Abstract
Linking the cognitive performance of wild animals with fitness consequences is crucial for understanding evolutionary processes that shape individual variation in cognition. However, the few studies that have examined these links revealed differing relationships between various cognitive performance measures and fitness proxies. To contribute additional comparative data to this body of research, we linked individual performance during repeated problem-solving and spatial learning ability in a maze with body condition and survival in wild grey mouse lemurs (Microcebus murinus). All four variables exhibited substantial inter-individual variation. Solving efficiency in the problem-solving task, but not spatial learning performance, predicted the magnitude of change in body condition after the harsh dry season, indicating that the ability to quickly apply a newly discovered motor technique might also facilitate exploitation of new, natural food resources. Survival was not linked with performance in both tasks, however, suggesting that mouse lemurs' survival might not depend on the cognitive performances addressed here. Our study is the first linking cognition with fitness proxies in a wild primate species, and our discussion highlights the importance and challenges of accounting for a species’ life history and ecology in choosing meaningful cognitive and fitness variables for a study in the wild.
This article is part of the theme issue ‘Causes and consequences of individual differences in cognitive abilities’.
Keywords: cognitive performance, fitness, survival, body condition, primate
1. Introduction
Observing animals around us, like a squirrel harvesting and caching nuts, it seems obvious that animals ought to benefit from cognitive abilities. Individuals of many species have to remember the location of food resources or shelters, respond flexibly to the presence of predators, potential mates or environmental changes, and could benefit from innovating new behavioural strategies in response to environmental change, for example. Cognitive abilities, i.e. the ability to acquire, process, store and respond appropriately to social and environmental information [1], should therefore be associated with individual fitness benefits, so that individuals that learn faster, remember better, behave more flexibly or innovate when confronted with new challenges, should on average also be in better body condition, produce more offspring and survive better. Nonetheless, not all animals have maximized cognitive capacities, but persistent individual differences in cognitive performance exist as higher cognitive performance is not only associated with fitness benefits but also with costs and therefore under-selection (reviewed in [2], and see below). However, we still know little about the evolutionary forces and trade-offs that shape cognitive abilities as the links between them and fitness outcomes have been investigated in only a few species, and these studies revealed differing relationships (see below). Here, we contribute to this body of research by presenting results of the first study of the cognition–fitness links in a wild primate species.
Investigating fitness consequences of variation in cognitive abilities requires the study of both sets of variables in wild animals, which can be time-consuming and challenging for many practical reasons, especially for long-lived species [2,3]. In humans, intelligence has been linked to fitness-related traits like education, health and longevity [4]. However, evidence for the predicted positive relationship between cognition and fitness measures from animals is still rare, especially from the wild (electronic supplementary material, table S1). Among invertebrates, learning speed of bumblebee (Bombus terrestris) colonies correlated positively with colonies' overall foraging success [5], but individual bumblebees’ learning ability did not correlate with daily foraging performance, and bees with better learning abilities foraged for fewer days, indicating a (neuronal) cost of enhanced learning ability [6]. In selected laboratory populations of fruit flies (Drosophila melanogaster), improved learning ability was also associated with a fitness cost and correlated with decreased larval competitive ability [7].
Among vertebrates, spatial learning accuracy in a maze correlated positively with reproductive success of captive rose bitterling males (Rhodeus ocellatus) in a sneaker role, but not in the dominant guarding role, the alternative male mating tactic in this fish species [8]. Performance in problem-solving tasks, in which animals are presented with novel problems like artificial foraging tasks, was used as a measure of cognition in several studies of birds. However, this approach has recently been criticized because performance in problem-solving tasks is likely also affected by non-cognitive factors, and because the involved cognitive processes are not well defined [3,9,10]. Nonetheless, in great tits (Parus major) [11–13] and house sparrows (Passer domesticus) [14], problem-solving performance correlated positively with measures of reproductive success, but problem-solvers also exhibited a higher probability of deserting their nests [11], suggesting associated fitness costs. Problem-solving performance of male satin bower birds (Ptilonorhynchus violaceus) in tasks closely related to natural display behaviour correlated positively with their mating success [15,16]. However, cognitive performance in a closely related species, the spotted bower birds (Ptilonorhynchus maculatus), did not correlate with male mating success when tested in a task battery addressing multiple cognitive abilities [17]. Moreover, performance in cognitive tasks was not consistently related to song repertoire size, a predictor of various fitness-related traits, in song sparrows (Melospiza melodia): whereas reversal learning performance correlated positively with male song repertoire size, performance in two other cognitive tasks did not and performance in a detour reaching task was negatively related to song repertoire size [18]. Pheasant chicks (Phasianus colchicus) that were slow to reverse learned associations were more likely to survive for 60 days in the wild. Moreover, heavy pheasants that were quick in learning associations had improved survival, but for light animals, slow associative learners were more likely to survive [19]. In Australian magpies (Cracticus tibicen dorsalis), group size was positively correlated with cognitive performance, and general cognitive performance in four different tasks predicted reproductive success in females [20]. Finally, wild male African striped mice (Rhabdomys pumilio) that were better in a long-term spatial memory task survived for longer, whereas female survival correlated negatively with the number of errors in a short-term spatial memory task [21].
Thus, links between cognition and fitness outcomes have only been studied in a small number of wild vertebrate species, often focusing on members of one sex and on a single pair of variables. Furthermore, the differing results of these studies indicate that trade-offs of cognitive abilities and their links with fitness are likely to also depend on the study design such as the chosen cognitive measures, the conditions in which fitness measures are assessed, or individual characteristics like the sex or reproductive tactic of study subjects. Previous studies also demonstrated that when studying the adaptive value of cognition it is important to bear in mind that cognition is not a unitary trait, and that many different cognitive processes are involved in shaping a given behavioural outcome [9]. Moreover, cognition is involved in various different contexts, and what is beneficial in one situation can be disadvantageous in another [9,22]. Furthermore, cognitive ability per se is likely to be associated with costs because neuronal tissue is energetically expensive and therefore also under selection [2,23]. Hence, average individual cognitive performance in a particular test may not necessarily be closely and positively correlated with any fitness measure [9], and detecting the underlying trade-offs is especially challenging in the wild (but see [11]). Nevertheless, stable inter-individual variation in cognitive abilities persists [24], and relating it to variation in multiple fitness outcomes provides a reasonable starting point for a better understanding of the evolution of cognition [10,25].
Primates stand out among mammals for their relatively large brains and social complexity, both of which have been linked to cognitive abilities [26–31]. Because primates also have relatively slow life histories and wild populations do not readily cooperate in cognitive tests (but see [32–34]), nothing is known to date about potential fitness consequences of inter-individual variation in their cognitive abilities. Grey mouse lemurs (Microcebus murinus) are ideally suited among primates for such a study for several reasons, however. They are small (60 g), nocturnal, solitary primates with large brains for their body size [35]. Grey mouse lemurs are omnivorous ecological generalists, responding flexibly to seasonal changes in food availability [36] while evading several types of predators [37]. In addition, juveniles have to complete growth and physiological preparations in time for several months of hibernation by the time they are about six months old [38]. Thus, grey mouse lemurs face multiple ecological challenges under which they are likely to benefit from relevant cognitive abilities [39]. As a practical advantage, mouse lemurs can be easily captured with live traps, enabling us to bring them into a field laboratory for short-term cognitive testing before returning them to their natural home ranges. They also have one of the fastest life histories among primates, reaching sexual maturity within their first year of life and living on average for 2–3 years [40,41], so that variation in fitness can be estimated within a few field seasons.
The specific aims of this study were, therefore, to test wild grey mouse lemurs in a problem-solving task and a maze and link test performance with fitness proxies. To this end, we measured problem-solving efficiency during repeated lid opening of an artificial foraging task and spatial learning by remembering a food location in a maze, and linked individual variation in test performance with body condition after the dry season, a strong predictor of survival and males' mating success [42] and with long-term survival. We expected performance in these two tasks to be ecologically meaningful and fitness proxies to be relevant because during the extended lean season that mouse lemurs face, spatial learning of available food resources and potential innovative foraging skills are likely to impact body condition and ultimately survival.
2. Material and methods
(a). Study population and general procedure
This study was conducted at the research station of the German Primate Centre in the Forêt de Kirindy/CNFEREF, a dry deciduous forest in central Western Madagascar [43]. The study site is characterized by pronounced seasonality, with a three- to four-month hot–wet season with high fruit and insect abundance followed by eight to nine months of a cool–dry season with reduced food abundance during which mouse lemurs enter daily torpor or hibernation [42]. Grey mouse lemurs living in a 10 ha study area have been regularly captured and monitored since 1994 [44]. For this study, we used animals captured during monthly capture sessions between March and November, respectively, between 2015 and 2017. All animals were individually marked with subdermal micro transponders, sexed and aged (juveniles: less than 10 months old) based on morphometric data collected at the time of first capture [45].
For cognitive testing, individuals were kept at the research station in 1 m3 cages containing a nest-box and a testing platform. Tests were conducted at night and video-recorded under dim red light. Small pieces of banana served as reward in the tests. After testing, individuals were fed with a 1.5 cm banana piece (minus the amount obtained in the tests) per night and water was provided ad libitum. After one to three nights in captivity, individuals were released in the evening at their specific site of capture and, if possible, recaptured after a minimum of 10 days for further cognitive testing. Cognitive tests were conducted at the beginning of the dry season, months before the start of the mating season, thus rendering it unlikely that individuals’ fitness was affected by the few days in captivity. Testing subjects in captivity provided more controlled conditions and excluded potential threats from predators during the time of testing. Mouse lemurs were initially shy, but they habituated quickly and participated voluntarily in the experiments. We are therefore confident that testing under short-term captive conditions did not affect performance per se. Subjects were first tested with a food extraction (FE) task and then in a maze, either during three consecutive nights or after being recaptured. Because not all individuals could be recaptured with the same frequencies, sample sizes for the cognitive tests and fitness measures vary. Videos were analysed with the help of the software BORIS [46]. We assessed inter-observer reliability with a second person naive to the research question scoring more than 10% of test sessions, which was excellent (intra-class correlation coefficient: FE task = 1, N = 10; maze = 0.998, N = 10).
(b). Food extraction task
In the FE task, animals had to solve a novel problem by removing a sliding cover on each of the six wells (5 × 4.5 cm) of a small box (6 × 12 cm) in order to access a small piece of banana in each compartment (electronic supplementary material, figure S1). Banana on top of the apparatus served as an initial incentive to start interacting with it. Subjects were presented with the task for a maximum of 20 min. If a subject did not appear on the test platform and interact with the box within 10 min (N = 16), the trial was not counted and repeated the following night. Fifteen of these subjects interacted with the box on the second attempt, resulting in a total sample size of 96 individuals for this task.
We recorded whether a subject opened at least one lid (general success: yes/no), the total number of successes (0–6) and the latency from first contact with the box to first success. For subjects that interacted with the box but did not succeed, we recorded their total duration of testing, starting with the first contact with the box (i.e. capped latencies). Moreover, we measured an individual's solving time, i.e. the mean time a subject spent per successful opening after having opened the first lid, thus reflecting a subject's efficiency in repeatedly opening the lids of of the novel motor task. For two subjects, we could not rate the total number of successes due to technical difficulties during testing. We were able to test part of the subjects repeatedly in the FE task with a time delay of 10–30 days and individuals' solving time was repeatable (intra-class correlation coefficient = 0.63, p = 0.044, N = 8; for other measures, see electronic supplementary material, table S2).
(c). Maze
In this spatial learning task, the ability of subjects to remember and retrieve the position of a food reward in a plus maze was tested. The maze consisted of four wooden arms (40 × 17 cm; electronic supplementary material, figure S2) with attached boxes (20 × 17 cm) at each arm's end. One of the boxes served as the starting point from where subjects were released into the maze, and either the arm to the left or the right led to the reward (goal box). After successfully finding the reward in the goal box, the box was closed and rebaited before subjects were returned to the starting position and released again. To avoid the use of olfactory cues, big pieces of banana were placed out of reach at the end of every arm, thus, masking the smell of the 2 mm3 reward inside the goal box. Each trial started with the release of the subject from the start box and ended with the subject consuming the reward in the goal box. After every third trial, the maze was cleaned with 70% ethanol in order to prevent individuals from using potential own odour trails as orientation cues. During an initial familiarization trial, all three boxes were rewarded, and subjects had to find all rewards to continue with the test trials. If subjects failed to find the food rewards within 10 min, testing was terminated and the familiarization trial was repeated on the following night. In total, 21 subjects did not complete the familiarization trial or stopped participating during the test session, but 12 of them could be retested on a subsequent day with eight subjects completing the test, resulting in a final sample size of 73 subjects.
During each of the 15 test trials, we recorded the number of errors subjects made, i.e. the number of times animals entered an unrewarded maze arm. More specifically, we rated a subject entering the box at the end of an unrewarded arm with a score of 1, entering a wrong arm with all four limbs, but not the box at the end, with a score of 0.5, and entering an arm with only part of the body with a score of 0.25. We defined a learning criterion, which was reached when a subject found the reward directly without any errors in three consecutive trials. For each subject, we determined whether it reached the learning criterion as well as the total number of errors it made until reaching the criterion or across all 15 trials.
(d). Body mass index
To estimate body condition, which reflects variation in energetic state in small mammals [47], we calculated a body mass index (BMI) by dividing body mass (g) by bizygomatic breadth (mm), the latter being a reliable measure of body size in this species [48]. Because body mass fluctuates seasonally [38], which may affect motivation to search for food rewards, we used individual's BMI measured up to two months prior testing and mean values for subjects that were measured several times in this time window. For a total of 44 subjects, we were also able to calculate the change in BMI between the end of the rainy season (mean of BMIs measured in March–May) and the end of the dry season (mean of BMIs measured in September–November) by subtracting the latter from the former.
(e). Survival
We estimated individual survival by determining the number of days alive between birth and the date of last capture, truncating the study period in November 2017. Birth dates for all individuals were set at the modal birth date 1 January of the year of first capture for juveniles and 1 year earlier for subjects first captured as adults (see [42]). This second estimate is reliable because natal dispersals occur within the first year of life [49], and the probability of not capturing a natal individual within the first year of life is presumably extremely small. To define death operationally for individuals not recaptured for longer periods, we determined the 95th percentile of the frequency distribution of 10 936 inter-capture intervals recorded between 1995 and 2017 as a cut-off point. Accordingly, study subjects were operationally considered dead if they were not recaptured within 161 days before 1 November 2017. In total, we could estimate survival for 84 individuals, excluding 11 juvenile males that presumably dispersed from the study area after their first test.
(f). Statistical analyses
To evaluate the potential effects of individual characteristics, such as age, sex and body condition (which might proximately affect motivation), on performance in the cognitive tasks, we fitted multiple models with the respective measure of test performance as response and sex and BMI at the time of testing as predictor variables. We could not implement age class in these models, as BMI and age class were correlated and thus collinear. Therefore, to test for age differences in subjects' general ability to succeed in the FE task and to reach criterion in the maze, we ran proportion tests. To assess the effects of BMI and sex on subjects’ probability to succeed in the FE task and on the probability to reach the learning criterion in the maze, we fitted generalized linear models (GLM) with binomial error structure and logit link function. To model the effect on the number of successes and failures per individual in the FE task as response, we fitted a logistic generalized linear mixed model (GLMM) with individual identity included as random effect (R package lme4 [50]). We used a general linear model (LM) to fit the effect of sex and BMI on solving time (log-transformed) in the FE task. We used Cox proportional hazards models (package survival in R [51]) to model the effect on success latencies in the FE task and on the number of errors until criterion in the maze, treating maximal latencies for subjects that did not succeed (FE task) and maximal errors for subjects that did not reach criterion (Maze) as censored observations.
To determine whether an individual's performance in one task also predicted its performance in the other task, we used Spearman rank correlations for continuous measures of performance and Cohen's kappa coefficients for qualitative measures (success: yes/no in FE task, reached criterion: yes/no in Maze). We interpreted κ values according to Landis & Koch [52].
To assess the effect of subjects' cognitive performance on their BMI change from the rainy to the dry season, we fitted LMs with BMI change as response. For the FE task, we implemented solving time (log-transformed) as predictor and age and sex as control predictors. For the maze, we used the number of errors until criterion and the two control predictors. In both models, we first also tested the interactions between sex and performance measure and age class and performance measure. These interactions were not significant, but the full null model comparisons with the interactions and main effect removed were significant, and we therefore removed them from the model.
We used Cox proportional hazards models to fit the effect of cognitive performance, sex and age class on survival (in days). We implemented age class and sex as control predictors as these factors were previously shown to influence survival in mouse lemurs [40]. We fitted one model for the FE task with solving time (log-transformed) and sex and age, and another model for the maze with the number of errors until criterion and the two control predictors. Again, interactions between test performance and age class and test performance and sex were removed from the models as they did not significantly explain individual survival. For all models, prior to fitting, we z-transformed covariates to a mean of zero and a standard deviation of 1 to facilitate interpretation of predictor estimates [53]. We checked the model assumptions ‘absence of collinearity’ using variance inflation factors [54] (package car in R [55]) and ‘absence of influential observations' using dfbetas in all models (package survminer in R for Cox models [56]). We controlled for the effect of potential outliers/influential cases by comparing model results fitted with and without these observations but retained the complete dataset in all models. For LMs, we visually checked normally distributed and homogeneous residuals and absence of overdispersion for the GLMM. For the Cox proportional hazards models, we checked the violation of proportional hazards. We always tested our full model against a null model containing the intercept-only or just control predictors with an F-test for general LMs and a likelihood ratio test for GLM, GLMM and Cox models. All analyses were conducted in R, v. 3.4.2 [57]. The level of significance was set at 0.05.
3. Results
(a). Inter-individual variation in test performance
(i). Food extraction task
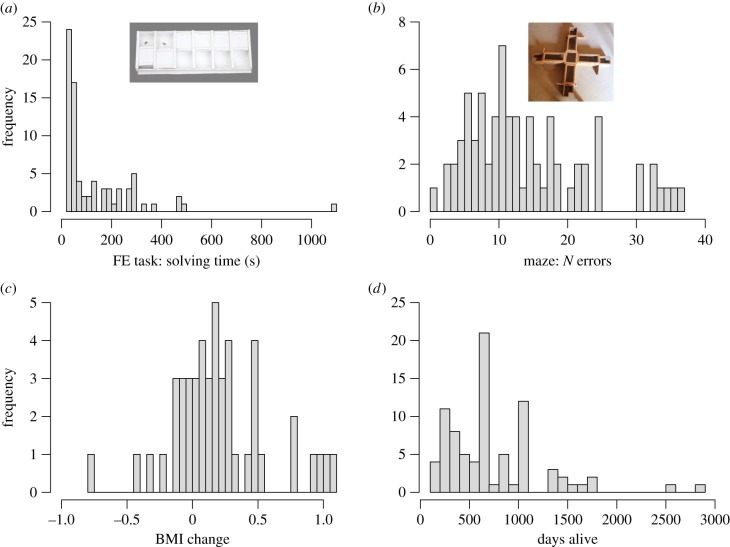
Overall, 88% of 96 subjects successfully solved the FE task, i.e. they opened at least one of the six lids. Subjects varied in the total number of lids opened (mean ± s.d. = 4.6 ± 2.15; CV = 46.74), their latency until the first success (mean ± s.d. = 207 ± 325 s; CV = 156.88) and solving time per successful opening after the first success (mean ± s.d. = 134 ± 161 s; CV = 120.53, figure 1a). An individual's BMI predicted its probability to open at least one lid (full null model comparison: X2= 9.63, d.f. = 2, p = 0.008; estimate ± s.d. = −1.13 ± 0.41, z = −2.77, p = 0.006, N = 96; electronic supplementary material, table S3), and also the total number of successes (full null model comparison: X2 = 6.73, d.f. = 2, p = 0.035; estimate ± s.e. = −2.26 ± 0.77, z = −2.94, p = 0.003, N = 94; electronic supplementary material, table S4): subjects with a lower BMI were more likely to solve the problem and opened more lids than subjects with a higher BMI. Moreover, subjects with a lower BMI had shorter latencies until first success (full null model comparison: X2 = 9.17, d.f. = 2, p = 0.010, estimate ± s.e. = −0.35 ± 0.12, z = −3.03, p = 0.002, N = 96; electronic supplementary material, table S5), but solving time per successful opening after the first success was not influenced by BMI (full null model comparison: F2,73 = 0.43, p = 0.655, estimate ± s.e. = 0.11 ± 0.12, t = 0.92, p = 0.359, N = 76; electronic supplementary material, table S6). Significantly more juveniles than adults were successful (proportion test, X2 = 7.7, d.f. = 1, p < 0.01, N = 97). Sex had no influence on any measure of performance in the FE task (probability of success: estimate ± s.e. = −0.93 ± 0.82, z = −1.13, p = 0.258, N = 96; electronic supplementary material, table S3; number of successes: estimate ± s.e. = −1.10 ± 1.38, z = −0.80, p = 0.423, N = 94; electronic supplementary material, table S4; latency first success: estimate ± s.e. = −0.28 ± 0.23, z = −1.19, p = 0.234, N = 96; electronic supplementary material, table S5; solving time: estimate ± s.e. = 0.10 ± 0.24, t = 0.43, p = 0.667, N = 76; electronic supplementary material, table S6).
Figure 1.
Inter-individual variation in performance in two cognitive tests and two fitness proxies. Depicted are histograms of the two main cognitive measures, (a) solving time of the FE task and (b) number of errors made until the learning criterion in the maze, and the two fitness proxies, (c) BMI change and (d) days alive. (Online version in colour.)
(ii). Maze
In the maze, 71% of 73 subjects reached the learning criterion within the 15 test trials. Individuals varied in their number of errors until reaching the learning criterion (mean ± s.d. = 14.24 ± 8.97; CV = 63.00, figure 1b), but juveniles and adults did not differ in their ability to reach the criterion (proportion test, X2 = 0.01, d.f. = 1, p = 0.95, N = 73). BMI and sex did not influence performance and learning in the maze (probability of reaching criterion: full null model comparison: X2 = 1.68, d.f. = 2, p = 0.431, BMI: estimate ± s.e. = −0.17 ± 0.28, z = −0.59, p = 0.558, sex: estimate ± s.e. = −0.73 ± 0.58, z = 1.26, p = 0.209, N = 73; electronic supplementary material, table S7; number of errors: full null model comparison: X2 = 0.81, d.f. = 2, p = 0.667, BMI: estimate ± s.e. = −0.09 ± 0.13, z = −0.71, p = 0.479, sex: estimate ± s.e. = −0.20 ± 0.82, z = −0.69, p = 0.49, N = 73; electronic supplementary material, table S8).
Individuals’ performance in the FE task and learning in the maze did not correlate between any performance measures (electronic supplementary material, table S9 and figure S3). However, there was a tendency for successful animals in the FE task to be more likely to reach the learning criterion in the maze (Cohen's κ = 0.019, N = 71; electronic supplementary material, table S9).
(iii). Relationship between test performance and fitness proxies
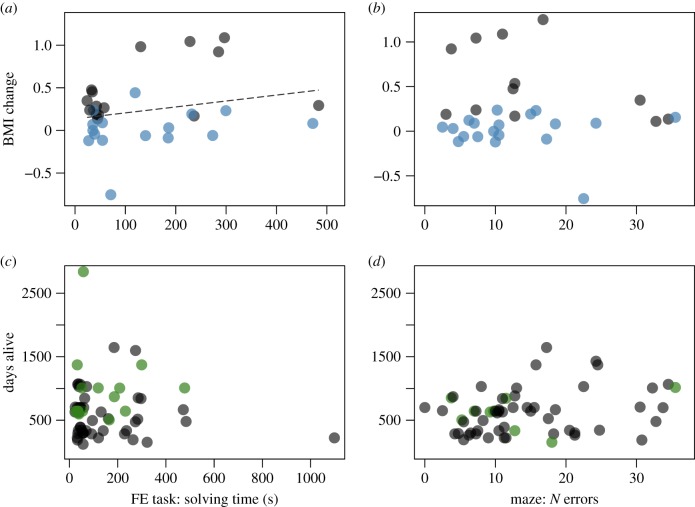
Grey mouse lemurs varied in the two fitness proxies: BMI change during the dry season (mean ± s.d.= 0.21 ± 0.37; CV = 176.19, figure 1c) and survival (mean ± s.d. = 750.8 ± 499.1 days; CV = 66.48, figure 1d). Individuals' solving time, the measure of performance in the FE task that was not affected by body condition during the time of testing, predicted BMI change (full null model comparison: F1,27 = 4.742, p = 0.038). Animals that were slower in opening the lids after mastering it for the first time lost more body mass during the dry season (estimate ± s.e. = 0.12 ± 0.05, t = 2.18, p = 0.038, figure 2a; electronic supplementary material, table S10). Moreover, BMI change was also affected by sex (females lost more body mass than males (estimate ± s.e. = −0.48 ± 0.11, t = −4.35, p < 0.001; electronic supplementary material, table S9), but not by age (estimate ± s.e. = −0.01 ± 0.11, t = −0.13, p = 0.900, N = 31; electronic supplementary material, table S10). Subjects’ number of errors in the maze did not significantly predict their BMI change (full null model comparison: F1,27 = 3.88, p = 0.059), but there was a trend for animals that made more errors in the maze to experience a smaller change in BMI (estimate ± s.e. = −0.12 ± 0.06, t = −1.97, p = 0.059, N = 31, figure 2b; electronic supplementary material, table S10).
Figure 2.
Relationship between BMI change and survival (number of days alive) and the cognitive performance measures, (a,c) solving time in the FE task and (b,d) number of errors in the maze. (a,b) BMI change: blue, males; grey, females; a positive BMI change corresponds to a decrease in BMI during the dry season, negative values reflect an increase in BMI from rainy to the end of dry season. (c,d) Survival: green, censored days alive for animals that are still alive; grey, dead animals. (Online version in colour.)
Subjects’ probability of survival was not predicted by their solving efficiency in the FE task (solving time: estimate ± s.e. = 0.09 ± 0.15, z = 0.62, p = 0.534, N = 64, figure 2c; electronic supplementary material, table S11), whereas age class and sex predicted survival (full null model comparison: likelihood ratio test: X2 = 25.97, d.f. = 3, p < 0.001). Specifically, juveniles and females had lower survival probabilities (age class: estimate ± s.e. = 1.87 ± 0.44, z = 4.28, p < 0.001; sex: estimate ± s.e. = −0.72 ± 0.31, z = −2.35, p = 0.019; electronic supplementary material, table S11). Mouse lemurs' survival probability was also not predicted by the number of errors they made in the spatial learning task (estimate ± s.e. = −0.04 ± 0.16, z = −0.23, p = 0.824, N = 62, figure 2d; electronic supplementary material, table S12).
4. Discussion
(a). Individual variation in test performance
Our study contributes the first data on the cognition–fitness link for primates and established the feasibility of conducting cognitive tests with wild individuals during short-term captivity. We found that individual mouse lemurs varied in the chosen fitness proxies and in the measures of test performance in the two tasks, which is an important prerequisite for linking performance with fitness outcomes. Individuals’ performance in the two tasks did not correlate, suggesting that there is no general factor underpinning performance in the two tasks, which presumably address different cognitive abilities (cf. [58]). Importantly, solving time in the FE task and the number of errors in the maze were not affected by a subject's body condition, age or sex. Thus, we attempted to minimize the confounding effect of non-cognitive factors on individual test performance by linking variation in these performance measures with our fitness proxies in the second part of the study.
Inter-individual variation in test performance can be due to differences in cognitive abilities, but also to variation in motivation, personality, sex and age [2,9]. Especially, problem-solving tasks have been criticized as a measure of cognitive performance because differences in test performance might also be caused by variation in neophobia, persistence and prior experience or simply by chance ([10,59], reviewed in [60]), and because the specific cognitive processes underlying problem-solving are not well defined [9,61]. We attempted to address this issue by testing the influence of several non-cognitive factors on test performance (see below), and by using a problem-solving design that allowed to test the repeated solving of the novel problem [10]. Thus, we not only measured performance during the criticized initial innovative problem-solving, but also solving efficiency after the first successful opening of the artificial feeding box. Subjects with a low solving time efficiently and quickly opened the lids repeatedly after the first discovery of the novel solution and we suggest that they were able to do so because they quickly learned the new motor actions and associated them with the reward (cf. [62]; electronic supplementary material, figure S4).
Performance in problem-solving and other cognitive tasks can also be impacted by dimensions of individual personality, such as persistence, willingness to approach novel objects and speed to explore environments ([63,64], reviewed in [2]). To control for these potential effects, we assessed neophilia in a novel object task and general activity as well as exploration in an open field task. Details of these tests are beyond the scope of the present analysis and reported elsewhere [65]. The two cognitive performance measures (solving time in the FE task and number of errors in the maze) in the present study were not affected by these personality traits, however [65]. Thus, variation in these personality measures does not predict inter-individual variation in the measures of test performance in our study and are unlikely to mediate the correlation between solving efficiency in the FE task and body condition change as fitness proxy.
Moreover, when testing animals in food-rewarded tasks, controlling for motivation is equally important albeit difficult to operationalize. Differences in feeding motivation can be reduced in captivity by controlling access to food or water during a certain time window before testing animals, but this level of control cannot be achieved with wild animals. However, body condition may present a good proxy for the energetic state of wild individuals and, hence, their motivation to feed in the experiment. In line with the idea that ‘necessity drives innovation’ suggesting that young, low-ranking individuals in poorer body condition are more likely to innovate ([66,67] but see [60,68]), in the FE task, the initial and total number of successes, as well as first success latency, were indeed affected by body condition at the time of testing, which differed widely between juveniles and adults. Juvenile mouse lemurs, which were tested during an important period of growth, had a lower BMI and appeared to be more motivated to solve the FE task than adults, which accumulated fat in the rainy season prior to testing. Yet, within a given age class, variation in BMI had no effect on test performance (F Huebner, C Fichtel, PM Kappeler 2018, unpublished data). In contrast to mammals, birds are limited in how much fat they can store [69], and motivation to feed (e.g. feeding latencies prior to testing [70]), but not body condition [60,71] had an effect on problem-solving performance. Thus, lineage-specific constraints need to be considered and more comparative data are required for a more general assessment of the links between body condition and motivation in cognitive tasks.
(b). Cognitive test performance and body mass index change
Changes in BMI across the austral winter should reflect the ability of grey mouse lemurs to cope with the energetic challenges of a long cool dry season with reduced food availability [45,72]. Individuals exhibiting greater reduction in BMI lost disproportionately more fat reserves, indicating that they used more and/or acquired less energy than others between subsequent measures. Body condition at the end of the dry season is functionally relevant because it influences male mating success [42] and females' mating strategies [73]. Hence, this measure may also be meaningful for other small mammals or species experiencing strong environmental seasonality.
In our study, solving time in the FE task predicted BMI change, indicating a link between this specific measure of efficient, repeated problem-solving and a fitness proxy. While necessity and motivation might drive initial innovations [70], after the initial discovery, associative learning and efficient reapplication of the new motor actions, as, for example, a novel behaviour to exploit new food resources, is crucial [60]. Especially under conditions where food resources are ephemeral, unpredictable and only seasonally available, innovation and efficient and swift associative learning of novel motor actions can be beneficial, as has been shown for several bird species [74,75]. A previous field experiment with our study population revealed that mouse lemurs rapidly exploited new artificial feeding resources and swiftly learned changes in spatial arrangements [32], suggesting that innovative foraging might be ecologically meaningful also under natural conditions.
Performance in the maze was not linked to BMI change in this study. We chose this test because we expected a positive correlation between an animal's ability to remember a food location in the maze and its ability to remember and find natural food resources, which, in turn, should affect body mass dynamics. Failure to demonstrate this link could be due to two reasons. First, females hibernate for several months during the dry season, whereas males only enter short daily torpor bouts [72,76]. Thus, remembering food locations may not be subject to strong selection in females. By contrast, males feed on tree gum and sugary secretions of colonial invertebrates during the lean dry season, which are both patchily distributed, so that remembering the location of these food resources might be beneficial. Despite this sex difference in natural foraging ecology, the effect of test performance on BMI change did not differ between males and females, however. Second, variation in motivation and explorative behaviour might have influenced the number of errors in the maze. There was indeed a trend indicating that subjects making more errors in the maze experienced smaller BMI changes. However, this trend could not be explained by the current BMI, our proxy for feeding motivation. Also, subjects were highly motivated to participate in all food-rewarded tasks, and we never observed any animal rejecting offered food. Unfortunately, we could not control for individual variation in exploratory behaviour during the trials in the maze. Imposing a cost for exploring the environment, as, for example, in the Morris water maze [77], might allow to evaluate this possibility in a future study.
(c). Cognitive test performance and survival
Grey mouse lemur's solving time in the FE task or spatial learning performance in the maze did not predict their subsequent survival in the current study. One possible explanation for our failure to find a relationship between these performance measures and survival might involve a lack of statistical power, even though our sample sizes were larger than those in most previous studies of primate cognition. However, we found a significant correlation between BMI change and performance in the FE task, and several recent studies with even smaller sample sizes could demonstrate a link between cognitive measures and fitness proxies (electronic supplementary material, table S1). Thus, it is possible that mouse lemurs' survival might not be predicted by the specific cognitive abilities addressed here.
While the two tests measure cognitive performances that ought to impact survival via body condition, mouse lemur survival is probably impacted more profoundly by predation risk. Among primates, mouse lemurs are exposed to one of the highest predation rates [78] and are preyed upon by various carnivores, owls, snakes and even another lemur species (reviewed in [79]). Predator avoidance has been shown to be linked with survival in striped mice: female survival was predicted by a faster response to predator stimuli, and male survival covaried positively with better long-term spatial memory of shelter locations. By contrast, female striped mice that made more errors in a maze testing short-term memory survived longer, and overall survival was not linked to performance in the spatial memory task [21], indicating that even when linking predator avoidance performance with survival, the direction of these links are not necessarily as predicted. Because grey mouse lemurs are nocturnal, certain anti-predator tactics, such as vigilance and subsequent fleeing to a distant shelter, are not effective [79]. Instead, grey mouse lemurs tend to freeze after detecting a predator [37], a behaviour that is more difficult to address in a laboratory cognitive task. Thus, a species’ sensory ecology and their actual specific behaviours in fitness-relevant contexts need to be taken into account when choosing appropriate cognitive tests and fitness proxies [3].
5. Conclusions
Our study indicates that links between experimental measures of cognitive test performance and fitness proxies of wild animals are not necessarily direct and easy to assess and interpret. It is essential to appreciate a species' life history and ecology in studying how selection shapes certain cognitive abilities, not only with regard to study design, but also with respect to the complex interactions among cognitive performance and confounding factors like personality, motivation, age and sex differences. Similarly, fitness proxies have been notoriously difficult to measure in behavioural ecology, especially when egg-counting is not an option, and this and most other mammal species offer examples for the practical challenges of identifying and operationalizing meaningful fitness proxies. Thus, more comprehensive study designs than bivariate correlations will be required in the long term to broaden our understanding of the evolutionary mechanisms underlying species-specific adaptations in cognitive abilities and their intra-specific variation.
Supplementary Material
Supplementary Material
Acknowledgements
We are very thankful to Bruno Tsiverimana, Léonard Razamanantsoa and all other members of the Kirindy research station team for their support in the field. Moreover, we thank Henning Lahmann for the administration of the long-term data, Lynne Werner for scoring videos for the inter-observer reliability and Roger Mundry for statistical advice. Finally, we thank Alex Thornton and two anonymous reviewers for their constructive comments.
Ethics
All research reported here is in compliance with animal care regulations and applicable national laws of Germany and Madagascar. The Ministry for the Environment, Water and Forests of Madagascar, MINEEF and CNFEREF Morondava authorized research in Kirindy, and our research was approved by the relevant German Animal Use and Care committees.
Data accessibility
The dataset supporting this article has been uploaded as part of the electronic supplementary material.
Authors' contributions
P.M.K., C.F. and F.H. conceived this study, F.H. conducted fieldwork, analysed the data, and wrote the paper together with C.F. and P.M.K. All authors approved the final version of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This study was funded by the Deutsche Forschungsgemeinschaft (awarded to P.M.K.: Ka 1082/33-1).
References
- 1.Shettleworth SJ. 2010. Cognition, evolution and behaviour, 2nd edn. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Morand-Ferron J, Cole EF, Quinn JL. 2015. Studying the evolutionary ecology of cognition in the wild: a review of practical and conceptual challenges. Biol. Rev. 91, 367–389. ( 10.1111/brv.12174) [DOI] [PubMed] [Google Scholar]
- 3.Cauchoix M, Chaine AS. 2016. How can we study the evolution of animal minds? Front. Psychol. 7, 358 ( 10.3389/fpsyg.2016.00358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plomin R, Deary IJ. 2014. Genetics and intelligence differences: five special findings. Mol. Psychiatry 20, 98–108. ( 10.1038/mp.2014.105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raine NE, Chittka L. 2008. The correlation of learning speed and natural foraging success in bumble-bees. Proc. R. Soc. B 275, 803–808. ( 10.1098/rspb.2007.1652) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans LJ, Smith KE, Raine NE. 2017. Fast learning in free-foraging bumble bees is negatively correlated with lifetime resource collection. Sci. Rep. 7, 496 ( 10.1038/s41598-017-00389-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mery F, Kawecki TJ. 2003. A fitness cost of learning ability in Drosophila melanogaster. Proc. R. Soc. Lond. B 270, 2465–2469. ( 10.1098/rspb.2003.2548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith C, Philips A, Reichard M. 2015. Cognitive ability is heritable and predicts the success of an alternative mating tactic. Proc. R. Soc. B 282, 20151046 ( 10.1098/rspb.2015.1046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rowe C, Healy SD. 2014. Measuring variation in cognition. Behav. Ecol. 25, 1287–1292. ( 10.1093/beheco/aru090) [DOI] [Google Scholar]
- 10.Thornton A, Isden J, Madden JR. 2014. Toward wild psychometrics: linking individual cognitive differences to fitness. Behav. Ecol. 25, 1299–1301. ( 10.1093/beheco/aru095) [DOI] [Google Scholar]
- 11.Cole EF, Morand-Ferron J, Hinks AE, Quinn JL. 2012. Cognitive ability influences reproductive life history variation in the wild. Curr. Biol. 22, 1808–1812. ( 10.1016/j.cub.2012.07.051) [DOI] [PubMed] [Google Scholar]
- 12.Cauchard L, Boogert NJ, Lefebvre L, Dubois F, Doligez B. 2013. Problem-solving performance is correlated with reproductive success in a wild bird population. Anim. Behav. 85, 19–26. ( 10.1016/j.anbehav.2012.10.005) [DOI] [Google Scholar]
- 13.Preiszner B, Papp S, Pipoly I, Seress G, Vincze E, Liker A, Bokony V. 2016. Problem-solving performance and reproductive success of great tits in urban and forest habitats. Anim. Cogn. 20, 53–63. ( 10.1007/s10071-016-1008-z) [DOI] [PubMed] [Google Scholar]
- 14.Wetzel DP. 2017. Problem-solving skills are linked to parental care and offspring survival in wild house sparrows. Ethology 123, 475–483. ( 10.1111/eth.12618) [DOI] [Google Scholar]
- 15.Keagy J, Savard J-F, Borgia G. 2009. Male satin bowerbird problem-solving ability predicts mating success. Anim. Behav. 78, 809–817. ( 10.1016/j.anbehav.2009.07.011) [DOI] [Google Scholar]
- 16.Keagy J, Savard J-F, Borgia G. 2011. Cognitive ability and the evolution of multiple behavioral display traits. Behav. Ecol. 23, 448–456. ( 10.1093/beheco/arr211) [DOI] [Google Scholar]
- 17.Isden J, Panayi C, Dingle C, Madden J. 2013. Performance in cognitive and problem-solving tasks in male spotted bowerbirds does not correlate with mating success. Anim. Behav. 86, 829–838. ( 10.1016/j.anbehav.2013.07.024) [DOI] [Google Scholar]
- 18.Boogert NJ, Anderson RC, Peters S, Searcy WA, Nowicki S. 2011. Song repertoire size in male song sparrows correlates with detour reaching, but not with other cognitive measures. Anim. Behav. 81, 1209–1216. ( 10.1016/j.anbehav.2011.03.004) [DOI] [Google Scholar]
- 19.Madden JR, Langley EJG, Whiteside MA, Beardsworth CE, van Horik JO. 2018. The quick are the dead: pheasants that are slow to reverse a learned association survive for longer in the wild. Phil. Trans. R. Soc. B 373, 20170297 ( 10.1098/rstb.2017.0297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashton BJ, Ridley AR, Edwards EK, Thornton A. 2018. Cognitive performance is linked to group size and affects fitness in Australian magpies. Nature 554, 364–367. ( 10.1038/nature25503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maille A, Schradin C. 2016. Survival is linked with reaction time and spatial memory in African striped mice. Biol. Lett. 12, 20160346 ( 10.1098/rsbl.2016.0346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ten Cate C. 2014. Towards fruitful interaction between behavioral ecology and cognitive science: a comment on Rowe and Healy. Behav. Ecol. 25, 1295–1296. ( 10.1093/beheco/aru121) [DOI] [Google Scholar]
- 23.Kotrschal A, Rogell B, Bundsen A, Svensson B, Zajitschek S, Brännström I, Immler S, Maklakov AA, Kolm N. 2013. Artificial selection on relative brain size in the guppy reveals costs and benefits of evolving a larger brain. Curr. Biol. 23, 168–171. ( 10.1016/j.cub.2012.11.058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cauchoix M, et al. 2018. The repeatability of cognitive performance: a meta-analysis. Phil. Trans. R. Soc. B 373, 20170281 ( 10.1098/rstb.2017.0281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thornton A, Lukas D. 2012. Individual variation in cognitive performance: developmental and evolutionary perspectives. Phil. Trans. R. Soc. B 367, 2773–2783. ( 10.1098/rstb.2012.0214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byrne RW, Whiten A. 1988. Machiavellian intelligence: social expertise and the evolution of intelligence in monkeys, apes and humans. Oxford, UK: Oxford University Press. [Google Scholar]
- 27.Dunbar RI. 1998. The social brain hypothesis. Evol. Anthropol. 6, 178–190. ( 10.1002/(SICI)1520-6505(1998)6:5%3C178::AID-EVAN5%3E3.0.CO;2-8) [DOI] [Google Scholar]
- 28.Reader SM, Laland KN. 2002. Social intelligence, innovation, and enhanced brain size in primates. Proc. Natl. Acad. Sci. USA 99, 4436–4441. ( 10.1073/pnas.062041299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deaner RO, van Schaik CP, Johnson V. 2006. Do some taxa have better domain-general cognition than others? A meta-analysis of nonhuman primate studies. Evol. Psychol. 4, 147470490600400 ( 10.1177/147470490600400114) [DOI] [Google Scholar]
- 30.Dunbar RI, Shultz S. 2007. Understanding primate brain evolution. Phil. Trans. R. Soc. B 362, 649–658. ( 10.1098/rstb.2006.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reader SM, Hager Y, Laland KN. 2011. The evolution of primate general and cultural intelligence. Phil. Trans. R. Soc. B 366, 1017–1027. ( 10.1098/rstb.2010.0342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lührs M-L, Dammhahn M, Kappeler PM, Fichtel C. 2009. Spatial memory in the grey mouse lemur (Microcebus murinus). Anim. Cogn. 12, 599–609. ( 10.1007/s10071-009-0219-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van de Waal E, Borgeaud C, Whiten A. 2013. Potent social learning and conformity shape a wild primate's foraging decisions. Science 340, 483–485. ( 10.1126/science.1232769) [DOI] [PubMed] [Google Scholar]
- 34.Huebner F, Fichtel C. 2015. Innovation and behavioral flexibility in wild redfronted lemurs (Eulemur rufifrons). Anim. Cogn. 18, 777–787. ( 10.1007/s10071-015-0844-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacLean EL, Barrickman NL, Johnson EM, Wall CE. 2009. Sociality, ecology, and relative brain size in lemurs. J. Hum. Evol. 56, 471–478. ( 10.1016/j.jhevol.2008.12.005) [DOI] [PubMed] [Google Scholar]
- 36.Dammhahn M, Kappeler PM. 2008. Small-scale coexistence of two mouse lemur species (Microcebus berthae and M. murinus) within a homogeneous competitive environment. Oecologia 157, 473–483. ( 10.1007/s00442-008-1079-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahlfs M, Fichtel C. 2010. Anti-predator behaviour in a nocturnal primate, the grey mouse lemur (Microcebus murinus). Ethology 116, 429–439. ( 10.1111/j.1439-0310.2010.01756.x) [DOI] [Google Scholar]
- 38.Schmid J, Kappeler PM. 1998. Fluctuating sexual dimorphism and differential hibernation by sex in a primate, the gray mouse lemur (Microcebus murinus). Behav. Ecol. Sociobiol. 43, 125–132. ( 10.1007/s002650050474) [DOI] [Google Scholar]
- 39.Roth TC, LaDage LD, Pravosudov VV. 2010. Learning capabilities enhanced in harsh environments: a common garden approach. Proc. R. Soc. B 277, 3187–3193. ( 10.1098/rspb.2010.0630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kraus C, Eberle M, Kappeler PM. 2008. The costs of risky male behaviour: sex differences in seasonal survival in a small sexually monomorphic primate. Proc. R. Soc. B 275, 1635–1644. ( 10.1098/rspb.2008.0200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hämäläinen A, Dammhahn M, Aujard F, Eberle M, Hardy I, Kappeler PM, Perret M, Schliehe-Diecks S, Kraus C. 2014. Senescence or selective disappearance? Age trajectories of body mass in wild and captive populations of a small-bodied primate. Proc. R. Soc. B 281, 20140830 ( 10.1098/rspb.2014.0830) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eberle M, Kappeler PM. 2004. Sex in the dark: determinants and consequences of mixed male mating tactics in Microcebus murinus, a small solitary nocturnal primate. Behav. Ecol. Sociobiol. 57, 77–90. ( 10.1007/s00265-004-0826-1) [DOI] [Google Scholar]
- 43.Kappeler PM, Fichtel C. 2012. A 15-year perspective on the social organization and life history of sifaka in Kirindy Forest. In Long-term field studies of primates (eds Kappeler PM, Watts DP), pp. 101–121. Berlin, Germany: Springer. [Google Scholar]
- 44.Eberle M, Kappeler P. 2002. Mouse lemurs in space and time: a test of the socioecological model. Behav. Ecol. Sociobiol. 51, 131–139. ( 10.1007/s002650100409) [DOI] [Google Scholar]
- 45.Dammhahn M, Kappeler PM. 2008. Comparative feeding ecology of sympatric Microcebus berthae and M. murinus. Int. J. Primatol. 29, 1567–1589. ( 10.1007/s10764-008-9312-3) [DOI] [Google Scholar]
- 46.Friard O, Gamba M. 2016. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 7, 1325–1330. ( 10.1111/2041-210X.12584) [DOI] [Google Scholar]
- 47.Schulte-Hostedde AI, Zinner B, Millar JS, Hickling GJ. 2005. Restitution of mass-size residuals: validating body condition indices. Ecology 86, 155–163. ( 10.1890/04-0232) [DOI] [Google Scholar]
- 48.Rasoloarison RM, Goodman SM, Ganzhorn JU. 2000. Taxonomic revision of mouse lemurs (Microcebus) in the western portions of Madagascar. Int. J. Primatol. 21, 963–1019. ( 10.1023/a:1005511129475) [DOI] [Google Scholar]
- 49.Schliehe-Diecks S, Eberle M, Kappeler PM. 2012. Walk the line—dispersal movements of gray mouse lemurs (Microcebus murinus). Behav. Ecol. Sociobiol. 66, 1175–1185. ( 10.1007/s00265-012-1371-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 51.Therneau T. 2015. A package for survival analysis in S. R package version 2.38.
- 52.Landis JR, Koch GG. 1977. The measurement of observer agreement for categorical data. Biometrics 33, 159 ( 10.2307/2529310) [DOI] [PubMed] [Google Scholar]
- 53.Schielzeth H. 2010. Simple means to improve the interpretability of regression coefficients. Methods Ecol. Evol. 1, 103–113. ( 10.1111/j.2041-210x.2010.00012.x) [DOI] [Google Scholar]
- 54.Fox J, Monette G. 1992. Generalized collinearity diagnostics. J. Am. Stat. Assoc. 87, 178–183. ( 10.1080/01621459.1992.10475190) [DOI] [Google Scholar]
- 55.Fox J, Weisberg S. 2011. An {R} companion to applied regression, 2nd edn. Thousand Oaks, CA: Sage; R package version 2.1.4. [Google Scholar]
- 56.Kassambara A, Kosinski M. 2017. Survminer: Drawing survival curves using ‘ggplot2’. R package version 0.4.0.
- 57.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 58.Shaw RC, Schmelz M. 2017. Cognitive test batteries in animal cognition research: evaluating the past, present and future of comparative psychometrics. Anim. Cogn. 20, 1003–1018. ( 10.1007/s10071-017-1135-1) [DOI] [PubMed] [Google Scholar]
- 59.van Horik JO, Madden JR. 2016. A problem with problem solving: motivational traits, but not cognition, predict success on novel operant foraging tasks. Anim. Behav. 114, 189–198. ( 10.1016/j.anbehav.2016.02.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Griffin AS, Guez D. 2014. Innovation and problem solving: a review of common mechanisms. Behav. Processes 109, 121–134. ( 10.1016/j.beproc.2014.08.027) [DOI] [PubMed] [Google Scholar]
- 61.Healy SD. 2012. Animal cognition: the trade-off to being smart. Curr. Biol. 22, R840–R841. ( 10.1016/j.cub.2012.08.032) [DOI] [PubMed] [Google Scholar]
- 62.Griffin AS, Diquelou M, Perea M. 2014. Innovative problem solving in birds: a key role of motor diversity. Anim. Behav. 92, 221–227. ( 10.1016/j.anbehav.2014.04.009) [DOI] [Google Scholar]
- 63.Carere C, Locurto C. 2011. Interaction between animal personality and animal cognition. Curr. Zool. 57, 491–498. ( 10.1093/czoolo/57.4.491) [DOI] [Google Scholar]
- 64.Dougherty L, Guillette LM. 2018. Linking personality and cognition: a meta-analysis. Phil. Trans. R. Soc. B 373, 20170282 ( 10.1098/rstb.2017.0282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huebner F, Fichtel C, Kappeler PM. 2018. In preparation. [Google Scholar]
- 66.Reader SM, Laland KN. 2003. Animal innovation: an introduction. In Animal innovation (eds Reader SM, Laland KN), pp. 3–35. Oxford, UK: Oxford University Press. [Google Scholar]
- 67.Clayton NS. 2004. Is necessity the mother of innovation? Trends Cogn. Sci. 8, 98–99. ( 10.1016/j.tics.2004.01.001) [DOI] [Google Scholar]
- 68.Reader SM, Laland KN. 2001. Primate innovation: sex, age and social rank differences. Int. J. Primatol. 22, 787–805. ( 10.1023/A:1012069500899) [DOI] [Google Scholar]
- 69.Witter MS, Cuthill IC. 1993. The ecological costs of avian fat storage. Phil. Trans. R. Soc. B 340, 73–92. ( 10.1098/rstb.1993.0050) [DOI] [PubMed] [Google Scholar]
- 70.Sol D, Griffin AS, Bartomeus I. 2012. Consumer and motor innovation in the common myna: the role of motivation and emotional responses. Anim. Behav. 83, 179–188. ( 10.1016/j.anbehav.2011.10.024) [DOI] [Google Scholar]
- 71.Shaw RC. 2017. Testing cognition in the wild: factors affecting performance and individual consistency in two measures of avian cognition. Behav. Processes 134, 31–36. ( 10.1016/j.beproc.2016.06.004) [DOI] [PubMed] [Google Scholar]
- 72.Schmid J. 1999. Sex-specific differences in activity patterns and fattening in the Gray mouse lemur (Microcebus murinus) in Madagascar. J. Mammal. 80, 749–757. ( 10.2307/1383244) [DOI] [Google Scholar]
- 73.Huchard E, Canale CI, Le Gros C, Perret M, Henry P-Y, Kappeler PM. 2011. Convenience polyandry or convenience polygyny? Costly sex under female control in a promiscuous primate. Proc. R. Soc. B 279, 1371–1379. ( 10.1098/rspb.2011.1326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sol D, Duncan RP, Blackburn TM, Cassey P, Lefebvre L. 2005. Big brains, enhanced cognition, and response of birds to novel environments. Proc. Natl Acad. Sci. USA 102, 5460–5465. ( 10.1073/pnas.0408145102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sol D, Lefebvre L, Rodriguez-Teijeiro JD. 2005. Brain size, innovative propensity and migratory behaviour in temperate Palaearctic birds. Proc. R. Soc. B 272, 1433–1441. ( 10.1098/rspb.2005.3099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rasoazanabary E. 2006. Male and female activity patterns in Microcebus murinus during the dry season at Kirindy forest, Western Madagascar. Int. J. Primatol. 27, 437–464. ( 10.1007/s10764-006-9017-4) [DOI] [Google Scholar]
- 77.D'Hooge R, De Deyn PP. 2001. Applications of the Morris water maze in the study of learning and memory. Brain Res. Rev. 36, 60–90. ( 10.1016/s0165-0173(01)00067-4) [DOI] [PubMed] [Google Scholar]
- 78.Fichtel C. 2012. Predation. In The evolution of primate societies (eds Mitani JC, Call J, Kappeler P, Palombit R, Silk J), pp. 169–194. Chicago, IL: Chicago University Press. [Google Scholar]
- 79.Fichtel C. 2016. Predation in the dark: antipredator strategies of Cheirogaleidae and other nocturnal primates. In The dwarf and mouse lemurs of Madagascar. Biology, behavior and conservation biogeography of the Cheirogaleidae (eds Lehman S, Radespiel U, Zimmermann E), pp. 366–380. Cambridge, UK: Cambridge University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset supporting this article has been uploaded as part of the electronic supplementary material.