Abstract
Estrogen receptor alpha (ERα) is detected in more than 70% of the cases of breast cancer. Nuclear activity of ERα, a transcriptional regulator, is linked to the development of mammary tumors, whereas the extranuclear activity of ERα is related to endocrine therapy resistance. ERα polyubiquitination is induced by the estradiol hormone, and also by selective estrogen receptor degraders, resulting in ERα degradation via the ubiquitin proteasome system. Moreover, polyubiquitination is related to the ERα transcription cycle, and some E3-ubiquitin ligases also function as coactivators for ERα. Several studies have demonstrated that ERα polyubiquitination is inhibited by multiple mechanisms that include posttranslational modifications, interactions with coregulators, and formation of specific protein complexes with ERα. These events are responsible for an increase in ERα protein levels and deregulation of its signaling in breast cancers. Thus, ERα polyubiquitination inhibition may be a key factor in the progression of breast cancer and resistance to endocrine therapy.
Keywords: Estrogen receptor alpha polyubiquitination, Breast cancer, Estrogen receptor alpha
Core tip: The inhibition of the estrogen receptor alpha polyubiquitination and degradation by several molecular mechanisms is related to the progression of breast cancer and resistance to endocrine therapy.
INTRODUCTION
Estrogen receptor alpha (ERα) protein, also known as nuclear receptor subfamily3 group A member 1 (NR3A1), comprises of 595 amino acids, organized in two activation function domains (AF-1 and AF-2), a DNA-binding domain (DBD), a ligand-binding domain (LBD) that recognize the 17beta-estradiol hormone (E2), and a hinge region that connects the DBD and the LBD[1-3] (Figure 1). Many nuclear functions of ERα are triggered by the binding of E2 to the receptor[4,5], inducing ERα homodimers to bind to estrogen responsive elements (ERE) within the enhancer and promoter regions of E2-target genes[6,7]. In these events, pioneer factors expose chromatin sections, facilitating the association of ERα with EREs[8]. Moreover, transcriptional coregulators are recruited by the AF-1 and AF-2 domains of the receptor for the remodeling of the chromatin structure[9,10] and promotion of chromatin loops that modulate E2-responsive gene expression[11,12]. In addition, there is crosstalk between ERα and other signaling pathways: ERα acts as a coregulator by interacting with other transcription factors, such as activator protein 1 (AP-1), specificity protein 1 (Sp1), and nuclear factor-κB (NF-κB)[3,5,13-17]. Additionally, ERα is phosphorylated and transcriptionally activated in response to growth factors such as the epidermal growth factor (EGF) and insulin-like growth factor (IGF)[13,14,18-20]. Recently, progesterone receptor (PR) was shown as an ERα interacting protein that modulates and re–directs the binding of ERα to the chromatin and the expression of specific genes in breast cancer cells [21] (Figure 2).
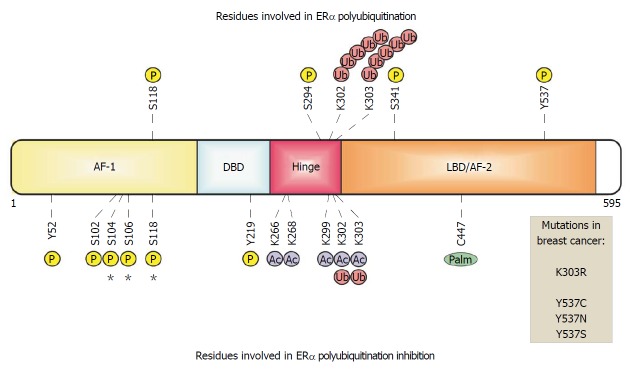
Figure 1.
Estrogen receptor α in breast cancer cells. ERα is organized in functional domains. The transactivation domains AF-1 and AF-2 recruit both coactivators and corepressors. The DNA-binding domain (DBD) recognizes and binds to estrogen response elements in enhancers or promoters. The ligand-binding domain (LBD) is recognized and activated by the 17 beta estradiol hormone. The hinge domain links LBD and DBD allowing the conformational changes of this receptor. Some residues are modified by phosphorylation, acetylation, ubiquitination and palmitoylation , which are related with ERα polyubiquitination. Sites of phosphorylation or mutations in ERα that have been identified in breast–cancer biopsy samples are indicated.
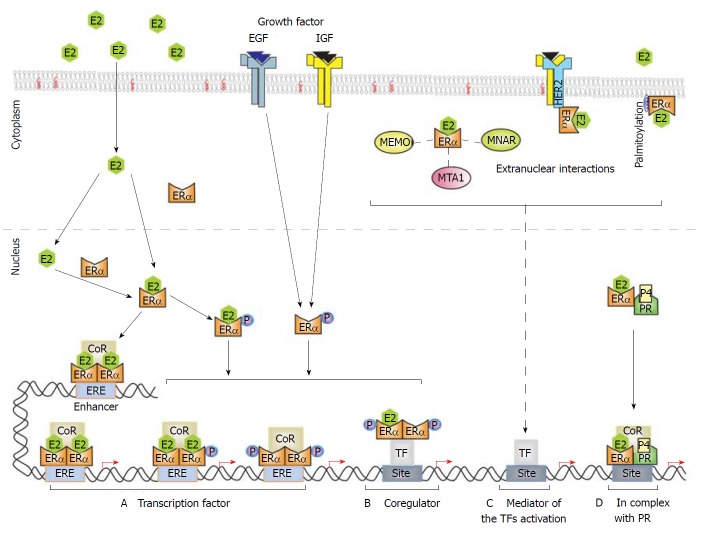
Figure 2.
Nuclear and extranuclear signaling of estrogen receptor α. E2 binds to ERα in the cytoplasm and/or nucleus. Then ERα forms homodimers that recognize the ERE sequence (AGGTCAnnnTGACCT) in target enhancers and promoters, recruiting coregulator (CoR) complexes such as coactivators to induce gene expression. ERα phosphorylation can be induced by E2 to modulate its activity as a transcription regulator. A and B: Growth factors (epidermal growth factor and insulin-like growth factor) also induce ERα phosphorylation in an E2-independent manner to promote ERα activity as a transcription factor or CoR for some transcription factors (i.e., AP-1, Sp1, and NF-κB); C: Cell membrane-associated ERα (via palmitoylation) associated with transmembranal receptors (i.e., HER2) or with cytoplasmatic proteins as (i.e., MEMO, MTA1 and MNAR). These extranuclear interactions can induce kinase–dependent signaling that could finalize in the activation of some transcription factors; D: PR can associate with ERα to coordinate the binding of ERα to the chromatin modulating the expression of specific genes.
ERα also exhibits extranuclear activity by associating with the cell membrane via palmitoylation, and with the help of protein complexes, linked to the cell membrane or cytoplasm[22] (Figure 2). Thereafter, ERα transduces rapid extranuclear signaling that can trigger second messengers such as calcium and cAMP, and activate kinases such as ERK/MAPK, PI3K/AKT, PKC and Src kinase[13,23,24]. Both nuclear and extranuclear signaling of ERα are connected and are critical in about 70% of breast cancer cases (ERα+ breast cancer)[13,24,25]. Consequently, ERα is a target for endocrine therapy via the use of selective estrogen receptor modulators (SERMs), such as tamoxifen (Tam), which competes with E2 by binding to ERα to inhibit its transcriptional activity, as well as, via the use of selective estrogen receptor degraders (SERDs) such as fulvestrant that decreases the ERα stability[8,14,26,27]. The acquisition of resistance to these treatments commonly occurs in ERα+ breast cancer, and although the mechanisms are unclear, the extranuclear signaling of ERα is strongly activated under this condition[19,20,26,28-31].
The activation or inhibition of ERα activity is modulated by its transcriptional coregulators, by phosphorylation induced by E2 hormones and growth factors, and by other posttranslational modifications such as ubiquitination. Remarkably, several studies have emerged to demonstrate that multiple mechanisms are activated in ERα+ breast cancers to inhibit ERα polyubiquitination, increasing its signaling pathways (Figure 2), which have crucial implications in the progression of this cancer type, as we will describe in the following sections.
GENERALITIES OF THE POSTTRANSLATIONAL MODIFICATION “UBIQUITINATION” FOR ERα IN BREAST CANCER CELLS
ERα is a monoubiquitination and polyubiquitination-target. However, fewer reports are available to demonstrate monoubiquitination of ERα, in comparison to those that exhibit polyubiquitination of this receptor. Nevertheless, these studies clearly show that ERα monoubiquitination is decreased by E2, and that, this modification is important, both for stability and for the transcriptional activity of this receptor in breast cancer. In contrast, polyubiquitination is induced by E2, resulting in a signal to direct ERα degradation via the UPS[14,32,33], facilitated by the concerted action of the enzymes E1 (ubiquitin activating enzyme), E2 (ubiquitin conjugating enzyme), and E3 (ubiquitin ligase)[32,33]. The specific covalent binding of ubiquitin to ERα lysine residues is mediated by several E3 ubiquitin ligases for ERα, that include CHIP[34], E6AP[35], BRCA1[36], BARD1[37], SKP2[38], MDM2[39], and Hbo1[40]. Importantly, E2 treatment induces ERα polyubiquitination, followed by its degradation by the UPS[14,17,33,41-43].
Although polyubiquitination leads to ERα downregulation through its degradation by the 26S proteasome, it is important to note that, this modification and the proteasome activity, have also been reported as elements required for the transcriptional cycle of ERα. Likewise, it has been evidenced that ERα bound to ERE can recruit coactivators, some of which possess E3-ubiquitin ligase activity, such as SKP2[17], E6AP, and RNF8. As coactivators enhance the activity of ERα, and the activity of E3-ubiquitin ligases mediate the downregulation of this receptor, the recruitment of these proteins with dual function may maintain a balance in the level and activity of ERα[17,44,45].
ERα residues, K302 and K303, have been suggested as the lysine targets for ubiquitination and degradation, in response to E2 and fulvestrant, but the same residues are also important for ERα stability in untreated breast cancer cells[46]. Against this background, it maybe envisaged that, several factors delicately modulate the stability and degradation of ERα, which may be altered in breast cancer.
Additionally, the ubiquitination of ERα is also related to its phosphorylation state. Several kinases, such as CDK11p58[47], cyclin E-CDK2[17], Src[35], PKC[42], p38MAPK[38], and ERK7[48] have been reported as modifiers of ERα in breast cancer. The main residues of ERα that are phosphorylated in E2-response, and have been associated with its polyubiquitination and degradation, are S118[49], S294[38], S341[17], and Y537[35]. A key example is the sequential modification of ERα, where, first, the ERα Y537 residue is phosphorylated by Src kinase in E2-treated cells, followed by E6AP, an E3-ubiquitin ligase, which induces ERα polyubiquitination and its degradation[35]. Thus, phosphorylation and ubiquitination of ERα are interconnected in order to control both, the abundance and the functions of this receptor.
IS ERα IN BREAST CANCER CELLS POLYUBIQUITINATED AND DEGRADED?
In recent years, several studies have emerged to demonstrate the inhibition of polyubiquitination of ERα and consequently, a decrease in its degradation via the UPS, increasing its protein stability in breast cancer cells, through several mechanisms and ERα-associated proteins. Here, we describe these evidences.
ERα polyubiquitination inhibitor proteins in breast cancer cells
ERα polyubiquitination inhibitor proteins (EPIP). There has been a progressive increase in the number of ERα polyubiquitination inhibitor proteins that have been discovered in breast cancer cells, which we have grouped and identified as EPIP. So far, it has been reported that proteins such as Mucin 1 (MUC1), PIN1, GSK3, LMTK3, RNF8, RNF31, RB, ABL, SHARPIN, and SMURF1 have the ability to interact with ERα, conferring it protection against polyubiquitination and degradation. Interestingly, not all of these proteins have related sequences and structures, but some of them are functionally similar.
MUC1 and Protein interacting with Never in mitosis A (PIN1), for example, induce the formation of stable transcription complexes on the DNA[49,50]. MUC1 interacts with ERα to inhibit its polyubiquitination and degradation, and recruits coactivators such as SRC1 and GRIP on E2-regulated promoters to enhance gene transcription linked to cellular proliferation, migration, tumorigenicity, and endocrine resistance[50-54]. Likewise, PIN1 interacts with ERα phosphorylated at S118, inducing its cis/trans isomerization. Moreover, PIN1 blocks the polyubiquitination and degradation of ERα by preventing its interaction with the E6AP E3 ligase, hence enhancing its stability, binding to EREs, and the subsequent transcriptional activity of ERα[10,49,55-57]. High levels of PIN1 and ERα, and low levels of E6AP are observed in endocrine resistance[49].
Other examples are GSK3, LMTK3, and ABL1 kinases that phosphorylate ERα to inhibit its polyubiquitination[58,59]. First, the glycogen synthase kinase-3 (GSK3) isoforms interact with and phosphorylate ERα at S102, S104, S106, and S118. GSK3 depletion decreases phosphorylation and E2-induced transcriptional activity by increasing polyubiquitination and degradation of this receptor[59-61]. Thereafter, LMTK3 (lemur tyrosine kinase 3) interacts with and phosphorylates ERα to protect it from polyubiquitination and degradation via the UPS in breast cancer cells[58]. Similarly, ABL (ABL proto-oncogene 1, non-receptor tyrosine kinase) interacts with and phosphorylates ERα at Y52 and Y219, increasing the ERα stability and resistance to Tam; both proteins are increased in breast tumor tissue samples[62,63].
On the other hand, RB induces the assembly of ERα with chaperone proteins[64]. Hence, retinoblastoma (RB) interacts with ERα, HSP90, and p23 in the cytoplasm to protect ERα from polyubiquitination and degradation by the UPS. ERα is highly ubiquitinated and degraded in RB-knockdown cells; however, its levels are restored with MG132 (a proteasome inhibitor) treatment in breast cancer[64].
Interestingly, E3 ubiquitin ligases such as RNF8, RNF31, SHARPIN, and SMURF1 interact with ERα to block its polyubiquitination and to promote the proliferation of breast cancer cells. RNF8, RNF31, and SHARPIN inhibit ERα polyubiquitination by catalyzing monoubiquitination of this receptor, and as a result, ERα protein levels and E2-dependent transcriptional activity are enhanced in breast cancer cells[65]. SHARPIN could monoubiquitinate the ERα K302/303, but whether these residues are also modified by RNF8 and/or RNF31 is unclear. Moreover, RNF8 also acts as a coactivator for ERα in breast cancer cells. Instead, SMURF1 apparently inhibits polyubiquitination of ERα, but the implicated mechanisms need to be studied[65-68].
Other proteins and modifications that inhibit ERα polyubiquitination
ERα polyubiquitination indirect inhibitors (EPII), intriguingly, the inhibition of ERα polyubiquitination also occurs with the help of other proteins that lack the ability to directly interact with ERα. For instance, it has been suggested that Src-dependent phosphorylation of ERα allows E6AP to polyubiquitinate and induce the degradation of this receptor. However, PEBP4 (phosphatidyl ethanolamine-binding protein 4) protein[69,70] interacts with Src, blocking the phosphorylation and degradation of ERα induced by Src[69].
Furthermore, although the mechanisms are unclear, it has been reported that ERα protein levels decrease in cells with low levels of REGγ (PA28γ, a nuclear proteasome coactivator), but when the proteasome is inhibited by MG132 treatment, ERα protein levels are recovered, suggesting that downregulation of REGγ promotes ERα polyubiquitination and degradation. High levels of REGγ and ERα in breast tumors correlated with poor prognosis in patients with breast cancer[69].
Additionally, some posttranslational modifications are also associated with ERα polyubiquitination inhibition. Hence, ERα acetylation induced by trichostatin (a deacetylase inhibitor) increases the p300 levels and the stability of the receptor in breast cancer cells, but the mechanisms implicated need to be investigated (Figure 1)[71]. Palmitoylation has also been linked to ERα polyubiquitination since it has been shown that the ERα mutants that cannot be palmitoylated are polyubiquitinated and degraded via UPS[72].
Mutations and modifications that affect ERα polyubiquitination detected in mammary tumors from patients
ERα polyubiquitination has a clinical relevance, since mutations and/or posttranslational modifications such as phosphorylation in residues of ERα have been identified in tumor tissues from samples of patients with breast cancer, and these residues have been linked to the polyubiquitination and downregulation by degradation of this receptor. Thus, the Y537 residue is required for the ERα phosphorylation, and this modification subsequently promotes polyubiquitination and degradation of the receptor[35]. However, mutations in the residues Y537N, Y537C, and Y537S are detected in mammary tumors of patients with metastasis and endocrine resistance. Accordingly, ERα polyubiquitination and degradation is prevented by experimentally induced mutations at the Y537 residue, and similarly, these mutations have been associated with the development of endocrine therapy resistance in breast cancer[15,73,74]. In the same way, the K303 residue is needed for ERα polyubiquitination and degradation, but this residue has been identified to be mutated as K303R in tumors of patients who have poor survival outcome and prognosis[46,74]. Other residues, such as S104, S106, S118, and S294, that seem to be related with ERα stability, have been found to be phosphorylated in breast tumor samples[15,73].
ERα POLYUBIQUITINATION INHIBITION IN BREAST CANCER AS A KEY FACTOR FOR THERAPEUTIC STRATEGY
ERα polyubiquitination for its downregulation via the UPS, is a central mechanism of some endocrine therapies with SERDs, such as fulvestrant[46,75]. Clearly, the induction of ERα polyubiquitination for its degradation decreases the abundance and pro-tumor activity of ERα, consequently novel drugs including AZD9496[76], GDC-0810[77], bazedoxifene[78], and RAD1901[79] have been synthetized as SERDs, but more studies are required. Despite the importance of SERDs in the therapy of breast cancer, EPIP are promising targets for the management of this disease. Remarkably, the proteins that inhibit the ERα polyubiquitination are enhanced in ERα+ breast cancers, contributing to disease progression. For this reason, EPIP may be useful as a biomarker for breast cancer and as a therapeutic target.
PIN1 is overexpressed in breast cancer and is related to mammary tumor growth, and epithelial-mesenchymal transition, and natural and synthetic inhibitors are being probed to control its activity[55,57,80-87]. Similarly, LMTK3 overexpression stimulates cellular proliferation and tumor formation, and correlates with shorter survival times in ERα+ breast cancer, and resistance to Tam treatment, but these events are reduced when LMTK3 expression is decreased[58,88-90]. Moreover, CG0009, is a GSK3 inhibitor that decreases proliferation of breast cancer cells[61,73,91-94].
Another molecule is RNF31, whose overexpression increases ERα protein levels, expression of ERα target genes and the growth of breast cancer cells, and these events are decreased when RNF31 is abated[65]. Lastly, the loss of RB expression seems to be related to the loss of ERα stability in ERα negative (ERα–) breast cancers and with poor responses to hormonal therapies in patients[64,95-98]. Thus, these proteins can be potential biomarkers and target for the treatment of ERα+ breast cancer.
Among EPIIs, PEBP4 inhibits ERα polyubiquitination and enhances its transcriptional activity in breast cancer cells. Because PEBP4 is overexpressed in breast cancer and competes with ERα for components of the UPS, this protein may be an important target for breast cancer. Additionally, specific posttranslational modifications, such as palmitoylation, acetylation and phosphorylation, as well as, mutations of sites linked to ERα polyubiquitination and degradation, demands more research to find new strategies for detection and treatment of breast cancer.
Muc1 is an EPIP in breast cancer
Mucin 1 (MUC1) is a heterodimeric glycoprotein conformed by MUC1 N-terminal (MUC1-N) and MUC1 C-terminal (MUC1-C) subunits[52]. MUC1-N is an extracellular glycosylated subunit and MUC1-C is a transmembrane subunit with a cytoplasmic domain that interacts with diverse proteins[54]. MUC1 is localized on the apical borders in normal mammary epithelium, but under breast cancer conditions, it also localizes to the nucleus. An aberrant expression of MUC1-C is detected in breast cancer cells through a regulation loop that implicates Rab31 protein inhibits the lysosomal degradation of MUC1-C, and Rab31 gene expression is induced by MUC1-C[52-54,99]. Furthermore, MUC1 is upregulated in 90% of breast cancers, where the expression of Rab31 gene and other genes associated with endocrine resistance are modulated by the MUC1-C/ERα complex. For these reasons, MUC1 has been suggested as a potential biomarker of breast cancer and predictor of resistance to Tam treatment[51,100,101] (Figure 3).
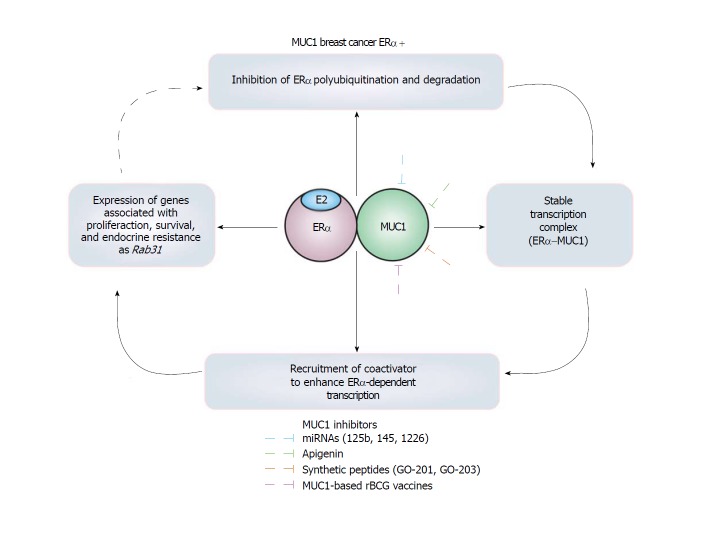
Figure 3.
Mucin 1 is an estrogen receptor α polyubiquitination inhibitor protein in breast cancer cells.
Interestingly, MUC1-C subunit interacts with DBD of ERα promoting (1) Inhibition of ERα polyubiquitination maintaining high levels of this receptor; (2) a stable complex between MUC1-C and ERα; and (3) an enhancement in the pro-tumor transcriptional activity of ERα since SRC1 and GRIP coactivators with histone acetyltransferase activity are recruited by MUC1[50]. Thus, MUC1-C increases the growth and survival induced by E2 in breast cancer cells, but also transformation, loss of cellular polarity, cellular proliferation and migration, anchorage-independent growth , and tumorigenicity in transgenic mouse models[51,99,102-104].
Remarkably, MUC1 is an EPIP involved in proliferation and endocrine resistance[50,53,54,100,105], inhibited by miR-125b[106], miR-145[104], miR-1226[103], and by specific siRNAs, inducing apoptosis, reducing cell proliferation, and increasing sensitivity to Tam[100]. Similarly, apigenin[107], and the synthetic peptides GO-201[54] and GO-203[100], affect localization and dimerization of MUC1, and as a result, tumor development is decreased, and sensitivity to Tam is increased[54,100,107]. Moreover, MUC1-based rBCG (Bacillus Calmette-Guerin) vaccines induce anti-MUC immune responses inhibiting the growth of tumors in mice[108,109]. Interestingly, high levels of Rab31 antigen have been associated with a proliferative status, a high tumor grade, and with poor 5-year disease-free survival in patients with ERα+ breast cancer. Consequently, the Rab31 antigen levels in mammary tumors have been suggested as a biomarker for ERα+ breast cancers that may also to be useful in the selection of patients for MUC1-targeted therapeutic strategies[110].
CONCLUSION
Several mechanisms seem to cooperate to inhibit ERα polyubiquitination, decreasing its degradation in ERα+ breast cancer cells. These cells become resistant to ERα polyubiquitination due to the evident upregulation of proteins, modifications, and mutations that protect it from ubiquitination. There is no pattern of the characteristics of the inhibitor or protector proteins for ERα polyubiquitination. Some of the reported EPIPs are MUC1, GSK3, LMTK3, RNF8, RNF31, SHARPIN, SMURF1, RB, and PIN1. All of them inhibit ERα polyubiquitination and its degradation in a dissimilar manner, via subcellular compartments or mechanisms. Some of them can be grouped as coactivators for ERα (MUC1, PIN1, and RNF8), kinases for ERα (GSK3, LMTK3, and ABL1), E3 ubiquitin ligase (RNF8, RNF31, SHARPIN, and SMURF1), and scaffold protein (RB). Amongst these different mechanisms, the participation of E3-ubiquitin ligases, such as RNF8, RNF31, and SHARPIN, are interesting, since they catalyze ERα monoubiquitination, suggesting a possible competition between monoubiquitination and polyubiquitination of this receptor.
Considering the findings described above, inhibition of ERα polyubiquitination, increases its abundance, and the expression of E2-dependent genes linked to proliferation and tumor development. In addition, inhibition of ERα polyubiquitination may have other serious implications, since it has been reported that this modification and proteasome activity are coupled to the transcriptional cycle of this receptor[45]. Moreover, it has been proposed that high ERα protein levels are related to ERα binding to other DNA regulatory regions of genes that are atypically activated under this condition[111]. Thus, inhibition of ERα polyubiquitination and its degradation increases the stability of this receptor, but also affects ERα/E2 signaling and its transcriptional activity, involved with the development of tumor and endocrine resistance[111,112] (Figure 4).
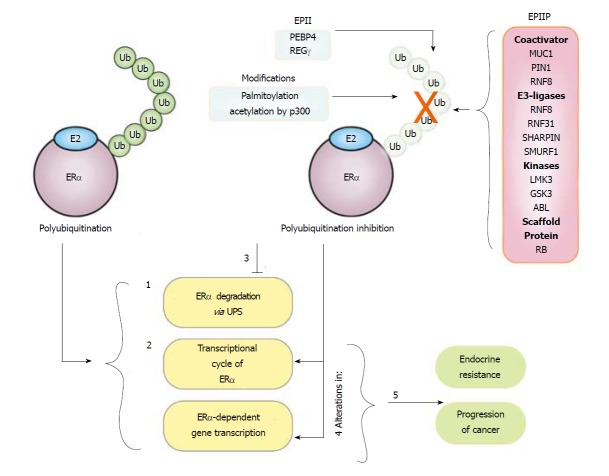
Figure 4.
Mechanisms implicated in the estrogen receptor α polyubiquitination inhibition. Half-life of estrogen receptor α protein oscillates between 3-5 h under basal condition. E2 treatment induces ERα polyubiquitination, and as result: (1) Degradation of this receptor is promoted, decreasing its protein levels starting from 1h after treatment; (2) the ERα transcriptional cycle is activated. ERα polyubiquitination inhibitor proteins (EPIP) and ERα polyubiquitination indirect inhibitors (EPII) and other modifications increased in breast cancer cells can inhibit the basal and E2-induced polyubiquitination of ERα; resulting in (3) the inhibition of its degradation and an enhancement in the ERα protein levels; (4) alterations in the transcription cycle of this receptor and the expression of its targets genes; and (5) these events seem to be associated with endocrine resistance and progression of breast cancer.
Importantly, there is an interplay between inhibition of ERα polyubiquitination and endocrine therapy resistance in ERα+ breast cancer, promoted by EPIP and EPII [49,50,58,65]. In contrast, in luminal B breast cancers or ERα– breast cancers, RB is commonly lost or dysfunctional, leading to high levels of polyubiquitination and degradation of ERα, with a poor prognosis for patients. Therefore, EPIP, EPII, and mutations and modifications that inhibit ERα polyubiquitination and degradation may act in a cooperative manner to enhance the stability of the receptor in the progression of breast cancer. Consequently, the mechanisms involved in the inhibition of ERα polyubiquitination represent useful biomarkers, therapeutic targets, and prognostic indicators of endocrine therapy in breast cancer.
In conclusion, EPIP, EPII, and mutations and modifications associated to ERα polyubiquitination inhibition, enhance the signaling pathways of this receptor. These findings represent a new field in breast cancer, for the establishment of potential biomarkers, as well as, in the design of effective therapeutic targets to control the progression of this disease. Integration between the molecular basis of ERα inhibition and its correlation with the progression of breast tumors remains to be elicited.
ACKNOWLEDGMENTS
We thank Dr. Bibiana Ortega Dominguez for her helpful review of the manuscript.
Footnotes
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Manuscript source: Invited manuscript
Peer-review started: April 27, 2018
First decision: June 15, 2018
Article in press: June 29, 2018
Specialty type: Oncology
Country of origin: Mexico
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Sukocheva OA S- Editor: Cui LJ L- Editor: A E- Editor: Tan WW
Contributor Information
Angeles C Tecalco-Cruz, Programa de Investigación de Cáncer de Mama (PICM), Departamento de Biología Molecular y Biotecnología, Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de México, México 04510, México. 12anget@gmail.com.
Josué O Ramírez-Jarquín, Instituto de Fisiología Celular, Universidad Nacional Autónoma de México, México 04510, México.
References
- 1.Germain P, Staels B, Dacquet C, Spedding M, Laudet V. Overview of nomenclature of nuclear receptors. Pharmacol Rev. 2006;58:685–704. doi: 10.1124/pr.58.4.2. [DOI] [PubMed] [Google Scholar]
- 2.Kumar R, Zakharov MN, Khan SH, Miki R, Jang H, Toraldo G, Singh R, Bhasin S, Jasuja R. The dynamic structure of the estrogen receptor. J Amino Acids. 2011;2011:812540. doi: 10.4061/2011/812540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng HW, Perkins R, Tong W, Hong H. Versatility or promiscuity: the estrogen receptors, control of ligand selectivity and an update on subtype selective ligands. Int J Environ Res Public Health. 2014;11:8709–8742. doi: 10.3390/ijerph110908709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manavathi B, Dey O, Gajulapalli VN, Bhatia RS, Bugide S, Kumar R. Derailed estrogen signaling and breast cancer: an authentic couple. Endocr Rev. 2013;34:1–32. doi: 10.1210/er.2011-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vrtačnik P, Ostanek B, Mencej-Bedrač S, Marc J. The many faces of estrogen signaling. Biochem Med (Zagreb) 2014;24:329–342. doi: 10.11613/BM.2014.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hah N, Kraus WL. Hormone-regulated transcriptomes: lessons learned from estrogen signaling pathways in breast cancer cells. Mol Cell Endocrinol. 2014;382:652–664. doi: 10.1016/j.mce.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hah N, Murakami S, Nagari A, Danko CG, Kraus WL. Enhancer transcripts mark active estrogen receptor binding sites. Genome Res. 2013;23:1210–1223. doi: 10.1101/gr.152306.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manavathi B, Samanthapudi VS, Gajulapalli VN. Estrogen receptor coregulators and pioneer factors: the orchestrators of mammary gland cell fate and development. Front Cell Dev Biol. 2014;2:34. doi: 10.3389/fcell.2014.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hervouet E, Cartron PF, Jouvenot M, Delage-Mourroux R. Epigenetic regulation of estrogen signaling in breast cancer. Epigenetics. 2013;8:237–245. doi: 10.4161/epi.23790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajbhandari P, Finn G, Solodin NM, Singarapu KK, Sahu SC, Markley JL, Kadunc KJ, Ellison-Zelski SJ, Kariagina A, Haslam SZ, et al. Regulation of estrogen receptor α N-terminus conformation and function by peptidyl prolyl isomerase Pin1. Mol Cell Biol. 2012;32:445–457. doi: 10.1128/MCB.06073-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He C, Wang X, Zhang MQ. Nucleosome eviction and multiple co-factor binding predict estrogen-receptor-alpha-associated long-range interactions. Nucleic Acids Res. 2014;42:6935–6944. doi: 10.1093/nar/gku327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu MH, Cheung E. Estrogen receptor-mediated long-range chromatin interactions and transcription in breast cancer. Mol Cell Endocrinol. 2014;382:624–632. doi: 10.1016/j.mce.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Acconcia F, Kumar R. Signaling regulation of genomic and nongenomic functions of estrogen receptors. Cancer Lett. 2006;238:1–14. doi: 10.1016/j.canlet.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Kerdivel G, Flouriot G, Pakdel F. Modulation of estrogen receptor alpha activity and expression during breast cancer progression. Vitam Horm. 2013;93:135–160. doi: 10.1016/B978-0-12-416673-8.00004-6. [DOI] [PubMed] [Google Scholar]
- 15.Le Romancer M, Poulard C, Cohen P, Sentis S, Renoir JM, Corbo L. Cracking the estrogen receptor’s posttranslational code in breast tumors. Endocr Rev. 2011;32:597–622. doi: 10.1210/er.2010-0016. [DOI] [PubMed] [Google Scholar]
- 16.Soltysik K, Czekaj P. Membrane estrogen receptors - is it an alternative way of estrogen action? J Physiol Pharmacol. 2013;64:129–142. [PubMed] [Google Scholar]
- 17.Zhou W, Srinivasan S, Nawaz Z, Slingerland JM. ERα, SKP2 and E2F-1 form a feed forward loop driving late ERα targets and G1 cell cycle progression. Oncogene. 2014;33:2341–2353. doi: 10.1038/onc.2013.197. [DOI] [PubMed] [Google Scholar]
- 18.Treviño LS, Weigel NL. Phosphorylation: a fundamental regulator of steroid receptor action. Trends Endocrinol Metab. 2013;24:515–524. doi: 10.1016/j.tem.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tecalco-Cruz AC, Pérez-Alvarado IA, Ramírez-Jarquín JO, Rocha-Zavaleta L. Nucleo-cytoplasmic transport of estrogen receptor alpha in breast cancer cells. Cell Signal. 2017;34:121–132. doi: 10.1016/j.cellsig.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Tecalco-Cruz AC, Ramírez-Jarquín JO. Mechanisms that Increase Stability of Estrogen Receptor Alpha in Breast Cancer. Clin Breast Cancer. 2017;17:1–10. doi: 10.1016/j.clbc.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Mohammed H, Russell IA, Stark R, Rueda OM, Hickey TE, Tarulli GA, Serandour AA, Birrell SN, Bruna A, Saadi A, et al. Corrigendum: Progesterone receptor modulates ERα action in breast cancer. Nature. 2015;526:144. doi: 10.1038/nature14959. [DOI] [PubMed] [Google Scholar]
- 22.Pedram A, Razandi M, Deschenes RJ, Levin ER. DHHC-7 and -21 are palmitoylacyltransferases for sex steroid receptors. Mol Biol Cell. 2012;23:188–199. doi: 10.1091/mbc.E11-07-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Acconcia F, Marino M. The Effects of 17β-estradiol in Cancer are Mediated by Estrogen Receptor Signaling at the Plasma Membrane. Front Physiol. 2011;2:30. doi: 10.3389/fphys.2011.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marino M, Galluzzo P, Ascenzi P. Estrogen signaling multiple pathways to impact gene transcription. Curr Genomics. 2006;7:497–508. doi: 10.2174/138920206779315737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyoshi Y, Murase K, Saito M, Imamura M, Oh K. Mechanisms of estrogen receptor-α upregulation in breast cancers. Med Mol Morphol. 2010;43:193–196. doi: 10.1007/s00795-010-0514-3. [DOI] [PubMed] [Google Scholar]
- 26.Cook KL, Shajahan AN, Clarke R. Autophagy and endocrine resistance in breast cancer. Expert Rev Anticancer Ther. 2011;11:1283–1294. doi: 10.1586/era.11.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magnani L, Brunelle M, Gévry N, Lupien M. Chromatin landscape and endocrine response in breast cancer. Epigenomics. 2012;4:675–683. doi: 10.2217/epi.12.64. [DOI] [PubMed] [Google Scholar]
- 28.de Leeuw R, Neefjes J, Michalides R. A role for estrogen receptor phosphorylation in the resistance to tamoxifen. Int J Breast Cancer. 2011;2011:232435. doi: 10.4061/2011/232435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson AB, O’Malley BW. ERasing breast cancer resistance through the kinome. Nat Med. 2011;17:660–661. doi: 10.1038/nm0611-660. [DOI] [PubMed] [Google Scholar]
- 30.Muluhngwi P, Klinge CM. Roles for miRNAs in endocrine resistance in breast cancer. Endocr Relat Cancer. 2015;22:R279–R300. doi: 10.1530/ERC-15-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–247. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helzer KT, Hooper C, Miyamoto S, Alarid ET. Ubiquitylation of nuclear receptors: new linkages and therapeutic implications. J Mol Endocrinol. 2015;54:R151–R167. doi: 10.1530/JME-14-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou W, Slingerland JM. Links between oestrogen receptor activation and proteolysis: relevance to hormone-regulated cancer therapy. Nat Rev Cancer. 2014;14:26–38. doi: 10.1038/nrc3622. [DOI] [PubMed] [Google Scholar]
- 34.Fan M, Park A, Nephew KP. CHIP (carboxyl terminus of Hsc70-interacting protein) promotes basal and geldanamycin-induced degradation of estrogen receptor-alpha. Mol Endocrinol. 2005;19:2901–2914. doi: 10.1210/me.2005-0111. [DOI] [PubMed] [Google Scholar]
- 35.Sun J, Zhou W, Kaliappan K, Nawaz Z, Slingerland JM. ERα phosphorylation at Y537 by Src triggers E6-AP-ERα binding, ERα ubiquitylation, promoter occupancy, and target gene expression. Mol Endocrinol. 2012;26:1567–1577. doi: 10.1210/me.2012-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eakin CM, Maccoss MJ, Finney GL, Klevit RE. Estrogen receptor alpha is a putative substrate for the BRCA1 ubiquitin ligase. Proc Natl Acad Sci USA. 2007;104:5794–5799. doi: 10.1073/pnas.0610887104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hashizume R, Fukuda M, Maeda I, Nishikawa H, Oyake D, Yabuki Y, Ogata H, Ohta T. The RING heterodimer BRCA1-BARD1 is a ubiquitin ligase inactivated by a breast cancer-derived mutation. J Biol Chem. 2001;276:14537–14540. doi: 10.1074/jbc.C000881200. [DOI] [PubMed] [Google Scholar]
- 38.Bhatt S, Xiao Z, Meng Z, Katzenellenbogen BS. Phosphorylation by p38 mitogen-activated protein kinase promotes estrogen receptor α turnover and functional activity via the SCF(Skp2) proteasomal complex. Mol Cell Biol. 2012;32:1928–1943. doi: 10.1128/MCB.06561-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saji S, Okumura N, Eguchi H, Nakashima S, Suzuki A, Toi M, Nozawa Y, Saji S, Hayashi S. MDM2 enhances the function of estrogen receptor alpha in human breast cancer cells. Biochem Biophys Res Commun. 2001;281:259–265. doi: 10.1006/bbrc.2001.4339. [DOI] [PubMed] [Google Scholar]
- 40.Iizuka M, Susa T, Tamamori-Adachi M, Okinaga H, Okazaki T. Intrinsic ubiquitin E3 ligase activity of histone acetyltransferase Hbo1 for estrogen receptor α. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93:498–510. doi: 10.2183/pjab.93.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kocanova S, Mazaheri M, Caze-Subra S, Bystricky K. Ligands specify estrogen receptor alpha nuclear localization and degradation. BMC Cell Biol. 2010;11:98. doi: 10.1186/1471-2121-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marsaud V, Gougelet A, Maillard S, Renoir JM. Various phosphorylation pathways, depending on agonist and antagonist binding to endogenous estrogen receptor alpha (ERalpha), differentially affect ERalpha extractability, proteasome-mediated stability, and transcriptional activity in human breast cancer cells. Mol Endocrinol. 2003;17:2013–2027. doi: 10.1210/me.2002-0269. [DOI] [PubMed] [Google Scholar]
- 43.Valley CC, Solodin NM, Powers GL, Ellison SJ, Alarid ET. Temporal variation in estrogen receptor-alpha protein turnover in the presence of estrogen. J Mol Endocrinol. 2008;40:23–34. doi: 10.1677/JME-07-0067. [DOI] [PubMed] [Google Scholar]
- 44.Lonard DM, Nawaz Z, Smith CL, O’Malley BW. The 26S proteasome is required for estrogen receptor-alpha and coactivator turnover and for efficient estrogen receptor-alpha transactivation. Mol Cell. 2000;5:939–948. doi: 10.1016/s1097-2765(00)80259-2. [DOI] [PubMed] [Google Scholar]
- 45.Reid G, Hübner MR, Métivier R, Brand H, Denger S, Manu D, Beaudouin J, Ellenberg J, Gannon F. Cyclic, proteasome-mediated turnover of unliganded and liganded ERalpha on responsive promoters is an integral feature of estrogen signaling. Mol Cell. 2003;11:695–707. doi: 10.1016/s1097-2765(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 46.Berry NB, Fan M, Nephew KP. Estrogen receptor-alpha hinge-region lysines 302 and 303 regulate receptor degradation by the proteasome. Mol Endocrinol. 2008;22:1535–1551. doi: 10.1210/me.2007-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, Zong H, Chi Y, Hong Y, Yang Y, Zou W, Yun X, Gu J. Repression of estrogen receptor alpha by CDK11p58 through promoting its ubiquitin-proteasome degradation. J Biochem. 2009;145:331–343. doi: 10.1093/jb/mvn177. [DOI] [PubMed] [Google Scholar]
- 48.Henrich LM, Smith JA, Kitt D, Errington TM, Nguyen B, Traish AM, Lannigan DA. Extracellular signal-regulated kinase 7, a regulator of hormone-dependent estrogen receptor destruction. Mol Cell Biol. 2003;23:5979–5988. doi: 10.1128/MCB.23.17.5979-5988.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajbhandari P, Schalper KA, Solodin NM, Ellison-Zelski SJ, Ping Lu K, Rimm DL, Alarid ET. Pin1 modulates ERα levels in breast cancer through inhibition of phosphorylation-dependent ubiquitination and degradation. Oncogene. 2014;33:1438–1447. doi: 10.1038/onc.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei X, Xu H, Kufe D. MUC1 oncoprotein stabilizes and activates estrogen receptor alpha. Mol Cell. 2006;21:295–305. doi: 10.1016/j.molcel.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 51.Ghosh SK, Uchida M, Yoo B, Ross AW, Gendler SJ, Gong J, Moore A, Medarova Z. Targeted imaging of breast tumor progression and therapeutic response in a human uMUC-1 expressing transgenic mouse model. Int J Cancer. 2013;132:1860–1867. doi: 10.1002/ijc.27872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haddon L, Hugh J. MUC1-mediated motility in breast cancer: a review highlighting the role of the MUC1/ICAM-1/Src signaling triad. Clin Exp Metastasis. 2015;32:393–403. doi: 10.1007/s10585-015-9711-8. [DOI] [PubMed] [Google Scholar]
- 53.Jin C, Rajabi H, Pitroda S, Li A, Kharbanda A, Weichselbaum R, Kufe D. Cooperative interaction between the MUC1-C oncoprotein and the Rab31 GTPase in estrogen receptor-positive breast cancer cells. PLoS One. 2012;7:e39432. doi: 10.1371/journal.pone.0039432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raina D, Ahmad R, Joshi MD, Yin L, Wu Z, Kawano T, Vasir B, Avigan D, Kharbanda S, Kufe D. Direct targeting of the mucin 1 oncoprotein blocks survival and tumorigenicity of human breast carcinoma cells. Cancer Res. 2009;69:5133–5141. doi: 10.1158/0008-5472.CAN-09-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu Z, Hunter T. Prolyl isomerase Pin1 in cancer. Cell Res. 2014;24:1033–1049. doi: 10.1038/cr.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rajbhandari P, Ozers MS, Solodin NM, Warren CL, Alarid ET. Peptidylprolyl Isomerase Pin1 Directly Enhances the DNA Binding Functions of Estrogen Receptor α. J Biol Chem. 2015;290:13749–13762. doi: 10.1074/jbc.M114.621698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang JZ, Liu BG, Zhang Y. Pin1-based diagnostic and therapeutic strategies for breast cancer. Pharmacol Res. 2015;93:28–35. doi: 10.1016/j.phrs.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 58.Giamas G, Filipović A, Jacob J, Messier W, Zhang H, Yang D, Zhang W, Shifa BA, Photiou A, Tralau-Stewart C, et al. Kinome screening for regulators of the estrogen receptor identifies LMTK3 as a new therapeutic target in breast cancer. Nat Med. 2011;17:715–719. doi: 10.1038/nm.2351. [DOI] [PubMed] [Google Scholar]
- 59.Grisouard J, Medunjanin S, Hermani A, Shukla A, Mayer D. Glycogen synthase kinase-3 protects estrogen receptor alpha from proteasomal degradation and is required for full transcriptional activity of the receptor. Mol Endocrinol. 2007;21:2427–2439. doi: 10.1210/me.2007-0129. [DOI] [PubMed] [Google Scholar]
- 60.Medina M, Wandosell F. Deconstructing GSK-3: The Fine Regulation of Its Activity. Int J Alzheimers Dis. 2011;2011:479249. doi: 10.4061/2011/479249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Medunjanin S, Hermani A, De Servi B, Grisouard J, Rincke G, Mayer D. Glycogen synthase kinase-3 interacts with and phosphorylates estrogen receptor alpha and is involved in the regulation of receptor activity. J Biol Chem. 2005;280:33006–33014. doi: 10.1074/jbc.M506758200. [DOI] [PubMed] [Google Scholar]
- 62.Dennis AP, Haq RU, Nawaz Z. Importance of the regulation of nuclear receptor degradation. Front Biosci. 2001;6:D954–D959. doi: 10.2741/dennis. [DOI] [PubMed] [Google Scholar]
- 63.He X, Zheng Z, Song T, Wei C, Ma H, Ma Q, Zhang Y, Xu Y, Shi W, Ye Q, et al. c-Abl regulates estrogen receptor alpha transcription activity through its stabilization by phosphorylation. Oncogene. 2010;29:2238–2251. doi: 10.1038/onc.2009.513. [DOI] [PubMed] [Google Scholar]
- 64.Caligiuri I, Toffoli G, Giordano A, Rizzolio F. pRb controls estrogen receptor alpha protein stability and activity. Oncotarget. 2013;4:875–883. doi: 10.18632/oncotarget.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu J, Zhao C, Kharman-Biz A, Zhuang T, Jonsson P, Liang N, Williams C, Lin CY, Qiao Y, Zendehdel K, et al. The atypical ubiquitin ligase RNF31 stabilizes estrogen receptor α and modulates estrogen-stimulated breast cancer cell proliferation. Oncogene. 2014;33:4340–4351. doi: 10.1038/onc.2013.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang H, Yu N, Xu J, Ding X, Deng W, Wu G, Li X, Hou Y, Liu Z, Zhao Y, et al. SMURF1 facilitates estrogen receptor ɑ signaling in breast cancer cells. J Exp Clin Cancer Res. 2018;37:24. doi: 10.1186/s13046-018-0672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhuang T, Yu S, Zhang L, Yang H, Li X, Hou Y, Liu Z, Shi Y, Wang W, Yu N, et al. SHARPIN stabilizes estrogen receptor α and promotes breast cancer cell proliferation. Oncotarget. 2017;8:77137–77151. doi: 10.18632/oncotarget.20368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang S, Luo H, Wang C, Sun H, Sun G, Sun N, Zeng K, Song H, Zou R, Zhou T, et al. RNF8 identified as a co-activator of estrogen receptor α promotes cell growth in breast cancer. Biochim Biophys Acta. 2017;1863:1615–1628. doi: 10.1016/j.bbadis.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 69.Chai F, Liang Y, Bi J, Chen L, Zhang F, Cui Y, Jiang J. REGγ regulates ERα degradation via ubiquitin-proteasome pathway in breast cancer. Biochem Biophys Res Commun. 2015;456:534–540. doi: 10.1016/j.bbrc.2014.11.124. [DOI] [PubMed] [Google Scholar]
- 70.Liu H, Qiu J, Li N, Chen T, Cao X. Human phosphatidylethanolamine-binding protein 4 promotes transactivation of estrogen receptor alpha (ERalpha) in human cancer cells by inhibiting proteasome-dependent ERalpha degradation via association with Src. J Biol Chem. 2010;285:21934–21942. doi: 10.1074/jbc.M110.109876. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Kim SH, Kang HJ, Na H, Lee MO. Trichostatin A enhances acetylation as well as protein stability of ERalpha through induction of p300 protein. Breast Cancer Res. 2010;12:R22. doi: 10.1186/bcr2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.La Rosa P, Pesiri V, Leclercq G, Marino M, Acconcia F. Palmitoylation regulates 17β-estradiol-induced estrogen receptor-α degradation and transcriptional activity. Mol Endocrinol. 2012;26:762–774. doi: 10.1210/me.2011-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Murphy LC, Seekallu SV, Watson PH. Clinical significance of estrogen receptor phosphorylation. Endocr Relat Cancer. 2011;18:R1–14. doi: 10.1677/ERC-10-0070. [DOI] [PubMed] [Google Scholar]
- 74.Thomas C, Gustafsson JÅ. Estrogen receptor mutations and functional consequences for breast cancer. Trends Endocrinol Metab. 2015;26:467–476. doi: 10.1016/j.tem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 75.Yeh WL, Shioda K, Coser KR, Rivizzigno D, McSweeney KR, Shioda T. Fulvestrant-induced cell death and proteasomal degradation of estrogen receptor α protein in MCF-7 cells require the CSK c-Src tyrosine kinase. PLoS One. 2013;8:e60889. doi: 10.1371/journal.pone.0060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Savi C, Bradbury RH, Rabow AA, Norman RA, Buttar D, Currie GS, Weir H, Donald C, Andrews D, MacFaul P, et al. Abstract 3650: Discovery of the clinical candidate AZD9496: a potent and orally bioavailable selective estrogen receptor downregulator and antagonist. Cancer Research. 2015;75:3650. [Google Scholar]
- 77.Lai A, Kahraman M, Govek S, Nagasawa J, Bonnefous C, Julien J, Douglas K, Sensintaffar J, Lu N, Lee KJ, et al. Identification of GDC-0810 (ARN-810), an Orally Bioavailable Selective Estrogen Receptor Degrader (SERD) that Demonstrates Robust Activity in Tamoxifen-Resistant Breast Cancer Xenografts. J Med Chem. 2015;58:4888–4904. doi: 10.1021/acs.jmedchem.5b00054. [DOI] [PubMed] [Google Scholar]
- 78.Wardell SE, Nelson ER, Chao CA, McDonnell DP. Bazedoxifene exhibits antiestrogenic activity in animal models of tamoxifen-resistant breast cancer: implications for treatment of advanced disease. Clin Cancer Res. 2013;19:2420–2431. doi: 10.1158/1078-0432.CCR-12-3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garner F, Shomali M, Paquin D, Lyttle CR, Hattersley G. RAD1901: a novel, orally bioavailable selective estrogen receptor degrader that demonstrates antitumor activity in breast cancer xenograft models. Anticancer Drugs. 2015;26:948–956. doi: 10.1097/CAD.0000000000000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim JA, Kim MR, Kim O, Phuong NT, Yun J, Oh WK, Bae K, Kang KW. Amurensin G inhibits angiogenesis and tumor growth of tamoxifen-resistant breast cancer via Pin1 inhibition. Food Chem Toxicol. 2012;50:3625–3634. doi: 10.1016/j.fct.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 81.Kim JH, Jung JH, Kim SH, Jeong SJ. Decursin exerts anti-cancer activity in MDA-MB-231 breast cancer cells via inhibition of the Pin1 activity and enhancement of the Pin1/p53 association. Phytother Res. 2014;28:238–244. doi: 10.1002/ptr.4986. [DOI] [PubMed] [Google Scholar]
- 82.Kotiyal S, Bhattacharya S. Breast cancer stem cells, EMT and therapeutic targets. Biochem Biophys Res Commun. 2014;453:112–116. doi: 10.1016/j.bbrc.2014.09.069. [DOI] [PubMed] [Google Scholar]
- 83.Li X, Li L, Zhou Q, Zhang N, Zhang S, Zhao R, Liu D, Jing Y, Zhao L. Synthesis of the novel elemonic acid derivatives as Pin1 inhibitors. Bioorg Med Chem Lett. 2014;24:5612–5615. doi: 10.1016/j.bmcl.2014.10.087. [DOI] [PubMed] [Google Scholar]
- 84.Moore JD, Potter A. Pin1 inhibitors: Pitfalls, progress and cellular pharmacology. Bioorg Med Chem Lett. 2013;23:4283–4291. doi: 10.1016/j.bmcl.2013.05.088. [DOI] [PubMed] [Google Scholar]
- 85.Potter A, Oldfield V, Nunns C, Fromont C, Ray S, Northfield CJ, Bryant CJ, Scrace SF, Robinson D, Matossova N, et al. Discovery of cell-active phenyl-imidazole Pin1 inhibitors by structure-guided fragment evolution. Bioorg Med Chem Lett. 2010;20:6483–6488. doi: 10.1016/j.bmcl.2010.09.063. [DOI] [PubMed] [Google Scholar]
- 86.Wei S, Kozono S, Kats L, Nechama M, Li W, Guarnerio J, Luo M, You MH, Yao Y, Kondo A, et al. Active Pin1 is a key target of all-trans retinoic acid in acute promyelocytic leukemia and breast cancer. Nat Med. 2015;21:457–466. doi: 10.1038/nm.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu GG, Slebodnick C, Etzkorn FA. Cyclohexyl ketone inhibitors of Pin1 dock in a trans-diaxial cyclohexane conformation. PLoS One. 2012;7:e44226. doi: 10.1371/journal.pone.0044226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stebbing J, Filipovic A, Ellis IO, Green AR, D’Silva TR, Lenz HJ, Coombes RC, Wang T, Lee SC, Giamas G. LMTK3 expression in breast cancer: association with tumor phenotype and clinical outcome. Breast Cancer Res Treat. 2012;132:537–544. doi: 10.1007/s10549-011-1622-z. [DOI] [PubMed] [Google Scholar]
- 89.Stebbing J, Filipovic A, Lit LC, Blighe K, Grothey A, Xu Y, Miki Y, Chow LW, Coombes RC, Sasano H, et al. LMTK3 is implicated in endocrine resistance via multiple signaling pathways. Oncogene. 2013;32:3371–3380. doi: 10.1038/onc.2012.343. [DOI] [PubMed] [Google Scholar]
- 90.Zhao G, Guo J, Li D, Jia C, Yin W, Sun R, Lv Z, Cong X. MicroRNA-34a suppresses cell proliferation by targeting LMTK3 in human breast cancer mcf-7 cell line. DNA Cell Biol. 2013;32:699–707. doi: 10.1089/dna.2013.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jacobs KM, Bhave SR, Ferraro DJ, Jaboin JJ, Hallahan DE, Thotala D. GSK-3β: A Bifunctional Role in Cell Death Pathways. Int J Cell Biol. 2012;2012:930710. doi: 10.1155/2012/930710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim HM, Kim CS, Lee JH, Jang SJ, Hwang JJ, Ro S, Choi J. CG0009, a novel glycogen synthase kinase 3 inhibitor, induces cell death through cyclin D1 depletion in breast cancer cells. PLoS One. 2013;8:e60383. doi: 10.1371/journal.pone.0060383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McCubrey JA, Davis NM, Abrams SL, Montalto G, Cervello M, Basecke J, Libra M, Nicoletti F, Cocco L, Martelli AM, et al. Diverse roles of GSK-3: tumor promoter-tumor suppressor, target in cancer therapy. Adv Biol Regul. 2014;54:176–196. doi: 10.1016/j.jbior.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 94.Mishra R. Glycogen synthase kinase 3 beta: can it be a target for oral cancer. Mol Cancer. 2010;9:144. doi: 10.1186/1476-4598-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ertel A, Dean JL, Rui H, Liu C, Witkiewicz AK, Knudsen KE, Knudsen ES. RB-pathway disruption in breast cancer: differential association with disease subtypes, disease-specific prognosis and therapeutic response. Cell Cycle. 2010;9:4153–4163. doi: 10.4161/cc.9.20.13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lehn S, Fernö M, Jirström K, Rydén L, Landberg G. A non-functional retinoblastoma tumor suppressor (RB) pathway in premenopausal breast cancer is associated with resistance to tamoxifen. Cell Cycle. 2011;10:956–962. doi: 10.4161/cc.10.6.15074. [DOI] [PubMed] [Google Scholar]
- 97.Treré D, Brighenti E, Donati G, Ceccarelli C, Santini D, Taffurelli M, Montanaro L, Derenzini M. High prevalence of retinoblastoma protein loss in triple-negative breast cancers and its association with a good prognosis in patients treated with adjuvant chemotherapy. Ann Oncol. 2009;20:1818–1823. doi: 10.1093/annonc/mdp209. [DOI] [PubMed] [Google Scholar]
- 98.Witkiewicz AK, Knudsen ES. Retinoblastoma tumor suppressor pathway in breast cancer: prognosis, precision medicine, and therapeutic interventions. Breast Cancer Res. 2014;16:207. doi: 10.1186/bcr3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alam M, Rajabi H, Ahmad R, Jin C, Kufe D. Targeting the MUC1-C oncoprotein inhibits self-renewal capacity of breast cancer cells. Oncotarget. 2014;5:2622–2634. doi: 10.18632/oncotarget.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kharbanda A, Rajabi H, Jin C, Raina D, Kufe D. Oncogenic MUC1-C promotes tamoxifen resistance in human breast cancer. Mol Cancer Res. 2013;11:714–723. doi: 10.1158/1541-7786.MCR-12-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pitroda SP, Khodarev NN, Beckett MA, Kufe DW, Weichselbaum RR. MUC1-induced alterations in a lipid metabolic gene network predict response of human breast cancers to tamoxifen treatment. Proc Natl Acad Sci USA. 2009;106:5837–5841. doi: 10.1073/pnas.0812029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alam M, Bouillez A, Tagde A, Ahmad R, Rajabi H, Maeda T, Hiraki M, Suzuki Y, Kufe D. MUC1-C Represses the Crumbs Complex Polarity Factor CRB3 and Downregulates the Hippo Pathway. Mol Cancer Res. 2016;14:1266–1276. doi: 10.1158/1541-7786.MCR-16-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jin C, Rajabi H, Kufe D. miR-1226 targets expression of the mucin 1 oncoprotein and induces cell death. Int J Oncol. 2010;37:61–69. doi: 10.3892/ijo_00000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sachdeva M, Mo YY. MicroRNA-145 suppresses cell invasion and metastasis by directly targeting mucin 1. Cancer Res. 2010;70:378–387. doi: 10.1158/0008-5472.CAN-09-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lacunza E, Baudis M, Colussi AG, Segal-Eiras A, Croce MV, Abba MC. MUC1 oncogene amplification correlates with protein overexpression in invasive breast carcinoma cells. Cancer Genet Cytogenet. 2010;201:102–110. doi: 10.1016/j.cancergencyto.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 106.Rajabi H, Jin C, Ahmad R, McClary C, Joshi MD, Kufe D. MUCIN 1 ONCOPROTEIN EXPRESSION IS SUPPRESSED BY THE miR-125b ONCOMIR. Genes Cancer. 2010;1:62–68. doi: 10.1177/1947601909357933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou Y, Rajabi H, Kufe D. Mucin 1 C-terminal subunit oncoprotein is a target for small-molecule inhibitors. Mol Pharmacol. 2011;79:886–893. doi: 10.1124/mol.110.070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yuan S, Shi C, Ling R, Wang T, Wang H, Han W. Immunization with two recombinant Bacillus Calmette-Guérin vaccines that combine the expression of multiple tandem repeats of mucin-1 and colony stimulating-factor suppress breast tumor growth in mice. J Cancer Res Clin Oncol. 2010;136:1359–1367. doi: 10.1007/s00432-010-0787-x. [DOI] [PubMed] [Google Scholar]
- 109.Yuan S, Shi C, Liu L, Han W. MUC1-based recombinant Bacillus Calmette-Guerin vaccines as candidates for breast cancer immunotherapy. Expert Opin Biol Ther. 2010;10:1037–1048. doi: 10.1517/14712598.2010.485185. [DOI] [PubMed] [Google Scholar]
- 110.Kotzsch M, Kirchner T, Soelch S, Schäfer S, Friedrich K, Baretton G, Magdolen V, Luther T. Inverse association of rab31 and mucin-1 (CA15-3) antigen levels in estrogen receptor-positive (ER+) breast cancer tissues with clinicopathological parameters and patients’ prognosis. Am J Cancer Res. 2017;7:1959–1970. [PMC free article] [PubMed] [Google Scholar]
- 111.Fowler AM, Solodin N, Preisler-Mashek MT, Zhang P, Lee AV, Alarid ET. Increases in estrogen receptor-alpha concentration in breast cancer cells promote serine 118/104/106-independent AF-1 transactivation and growth in the absence of estrogen. FASEB J. 2004;18:81–93. doi: 10.1096/fj.03-0038com. [DOI] [PubMed] [Google Scholar]
- 112.Fowler AM, Solodin NM, Valley CC, Alarid ET. Altered target gene regulation controlled by estrogen receptor-alpha concentration. Mol Endocrinol. 2006;20:291–301. doi: 10.1210/me.2005-0288. [DOI] [PubMed] [Google Scholar]