were 50~280 ng/ml (1382–7739.2 nmol/L), 50–200 pg/ml (272.3–1092 pmol/L), and 100–570 pg/ml (591–3369 pmol/L) respectively. Strict quality controls were applied throughout all these assays.
Statistical analysis
The latent class model was used to assess the performance of plasma adrenaline/noradrenaline, cortisol, and SHSQ-25 for diagnosis of SHS. LCA was performed using a statistical model to define a latent variable that could be referred as a gold standard. LCA seeks to stratify the cross-classification table of observed (manifest) variables by an unobserved (latent) unordered categorical variable that eliminates all confounding between the manifest variables [20, 24]. The latent classes were estimated and characterized using two parameters: item-response probabilities and class prevalence, which is the probability of belonging to a latent class.
Supposing that the diagnosis of SHS by SHSQ-25, plasma cortisol, and adrenaline was initially recognized as the manifest variables A, B, and C, and the corresponding values were coded as i, j, and k, respectively. X was used to denote the latent variable (true SHS), and its classification was expressed as t. The formula for posterior probability P(A|X) that each individual response to manifest variable A, belongs to, conditional on latent class of true SHS,
In which, denotes the probability of an observation i belonging to a specific latent class t. The sensitivity (Se) and specificity (Sp) of the variable A can be calculated as [25]:
Similarly, the sensitivity and specificity of variables B and C can be obtained. The estimate was performed using “proc (proceed) LCA” process in SAS 9.3 with the maximum likelihood (expectation-maximization, EM) algorithm [26].
Results
Participant characteristics
This study obtained completed data of 868 participants including questionnaires and laboratory tests. Table 1 shows the characteristics of participants. The average age of the total participants was 44.3 years (SD, 7.8), and 55.3% were women. Most of them had a university degree (76.1%) and were administrative workers (85.3%).
Table 1.
Demographic characteristics of participants (n = 868)
| Variable | Number | Percent |
|---|---|---|
| Gender | ||
| Female | 480 | 55.3 |
| Male | 388 | 44.7 |
| Age | ||
| 18–30 years | 49 | 5.6 |
| 31–40 years | 203 | 23.4 |
| 41–50 years | 424 | 48.8 |
| 51–60 years | 192 | 22.1 |
| Highest education level | ||
| Compulsory school (through grade 9) | 31 | 3.6 |
| High school graduation | 142 | 16.4 |
| University/college degree | 695 | 80.1 |
| Occupation | ||
| Administrative worker | 754 | 86.9 |
| Blue-collar worker | 114 | 13.1 |
Determination of the initial classification of each variable
The LCA model is commonly used to describe the relationship among a group of categorical variables. In this study, the measurements of the four variables (SHSQ-25 score, cortisol, adrenaline, and noradrenaline) are quantitative data. Before using the LCA model, all the subjects should be divided into two categories according to the four variables respectively.
In our previous study, based on the five major risk factors for SHS, the lower risk reference for SHS was screened and identified [9]. These factors included “competition in work” (never/almost never or occasionally), “receive work beyond ability” (never/almost never or occasionally), “physical exercise” (yes), “there is a friend to confabulate with when you are in a bad mood” (often or always), and “you can have a short rest during work” (often or always). The 90% quantiles upper level of the SHSQ-25 score among lower risk population was set as the cutoff point for SHS [9].
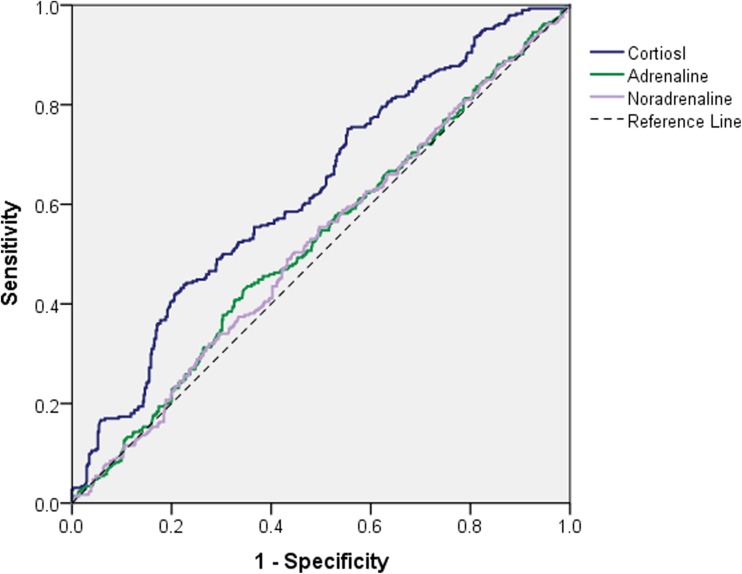
The plasma concentrations of stress hormones of the participants were listed in Table 2. In the current study, the initial classification of SHS according to cortisol and adrenaline/noradrenaline were determined using receiver operating characteristic (ROC) curve with SHSQ-25 score ≥ 35 as the diagnosis criteria (Fig. 1). The respective cutoff values were 173.0 ng/ml (4781.7 nmol/L) for cortisol (AUC = 0.629, 95% CI 0.590–0.668), 134.0 pg/ml (731.4 pmol/L, AUC = 0.524, 95% CI 0.483–0.565) for adrenaline, and 375.0 pg/ml (2216.6 pmol/L, AUC = 0.518, 95% CI 0.477–0.559) for noradrenaline (Fig. 1). Since the concentrations of plasma adrenaline and noradrenaline were closely correlated (r = 0.959, P < 0.001), only adrenaline was used in further analysis. The original data were converted into a cross-classification table of the manifest variables (Table 3).
Table 2.
Distribution of SHSQ-25 and stress hormones in study participants
| Variables | Total subjects | Health groupa | SHS groupa | |
|---|---|---|---|---|
| Means ± SD | Median (P25–P75) | Means ± SD | Means ± SD | |
| SHSQ-25 | 29.74 ± 13.60 | 29 (21−38) | 25.89 ± 12.48 | 37.09 ± 12.60* |
| Cortisol (ng/ml) | 176.91 ± 35.33 | 170 (155.0 −196.24) | 161.80 ± 7.79 | 205.80 ± 29.82* |
| A (pg/ml) | 137.21 ± 48.07 | 133.79 (99.9 −171.87) | 126.76 ± 5.58 | 157.21 ± 46.41* |
| NA (pg/ml) | 380.54 ± 91.93 | 373.76 (311.90−444.96) | 361.82 ± 88.19 | 416.35 ± 88.40* |
A adrenaline, NA noradrenaline, P percentile, SHS suboptimal health status. aClassification of SHS group and health group was based on latent class analysis, *P < 0.001
Fig. 1.
Receiver operating characteristic (ROC) curve analysis for plasma cortisol, adrenaline, and noradrenaline with SHSQ-25 score ≥ 35 as the initial diagnosis criteria for suboptimal health status (SHS)
Table 3.
Initial cross-classification table of SHS by SHSQ-25, plasma cortisol, and adrenaline
| SHSQ-25 | Cortisol | Adrenaline | Total | ||
|---|---|---|---|---|---|
| − | + | − | + | ||
| − | 34 | 233 | 297 | 277 | 574 |
| + | 114 | 180 | 140 | 154 | 294 |
| Total | 455 | 413 | 437 | 431 | 868 |
− negative (lower than the cutoff value), + positive (higher than the cutoff value)
LCA model fitting and parameter estimation
Beginning with arbitrary initial values, the EM algorithm was proceeded iteratively assuming that the number of sub-classes of the latent variable was 2 (SHS and health status). In the maximization step, parameter estimates including conditional probability of each variable and probability of latent class were updated by maximizing the log-likelihood function. Preferred models are those that can minimize values of Akaike information criterion (AIC). The final AIC was 14.11 and consistent AIC (CAIC) was 54.48, indicating that the model was well fitted. Like AIC, those statistics of posterior probability were outputted automatically after running “proc LCA” process [24, 26].
After parameterization of the model, combined with the latent class conditional probability of SHSQ-25, cortisol, and adrenaline, the latent class 1 was defined as the healthy status (class 1) and 2 was defined as the SHS (class 2). Identification of SHS was according to a high posterior probability of SHSQ-25 (0.5840), cortisol (0.8261), and adrenaline (0.5745) (Table 4). Finally, each participant was categorized into a specific subgroup according to the higher posterior probability of each latent class (Table 4). Of the 868 subjects in the present study, 298 were defined as SHS and the other 570 were in the health status.
Table 4.
Conditional probability of SHS response to SHSQ-25, plasma cortisol, and adrenaline
| Variables | Negative/positive | Class 1 | Class 2 |
|---|---|---|---|
| SHSQ-25 | − | 0.7922 | 0.4160 |
| + | 0.2078 | 0.5840 | |
| Cortisol | − | 0.7111 | 0.1739 |
| + | 0.2889 | 0.8261 | |
| Adrenaline | − | 0.5451 | 0.4255 |
| + | 0.4549 | 0.5745 | |
| Probability of latent class | 0.6522 | 0.3478 |
Class 1 healthy state, class 2 suboptimal health status
The diagnostic accuracy of the variables
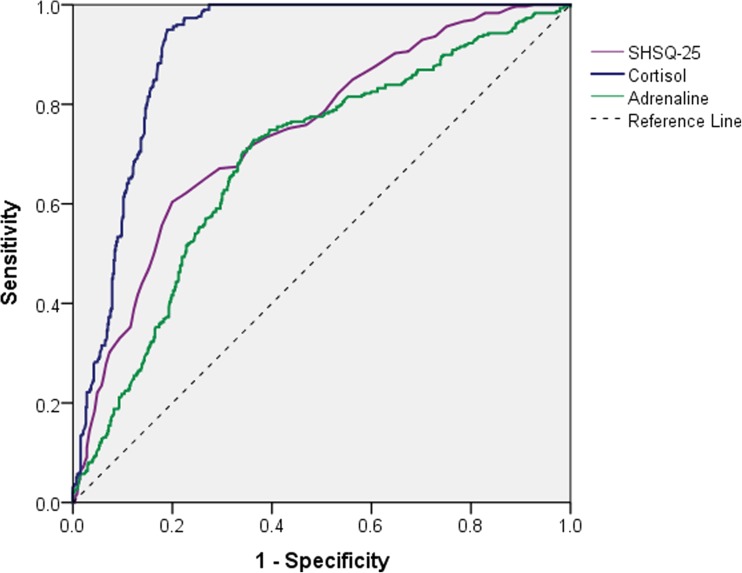
Based on the model fitting, the diagnostic accuracy of SHSQ-25, cortisol, and adrenaline were also estimated (Table 5 and Fig. 2). In the surveyed population, the prevalence of SHS was 34.78% (95% CI 31.61–37.95%). Among the three variables, the performance of plasma cortisol was the best, with the sensitivity and specificity were 0.836 (95% CI 0.811–0.861) and 0.840 (95% CI 0.816–0.864), respectively, indicating that cortisol is a suitable biomarker for recognizing SHS. The cutoff value of plasma cortisol was 180 ng/ml (4975.2 nmol/L) for identifying SHS. The AUC of SHSQ-25 is 0.743 (95% CI 0.709–0.777) with moderate sensitivity and specificity, and the cutoff value of the SHSQ-25 score was 30. The SHSQ-25 is more suitable for screening SHS than plasma cortisol and adrenaline from the perspective of cost-effectiveness at a large population-based survey. The AUC of plasma adrenaline was 0.688 (95% CI 0.651–0.725), indicating its accuracy and applicability are inferior to cortisol and SHSQ-25, respectively. The average levels of SHSQ-25 and plasma hormones including cortisol, adrenaline, and noradrenaline were significantly different between health group and SHS group (Table 2).
Table 5.
Estimated performance of SHSQ-25, plasma cortisol, and plasma adrenaline for recognition of suboptimal health status based on latent class analysis
| Variable | AUC (95% CI) | Se (95% CI) | Sp (95% CI) | Cutoff value |
|---|---|---|---|---|
| SHSQ | 0.743 (0.709, 0.777) |
71.5% (68.5, 74.5) |
64.6% (61.4, 67.7) |
30 |
| Cortisol | 0.905 (0.885, 0.925) |
83.6 (81.1, 86.1) |
84.0 (81.6, 86.4) |
180 ng/ml 4975.2 nmol/L |
| Adrenaline | 0.688 (0.651, 0.725) |
71.8 (68.8, 74.8) |
64.0 (60.9, 67.2) |
136.0 pg/ml 742.3 pmol/L |
Se sensitivity, Sp specificity
Fig. 2.
ROC plot for the estimated performance of SHSQ-25, plasma cortisol, and plasma adrenaline for recognition of SHS based on latent class analysis
Discussion
It is generally believed that SHS is an early stage of chronic diseases, and a condition can be reversible, which provides a potential in term of predictive and preventive medicine [27]. The development of SHS reflects the concept that chronic diseases can be effectively prevented from occurrence and development. Reasonable evaluation and effective intervention measures can slow down or block the transformation from SHS to chronic diseases. Simple but reliable methods for identification of the individuals with SHS are of great importance for early prediction and reversal of unfavorable health state by prompt application of the particular health-supporting activities or procedures. In previous studies, based on the influence of chronic stress on main organs and systems of human body, perceived discomfort was measured by SHSQ-25 [7, 11–14, 22].
Under stress condition, cells of the paraventricular nucleus of the hypothalamus are activated to produce corticotropin-releasing factor (CRF), the major coordinator of the stress response. CRF acts on the anterior pituitary to promote the secretion of adrenocorticotropic hormone (ACTH), which in turn acts on the inner adrenal to initiate the synthesis and release of glucocorticoid (GC) hormones (cortisol in humans) [28]. Cortisol has strong effects in energy metabolism and immune inhibition and plays a role in the coordination of emotion and cognition [29]. At the same time, the sympathetic nervous system is activated to release catecholamines which promote glycogen and fat decomposition and blood flow redistribution [29]. These reactions are important protectors of the body in the short run. Yet, when the stress is repeated over a period of weeks, chronically elevated cortisol can cause damage and accelerate the process of disease [3–6, 29]. To date, SHS has been proved to be associated with increased blood pressure, plasma glucose, and hyperlipoidemia and contributes to the development of cardiovascular disease [11, 13, 30].
Investigation in an occupational population showed that significantly higher levels of plasma cortisol and decreased mRNA expression of glucocorticoid receptor (GRα) were observed among the high SHS score group [12], indicating that reducing excessive responses of cells to cortisol stress is an important way of intervention on SHS. Given the evidences, we assumed that plasma cortisol may be an important biomarker of SHS from the perspective of predictive and preventive medicine. Salivary cortisol has been used increasingly as a biomarker of stress in a research setting, especially in studies examining psychological stress with repeated measurements [19]. We measured the plasma stress hormones because it is convenient to obtain blood samples during routine physical examination especially for follow-up studies.
LCA is a statistical method combining latent variable theory with classification variables. Its merit is to use the latent class to explain complex associations between a number of manifest categorical variables. LCA determines the least number of latent classes with manifest variables which are assumed to be statistically independent in a given data set [24, 25]. Typical LCA assumes that the observed data obtained by the investigators belongs to a certain level of a latent class variable x, and all levels are mutually exclusive and independent.
There are two parameters estimated by LCA, including probability of observations in each latent class and the conditional probabilities of each individual response to each manifest variable, conditional on latent class. Given the parameters, the posterior probability of individual belonging to each class can be calculated according to observed values of the manifest variables.
In this study, we applied LCA to classify SHS with multivariate data including measures of questionnaire and stress hormones. Based on the sensitivity and specificity obtained in this current study, the diagnostic performance of plasma cortisol is the best when compared with SHSQ-25 and plasma adrenaline, suggesting that the cortisol is a potential valuable biomarker for diagnosis of SHS. The cutoff value of plasma cortisol is 180 ng/ml (4975.2 nmol/L), which is higher than the initial cutoff value (173 ng/ml). Both sensitivity and specificity of SHSQ-25 are lower than those of cortisol, indicating it is more suitable for screening rather than diagnosing of SHS. The cutoff value of SHSQ-25 score is 30, which is lower than the initial cutoff value (35).
Early recognizing SHS by using SHSQ-25 and plasma cortisol are of economic advantage and operational applicability in the wide population. However, there are several limitations in our study. A single-point determination of stress hormones was not sufficient to be considered a definitive measure of HPA axis and SNS activity. Secondly, the measurement accuracy of plasma catecholamines might be influenced by some lifestyle factors such as exercise, smoking, and caffeine/alcohol using. Additionally, both acute and chronic stress has been shown to be linked to “dry mouth” syndrome (estimated by Dodds xerostomia questionnaire) and other symptoms and biomarkers [31]. “Multi-level diagnostics” may improve individualized prediction and medical services in the context of personalized prediction and prevention medicine.
Conclusion
By using LCA, we identified that plasma cortisol is a valuable biomarker for SHS detection, whereas SHSQ-25 is more suitable for SHS screening in the population-based health survey. Future studies are needed for the identification of other possible objective tests, besides cortisol (such as metabonomics, proteomics, and transcriptomics), in order to find “the gold standard” of SHS. Further, optimal mathematical methods and models for data analysis and data mining might be necessary in order to empower the methods of prediction and early diagnostics, especially for personalized prediction.
Acknowledgements
This study was supported by the National Natural Science Foundation (81102208, 81573214), the Beijing Municipal Natural Science Foundation (7162020), and the Scientific Research Project of Beijing Municipal Educational Committee (KM201510025006).
Abbreviations
- ACTH
adrenocorticotropic hormone
- AIC
Akaike information criterion
- CAIC
consistent Akaike information criterion
- CRF
corticotropin-releasing factor
- GC
glucocorticoid
- HPA
hypothalamus-pituitary-adrenal
- LCA
latent class analysis
- PPPM
predictive, preventive and personalized medicine
- ROC
receiver operating characteristic
- SHS
suboptimal health status
- SHSQ-25
suboptimal health status questionnaire-25
- SNS
sympathetic nervous system
Author contributions
Yu-Xiang Yan and Wei Wang designed the study; Jing Dong and Shuo Wang collected the data; Yu-Xiang Yan and Li-Juan Wu conducted the experiments’ statistical analyses. Yu-Xiang Yan and Huan-Bo Xiao conducted the experiments. All authors interpreted the data, and all authors contributed to writing. All authors have approved the final manuscript.
Compliance with ethical standards
The study was approved by the Ethical Committee of Capital Medical University and was conducted in accordance with Good Clinical Practice within the tenets of the Declaration of Helsinki. Each participant was required to sign an informed consent form before being enrolled in the study and prior to any measurements being taken.
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Yan YX, Wang W. Advances in research of suboptimal health status. Chin J Public Health (in Chin) 2008;24:1037–1038. [Google Scholar]
- 2.Chen SW. Job stress models, depressive disorders and work performance of engineers in microelectronics industry. Int Arch Occup Environ Health. 2011;84:91–103. doi: 10.1007/s00420-010-0538-y. [DOI] [PubMed] [Google Scholar]
- 3.Grynderup MB, Mors O, Hansen ÅM, Andersen JH, Bonde JP, Kærgaard A, Kærlev L, Mikkelsen S, Rugulies R, Thomsen JF, Kolstad HA. Work-unit measures of organisational justice and risk of depression: a 2-year cohort study. Occup Environ Med. 2013;70:380–385. doi: 10.1136/oemed-2012-101000. [DOI] [PubMed] [Google Scholar]
- 4.Pranita A, Balsubramaniyan B, Phadke AV, Tambe DB, Apte GM, Kharche JS, Godbole G, Joshi AR. Association of occupational & prediabetes statuses with obesity in middle aged women. J Clin Diagn Res. 2013;7:1311–1313. doi: 10.7860/JCDR/2013/5466.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang YZ, Dong J, Zhang J, Wang S, He Y, Yan YX. Identification of neuroendocrine stress response-related circulating microRNAs as biomarkers for type 2 diabetes mellitus and insulin resistance. Front Endocrinol. 2018;9:132. doi: 10.3389/fendo.2018.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du CL. Workplace justice and psychosocial work hazards in association with return to work in male workers with coronary heart diseases: a prospective study. Int J Cardiol. 2013;166:745–747. doi: 10.1016/j.ijcard.2012.09.176. [DOI] [PubMed] [Google Scholar]
- 7.Park J, Kim Y, Cheng Y, Horie S. A comparison of the recognition of overwork-related cardiovascular disease in Japan, Korea, and Taiwan. Ind Health. 2012;50:17–23. doi: 10.2486/indhealth.MS1317. [DOI] [PubMed] [Google Scholar]
- 8.Yan YX, Liu YQ, Li M, Hu PF, Guo AM, Yang XH, Qiu JJ, Yang SS, Shen J, Zhang LP, Wang W. Development and evaluation of a questionnaire for measuring suboptimal health status in urban Chinese. J Epidemiol. 2009;19:333–341. doi: 10.2188/jea.JE20080086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan YX, Dong J, Li M, Yang SS, Wang W. Establish the cut off point for suboptimal health status using SHSQ-25. Chin Health Stat (in Chin) 2011;28:256–258. [Google Scholar]
- 10.Wang Y, Ge S, Yan Y, Wang A, Zhao Z, Yu X, Qiu J, Alzain MA, Wang H, Fang H, Gao Q, Song M, Zhang J, Zhou Y, Wang W. China suboptimal health cohort study: rationale, design and baseline characteristics. J Transl Med. 2016;14:291. doi: 10.1186/s12967-016-1046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kupaev V, Borisov O, Marutina E, Yan YX, Wang W. Integration of suboptimal health status and endothelial dysfunction as a new aspect for risk evaluation of cardiovascular disease. EPMA J. 2016;7:19. doi: 10.1186/s13167-016-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan YX, Dong J, Liu YQ, Zhang J, Song MS, He Y, Wang W. Association of suboptimal health status with psychosocial stress, plasma cortisol and mRNA expression of glucocorticoid receptor alpha/beta in lymphocyte. Stress. 2015;18:29–34. doi: 10.3109/10253890.2014.999233. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Liu X, Qiu J, Wang H, Liu D, Zhao Z, Song M, Song Q, Wang X, Zhou Y, Wang W. Association between ideal cardiovascular health metrics and suboptimal health status in Chinese population. Sci Rep. 2017;7:14975. doi: 10.1038/s41598-017-15101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alzain MA, Asweto CO, Zhang J, Fang H, Zhao Z, Guo X, Song M, Zhou Y, Chang N, Wang Y, Wang W. Telomere length and accelerated biological aging in the China suboptimal health cohort: a case-control study. OMICS. 2017;21:333–339. doi: 10.1089/omi.2017.0050. [DOI] [PubMed] [Google Scholar]
- 15.McEwen BS. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nonogaki K, Iguchi A. Stress, acute hyperglycemia, and hyperlipidemia role of the autonomic nervous system and cytokines. Trends Endocrinol Metab. 1997;8:192–197. doi: 10.1016/S1043-2760(97)00038-6. [DOI] [PubMed] [Google Scholar]
- 17.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34:163–171. doi: 10.1016/j.psyneuen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Inder WJ, Dimeski G, Russell A. Measurement of salivary cortisol in 2012 - laboratory techniques and clinical indications. Clin Endocrinol. 2012;77:645–651. doi: 10.1111/j.1365-2265.2012.04508.x. [DOI] [PubMed] [Google Scholar]
- 20.van Smeden M, Naaktgeboren CA, Reitsma JB, Moons KG, de Groot JA. Latent class models in diagnostic studies when there is no reference standard - a systematic review. Am J Epidemiol. 2013;179:423–431. doi: 10.1093/aje/kwt286. [DOI] [PubMed] [Google Scholar]
- 21.Dong J, Liang YZ, Zhang J, Wu LJ, Wang S, Hua Q, Yan YX. Potential role of lipometabolism-related microRNAs in peripheral blood mononuclear cells as biomarkers for coronary artery disease. J Atheroscler Thromb. 2017;24:430–441. doi: 10.5551/jat.35923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang YZ, Chu X, Meng SJ, Zhang J, Wu LJ, Yan YX. Relationship between stress-related psychosocial work factors and suboptimal health among Chinese medical staff: a cross-sectional study. BMJ Open. 2018;8:e018485. doi: 10.1136/bmjopen-2017-018485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becker AJ, Uckert S, Stief CG, Truss MC, Machtens S, Scheller F, et al. Plasma levels of cavernous and systemic norepinephrine and epinephrine in men during different phases of penile erection. J Urol. 2000;164:573–577. doi: 10.1016/S0022-5347(05)67425-3. [DOI] [PubMed] [Google Scholar]
- 24.Drew L, Lewis JB. poLCA: an R package for polytomous variable latent class analysis. J Stat Softw. 2011;42:1–29. [Google Scholar]
- 25.Collins J, Huynh M. Estimation of diagnostic test accuracy without full verification: a review of latent class methods. Stat Med. 2014;33:4141–4169. doi: 10.1002/sim.6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lanza ST, Collins LM, Lemmon DR, Schafer JL. PROC LCA: a SAS procedure for latent class analysis. Struct Equ Model. 2007;14:671–694. doi: 10.1080/10705510701575602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, et al. Medicine in the early twenty-first century: paradigm and anticipation - EPMA position paper 2016. EPMA J. 2016;7:23. doi: 10.1186/s13167-016-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- 30.Yan YX, Dong J, Liu YQ, Yang XH, Li M, Shia G, Wang W. Association of suboptimal health status and cardiovascular risk factors in urban Chinese workers. J Urban Health. 2012;89:329–338. doi: 10.1007/s11524-011-9636-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gholami N, Hosseini Sabzvari B, Razzaghi A, Salah S. Effect of stress, anxiety and depression on unstimulated salivary flow rate and xerostomia. J Dent Res Dent Clin Dent Prospects. 2017;11:247–252. doi: 10.15171/joddd.2017.043. [DOI] [PMC free article] [PubMed] [Google Scholar]