Abstract
Arrhythmia recurrences after catheter ablation of atrial fibrillation (AF) cause intensive treatment costs. Left atrial electro-anatomical remodeling measured as low voltage areas (LVA) during catheter ablation indicates advanced disease stage and is associated with poor ablation success. The aim of this study was to analyze the prediction of LVA and arrhythmia recurrences using APPLE, DR-FLASH and MB-LATER scores. APPLE, DR-FLASH scores were calculated at baseline and MB-LATER at 3 months post-ablation in AF patients undergoing first catheter ablation. LVA was determined using high-density maps and defined as <0.5 mV. Early (ERAF, <3 months) and late (LRAF, 3–12 months) were analyzed during follow-up. The study population included 241 patients (age 64 ± 11 years, 59% males, 59% persistent AF, 27% LVA, 27% LRAF). LVA were significantly associated with recurrences (OR 2.081, p = 0.026). While on univariable analysis, all scores were significantly associated with LVA, on multivariable analysis only APPLE (OR 1.789, p < 0.001) and DR-FLASH (OR 2.144, p < 0.001) remained significant predictors. However, MB-LATER (OR 1.445, p = 0.034) and ERAF (OR 5.078, p < 0.001) remained associated with LRAF on the multivariable analysis. These results were validated in a subgroup of 873 patients (age 61 ± 10, 63% males, 39% persistent AF, 34% LRAF, 27% LVA) from The Leipzig Heart Center AF Ablation Registry. All scores were significantly associated with recurrences. However, ERAF was the most powerful predictor for later rhythm outcomes. Summarizing, a clinical score useful for prediction for both LVA and rhythm outcomes in AF patients remains a clinical unmet need.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia, which is associated with an increased risk of dementia, heart failure and thromboembolism, leading to an increased mortality1. Pathophysiological, AF results in electrical and later structural remodeling of the atrial myocardium (inflammation, fibrosis, and atrial dilatation)2.
Left atrial low voltage areas (LVA), also known as electro-anatomical substrate, represent these atrial remodeling processes and are considered to play an important role in AF progression3,4. LVA can be found in 10% of patients with paroxysmal AF and in 35% of patients with persistent AF4. However, performing individually tailored substrate modification, a significantly higher arrhythmia-free survival rate compared with a conventional approach can be achieved5.
Nevertheless, the prediction of LVA using clinical scores is understudied. Ribo et al. analyzed prediction of electro-anatomical substrate using the CHA2DS2-VASc score and demonstrated that high scores were associated with extensive AF substrate and higher recurrence rates after catheter ablation of persistent AF6. The DR-FLASH score (based on Diabetes mellitus, Renal dysfunction, persistent AF type, LA diameter >45 mm, Age >65 years, female Sex, and Hypertension) was recently introduced to predict LVA and demonstrated a high predictive ability (AUC = 0.801, p < 0.001)7. Recently, we introduced the APPLE score (one point for Age >65 years, Persistent AF, imPaired eGFR (<60 ml/min/1.73 m2), LA diameter ≥43 mm, EF <50%) for the prediction of AF recurrences after catheter ablation in patients undergoing first and re-do intervention8,9. Later, we demonstrated that the APPLE score was useful to predict LVA, too10. The MB-LATER score (one point for Male gender, Bundle branch block or QRS >120 ms, LA diameter ≥47 mm, AF Type (persistent AF), Early Recurrence <3 months) had been developed as the rhythm outcomes score for the prediction of very late arrhythmia recurrences (>12 months) after catheter ablation11, but its potential to predict the presence of LVA during catheter ablation is unknown. Therefore, the aim of the current study was to compare prediction of LVA and arrhythmia recurrences using the APPLE, MB-LATER and DR-FLASH scores in AF patients undergoing first catheter ablation.
Methods
The study population included 241 AF patients from the BioAF cohort initiated at the Heart Center Leipzig between 2015 and 2017 as previously described12. All patients underwent first AF radiofrequency catheter ablation and were detailed phenotyped including clinical, imaging, peri-interventional, follow-up data and biodata (blood and plasma). Furthermore, a subgroup of 873 patients from The Leipzig Heart Center AF Ablation Registry (2007–2011) had been used to validate the results8,13. Exclusion criteria in both cohorts were pregnancy, age <18 or >75, valvular AF, cancer, acute or systemic inflammatory diseases. The study was approved by the local Ethical Committee (Medical Faculty, Leipzig University) and all patients provided written informed consent for participation. All methods were performed in accordance with the relevant guidelines and regulations.
Paroxysmal and persistent AF were defined according to current guidelines1. Paroxysmal AF was defined as self-terminating within 7 days after onset. Persistent AF lasted longer than 7 days or required drugs or electrical cardioversion for termination. In all patients, transthoracic and transoesophageal echocardiography were performed prior to the ablation. All class I or III antiarrhythmic medications with exception of amiodarone were discontinued for at least 5 half-lives before the AF ablation procedure.
Catheter ablation
The electro-anatomical mapping was performed in sinus rhythm. In patients presenting with AF, the arrhythmia was terminated by electrical cardioversion and the mapping was further performed in sinus rhythm. End-point of the catheter ablation was isolation of the pulmonary veins with proof of both exit and entrance block. The electro-anatomical voltage maps of the left atrium excluding the pulmonary veins were created using multielectrode spiral catheter with interelectrode distance 2-5-2 or ablation catheter with a 3.5 mm electrode tip and contact measurement properties (SmartTouch Thermocool, Biosense Webster, Diamond Bar, CA, USA and TactiCath, St Jude Medical (SJM), Saint Paul, MN, USA) as mapping catheter. Electro-anatomical mapping was performed using 3-D electro-anatomical mapping systems (Carto, Biosense Webster, Diamond Bar, CA, USA or EnSite Precision, SJM). In both mapping systems the cut-off values for defining LVA were identical: <0.5 mV for low voltage and <0.2 mV for dense scar. Patients underwent high density mapping of left atrial voltage using multipolar catheters in combination with auto-annotation algorithms (AutoMap in Precision and ConfiDense in Carto 3). Here the number of points was >1000. Points with insufficient catheter-to-tissue contact or inside ablation lines were excluded. At the end of the procedure, an attempt to induce AF or left atrial macro-reentry tachycardia (LAMRT) was performed using a standardized protocol (burst stimulation with 300, 250, 200 ms from coronary sinus). According to the underlying LVA and inducible LAMRT additional ablation lines were applied.
In the subgroup from The Leipzig Heart Center AF Ablation Registry, LA catheter ablation was performed using a well-documented approach13. Briefly, in all patients, circumferential LA ablation lines were placed around the antrum of the ipsilateral pulmonary veins (irrigated tip catheter, pre-selected tip temperature of 48 °C, and maximum power of 30–50 W). In patients with persistent AF, additional linear lesions were added at the LA roof, the basal posterior wall and the LA (mitral) isthmus. At the end of procedure, linear block was confirmed across the roof and the mitral isthmus. The isolation of all pulmonary veins with bidirectional block was verified with a multipolar circular mapping catheter and was defined as the procedural endpoint.
In both cohorts, after ablation class I and III antiarrhythmic drugs were not routinely initiated. Only patients with failed sinus rhythm restoration received previous antiarrhythmic medication during blanking period and thereafter dependent on the rhythm in Holter ECG during follow-up. Proton pump inhibitors were added for 4 weeks. According to the guidelines1, oral anticoagulation was prescribed for 3–6 months after catheter ablation depending on risk stratification of stroke using the CHA2DS2-VASc score thereafter.
Follow-up
All patients were followed in the outpatient clinic after catheter ablation. During the follow-up period, 4-days (in the BioAF cohort) or 7-days (in The Leipzig Heart Center AF Ablation Registry) Holter ECG recordings were performed at 3, 6 and 12 months. Additional ECGs and Holter ECG recordings were obtained when patients’ symptoms were suggestive of AF. Early arrhythmia recurrences (ERAF) were defined as any atrial arrhythmia lasting longer than 30 seconds and occurring within the first 3 months after procedure. Late arrhythmia recurrences (LRAF) were any atrial arrhythmia lasting >30 s between 3 and 12 months after ablation. If electrical or pharmacologic cardioversion and/or repeat procedure were needed after 3 months blanking period, this was also considered as an arrhythmia recurrence, i.e. study endpoint.
Statistical analysis
Data are presented as mean and standard deviation (SD) if normally distributed or as median [interquartile range] for skewed continuous variables and as proportions for categorical variables. Continuous variables were tested for normal distribution using the Kolmogorov-Smirnov test. The differences between continuous values were assessed using an unpaired t-test if normally distributed or Mann–Whitney test if skewed continuous variables, and a chi-square test for categorical variables.
Multivariable logistic regression analysis (MV), which included variables with a p-value < 0.1 found on univariable analysis, was performed to identify predictors for LVA and arrhythmia recurrences. We performed multivariable analyses separately for APPLE, MB-LATER and DR-FLASH scores with adjustment for the variables, which were significant in univariable model, but were not included into the scores: Model 1 for the APPLE with adjustment for gender, hypertension, diabetes, bundle brunch block, ERAF; Model 2 for the MB-LATER with adjustment for hypertension, diabetes, EF, eGFR; Model 3 for the DR-FLASH with adjustment for EF, bundle brunch block, ERAF. In patients with available LVA data, LVA was adjusted to each model analyzing prediction of arrhythmia recurrences.
ROC (receiver operating characteristic) curves were generated for graphical illustration of APPLE, MB-LATER and DR-FLASH scores’ performance in predicting LVA and arrhythmia recurrences, with the area under the curve (AUC) being equivalent to the c-index for determining the predictive value for a score.
A p-value < 0.05 was considered statistically significant. All analyses were performed with IBM SPSS Statistics for Windows Version 23 (IBM Corp, Armonk, NY, USA).
Results
Study cohorts
The study population consisted of two cohorts: 241 patients from BioAF cohort with available LVA and rhythm outcomes data and 873 patients from The Leipzig Heart Center AF Ablation Registry used for the results validation (215 with available LVA data). The baseline characteristics of both cohorts are presented in Tables 1 and 2. Patients in the BioAF cohort were significantly older, had more often persistent AF, worse renal function and left ventricular EF, higher BMI, larger LA diameter, hypertension as well as higher APPLE and DR-FLASH scores than patients from The Leipzig Heart Center AF Ablation Registry (all p < 0.05, Suppl. Table 1). Patients in The Leipzig Heart Center AF Ablation Registry had significantly higher incidence for ERAF and LRAF.
Table 1.
Baseline characteristics of the BioAF cohort (n = 241).
| LVA | p-value | Recurrences | p-value | |||
|---|---|---|---|---|---|---|
| Yes (n = 65) | No (n = 176) | Yes (n = 62) | No (n = 179) | |||
| Age, years | 69 (64–75) | 63 (55–71) | <0.001 | 64 (56–69) | 65 (58–73) | 0.112 |
| Females | 55 | 35 | 0.005 | 40 | 38 | 0.763 |
| Persistent AF | 82 | 51 | <0.001 | 68 | 54 | 0.044 |
| Electro-anatomical substrate | — | — | — | 36 | 21 | 0.024 |
| eGFR, ml/min/1.73 m2 | 68 (57–82) | 79 (68–93) | <0.001 | 79 (66–89) | 76 (63–89) | 0.390 |
| BMI, kg/m2 | 30 (26–33) | 29 (26–33) | 0.255 | 31 (27–34) | 28 (26–33) | 0.025 |
| LA diameter, mm | 42 (45–49) | 43 (39–48) | 0.028 | 45 (39–48) | 44 (40–48) | 0.765 |
| EF, % | 60 (50–65) | 58 (50–65) | 0.785 | 60 (50–65) | 59 (50–65) | 0.694 |
| CHA2DS2-VASc score | 3 (3–4) | 2 (1–4) | <0.001 | 3 (1–4) | 3 (1–4) | 0.996 |
| APPLE score | 3 (2–4) | 2 (1–2) | <0.001 | 2 (1–3) | 2 (1–3) | 0.579 |
| DR-FLASH score | 5 (4–5) | 3 (2–4) | <0.001 | 4 (3–5) | 4 (2–5) | 0.214 |
| MB-LATER score | 2 (1–3) | 2 (1–2) | 0.053 | 2 (1–3) | 2 (1–3) | 0.018 |
| Recurrences | 22 (34) | 37 (21) | 0.024 | — | — | — |
Data presented as mean (IQR) or %.
Abbreviations: LVA – low voltage areas; AF – atrial fibrillation; BMI – body mass index; eGFR – estimated glomerular filtration rate; LA – left atrial; EF – ejection fraction; CHA2DS2-VASc score – congestive heart failure, hypertension, age ≥75 y, diabetes, stroke/thromboembolism, vascular disease (s), age 65–74 y, females; APPLE score – Age > 65 years, Persistent AF, imPaired eGFR (<60 ml/min/1.73 m2), LA diameter ≥43 mm, EF <50%; DR-FLASH score – diabetes mellitus, renal dysfunction, persistent form of AF, LA diameter >45 mm, age >65 years, female sex, and hypertension; MB-LATER score – Male gender, Bundle branch block or QRS >120 ms, LA diameter ≥47 mm, AF Type (persistent AF), Early Recurrence <3 months.
Table 2.
Baseline characteristics of the validation cohort from The Leipzig Heart Center AF Ablation Registry (n = 873).
| Recurrences | p-value | LVA* | p-value | |||
|---|---|---|---|---|---|---|
| Yes (n = 300) | No (n = 573) | Yes (n = 58) | No (n = 157) | |||
| Age, years | 63 (55–70) | 61 (54–68) | 0.007 | 69 (61–72) | 60 (53–67) | <0.001 |
| Females | 39 | 35 | 0.212 | 36 | 19 | 0.009 |
| Persistent AF | 54 | 31 | <0.001 | 86 | 57 | <0.001 |
| eGFR, ml/min/1.73 m2 | 95 (76–116) | 97 (81–119) | 0.100 | 72 (62–86) | 83 (71–94) | <0.001 |
| BMI, kg/m2 | 28 (26–31) | 28 (25–31) | 0.325 | 29 (27–31) | 28 (27–31) | 0.123 |
| LA diameter, mm | 44 (40–48) | 42 (38–46) | <0.001 | 47 (40–49) | 42 (39–47) | 0.013 |
| EF, % | 60 (54–65) | 60 (55–65) | 0.040 | 58 (50–62) | 60 (54–64) | 0.145 |
| CHA2DS2-VASc score | 2 (1–3) | 2 (1–3) | 0.004 | 3 (2–4) | 1 (1–2) | <0.001 |
| APPLE score | 2 (1–3) | 1 (1–2) | <0.001 | 3 (2–4) | 2 (1–2) | <0.001 |
| DR-FLASH score | 3 (2–4) | 3 (2–4) | <0.001 | 5 (4–5) | 3 (2–4) | <0.001 |
| MB-LATER score | 2 (1–3) | 2 (1–2) | <0.001 | 3 (2–3) | 2 (1–3) | 0.035 |
| Recurrences, % | — | — | 43 | 35 | 0.277 | |
*Subgroup from the Leipzig Heart Center AF Ablation Registry with available LVA data (n = 215).
Prediction of LVA
LVA were significantly associated with LRAF (OR 2.081, 95% CI 1.092–3.965, p = 0.026). On the univariable analysis (Suppl. Table 2), age, female sex, persistent AF, hypertension, diabetes, renal function, LA diameter and all scores were significantly associated with LVA. However, on the multivariable analysis, only APPLE (OR 1.789, 95% CI 1.316–2.432, p < 0.001) and DR-FLASH (OR 2.144, 95% CI 1.587–2.896, p < 0.001) scores remained significant predictors for LVA, while MB-LATER did not reach significance (Suppl. Table 2).
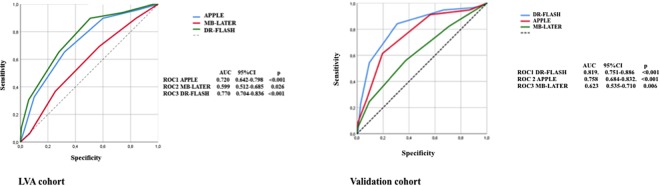
In the ROC curve analyses, all scores showed significant predictive value for LVA (APPLE AUC 0.720, 95% CI 0.642–0.798, p < 0.001, DR-FLASH AUC 0.770, 95% CI 0.704–0.836, p < 0.001, MB-LATER AUC 0.599, 95% CI 0.512–0.685, p = 0.026, Fig. 1).
Figure 1.
Prediction of LVA using APPLE, DR-FLASH and MB-LATER scores.
In the validation cohort, all scores were significantly associated with LVA (Suppl. Table 3).
Prediction of arrhythmia recurrences
BioAF cohort
There were 64 patients (27%) with LRAF in the BioAF cohort. On univariable analysis, persistent AF, LVA, ERAF and MB-LATER were significantly associated with rhythm outcomes within first 12 months after catheter ablation (Suppl. Table 4). On multivariable analyses, MB-LATER (OR 1.445, 95% CI 1.028–2.030, p = 0.034, MV Model 2) and ERAF (OR 5.078 in MV Model 1 and OR 5.066 in MV Model 3, both p < 0.001) remained significant predictors for LRAF.
The Leipzig Heart Center AF Ablation Registry
For the validation of predictive value of all scores we performed similar analyses in a subgroup of AF patients from The Heart Center Leipzig AF Ablation Registry. In total, 873 patients (age 61 ± 10, 63% males, 39% persistent AF, 34% LRAF) with available data for all scores’ calculation were included into analyses (Table 2).
On the univariable analysis (Suppl. Table 5), age, persistent AF, LA diameter, ERAF and all scores were significantly associated with LRAF. On the multivariable analysis, the APPLE (OR 1.550, 95% CI 1.333–1.803, p < 0.001), MB-LATER (OR 1.747, 95% CI 1.527–1.998, p < 0.001) and DR-FLASH (OR 1.242, 95% CI 1.118–1.381, p = 0.001) scores were significant predictors for LRAF. Similarly, as with LRAF prediction in the BioAF cohort, ERAF was the most powerful predictor for rhythm outcomes within 3–12 months with almost 4.5-fold risk for LRAF (OR 4.436 in MV Model 1 and OR 4.485 in MV Model 3, both p < 0.001).
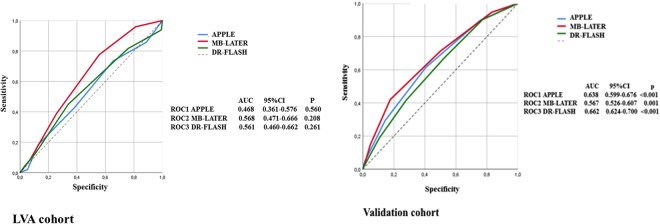
In the ROC curve analysis, all scores demonstrated a significant predictive ability for LRAF: AUC 0.638, 95% CI 0.599–0.676, p < 0.001 for the APPLE score, AUC 0.662, 95% CI 0.624–0.700, p < 0.001 for DR-FLASH, and AUC 0.567, 95% CI 0.526–0.607, p = 0.001 for MB-LATER (Fig. 2).
Figure 2.
Prediction of recurrences using APPLE, DR-FLASH and MB-LATER scores.
Discussion
To the best of our knowledge, this is the first analysis comparing the prediction for electro-anatomical remodelling measured as LVA and rhythm outcomes using APPLE, MB-LATER and DR-FLASH scores. First, the APPLE and DR-FLASH scores were significant predictors for LVA. Furthermore, all scores significantly predicted arrhythmia recurrences within 3–12 months after catheter ablation in a validation cohort from The Leipzig Heart Center AF Ablation Registry. However, the most powerful predictor for LRAF remained ERAF in both cohorts.
Prediction of electro-anatomical substrate
Left atrial structural remodelling plays a major role in AF pathogenesis and up to date could be detected invasively using LA voltage mapping during catheter ablation4,14 or non-invasively using cardiac MRI15. Voltage-guided substrate modification by targeting LVA in addition to PVI is more effective than conventional PVI ablation approaches concerning arrhythmia freedom after the ablation4,5,14. Recently, Yagishita et al. showed that a LA voltage cut-off of <1.1 mV for electro-anatomic voltage mapping in sinus rhythm can be seen as an independent predictor for recurrences in patients without LVA (<0.5 mV)16. Although LVA is an important risk factor for post-procedural AF4,5, there are no standardized methods to predict LVA non-invasively before catheter ablation.
Some studies have analysed the prediction of electro-anatomical substrate using CHADS2 and CHA2DS2-VASc scores6, as both scores include the most important risk factors associated with cardiovascular complications in AF patients. Furthermore, the DR-FLASH score was developed to predict LVA in patients with AF undergoing radiofrequency catheter ablation7. However, clinical variables used by this score are mostly the same as in the widely used CHA2DS2-VASc score to predict thromboembolic risk. Nevertheless, it also includes variables as LA diameter and AF type, which are associated with AF progression.
The APPLE and MB-LATER scores were both introduced as rhythm outcomes predicting scores. While the APPLE score predicted arrhythmia recurrences within one year in patients undergoing first and repeat ablations8,9, the MB-LATER score had been developed for the prediction of arrhythmia recurrences over 12 months in patients with arrhythmia-free survival within first year11.
In the current study, we found that APPLE and DR-FLASH significantly predicted LVA before catheter ablation. The prediction of electro-anatomical substrate using DR-FLASH score was the best with more than 2-fold risk for LVA, however, rather expectable, as it is the score developed for the prediction of underlying electro-anatomical substrate in AF patients7. Of note, female gender – as a component of DR-FLASH score – was recently considered as a risk factor for substrate in AF patients. Indeed, females have a 2-fold risk for LVA17 and an almost 3-fold increased risk for AF recurrence following catheter ablation3. Females might probably present with clinical AF in a later state of fibro-fatty infiltration, which could explain a higher presence of electro-anatomical substrate and worse rhythm outcomes after catheter ablation17. These findings had been also confirmed in our current analysis.
In contrast, the APPLE had been developed as the rhythm outcomes prediction score and included such relevant components for AF progression as age, AF type and LA diameter8,9. Other components – like EF (an indicator for heart failure) and renal dysfunction – are also associated with an electro-anatomical substrate18,19. Therefore, the association between the APPLE score and underlying substrate in AF patients is explainable. This had been also recently confirmed10.
The MB-LATER score, however, did not reach significance predicting LVA. Although bundle branch block (BBB) – as the one of the parameters included in MB-LATER score – could be considered as a predictor for cardiac fibrotic turn-over in the ventricles mirroring remodelling processes in the atria, on multivariable analyses this parameter was not significantly associated with LVA. Interestingly, on multivariable analyses, hypertension was associated with almost 4-fold risk for LVA. Hypertension – as one of the most common cardiac diseases – leads to the cardiomyocyte hypertrophy and resulting pro-fibrotic changes20,21. Although in multivariable analyses it does not reach significance, importance of hypertension prevention and treatment in AF patients is obvious.
Prediction of arrhythmia recurrences
Arrhythmia recurrences still remain a common issue after catheter ablation because of the impairment of the quality of life, higher hospitalization rates and consequently intensive treatment costs1. Therefore, on the one hand, there is a considerable clinical interest to predict the risk for recurrences already before procedure; on the other one – to shape personalized therapeutic strategies.
There have been multiple studies analysing the impact of different scores on recurrence prediction. First, the thromboembolic risk predicting CHADS2 and CHA2DS2-VASc scores demonstrated only modest prediction for arrhythmia recurrences21,22. This had led to the development of specific rhythm outcomes prediction scores as ALARMEC, BASE-AF2, CAAP-AF and ATLAS23–26. However, the results of these studies are partly difficult to interpret because of some non-standardized definitions (especially renal dysfunction and metabolic syndrome) and relatively small study populations. The APPLE score had been established for recurrences within 12 months after first ablation using 1.145 patients of The Leipzig Heart Center AF Ablation Registry8 as well as after repeat catheter ablation in 379 patients9. In both groups, APPLE score showed better ability to predict rhythm outcomes than CHADS2 and CHA2DS2-VASc. The MB-LATER score had been introduced to predict very late arrhythmia recurrences in patients with arrhythmia-free survival within 3–12 months after ablation11. It was developed using small retrospective cohort (n = 133), compared against other clinical scores (ALARMEc, BASE-AF2, APPLE, CHADS2, CHA2DS2–VASc, or HATCH), and demonstrated significantly better VLRAF prediction than most scores, apart from the APPLE score. Beside its predictive value for LVA, DR-FLASH was also associated with AF recurrences within 12 months after catheter ablation7, however in the initial study only cut-off had been shown as predictive.
In the current study, the ERAF demonstrated the best association with LRAF in the BioAF cohort and in the retrospective validation subgroup from The Leipzig Heart Center AF Ablation Registry. Of note, among all analysed scores, only MB-LATER included early recurrences11. Previously, we demonstrated that patients with ERAF had almost 4-fold risk for LRAF occurrence during follow-up13. This had been also confirmed by a meta-analysis27. However, ERAF is not available at baseline, therefore, MB-LATER cannot be calculated before catheter ablation.
Prediction of electro-anatomical substrate and arrhythmia recurrences
In the current study, we found that the APPLE score demonstrated robust predictive value for both LVA and rhythm outcomes. On the one hand, it is a simple calculated score with easy obtainable clinical variables; on the other hand, it can be calculated already at baseline. Nevertheless, it was not significant for the prediction of arrhythmia recurrences in the BioAF cohort. Although both cohorts had been initiated in the same high-volume ablation center and performed using radiofrequency ablation approach, the BioAF cohort was initiated almost a decade later. This partly explains significant differences in baseline characteristics between both cohorts. While 10 years ago the guidelines recommended AF ablation only by failed rhythm and/or frequency control, during the last decade we got hard data presenting catheter ablation benefits on morbidity and mortality, what had led to the update of currently available AF management guidelines1. Also, during the last years catheter ablation had been more frequently recommended in older and comorbid patients. These observations explain why patients in the BioAF cohort were significantly older and had more co-morbidities. Also, there were significant differences in ERAF and LRAF occurrence between both cohorts. On the one hand, this could be explained by the technical development and application of better catheters and mapping systems in the BioAF cohort, on the other hand – by the shorter duration of ECG Holter monitoring during follow-up. Both issues might be the reason, why we failed to demonstrated significant prediction of arrhythmia outcomes using the APPLE score in BioAF cohort.
In general, prediction of arrhythmia recurrences remains complex as not only disease stage and patients’ comorbidities play a role, but also operators experience and continuity of the rhythm control. Still, prediction of electro-anatomical remodelling and rhythm outcomes remains challenging and obvious clinical unmet need. As both are very likely associated with pro-fibrotic changes in atrial myocardium, the prediction of atrial fibrosis using non-invasive tools (MRI, blood biomarkers) could influence therapy decisions and help to tailor individualized treatment strategy. Further translational and interdisciplinary studies are needed to elucidate the true role of atrial fibrosis and its’ prediction in AF patients.
Study limitations
This study is limited by its observational, retrospective design in a small single-center cohort and retrospective results validation. Furthermore, the majority of the patients were not monitored by loop recorders; asymptomatic episodes of silent AF could have been missed. Also, there were only patients undergoing first radiofrequency AF catheter ablation. The results could differ in patients with repeated procedures or with ablation using other sources. Finally, in The Leipzig Heart Center AF Ablation Registry conducted between 2007 and 2011, the LVA data were available only in 214 patients.
Conclusion
APPLE and DR-FLASH scores were significant predictors for LVA, while the most powerful predictor for the rhythm outcomes was ERAF. A clinical score useful for prediction of both LVA and rhythm outcomes after catheter ablation remains a clinical unmet need.
Electronic supplementary material
Comparison of baseline characteristics between BioAF cohort and the validation cohort from The Leipzig Heart Center AF Ablation Registry
Prediction of LVA in the Leipzig Heart Center AF Ablation Registry
Prediction of arrhythmia recurrences in the BioAF cohort
Prediction of arrhythmia recurrences in the subgroup from the Leipzig Heart Center AF Ablation Registry
Acknowledgements
Katja Schumacher is supported by the German Cardiac Society Otto Hess Scholarship 2017.
Author Contributions
J.K. – concept/design; J.K., K.S., B.D., F.K. – data collection; J.K. – analysis and interpretation; J.K. – statistics; J.K., K.S. – drafting article; B.D., P.S., A.B., G.Y.H.L. – critical revision of article; B.D., P.S., A.A., D.H., A.B. – patient inclusion; J.K., G.H. – approval of article.
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-31133-x.
References
- 1.Kirchhof P, et al. ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–1678. doi: 10.1093/europace/euw295. [DOI] [PubMed] [Google Scholar]
- 2.Iwasaki YK, et al. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124:2264–2274. doi: 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- 3.Dinov B, et al. Impact of metabolic syndrome on left atrial electroanatomical remodeling and outcomes after radiofrequency ablation of nonvalvular atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:483–489. doi: 10.1161/CIRCEP.113.001185. [DOI] [PubMed] [Google Scholar]
- 4.Rolf S, et al. Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:825–833. doi: 10.1161/CIRCEP.113.001251. [DOI] [PubMed] [Google Scholar]
- 5.Kircher, S. et al. Individually tailored vs. standardized substrate modification during radiofrequency catheter ablation for atrial fibrillation: a randomized study. Europace, 10.1093/europace/eux310 (2017). [DOI] [PubMed]
- 6.Ribo T, et al. Impact of CHA2DS2 VASc score on substrate for persistent atrial fibrillation and outcome post catheter ablation of atrial fibrillation. Zhonghua Xin Xue Guan Bing Za Zhi. 2015;43:695–699. [PubMed] [Google Scholar]
- 7.Kosiuk J, et al. Prospective, multicenter validation of a clinical risk score for left atrial arrhythmogenic substrate based on voltage analysis: DR-FLASH score. Heart Rhythm. 2015;12:2207–2212. doi: 10.1016/j.hrthm.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Kornej J, et al. The APPLE score: a novel and simple score for the prediction of rhythm outcomes after catheter ablation of atrial fibrillation. Clin Res Cardiol. 2015;104:871–876. doi: 10.1007/s00392-015-0856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kornej J, et al. The APPLE Score - A Novel Score for the Prediction of Rhythm Outcomes after Repeat Catheter Ablation of Atrial Fibrillation. Plos One. 2017;12:e0169933. doi: 10.1371/journal.pone.0169933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kornej, J. et al. Prediction of electro-anatomical substrate using APPLE score and biomarkers. Europace., 10.1093/europace/euy120 (2018). [DOI] [PubMed]
- 11.Mujović N, et al. Prediction of very late arrhythmia recurrence after radiofrequency catheter ablation of atrial fibrillation: The MB-LATER clinical score. Sci Rep. 2017;7:40828. doi: 10.1038/srep40828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Büttner, P. et al. Role of NT-proANP and NT-proBNP in patients with atrial fibrillation: Association with atrial fibrillation progression phenotypes. Heart Rhythm., 10.1016/j.hrthm.2018.03.021 (2018). [DOI] [PubMed]
- 13.Kornej J, et al. Comparison of CHADS2, R2CHADS2, and CHA2DS2-VASc scores for the prediction of rhythm outcomes after catheter ablation of atrial fibrillation: the Leipzig Heart Center AF Ablation Registry. Circ Arrhythm Electrophysiol. 2014;7:281–287. doi: 10.1161/CIRCEP.113.001182. [DOI] [PubMed] [Google Scholar]
- 14.Blandino A, et al. Left Atrial Substrate Modification Targeting Low-Voltage Areas for Catheter Ablation of Atrial Fibrillation: A Systematic Review and Meta-Analysis. Pacing Clin Electrophysiol. 2017;40:199–212. doi: 10.1111/pace.13015. [DOI] [PubMed] [Google Scholar]
- 15.Marrouche NF, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014;311:498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 16.Yagishita A, et al. Identification and electrophysiological characterization of early left atrial structural remodeling as a predictor for atrial fibrillation recurrence after pulmonary vein isolation. J Cardiovasc Electrophysiol. 2017;28:642–650. doi: 10.1111/jce.13211. [DOI] [PubMed] [Google Scholar]
- 17.Huo, Y. et al. Prevalence and predictors of low voltage zones in the left atrium in patients with atrial fibrillation. Europace, 10.1093/europace/eux082 (2017). [DOI] [PubMed]
- 18.Akkaya M, et al. Higher degree of left atrial structural remodeling in patients with atrial fibrillation and left ventricular systolic dysfunction. J Cardiovasc Electrophysiol. 2013;24:485–491. doi: 10.1111/jce.12090. [DOI] [PubMed] [Google Scholar]
- 19.Chao TF, et al. Associations between renal function, atrial substrate properties and outcome of catheter ablation in patients with paroxysmal atrial fibrillation. Circ J. 2011;75:2326–2332. doi: 10.1253/circj.CJ-11-0178. [DOI] [PubMed] [Google Scholar]
- 20.Neilan TG, et al. Myocardial extracellular volume expansion and the risk of recurrent atrial fibrillation after pulmonary vein isolation. JACC Cardiovasc Imaging. 2014;7:1–11. doi: 10.1016/j.jcmg.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau DH, et al. Hypertension and atrial fibrillation: evidence of progressive atrial remodeling with electrostructural correlate in a conscious chronically instrumented ovine model. Heart Rhythm. 2010;7:1282–1290. doi: 10.1016/j.hrthm.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Letsas KP, et al. CHADS2 and CHA2DS2-VASc scores as predictors of left atrial ablation outcomes for paroxysmal atrial fibrillation. Europace. 2014;16:202–207. doi: 10.1093/europace/eut210. [DOI] [PubMed] [Google Scholar]
- 23.Wójcik M, et al. Repeated catheter ablation of atrial fibrillation: how to predict outcome? Circ J. 2013;77:2271–2279. doi: 10.1253/circj.CJ-13-0308. [DOI] [PubMed] [Google Scholar]
- 24.Canpolat U, et al. A proposal for a new scoring system in the prediction of catheter ablation outcomes: promising results from the Turkish Cryoablation Registry. Int J Cardiol. 2013;169:201–206. doi: 10.1016/j.ijcard.2013.08.097. [DOI] [PubMed] [Google Scholar]
- 25.Winkle RA, et al. Predicting atrial fibrillation ablation outcome: The CAAP-AF score. Heart Rhythm. 2016;13:2119–2125. doi: 10.1016/j.hrthm.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 26.Mesquita, J. et al. Development and validation of a risk score for predicting atrial fibrillation recurrence after a first catheter ablation procedure - ATLAS score. Europace, 10.1093/europace/eux265 (2017). [DOI] [PubMed]
- 27.D’Ascenzo F, et al. Which are the most reliable predictors of recurrence of atrial fibrillation after transcatheter ablation?: a meta-analysis. Int J Cardiol. 2013;167:1984–1989. doi: 10.1016/j.ijcard.2012.05.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of baseline characteristics between BioAF cohort and the validation cohort from The Leipzig Heart Center AF Ablation Registry
Prediction of LVA in the Leipzig Heart Center AF Ablation Registry
Prediction of arrhythmia recurrences in the BioAF cohort
Prediction of arrhythmia recurrences in the subgroup from the Leipzig Heart Center AF Ablation Registry
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).