Abstract
A set of reference genes expressing stably under aerobic and anaerobic conditions in rice is essential to execute omics studies relating to aerobic adaptations. Stability of expression of ten rice reference genes, viz. Actin, eEF-1a, eIF-5C, Exp1, Exp2, Memp, SKP1A, TF-SUI1, TPH, and UBQ5 was validated across six experimental sets in shoot and root tissues at seedling, tillering, and panicle initiation stages. Comprehensively, Memp (Membrane protein), TPH (Tumor protein homolog), and Exp1 (Expressed protein) were revealed as the most stable set with acceptable M and V value according to the gold standards of qRT-PCR using various algorithms/tools. The identified set of reference genes was validated using root trait genes, which showed concurrence with the functional expression patterns in the aerobic and anaerobic adapted cultivars. The Memp (Membrane protein), TPH (Tumor protein homolog), and Exp1 (Expressed protein) genes are the most stable reference genes across the root and shoot at various developmental stages under aerobic and anaerobic conditions in rice. This is the first study for accurate and reliable relative gene expression analysis in rice grown in aerobic and anaerobic conditions.
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1406-9) contains supplementary material, which is available to authorized users.
Keywords: Aerobic, Anaerobic, Gene expression, qRT-PCR, Reference gene, Rice
Introduction
Changing climate along with ever-increasing population pose immense challenges to plant biologists for improvement of economic traits and development of climate-smart next-generation crop varieties suitable for sustainable agriculture. Rice, a major staple food crop, is widely cultivated across various eco-systems such as fully anaerobic (irrigated rice), temporarily rainfed (lowland rice or floating rice), or aerobic (upland rice). Conventionally, rice is puddled transplanted and requires standing water which is a major concern with respect to water availability. As an alternative to this method, aerobic and dry direct-seeded system of cultivation has emerged wherein the water requirement is reduced to a significant extent with proper management practices. This kind of shift in rice cultivation is becoming popular to cope up with water scarcity as well as for maintaining the ground water table (Pathak et al. 2011). There have been several reports on yield, morphological, and adaptability differences in anaerobic and aerobic systems of cultivation (Kato and Okami 2010; Patel et al. 2010; Sandhu et al. 2013), but sparse information is available on the genes responsible for the traits conferring aerobic adaptation. The morphological and physiological traits are very much essential for adaption to such a variation with concomitant changes in the molecular mechanism and expression of the genes responsible. It has been observed that the genotypes which are adapted to anaerobic conditions show reduced yields in the aerobic conditions and vice versa (Dixit et al. 2015). With limited understanding, it is certain that traits related to root architecture, root establishment, uptake and transport of macro/micro elements at seedling, vegetative and reproductive stages of the life cycle are responsible for the desired adaptation to aerobic and anaerobic systems of cultivations. To accurately estimate expression of various genes under such adaptations, there is a need to identify the genes whose expression is constant across the conventional anaerobic and aerobic systems of cultivation.
The qRT-PCR is a powerful tool for estimation of relative expression values in a given set of samples subjected to a particular condition at a given time (Bustin et al. 2009). The reliable quantification requires the control and normalization of a number of variables, such as initial sample size, RNA recovery and integrity, the enzymatic efficiency of cDNA synthesis and PCR amplification, and transcriptional activity of the tissues or cells (Bustin 2002; Ginzinger 2002). Many such variables are controlled using reference or internal control gene(s), whose expression is constant for normalizing gene expression (Schmidt and Delaney 2010). Such normalization is thus an essential step in any qRT-PCR assay as it takes care of the variations in experimental protocols. The Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines impresses that, the reliability and reproducibility of the gene expression at a given time and space largely depends on the appropriate choice of the reference gene(s) (Bennett et al. 2015).
Till date, many reports are available on the reference gene validation in rice at different experimental conditions, but there are no reports suggesting the suitable reference genes for gene expression studies for the aerobic/anaerobic system (Moraes et al. 2015; Xu et al. 2015; Pabuayon et al. 2016; Zhang et al. 2017). Reference genes such as actin, ubiquitin, elongation factor, GAPDH, and 18s rRNA have been traditionally used for normalization. However, there has been observations that above mentioned reference genes are not stable regarding expression in all the developmental stages, tissues as well as under various stress conditions. Also, to analyze the stability and reliability of reference genes, several on-line and off-line (excel) statistical algorithms/tools deploying Ct values (cycle threshold), standard deviations (SD), etc., are available. In the present study, we analyzed the expression stability of ten reference genes, viz. Actin, eEF-1a, eIF-5C, Exp1, Exp2, Memp, SKP1A, TF-SUI1, TPH, and UBQ5 in four genotypes, two each adapted for anaerobic and aerobic systems of cultivation for accurate assessment of the relative gene expression through qRT-PCR. The ten genes used in the present study have been shown to be relatively stable in microarray experiments (Narsai et al. 2010) and thus were thought to be used as endogenous genes in rice relative gene expression studies.
Materials and methods
Plant material and treatments
Rice (Oryza sativa L.) genotypes, viz. BPT 5204, MTU 1010 (flooding mega varieties), CR Dhan 202 (aerobic adapted), and Birsa Vikas Dhan 111 (upland) were used in this study. The seeds of genotypes were sown in polythene bags containing 15 kg soil under aerobic and anaerobic conditions in the greenhouse with 28/20 °C day/night temperature. After three days of germination, each plant per bag was grown in 12 replications per cultivar in each condition. A layer of 2–5 cm water above the soil was maintained for 100–120 days for the anaerobic condition. For maintaining aerobic condition, moisture at field capacity and well-drained soil were retained. Need-based irrigation, i.e., water in measured volume as and when required to maintain the above said condition was given. The nutrients (NPK) equivalent for 15 kg soil in split doses was applied following regular recommendations. The shoot and root tissues (48 samples) were harvested from three different growth stages, viz. seedling i.e., 14 days after germination (dag), tillering (40–50 dag) and panicle initiation stages (60–70 dag) in three replications and stored immediately in liquid nitrogen until RNA isolation at -80 °C (Supplementary Table 1). The 48 samples were grouped and designated as six experimental sets, viz. (1) all tissues (2) root tissue (3) shoot tissue (4) seedling (root and shoot tissues) (5) tillering (root and shoot tissues) and (6) panicle initiation (PI) (root and shoot tissues).
Total RNA isolation and first strand cDNA synthesis
Total RNA was isolated from rice root and shoot tissues of the four genotypes (48) in three replications using NucleoSpin RNA plant kit (Macherey–Nagel, Duren, Germany) according to the manufacturer’s protocol with slight modifications. The RNA concentration and purity were assessed using ND1000 Spectrophotometer (Thermo Scientific), and integrity was checked by 1% (w/v) agarose gel. The total RNA samples (800) ng each with 260/280 ratio between 2.0 and 2.1 and 260/230 ratio between 2.1 and 2.3 were used for cDNA synthesis for all the samples (Chomczynski and Sacchi 1987). First strand cDNA was synthesized (three replicates/sample) using the PrimeScript™ first strand cDNA synthesis kit (Takara, Japan), following the manufacturer’s instructions in a final volume of 20 µl. The final cDNA samples were diluted three folds and used as template in qRT-PCR.
Selection of reference genes, target genes, and primer designing
The ten reference genes were selected based on the previous studies on endogenous genes in rice for various stress conditions, tissues and developmental stages (Jain et al. 2006; Narsai et al. 2010) (Table 1). For validation of gene expression, a total of 27 genes were selected based on root genetic traits in rice (Bu 2011; Wu and Cheng 2014; Li et al. 2015; Fan et al. 2016) (Supplementary Table 2). For all the genes, primers were designed using high throughput qRT-PCR tool, QuantPrime (http://quantprime.mpimp-golm.mpg.de/) available online using the default parameters. The primers were further checked online for secondary structure/hairpin/duplex formation using OligoAnalyzer 3.1, IDT (https://eu.idtdna.com/calc/analyzer).
Table 1.
Primer details of the candidate reference genes
| S. no | Gene symbol | Gene description | Accession no./locus information | Primer sequence 5′–3′ (forward/reverse) | Amplicon length (bp) | Tm (°C) |
|---|---|---|---|---|---|---|
| 1 | ACT | Actin | – | CAGCCACACTGTCCCCATCTA AGCAAGGTCGAGACGAAGGA |
80 | 60 |
| 2 | eEF-1a | Eukaryotic elongation factor 1-alpha | AK061464 | TTTCACTCTTGGTGTGAAGCAGAT GACTTCCTTCACGATTTCATCGTAA |
103 | 60 |
| 3 | eIF-5C | Eukaryotic translation initiation factor 5C | LOC_Os11g21990.1 | CACGTTACGGTGACACCTTTT GACGCTCTCCTTCTTCCTCAG |
90 | 60 |
| 4 | Exp1 | Expressed protein | LOC_Os06g47230.1 | GCCGAGAAGAAGGAGTACGA CTTGGCCTTAAGCTCCTTCA |
90 | 60 |
| 5 | Exp2 | Expressed protein | LOC_Os07g02340.1 | AGGAACATGGAGAAGAACAAGG CAGAGGTGGTGCAGATGAAA |
112 | 60 |
| 6 | Memp | Membrane protein | LOC_Os12g32950.1 | GAGCGCAAAGTTCCAGAAGAA CGCCACTAGTTGCCGTCCTGAT |
164 | 60 |
| 7 | SKP1A | SKP1-like protein 1A | LOC_Os11g26910.1 | GACGCCGACTTCGTCAAG CAACCCCTTGATGTTGAGGT |
81 | 60 |
| 8 | TF-SUI1 | Translation factor SUI1 | LOC_Os07g34589.1 | GCTGCAATGGTACTGTTGTCC CCGGCCTGAACAAGAAAAT |
100 | 60 |
| 9 | TPH | Tumor protein homolog | LOC_Os11g43900.1 | CATTGGTGCCAACCCATC AAGGAGGTTGCTCCTGAAGA |
113 | 60 |
| 10 | UBQ5 | Ubiquitin 5 | AK061988 | ACCACTTCGACCGCCACTACT ACGCCTAAGCCTGCTGGTT |
69 | 60 |
Quantitative real-time PCR (qRT-PCR)
The qRT-PCR reactions were performed on a Light Cycler 96 system (Roche, USA) using the SYBR premix ExTaq™ II (Takara, Japan) in 96-well optical reaction Roche plates. Each reaction was performed in triplicate containing 5 µl SYBR Green Master, 0.8 µl template cDNA, 0.4 µl each of the primers (10 µM), and 3.4 µl RNase-free water with a total volume of 10 µl. The qRT-PCR profile for reference genes was as follows, 95 °C (2 min), 40 cycles of 95 °C (5 s), 60 °C (30 s) with fluorescent signal recording and 72 °C for 30 s. The melting curve was obtained using a high-resolution melting profile performed after the last PCR cycle, 95 °C for 15 s followed by a constant increase in the temperature between 65 °C (15 s) and 95 °C (1 s). For root trait-related genes, the thermal profiling was similar except melting temperature (Tm) of 62 °C (30 s) with fluorescent signal recording. All the samples in two biological replicates and three technical replicates were repeated for the confirmation of results. The primer specificity and amplicon length were confirmed by electrophoresis of products on a 2% agarose gel with ethidium bromide staining.
Gene expression stability analysis
The stability of ten reference genes under aerobic and anaerobic conditions across six experimental sets in shoot and root tissues was analyzed using four different algorithms, viz. geNorm (Vandesompele et al. 2002), NormFinder (Andersen et al. 2004), BestKeeper (Pfaffl et al. 2004), and RefFinder (Xie et al. 2012) (Supplementary Fig. 1). The selected most stable reference genes were searched in the Rice Genome Annotation Project Database (http://rice.plantbiology.msu.edu/) for knowing the in-depth function, structural domains and in silico expression.
Validation of reference genes
Twenty-seven root trait-related genes reported for root growth and development were selected for the validation of reference gene(s) in root tissue at panicle initiation stage in two genotypes viz. BPT 5204 (flooding mega varieties) and CR Dhan 202 (aerobic adapted). The genes were selected based on its significance in root growth, development, nutrient uptake, and stress tolerance-related functions (Wu and Cheng 2014; Mai et al. 2014). Primer designing and qRT-PCR thermal profiling were followed as mentioned in the above section. The relative expression levels of genes in the samples were recorded using stable reference gene(s) in root tissues at panicle initiation stage using qBase plus software (Hellemans et al. 2007).
Results and discussion
Relative quantification of gene expression through qRT-PCR calls for minimum errors in experiment with the selection of suitable reference genes (Yang et al. 2014). Various studies have reported suitable reference gene(s) in rice (Kim et al. 2003; Jain et al. 2006; Li et al. 2009; Narsai et al. 2010; Maksup et al. 2013; Bevitori et al. 2014; Moraes et al. 2015; Xu et al. 2015; Pabuayon et al. 2016) at different developmental stages and under various stress or combined stress conditions. Thus, under aerobic and anaerobic conditions it is necessary to carefully select the reference gene(s) with stable expression in root and shoot tissues (Udvardi et al. 2008; Bustin et al. 2010).
Expression stability and optimal number of reference genes for normalization using geNorm
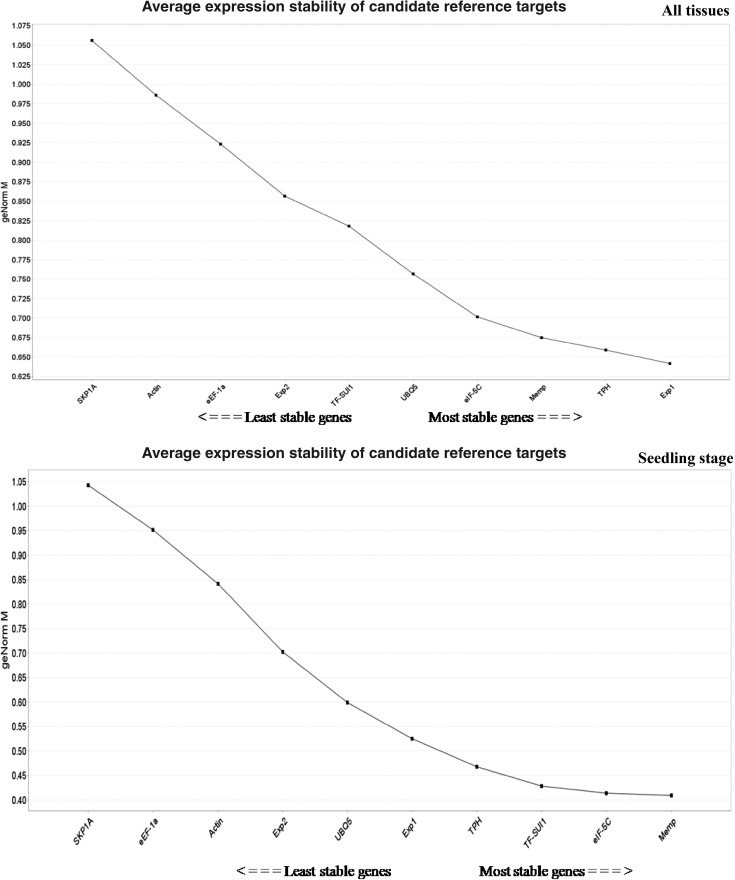
The geNorm algorithm in qBase plus is widely used for analyzing candidate reference gene expression (Vandesompele et al. 2002). According to the geNorm, genes showing the lowest stability value (M value) are considered as the most stable, whereas genes showing the highest stability value (M value) are least stable (Fig. 1). Based on M values, three best reference genes were Exp1, Memp, and TPH and least stable was SKP1A in all tissue set across all the experimental sets (Table 2 and Supplementary Table 3–7).
Fig. 1.
Gene expression stability and ranking of ten candidate reference genes using geNorm algorithm. Gene expression stability graph based on average expression stability values (M value) calculated using stepwise exclusion process. The lower the M value indicates higher the stability of gene. The direction of the arrow indicates the least and most stable candidate reference genes
Table 2.
Stability ranking of candidate reference genes in all tissues set
| Algorithm | geNorm | NormFinder | BestKeeper | RefFinder | ||||
|---|---|---|---|---|---|---|---|---|
| Ranking | Genes | Stability value (M) | Genes | Stability value (SV) | Genes | Coeff. of corr. (r) | Genes | Geomean of ranking values |
| 1 | Exp1 | 0.64 | TPH | 0.50 | TPH | 0.98 | Exp1 | 1.41 |
| 2 | TPH | 0.66 | Exp1 | 0.50 | eIF-5C | 0.98 | TPH | 1.78 |
| 3 | Memp | 0.68 | Memp | 0.50 | Exp1 | 0.97 | Memp | 2.28 |
| 4 | eIF-5C | 0.70 | eIF-5C | 0.52 | Memp | 0.97 | UBQ5 | 4.40 |
| 5 | UBQ5 | 0.76 | UBQ5 | 0.68 | eEF-1a | 0.96 | eIF-5C | 4.43 |
| 6 | TF-SUI1 | 0.82 | TF-SUI1 | 0.80 | UBQ5 | 0.95 | TF-SUI1 | 5.63 |
| 7 | Exp2 | 0.86 | Exp2 | 0.80 | Exp2 | 0.94 | Exp2 | 6.74 |
| 8 | eEF-1a | 0.92 | eEF-1a | 0.90 | TF-SUI1 | 0.93 | eEF-1a | 8.24 |
| 9 | Actin | 0.99 | Actin | 1.00 | Actin | 0.91 | Actin | 9.24 |
| 10 | SKP1A | 1.06 | SKP1A | 1.14 | SKP1A | 0.88 | SKP1A | 9.46 |
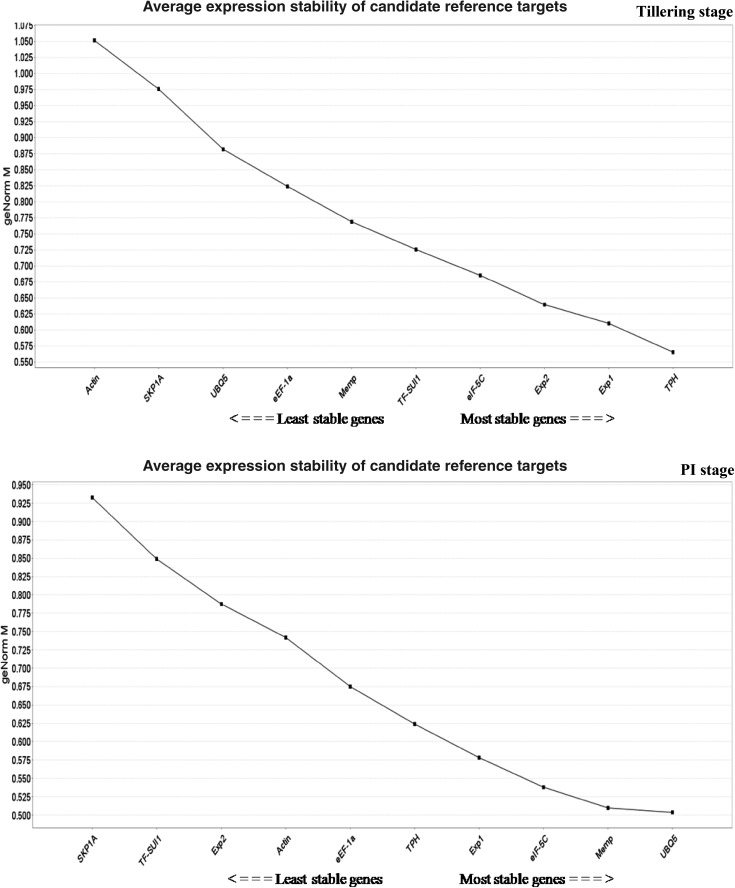
Pairwise variation (Vn/Vn + 1) between two normalization factors (NFn and NFn + 1) determined the optimal number of genes for precise normalization (Vandesompele et al. 2002). The V value cut-off below 0.15 signifies the exclusion of any further reference gene (Vandesompele et al. 2002). The pairwise variation values (V3/4) were less than the cut-off value of 0.15, in four experimental sets, viz. all tissue, seedling set, panicle initiation set, and root tissue set. This indicated that three most stable reference genes were optimal for its normalization except at tillering stage and shoot tissue set (Fig. 2). The expression of stable genes validated by geNorm algorithm correlated with NormFinder, BestKeeper, and RefFinder algorithms. Moreover, the coefficient of variation (CV) was observed to be lower than that of the most traditionally employed reference genes in rice (Jain et al. 2006).
Fig. 2.
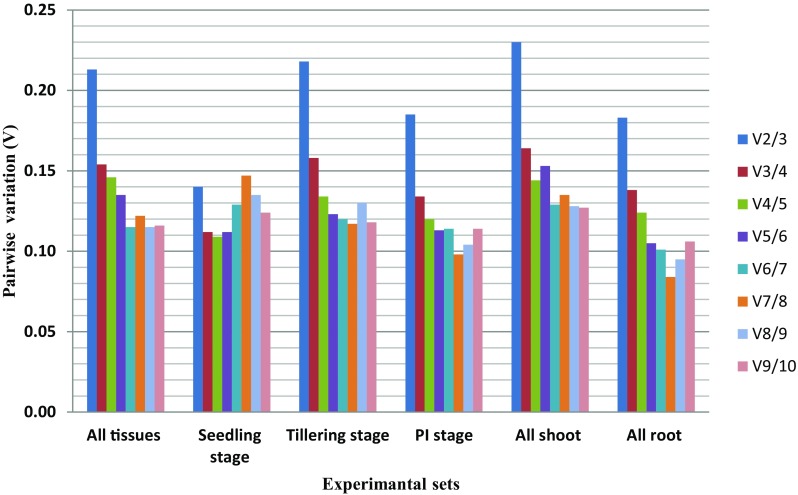
Determination of the optimal number of candidate reference genes required for accurate normalization in each experimental sets of rice. The pairwise variation (Vn/Vn + 1) was calculated between normalization factors NFn and NFn + 1 by geNorm algorithm to determine (V < 0.15) the optimal number of candidate reference genes
With regards to validation of reference genes viz. Memp, TPH, and Exp1, 27 root trait-related genes were selected for relative gene expression in root tissues at panicle initiation stage under aerobic and anaerobic conditions. Although three genes were selected, only two genes, viz. Memp and TPH were taken into consideration on the basis of stability value (M < 0.5) and V value of V2/3 reflecting the sufficiency of only two genes, viz. Memp and TPH for relative expression.
Stability values of reference genes using NormFinder
NormFinder performs a separate analysis of sample subgroups and estimates intra and inter-expression variation calculating the stability value (SV) for each reference gene wherein, lesser average stability value (SV) indicates higher stability of genes (Andersen et al. 2004). NormFinder algorithms identified Exp1 (SV values ranged from 0.32 to 0.58), Memp (SV values ranged from 0.34 to 0.66), TPH (SV values ranged from 0.38 to 0.60) as the most stable genes, and SKP1A as the least stable in all sets coinciding with the results of geNorm algorithm (Table 2 and Supplementary Table 3–7).
Pairwise co-relation using BestKeeper
The BestKeeper algorithm evaluates the suitability of reference genes based on geometric means of Ct values determined as coefficient of correlation (r) (Pfaffl et al. 2004). Higher coefficient of correlation (r) value indicates stable expression of gene (Table 2 and Supplementary Table 3–7). First two reference genes were similar to the results obtained from geNorm and NormFinder in all six experimental sets (Table 2 and Supplementary Table 3–7). BestKeeper analysis of the all tissue set revealed the best correlation for TPH (r = 0.98), eIF-5C (r = 0.98), Exp1 (r = 0.97), with p value of 0.001 and SD < 1 (standard deviation) (Table 2).
Analysis using RefFinder
RefFinder is a web-based tool (http://150.216.56.64/referencegene.php?type=reference) that integrates geNorm, NormFinder, BestKeeper, and delta (∆Ct) method to comprehensively rank reference genes based on its geometric means emanating from each program (Xie et al. 2012). The three most stable genes, viz. Exp1, TPH, and Memp comprehensively ranked by RefFinder were similar to the first three genes by geNorm and NormFinder with varying order except BestKeeper in all tissues set. It was observed that Exp1, TPH, and Memp were the most stable genes in all tissue sets, tillering stage, and all shoot sets. The eIF-5C, Memp, and TF-SUI1 were the most stable genes at the seedling stage. The most stable genes in panicle initiation stage set were eIF-5C, Memp, and Exp1 whereas eIF-5C, TF-SUI1, and Memp were the most stable genes in all root tissue. The comprehensive ranking in all six experimental sets revealed that SKP1A was the least stable gene while in tillering stage, Actin was the least stable gene (Table 2 and Supplementary Tables 3–7).
Best set of reference genes
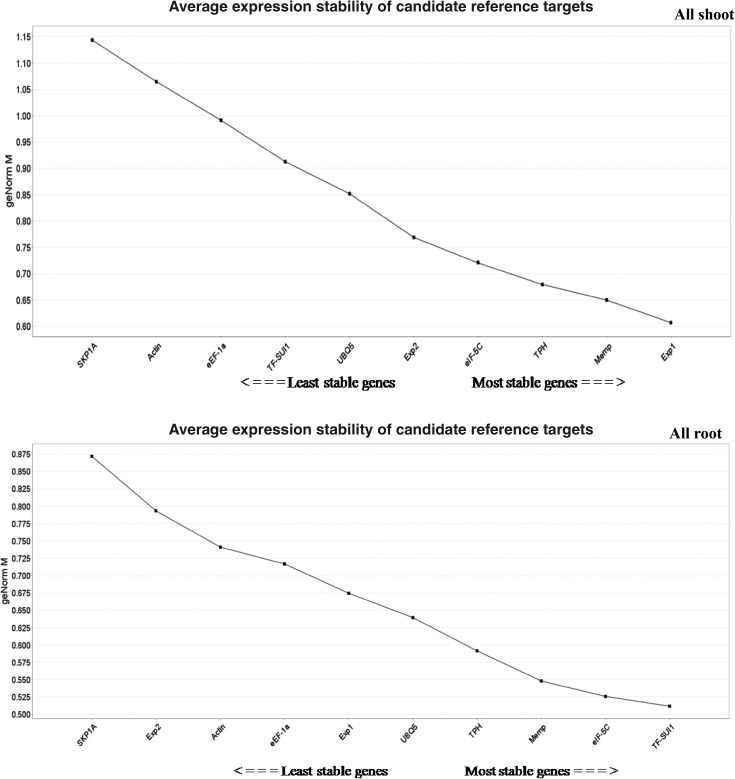
The set of best reference genes is expected to be almost stable across all the developmental stages (seedling, tillering and panicle initiation) subjected to aerobic and anaerobic systems of cultivation. In our study, in the set consisting of all tissues, all shoot set, the most consistent reference genes were Exp1, TPH, and Memp on the basis of three algorithms except BestKeeper which ranked Memp gene at fourth position (Table 2 and Supplementary Table 6).
Similarly, in seedling stage set and all root set, Memp, eIF-5C, and TF-SUI1 were found to be the most stable genes in all the four algorithms, while BestKeeper ranked Memp and TF-SUI1 genes at fourth and fifth position (Supplementary Table 3). In tillering stage set, Exp1 and TPH were observed to be the most stable genes, but the gene at third position varied in all the four algorithms (Supplementary Table 4). The genes, UBQ5, Memp, and eIF-5C were found to be the three most stable genes in all four algorithms in panicle initiation (PI) stage set (Supplementary Table 5).
It is anticipated that selection of the best genes at all the stages would be very much cost-effective, user-friendly, and efficient for unveiling the expression of genes in understanding the specific adaptations of rice growing in a different system of cultivation. Hence, we have given much attention to selection of reference genes suitable for studies at all the developmental stages across root and shoot tissues. Nevertheless, the set of best reference genes at each stage, i.e., seedling, tillering, and panicle initiation across root and shoot tissues can be selected as per the purpose and experimental design of the study. Comprehensively, the best set of reference genes, viz. Memp, TPH, and Exp1 as evidenced from our study is expected to suit well for gene expression studies under aerobic and anaerobic conditions. Interestingly, all the three genes have functions in membranes of mitochondria, cytosol, and endoplasmic reticulum. The Memp (membrane protein) belongs to the ribosome-associated membrane protein (RAMP4) which translocates across the endoplasmic membrane; TPH (Tumor protein homolog) is involved in calcium binding and microtubule stabilization, whereas Exp1 (Expressed protein) is a coiled-coil domain containing protein associated with translation machinery. The role of TPH protein in rice has been reported for imparting mercury tolerance by Wang et al. (2015) but not yet reported as a reference gene in a given set of conditions. Wang et al. (2010) reported the Exp1 as one of the most stable genes during developmental stages in rice irrespective of the genetic background. It can be stated that the three best reference genes are responsible for maintaining the membrane-related functions with basal metabolic activities required for growth under aerobic and anaerobic conditions.
Validation of reference genes for relative gene expression
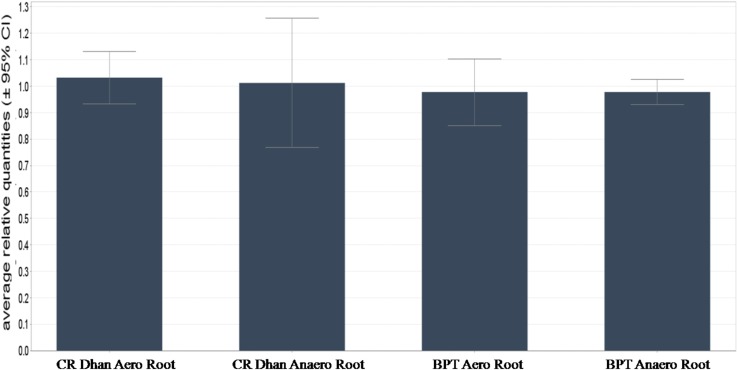
Roots are implied to be the most crucial organ responsible for initial establishment of the seedling, anchorage, water/nutrient uptake, and also confer adaptability in various stress situations on account of its plastic nature. We critically selected 27 genes related to root traits (involved in root development, root elongation, nutrient uptake, and stress tolerance) for expression analysis under aerobic and anaerobic conditions using most stable genes, viz. Memp, TPH, and Exp1 (average M value of 0.36 and CV value of 0.14) in root tissue at panicle initiation stage in BPT 5204 and CR Dhan 202. The expression of three most stable reference genes remained constant and was in line with the standard guidelines prescribed for relative gene expression (Bustin et al. 2009). The expression of Dro1 (Deeper rooting 1) was comparatively higher in CR Dhan 202 aerobic than CR Dhan 202 anaerobic. However, the difference was not that noticeable in BPT 5204 under aerobic and anaerobic conditions (Fig. 3). The Dro1, an early auxin response gene is known for deeper rooting by inducing cell elongation in the root tip, imparts drought tolerance, and contributes to the grain yield (Uga et al. 2011). As opposed to the complete absence of irrigation in drought, the aerobic condition is substantiated by need-based irrigation (water limiting condition) at proper intervals and without any standing water. In aerobic condition, the rice roots may grow deeper into the soil to access the available ground water and nutrients. In this process of water and nutrient acquisition, it can be postulated that there may exist some similarity in the mechanism of drought and aerobic conditions. The higher expression of Dro1 gene under the aerobic condition compared to the anaerobic condition in the genotype (CR Dhan 202) suggests its role in need-based adaptation. Among the root development-related genes, CRL2/CRL3 and OsYUCCA1 were highly expressed in BPT 5204 under anaerobic condition but in CR Dhan 202 did not show significant difference under aerobic and anaerobic conditions. The genes, OsWRKY31, and OsEXPB5 were highly expressed under anaerobic condition in both the genotypes. The expression OsIAA13 was higher in BPT 5204 under anaerobic condition and CR Dhan 202 under aerobic condition (Supplementary Fig. 2a). The root elongation-related gene, OsPIN3t, was highly expressed under aerobic condition in both the genotypes. The OsARF12 and OsGlu3 were highly expressed under aerobic condition in CR Dhan 202 while in BPT 5204 highly expressed under anaerobic condition. The OsSPR1 was highly expressed under anaerobic condition while OsRR2 was highly expressed under aerobic condition in both the genotypes (Supplementary Fig. 2b). The genes for crown roots formation, lateral root formation, and root hair formation were highly expressed under anaerobic condition than aerobic. The relative expression pattern corroborated with our root physiological studies wherein higher number of roots were recorded under anaerobic condition than in aerobic condition (data not published).
Fig. 3.
Validation of stable candidate reference genes (Memp, TPH, and Exp1) using Dro1 under aerobic and anaerobic conditions. Bar on top indicates standard error of three technical replicates
The OsbHLH133 (iron utilization) was highly expressed under anaerobic condition in both the genotypes (Supplementary Fig. 2c). Among the nitrogen (N) utilization genes, NRR and OsAMT3.3 were highly expressed under aerobic condition in both the genotypes. The OsAMT1.1, OsAMT2.1, and OsAMT2.3 were highly expressed under anaerobic condition in both the genotypes. The OsAMT3.1 was highly expressed under anaerobic condition in CR Dhan 202 while in BPT 5204, it was highly expressed under aerobic condition. The OsAMT1.3 and OsNPF2.2 were highly expressed under anaerobic condition in CR Dhan 202 while in BPT 5204 higher expression was recorded under aerobic condition (Supplementary Fig. 2c). The OsNRT2.3 was highly expressed under aerobic condition in CR Dhan 202, while in BPT 5204, it was highly expressed under anaerobic condition. The OsNPF2.2 was highly expressed under anaerobic condition in CR Dhan 202, while in BPT 5204 highly expressed under aerobic condition (Supplementary Fig. 2c). The OsNRT2.3 is known to be involved in high-affinity nitrate transport system and nitrogen acquisition (Fan et al. 2016). Under the anaerobic condition, significant nitrification on root surface has been observed when NH4+ dominates which may be responsible for its relative high expression in anaerobic compared to aerobic condition (Singh et al. 2016). Another gene OsNPF2.2 has is known to be vascular specific transporter that unloads nitrate from xylem affecting root-to-shoot nitrate transport was highly expressed in aerobic condition (Li et al. 2015). The uptake, transport, and mobilization of macro and micro nutrients are highly dependent on plants strategies for acquisition. The gene related to phosphorus uptake, viz. Pup1, was highly expressed under aerobic condition in CR Dhan 202, while in BPT 5204 highly expressed under anaerobic condition. The OsSPX1 (P uptake) and OsMGT (Mg uptake) were highly expressed under aerobic condition in both the genotypes, while OsHKT1.4 (K and Na uptake) was highly expressed under anaerobic condition in both the genotypes (Supplementary Fig. 2c). The abiotic stress tolerance genes, viz. OsMSR2 and OsNAC9 were highly expressed under anaerobic condition in both the genotypes (Supplementary Fig. 2d). It was observed that the roots sense its surrounding and accordingly respond as a signal relay for growth and development of the above ground parts. Thus, under the aerobic and anaerobic conditions, the roots adapt and respond by upregulating or downregulating certain functional classes of genes. It can be established that roots play a critical role in response to adaptation to water availability.
Conclusion
The present study comprehensively revealed that three reference genes, viz. Memp, Exp1, and TPH are ideal for relative gene expression studies in rice under aerobic and anaerobic conditions in root and shoot tissues at different developmental stages. The reference genes were validated using genes related to root traits at panicle initiation stage which depicted the role of root-related genes in adaptation under aerobic and anaerobic systems of cultivation. This investigation is expected to give boost to molecular studies for exploring aerobic adaptations that is gaining momentum in the realm of climate change, identification of genes responsible for such adaptation, and to accelerate the development of suitable rice genotypes for aerobic condition.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are thankful to Director, ICAR-IIRR (Institute Research Council Project Codes ABR/CI/BT/12 and ABR/CI/BT/15) and Director, Institute of Biotechnology, PJTSAU for providing necessary facilities for research. Amol S. Phule acknowledges the University Grant Commission (UGC), New Delhi, India for providing National Fellowship for furnishing the doctoral programme.
Abbreviations
- qRT-PCR
Quantitative real-time polymerase chain reaction
- CT
Cycle threshold
- CV
Coefficient of variation
- DAG
Days after germination
- Dro1
Deeper rooting 1
- Exp1
Expressed protein
- Memp
Membrane protein
- PI stage
Panicle initiation stage
- SD
Standard deviation
- SV
Stability value
- TPH
Tumor protein homolog
Author contributions
PAK, MSM, and KMB conceived, planned, and designed study. ASP, KMB, PS, and MBB conducted experiments. KMB and ASP analyzed the data and wrote the manuscript. PAK and MSM critically edited manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Andersen C, Jensen J, Orntoft T. Normalization of real-time quantitative reverse transcription PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245. doi: 10.1158/0008. [DOI] [PubMed] [Google Scholar]
- Bennett J, Hondred D, Register JC. Keeping qRT-PCR rigorous and biologically relevant. Plant Cell Rep. 2015;34(1):1–3. doi: 10.1007/s00299-014-1692-6. [DOI] [PubMed] [Google Scholar]
- Bevitori R, Oliveir MB, Grossi-de-Sá MF, Lanna AC, da Silveira RD, Petrofeza S. Selection of optimized candidate reference genes for qRT-PCR normalization in rice (Oryza sativa L.) during Magnaporthe oryzae infection and drought. Genet Mol Res. 2014;13(4):9795–9805. doi: 10.4238/2014.November.27.7. [DOI] [PubMed] [Google Scholar]
- Bu Y. Research progress of ammonium transporter in rice plants in plants. Genomics. 2011;2(3):19–23. doi: 10.5376/gab.2011.02.0003. [DOI] [Google Scholar]
- Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol. 2002;29:23–39. doi: 10.1677/jme.0.0290023. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Shipley GL. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55(4):611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Beaulieu J, Huggett J, Jaggi R, Kibenge FSB, Olsvik PA, Toegel S. MIQE précis: practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BMC Mol Biol. 2010;11:74. doi: 10.1186/1471-2199-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem. 1987;162(1):156–159. doi: 10.1016/0003-2697(87)90021-2. [DOI] [PubMed] [Google Scholar]
- Dixit S, Grondin A, Lee C, Henry A, Olds T. Understanding rice adaptation to varying agro-ecosystems: trait interactions and quantitative trait loci. BMC Genet. 2015 doi: 10.1186/s12863-015-0249-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Tang Z, Tan Y, Zhang Y, Luo B, Yang M, Xu G. Overexpression of a pH-sensitive nitrate transporter in rice increases crop yields. Proc Natl Acad Sci. 2016 doi: 10.1073/pnas.1525184113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzinger DG. Gene quantification using real-time quantitative PCR: an emerging technology hits the mainstream. Exp Hematol. 2002;30:503–512. doi: 10.1016/S0301-472X(02)00806-8. [DOI] [PubMed] [Google Scholar]
- Hellemans J, Mortier G, Paepe A, De Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007;8:R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M, Nijhawan A, Tyagi AK, Khurana JP. Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem Biophys Res Commun. 2006;345(2):646–651. doi: 10.1016/j.bbrc.2006.04.140. [DOI] [PubMed] [Google Scholar]
- Kato Y, Okami M. Root growth dynamics and stomatal behaviour of rice (Oryza sativa L.) grown under aerobic and flooded conditions. Field Crops Res. 2010;117(1):9–17. doi: 10.1016/j.fcr.2009.12.003. [DOI] [Google Scholar]
- Kim BR, Nam HY, Kim SU, Kim SI, Chang YJ. Normalization of reverse transcription quantitative-PCR with housekeeping genes in rice. Biotechnol Lett. 2003;25(21):1869–1872. doi: 10.1023/A:1026298032009. [DOI] [PubMed] [Google Scholar]
- Li QF, Sun SSM, Yuan DY, Yu HX, Gu MH, Liu QQ. Validation of candidate reference genes for the accurate normalization of real-time quantitative RT-PCR data in rice during seed development. Plant Mol Biol Rep. 2009;28(1):49–57. doi: 10.1007/s11105-009-0124-1. [DOI] [Google Scholar]
- Li Y, Ouyang J, Wang YY, Hu R, Xia K, Duan J, Zhang M. Disruption of the rice nitrate transporter OsNPF2.2 hinders root-to-shoot nitrate transport and vascular development. Sci Rep. 2015;5:9635. doi: 10.1038/srep09635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai CD, Phung NTP, To HTM, Gonin M, Hoang GT, Nguyen KL, Do VN, Courtois B, Gantet P. Genes controlling root development in rice. Rice. 2014;7:1–11. doi: 10.1186/s12284-014-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksup S, Supaibulwatana K, Selvaraj G. High-quality reference genes for quantifying the transcriptional responses of Oryza sativa L. (ssp. indica and japonica) to abiotic stress conditions. Chi Sci Bull. 2013;58(16):1919–1930. doi: 10.1007/s11434-013-5726-1. [DOI] [Google Scholar]
- Moraes GP, Benitez LC, do Amaral MN, Vighi IL, Auler PA, da Maia LC, Braga EJB. Evaluation of reference genes for RT-qPCR studies in the leaves of rice seedlings under salt stress. Genet Mol Res. 2015;14(1):2384–2398. doi: 10.4238/2015.March.27.24. [DOI] [PubMed] [Google Scholar]
- Narsai R, Ivanova A, Ng S, Whelan J. Defining reference genes in Oryza sativa using organ, development, biotic and abiotic transcriptome datasets. BMC Plant Biol. 2010;10:56. doi: 10.1186/1471-2229-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabuayon IM, Yamamoto N, Trinidad JL, Longkumer T, Raorane ML, Kohli A. Reference genes for accurate gene expression analyses across different tissues, developmental stages and genotypes in rice for drought tolerance. Rice. 2016;9(1):32. doi: 10.1186/s12284-016-0104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel DP, Das A, Munda GC, Ghosh PK, Sandhya J, Kumar M. Evaluation of yield and physiological attributes of high-yielding rice varieties under aerobic and flood-irrigated management practices in mid-hills ecosystem. Agric Water Manag. 2010;97(9):1269–1276. doi: 10.1016/j.agwat.2010.02.018. [DOI] [Google Scholar]
- Pathak H, Tewari AN, Sankhyan S, Dubey DS, Mina U, Singh VK, Bhatia A. Direct-seeded rice: potential, performance and problems—a review. Curr Adv Agric Sci. 2011;3(2):77–88. [Google Scholar]
- Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper-Excel-based tool using pair-wise correlations. Biotechnol Lett. 2004;26(6):509–515. doi: 10.1023/B:BILE.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- Sandhu N, Jain S, Kumar A, Mehla BS, Jain R. Genetic variation, linkage mapping of QTL and correlation studies for yield, root, and agronomic traits for aerobic adaptation. BMC Genet. 2013;14:104. doi: 10.1186/1471-2156-14-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt GW, Delaney SK. Stable internal reference genes for normalization of real-time RT-PCR in tobacco (Nicotiana tabacum) during development and abiotic stress. Mol Genet Genom. 2010;283(3):233–241. doi: 10.1007/s00438-010-0511-1. [DOI] [PubMed] [Google Scholar]
- Singh A, Kumar P, Gautam V, Rengasamy B, Adhikari B, Udayakumar M, Sarkar AK. Root transcriptome of two contrasting indica rice cultivars uncovers regulators of root development and physiological responses. Sci Rep. 2016;6:39266. doi: 10.1038/srep39266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udvardi MK, Czechowski T, Scheible WR. Eleven golden rules of quantitative RT-PCR eleven golden rules of quantitative RT-PCR. Plant Cell. 2008;20:1736–1737. doi: 10.1105/tpc.108.061143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uga Y, Okuno K, Yano M. Dro1, a major QTL involved in deep rooting of rice under upland field conditions. J Exp Bot. 2011;62(8):2485–2494. doi: 10.1093/jxb/erq429. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Xie W, Chen Y, Tang W, Yang J, Ye R, Zhang Q. A dynamic gene expression atlas covering the entire life cycle of rice. Plant J. 2010;61(5):752–766. doi: 10.1111/j.1365-313X.2009.04100.x. [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Li GZ, Gong QQ, Li GX, Zheng SJ. OsTCTP, encoding a translationally controlled tumor protein, plays an important role in mercury tolerance in rice. BMC Plant Biol. 2015;15:123. doi: 10.1186/s12870-015-0500-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Cheng S. Root genetic research, an opportunity and challenge to rice improvement. Field Crops Res. 2014;165:111–124. doi: 10.1016/j.fcr.2014.04.013. [DOI] [Google Scholar]
- Xie F, Xiao P, Chen D, Xu L, Zhang B. miRDeepFinder: a miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol Biol. 2012;80(1):75–84. doi: 10.1007/s11103-012-9885-2. [DOI] [PubMed] [Google Scholar]
- Xu H, Bao JD, Dai JS, Li Y, Zhu Y. Genome-Wide Identification of new reference genes for qRT-PCR normalization under high temperature stress in rice endosperm. PLoS One. 2015;10(11):e0142015. doi: 10.1371/journal.pone.0142015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Liu J, Huang S, Guo T, Deng L, Hua W. Selection and evaluation of novel reference genes for quantitative reverse transcription PCR (qRT-PCR) based on genome and transcriptome data in Brassica napus L. Gene. 2014;538(1):113–122. doi: 10.1016/j.gene.2013.12.057. [DOI] [PubMed] [Google Scholar]
- Zhang X, Jiang H, Wang H, Cui J, Wang J, Hu J, Guo L, Qian Q, Xue D. Transcriptome analysis of rice seedling roots in response to potassium deficiency. Sci Rep. 2017;7:5523. doi: 10.1038/s41598-017-05887-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.