Abstract
Drinking soursop (Annona muricata) tea has become popular in Thailand due to recent findings about the medicinal properties of soursop tea regarding anti-cancer in particular. Consequently, numerous A. muricata tea products were found to be sold on markets and relatively expensive. It is almost impossible to identify the plant species component in the tea bag or powder products using traditional methods which are based on morphological characters. Therefore, a main objective of this study is to develop a molecular method called Bar-HRM (DNA barcoding coupled with High Resolution Melting) for authenticating A. muricata products. Three chloroplast regions including matK, rbcL and trnL were selected for in silico analyses. The findings show that rbcL is the most suitable region to be used for species identification in HRM analysis. Eleven A. muricata herbal products were purchased and tested with rbcL primers. Results from melting profile indicated that three out of eleven tested products were adulterated with other Annona species. It is believed that the Annona products are adulterated to increase the quantity and to make more profit. Notably, all of the tested products purchased from local producers were found to contain herbal species that differ from the species indicated by the seller.
Introduction
Traditional medicines have played an important role in human healthcare for thousands of years and are still widely used today. Medicinal plants have been the basis for traditional remedies, a source of nutrition around the world1. Plants with medical potentials have been used as alternative medicines for various treatments of diseases in both developed and developing countries. Thus, pharmacological studies about traditional herbal medicine are now a critical part in global health. This is not to mention that around 80% of current drugs used are related to the active compounds of the medicinal plants2–5. It is therefore, unsurprising that traditional herbal medicines are getting significant attention worldwide. However, as attention and research funding for international traditional herbal medicine grows, the safety of the global herbal market should not be overlooked. The majority of alternative medicines currently available on the market are in processed form which are derived from natural products, mainly from higher plants. Depending on the particular country and existing legislation, herbal products are normally regulated. The standard for the assessment of herbal medicines was set and proposed by several organisations with accessible guidelines6,7. A large number of counterfeiting and adulteration in herbal products was reported. The perception that herbal drugs are very safe and free from side effects is not the reality. Generally, plants have hundreds of constituents and some are very toxic. Taking the wrong herbal drugs could have adverse effects.
Annona muricata is a member of the Annonaceae. It is known as soursop with Thai native vernacular name as ‘Thu-Rein-Thed’. A. muricata is a tropical and subtropical plant species known for its edible fruit which used in several different ways such as in the field of culinary (e.g. syrup, ice cream, candies) and particularly medical treatments8,9. Based on numerous pharmacological studies, the A. muricata was found to possess anti-cancer, anti-tumor, anti-bacterial, anti-inflammatory, anti-parasitic, anti-malarial and anti-ulcer activities10–12. Recently, A. muricata has become increasingly popular due to its anti-cancer properties. From 2012 to 2014, cancer overtook heart disease as the main cause of death in Thailand with huge increase each year13. Potential studies of A. muricata on cancer curing were not only limited to in vitro and in vivo evaluations. Consumption of boiled water infusion of the A. muricata leaves resulted a stabilisation of the metastatic breast cancer in a case study of a 66 year old woman for 5 year14. Herbal tea is a beverage brewed from dried leaves, seeds, flowers, stems or roots of plants species rather than Camellia sinensis, which has been gaining popularity in Thailand. A. muricata is now made into a tea due to the promising anti-cancer and anti-tumor activity. There is inevitable adulteration when the demand and prices increase and thus, reliable quality control methods for medicinal plant materials become necessary.
DNA barcoding is a method using a short fragment of DNA (~500 base pair) for species identification and taxonomic classification. DNA barcodes have been successfully used in animal species identification using cytochrome c oxidase subunit I (COI) in mitochondrial genome15–17. Regions in the chloroplast and nuclear genome were recommended in plant including coding regions (rbcL and matK) and non-coding regions (trnL and trnH-psbA in chloroplast genome and internal transcribed spacers in nuclear genome)18,19. The DNA barcoding approach has been applied to use for detection of adulteration in food and herbal products with success20–22. However, some limitations of the method mean it is not fully practical in some developing countries like Thailand. Main drawbacks are likely to be that it is time-consuming and its high costs due to outsourced sequencing. Recently, DNA barcoding has been applied to be used in combination with High Resolution Melting analysis, called Bar-HRM. The Bar-HRM was proved to be a good compromise for counterfeit herbal products and adulteration detection. The Bar-HRM is a sequencing free method using fluorescent dye for detection of double-stranded DNA in real-time PCR. Increasing temperature during the process lead to denaturation of double-stranded DNA into single-stranded DNA and melting temperature (Tm) is measured. The Bar-HRM analysis is not only rapid, cheap (in long term and large scale investigation), and feasible for accurately species discrimination in plants23–27.
In consequence, the Bar-HRM is one of many promising techniques for counterfeit and adulterant detection in soursop commercial products sold on markets in developing country like Thailand. The majority of soursop products are very expensive and available in tea bags. Here, the replacement of A. muricata with lower grade or cheaper substitutes such as custard apple leaves (Annona squamosa) and jackfruits leaves (Artocarpus heterophyllus) in commercial products was investigated. In addition, this work evaluated three chloroplast regions including matK, rbcL and trnL to find out which DNA barcode region is the most suitable for identifying the three plants species (A. muricata, A. squamosal and A. heterophyllus).
Results and Discussion
Data mining and in silico analyses
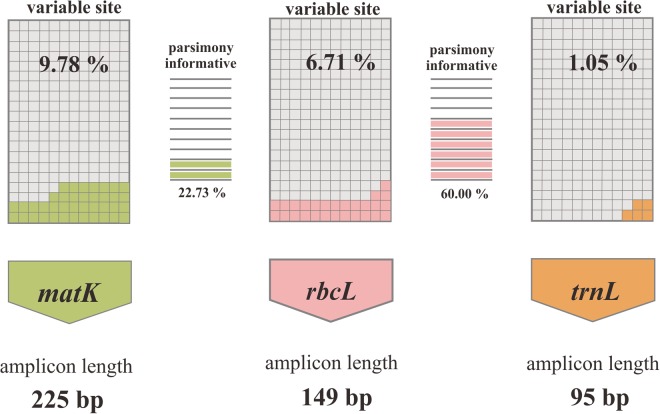
The number of DNA sequence records of Annona species retrieved from GenBank of each barcode region are 16, 25 and 12 species for matK, rbcL and trnL, respectively. All retrieved sequences were then analysed using MEGA 6 program for conserved site (%), variable site (%), parsimony-informative site (%), singleton site (%), conserved forward primer/total (%) and conserved reverse primer/total (%). The longest and shortest sequence of each region was found to be 566–1,763 bp of matK, 550–1,469 of rbcL and 864–947 for trnL and after filtering and trimming the analysed sequences length were reduced to 507, 466 and 873 bp of matK, rbcL and trnL, respectively. As can be seen from Table 1, the analysed matK fragment was observed to have higher nucleotide variation (9.78%) than both rbcL (6.71%) and trnL (1.05%). It is indicated that trnL is least suitable to be used for our tested species identification whereas matK is likely to be the best region for this study. However, further investigation revealed that matK was not as good as rbcL region for HRM analysis. Although the analysis of matK sequences showed higher number of nucleotide variation than that found in rbcL, most of variable sites of the rbcL are parsimony-informative type (Fig. 1).
Table 1.
Characteristics records from in silico analysis of each region.
| Markers | matK | rbcL | trnL |
|---|---|---|---|
| Available species | 16 | 25 | 12 |
| Shortest and longest sequence (bp) | 566–1,763 | 550–1,469 | 864–947 |
| Characters used in analysis (bp) | 507 | 466 | 873 |
| Product size (bp) | 225 | 149 | 95 |
| Variable site/total (%) | 22/225 (9.78) | 10/149 (6.71) | 1/95 (1.05) |
| Parsimony-informative site/total (%) | 5/22 (22.73) | 6/10 (60.00) | 1/100 (100) |
| Singleton site/total (%) | 17/22 (77.27) | 4/10 (40.00) | 0/1 (0.00) |
Figure 1.
Variable sites found in each analysed region (matK, rbcL and trnL) and parsimony-informative type of matK and rbcL amplicons.
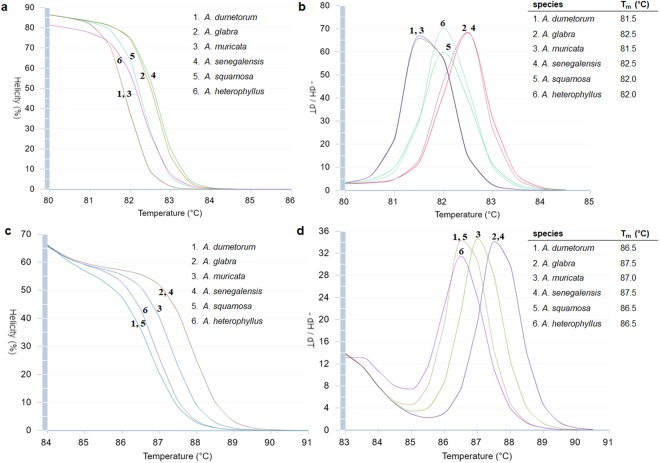
Thus, interspecific variation of matK are lower than rbcL for discrimination of Annona species. In addition, our target species (A. muricata) could be discriminated from other tested species in simulated HRM (uMeltSM) only by rbcL marker not matK (Fig. 2a–d). The different barcode regions tend to work well in different plant groups so the choice of barcode used in each experiment depends on the plant group studied28. Previously, the rbcL had been used for Annona species identification by the number of studies, although some suggested the use of both matK and rbcL29,30. Primer pairs should yield an amplicon of 100 to 300 bp for effective HRM analysis31. In this study, amplicon products derived from rbcL primers were found to be 149 bp in length which is suitable for HRM analysis.
Figure 2.
Normalised curves and Tm generated from simulated HRM analyses (uMeltSM). (a) and (b) matK primers, (c) and (d) rbcL primers with sequences from six species included A. dumetorum, A. glabra, A. muricata, A. senegalensis, A. squamosa and A. heterophyllus.
Real-time PCR for high resolution melting (HRM) analysis
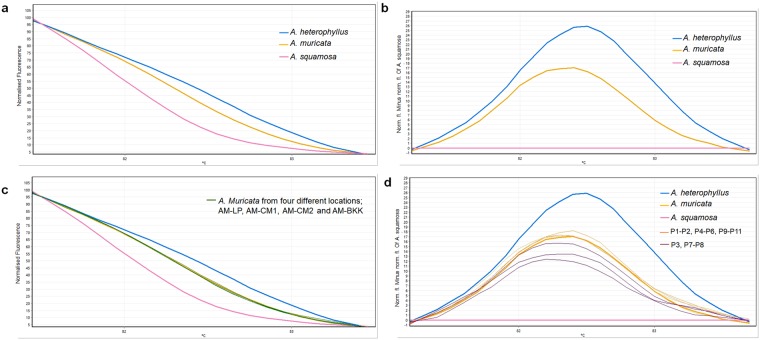
The real-time PCR and HRM analysis result of the rbcL is shown as melting curves. This indicates the rbcL has an adequate ability to be used for discriminating all the three reference plant species (A. muricata, A. squamosa and A. heterophyllus) (Fig. 3a,b). The melting curves of the three tested species were clearly separated whereas the curves of samples from same species even collecting from different location are identical (Fig. 3c). Melting temperatures (Tm) of A. muricata, A. squamosa and A. heterophyllus were 82.36 ± 0.06, 81.86 ± 0.03 and 82.58 ± 0.04, respectively. Genotype confidence percentage (GCP) values were calculated, and a cut-off value of 90% was used to assign a genotype. A cut off value for all three genotype based on SD subtracted from the mean GCP were as followed; 95.15 for A. muricata, 94.31 for A. squamosal and 98.01 for A. heterophyllus. Therefore, the melting curves of the three tested species were then used as references in adulteration test.
Figure 3.
Melting curve profiles of amplicons obtained from rbcL primers (a) Normalised melting curves, (b) Difference melting curves from the three reference species plants, (c) Melting curves of A. muricata collected from four locations (AM-LP, AM-CM1, AM-CM2 and AM-BKK) compared with the reference curves, (d) Difference melting curves of all eleven tested products compared with the three reference species plants.
An investigation of substitution or adulteration in eleven soursop tea commercials products was then carried out. Real-time PCR and HRM analysis using the rbcL primers revealed that substitution or adulteration occurred in three out of the eleven tested products (Fig. 3d). The melting curves of the three (P3, P7 and P8) were shifted from the curve of pure A. muricata DNA. The curves were close to the curves of A. squamosa DNA, so it is expected that the substitution or adulteration material would be the A. squamosa.
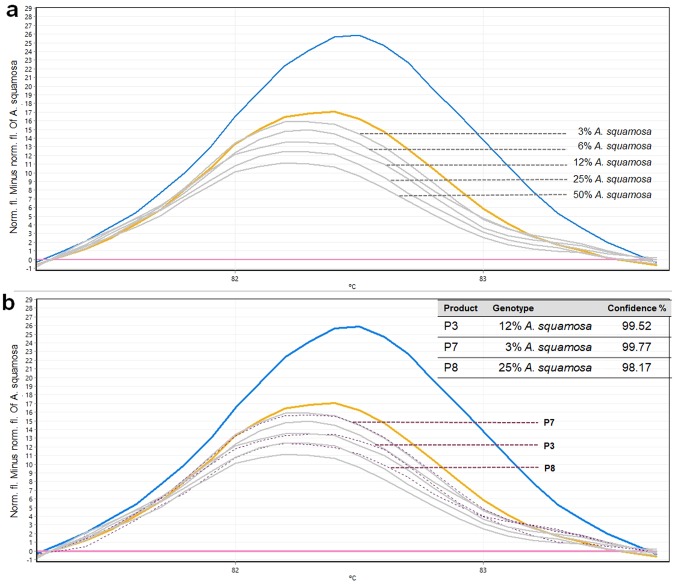
Detecting adulteration in A. muricata products was then tested using the developed method. The results of the validation method with A. muricata spiked with A. squamosa in different proportions (6%, 12%, 25% and 50%) were shown in Fig. 4a. These results show the analysis for one experiment as all three experiments gave similar results, thus showing very good reproducibility. As expected, the level of contamination resulting from adulteration alters the shape and shifts proportionally the melting curve, compared to the curve of pure A. muricata DNA. This happens as the presence of increasing quantity of A. squamosa into the A. muricata DNA. Real-time PCR and HRM analysis using the rbcL primers revealed the levels of substitution or adulteration in the three tested products (P3, P7 and P8) (Fig. 4b). It is indicated that there is at least 12% of A. squamosa in P3, 3% of A. squamosa in P7 and 25% of A. squamosa in P8 with >95% confidence (Fig. 4b). It is therefore undeniable that the A. muricata products sold on market are substituted with the inferior or superficially similar species which may or may not have any thereupatic potential. In this case, the products might be adulterated to increase the quantity and to make more profit. All three adulterated products (P3, P7 and P8) were purchased from local producers and thus there is no proper packaging or labeling of the products. In contrast, other tested products (P1-P2, P4-P6 and P9-P11) were purchased from drug stores or supermarkets showing no substitution or adulteration. The results here are similar to those from previous studies of different herbal species32–35.
Figure 4.
Melting curves obtained by high resolution melting analysis of the mixtures of A. muricata and A. squamosa, (a) Specific amplicons and applied to reference mixtures containing 6, 12, 25 and 50% of A. squamosa in A. muricata, (b) Difference curves of the three tested products comparing with the melting profiles of the mixtures.
Conclusion
Based on the forecast in silico analysis in finding suitable DNA barcode region for the HRM analysis, there is almost guaranteed success of using Bar-HRM approach for detection of adulteration or substitution in herbal product. Here, the rbcL region is the most suitable for discriminating between Annona plant species and the Bar-HRM method using rbcL primers can be used to investigate the A. muricata products sold in processed form such as in tea bags. The relatively high cost is a result of the high market demand of A. muricata products and it could be one of the reasons of adulteration found in this study.
Methods
Collection of plants samples and tested commercial products
Leaves of the reference plant species including A. muricata (soursop), A. squamosa (custard apple), and A. heterophyllus (jackfruit) were collected in areas of Chiang Mai, Bangkok and Lampoon provinces, Thailand by a taxonomic expert from Chiang Mai University. Eight commercial products, clearly labelled as A. muricata (P1-P2, P4-6 and P9-P11) were bought from drug stores or supermarkets and three products (P3 and P7-P8) were bought from local producers and were then used in the adulteration/substitution test.
Data mining, sequence analyses and simulated high resolution melting (HRM) analysis
The DNA barcode sequences of plant species in genus Annona were extracted from GenBank on NCBI (National Center for Biotechnology Information) website using the keyword “Annona and each selected barcode region” (matK, rbcL and trnL). Sequence alignment and analysis were done using the MEGA 6 program36. The following characteristics were recorded: conserved site (%), variable site (%), average GC content, conserved forward primer/total (%) and conserved reverse primer/total (%). The suitable regions were then used in simulated analysis by uMeltSM application to predict fluorescent high-resolution DNA melting curves of PCR products37.
DNA extraction
The plant material (of both fresh leaves and tea products) was ground with liquid nitrogen, and 100 mg of fine powder was then used for DNA extraction with the Nucleospin Plant® II kit (Macherey-Nagel, Germany) following the manufacturer’s instructions. DNA concentrations of all samples were adjusted equally (20 ng/µL). The DNA was stored at −20 °C for further use.
Real-time PCR amplification and high resolution melting (HRM) analysis
In order to distinguish the tested plant species, it was necessary to determine the characteristic melting temperature (Tm) for each sample. Then, PCR amplification, DNA melting, and endpoint fluorescence level acquiring PCR amplifications were performed in a total volume of 20 µL on a Rotor-Gene Q. The reaction mixture contained 20 ng genomic DNA, 10 µL of MeltDoctor™ HRM Master Mix (Applied Biosystems, California, USA), 0.2 µL of 10 mM rbcL_F 5′-GGTACATGGACAACTGTGTGGA-3′ and rbcL_R 5′-ACAGAACCTTCTTCAAAAAGGTCTA-3′ primers28. The real-time PCR reaction conditions are as follows; an initial denaturing step at 95 °C for 5 min followed by 35 cycles of 95 °C for 30 s, 57 °C for 30 s, and 72 °C for 20 s. Subsequently, the PCR amplicons were denatured for HRM at 95 °C for 15 s, and then annealed at 50 °C for 15 s to form random DNA duplexes. Melting curves were generated after the last extension step. The temperature increased from 55 to 95 °C, at 0.1 °C/s. Each species was set as a ‘genotype’ (reference species including A. muricata, A. squamosa, and A. heterophyllus) and the average HRM genotype confidence percentages (GCPs) for the replicates were recorded. The means of the confidence percentage of the species replicates assigned to a representative genotype, together with the standard deviation, were generated.
Adulteration/Substitution in soursop commercial product test
Eight soursop (A. Muricata) tea products (P1-P2, P4-6 and P9-P11) purchased from drug stores or supermarkets and three (A. Muricata) tea products (P3 and P7-P8) purchased from local producers were used in the detection of authentication test. DNA isolation and HRM analysis were performed according to the description in the above sections (DNA extraction and Real-time PCR amplification and high resolution melting (HRM) analysis).
Electronic supplementary material
Acknowledgements
This research was financially supported by National Research Council of Thailand and The Thailand Research Fund – DBG6080012. I thank the botanical garden organization, ministry of natural resources and environment, Thailand for providing and identifying the samples. I am thankful to my colleagues and my students, for every little help from them and also Dr Lauren R. Clark for English editing.
Author Contributions
M.O. conceived the project, designed the experiments, collected samples, performed the data analysis and wrote the manuscript.
Competing Interests
The author declares no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-31127-9.
References
- 1.WHO. WHO Traditional Medicine Strategy 2002–2005. Geneva (2002).
- 2.Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environmental Health Perspectives. 2001;109(Suppl 1):69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gajalakshmi S, Divya R, Divya VD, Mythili S, Sathiavelu A. Pharmacological activities of annona squamosa: a review. International Journal of Pharmaceutical Sciences Review and Research. 2011;10(3):24–29. [Google Scholar]
- 4.Hosseinzadeh H, Nassiri-Asl M. Pharmacological Effects of Glycyrrhiza spp. and Its Bioactive Constituents: Update and Review. Phytotherapy Research. 2015;29(12):1868–1886. doi: 10.1002/ptr.5487. [DOI] [PubMed] [Google Scholar]
- 5.Struthers R, Eschiti VS, Patchell B. Traditional indigenous healing: Part I. Complement Ther Nurs Midwifery. 2004;10(3):141–149. doi: 10.1016/j.ctnm.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Bulletin of the World Health Organization. Research guidelines for evaluating the safety and efficacy of herbal medicine: Geneva. (1993).
- 7.WHO. WHO guidelines for assessing quality of herbal medicines with reference to contaminants and residues. Spain (2007).
- 8.Coria-Téllez, A. V., Montalvo-Gónzalez, E., Yahia, E. M. & Obledo-Vázquez, E. N. Annona muricata: A comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity. Arabian Journal of Chemistry, 10.1016/j.arabjc.2016.01.004 (2016).
- 9.Moghadamtousi SZ, et al. Annona muricata (Annonaceae): A Review of Its Traditional Uses, Isolated Acetogenins and Biological Activities. Int J Mol Sci. 2015;16(7):15625–15658. doi: 10.3390/ijms160715625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamizah S, et al. Chemopreventive potential of Annona muricata L leaves on chemically-induced skin papillomagenesis in mice. Asian Pac J Cancer Prev. 2012;13(6):2533–2539. doi: 10.7314/APJCP.2012.13.6.2533. [DOI] [PubMed] [Google Scholar]
- 11.Kedari TS, Khan AA. Guyabano (Annona Muricata): A review of its traditional uses phytochemistry and pharmacology. American Journal of Research Communication. 2014;2(10):247–268. [Google Scholar]
- 12.Suneel KA, Venkatarathanamma V, Naga SV. Phytochemical and phytotherapeutic properties of Annona squamosa, Annona reticulata and Annona muricata: A review. Asian Journal of Plant Science and Research. 2015;5(8):28–33. [Google Scholar]
- 13.Bureau of Non Communicable Disease. Annual Report 2015: The War Veterans Organization of Thailand Under Royal Patronage of His Majesty the King (2015).
- 14.Hansra DM, Silva O, Mehta A, Ahn E. Patient with metastatic breast cancer achieves stable disease for 5 years on graviola and xeloda after progressing on multiple lines of therapy. Adv. Breast Cancer Res. 2014;3:84–87. doi: 10.4236/abcr.2014.33012. [DOI] [Google Scholar]
- 15.Fazekas AJ, et al. Multiple Multilocus DNA Barcodes from the Plastid Genome Discriminate Plant Species Equally Well. PLoS ONE. 2008;3(7):e2802. doi: 10.1371/journal.pone.0002802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollingsworth PM, Graham SW, Little DPC. and Using a Plant DNA Barcode. PLoS ONE. 2011;6(5):e19254. doi: 10.1371/journal.pone.0019254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newmaster SG, Fazekas AJ, Ragupathy S. DNA barcoding in land plants: evaluation of rbcL in a multigene tiered approach. Canadian Journal of Botany. 2006;84(3):335–341. doi: 10.1139/b06-047. [DOI] [Google Scholar]
- 18.de Vere, N., Rich, T. C. G., Trinder, S. A. & Long, C. DNA Barcoding for Plants. In J. Batley (Ed.), Plant Genotyping: Methods and Protocols (pp. 101–118). New York, NY: Springer New York (2015). [DOI] [PubMed]
- 19.Taberlet P, et al. Power and limitations of the chloroplast trnL (UAA) intron for plant DNA barcoding. Nucleic Acids Research. 2007;35(3):e14–e14. doi: 10.1093/nar/gkl938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Little DP. Authentication of Ginkgo biloba herbal dietary supplements using DNA barcoding. Genome. 2014;57(9):513–516. doi: 10.1139/gen-2014-0130. [DOI] [PubMed] [Google Scholar]
- 21.Little DP, Jeanson ML. DNA Barcode Authentication of Saw Palmetto Herbal Dietary Supplements. Scientific Reports. 2013;3:3518. doi: 10.1038/srep03518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newmaster SG, Grguric M, Shanmughanandhan D, Ramalingam S, Ragupathy S. DNA barcoding detects contamination and substitution in North American herbal products. BMC Medicine. 2013;11(1):222. doi: 10.1186/1741-7015-11-222. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Ganopoulos I, Madesis P, Darzentas N, Argiriou A, Tsaftaris A. Barcode High Resolution Melting (Bar-HRM) analysis for detection and quantification of PDO “Fava Santorinis” (Lathyrus clymenum) adulterants. Food Chemistry. 2012;133(2):505–512. doi: 10.1016/j.foodchem.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Madesis P, Ganopoulos I, Bosmali I, Tsaftaris A. Barcode High Resolution Melting analysis for forensic uses in nuts: A case study on allergenic hazelnuts (Corylus avellana) Food Research International. 2013;50(1):351–360. doi: 10.1016/j.foodres.2012.10.038. [DOI] [Google Scholar]
- 25.Osathanunkul M, et al. Hybrid analysis (barcode-high resolution melting) for authentication of Thai herbal products, Andrographis paniculata (Burm.f.) Wall.ex Nees. Pharmacognosy Magazine. 2016;12(45):71–75. doi: 10.4103/0973-1296.176112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osathanunkul M, et al. Refining DNA Barcoding Coupled High Resolution Melting for Discrimination of 12 Closely Related Croton Species. PLoS ONE. 2015;10(9):e0138888. doi: 10.1371/journal.pone.0138888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song M, Li J, Xiong C, Liu H, Liang J. Applying high-resolution melting (HRM) technology to identify five commonly used Artemisia species. Scientific Reports. 2016;6:34133. doi: 10.1038/srep34133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osathanunkul M, Suwannapoom C, Osathanunkul K, Madesis P, de Boer H. Evaluation of DNA barcoding coupled high resolution melting for discrimination of closely related species in phytopharmaceuticals. Phytomedicine. 2016;23(2):156–165. doi: 10.1016/j.phymed.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 29.Larranaga, N. & Hormaza, J. DNA barcoding of perennial fruit tree species of agronomic interest in the genus Annona (Annonaceae). [Original Research]. Frontiers in Plant Science, 6 (589) (2015). [DOI] [PMC free article] [PubMed]
- 30.Rahman MSM, Yamada M, Yoshida M. Relationship of Annona species as revealed by PCR-RFLP analysis. Breeding Science. 1997;47:335–339. [Google Scholar]
- 31.Reed GH, Wittwer CT. Sensitivity and Specificity of Single-Nucleotide Polymorphism Scanning by High-Resolution Melting Analysis. Clinical Chemistry. 2004;50(10):1748. doi: 10.1373/clinchem.2003.029751. [DOI] [PubMed] [Google Scholar]
- 32.Osathanunkul M, Madesis P, de Boer H. Bar-HRM for Authentication of Plant-Based Medicines: Evaluation of Three Medicinal Products Derived from Acanthaceae Species. PLoS ONE. 2015;10(5):e0128476. doi: 10.1371/journal.pone.0128476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singtonat S, Osathanunkul M. Fast and reliable detection of toxic Crotalaria spectabilis Roth. Thunbergia laurifolia Lindl. herbal products using DNA barcoding coupled with HRM analysis. BMC Complementary and Alternative Medicine. 2015;15(1):1–8. doi: 10.1186/s12906-015-0692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osathanunkul, M., Ounjai, S., Osathanunkul, R. & Madesis, P. Evaluation of a DNA-based method for spice/herb authentication, so you do not have to worry about what is in your curry, buon appetito! PLoS ONE12(10), e0186283 (2017). [DOI] [PMC free article] [PubMed]
- 35.Osathanunkul, M., Osathanunkul, R. & Madesis, P. Species identification approach for both raw materials and end products of herbal supplements from Tinospora species. BMC Complementary and Alternative Medicine18 (111), 1–6 (2018). [DOI] [PMC free article] [PubMed]
- 36.Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol, 10.1093/molbev/mst197 (2013). [DOI] [PMC free article] [PubMed]
- 37.Dwight Z, Palais R, Wittwer CT. 2011. uMELT: prediction of high-resolution melting curves and dynamic melting profiles of PCR products in a rich web application. Bioinformatics. 2015;27(7):1019–1020. doi: 10.1093/bioinformatics/btr065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.