Abstract
Individuals vary in their cognitive performance. While this variation forms the foundation of the study of human psychometrics, its broader importance is only recently being recognized. Explicitly acknowledging this individual variation found in both humans and non-human animals provides a novel opportunity to understand the mechanisms, development and evolution of cognition. The papers in this special issue highlight the growing emphasis on individual cognitive differences from fields as diverse as neurobiology, experimental psychology and evolutionary biology. Here, we synthesize this body of work. We consider the distinct challenges in quantifying individual differences in cognition and provide concrete methodological recommendations. In particular, future studies would benefit from using multiple task variants to ensure they target specific, clearly defined cognitive traits and from conducting repeated testing to assess individual consistency. We then consider how neural, genetic, developmental and behavioural factors may generate individual differences in cognition. Finally, we discuss the potential fitness consequences of individual cognitive variation and place these into an evolutionary framework with testable hypotheses. We intend for this special issue to stimulate researchers to position individual variation at the centre of the cognitive sciences.
This article is part of the theme issue ‘Causes and consequences of individual differences in cognitive abilities’.
Keywords: cognition, evolution, heritability, individual differences, personality, plasticity
1. Introduction
All animals learn, remember and integrate information in order to reach decisions and behave appropriately, but how, why and when these cognitive abilities evolve remains uncertain. One reason for this uncertainty is that research in animal cognition has frequently ignored individual differences and this precludes our understanding of how natural selection sifts individual differences leading to evolutionary changes. Instead, the study of cognition in animals has traditionally taken one of three (non-exclusive) forms. First, particular model species (e.g. pigeons or rats) have been used to investigate the mechanisms underpinning specific cognitive processes. This has typically used laboratory paradigms involving prolonged training of batches of individuals to complete tasks aimed to elucidate fundamental learning principles. Second, the comparative approach tests species or populations with the same, or purportedly similar, tasks to understand when in evolutionary history particular cognitive processes may have emerged and what ecological or social conditions may facilitate these processes. The abilities of a sample of individuals within a population or species are pooled and considered representative of the whole grouping. Finally, within a species, the abilities of particular, often highly enculturated, ‘genius’ individuals are explored in great detail in order to establish the presence of, or limits to, particular cognitive capacities. From these instances of presence or absence, broader patterns of evolution may be suggested and inferences drawn about the adaptive benefits of possessing such cognitive abilities for the species, based on its ecology and social behaviour. While progress has been made in understanding both fine-scale cognitive mechanisms and broad-scale evolutionary patterns using these methods, it is hard to understand the evolution of cognitive abilities through natural selection when we ignore inter- and intra-individual variation in cognitive abilities.
While individual differences have been central to human psychology since the early 20th century [1], research on non-human animals has, until recently, tended to ignore the variation amongst individuals. Over the last decade, there has been a growing focus on intraspecific variation in non-human animals [2]. This is perhaps influenced by (i) the behavioural ecology approach originating in the 1980s, which explicitly considered natural selection on individual phenotypes [3]; (ii) the more recent studies of animal personality, which emphasize individual differences across correlated suites of behaviours [4]; (iii) the development of statistical (mixed) modelling techniques that permit explicit consideration of individual differences; and (iv) the development of technology permitting fine-scale tracking of individuals’ movements and interactions [5]. This special issue draws together recent theoretical and empirical developments in the emerging field of individual variation in cognition.
As cognitive abilities cannot be directly observed, they must be inferred through careful experimentation. Measuring individual cognitive variation poses particular logistical and analytical challenges because it requires repeated testing of known individuals under standardized conditions, in a way that allows for noise caused by differences in, for example, motivation, attention and prior experience to be identified, quantified and/or removed (experimentally or statistically). Papers in the first section of this special issue explore these methodological aspects further [6,7]. Given these difficulties likely to be encountered when measuring individual differences in cognitive abilities, why should we go to the trouble of doing so?
By measuring the cognitive abilities of individuals, we can address otherwise intractable questions regarding the mechanisms, development and evolution of cognition. For instance, how is the ontogeny of cognitive phenotypes shaped by the physical or social environment? What is the relationship between personality and cognitive performance? Does an individual’s ability to solve cognitive problems influence its ability to survive and reproduce? This special issue examines both the causes and consequences of individual variation in cognitive abilities.
The second section of the special issue examines a range of causes of individual differences. First, by understanding the genetic heritage of an individual [8,9] and fine-scale aspects of their brain morphology and neurology in conjunction with an understanding of their unique developmental history [10–13], we can begin to understand how individual differences within a population originated. An individual’s experiences throughout its life may constrain its ability to invest in the growth and maintenance of neuronal tissues [14,15], influence its life-history trajectory and provide differential opportunities to experience cues, acquire information and sample rewards, which may shape a range of cognitive processes [14,16]. Therefore, the current physical and/or social environment may have both immediate and long-term effects on an individual's cognitive abilities [10–13]. It is not clear whether such effects are fixed or plastic and susceptible to later changes in the environment. We encourage researchers to track changes in an individual's cognitive abilities over time under a variety of relevant environmental conditions (e.g. [17]).
The third set of papers in the special issue explores the evolutionary consequences of individual cognitive variation, by considering whether individuals' cognitive abilities influence their fitness [18–20] and thus whether natural selection may act on these abilities and contribute to their evolution (if they are heritable) [21,22]. Currently, most studies have tested relationships between individual cognitive performance and single assays of fitness (but see [23–25] for examples where multiple assays of cognitive performance have been related to fitness), and in most studies the relationship has been positive (summarized in [20]). Although here we conveniently separate cause from consequence, we recognize that there may be feedback loops between the causes of individual cognitive variation and their fitness consequences in a dynamic, evolutionary landscape. For example, an individual’s social environment may shape its cognitive performance, which in turn influences its reproductive success [13,18], which could then shape the social environment of its offspring.
In the sections below we describe the three main focuses of our special issue and their accompanying papers in more detail, and provide suggestions for fruitful progress in the field of individual differences in cognition.
2. Quantifying individual differences in cognitive performance
Quantifying individual differences in cognitive ability is difficult because it requires inferring psychological processes and abilities from observing behaviours, usually involving unnatural, experimental test apparatus or conditions. Attempts to measure individual differences in cognitive abilities require careful experimental and analytical design [26–28].
(a). Reducing noise
One common approach to quantifying individual differences has been to administer ‘problem-solving tasks' that measure whether an animal does or does not perform a novel action, often to gain access to food. However, performance on problem-solving tasks can be difficult to interpret because the putative cognitive processes involved have not been clearly identified (for extensive discussion of these issues, see [28]). In contrast, in psychometric tests, individuals must make several decisions, where correct and incorrect choices are defined a priori [29], such that learning or memory can be demonstrated by deviations from random probabilities [29,30]. Typically, each task explicitly targets one cognitive process that has been well-described in the psychological literature. Nevertheless, individual variation in performance may also be confounded by non-cognitive factors (e.g. hunger, motivation, breeding status, environmental conditions). Many recent papers have pointed out the importance of distinguishing signal from noise by controlling for such factors by standardizing conditions or explicitly accounting for their effects in statistical analyses [21,26,31]. In comparative studies, noise can be reduced by averaging across multiple individuals, assuming that each differs randomly in, for example, their attention, motivation or prior experience. Critically, averaging is not possible when considering the performance of individuals and consequently it is important that non-cognitive sources of individual variation are controlled or accounted for. For instance, in captive studies one can ensure individuals have been food-deprived for an equivalent amount of time (e.g. [32–34]; although this does not control for inter-individual differences in basal metabolic rate [34]). In the wild it is sometimes possible to account for differences in food intake or body mass [18]. We can also attempt to control for the effects of prior experience by presenting stimuli or contingencies that are likely to be novel to all subjects [26]. One way to increase subjects' attention to the cognitive task at hand and reliance on cognitive processing might be to increase the costs of making mistakes. For example, in a spatial memory task, increasing the costs of exploration (e.g. by weighing down lids covering food wells [35]) may increase the benefits of relying on memory rather than random search. The level of control over testing conditions required means that working with wild populations can be especially problematic. Therefore, it is encouraging that in at least one species, the performance of captive individuals on a reversal task closely matches that of their wild conspecifics [36], suggesting that both test contexts may offer viable alternatives. An important next step is to determine whether, at the individual level, performance is consistent when tested in captivity versus in the wild (c.f. [37]).
(b). Repeatability
One means of addressing (although not eliminating) confounds is to assess individual consistency through repeated testing [31]. If we measure an individual once, and it makes more errors in remembering a rewarded location than a conspecific, this could be because it has ‘worse’ spatial memory or because it happened to be distracted. However, if we measure individuals repeatedly and we find that some individuals perform consistently better than others, this may be indicative of a stable phenotypic trait that selection can act upon. It is, therefore, vital that individuals are tested repeatedly to determine genuine among-individual phenotypic variation. However, certain confounds may also be consistent over time and so these would also consistently confound performance. This is an issue that has received a great deal of attention in the literature on animal personality [38], yet is only just beginning to be recognized in studies of animal cognition.
Measures of repeatability provide a way of assessing individual differences by quantifying among- and within-individual variation [39]. Cauchoix et al. [6] provide the first meta-analysis of recently published studies and a large number of unpublished datasets reporting individual repeatability on a variety of cognitive tasks. They find low to moderate levels of repeatability when individuals are measured several times on the same task (temporal repeatability; e.g. [40]). These modest estimates (R = 0.15–0.28) may be due in part to carry-over effects of learning and memory. For example, if all individuals learn over repeated attempts at the same task, then this can reduce among-individual variance and thus repeatability. Another approach is to present individuals with tasks that differ in their physical characteristics but are designed to measure the same cognitive trait by having the same causal contingencies (contextual repeatability; e.g. [7,14]). Indeed, Cauchoix et al. [6] find slightly, though not significantly, higher estimates of contextual repeatability (R = 0.20–0.27). Most studies have conducted only single repeats of tests; it remains to be determined how adding further repeat variants of a test increases the reliability of the measure of cognitive ability.
(c). Assessing test validity
Developing different tasks that measure the same cognitive ability is more difficult than it appears. For instance, in this issue Völter et al. [7] explicitly examine individual performance in two tasks that are widely assumed to measure inhibitory control (or self-control, i.e. the ability to inhibit pre-potent behavioural responses): the detour-reaching task and the A-not-B task. A recent high-profile study [41] found that across species, average performance on the two tasks was positively correlated and showed a strong, positive correlation with average brain size. This was interpreted as suggesting that increases in brain size across evolutionary time underlie the evolution of increased self-control [41]. To determine whether the tasks genuinely measured the same cognitive trait, Völter et al. [7] re-analysed these and other datasets at the individual level, consistently finding no correlation between individual performance on tasks measuring inhibitory control. This suggests that (i) correlations across species do not necessarily imply that the same pattern holds within species and (ii) that, contrary to common assumption, these two tasks do not necessarily measure the same ability (see also [42]). Völter et al. [7] advocate triangulating across batteries of tasks by measuring individuals' patterns of mistakes as a marker of the limits of their abilities in a particular cognitive domain (see also [43]). It remains unclear whether the lack of correlation between performances in tasks deemed a priori to test the same cognitive process is due to differential demands on cognitive processes or differential effects on attention, or non-cognitive factors such as motivation.
(d). Domain-generality versus modularity
A related issue is whether individuals are also consistent in their performance across different cognitive domains. As Dubois et al. [44] highlight in this issue, human psychometric studies consistently show strong positive correlations in individuals' performance on a disparate range of tasks (e.g. verbal comprehension, reasoning, working memory). These results suggest that common information processing mechanisms may underlie performance across different cognitive domains, often referred to as general intelligence or ‘g’ [29]. In contrast, the animal cognition literature has tended to favour a modular approach, emphasizing specific cognitive adaptations to specific ecological problems (e.g. spatial memory as an adaptation to the challenges of food cache recovery [45,46]). However, in recent years, researchers have also begun to turn their attention to the potential for cross-domain individual consistency in non-human animals [30]. Although this consistency in non-human animals is also sometimes referred to as ‘g’, it is important to note that animal psychometric tests often incorporate very different types of tasks, methodologies and statistical approaches to human psychometrics. Indeed, it has been suggested that in humans, test batteries primarily test reasoning or rule extraction while those used with animals primarily focus on associative learning and memory [47]. Thus, while there are superficial similarities between human general intelligence and animal ‘general cognitive performance’ [48,49], these may not necessarily reflect the same underlying cognitive architecture. Interpretation is made harder because the likelihood of extracting a single component indicative of a general ability is highly susceptible to the exact set of tasks that are included in the test battery [50].
3. Considering causes of individual differences in cognitive abilities
Although unwanted noise may explain some of the variation observed between individuals in their cognitive performance, a growing number of studies have now provided evidence for consistent inter-individual variation in cognitive performance [6]. However, the exact mechanisms underlying individual differences in cognition are not yet well understood in any species.
(a). Neural correlates
As cognition is a manifestation of neuronal processing, one may expect individual differences in cognitive performance to reflect individual differences in the brain. Total brain volume is a widely reported correlate of intelligence in humans, but as Dubois et al. [44] point out, this tells us little about the neuronal mechanisms involved. Instead, they investigated whether variation in intelligence in humans may be due to differences in brain activity. They found that 20 per cent of the variance in general intelligence could be explained by differences in resting-state activity patterns distributed across brain networks. Critically, these individual differences were not due to activity in any particular anatomical structure or network, but resulted from connectivity across the brain as a whole. The extent to which individual cognitive differences in other species are reflected in similar brain connectivity patterns is yet to be determined (but see [51]). Moreover, it is important to note that correlations between individual differences in brain activity and cognitive performance do not in themselves reveal the mechanisms underlying this variation. Ideally, one could test causal hypotheses with experimental manipulations of brain structure/functioning, but of course this would not be ethically feasible in humans.
(b). Heritability
One important source of consistent individual differences in cognition is genetic inheritance. Studies on humans have generated extensive evidence for heritability of general intelligence [52,53], and there is a small but growing number of studies exploring the heritability of neural and cognitive phenotypes in other animals [54,55]. Moreover, several experimental studies have shown that artificial selection for cognitive traits or brain size can drive directional changes across generations [56–59]. However, understanding the heritability of cognitive performance (and its evolutionary consequences; see below) is far from straightforward, as discussed by two papers in this special issue [8,9]. As heritability is assessed by partitioning variance between environmental and genetic components, heritability estimates are highly dependent on the local environment. For example, Sauce et al. [8] show strong heritability of cognitive performance in mice raised in standard laboratory conditions, while in groups of mice exposed to environmental enrichment, the increased environmental variance component leads to heritability estimates of effectively zero. Heritability estimates may also be highly specific to particular cognitive traits. For example, Sorato et al. [9] show that in red junglefowl, Gallus gallus, reversal learning performance is moderately heritable, while discrimination learning and cognitive judgement biases (i.e. optimism/pessimism) are not. Exploring the genetic covariance between multiple behavioural and cognitive traits is an important avenue for future research [60] to understand the evolutionary implications of individual differences in cognition.
(c). Influences of the physical environment
Laboratory research on non-human animals may provide an important avenue to understand cognitive differences, because we can experimentally control the physical environment in which individuals develop and are tested. This is critical because the physical environment can alter neural development and cognitive abilities. In this issue, Pike et al. [12] tested the effect of environmentally induced brain plasticity on cognitive performance in juvenile wild-caught sticklebacks, Gasterosteus aculeatus. Fish reared in a visually restricted environment grew relatively larger olfactory bulbs but smaller optic tecta than fish reared in clear water. When fish were provided with conflicting chemical and visual information regarding the location of neighbouring shoals of different sizes, fish with larger olfactory bulbs relied more on the chemical information, while fish with relatively larger optic tecta relied more on visual information. This illustrates how the physical environment in which an individual develops can mould brain structure and, consequently, their acquisition and use of information.
More subtle aspects of the physical environment may also prove influential. Food availability and stressors may shape the microbial communities in an individual's gut. As Davidson et al. [61] point out in this issue, the gut microbiome could in turn have profound but as yet poorly understood influences on the development and maintenance of brain function, and hence on cognitive performance.
Effects of the physical environment can generate inter-individual differences in cognitive performance even over relatively short time scales. For instance, as Sauce et al. [8] show in this issue, only 16 days of environmental enrichment were sufficient to generate a substantial improvement in cognitive performance in laboratory mice when compared to control mice reared in standard laboratory conditions. These findings illustrate the importance of taking individuals' developmental histories (including previous experiences with other experimental tests [2], and natal habitat in the wild [55]) into consideration when interpreting data on individual cognitive differences.
(d). Influences of the social environment
For many animals, the development of cognitive performance is also likely to be influenced by their social environment. The negative effects of social isolation on cognition are well established [62]. In this special issue, Dalesman [11] shows that the effects of social isolation may be contingent on previous social experiences: in tests of long-term memory in pond snails, only those populations that had been maintained in groups suffered weakened long-term memory formation following social isolation.
In highly social species, it is not just the presence or absence of conspecifics that is important for cognitive development, but the diversity and range of social experiences individuals are exposed to throughout their lives. Sociality has long been seen as a key driver of cognitive evolution; the Social Intelligence Hypothesis [63,64] argues that the need to navigate the challenges of social life generates selection for increased brain size and elevated cognitive performance in species that show differentiated social relationships. In this issue, Ashton et al. [18] argue that these social challenges may have effects not only over evolutionary time, but also within the lifetimes of individuals. They describe how, for Australian magpies Cracticus tibicen dorsalis, growing up in a larger group seems to generate challenges that promote cognitive development [23]. While the Social Intelligence Hypothesis typically emphasizes the importance of social challenges for the evolution of social cognition (e.g. third-party recognition, transitive inference, theory of mind), Ashton et al.'s [23] findings show that social factors may also influence more domain-general cognitive traits (e.g. associative and reversal learning, spatial memory and inhibitory control).
It is not just the size of groups that may influence cognitive abilities but also the fine-scale patterns of association and interaction occurring within them. For example, developmentally stressed zebra finches, Taeniopygia guttata, exclusively copied unrelated adults whereas control birds copied their parents in learning to solve a novel food puzzle [65,66]. In this special issue, Boogert et al. [10] show that these effects also extend to the acquisition of a sexually selected trait: song, which male zebra finches typically learn from their fathers. While overall song copying accuracy did not vary across experimental conditions, developmentally stressed chicks spent more time with non-kin and copied their fathers' songs less accurately. These results emphasize that social factors can influence cognitive performance by facilitating inter-individual differences in strategies of information acquisition and use. More broadly, given the critical role of song learning in reproductive fitness, they illustrate how developmental conditions influence cognitive processes which in turn shape the cognitive and behavioural phenotypes which then come under selection.
One crucial yet often neglected point is that the causal arrow between social factors and cognitive performance may point both ways. In this issue, Wascher et al. [13] suggest that an individual's cognitive performance and/or knowledge state may both influence its position within its social network and be influenced by the social position that an individual occupies. We consider that the interplay between cognitive ability and the social environment is one particular area where studies of individual differences in cognitive abilities provide a unique perspective, not available to more traditional comparative studies. Crucially, the reviews in this special issue demonstrate that when assaying individual differences in cognitive ability, we need to account for the social environment, both current and past, and this environment comprises both coarse measures (group size) and more subtle measures (specific dyadic relationships/network structure), all of which can both shape cognitive abilities and arise from the abilities of individuals. In studies of free-living animals, our ignorance of fine-scale details of an individual's social history may account for some of the unexplained variation in cognitive abilities we observe between individuals. However, the social interactions observed may be more representative of those genuinely experienced by animals subjected to selection in the wild.
(e). Personality
Several authors have suggested that personality differences, typified by stable, consistent, individual differences in behaviour across time and contexts [38], are likely to generate variation in the ways in which individuals gather and act on information, resulting in ‘cognitive styles’ [31,67]. For example, fast explorers have been suggested to rapidly form associations when exposed to a new set of cues, resulting in the formation of inflexible behavioural routines. They are thus expected to outperform slow explorers in initial discriminations between stimuli, but to be outperformed in reversal learning tests. Although there has been some initial support for these predictions, the meta-analyses by Dougherty & Guillette [68] in this special issue show that results are inconsistent across species. Relationships between cognitive performance and personality traits may even vary at the intraspecific level: Dalesman [11] shows that, in pond snails, the relationship between exploratory behaviour and memory formation varies both across populations (i.e. wild-caught versus laboratory-reared) and social test conditions (isolation versus group). Aspects of an individual's personality are suspected to correspond to their cognitive abilities, but given these variable results, it is important not to assume that there are relationships which consistently take a particular form or are always consistent within the same population across time [55]. Instead, covariance should be measured and tested explicitly in each study population, ideally at multiple points in time.
Personality differences may also be relevant when considering what we are measuring in cognitive tests. For example, when an individual makes multiple ‘mistakes’ during a test of reversal learning, the common interpretation is that it must not have learned the new stimulus-reward contingency. However, an alternative interpretation is that differences in success in a reversal learning task reflect not (just) differences in learning performance, but individual differences in information gathering strategies. Future studies would, therefore, benefit from considering whether individuals exhibit stable differences in their information sampling strategies, and if so, how these relate to measures of both personality and cognitive test performance.
4. What are the consequences of individual variation in cognition?
A central reason for focusing on individual differences in cognition is that this variation provides the raw material on which natural selection can act. If individual differences are stable and heritable, phenotypes that enhance fitness may evolve through natural selection. Several papers in this special issue, therefore, examine relationships between individual cognitive performance and proxies of fitness, including reproductive success [23], survival [19,20] and body condition [19]. Huebner et al. [19] studied problem-solving and spatial memory, and the relationships with body mass index and survival in a wild population of grey mouse lemurs (Microcebus murinus). While body mass index at the time of testing did not impact problem-solving speed, faster problem-solvers gained more mass during the dry season compared with slower solvers, suggesting a possible causal link between this metric and foraging success in the wild. However, the number of errors individuals made in a maze did not predict body mass index or its change, nor survival, thereby providing no support for a role of spatial learning ability in determining survival during the dry season. Madden et al.'s [20] findings remind us that elevated performance on cognitive tests will not always result in increases in fitness, and may be maladaptive, as the net benefits depend on prevailing conditions [69]. Indeed, reversal learning performance was negatively related with survival in captive-reared pheasants, Phasianus colchicus, released into the wild. Interestingly, this result is in line with predictions from models on the adaptive value of learning (e.g. [70]), because the tested pheasants had stable access to consistent artificial feeders, which may have reduced the value of information about food. Ashton et al. [18] discuss the relationship between performance on a battery of cognitive tasks and female reproductive success in wild Australian magpies [23]. Individual differences in cognitive performance were highly repeatable, and were positively associated with reproductive success; these elements thus set the scene for possible selection on general cognitive abilities. However, the process of selection may not be straightforward. Differences among individual magpies only appeared at 200 days of age, indicating an important role of plasticity (in this case the size of group in which the juvenile was raised) in determining cognitive phenotypes. It is thus possible that general cognitive performance exhibits low heritability and does not lead to selection despite links with reproductive success, or is heritable but is heavily moderated by the developmental environment, for instance, via strong genotype × environment interactions.
(a). Evolutionary implications
The implications of linear relationships between ecological/social conditions, cognitive performance and fitness are important: if a cognitive phenotype is truly advantaged (or disadvantaged) over other phenotypes in the population, there could be directional selection for traits underlying the observed variation in cognitive performance. However, strong directional selection on any trait is rarely detected in the wild [71]; the proportion of studies expected to detect a significant linear relationship between cognitive performance and fitness over a reasonable number of reproductive events is thus quite low. It is perhaps surprising that proportionally so many (published) studies (summarized in [20]) exploring this relationship report positive relationships even when sample sizes are generally small and cognitive assays likely rather noisy. Instead of directional selection, given the costs associated with information gathering and processing, stabilizing selection on cognitive traits may occur more commonly in natural populations. This suggests that, in addition to examining linear relationships between fitness proxies and cognitive performance, researchers should also investigate quadratic relationships, although statistical power may often be prohibitive [22,72].
Linear relationships between cognitive performance and fitness lead to an interesting question: if the best (or worst) performers enjoy fitness benefits, why is there still variation in the population? For instance, was there a recent change in the costs–benefits balance associated with a particular cognitive trait and hence the response to selection is still ongoing? Is the population facing fluctuating selection, such that contrasting phenotypes are advantaged in different years? Alternatively, the observed covariation between cognitive performance and single fitness proxies may not correspond to overall fitness outcomes. Field researchers can only very rarely measure lifetime reproductive success because tracking known individuals throughout their lifetime is logistically challenging. Laboratory-based researchers may find it hard to collect ecologically relevant measures of fitness. Consequently, a given fitness component that is measured could well be traded-off against another component that is not measured (e.g. offspring quantity versus quality). Furthermore, a given cognitive trait may positively affect some component of fitness but act to reduce another, resulting in no net selection (e.g. [73]).
Cognitive traits may also fail to respond to selection if they are correlated with other aspects of the phenotype that bear different fitness consequences. For example, neural structures such as large brains or regions underlying cognitive abilities and information processing itself are energetically expensive [74]. Therefore, the benefits derived from improved cognitive abilities must offset the costs imposed by their underlying physical bases. Furthermore, organisms are under multivariate selection pressures; selection on correlated traits can impact evolutionary change of any single trait, and these correlations can themselves be under selection [75] An important task for students of individual variation in cognitive abilities is thus to map correlations between multiple cognitive traits (e.g. [9]), and between cognitive and other traits (e.g. behavioural: [76]; physiological: [10,61]; neurological: [44]), and eventually, to examine multivariate selection on a set of traits [77]. In this special issue, Madden et al. [20] report on the moderating effect of body mass on the link between associative learning performance and survival. This highlights the importance of considering other important (non-cognitive) determinants of fitness in conjunction with cognitive abilities when assessing micro-evolutionary patterns for cognitive traits.
The resolution of optimal solutions within populations can be achieved using a game theory approach in which the best individual solution depends on the behaviour of others in the population. Models have examined the evolutionary emergence of learning polymorphisms in the context of the producer-scrounger game. In some theoretical treatments ([78], but see [79]), the equilibrium population was composed of only a fraction of learners who collect information about the value of behavioural alternatives before deciding which one to play. When environmental conditions change, the behavioural adjustments of learners are sufficient to restore equilibrium proportions of producer and scroungers, thereby preventing the fixed phenotypes from paying a cost for their lack of plasticity. As is the case for a variety of behaviours (e.g. aggression, cooperation) the adaptive value of cognitive abilities may depend on the behaviour of others. Consequently, frequency dependence may be an important cause for the evolutionary maintenance of individual differences in cognition. This would appear to be a fruitful area for further research utilizing both empirical and modelling approaches.
One well-established approach to testing evolutionary hypotheses that has been productive in studying non-cognitive traits involves the consideration of life-history theory. This approach attempts to quantify fitness peaks associated with different investment strategies. Trade-offs inevitably occur when time and energy are limited; some trade-offs seem common across taxa and traits, including investment in current versus future reproduction, offspring quality versus quantity and survival versus reproduction [80]. A range of phenotypic traits related to cognitive abilities can map onto these trade-offs, including morphology, physiology and behaviour. For instance, bird species that develop more slowly tend to live longer, possess larger brains relative to their body mass and are more innovative than species that develop rapidly [81]. These trade-offs can also be observed at the individual level. For instance, female guppies, Poecilia reticulata, artificially selected for increases in brain size enjoyed a survival advantage in the presence of predators [82], but this investment in the nervous system seemed to be traded-off against investment in the innate immune system [83] and the size of the digestive tract (cf. expensive tissue hypothesis [56]). Trade-offs can thus lead to the emergence of alternative strategies, which outcompete others on some fitness components but not others, thereby leading to the maintenance of variation within populations. Alternative strategies would be predicted to result in a statistical interaction between cognitive performance and a correlated trait when explaining fitness; for instance, learning may be more positively associated with survival in subordinate individuals compared with dominants if subordinates gain more from learned information due to reduced access to resources (i.e. ‘necessity drives innovation’ hypothesis [84]). Because life-history trade-offs can occur under any selection regime (positive, negative, or stabilizing selection), they represent an important theoretical framework that can be co-opted to derive predictions on contrasting links between cognitive performance and fitness components.
5. Conclusion
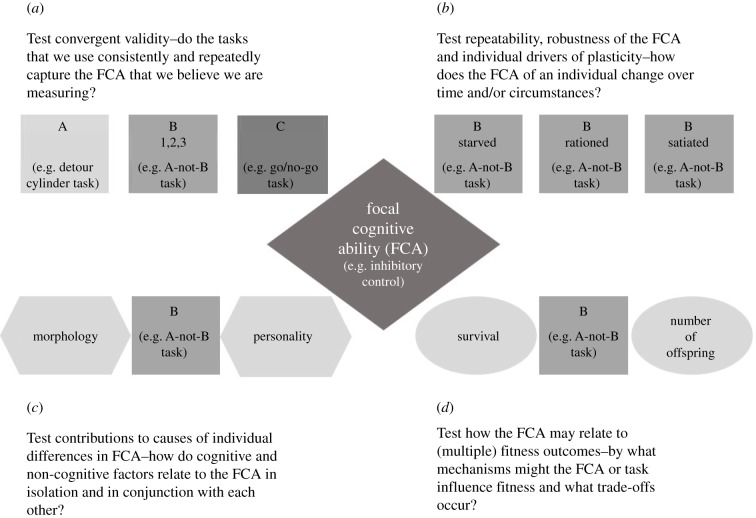
Acknowledging and appreciating the variation in cognitive abilities between individuals, including the factors that cause it and the consequences that it imparts, provides the basis for a novel and powerful approach to understanding the evolution of cognition, whether focusing on general cognitive abilities or performance in a specific cognitive domain. However, as we and other authors in this special issue highlight, the measurement of the cognitive abilities of individuals is inherently difficult and highly susceptible to extrinsic factors both at the time of testing and during earlier development. In addition, the fitness gains manifest specifically through cognitive abilities may be hard to determine and be highly dependent on particular elements of the selection environment. Consequently, we suggest that in order to progress, research programmes wishing to measure individual cognitive abilities and determine their causes and consequences should use the following approaches (figure 1): (1) Multiple tests to assess convergent validity of the test: although it is usually not possible to determine that a given cognitive process has been targeted effectively, the use of different tasks, stimuli dimensions and sets of cues, should reduce the probability of misspecification and may also help to reduce the impact of confounding variables. (2) Multiple internal and external states: this is akin to the method of ‘systematic variation’ [85] and examines the robustness of individual ranks in performance in a given task when variables related to internal state (e.g. hunger, reproductive status) and external context (e.g. test room, laboratory versus field) vary. Unfortunately, this type of experiment is rarely done, possibly because of issues associated with estimating repeatability of cognitive performance [6,31]. (3) Multiple traits: cognitive traits may covary with other aspects of the phenotype, including personality traits. Exploring the different hypotheses for this covariation (e.g. genetic correlation, common underlying physiological trait or confounding variable) will be critical for our understanding of the evolution of cognitive traits under constraints stemming from multivariate selection pressures. (4) Multiple fitness proxies: this approach allows tests of hypotheses based on life-history trade-offs, and thus can reveal complex ways in which cognitive abilities influence survival, reproduction and the transmission of genes between generations.
Figure 1.
Conceptual representation of the four research approaches that can inform our understanding of the causes and consequences of individual variation in cognitive abilities. (a) Multiple tests: several variants of a test (A, B, C) are used in order to assess convergent validity. Repeatability of performance is also estimated, at least for the focal test (B1, B2, B3). (b) Multiple states: a test (B) is administered under varying levels of internal or external states. These could be experimentally manipulated (e.g. food deprivation) or observational (e.g. samples at 0, 100 and 200 days during development), in order to assess the robustness of individual ranks in performance and identify drivers of plasticity in cognitive ability. (c) Multiple traits: other traits are quantified, in order to identify phenotypic and genetic correlations with the cognitive ability of interest (B). (d) Multiple fitness proxies: several components of lifetime reproductive success are measured in order to examine potential trade-offs amongst them, or contrasting links between fitness proxies with the cognitive ability of interest (B).
While implementing all four approaches may be difficult for any single research group, we argue that each of them is worth pursuing on its own, because findings along any one of these lines of research will benefit understanding and advancement in others. Furthermore, we suggest that relationships between cognitive abilities and fitness measures are not always intuitive, and neither are the factors which may cause individual variation in such abilities. Consequently, we can see value in hypothesis-driven exploratory studies examining potential correlations between cognitive performances, potentially plausible causal factors and putative fitness outcomes. Clearly such correlations must be treated with caution, yet they may provide important novel insights and route maps that can help develop further hypotheses on the causes and consequences of individual variation in cognition. We can also see great value in methodological studies that improve the precision and accuracy of cognitive tests, and confirm the relationships between behaviours in the tests and the underlying cognitive processes that they are intended to reveal. As this young field develops, so too will the underlying theory and experimental techniques. Methods, and to a lesser extent empirical results, have already been the subject of reviews and some debate [21,22,26–28,46]. Critically, it is now imperative to draw together empirical findings and start to formulate and develop explicit hypotheses and overarching conceptual and theoretical frameworks explaining the causes and consequences of individual differences in cognitive abilities.
Acknowledgements
We thank all participants of the workshop on Causes and Consequences of Individual Differences in Cognition, October 2017. J.M.F. would like to thank Gabrielle Davidson for discussions on hypothesis testing during the workshop. John Quinn and one anonymous reviewer provided helpful comments on this manuscript.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
N.J.B. is funded by a Royal Society Dorothy Hodgkin Fellowship. A.T. is funded by a Human Frontiers Research Program grant (RGP00049). J.R.M. is funded by an ERC Consolidator Award (616474). J.M.F. is funded by a HFSP grant (RGP0006) and a Natural Sciences and Engineering Research Council of Canada Discovery Grant (435596).
References
- 1.Carroll JB, Maxwell SE. 1979. Individual differences in cognitive abilities. Annu. Rev. Psychol. 30, 603–640. ( 10.1146/annurev.ps.30.020179.003131) [DOI] [PubMed] [Google Scholar]
- 2.Thornton A, Lukas D. 2012. Individual variation in cognitive performance: developmental and evolutionary perspectives. Phil. Trans. R. Soc. B 367, 2773–2783. ( 10.1098/rstb.2012.0214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krebs JR, Davies NB. Behavioural ecology: an evolutionary approach: John Wiley & Sons, 2009. [Google Scholar]
- 4.Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. 2007. Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318. ( 10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- 5.Kays R, Crofoot MC, Jetz W, Wikelski M. 2015. Terrestrial animal tracking as an eye on life and planet. Science 348, aaa2478 ( 10.1016/j.anbehav.2018.05.020) [DOI] [PubMed] [Google Scholar]
- 6.Cauchoix M, et al. 2018. The repeatability of cognitive performance: a meta-analysis. Phil. Trans. R. Soc. B 373, 20170281 ( 10.1098/rstb.2017.0281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Völter C, Tinklenberg B, Call J, Seed AM. 2018. Comparative psychometrics: establishing what differs is central to understanding what evolves. Phil. Trans. R. Soc. B 373, 20170283 ( 10.1098/rstb.2017.0283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sauce B, Bendrath S, Herzfeld M, Siegel D, Style C, Rab S, Korabelnikov J, Matzel LD. 2018. The impact of environmental interventions among mouse siblings on the heritability and malleability of general cognitive ability. Phil. Trans. R. Soc. B 373, 20170289 ( 10.1098/rstb.2017.0289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorato E, Zidar J, Garnham L, Wilson A, Løvlie H. 2018. Heritabilities and co-variation among cognitive traits in red junglefowl. Phil. Trans. R. Soc. B 373, 20170285 ( 10.1098/rstb.2017.0285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boogert NJ, Lachlan RF, Spencer KA, Templeton CN, Farine DR. 2018. Stress hormones, social associations and song learning in zebra finches. Phil. Trans. R. Soc. B 373, 20170290 ( 10.1098/rstb.2017.0290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalesman S. 2018. Habitat and social context affect memory phenotype, exploration and covariance among these traits. Phil. Trans. R. Soc. B 373, 20170291 ( 10.1098/rstb.2017.0291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pike TW, Ramsey M, Wilkinson A. 2018. Environmentally induced changes to brain morphology predict cognitive performance. Phil. Trans. R. Soc. B 373, 20170287 ( 10.1098/rstb.2017.0287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wascher CAF, Kulahci IG, Langley EJG, Shaw RC. 2018. How does cognition shape social relationships? Phil. Trans. R. Soc. B 373, 20170293 ( 10.1098/rstb.2017.0293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchanan KL, Grindstaff JL, Pravosudov VV. 2013. Condition dependence, developmental plasticity, and cognition: implications for ecology and evolution. Trends Ecol. Evol. 28, 290–296. ( 10.1016/j.tree.2013.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lupien SJ, McEwen BS, Gunnar MR, Heim C. 2009. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 10, 434–445. ( 10.1038/nrn2639) [DOI] [PubMed] [Google Scholar]
- 16.Reader SM. 2015. Causes of individual differences in animal exploration and search. Top. Cogn. Sci. 7, 451–468. ( 10.1111/tops.12148) [DOI] [PubMed] [Google Scholar]
- 17.Langley EJ, van Horik JO, Whiteside MA, Madden JR. 2018. Individuals in larger groups are more successful on spatial discrimination tasks. Anim. Behav. 142, 87–93. ( 10.1016/j.anbehav.2018.05.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashton BJ, Thornton A, Ridley AR. 2018. An intraspecific appraisal of the social intelligence hypothesis. Phil. Trans. R. Soc. B 373, 20170288 ( 10.1098/rstb.2017.0288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huebner F, Fichtel C, Kappeler PM. 2018. Linking cognition with fitness in a wild primate: fitness correlates of problem-solving performance and spatial learning ability. Phil. Trans. R. Soc. B 373, 20170295 ( 10.1098/rstb.2017.0295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madden JR, Langley EJG, Whiteside MA, Beardsworth CE, van Horik JO. 2018. The quick are the dead: pheasants that are slow to reverse a learned association survive for longer in the wild. Phil. Trans. R. Soc. B 373, 20170297 ( 10.1098/rstb.2017.0297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thornton A, Isden J, Madden JR. 2014. Toward wild psychometrics: linking individual cognitive differences to fitness. Behav. Ecol. 25, 1299–1301. ( 10.1093/beheco/aru095) [DOI] [Google Scholar]
- 22.Morand-Ferron J, Quinn JL. 2015. The evolution of cognition in natural populations. Trends Cogn. Sci. 19, 235–237. ( 10.1016/j.tics.2015.03.005) [DOI] [PubMed] [Google Scholar]
- 23.Ashton BJ, Ridley AR, Edwards EK, Thornton A. 2018. Cognitive performance is linked to group size and affects fitness in Australian magpies. Nature 554, 364–367. ( 10.1038/nature25503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isden J, Panayi C, Dingle C, Madden J. 2013. Performance in cognitive and problem-solving tasks in male spotted bowerbirds does not correlate with mating success. Anim. Behav. 86, 829–838. ( 10.1016/j.anbehav.2013.07.024) [DOI] [Google Scholar]
- 25.Keagy J, Savard J-F, Borgia G. 2011. Complex relationship between multiple measures of cognitive ability and male mating success in satin bowerbirds, Ptilonorhynchus violaceus. Anim. Behav. 81, 1063–1070. ( 10.1016/j.anbehav.2011.02.018) [DOI] [Google Scholar]
- 26.Rowe C, Healy SD. 2014. Measuring variation in cognition. Behav. Ecol. 25, 1287–1292. ( 10.1093/beheco/aru090) [DOI] [Google Scholar]
- 27.Pritchard DJ, Hurly TA, Tello-Ramos MC, Healy SD. 2016. Why study cognition in the wild (and how to test it)? J. Exp. Anal. Behav. 105, 41–55. ( 10.1002/jeab.195) [DOI] [PubMed] [Google Scholar]
- 28.Morand-Ferron J, Cole EF, Quinn JL. 2016. Studying the evolutionary ecology of cognition in the wild: a review of practical and conceptual challenges. Biol. Rev. 91, 367–389. ( 10.1111/brv.12174) [DOI] [PubMed] [Google Scholar]
- 29.Deary IJ, Penke L, Johnson W. 2010. The neuroscience of human intelligence differences. Nat. Rev. Neurosci. 11, 201–211. ( 10.1038/nrn2793) [DOI] [PubMed] [Google Scholar]
- 30.Shaw RC, Schmelz M. 2017. Cognitive test batteries in animal cognition research: evaluating the past, present and future of comparative psychometrics. Anim. Cogn. 20, 1003–1018. ( 10.1007/s10071-017-1135-1) [DOI] [PubMed] [Google Scholar]
- 31.Griffin AS, Guillette LM, Healy SD. 2015. Cognition and personality: an analysis of an emerging field. Trends Ecol. Evol. 30, 207–214. ( 10.1016/j.tree.2015.01.012) [DOI] [PubMed] [Google Scholar]
- 32.van Horik JO, Madden JR. 2016. A problem with problem solving: motivational traits, but not cognition, predict success on novel operant foraging tasks. Anim. Behav. 114, 189–198. ( 10.1016/j.anbehav.2016.02.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boogert NJ, Anderson RC, Peters S, Searcy WA, Nowicki S. 2011. Song repertoire size in male song sparrows correlates with detour reaching, but not with other cognitive measures. Anim. Behav. 81, 1209–1216. ( 10.1016/j.anbehav.2011.03.004) [DOI] [Google Scholar]
- 34.Careau V, Thomas D, Humphries M, Réale D. 2008. Energy metabolism and animal personality. Oikos 117, 641–653. ( 10.1111/j.0030-1299.2008.16513.x) [DOI] [Google Scholar]
- 35.Sanford K, Clayton NS. 2008. Motivation and memory in zebra finch (Taeniopygia guttata) foraging behavior. Anim. Cogn. 11, 189–198. ( 10.1007/s10071-007-0106-3) [DOI] [PubMed] [Google Scholar]
- 36.Cauchoix M, Hermer E, Chaine A, Morand-Ferron J. 2017. Cognition in the field: comparison of reversal learning performance in captive and wild passerines. Sci. Rep. 7, 12945 ( 10.1038/s41598-017-13179-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morand-Ferron J, Cole EF, Rawles JE, Quinn JL. 2011. Who are the innovators? A field experiment with 2 passerine species. Behav. Ecol. 22, 1241–1248. ( 10.1093/beheco/arr120) [DOI] [Google Scholar]
- 38.Dall SRX, Houston AI, McNamara JM. 2004. The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 7, 734–739. ( 10.1111/j.1461-0248.2004.00618.x) [DOI] [Google Scholar]
- 39.Nakagawa S, Schielzeth H. 2010. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev. 85, 935–956. ( 10.1111/j.1469-185X.2010.00141.x) [DOI] [PubMed] [Google Scholar]
- 40.Cole EF, Cram DL, Quinn JL. 2011. Individual variation in spontaneous problem-solving performance among wild great tits. Anim. Behav. 81, 491–498. ( 10.1016/j.anbehav.2010.11.025) [DOI] [Google Scholar]
- 41.MacLean EL, et al. 2014. The evolution of self-control. Proc. Natl Acad. Sci. USA 111, E2140–E21E8. ( 10.1073/pnas.1323533111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Horik JO, Langley EJG, Whiteside MA, Laker PR, Beardsworth CE, Madden JR. 2018. Do detour tasks provide accurate assays of inhibitory control? Proc. R. Soc. B 285, 20180150 ( 10.1098/rspb.2018.0150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seed A, Seddon E, Greene B, Call J. 2012. Chimpanzee ‘folk physics’: bringing failures into focus. Phil. Trans. R. Soc. B 367, 2743–2752. ( 10.1098/rstb.2012.0222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dubois J, Galdi P, Paul LK, Adolphs R. 2018. A distributed brain network predicts general intelligence from resting-state human neuroimaging data. Phil. Trans. R. Soc. B 373, 20170284 ( 10.1098/rstb.2017.0284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shettleworth SJ. 2012. Modularity, comparative cognition and human uniqueness. Phil. Trans. R. Soc. B 367, 2794–2802. ( 10.1098/rstb.2012.0211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Healy SD, Bacon IE, Haggis O, Harris AP, Kelley LA. 2009. Explanations for variation in cognitive ability: Behavioural ecology meets comparative cognition. Behav. Processes 80, 288–294. ( 10.1016/j.beproc.2008.10.002) [DOI] [PubMed] [Google Scholar]
- 47.van Horik JO, Lea SE. 2017. Disentangling learning from knowing: does associative learning ability underlie performances on cognitive test batteries? Behav. Brain Sci. 40, e220 ( 10.1017/S0140525X16001795) [DOI] [PubMed] [Google Scholar]
- 48.Burkart JM, Schubiger MN, van Schaik CP. 2017. The evolution of general intelligence. Behav. Brain Sci. 40, e195 ( 10.1017/S0140525X16000959). [DOI] [PubMed] [Google Scholar]
- 49.Shaw RC, Boogert NJ, Clayton NS, Burns KC. 2015. Wild psychometrics: evidence for ‘general’ cognitive performance in wild New Zealand robins, Petroica longipes. Anim. Behav. 109, 101–111. ( 10.1016/j.anbehav.2015.08.001) [DOI] [Google Scholar]
- 50.van Horik JO, Langley EJ, Whiteside MA, Laker PR, Madden JR. 2018. Intra-individual variation in performance on novel variants of similar tasks influences single factor explanations of general cognitive processes. R. Soc. open sci. 5, 171919 ( 10.1098/rsos.171919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shanahan M, Bingman V, Shimizu T, Wild M, Güntürkün O. 2013. Large-scale network organization in the avian forebrain: a connectivity matrix and theoretical analysis. Front. Comput. Neurosci. 7, 89 ( 10.3389/fncom.2013.00089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deary IJ, Johnson W, Houlihan LM. 2009. Genetic foundations of human intelligence. Hum. Genet. 126, 215–232. ( 10.1007/s00439-009-0655-4) [DOI] [PubMed] [Google Scholar]
- 53.Galsworthy MJ, Paya-Cano JL, Liu L, Monleón S, Gregoryan G, Fernandes C, Schalkwyk LC, Plomin R. 2005. Assessing reliability, heritability and general cognitive ability in a battery of cognitive tasks for laboratory mice. Behav. Genet. 35, 675–692. ( 10.1007/s10519-005-3423-9) [DOI] [PubMed] [Google Scholar]
- 54.Croston R, Branch CL, Kozlovsky DY, Dukas R, Pravosudov VV. 2015. Heritability and the evolution of cognitive traits. Behav. Ecol. 26, 1447–1459. ( 10.1093/beheco/arv088) [DOI] [Google Scholar]
- 55.Quinn JL, Cole EF, Reed TE, Morand-Ferron J. 2016. Environmental and genetic determinants of innovativeness in a natural population of birds. Phil. Trans. R. Soc. B 371, 20150184 ( 10.1098/rstb.2015.0184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kotrschal A, Rogell B, Bundsen A, Svensson B, Zajitschek S, Brännström I, Immler S, Maklakov AA, Kolm N. 2013. Artificial selection on relative brain size in the guppy reveals costs and benefits of evolving a larger brain. Curr. Biol. 23, 168–171. ( 10.1016/j.cub.2012.11.058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mery F, Kawecki TJ. 2003. A fitness cost of learning ability in Drosophila melanogaster. Proc. R. Soc. B 270, 2465–2469. ( 10.1098/rspb.2003.2548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burger JMS, Kolss M, Pont J, Kawecki TJ. 2008. Learning ability and longevity: a symmetrical evolutionary trade-off in Drosophila. Evolution 62, 1294–1304. ( 10.1111/j.1558-5646.2008.00376.x) [DOI] [PubMed] [Google Scholar]
- 59.Zwoinska MK, Lind MI, Cortazar-Chinarro M, Ramsden M, Maklakov AA. 2016. Selection on learning performance results in the correlated evolution of sexual dimorphism in life history. Evolution 70, 342–357. ( 10.1111/evo.12862) [DOI] [PubMed] [Google Scholar]
- 60.Thornton A, Wilson AJ. 2015. In search of the Darwinian Holy Trinity in cognitive evolution: a comment on Croston et al. Behav. Ecol. 26, 1460–1461. ( 10.1093/beheco/arv119) [DOI] [Google Scholar]
- 61.Davidson GL, Cooke AC, Johnston CN, Quinn JL. 2018. The gut microbiome as a driver of individual variation in cognition and functional behaviour. Phil. Trans. R. Soc. B 373, 20170286 ( 10.1098/rstb.2017.0286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thornton A, Boogert NJ. In press. The nature and nurturing of animal minds. In Genes and behaviour beyond nature nurture (eds Hosken D, Wedell N, Hunt J). Wiley. [Google Scholar]
- 63.Humphrey N. 1976. The social function of intellect. In Growing points in ethology (eds Bateson P, Hinde R), pp. 307–317. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 64.Dunbar RIM. 1998. The social brain hypothesis. Evol. Anthropol. 6, 178–190. ( 10.1002/(SICI)1520-6505(1998)6:5%3C178::AID-EVAN5%3E3.0.CO;2-8) [DOI] [Google Scholar]
- 65.Boogert NJ, Farine DR, Spencer KA. 2014. Developmental stress predicts social network position. Biol. Lett. 10, 20140561 ( 10.1098/rsbl.2014.0561) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Farine DR, Spencer KA, Boogert NJ. 2015. Early-life stress triggers juvenile zebra finches to switch social learning strategies. Curr. Biol. 25, 2184–2188. ( 10.1016/j.cub.2015.06.071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sih A, Del Giudice M. 2012. Linking behavioural syndromes and cognition: a behavioural ecology perspective. Phil. Trans. R. Soc. B 367, 2762–2772. ( 10.1098/rstb.2012.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dougherty LR, Guillette LM. 2018. Linking personality and cognition: a meta-analysis. Phil. Trans. R. Soc. B 373, 20170282 ( 10.1098/rstb.2017.0282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rice AM, McQuillan MA. 2018. Maladaptive learning and memory in hybrids as a reproductive isolating barrier. Proc. R. Soc. B 285, 20180542 ( 10.1098/rspb.2018.0542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dunlap AS, Stephens DW. 2016. Reliability, uncertainty, and costs in the evolution of animal learning. Curr. Opin. Behav. Sci. 12, 73–79. ( 10.1016/j.cobeha.2016.09.010) [DOI] [Google Scholar]
- 71.Kingsolver JG, et al. 2001. The strength of phenotypic selection in natural populations. Am. Nat. 157, 245–261. ( 10.1086/319193) [DOI] [PubMed] [Google Scholar]
- 72.Kingsolver JG, Diamond SE, Siepielski AM, Carlson SM. 2012. Synthetic analyses of phenotypic selection in natural populations: lessons, limitations and future directions. Evol. Ecol. 26, 1101–1118. ( 10.1007/s10682-012-9563-5) [DOI] [Google Scholar]
- 73.Cole EF, Morand-Ferron J, Hinks AE, Quinn JL. 2012. Cognitive ability influences reproductive life history variation in the wild. Curr. Biol. 22, 1808–1812. ( 10.1016/j.cub.2012.07.051) [DOI] [PubMed] [Google Scholar]
- 74.Laughlin SB, van Steveninck R, Anderson JC. 1998. The metabolic cost of neural information. Nat. Neurosci. 1, 36–41. ( 10.1038/236) [DOI] [PubMed] [Google Scholar]
- 75.Sgro C, Hoffmann A. 2004. Genetic correlations, tradeoffs and environmental variation. Heredity 93, 241–248. ( 10.1038/sj.hdy.6800532) [DOI] [PubMed] [Google Scholar]
- 76.Seppänen J-T, Forsman JT, Mönkkönen M, Krams I, Salmi T. 2011. New behavioural trait adopted or rejected by observing heterospecific tutor fitness. Proc. R. Soc. B 278, 1736–1741. ( 10.1098/rspb.2010.1610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wolf M, Weissing FJ. 2012. Animal personalities: consequences for ecology and evolution. Trends Ecol. Evol. 27, 452–461. ( 10.1016/j.tree.2012.05.001) [DOI] [PubMed] [Google Scholar]
- 78.Dubois F, Morand-Ferron J, Giraldeau L-A. 2010. Learning in a game context: strategy choice by some keeps learning from evolving in others. Proc. R. Soc. B 277, 3609–3616. ( 10.1098/rspb.2010.0857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Katsnelson E, Motro U, Feldman MW, Lotem A. 2012. Evolution of learned strategy choice in a frequency-dependent game. Proc. R. Soc. B 279, 1176–1184. ( 10.1098/rspb.2011.1734) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roff D. 2002. Life history evolution. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 81.Sol D, Sayol F, Ducatez S, Lefebvre L. 2016. The life-history basis of behavioural innovations. Phil. Trans. R. Soc. B 371, 20150187 ( 10.1098/rstb.2015.0187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kotrschal A, Buechel SD, Zala SM, Corral-Lopez A, Penn DJ, Kolm N. 2015. Brain size affects female but not male survival under predation threat. Ecol. Lett. 18, 646–652. ( 10.1111/ele.12441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kotrschal A, Kolm N, Penn DJ. 2016. Selection for brain size impairs innate, but not adaptive immune responses. Proc. R. Soc. B 283, 20152857 ( 10.1098/rspb.2015.2857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reader SM, Laland KN. 2003. Animal innovation: an introduction in animal innovation. Oxford, UK: Oxford University Press. [Google Scholar]
- 85.Bitterman ME. 1975. The comparative analysis of learning. Science 188, 699–709. ( 10.1126/science.188.4189.699) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.