Abstract
With immunohistochemical and Western blot techniques, P2X1 receptors were detected in the whole mouse gastrointestinal tract and pancreatic islets of mouse and human. (1) δ Cells containing somatostatin (SOM) in the stomach corpus, small intestines, distal colon, pancreatic islets of both mouse and human express P2X1 receptors; (2) strong immunofluorescence of P2X1 receptors was detected in smooth muscle fibers and capillary networks of the villus core of mouse intestine; and (3) P2X1 receptor-immunoreactive neurons were also detected widely in both mouse myenteric and submucosal plexuses, all of which express SOM. The present data implies that ATP via P2X1 receptors is involved in SOM release from pancreatic δ cells, enteric neurons, and capillary networks in villi.
Keywords: P2X1 receptor, Gastrointestinal tract, Pancreatic islet, Mouse, Human
Introduction
ATP and its derivatives, via purinergic receptors, regulate the functions of smooth muscle, enteric plexuses, blood vessels, and mucosal epithelium in the gastrointestinal tract [4]. Pharmacological and morphological data have shown that ATP and its derivatives regulate smooth muscle functions in the gastrointestinal tract via purinergic receptors, including adenosine receptors (A1, A2), P2X receptors (P2X1, P2X2, P2X3) and P2Y receptors (P2Y1, P2Y2, P2Y4 and P2Y14). In addition to the roles of ATP and its derivatives on smooth muscle, they also affect the functions of enteric plexuses. Pharmacological studies have shown that 90% of cultured myenteric neurons of the guinea pig small intestine respond to ATP via P2X purinoceptors, including P2X1, P2X2, P2X4, P2X5 and P2X6 receptors [3, 34]. Immunohistochemical studies have shown that P2X2 (mouse, rat, guinea pig), P2X3 (mouse, rat, guinea pig), P2X5 (mouse), and P2X6 (rat) receptors are expressed on neurons of the submucosal and myenteric ganglia in the gastrointestinal tract [5, 6, 20, 22, 26, 30, 31, 33]. In a report from Van Crombruggen et al. [25], P2X4 receptor staining was found only in macrophages in the rat distal colon and P2X6 receptor staining only in enteric glial cells.
At present, there has not been a systematical study of the expression of P2X1 receptors in the gastrointestinal tract. Here, we report that the P2X1 receptor highly expresses in the capillary nets and smooth muscle fibers in the villus of the small intestine, somatostatinergic endocrine cells in the stomach, small intestine and colon, and pancreatic islets. P2X1 receptors are also expressed in the submucosal and myenteric ganglia of the enteric nervous system of the mouse.
Materials and methods
All experimental procedures were approved by the Institutional Animal Care and Use Committee at Second Military Medical University. Six mice (30–35 g) were used. The mice were anesthetized with sodium pentobarbitone and perfused through the aorta with 0.9% NaCl solution and 4% paraformaldehyde in 0.1 mol/l phosphate buffer pH 7.4. The digestive tracts were removed and washed with phosphate-buffered saline (PBS). For the whole-mount sections of gastrointestinal tract, one end of the segment was knotted with a silk thread and fixative was injected into the lumen to fill it and the open end was also knotted with a silk thread. The fixative-filled segment was then immersed in 4% paraformaldehyde in 0.1 mol/l phosphate buffer, pH 7.4, for 4–6 h. The mucosa was discarded and the submucosal and myenteric plexuses of the gastric corpus, jejunum, ileum, and proximal and distal colon of the mice were used as whole-mount preparations. For the section specimens, segments of gastrointestinal tract and pancreas of the mouse and human were post-fixed in 4% paraformaldehyde in PBS for 4–6 h and then transferred to 25% sucrose in PBS and kept in the solution until they sank to the bottom. Thereafter, the tissue blocks were rapidly frozen and coronal sections (10 μm in thickness) were cut with a Leica cryostat and thawed on gelatine-coated slides. The sections of human pancreatic tissue adjacent to cancer tissues from three patients were kindly supplied by the Department of Anesthesiology and Intensive Care Medicine, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, Shanghai, China, with informed consent from the patients.
Immunohistochemistry
The following protocol was used for P2X1 receptor or P2X1 receptor with NeuN (neuronal marker), smooth-specific muscle actin (SPMA, smooth muscle marker), intercellular cell adhesion molecule-1 (ICAM1, blood vessel endothelial marker), and somatostatin (SOM). The preparations were washed 3 × 5 min in PBS and then preincubated in antiserum solution 1 (10% normal bovine serum, 0.2% Triton X-100, 0.4% sodium azide in 0.01 mol/L PBS pH 7.2) for 30 min, followed by incubation with different combinations of P2X1 antibody (Alomone, Israel, rabbit anti-rat) diluted 1:200, SPMA antibody (mouse anti-rat; Santa Cruz) diluted 1:200, ICAM1 antibody (mouse anti-rat; Proteintech) diluted 1:100, NeuN antibody (mouse anti-rat; Chemicon) diluted 1:200, SOM antibody (mouse anti-rat; Abgent) diluted 1:200 in antiserum solution 2 (1% normal bovine serum, 0.2% Triton X-100, 0.4% sodium azide in 0.01 mol/L PBS pH 7.2), at room temperature. Subsequently, the preparations were incubated with Cy3- or FITC-conjugated donkey anti-rabbit IgG diluted 1:400 or 200 for P2X1 and FITC- or Cy3-conjugated donkey anti-mouse IgG diluted 1:200 or 400 for NeuN, SPMA, ICAM1, and SOM antibodies in antiserum solution 2 for 2 h at room temperature. The secondary antibodies are specialized for multiple immunostaining from the Jackson ImmunoResearch Lab. All the incubations and reactions were separated by 3 × 10 min washes in PBS.
Triple labeling immunofluorescence was carried out on the mouse and human pancreas to detect P2X1 receptors, glucogen, and insulin. As both P2X1 receptors and glucogen antibodies were from the same animal, rabbit, a tyramide signal amplification system was used in this protocol to first visualize P2X1 receptor-immunoreactive (-ir) (green), and afterwards glucogen and insulin were visualized. Usually, two primary antibodies from the same species of animals cannot be used to detect their antigens at the same time as the cross-reactions of primary and secondary antibodies will occur. So simultaneous detection of two antigens by immunostaining usually requires primary antibodies from two different species. However, a novel double labeling immunostaining method for detection of two independent antigens using two antibodies from the same species of animals has been described [24]. The principle of the method was that the first antigen is detected by the first primary antibody that is diluted so extensively (in this study, P2X1 receptor antibody is diluted to 1:10000) that it cannot be detected with conventional immunostaining, but can be detected by the highly sensitive tyramide signal amplification (TSA) system; the second antigen is stained with the secondary primary antibody and detected by conventional immunostaining. The secondary-secondary antibody will bind to the first primary antibody, but the immunostaining reactions would not be detected by the conventional immunostaining as the first primary antibody diluted so extensively. The protocol was modified from previous protocols [24, 32]. Endogenous peroxidase was blocked by 1% H2O2 in PBS for 30 min. The sections were preincubated in 10% normal horse serum (NHS), 0.2% Triton X-100 in PBS for 30 min, followed by incubation with the P2X1 antibody, diluted 1:10000 in antiserum solution1 overnight at 4 °C. Subsequently, the sections were incubated with biotinylated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories) at a dilution of 1:500 in PBS containing 1% NHS for 1 h. The sections were then incubated in extravidin peroxidase (Sigma) diluted 1:1000 in PBS for 30 min at room temperature. P2X1 receptor-immunoreactivity was visualized by the TSA fluorescein system (NEL701, NEN, USA). After visualization, the sections were incubated with the second primary antibodies for glucogen diluted 1:400 and insulin (mouse monoclonal, Proteintech) diluted 1:600 in antiserum solution1 overnight at 4 °C. Subsequently, the sections were incubated with FITC-conjugated donkey anti-rabbit for glucogen and AMCA-conjugated donkey anti-mouse for insulin (Jackson ImmunoResearch Laboratories) diluted 1:200 or 1:100 in antiserum solution 2 for 1 h at room temperature. All the incubations and reactions were separated by 3 × 10 min washes in PBS.
Western blot
Western blot analysis was carried out to further study the expression of P2X1 receptors in different parts of the gastrointestinal tract and pancreas. Mice were deeply anesthetized by sodium pentobarbital (60 mg/kg) and killed by decapitation. The different segments of gastrointestinal tract and islets were rapidly removed, washed with ice-cold PBS and lysed with 20 mM Tris-HCl buffer, pH 8.0, containing 1% NP-40, 150 mM NaCl, 1 mM EDTA, 10% glycerol, 0.1% mercaptoethanol, 0.5 mM dithiothreitol, and a mixture of proteinase and phosphatase inhibitors (Sigma). Protein concentration was determined by the BCA protein assay method using bovine serum albumin as a standard (BCA protein assay kit from Beyotime). Protein samples (100 μg) from the differential segments of the gastrointestinal tract and pancreas were loaded per lane, separated by SDS-PAGE (10% polyacrylamide gels) and then were electrotransferred onto nitrocellulose membranes. The membranes were blocked with 10% nonfat dry milk in Tris-buffered saline for 1 h and incubated overnight at 4 °C with P2X1 (Alomone) diluted 1:500 in 2% BSA in PBS. The membranes were then incubated with alkaline phosphatase-conjugated goat anti-rabbit IgG (Sigma) diluted 1:5000 in 2% BSA in PBS for 1 h at room temperature. The color development was performed with 400 μg/ml nitro-blue tetrazolium, 200 μg/ml 5-bromo-4-chloro-3-indolyl phosphate and 100 mg/ml levamisole in TSM2 (0.1 mol/l Tris–HCl2 buffer, pH 9.5, 0.1 mol/l NaCl and 0.05 mol/l MgCl2) in the dark. Bands were scanned using a densitometer (GS-700; Bio-Rad Laboratories).
Quantitative analysis
P2X1 receptor-ir cells in some preparations were counterstained with NeuN antiserum in order to assess the percentage of P2X1 receptor-ir cells compared to the total number of neurons in the enteric plexuses. The whole-mount preparations were used to perform a quantitative analysis as described previously [26]. Briefly, positively stained neuron bodies in the submucosal and myenteric plexuses were counted per visual field (area of 0.36 mm2). Ten randomly chosen fields in each whole-mount preparation and three preparations of each mouse were analyzed. At least four mice were used for each marker. The percentage of neurons immunoreactive for P2X1 receptors that were also positive for the NeuN marker was calculated and expressed as mean ± standard error of the mean.
Photomicroscopy
Images were taken with a Nikon digital camera DXM1200 (Nikon, Japan) attached to a Nikon Eclipse E600 microscope (Nikon). Images were imported into a graphics package (Adobe Photoshop).
Results
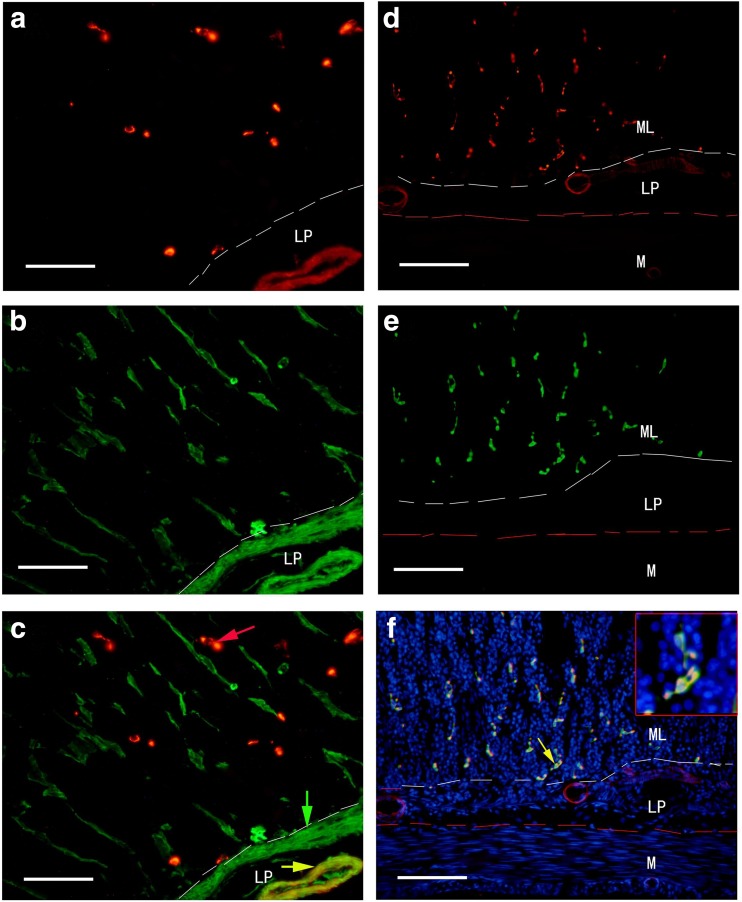
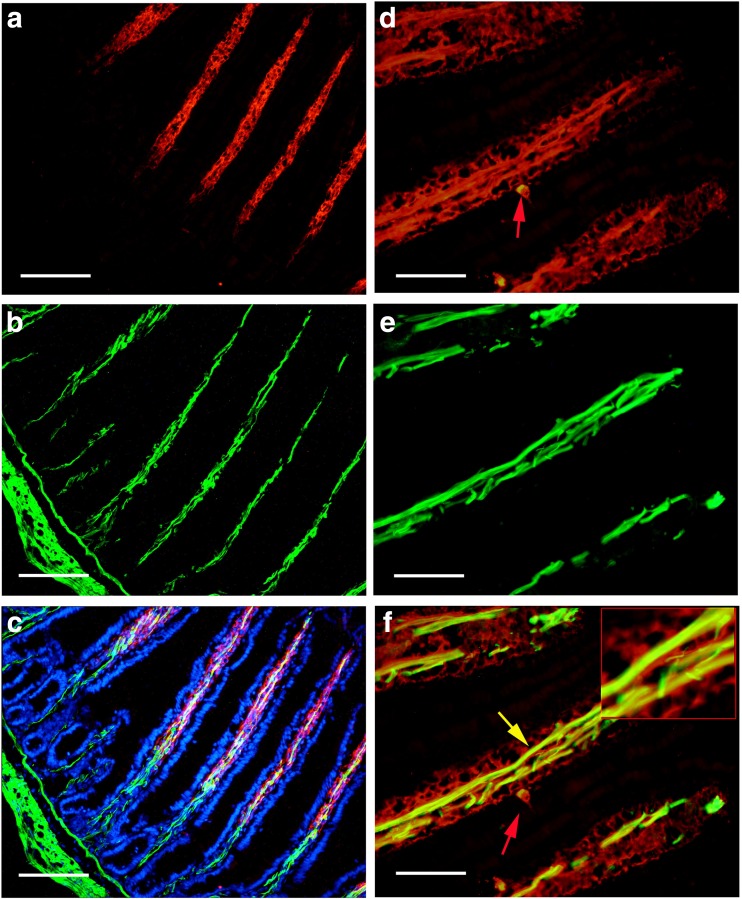
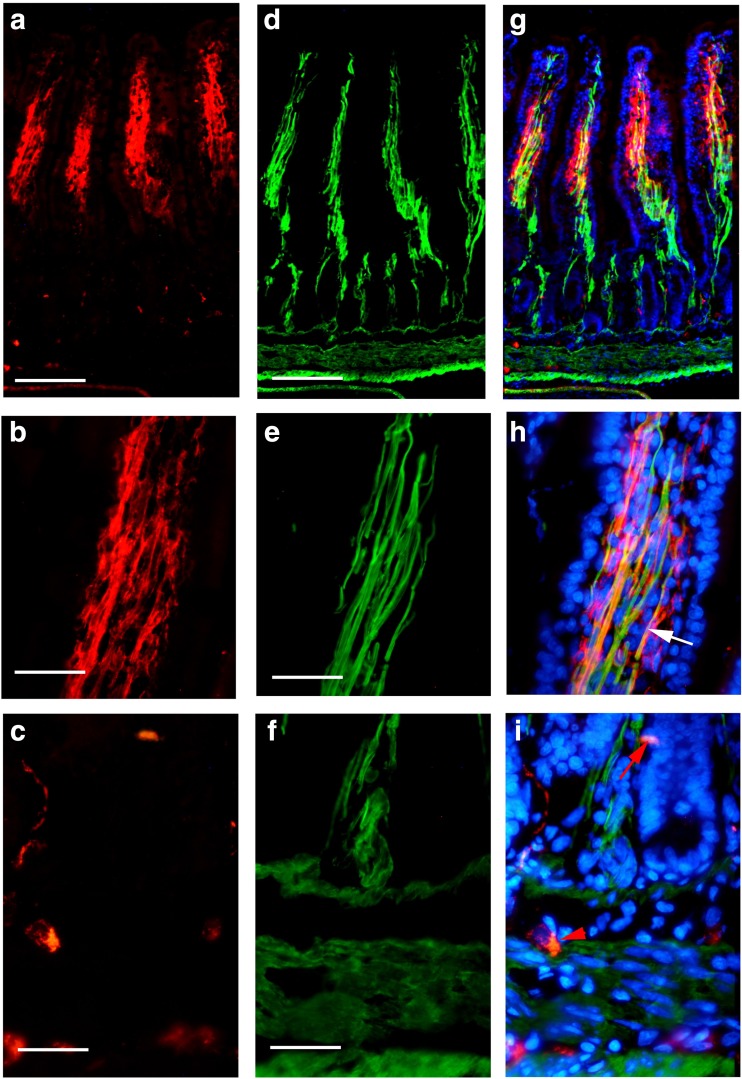
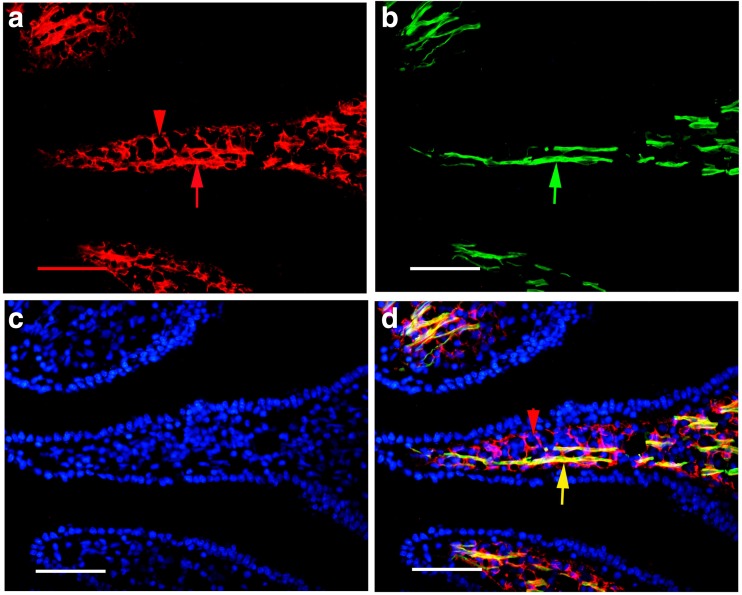
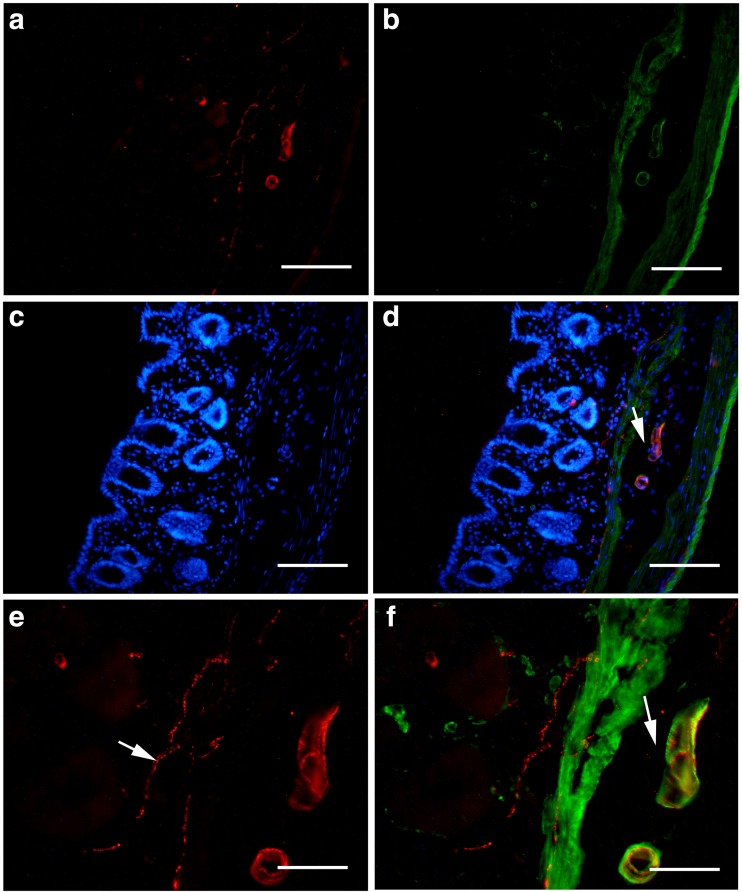
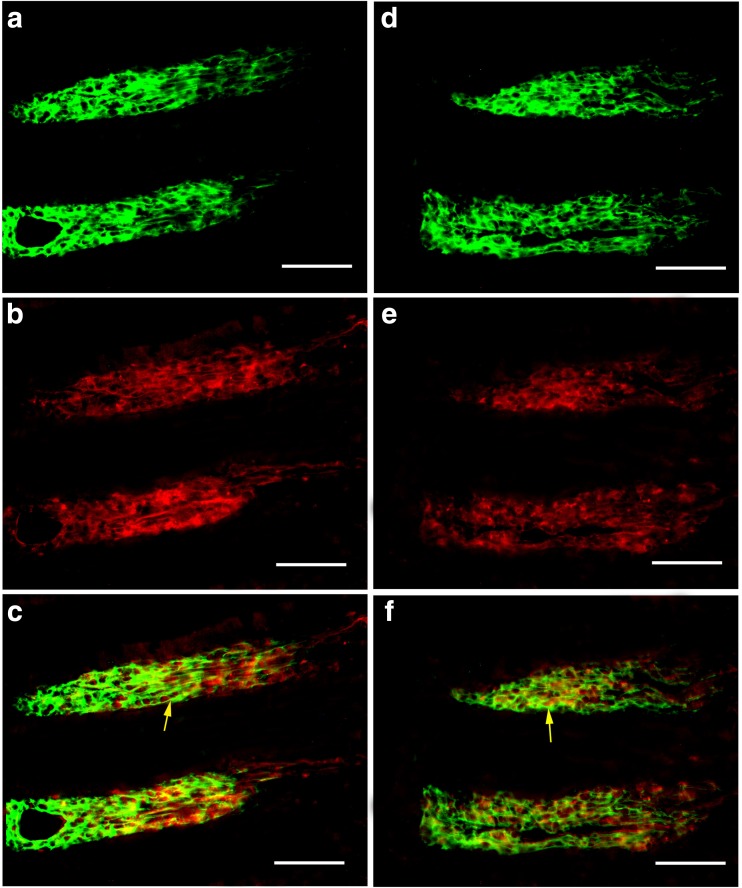
With immunohistochemical and Western blot techniques, P2X1 receptors were detected in the whole mouse gastrointestinal tract and pancreatic islet of mouse and human. In the body of the stomach, P2X1 receptor-immunoreactivity occurred in the cells in the mucosal layer and blood vessels in the submucosal layer (Fig. 1). P2X1-ir blood vessel was detected with SPMA immunoreactivity (Fig. 1c) and scattered P2X1-ir cells in the mucosal layer were found to coexist with SOM immunoreactivity (Fig. 1f). In the small intestine, strong immunofluorescence of P2X1 receptors was detected in the central region of the villus and scattered P2X1-ir cells were detected in the epithelium, intestinal glands, and enteric plexuses (Figs. 2 and 3). In the villus of the small intestine, very long cells with P2X1 immunoreactivity were also detected with SPMA immunoreactivity (Figs. 2f, 3c, f, and 4). In the colon, scattered cells, fibers, and blood vessels were detected with P2X1 receptor-immunoreactivity in the mucosal, submucosal, and muscular layers (Fig. 5). Blood vessels with P2X1 receptor immunoreactivity and SPMA immunoreactivity were detected (Fig. 5f). In order to confirm whether the nets of P2X1 receptor-ir structures were capillary nets, double immunofluorescence labeling of P2X1 receptors and ICAM1 was carried out. The result showed that most of the P2X1 receptor-ir structures were also labeled with ICAM1-ir (Fig. 6).
Fig. 1.
Expression of P2X1 receptor-ir (a, d, red), SPMA-ir (b, green), and SOM-ir (e, green) in the mouse stomach corpus. a P2X1 receptor-ir detected in the mucosal lamina propria and blood vessels. b SPMA-labeled smooth muscle in the same region of a. Positive cells are located in the mucosal lamina propria, mucosal muscle, and blood vessels. c The merged image of a and b. A yellow arrow indicates a double-labeled vessel with both P2X1 receptor-ir and SPMA-ir (yellow in color). A green arrow indicates a single labeled mucosal muscle layer and a red arrow indicates a single labeled cell with P2X1 immunoreactivity. d P2X1 receptor-ir in the stomach corpus. e SOM-ir in the same region of d. f The merged image of d, e and an image (blue stain) of the same region counterstained by DAPI. Note that all the P2X1 receptor-ir cells were labeled with SOM-ir. The inserted figure is a higher magnification of the region indicated by a yellow arrow. ML, mucosal layer; LP, mucosal lamina propria; M, muscle layer. The scale bars in a–c = 60 μm, in d–f = 120 μm, in inserted figure = 40 μm
Fig. 2.
Expression of P2X1 receptors and SPMA in the mouse jejunum. a P2X1 receptor-ir at low magnification. Note that strong P2X1 receptor-ir was detected in the villus core. b SPMA-ir in the same region of a. c is the merged image of a and b. d P2X1 receptor-ir in the villus core. e SPMA-ir in the same region of d. f The merged image of d and e. Note that all smooth muscle was double-labeled with P2X1 receptor-ir and SPMA-ir. The inserted image is a higher magnification of the region indicated by a yellow arrow in f. A red arrow indicates a P2X1 receptor-ir cell in the epithelium of the villus. The scale bars in a–c = 240 μm, in d–f = 60 μm, in inserted figure = 20 μm
Fig. 3.
Expression of P2X1 receptors and SPMA in the mouse ileum. a–c P2X1 receptor-ir. d–f SPMA-ir. g The merged image of a and d and counterstained with DAPI. Note that strong P2X1 receptor-ir was detected in the villus core. h The merged image of b and e, counterstained with DAPI in the villus. Note that the smooth muscle was labeled with SPMA-ir and P2X1 receptor-ir. An arrow indicates a typical labeled smooth muscle fiber. i The merged image of c and f and counterstained with DAPI. A red arrow indicates a P2X1 receptor-ir cell in the gland epithelium and a red arrow head indicates a P2X1 receptor-ir cell in the submucosal layer. The scale bars in a, d, g = 240 μm, in b, c, e, f, h, i = 60 μm
Fig. 4.
Expression of P2X1 receptor-ir and SPMA-ir in the mouse ileum villus under confocal microscope. a P2X1 receptor-ir. b SPMA-r. c DAPI. d The merged image of a–c. An arrow indicates a typical smooth muscle fiber double labeled with P2X1 receptor-ir and SPMA-ir in d. A red arrow head indicates a capillary-like structure in d. All the scale bars = 60 μm
Fig. 5.
Expression of P2X1 receptors and SPMA in the mouse distal colon. a P2X1 receptor-ir. b SPMA-ir in the same region of a. c DAPI staining in the same region of a. d The merged image of a–c. e A high magnification of the region indicated by an arrow in d, a white arrow indicates a nerve fiber with P2X1 receptor-ir. f The double-labeled image of P2X1 receptor-ir and SPMA-ir in the same region of e, an arrow indicates a double-labeled blood vessel. The scale bars in a–d = 120 μm, in e, f = 60 μm
Fig. 6.
Expression of P2X1 receptor-ir (green) and ICAM1-ir (red) in the mouse ileum villus. a P2X1 receptor-ir. b ICAM1-ir. c The merged image of a, b. A yellow arrow indicates a double labeling capillary-like structure. d P2X1 receptor-ir. e ICAM1-ir. f The merged image of d, e. A yellow arrow indicates a double labeling capillary-like structure. All the scale bars = 60 μm
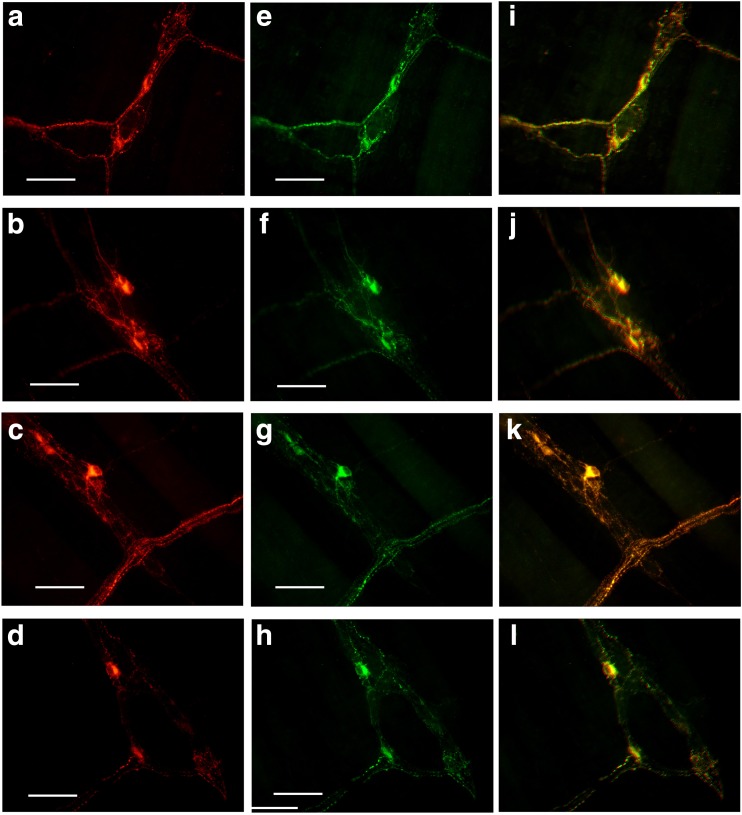
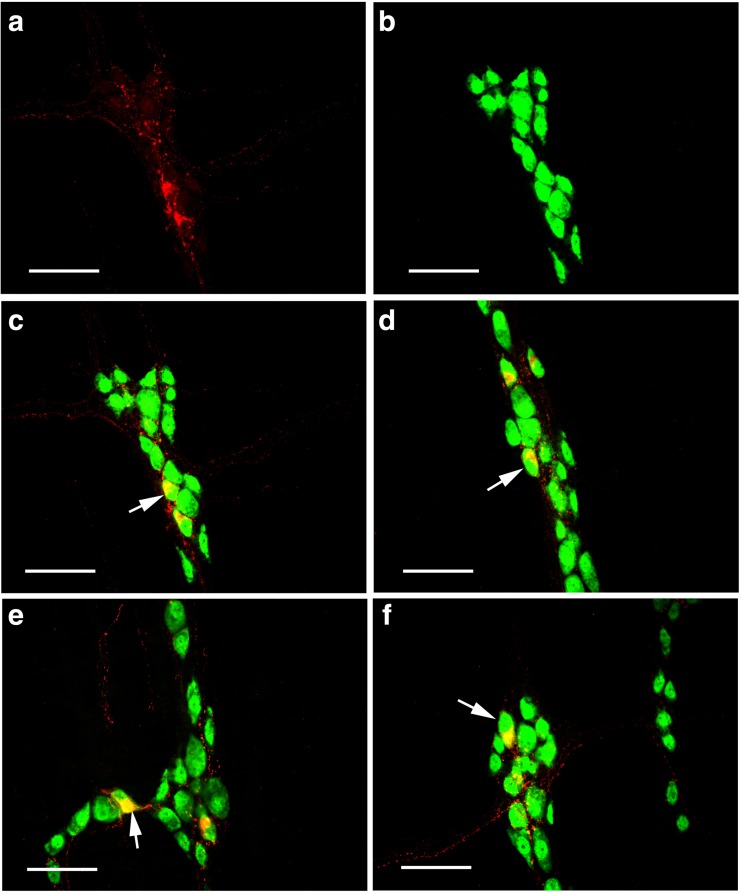
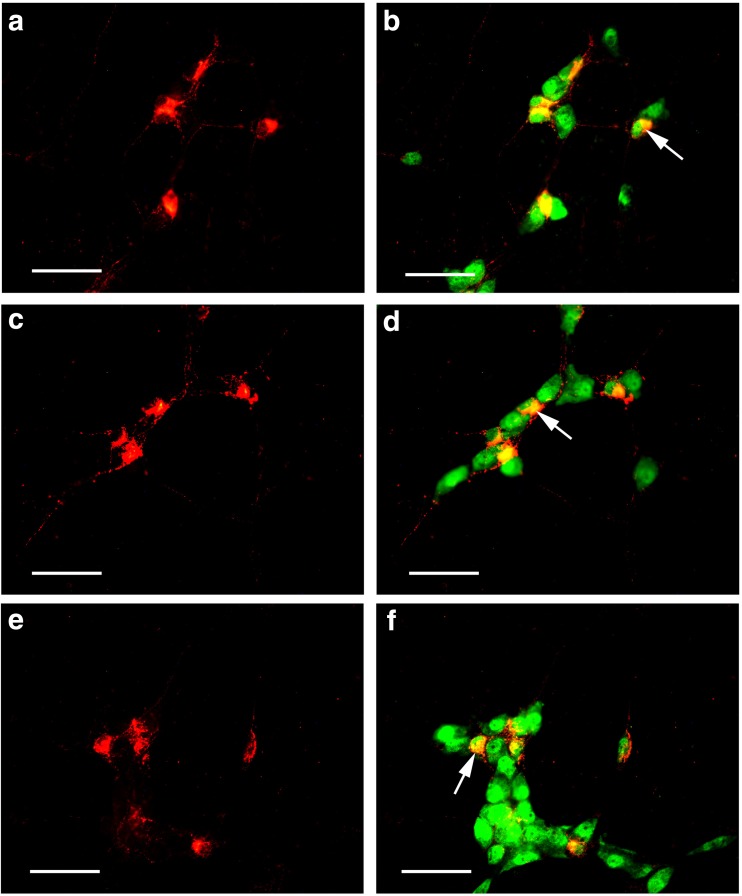
Scattered P2X1 receptor-ir cells were detected in the enteric plexuses of the whole-mount preparations of the stomach, jejunum, ileum, and distal colon (Figs. 7 and 8). In the myenteric plexus of all the segments examined, scattered P2X1 receptor-ir cells were detected and all the positive cells were also labeled with SOM immunoreactivity (Fig. 7). In the submucosal plexus of the jejunum, ileum, and distal colon, the number of P2X1 receptor-ir cells were greater than that in the myenteric plexus and also all the P2X1 receptor-ir cells were also labeled with SOM immunoreactivity (Fig. 8).
Fig. 7.
Expression of P2X1 receptors and SOM in the myenteric plexus of the mouse gastrointestinal tract. a–d P2X1 receptor-ir in stomach corpus, jejunum, ileum and distal colon, respectively. e–h are SOM-ir in the same regions of a–d, respectively. i–l The merged images of a and e, b and f, c and g, and d and h, respectively. Note that the scattered P2X1 receptor-ir neurons were all labeled with SOM-ir. All the scale bars = 60 μm
Fig. 8.
Expression of P2X1 receptors and SOM in the submucosal plexus of the mouse gastrointestinal tract. a–c P2X1 receptor-ir in the jejunum, ileum, and distal colon, respectively. d–f SOM-ir in the same regions of a–c, respectively. g–i are the merged images of a and d, b and e, and c and f, respectively. Note that the P2X1 receptor-ir neurons were all labeled with SOM-ir. All the scale bars = 60 μm
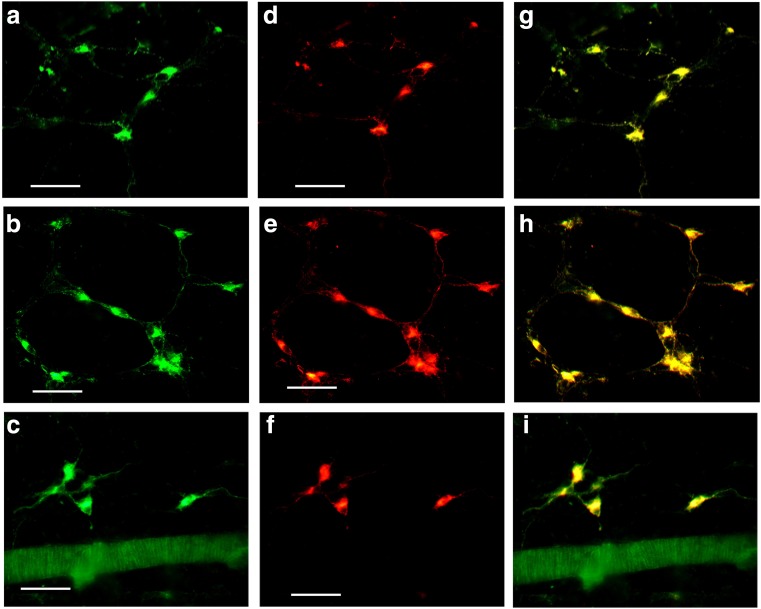
The percentages of P2X1 receptor-ir cells of the total number of neurons labeled with NeuN immunoreactivity are summarized in Table 1. Double labeling of P2X1 and NeuN are shown in the Figs. 9 and 10.
Table 1.
Quantitative analysis of double labeling studies between P2X1 receptors and NeuN in the myenteric plexus of the stomach, jejunum, ileum, and distal colon and the submucosal plexus of the jejunum, ileum, and distal colon. The first column shows the mean number of P2X1-ir neurons; the second column shows the mean number of NeuN-ir neurons; the third column shows the mean number of P2X1 receptor-ir neurons also labeled with NeuN-ir; and the fourth column shows the mean percentages of P2X1 receptor-ir neurons over the total number of NeuN-ir neurons
| Region | P2X1R-ir+ | NeuN-ir+ | P2X1R-ir+/NeuN-ir+ | percentage | |
|---|---|---|---|---|---|
| Stomach | MP | 2 ± 1 | 33 ± 7 | 2 ± 1 | 6.1 ± 3.0 |
| Jejunum | MP | 3 ± 2 | 36 ± 6 | 3 ± 2 | 8.3 ± 5.5 |
| SMP | 9 ± 4 | 28 ± 6 | 9 ± 4 | 32.1 ± 14.3 | |
| Ileum | MP | 3 ± 1 | 34 ± 5 | 3 ± 1 | 8.8 ± 2.9 |
| SMP | 10 ± 4 | 30 ± 6 | 10 ± 4 | 33.3 ± 13.3 | |
| Colon | MP | 3 ± 2 | 38 ± 5 | 3 ± 2 | 7.9 ± 5.3 |
| SMP | 8 ± 3 | 32 ± 6 | 8 ± 3 | 25.0 ± 9.4 | |
MP myenteric plexus, SMP submucosal plexus, Colon distal colon, P2X1-ir+ P2X1 receptor-ir neurons, NeuN-ir+ NeuN-ir neurons, P2X1-ir+/NeuN-ir+ P2X1 receptor-ir neurons also labeled with NeuN-ir, percentage the mean percentage of P2X1 receptor-ir neurons over the total number of NeuN-ir neurons
Fig. 9.
Double labeling of P2X1 receptor-ir and NeuN-ir in the myenteric plexus of the stomach corpus, jejunum, ileum, and distal colon of the mouse. a P2X1 receptor-ir in the myenteric plexus of the stomach. b NeuN-ir in the same region of a. c The merge image of a and b. d–f The merged images of P2X1 receptor-ir and NeuN-ir double labeling in the jejunum, ileum, and distal colon. An arrow indicates a double-labeled neuron. All the scale bars = 60 μm
Fig. 10.
Double labeling of P2X1 receptor-ir and NeuN-ir in the submucosal plexus of the jejunum, ileum, and distal colon of the mouse. a, c, e P2X1 receptor-ir neurons (red) in the jejunum, ileum, and distal colon, respectively. b, d, f The merged images of a, c, and e and NeuN-ir neurons (green) in the same regions of a, c, and e, respectively. An arrow indicates a double-labeled neuron. All the scale bars = 60 μm
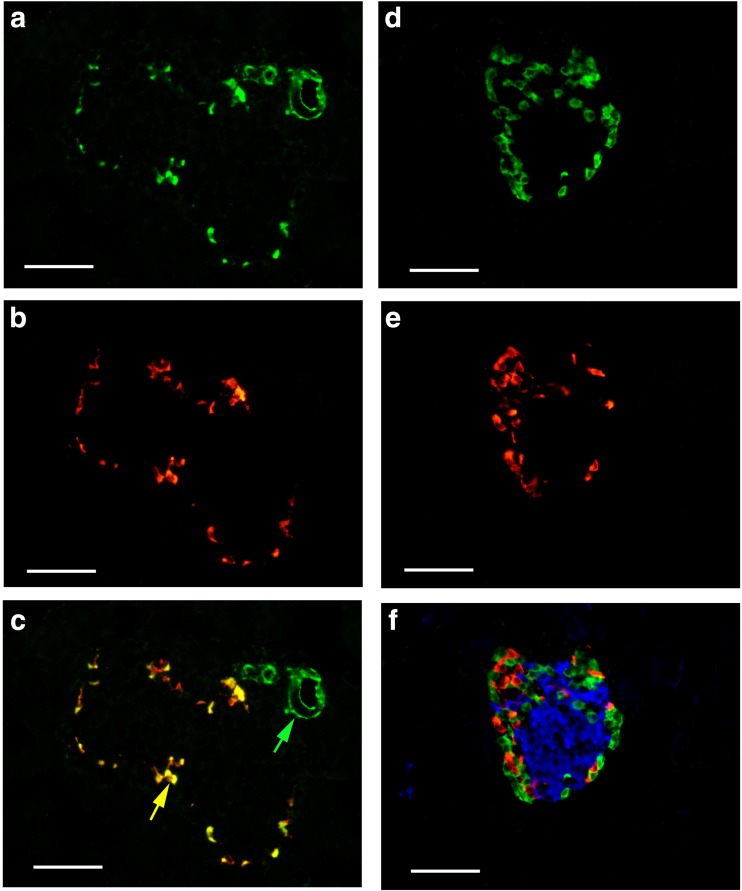
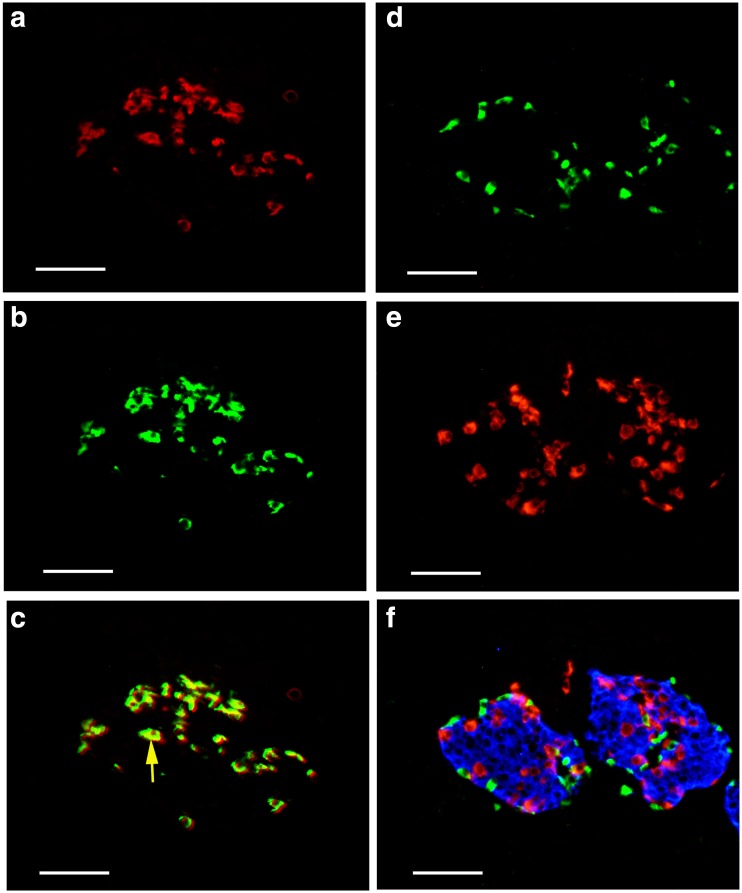
Scattered P2X1 receptor-ir cells were detected around the islets of both mouse (Fig. 11) and human pancreas (Fig. 12). Double labeling immunofluorescence showed that P2X1 receptor-ir cells were also labeled with SOM immunoreactivity, but not with insulin immunoreactivity or glucogen (Figs. 11f and 12f).
Fig. 11.
Expression of P2X1 receptors, SOM, glucagon, and insulin in mouse pancreatic islets. a P2X1 receptor-ir. b SOM-ir in the same region of a. c The merged image of a and b. Note that all the SOM-ir cells were labeled with P2X1 receptor-ir. A yellow arrow indicates a typical double-labeled cell. A green arrow indicates a small blood vessel with single labeling for P2X1 receptor-ir. d P2X1 receptor-ir. e Glucagon-ir in the same region of d. f The merged image of d and e and further immunostained with an antibody to insulin (blue color) in the same region of d and e. Note that no double or triple labeling in cells was detected. All the scale bars = 60 μm
Fig. 12.
Expression of P2X1 receptors, SOM, glucagon, and insulin in human pancreatic islets. a P2X1 receptor-ir. b SOM-ir in the same region of a. c The merged image of a and b. Note that all the SOM-ir cells were labeled with P2X1 receptor-ir. A yellow arrow indicates a typical double-labeled cell. d P2X1 receptor-ir. e Glucagon-ir in the same region of d. f The merged image of d and e and further immunostained with an antibody to insulin (blue color) in the same region of d and e. Note that no double or triple labeling in cells was detected. All the scale bars = 60 μm
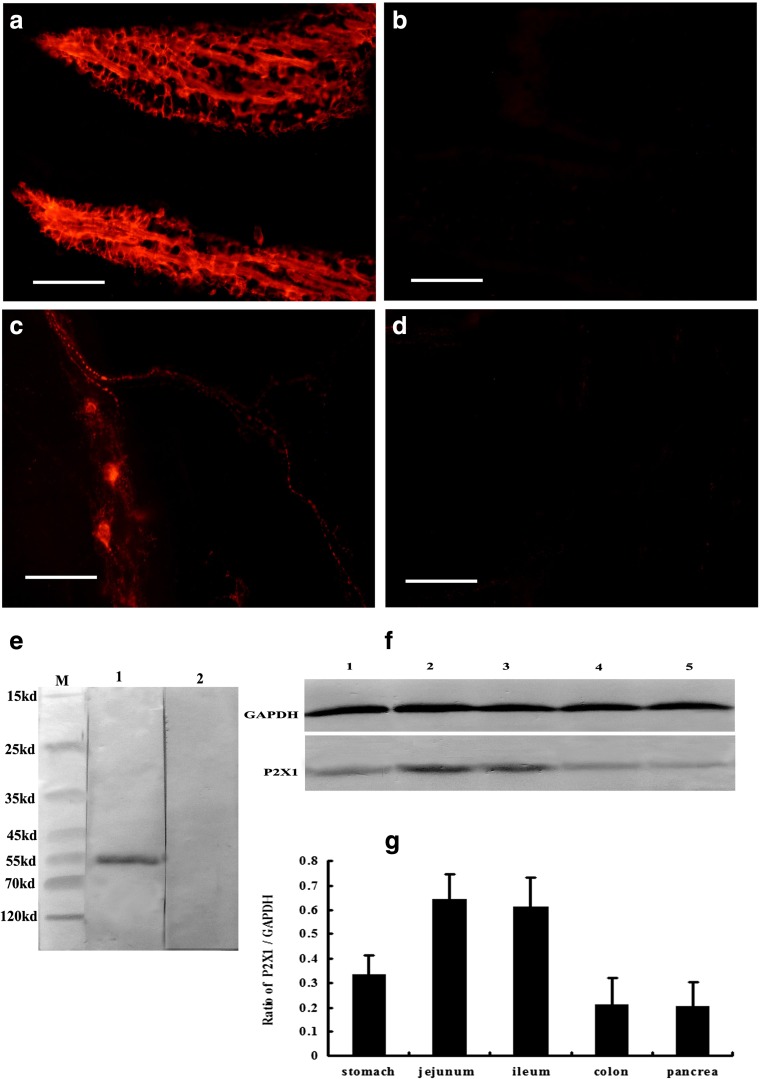
Western blotting, performed on tissue extracts derived from the mouse ileum (Fig. 13e), assessed the specificity of the polyclonal P2X1. An immunoreactive band was detected at about ~ 55 kDa (Fig. 13e, lane 1. Preabsorption of the antiserum with the peptide antigen resulted in the absence of the band (Fig. 13e, lane 2), indicating that the antibodies detected the appropriate antigen sequence. The result also showed that high levels of P2X1 receptor protein were detected in the jejunum and ileum and medium levels in the stomach and low levels in the distal colon and pancreas (Fig. 13f, g).
Fig. 13.
The results of peptide-absorptive control experiments and Western blot. a, c P2X1 receptor-ir in the villus core and myenteric plexus of mouse ileum, respectively. b, d The results of P2X1 receptor antibody and peptide-absorptive control experiments in the villus core and myenteric plexus of mouse ileum. Note that no P2X1 receptor-ir was detected in the villus core and myenteric plexus in the specimens of the peptide-absorptive control experiment. e The expression of P2X1 receptors detected by Western blotting from mouse ileum extracts. M molecular weight marker. Lane 1 is the P2X1 receptor-ir band located at about 55 kDa. Lane 2 is the preabsorption of the P2X1 receptor antisera with its peptide antigen, which resulted in the absence of the band. f is the expression of P2X1 receptors detected by Western blotting from mouse gastrointestinal tract and pancreas extracts. Lanes 1 to 5 are representative levels of P2X1 receptor-ir from the stomach corpus, jejunum, ileum, distal colon, and pancreas of the mouse. g The summary of P2X1 receptor/GAPDH ratios. The scale bars in a–d = 60 μm
Control experiments were also carried out with P2X1 preabsorbed with P2X1 peptides. No staining was observed in those preparations incubated with antiserum solutions preabsorbed with P2X1 (Fig. 13b, d).
Discussion
In the present study, the distribution patterns and morphological characteristics of P2X1 receptor-ir neurons have been studied systematically in all the major regions of the mouse gastrointestinal tract and pancreatic islets of both mouse and human. The results show that P2X1 receptor-ir cells are distributed widely in the all segments of the stomach, jejunum, ileum and colon, and islets of mouse and human pancreas.
There is much less data for P2X1 receptor expression in the gastrointestinal tract. P2X1 is usually expressed in smooth muscle cells of arteries, bladder, ureter and vas deferens [14, 29]. Morphological data showed that P2X1 receptor immunoreactivity was not detected in the smooth muscle of the mouse ileum [28]. RT-PCR also showed that no P2X1 receptor mRNA was detected in the canine colon circular and longitudinal myocytes [15]. P2X1 receptor immunoreactivity was not detected in the circular and longitudinal smooth muscle of the gastrointestinal tract wall. There was very strong P2X1 receptor immunofluorescence detected in the villus core of the whole small intestine of the mouse, which support and expand the results reported by Groschel-Stewart et al. [10]. Furthermore, this study confirmed that smooth muscle fibers in the villus of the small intestine also express P2X1 receptors, as did the endothelium of the capillary network.
The P2 receptor subtypes involved in gastric motility are still not entirely clear [18]. Most reports claim that the P2Y1 receptor is involved in relaxation (refs). It seems likely that a P2X receptor is involved in contraction [16]. P2Y1 receptors mediate NANC inhibitory transmission to intestinal smooth muscle of laboratory animals and humans. α,β-Methylene ATP (α,β-meATP) has a potent relaxant action in some preparations. It seems likely that α,β-meATP is acting on P2X3 receptors on nerve varicosities to release ATP, which then acts on P2Y1 receptors on smooth muscle eliciting relaxation. P2Y2 and/or P2Y4 receptors mediate smooth muscle contraction in the small intestine, since they are activated by UTP as well as by ATP [4]. Previous data have also shown that contraction of rat duodenal muscularis mucosae smooth muscle is mediated by P2X receptors [12, 13]. In this study, smooth muscle fibers in the villus of mouse small intestine were found to express P2X1 receptors at a high level. It implies that ATP plays a role in regulating the dynamics of the small intestinal villus. It is possible that ATP, via P2X1 receptors, either directly or indirectly regulates the villus dynamics and the absorptive capacity of the lymph and blood capillaries and thus influences the transport of absorbed nutrients into the body circulation, although this still needs to be confirmed.
This study showed for the first time that all the SOM endocrine cells and enteric neurons in the gastrointestinal tract and pancreatic islets express P2X1 receptors. This suggests that the physiological function of these SOM endocrine cells may be regulated by ATP via P2X1 receptors.
SOM endocrine cells are distributed widely in gland and villus epithelial endocrine cells and enteric neurons of the gastrointestinal tract [1, 2, 7, 11, 19, 21]. Extensive functional and morphological research has demonstrated the pivotal role of SOM in the regulation of a wide variety of gastrointestinal activities. SOM has inhibitory effects on gastrointestinal motility and exocrine and endocrine secretion processes along the entire gastrointestinal tract [27].
The pancreas is an important endocrine organ, the hormones is produces are secreted directly into the blood by (at least) five types of cells. In rat islets, the endocrine cell subsets are as follows: α cells producing glucagon (20% of total islet cells), β cells producing insulin and amylin (≈ 70%), δ cells producing somatostatin (< 10%), γ cells producing pancreatic polypeptide (< 5%), and ε cells producing ghrelin (< 1%) [8]. Morphological and pharmacological data showed that P2Y1, P2Y2, P2Y4, P2Y6, and P2X subtypes and A1 receptors are all expressed on insulin-secreting β cells, P2X7 and A2 receptors on glucagon-secreting α cells and possibly P2Y1 receptors on δ cells [17]. This study has shown that P2X1 receptors are expressed on the δ cells of mouse and human pancreatic islets for the first time. It is possible that ATP via P2X1 receptors regulates SOM release from δ cells, which in turn affects the release of insulin from β cells and glucagon from α cells [23].
Previous data showed that SOM neurons were found to be widely distributed in both the myenteric plexus, where they represented 4.7% of the total population of neurons, and in the submucous plexus, where they formed 17.4% of the total population, although these neurons only account for a small part of the total number of neurons [7, 21]. SOM neurons in the myenteric plexus are descending interneurons, axons of which extend anally along the gut. Descending interneurons express both choline acetyltransferase and SOM and are involved in the conduction of migrating myoelectric complexes, which forms the inhibitory reflex of peristalsis in the small intestine [7, 9]. SOM neurons in the submucosal plexus are cholinergic secretomotor (non-vasodilator) neurons, which are involved in local absorption of electrolytes [9]. This study showed that all the SOM neurons in both myenteric and submucosal plexuses express P2X1 receptors. This suggests that ATP via P2X1 receptors may regulate SOM release and in turn regulate the inhibitory reflex of peristalsis and absorption of electrolytes in the small intestine, although this hypothesis needs to be shown experimentally.
In conclusion, P2X1 receptors were detected in the entire mouse gastrointestinal tract and pancreatic islets of both mouse and human. Strong immunofluorescence for P2X1 receptors was detected in the smooth muscle and capillary epithelial networks of the villus core of the small intestine. All δ cells containing SOM in the stomach corpus, small intestines, distal colon and pancreatic islets of mouse, and islets of human pancreas were found to express P2X1 receptors. P2X1 receptor-ir neurons containing SOM were also distributed widely in both the myenteric plexus of stomach corpus, small intestine and distal colon, and the submucosal plexus of small intestine and distal colon. These data imply that ATP via P2X1 receptors may regulate SOM release from δ cells in the gastrointestinal tract and pancreatic islets, regulate the dynamics of the villus and the transport of absorbed nutrients into the circulation in the small intestine, and regulate the inhibitory reflex of peristalsis and absorption of electrolytes in the gastrointestinal tract.
Funding information
This work was supported by the National Natural Science Foundation of P. R. China (81471260 to Z Xiang), by the Qingnian Science Foundation of Changzheng Hospital, Shanghai, People’s Republic of China (2016CZQN05 to R. Ji) and by the Qiannian Science Foundation of Second Military Medical University of P.L.A (2017QN15).
Conflicts of interest
Ruihua Ji declares that she has no conflict of interest.
Jiao Zhu declares that she has no conflict of interest.
Dan Wang declares that she has no conflict of interest.
Qian-Qian Sui declares that she has no conflict of interest.
Gillian E. Knight declares that she has no conflict of interest.
Geoffrey Burnstock declares that he has no conflict of interest.
Hongbin Yuan declares that she has no conflict of interest.
Zhenghua Xiang declares that she has no conflict of interest.
Ethical approval
All experimental procedures were approved by the Institutional Animal Care and Use Committee at Second Military Medical University and conformed to the UK Animals (Scientific Procedures) Act 1986 and associated guidelines on the ethical use of animals.
Contributor Information
Hongbin Yuan, Phone: (8621)81885822, Email: moc.nuyila@yyzcjfj.com.
Zhenghua Xiang, Phone: (8621) 81871044-8, Email: zhxiang@hotmail.com.
References
- 1.Alumets J, Ekelund M, El Munshid HA, Hakanson R, Loren I, Sundler F. Topography of somatostatin cells in the stomach of the rat: possible functional significance. Cell Tissue Res. 1979;202:177–188. doi: 10.1007/BF00232233. [DOI] [PubMed] [Google Scholar]
- 2.Arimura A, Sato H, Dupont A, Nishi N, Schally AV. Somatostatin: abundance of immunoreactive hormone in rat stomach and pancreas. Science. 1975;189:1007–1009. doi: 10.1126/science.56779. [DOI] [PubMed] [Google Scholar]
- 3.Barajas-Lopez C, Huizinga JD, Collins SM, Gerzanich V, Espinosa-Luna R, Peres AL. P2x-purinoceptors of myenteric neurones from the guinea-pig ileum and their unusual pharmacological properties. Br J Pharmacol. 1996;119:1541–1548. doi: 10.1111/j.1476-5381.1996.tb16070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnstock G. Purinergic signalling in the gastrointestinal tract and related organs in health and disease. Purinergic Signal. 2014;10:3–50. doi: 10.1007/s11302-013-9397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castelucci P, Robbins HL, Poole DP, Furness JB. The distribution of purine P2X(2) receptors in the guinea-pig enteric nervous system. Histochem Cell Biol. 2002;117:415–422. doi: 10.1007/s00418-002-0404-4. [DOI] [PubMed] [Google Scholar]
- 6.Castelucci P, Robbins HL, Furness JB. P2X(2) purine receptor immunoreactivity of intraganglionic laminar endings in the mouse gastrointestinal tract. Cell Tissue Res. 2003;312:167–174. doi: 10.1007/s00441-003-0715-3. [DOI] [PubMed] [Google Scholar]
- 7.Costa M, Furness JB, Smith IJ, Davies B, Oliver J. An immunohistochemical study of the projections of somatostatin-containing neurons in the guinea-pig intestine. Neuroscience. 1980;5:841–852. doi: 10.1016/0306-4522(80)90153-0. [DOI] [PubMed] [Google Scholar]
- 8.Elayat AA, el-Naggar MM, Tahir M. An immunocytochemical and morphometric study of the rat pancreatic islets. J Anat. 1995;186(Pt 3):629–637. [PMC free article] [PubMed] [Google Scholar]
- 9.Furness JB. Types of neurons in the enteric nervous system. J Auton Nerv Syst. 2000;81:87–96. doi: 10.1016/S0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- 10.Groschel-Stewart U, Bardini M, Robson T, Burnstock G. P2X receptors in the rat duodenal villus. Cell Tissue Res. 1999;297:111–117. doi: 10.1007/s004410051338. [DOI] [PubMed] [Google Scholar]
- 11.Hokfelt T, Efendic S, Hellerstrom C, Johansson O, Luft R, Arimura A. Cellular localization of somatostatin in endocrine-like cells and neurons of the rat with special references to the A1-cells of the pancreatic islets and to the hypothalamus. Acta Endocrinol Suppl (Copenh) 1975;200:5–41. [PubMed] [Google Scholar]
- 12.Ivancheva C, Rahamimoff R, Radomirov R. Apamin-sensitive nitric oxide- and ATP-mediated motor effects on the guinea pig small intestine. Gen Physiol Biophys. 2001;20:97–108. [PubMed] [Google Scholar]
- 13.Johnson CR, Charlton SJ, Hourani SM. Responses of the longitudinal muscle and the muscularis mucosae of the rat duodenum to adenine and uracil nucleotides. Br J Pharmacol. 1996;117:823–830. doi: 10.1111/j.1476-5381.1996.tb15267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HY, Bardini M, Burnstock G. Distribution of P2X receptors in the urinary bladder and the ureter of the rat. J Urol. 2000;163:2002–2007. doi: 10.1016/S0022-5347(05)67618-5. [DOI] [PubMed] [Google Scholar]
- 15.Lee HK, Ro S, Keef KD, Kim YH, Kim HW, Horowitz B, Sanders KM. Differential expression of P2X-purinoceptor subtypes in circular and longitudinal muscle of canine colon. Neurogastroenterol Motil. 2005;17:575–584. doi: 10.1111/j.1365-2982.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 16.Murthy KS, Makhlouf GM. Coexpression of ligand-gated P2X and G protein-coupled P2Y receptors in smooth muscle. Preferential activation of P2Y receptors coupled to phospholipase C (PLC)-beta1 via Galphaq/11 and to PLC-beta3 via Gbetagammai3. J Biol Chem. 1998;273:4695–4704. doi: 10.1074/jbc.273.8.4695. [DOI] [PubMed] [Google Scholar]
- 17.Novak I. Purinergic receptors in the endocrine and exocrine pancreas. Purinergic Signal. 2008;4:237–253. doi: 10.1007/s11302-007-9087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otsuguro K, Ohta T, Ito S, Nakazato Y. Two types of relaxation-mediating P2 receptors in rat gastric circular muscle. Jpn J Pharmacol. 1998;78:209–215. doi: 10.1254/jjp.78.209. [DOI] [PubMed] [Google Scholar]
- 19.Polak JM, Pearse AG, Grimelius L, Bloom SR. Growth-hormone release-inhibiting hormone in gastrointestinal and pancreatic D cells. Lancet. 1975;1:1220–1222. doi: 10.1016/S0140-6736(75)92198-4. [DOI] [PubMed] [Google Scholar]
- 20.Poole DP, Castelucci P, Robbins HL, Chiocchetti R, Furness JB. The distribution of P2X3 purine receptor subunits in the guinea pig enteric nervous system. Auton Neurosci. 2002;101:39–47. doi: 10.1016/S1566-0702(02)00179-0. [DOI] [PubMed] [Google Scholar]
- 21.Portbury AL, Pompolo S, Furness JB, Stebbing MJ, Kunze WA, Bornstein JC, Hughes S. Cholinergic, somatostatin-immunoreactive interneurons in the guinea pig intestine: morphology, ultrastructure, connections and projections. J Anat. 1995;187(Pt 2):303–321. [PMC free article] [PubMed] [Google Scholar]
- 22.Ruan HZ, Burnstock G. The distribution of P2X5 purinergic receptors in the enteric nervous system of mouse. Cell Tissue Res. 2005;319:191–200. doi: 10.1007/s00441-004-1002-7. [DOI] [PubMed] [Google Scholar]
- 23.Strowski MZ, Parmar RM, Blake AD, Schaeffer JM. Somatostatin inhibits insulin and glucagon secretion via two receptors subtypes: an in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Endocrinology. 2000;141:111–117. doi: 10.1210/endo.141.1.7263. [DOI] [PubMed] [Google Scholar]
- 24.Teramoto N, Szekely L, Pokrovskaja K, Hu LF, Yoshino T, Akagi T, Klein G. Simultaneous detection of two independent antigens by double staining with two mouse monoclonal antibodies. J Virol Methods. 1998;73:89–97. doi: 10.1016/S0166-0934(98)00048-2. [DOI] [PubMed] [Google Scholar]
- 25.Van Crombruggen K, Van Nassauw L, Timmermans JP, Lefebvre RA. Inhibitory purinergic P2 receptor characterisation in rat distal colon. Neuropharmacology. 2007;53:257–271. doi: 10.1016/j.neuropharm.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Van Nassauw L, Brouns I, Adriaensen D, Burnstock G, Timmermans JP. Neurochemical identification of enteric neurons expressing P2X(3) receptors in the guinea-pig ileum. Histochem Cell Biol. 2002;118:193–203. doi: 10.1007/s00418-002-0447-6. [DOI] [PubMed] [Google Scholar]
- 27.Van Op den Bosch J, Adriaensen D, Van Nassauw L, Timmermans JP. The role(s) of somatostatin, structurally related peptides and somatostatin receptors in the gastrointestinal tract: a review. Regul Pept. 2009;156:1–8. doi: 10.1016/j.regpep.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Vial C, Evans RJ. Smooth muscle does not have a common P2x receptor phenotype: expression, ontogeny and function of P2x1 receptors in mouse ileum, bladder and reproductive systems. Auton Neurosci. 2001;92:56–64. doi: 10.1016/S1566-0702(01)00319-8. [DOI] [PubMed] [Google Scholar]
- 29.Vulchanova L, Arvidsson U, Riedl M, Wang J, Buell G, Surprenant A, North RA, Elde R. Differential distribution of two ATP-gated channels (P2X receptors) determined by immunocytochemistry. Proc Natl Acad Sci U S A. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiang Z, Burnstock G. Development of nerves expressing P2X3 receptors in the myenteric plexus of rat stomach. Histochem Cell Biol. 2004;122:111–119. doi: 10.1007/s00418-004-0680-2. [DOI] [PubMed] [Google Scholar]
- 31.Xiang Z, Burnstock G. P2X2 and P2X3 purinoceptors in the rat enteric nervous system. Histochem Cell Biol. 2004;121:169–179. doi: 10.1007/s00418-004-0620-1. [DOI] [PubMed] [Google Scholar]
- 32.Xiang Z, Burnstock G. Distribution of P2Y2 receptors in the guinea pig enteric nervous system and its coexistence with P2X2 and P2X3 receptors, neuropeptide Y, nitric oxide synthase and calretinin. Histochem Cell Biol. 2005;124:379–390. doi: 10.1007/s00418-005-0043-7. [DOI] [PubMed] [Google Scholar]
- 33.Yu Q, Zhao Z, Sun J, Guo W, Fu J, Burnstock G, He C, Xiang Z. Expression of P2X6 receptors in the enteric nervous system of the rat gastrointestinal tract. Histochem Cell Biol. 2010;133:177–188. doi: 10.1007/s00418-009-0659-0. [DOI] [PubMed] [Google Scholar]
- 34.Zhou X, Galligan JJ. P2X purinoceptors in cultured myenteric neurons of guinea-pig small intestine. J Physiol. 1996;496(Pt 3):719–729. doi: 10.1113/jphysiol.1996.sp021722. [DOI] [PMC free article] [PubMed] [Google Scholar]