Abstract
Natural selection can act on between-individual variation in cognitive abilities, yet evolutionary responses depend on the presence of underlying genetic variation. It is, therefore, crucial to determine the relative extent of genetic versus environmental control of these among-individual differences in cognitive traits to understand their causes and evolutionary potential. We investigated heritability of associative learning performance and of a cognitive judgement bias (optimism), as well as their covariation, in a captive pedigree-bred population of red junglefowl (Gallus gallus, n > 300 chicks over 5 years). We analysed performance in discriminative and reversal learning (two facets of associative learning), and cognitive judgement bias, by conducting animal models to disentangle genetic from environmental contributions. We demonstrate moderate heritability for reversal learning, and weak to no heritability for optimism and discriminative learning, respectively. The two facets of associative learning were weakly negatively correlated, consistent with hypothesized trade-offs underpinning individual cognitive styles. Reversal, but not discriminative learning performance, was associated with judgement bias; less optimistic individuals reversed a previously learnt association faster. Together these results indicate that genetic and environmental contributions differ among traits. While modular models of cognitive abilities predict a lack of common genetic control for different cognitive traits, further investigation is required to fully ascertain the degree of covariation between a broader range of cognitive traits and the extent of any shared genetic control.
This article is part of the theme issue ‘Causes and consequences of individual differences in cognitive abilities’.
Keywords: affective state, animal cognition, cognitive judgement bias, heritability, learning, cognitive repeatability
1. Introduction
Cognition (i.e. how individuals perceive, process, store and act on environmental information [1]) is a defining feature of complex animals and has been the focus of much psychological, neurobiological and ethological research. Traditionally, cognitive abilities are investigated at a species level (e.g. comparative studies [2,3]), with between-individual variation being mainly disregarded as statistical noise [4]. More recently, however, individual cognitive abilities have come under focus [4], paralleling burgeoning interest in animal personality [5]. Importantly, if among-individual variation in cognitive abilities is associated with differences in fitness, cognitive traits will be under selection and may thus evolve given the presence of additive genetic variation and associated heritability [6,7].
Quantifying the heritability of cognitive traits thus represents a fundamental step for understanding the causes of individual variation in cognitive abilities, and for assessing their evolutionary potential [8,9]. Despite this, the number of studies investigating the genetics of cognitive traits is still limited, partly due to difficulty in meeting the demands for substantial sampling effort and the genetic information required (e.g. known relatedness). Moreover, since most research has used humans or a few laboratory strains of animals (reviewed in [6]), current understanding may be limited by a narrow taxonomic focus and biased towards study populations potentially suffering from founder effects, inbreeding and artificial selection. With this in mind, available estimates indicate moderate to high heritabilities within most cognitive domains (e.g. learning, memory and attention [6,8,10]). The highest values are typically provided by human studies of general cognitive ability ('g'), which represents the main dimension of covariation between cognitive traits ([11,12], but see [13–15]). However, whether other animals possess a general cognitive ability remains debated [4,16–19].
Evidence for the alternative view, that different cognitive domains are governed by distinct developmental processes and genetic mechanisms, and thereby may evolve independently under diverse selection pressures, has been found in non-human primates and birds (e.g. [16,19]). Thus, given the uncertainty still surrounding the genetic architecture of cognitive traits, a statistically robust approach entailing multivariate genetic analysis [20,21] is conducive to evaluating these two hypotheses. Notably, multivariate animal models allow estimation of additive genetic components, and associated heritabilities, for each cognitive trait, and also permit partitioning of pairwise phenotypic correlations into genetic and environmental components [11,22].
Learning has traditionally held a central place in cognition research due to its widespread taxonomic occurrence and its involvement in behavioural flexibility under variable environmental conditions [1]. Particularly, associative learning may have far-reaching fitness consequences, as it mediates adaptive individual responses to environmental contingencies [23]. Nonetheless, research on the heritability of associative learning has been largely limited to a few model species (e.g. honeybees [24], fruit flies, [25], reviewed by [23]). Importantly, associative learning includes distinct facets such as discriminative learning (i.e. the process by which animals learn to respond differently to different stimuli) and reversal learning (i.e. the extinction of a previously learnt association and the formation of a novel one [1]). Reversal learning is tightly linked to behavioural flexibility and typically associated with behavioural inhibition (i.e. impulse control [26]). Because discriminative and reversal learning may depend on different neural processes involving different brain regions [27–29], individual abilities in these facets of associative learning may not be positively correlated. Empirical research has, so far, provided mixed results. Some studies show a positive association between discriminative and reversal learning, consistent with a general underlying cognitive ability (e.g. [28,30–32]). Other studies indicate a lack of (e.g. [33]), or negative association between the two (e.g. [34,35]). The extent to which these disparate findings are due to different evolutionary history of species, or methodological differences between studies, is unresolved. While limited statistical power could explain a lack of association, evidence for a negative association between discriminative and reversal learning agrees with theoretical models predicting speed–accuracy trade-offs in information gathering and decision-making [36,37]. Speed–accuracy trade-offs may occur within-individuals (e.g. due to changes in cost of errors [36]) and among-individuals (e.g. [38]). In the latter case, individuals are predicted to exhibit different cognitive styles, associated with different behavioural types [35,37,39]. While empirical evidence provides some support for the existence of cognitive styles [40–42], studies investigating the extent of genetics versus environment in their control are, to our knowledge, lacking.
The interplay between learning and other cognitive traits may also involve trade-offs, which may be genetically mediated. Although this would have important evolutionary consequences, available evidence is limited [10]. Past research has mainly considered links between learning abilities, memory formation and problem-solving (e.g. [43,44]), while relationships with other cognitive domains have remained largely unexplored. Among these, judgement biases have received increasing attention over the past decade, particularly within the field of applied ethology and animal welfare [45,46]. Cognitive judgement biases are consistent deviations from an accurate judgement of situations [47] typically implied to reflect individual affective state (i.e. emotions or mood [45]). Optimism and pessimism are examples of judgement biases; optimistic individuals overestimate the chances that they will benefit from a situation, pessimistic individuals overestimate that the situation will have adverse consequences [46]. Judgement biases may arise from long-lasting effects of early life conditions [48] and be associated with personality traits (e.g. [49–51]). Theoretical models predict that judgement biases may constitute stable individual traits [47,49], with a heritable component, and therefore may respond to natural selection [47]. Interestingly, theory predicts that varying selection pressures associated with spatio-temporal environmental heterogeneity may lead to genetically based individual differences in both judgement biases and learning abilities [47,52]. Unpredictable environmental variation may select for either optimism or pessimism, depending on the extent of ecological variability and movements between habitat patches [52], and at the same time favour behavioural flexibility [53]. Thus, we may expect covariation between these cognitive domains. At a proximate level, variation in the monoaminergic systems (e.g. dopamine and serotonin) is associated with both learning performance [54,55] and judgement bias [56]. For instance, dopaminergic function is implicated in the establishment of stimulus–reward associations during learning and is positively associated with optimism in mammals [57,58], birds [59] and insects [56,60]. Nonetheless, inter-relationships between learning abilities and judgement biases are still largely unexplored (but see [61]). In particular, how reversal learning abilities may map onto among-individual differences in judgement, and if these traits may be under shared genetic control, is unclear.
Here we explore the inter-relationships between different cognitive traits and assess their underlying genetic components, using as a captive population of red junglefowl (Gallus gallus), the wild ancestor of the domestic chicken [62]. Specifically, we investigated: (i) the associations between individual performance across a discriminative learning-, a reversal learning- and judgement bias test and (ii) narrow-sense heritabilities of these three cognitive traits.
2. Methods
(a). Study population
We tested chicks (n > 300, 2013–2017) from a captive population of red junglefowl housed at Linköping University, pedigree bred since 2011 and spanning six generations (see electronic supplementary material, S1). To reduce the expected influence of maternal effects, all eggs were artificially incubated. To minimize environmental contribution to between-individual differences, all chicks were raised in a laboratory environment (for details, see [63–65]). Chicks were individually tagged, kept on a 12 L : 12 D cycle (7–19 local time) and observations were carried out 8–18.
(b). Associative learning
Learning tests followed earlier described work using the same population [63,64]. In short, all birds were tested alone, in arenas (46 × 36 × 18 cm, L × W × H). Cues consisted of coloured bowls (5 × 3 cm, Ø × H) and laminated cards (9 cm2) of the same colour (2013, blue and green; 2014–2017, black and white [63,64]). Before testing, chicks were familiarized with being alone in the arena [63,64]. Initially, chicks were encouraged to approach the cues by the observer. A chick was regarded to have made a choice if it moved towards a cue without help and had its head within 2 cm of it. Correct choices were rewarded with one-third of a mealworm placed inside the bowl. In 2013, chicks were allowed to eat the reward even if the unrewarded cue was chosen, while for 2014–2017 the set-up was refined and the chick was collected immediately after choosing the unrewarded cue. We statistically controlled for effects of these methodological differences (see §2c below). In addition, sub-analyses specific to each of the two study set-ups provided similar heritability estimates. A new ‘trial’ started immediately after a choice had been made. A test ‘session’ lasted for a maximum of 15 min and was terminated earlier if the chick had lost motivation, with an interval of at least 1 h between test sessions [64].
(i). Discriminative learning
At 3–6 days old, chicks were trained to discriminate between a rewarded and an unrewarded cue (2013: half of the birds were rewarded on blue and half on green; 2014: half of the birds were rewarded on black and half on white; 2015–2017: all were rewarded on white). In 2013, the side of the rewarded cue alternated between subsequent trials, while for 2014–2017 the test was refined and the side on which the reward was presented varied according to a predetermined, pseudorandom schedule. Chicks were categorized as having learnt the discrimination once they chose the rewarded cue five (for 2013) or six (for 2014–2017) consecutive times. Even with the less stringent criterion of five correct choices, the chance of putative learners being false positives is low (electronic supplementary material, S2). ‘Learning speed’ was measured as the total number of trials needed to reach learning criterion. Ten birds did not learn to discriminate between the two cues due to lack of motivation to engage in the test (e.g. trying to escape the test arena). These individuals were therefore removed from the sample and not analysed further.
(ii). Reversal learning
After passing the discriminative learning test, chicks took part in a reversal learning test at around 5–7 days of age. If more than 7 h had passed since the final discriminative learning session, the chick was exposed to a ‘refresh’ session in which it had to again reach the learning criterion, before continuing to the reversal learning test. This was done to ensure that the association between the previously learned cue and the reward was still salient before performing reversal learning. In the reversal learning test, the previously rewarded cue was unrewarded, while the previously unrewarded cue was rewarded [64]. For this test, birds were not helped by the observer. Learning criterion and learning speed were measured as described for discriminative learning (above). Twenty-five birds did not pass this test due to lack of motivation to engage in the test, and so were removed from the sample.
(iii). Cognitive judgement bias
In 2014–2017, at 12–13 days old, chicks were exposed to a judgement bias test (for further details, see [66]). Briefly, individuals were first given a ‘refresh’ version of the reversal learning test to confirm that the previously learnt association had not been extinguished. Immediately following the refresh, chicks were then presented with five different colour cues, one at the time and in a predetermined, pseudorandom order. The cues were the previously learnt white (‘positive’, i.e. rewarded) and black (‘negative’, i.e. unrewarded) cues, and three novel, unrewarded, grey cues ('ambiguous’), intermediate in colour between the black and white cues (25%white/75%black, 50%white/50%black, 75%white/25%black). Chicks that were more likely to approach ambiguous cues and had a shorter latency to do so were considered optimistic. Individuals were exposed to each type of ambiguous cue three times in 2014 and 2017 (i.e. nine ambiguous cues interspersed between 24 positive and negative cues) and twice in 2015–2016 (i.e. six ambiguous cues interspersed between 16 positive and negative cues), due to time constraints arising from other ongoing experiments. Whether the chick approached the cue (yes/no) and the latency to approach (in seconds) were recorded. Maximum time per trial was set to 30 s.
(c). Statistical analyses
All analyses were conducted in RStudio (v. 1.1.383).
We analysed factors affecting learning speed in discriminative and reversal learning, two measures of judgement bias (i.e. probability of, and latency to approach ambiguous cues) and their associations, using univariate and multivariate mixed models implemented in the statistical software ASREML-R [67]. Additive genetic variances and corresponding heritabilities were estimated using a standard animal model approach by including individual genetic merit as a random effect and using the inverse of the pedigree-derived additive genetic relatedness matrix (see [22]; electronic supplementary material, S3 gives a brief overview of this approach and its advantages over classical techniques). For measures with repeated individual observations (i.e. judgement bias), we fitted a random permanent environment effect ('pe') as well as the additive genetic merit (G). Significance of heritability estimates was assessed via likelihood ratio tests (LRT). Fixed effects for each trait (described below) were selected based on the results of previous studies on the same population (e.g. [63–65]). Categorical factors were numerically coded by n − 1 (n = number of levels of the factor) dummy (0/1) variables. To aid model interpretation and numerical convergence, all predictors were centred by subtracting population mean values, and continuous variables were standardized by dividing centred values by twice their standard deviation. Correlation between individual learning speed in the discriminative and reversal tests was evaluated by calculation of Spearman's rank order correlation coefficient. Pairwise associations between each learning speed and individual judgement bias were estimated from bivariate mixed models (see below).
(i). Discriminative and reversal learning
Learning speeds in the discriminative and reversal tests were analysed separately, following log-transformation to achieve normality, by conducting animal models (Gaussian distribution and identity link function; see electronic supplementary material, S3-M1,2 for model syntax) to allow estimation of heritabilities (h2). ‘Sex’ (male and female) and the colour of the rewarded cue ('cue type') were included as fixed effects. Because cue type was associated with year (i.e. 2013: green/blue, 2014–2017: black/white), inclusion of cue type (four-level factor) as a fixed effect allowed us to control for the effect of methodological differences between the first and subsequent years (electronic supplementary material, S4). Excluding data from the first study year yielded virtually identical heritability estimates.
(ii). Cognitive judgement bias
Since in many trials chicks did not approach within the given 30 s period, approach latencies constituted a censored variable with a neat bimodal distribution. We therefore analysed two measures of individuals' responses in the judgement bias test: (i) approach probability and (ii) approach latency to cues, if an approach had occurred.
We first considered responses to all the five cues (i.e. positive, negative, and each of the three intermediate, ambiguous cues) and fitted models with cue-specific individual random effects (i.e. ‘5-cues models’ with a 5 × 5 covariance matrix for individual identity, to calculate repeatabilities for each cue type, and correlations of individual responses across cue types; see below). For approach probability, we specified univariate models including cue type as a fixed effect and its interactions with other predictors (electronic supplementary material, S3-M3). For approach latencies, we conducted multivariate models (five response variables, one for each cue; electronic supplementary material, S3-M4) to allow cue-specific residual variances (i.e. 5 × 5 diagonal error matrix to model heteroscedasticity of error terms across cues). This approach allowed assessment of judgement bias at the population level (mean level effects; see electronic supplementary material, S5), calculation of cue-specific repeatabilities (‘R’, adjusted for fixed effects, [68]) and evaluation of individual consistency of responses across the five cue types (pairwise correlations between individual responses to each cue type: ‘rbw’).
We analysed the probability of approaching cues using binomial (bernoulli) mixed effects models (employing the Penalized Quasi-Likelihood algorithm), with a binary response variable (1/0 for approaching versus not) and a logit link function. ‘Cue type’ (‘POS', ‘NEG’, ‘NearNEG’, ‘MID’, ‘NearPOS’) was predictor in all models, allowing the quantification of how approach probability differed between positive, negative and the three ambiguous cues. ‘Sex’ was included as a fixed effect term. In addition, to assess whether approach probability may have been affected by repeated exposure to ambiguous cues and by changes in emotional state (i.e. following recent access to a reward), we considered ‘Trial number’ (1–33), and whether the previous cue was rewarded or not (i.e. ‘Previous cue rewarded’) as additional predictors. To further evaluate whether ‘Sex’, ‘Trial number’ or ‘Previous cue rewarded’ may have affected approach responses differently according to cue type, all two-way interactions involving cue type were considered. Approach latency was analysed including only trials in which the focal individual approached a cue within the trial max duration (30 s) and following log-transformation to achieve normality. Fixed effects included ‘Sex’, ‘Trial number’, ’Previous cue rewarded’ and two-way pairwise interactions, as for the previous modelling on approach probability (see electronic supplementary material, S5 for the results of mean level effects).
Having verified the similarity of repeatabilities of responses to ambiguous cues, and a strong consistency in individual response across the three types of ambiguous cues (see §3), we subsequently re-ran models on ambiguous cue only to estimate overall random effects on pooled ambiguous cues (‘ambiguous cues models', electronic supplementary material, S3). By doing so, we obtained repeatability estimates (Rambiguous) for responses to ambiguous cues (one for approach probability and one for latency; see electronic supplementary material, S3-M5,7 for detailed model formulation) and corresponding heritability estimates 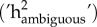 , as well as the proportion of repeatability explained by permanent environmental effects (
, as well as the proportion of repeatability explained by permanent environmental effects (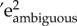 ’; electronic supplementary material, S3-M6,8). Note that significance values are reported only for approach latencies, because LRT tests are not applicable to binomial mixed effects models. For the latter, significance can be approximately inferred from confidence intervals (i.e. whether 0 is included in ±2 s.e., [22]). We then analysed the association between individual approach probability and approach latency to ambiguous cues, to assess whether individuals that were more likely to approach a cue were also on average faster to do so. We specified a bivariate mixed model, with approach probability and approach latency as the two dependent variables, ‘Individual identity’ as a random term and previously fitted predictors as added fixed effects (i.e. ‘Cue type’, ‘Trial number’ and ‘Previous cue rewarded’). Correlations between individual approach probabilities and latencies were estimated based on model variance–covariance matrixes [22]. This analysis was restricted to the phenotypic level, because sample size did not yield the power necessary for calculation of a genetic correlation.
’; electronic supplementary material, S3-M6,8). Note that significance values are reported only for approach latencies, because LRT tests are not applicable to binomial mixed effects models. For the latter, significance can be approximately inferred from confidence intervals (i.e. whether 0 is included in ±2 s.e., [22]). We then analysed the association between individual approach probability and approach latency to ambiguous cues, to assess whether individuals that were more likely to approach a cue were also on average faster to do so. We specified a bivariate mixed model, with approach probability and approach latency as the two dependent variables, ‘Individual identity’ as a random term and previously fitted predictors as added fixed effects (i.e. ‘Cue type’, ‘Trial number’ and ‘Previous cue rewarded’). Correlations between individual approach probabilities and latencies were estimated based on model variance–covariance matrixes [22]. This analysis was restricted to the phenotypic level, because sample size did not yield the power necessary for calculation of a genetic correlation.
(iii). Relationship between learning speeds and judgement bias
To investigate associations between individual learning speed (in discriminative and reversal tests) and degree of optimism towards ambiguous cues, we fitted a series of bivariate Gaussian mixed models, with one dependent variable being either discriminative or reversal learning speed (log-transformed values) and the other either approach probability (binomial variable: 0/1) or approach latency (log-transformed). Fixed effects were specified as in previous models for learning speed and judgement bias. In all models, individual identity was included as a random term in a 2 × 2 covariance matrix, allowing us to calculate correlations (±s.e.) from estimated variances and covariances. As models with approach probability assumed an underlying Gaussian error distribution, corresponding uncertainty estimates (s.e.) of correlations are approximate. Likewise, because likelihood ratio test assumptions are not met with binomial variables, corresponding p-values should be treated with caution and considered as indicative only. By doing so, we evaluated associations between task-specific individual learning performances and individual optimism. Hence, covariation was evaluated on the four combinations between measures of learning speed (discriminative and reversal tests) and cognitive judgement bias (approach probability and latency).
3. Results
(a). Associative learning
(i). Individual consistency across learning tests
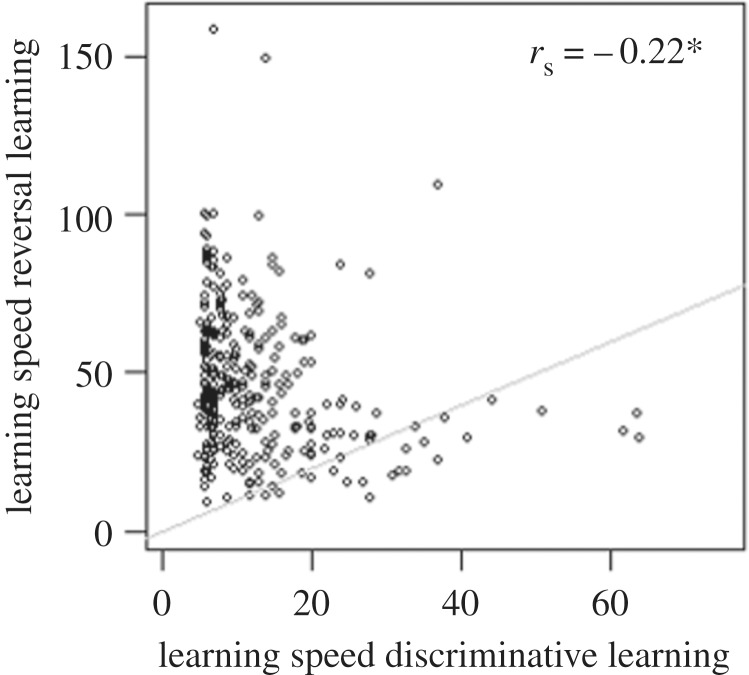
Individuals were not consistent in their learning speed across tests; to the contrary, learning speed in the discriminative learning test was weakly, but significantly, negatively correlated with learning speed in the reversal test (rs = −0.22, p < 0.001, N = 317; figure 1).
Figure 1.
Relationship between learning speed in discriminative and reversal learning tests in red junglefowl chicks. Learning speed is measured as trials until criterion reached (hence higher values indicate slower learning; see main text for further details). Each point represents an individual bird. Grey line marks equal speed in the two tasks. Asterisk (*) symbolizes significant value.
(ii). Discriminative learning
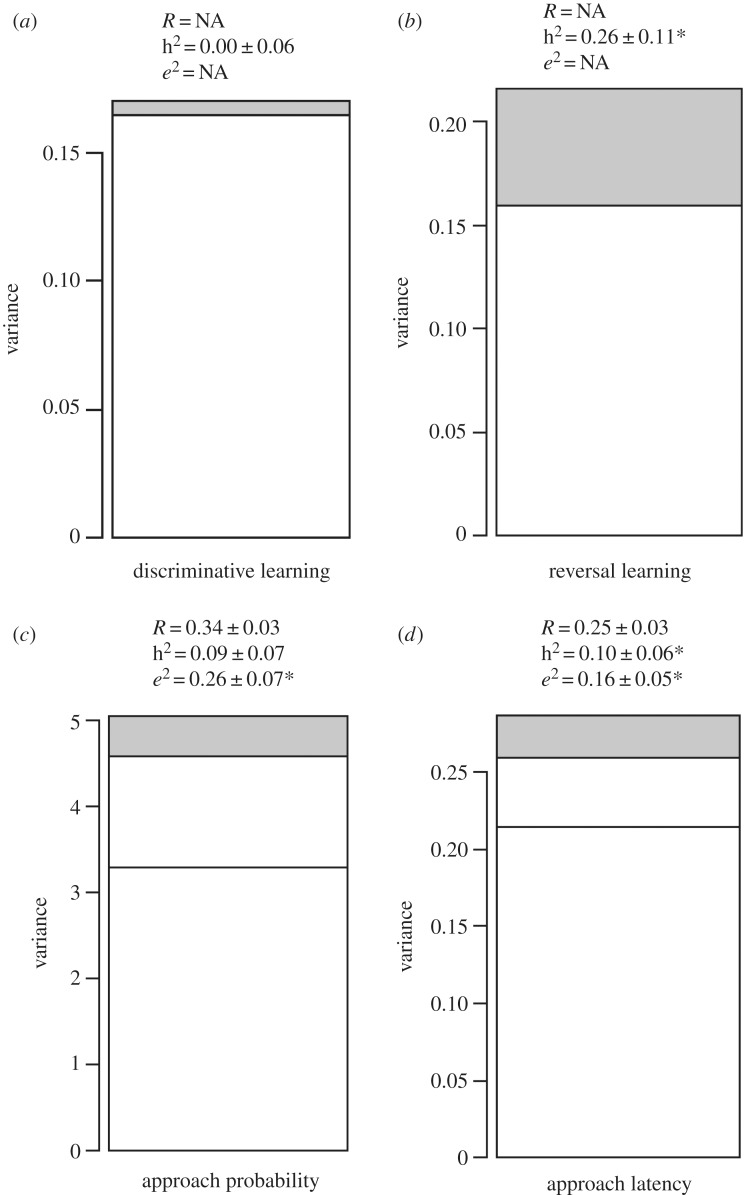
The number of trials that individuals needed to reach the set learning criterion for discrimination between two colour cues (learning speed) averaged 23.4 ± 11.1 (s.d.) (range = 8–70). Learning speed did not differ between the sexes (males = 23.1 ± 0.8 (s.e.); females = 23.5 ± 0.9; electronic supplementary material, table S4a) but varied according to the colour cue associated with the reward (2013 colour cues: blue = 26.5 ± 1.9, green = 34.9 ± 2.6; 2014–2017 colour cues: black = 21.2 ± 0.6, white = 28.1 ± 2.2; electronic supplementary material, table S4a). There was no evidence for heritability of learning speed in the discriminative test (h2 = 0.00 ± 0.06, p = 0.49; figure 2a). Given the absence of detectable additive genetic variance for discriminative learning we did not attempt to estimate a genetic correlation between this and reversal learning (see below).
Figure 2.
Variance components and heritability for performance of red junglefowl chicks in cognitive tasks. (a) Learning speed in a discriminative learning test, (b) learning speed in a reversal test, (c) approach probability to ambiguous cues, (d) approach latency to ambiguous cues. Stacked bars show, from bottom to top: residual variance, permanent environmental effects variance (limited to judgement bias), additive genetic variance (h2, grey bars). Estimates for approach probability are on the latent scale (logit). Asterisk (*) symbolizes significant values.
(iii). Reversal learning
Learning speed in the reversal learning test averaged 46.2 ± 21.7 (s.d.) (range = 9–158), did not differ between male and female chicks (males = 47.2 ± 1.8 (s.e.); females = 45.1 ± 1.7; electronic supplementary material, table S4b) and varied according to colour cue/year (blue = 28.9 ± 1.8; green = 33.9 ± 2.4; black = 45.7 ± 4.9; white = 49.9 ± 1.4; electronic supplementary material, table S4b). Contrary to the discriminative test, there was significant heritable variation in reversal learning speed (h2 = 0.26 ± 0.11, p < 0.01; figure 2b). Restricting the analysis to the years 2014–2017, to match the sample available for the judgement bias (see below) and remove methodological differences between years, yielded virtually the same heritability estimate (h2 = 0.25 ± 0.12).
(b). Cognitive judgement bias
Individuals differed in their probability of approaching cues across the entire range of cue types (i.e. repeatabilities: median = 0.44, range = 0.36–0.58; table 1, diagonal). Further, there was a high individual consistency in approach probability across cue types (i.e. between-individual correlations: rbw; all greater than 0.77; table 1). We therefore pooled ambiguous cues to increase power and accuracy of estimates. Overall, repeatability of probability of approach to ambiguous cues was moderate (Rambiguous = 0.34 ± 0.03). Between-individual variation in probability of approaching ambiguous cues was driven by environmental effects (e2 = 0.26 ± 0.07) (figure 2c), while the heritable component was low (h2 = 0.09 ± 0.07) (figure 2c).
Table 1.
Cue-specific repeatabilities and individual behavioural consistency across cue types for red junglefowl chicks in a judgement bias test. Repeatabilities (‘R’ by cue type, grey cells) and pairwise individual-level correlations (‘rbw’ between cue types, white cells) for: (i) ‘approach probability’ (i.e. probability of approaching a cue) above the diagonal line; (ii) ‘approach latency’ (i.e. latency to approach a cue) below the diagonal line. Repeatabilities and individual-level correlations were calculated from model variance–covariance estimates. POS, positive, i.e. familiar rewarded cue; NearPOS, ambiguous unfamiliar and unrewarded cue, most similar to the positive cue; MID, ambiguous unfamiliar and unrewarded cue, intermediate between positive and negative cues; NearNEG, ambiguous, unfamiliar and unrewarded cue, most similar to the negative cue; NEG, negative, i.e. familiar unrewarded cue. Estimate standard errors are provided in parenthesis.
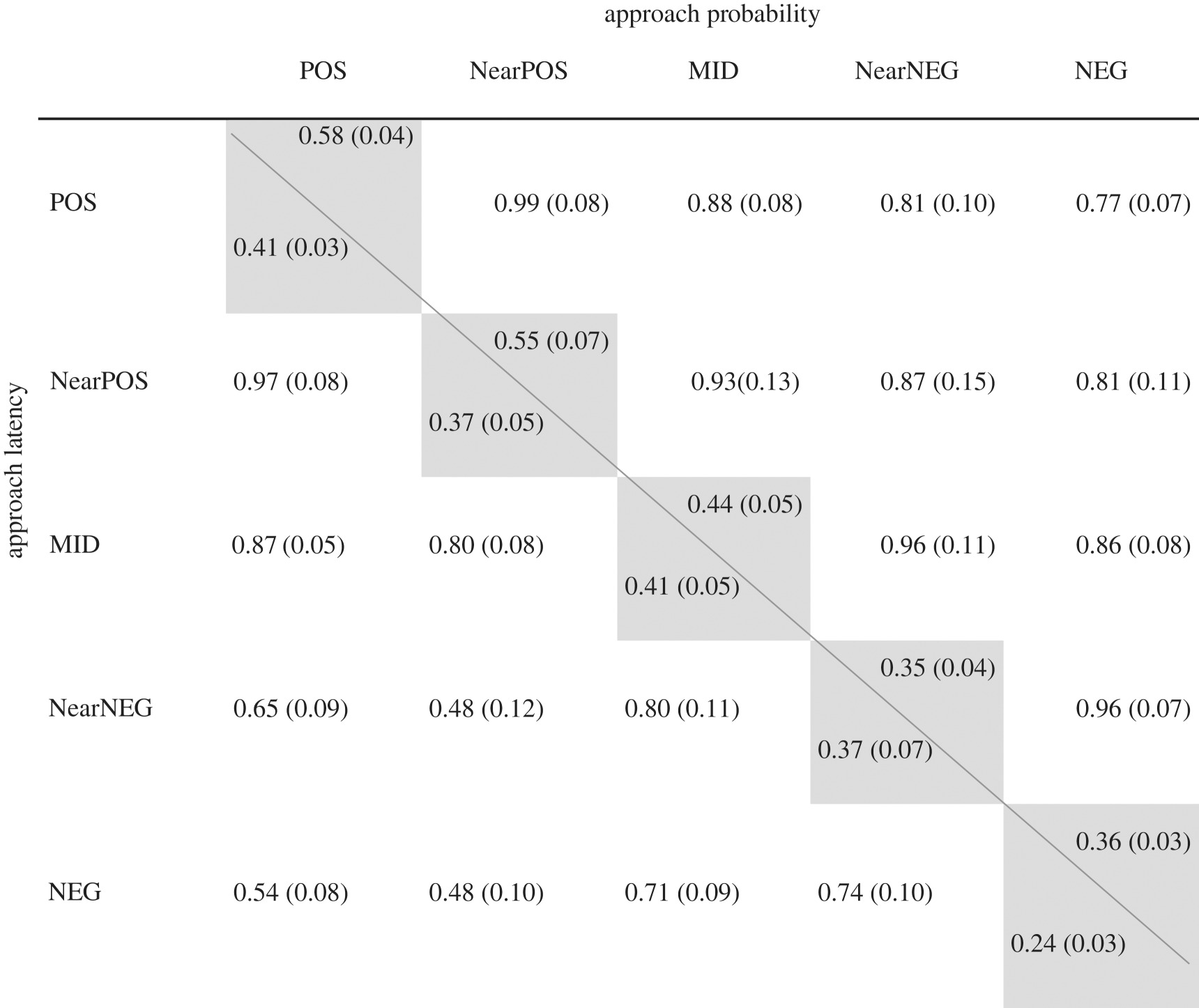 |
Individual repeatabilities in approach latency were similar across all cue types, apart from the negative cue for which repeatability was lowest (table 1, diagonal). Across cue types, there was an overall high individual consistency in approach latency, particularly between contiguous cues (POS–NearPOS, NearPOS–MID, MID–NearNEG, NearNEG–NEG: all rbw > 0.70; table 1). Overall, repeatability of approach latency to ambiguous cues was moderate (Rambiguous = 0.25 ± 0.03). Similar to approach probability, the repeatability was mainly driven by environmental effects (e2 = 0.16 ± 0.05, p < 0.01; figure 2d), while the heritable component was again low (h2 = 0.10 ± 0.06, p = 0.04). Finally, individuals that were more likely to approach ambiguous cues were also faster in doing so (rbw = −0.59 ± ∼0.09, p < 0.01).
(c). Association between learning speed and individual judgement bias
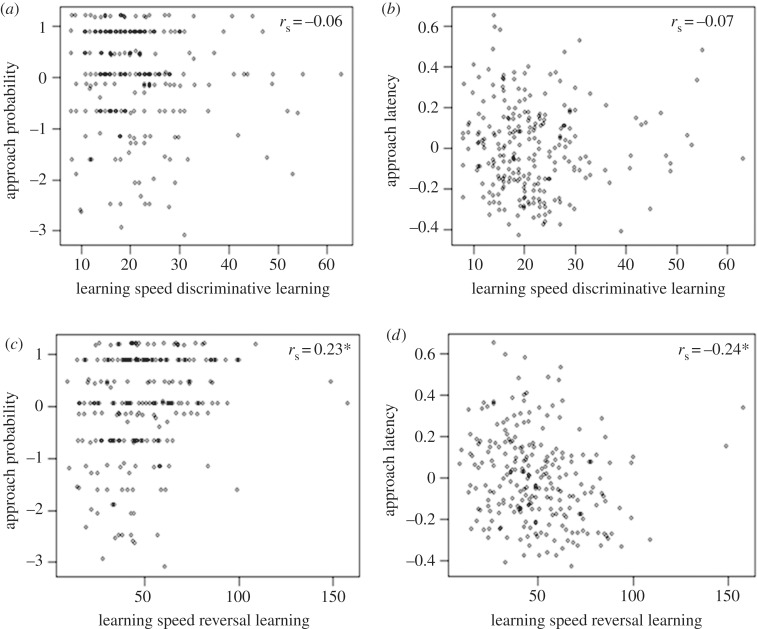
Learning speed in the discriminative learning test was neither associated with individual approach probability, nor individual latency to approach ambiguous cues in the judgement bias test (approach probability: r = −0.02 ± ∼0.08, p ∼ 0.80; latency to approach: r = −0.07 ± 0.08, p = 0.39; figure 3a,b). However, there was an association between learning speed in the reversal test and both approach probability and latency to approach ambiguous cues (approach probability: r = 0.28 ± ∼0.07, p < 0.01; latency to approach: r = −0.24 ± 0.08, p < 0.01; figure 3c,d). Individuals that were less likely, and slower, to approach ambiguous cues (i.e. less optimistic) tended to learn the reversal test faster than more optimistic chicks.
Figure 3.
Relationship between performance of red junglefowl chicks in various cognitive tests. Associations between: (a) learning speed in a discriminative learning test and approach probability to ambiguous cues, (b) learning speed in a discriminative learning test and approach latency to ambiguous cues, (c) learning speed in a reversal task and approach probability to ambiguous cues, (d) learning speed in a reversal task and approach latency to ambiguous cues. Learning speed is measured as trials until criterion is reached. Points represent individual best linear unbiased predictors (BLUPs) estimates from bivariate mixed models. Correlations were calculated from model 2 × 2 covariance matrixes of individual random effects. Significance (*) was assessed via LRT.
4. Discussion
We examined associations between performance across cognitive tests, and their heritabilities, in the red junglefowl. Our analysis revealed weak covariation between measured cognitive traits. Heritability estimates of performance across tests ranged from virtually null to moderate. Reversal learning yielded the highest heritability, while discriminative learning performance was not heritable. Individual optimism, inferred from responses to ambiguous cues, showed low heritability and was predominantly governed by environmental effects. Less optimistic chicks learnt the reversal, but not the discriminative test, faster. Finally, performance did not differ between the sexes in any cognitive test, matching the absence of sexual dimorphism in young junglefowl.
(a). Discriminative versus reversal learning
Individual performance was not consistent across the two learning contexts we assayed (discriminative and reversal associative learning). To the contrary, we demonstrated a weak negative association between learning speed in the discriminative—and in the reversal test, suggestive of speed–accuracy trade-offs and resulting individual cognitive styles [37]. The proximate control of these putative cognitive styles is presently unclear. A possible mechanism could entail among-individual differences in strength of instantiation of initial associations between cues and rewards. Strong instantiation may lead to fast learning of novel associations, which would, presumably, be mostly adaptive under stable environments. Strong instantiation could also be expected to increase the threshold for extinguishing previously learnt responses, should environmental conditions change (as required by reversal learning). Such a trade-off between rapid learning and behavioural flexibility has been demonstrated in invertebrates [29] and may also underlie speed–accuracy trade-offs found across vertebrate species.
Irrespective of mechanism, the lack of heritable variation underpinning individual differences in discriminative learning performance does not seem to support a genetically encoded trade-off. Notably, despite the absence of heritable variation in discriminative learning, we have previously found, in the same population, a high degree of temporal consistency in individual performance (from chick-stage to sexual maturity, repeatability: R > 0.4 [69]). Thus, long-lasting between-individual differences in discriminative learning performance may arise through environmental effects acting during development, and/or parental effects mediated by the gametes. Disentangling the pathways leading to these individual differences will require experimental manipulations of the environment experienced by young individuals and their parents. Regardless, the lack of heritable variation implies that selection on individual discriminative learning performance would not lead to an evolutionary change. Further, the lack of additive genetic variation does not seem to support that discriminative learning ability is part of a general intelligence ('g'), because the latter is typically explained by a common genetic underpinning (i.e. high heritability of ‘g’ [11]). Yet, the presence of ‘g’ cannot be presently ruled out in the junglefowl and its assessment will require further testing using a battery of cognitive assays encompassing a wide range of cognitive abilities and domains (e.g. mice studies [70,71]).
Conversely, we demonstrated a moderate heritability for performance in the reversal learning test, of similar strength to estimates available from other species (e.g. bees [24,72] and mice [73]). While the lack of test repeats precludes direct calculation of between-individual variation, heritability sets a lower bound for repeatability [74]. Accordingly, we can infer moderate to high between-individual differences in reversal learning abilities, with a substantial genetic component. Therefore, contrary to discriminative learning, between-individual differences in reversal learning abilities show the potential for microevolutionary responses to changing selection forces. Why performance in reversal, but not discriminative learning was heritable, is unclear. A possible explanation is that reversal learning performance is affected by individual differences in inhibitory control [26], a trait under genetic control in humans [75,76] and other animals (e.g. mice [77–79]). Then if, for example, spatially or temporally varying selection maintains genetic variation in inhibitory control, among-individual differences in reversal learning performance may be indirectly selected for (or vice versa if reversal learning is under selection).
Generally, the degree to which different cognitive abilities are heritable and genetically correlated to other cognitive and non-cognitive traits has important implications for their evolvability [80]. For example, strong positive genetic covariation between cognitive traits, as in the case of general intelligence, implies that selection on a single cognitive trait may cause evolutionary changes in other cognitive traits, even if these are not strongly associated with fitness. On the other hand, negative genetic correlations may place constraints on evolvability of certain cognitive traits, for example, if these are traded-off with other cognitive abilities under strong positive selection [81]. Finally, if different cognitive traits are underpinned by largely independent genetic control, evolutionary trajectories are most likely to differ, leading to individual and population differences in the association between cognitive abilities (such as modular cognitive structure and mosaic evolution [82]).
(b). Cognitive judgement bias
Overall, red junglefowl chicks appeared to behave optimistically and inspected ambiguous cues in more than 60% of test trials. This high approach probability was most likely a consequence of no cost (i.e. no punishment) of sampling non-positive/unrewarded cues, aside from the negligible energetic expenditure of approaching the cue [50,83]. Chicks differed in their probability of approaching ambiguous cues, and across individuals, approach probabilities to different cue types were strongly correlated. Similar results were obtained using latencies. Together these findings suggest that approach probability and latency similarly captured individual differences in judgement.
Heritability estimates for approach probability and latency were similarly low, with estimates of additive genetic variation two to three times lower than environmental variance. Therefore, between-individual differences in judgement of ambiguous cues seemed to be driven by environmental effects. Importantly, because individual consistency in judgement was assessed over a single testing session (duration up to 15 min), these environmental effects could have been the result of transient between-individual differences in affective state (e.g. mood [66]). Alternatively, between-individual differences in judgement may have resulted from long-lasting effects of developmental conditions or maternal effects, and thus underpin stable between-individual differences in judgement, possibly associated with personality [49,51]. The low heritability of judgement bias we observe here is compatible with both scenarios, provided that any long-term stability of individual optimism is driven by permanent environmental effects. However, the relatively limited number of individuals tested to date resulted in substantial uncertainty for our heritability estimates, with 95% confidence intervals ranging 0–0.2. Thus, at one extreme there may have been minor heritable variation underlying between-individual differences in judgement, while at the other extreme individual differences in optimism may have been associated with low-to-moderate heritability. Since heritable variation is a prerequisite for the occurrence of evolutionary responses to selection [6,9,22], distinguishing between these two alternatives should represent a priority for future research.
(c). Covariation between learning performance and judgement bias
Individual judgement bias was weakly associated with learning performance in the reversal test: less optimistic individuals were faster in reversing the association between colour cue and reward. There was no association between judgement biases and discriminative learning performance.
Why individual optimism may correlate with one facet of associative learning but not another is an unanswered question. To date, only a few studies have examined covariation between performance in discriminative learning and judgement biases [61,84] and have mostly reported no association between these two cognitive traits, similar to our results. However, to the best of our knowledge, links between judgement biases and reversal learning have not previously been empirically investigated.
To understand interplays between learning and judgement biases, it is useful to evaluate different causal pathways that may give rise to associations between learning performance and judgement. First, common traits may be causally linked to both performance in reversal learning and individual optimism. For example, speed–accuracy trade-offs underlying different cognitive styles, and typically associated with personality types (e.g. coping styles, [37]) may also underpin associations between learning performance and responses to ambiguous cues. Optimism may, thus, represent an individual cognitive trait, likely with genetic underpinnings. Yet, the lack of association between discriminative learning speed and optimism in the junglefowl is not easily reconciled with a speed–accuracy trade-off framework, which predicts that fast/proactive individuals should learn discriminative tests faster [37] and be at the same time more prone to impulsively approach ambiguous cues. Nevertheless, the negative association between optimism and performance in the reversal learning tests is compatible with individual differences in cognitive and coping styles. This is because reactive/slow types are considered to be both more competent in reversal learning and more susceptible to stress [26,85]. In turn, both acute and chronic stress have been linked to negative affective states, and, thereby, pessimistic-like behaviour [56,86–88]. Another possible explanation for our findings may entail between-individual differences in persistence underlying both reversal learning [89] and optimistic response to ambiguous cues [90]. Under this hypothesis, more persistent individuals are expected to continue responding during extinction (i.e. when presented with unrewarded cues) for longer and are therefore predicted to be both slower in reversing previously learnt associations and more persistent in approaching when exposed to ambiguous or negative cues. Individual differences in extinction, associated with personality and emotional traits, have been demonstrated in human infants and mice [91].
Finally, an alternative mechanism may involve a direct causal relationship, with individual affective state modulating learning performance. The affect-as-information hypothesis [92,93] posits that negative mood suppresses impulsive behaviour conducive to negative fitness consequences under challenging conditions, and favours instead inhibitory control [92]. Since inhibition is also implicated in reversal learning, it follows that individuals in a negative affective state (i.e. less optimistic) may show enhanced performance in a reversal learning test.
Fully distinguishing between these hypotheses will require an appraisal of temporal consistency of individual optimism, interplays with personality traits and experimental manipulations of mood to evaluate resulting changes in cognitive performance. Primarily, more data are required to ascertain the extent to which the phenotypic correlation between individual optimism and reversal learning may arise from shared genetic control (i.e. pleiotropy or genetic linkage).
5. General conclusion
To summarize, we have demonstrated genetic variation underlying individual differences in reversal learning performance, and a lack of genetic effects for discriminative learning. Between-individual variation in judgement of ambiguous cues was mainly driven by environmental effects and showed low heritability. Thus, the examined cognitive traits do not seem to have a shared genetic control. Importantly, our findings suggest that in the junglefowl, reversal but not discriminative learning abilities may evolve in response to selection. The proximate mechanisms behind differences in the genetic control of these two facets of associative learning are unclear. Additive genetic variation in individual inhibitory control provides a possible explanation to this conundrum. Understanding what maintains heritable individual differences in reversal learning will require linking performance in reversal learning with fitness [94]. Further work should also aim at elucidating the extent to which optimism may be heritable, and what mechanisms are driving covariation between learning abilities and judgement biases.
Supplementary Material
Supplementary Material
Acknowledgements
We would like to thank several students in the Lovlie group over the years, particularly Emilie Jansson, Ann-Marie Malmqvist, Sabina Ahlgren Porthén and Charlotte Rosher for help with behavioural testing, Lejla Bektic and Julia Buskas for animal care. The work was carried out within the Centre of Excellence in Animal Welfare Science, a collaborative research environment.
Ethics
The study followed ethical requirements in Sweden and the study was approved by Linköping ethical committee (permit numbers 122-10 and 50-13).
Data accessibility
Data are available as electronic supplementary material.
Authors' contributions
H.L. coordinated and funded the study; E.S. carried out statistical analyses with input from A.W.; E.S., L.G., J.Z. and H.L. collected behavioural data; E.S. and H.L. drafted the manuscript with input from J.Z., L.G. and A.W. All authors gave final approval for publication.
Competing interests
The authors declare no competing interests.
Funding
Funding was awarded (to H.L.) from Carl Trygger's foundation, ‘Future research leader’ at LiU and the Swedish Research council Formas.
References
- 1.Shettleworth SJ. 2009. Cognition, evolution, and behavior, 2nd edn Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Wasserman EA, Zentall TR. 2009. Comparative cognition. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Cauchoix M, Chaine AS. 2016. How can we study the evolution of animal minds? Front. Psychol . 7, 1–18. ( 10.3389/fpsyg.2016.00358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thornton A, Lukas D. 2012. Individual variation in cognitive performance: developmental and evolutionary perspectives. Phil. Trans. R. Soc. B 367, 2773–2783. ( 10.1098/rstb.2012.0214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carere C, Maestripieri D. 2013. Animal personalities: behavior, physiology, and evolution. Chicago, IL: The University of Chicago Press. [Google Scholar]
- 6.Croston R, Branch CL, Kozlovsky DY, Dukas R, Pravosudov VV. 2015. Heritability and the evolution of cognitive traits. Behav. Ecol. 26, 1447–1459. ( 10.1093/beheco/arv088) [DOI] [Google Scholar]
- 7.Versace E. 2015. Experimental evolution, behavior and genetics: associative learning as a case study. Curr. Zool. 61, 226–241. ( 10.1093/czoolo/61.2.226) [DOI] [Google Scholar]
- 8.Dukas R. 2004. Evolutionary biology of animal cognition. Annu. Rev. Ecol. Evol. Syst. 35, 347–374. ( 10.1146/annurev.ecolsys.35.112202.130152) [DOI] [Google Scholar]
- 9.Croston R, Branch CL, Kozlovsky DY, Dukas R, Pravosudov VV. 2015. The importance of heritability estimates for understanding the evolution of cognition: a response to comments on Croston et al. Behav. Ecol. 26, 1463–1464. ( 10.1093/beheco/arv192) [DOI] [Google Scholar]
- 10.Morand-Ferron J, Cole EF, Quinn JL. 2016. Studying the evolutionary ecology of cognition in the wild: a review of practical and conceptual challenges. Biol. Rev. 91, 367–389. ( 10.1111/brv.12174) [DOI] [PubMed] [Google Scholar]
- 11.Plomin R, Spinath FM. 2004. Intelligence: genetics, genes, and genomics. J. Pers. Soc. Psychol. 86, 112–129. ( 10.1037/0022-3514.86.1.112) [DOI] [PubMed] [Google Scholar]
- 12.Sauce B, Bendrath S, Herzfeld M, Siegel D, Style C, Rab S, Korabelnikov J, Matzel LD. 2018. The impact of environmental interventions among mouse siblings on the heritability and malleability of general cognitive ability. Phil. Trans. R. Soc. B 373, 20170289 ( 10.1098/rstb.2017.0289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plomin R. 1999. Genetics and general cognitive ability. Nature 402, C25–C29. ( 10.1038/35011520) [DOI] [PubMed] [Google Scholar]
- 14.Locurto C, Fortin E, Sullivan R. 2003. The structure of individual differences in Heterogeneous Stock mice across problem types and motivational systems. Genes, Brain Behav. 2, 40–55. ( 10.1034/j.1601-183X.2003.00006.x) [DOI] [PubMed] [Google Scholar]
- 15.Zhu Q, Song Y, Hu S, Li X, Tian M, Zhen Z, Dong Q, Kanwisher N, Liu J. 2010. Heritability of the specific cognitive ability of face perception. Curr. Biol. 20, 137–142. ( 10.1016/j.cub.2009.11.067) [DOI] [PubMed] [Google Scholar]
- 16.Amici F, Barney B, Johnson VE, Call J, Aureli F. 2012. A modular mind? A test using individual data from seven primate species. PLoS ONE 7, e51918 ( 10.1371/journal.pone.0051918) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burkart JM, Schubiger MN, van Schaik CP. 2016. The evolution of general intelligence. Behav. Brain Sci. 40, 1–65. ( 10.1017/S0140525X16000959) [DOI] [PubMed] [Google Scholar]
- 18.Huber L. 2017. Where is the evidence for general intelligence in nonhuman animals? Behav. Brain Sci. 40, e206 ( 10.1017/S0140525X16001667) [DOI] [PubMed] [Google Scholar]
- 19.Shaw RC, Schmelz M. 2017. Cognitive test batteries in animal cognition research: evaluating the past, present and future of comparative psychometrics. Anim. Cogn. 20, 1003–1018. ( 10.1007/s10071-017-1135-1) [DOI] [PubMed] [Google Scholar]
- 20.Lynch M, Walsh B. 1998. Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer Associates, Inc. [Google Scholar]
- 21.Plomin R. 2001. The genetics of G in human and mouse. Nat. Rev. Neurosci. 2, 136–141. ( 10.1038/35053584) [DOI] [PubMed] [Google Scholar]
- 22.Wilson AJ, Réale D, Clements MN, Morrissey MM, Postma E, Walling CA, Kruuk LEB, Nussey DH. 2010. An ecologist's guide to the animal model. J. Anim. Ecol. 79, 13–26. ( 10.1111/j.1365-2656.2009.01639.x) [DOI] [PubMed] [Google Scholar]
- 23.Morand-Ferron J. 2017. Why learn? The adaptive value of associative learning in wild populations. Curr. Opin. Behav. Sci. 16, 73–79. ( 10.1016/j.cobeha.2017.03.008) [DOI] [Google Scholar]
- 24.Ferguson HJ, Cobey S, Smith BH. 2001. Sensitivity to a change in reward is heritable in the honeybee, Apis mellifera. Anim. Behav. 61, 527–534. ( 10.1006/anbe.2000.1635) [DOI] [Google Scholar]
- 25.Kawecki TJ. 2010. Evolutionary ecology of learning: insights from fruit flies. Popul. Ecol. 52, 15–25. ( 10.1007/s10144-009-0174-0) [DOI] [Google Scholar]
- 26.Coppens CM, de Boer SF, Koolhaas JM. 2010. Coping styles and behavioural flexibility: towards underlying mechanisms. Phil. Trans. R. Soc. B 365, 4021–4028. ( 10.1098/rstb.2010.0217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Izquierdo A, Jentsch JD. 2012. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology (Berl) 219, 607–620. ( 10.1007/s00213-011-2579-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raine NE, Chittka L. 2012. No trade-off between learning speed and associative flexibility in bumblebees: a reversal learning test with multiple colonies. PLoS ONE 7, e45096 ( 10.1371/journal.pone.0045096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Remmelink E, Smit AB, Verhage M, Loos M. 2016. Measuring discrimination- and reversal learning in mouse models within 4 days and without prior food deprivation. Learn. Mem. 23, 660–667. ( 10.1101/lm.042085.116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boogert NJ, Anderson RC, Peters S, Searcy WA, Nowicki S. 2011. Song repertoire size in male song sparrows correlates with detour reaching, but not with other cognitive measures. Anim. Behav. 81, 1209–1216. ( 10.1016/j.anbehav.2011.03.004) [DOI] [Google Scholar]
- 31.Guillette LM, Hahn AH, Hoeschele M, Przyslupski AM, Sturdy CB. 2015. Individual differences in learning speed, performance accuracy and exploratory behaviour in black-capped chickadees. Anim. Cogn. 18, 165–178. ( 10.1007/s10071-014-0787-3) [DOI] [PubMed] [Google Scholar]
- 32.Nettle D, Andrews CP, Monaghan P, Brilot BO, Bedford T, Gillespie R, Bateson M. 2015. Developmental and familial predictors of adult cognitive traits in the European starling. Anim. Behav. 107, 239–248. ( 10.1016/j.anbehav.2015.07.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guido JM, Biondi LM, Vasallo AI, Muzio RN. 2017. Neophobia is negatively related to reversal learning ability in females of a generalist bird of prey, the Chimango Caracara, Milvago chimango. Anim. Cogn. 20, 591–602. ( 10.1007/s10071-017-1083-9) [DOI] [PubMed] [Google Scholar]
- 34.Shaw CL, Watson GDR, Hallock HL, Cline KM, Griffin AL. 2013. The role of the medial prefrontal cortex in the acquisition, retention, and reversal of a tactile visuospatial conditional discrimination task. Behav. Brain Res. 236, 94–101. ( 10.1016/j.bbr.2012.08.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bebus SE, Small TW, Jones BC, Elderbrock EK, Schoech SJ. 2016. Associative learning is inversely related to reversal learning and varies with nestling corticosterone exposure. Anim. Behav. 111, 251–260. ( 10.1016/j.anbehav.2015.10.027) [DOI] [Google Scholar]
- 36.Chittka L, Skorupski P, Raine NE. 2009. Speed–accuracy tradeoffs in animal decision making. Trends Ecol. Evol. 24, 400–407. ( 10.1016/j.tree.2009.02.010) [DOI] [PubMed] [Google Scholar]
- 37.Sih A, Del Giudice M. 2012. Linking behavioural syndromes and cognition: a behavioural ecology perspective. Phil. Trans. R. Soc. B 367, 2762–2772. ( 10.1098/rstb.2012.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moiron M, Mathot KJ, Dingemanse NJ. 2016. A multi-level approach to quantify speed–accuracy trade-offs in great tits (Parus major). Behav. Ecol. 27, 1539–1546. ( 10.1093/beheco/arw077) [DOI] [Google Scholar]
- 39.Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MAW, Blokhuis HJ. 1999. Coping styles in animals: current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 23, 925–935. ( 10.1016/S0149-7634(99)00026-3) [DOI] [PubMed] [Google Scholar]
- 40.Brust V, Guenther A. 2015. Domestication effects on behavioural traits and learning performance: comparing wild cavies to guinea pigs. Anim. Cogn. 18, 99–109. ( 10.1007/s10071-014-0781-9) [DOI] [PubMed] [Google Scholar]
- 41.Baragli P, Vitale V, Sighieri C, Lanata A, Palagi E, Reddon AR. 2017. Consistency and flexibility in solving spatial tasks: different horses show different cognitive styles. Sci. Rep. 7, 16557 ( 10.1038/s41598-017-16729-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lermite F, Peneaux C, Griffin AS. 2017. Personality and problem-solving in common mynas (Acridotheres tristis). Behav. Processes. 134, 87–94. ( 10.1016/j.beproc.2016.09.013) [DOI] [PubMed] [Google Scholar]
- 43.Isden J, Panayi C, Dingle C, Madden J. 2013. Performance in cognitive and problem-solving tasks in male spotted bowerbirds does not correlate with mating success. Anim. Behav. 86, 829–838. ( 10.1016/j.anbehav.2013.07.024) [DOI] [Google Scholar]
- 44.Feyissa DD, Aher YD, Engidawork E, Höger H, Lubec G, Korz V. 2017. Individual differences in male rats in a behavioral test battery: a multivariate statistical approach. Front. Behav. Neurosci. 11, 26 ( 10.3389/fnbeh.2017.00026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mendl M, Burman OHPP, Parker RMAA, Paul ES. 2009. Cognitive bias as an indicator of animal emotion and welfare: emerging evidence and underlying mechanisms. Appl. Anim. Behav. Sci. 118, 161–181. ( 10.1016/j.applanim.2009.02.023) [DOI] [Google Scholar]
- 46.Bateson M. 2016. Optimistic and pessimistic biases: a primer for behavioural ecologists. Curr. Opin. Behav. Sci. 12, 115–121. ( 10.1016/j.cobeha.2016.09.013) [DOI] [Google Scholar]
- 47.Fawcett TW, Fallenstein B, Higginson AD, Houston AI, Mallpress DEW, Trimmer PC, McNamara JM. 2014. The evolution of decision rules in complex environments. Trends Cogn. Sci. 18, 153–161. ( 10.1016/j.tics.2013.12.012) [DOI] [PubMed] [Google Scholar]
- 48.Bateson M, Emmerson M, Ergün G, Monaghan P, Nettle D. 2015. Opposite effects of early-life competition and developmental telomere attrition on cognitive biases in juvenile European starlings. PLoS ONE 10, 1–23. ( 10.1371/journal.pone.0132602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asher L, Friel M, Griffin K, Collins LM. 2016. Mood and personality interact to determine cognitive biases in pigs. Biol. Lett. 12, 20160402 ( 10.1098/rsbl.2016.0402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roelofs S, Boleij H, Nordquist RE, Van Der Staay FJ, Heath C. 2016. Making decisions under ambiguity: judgment bias tasks for assessing emotional state in animals. Front. Behav. Neurosci. 10, 1–16. ( 10.3389/fnbeh.2016.00119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.d'Ettorre P, Carere C, Demora L, Le Quinquis P, Signorotti L, Bovet D, Le P, Signorotti L, Bovet D. 2017. Individual differences in exploratory activity relate to cognitive judgement bias in carpenter ants. Behav. Processes 134, 63–69. ( 10.1016/j.beproc.2016.09.008) [DOI] [PubMed] [Google Scholar]
- 52.McNamara JM, Trimmer PC, Houston AI. 2012. It is optimal to be optimistic about survival. Biol. Lett. 8, 516–519. ( 10.1098/rsbl.2012.0010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McNamara JM, Trimmer PC, Eriksson A, Marshall JAR, Houston AI. 2011. Environmental variability can select for optimism or pessimism. Ecol. Lett. 14, 58–62. ( 10.1111/j.1461-0248.2010.01556.x) [DOI] [PubMed] [Google Scholar]
- 54.Buhot MC. 1997. Serotonin receptors in cognitive behaviors. Curr. Opin. Neurobiol. 7, 243–254. ( 10.1016/S0959-4388(97)80013-X) [DOI] [PubMed] [Google Scholar]
- 55.Wise RA. 2004. Dopamine, learning and motivation. Nat. Rev. Neurosci. 5, 483–494. ( 10.1038/nrn1406) [DOI] [PubMed] [Google Scholar]
- 56.Bateson M, Desire S, Gartside SE, Wright GA. 2011. Agitated honeybees exhibit pessimistic cognitive biases. Curr. Biol. 21, 1070–1073. ( 10.1016/j.cub.2011.05.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharot T, Guitart-Masip M, Korn CW, Chowdhury R, Dolan RJ. 2012. How dopamine enhances an optimism bias in humans. Curr. Biol . 22, 1477–1481. ( 10.1016/j.cub.2012.05.053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kregiel J, Golebiowska J, Popik P, Rygula R. 2016. Dopamine induces an optimism bias in rats—pharmacological proof for the translational validity of the ambiguous-cue interpretation test. Behav. Brain Res. 297, 84–90. ( 10.1016/j.bbr.2015.10.020) [DOI] [PubMed] [Google Scholar]
- 59.Zidar J, Campderrich I, Jansson E, Wichman A, Winberg S, Keeling L, Løvlie H. 2018. Environmental complexity buffers against stress-induced negative judgement bias in female chickens. Sci. Rep . 8, 5404 ( 10.1038/s41598-018-23545-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perry CJ, Baciadonna L, Chittka L. 2016. Unexpected rewards induce dopamine-dependent positive emotion-like state changes in bumblebees. Science 353, 1529–1531. ( 10.1126/science.aaf4454) [DOI] [PubMed] [Google Scholar]
- 61.Roelofs S, Murphy E, Ni H, Gieling E, Nordquist RE, van der Staay FJ. 2017. Judgement bias in pigs is independent of performance in a spatial holeboard task and conditional discrimination learning. Anim. Cogn. 20, 739–753. ( 10.1007/s10071-017-1095-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fumihito A, Miyake T, Sumi S, Takada M, Ohno S, Kondo N. 1994. One subspecies of the red junglefowl (Gallus gallus gallus) suffices as the matriarchic ancestor of all domestic breeds. Proc. Natl Acad. Sci. USA 91, 12 505–12 509. ( 10.1073/pnas.91.26.12505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zidar J, Balogh A, Favati A, Jensen P, Leimar O, Løvlie H. 2017. A comparison of animal personality and coping styles in the red junglefowl. Anim. Behav. 130, 209–220. ( 10.1016/j.anbehav.2017.06.024) [DOI] [Google Scholar]
- 64.Zidar J, Sorato E, Malmqvist AM, Jansson E, Rosher C, Jensen P, Favati A, Løvlie H. 2017. Early experience affects adult personality in the red junglefowl: a role for cognitive stimulation? Behav. Processes. 134, 78–86. ( 10.1016/j.beproc.2016.06.003) [DOI] [PubMed] [Google Scholar]
- 65.Favati A, Zidar J, Thorpe H, Jensen P, Løvlie H. 2016. The ontogeny of personality traits in the red junglefowl, Gallus gallus. Behav. Ecol. 27, 484–493. ( 10.1093/beheco/arv177) [DOI] [Google Scholar]
- 66.Zidar J, Campderrich I, Jansson E, Wichman A, Winberg S, Keeling L, Løvlie H. 2018 Environmental complexity buffers against stress-induced negative judgment bias in female chickens. Sci. Rep. 8, 5404 ( 10.1038/s41598-018-23545-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gilmour AR, Gogel BJ, Cullis BR, Thompson R.2009. ASReml user guide release 3.0. VSN Int. Ltd., 275. (doi:10.1017/CBO9781107415324.004)
- 68.Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. ( 10.1111/j.2041-210x.2012.00261.x) [DOI] [Google Scholar]
- 69.Cauchoix M, et al. 2018. The repeatability of cognitive performance: a meta-analysis. Phil. Trans. R. Soc. B 373, 20170281 ( 10.1098/rstb.2017.0281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Galsworthy MJ, Paya-Cano JL, Liu L, Monleón S, Gregoryan G, Fernandes C, Schalkwyk LC, Plomin R. 2005. Assessing reliability, heritability and general cognitive ability in a battery of cognitive tasks for laboratory mice. Behav. Genet. 35, 675–692. ( 10.1007/s10519-005-3423-9) [DOI] [PubMed] [Google Scholar]
- 71.Light KR, Kolata S, Wass C, Denman-Brice A, Zagalsky R, Matzel LD. 2010. Working memory training promotes general cognitive abilities in genetically heterogeneous mice. Curr. Biol. 20, 777–782. ( 10.1016/j.cub.2010.02.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chandra SBC, Hunt GJ, Cobey S, Smith BH. 2001. Quantitative trait loci associated with reversal learning and latent inhibition in honeybees (Apis mellifera). Behav. Genet. 31, 275–285. ( 10.1023/A:1012227308783) [DOI] [PubMed] [Google Scholar]
- 73.Laughlin RE, Grant TL, Williams RW, Jentsch JD. 2011. Genetic dissection of behavioral flexibility: reversal learning in mice. Biol. Psychiatry 69, 1109–1116. ( 10.1016/j.biopsych.2011.01.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Riska B, Prout T, Turelli M. 1989. Laboratory estimates of heritabilities and genetic correlations in nature. Genetics 123, 865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anokhin AP, Golosheykin S, Grant JD, Heath AC. 2011. Heritability of delay discounting in adolescence: a longitudinal twin study. Behav. Genet. 41, 175–183. ( 10.1007/s10519-010-9384-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anokhin AP, Grant JD, Mulligan RC, Heath AC. 2015. The genetics of impulsivity: evidence for the heritability of delay discounting. Biol. Psychiatry 77, 887–894. ( 10.1016/j.biopsych.2014.10.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cervantes MC, Laughlin RE, Jentsch JD. 2013. Cocaine self-administration behavior in inbred mouse lines segregating different capacities for inhibitory control. Psychopharmacology (Berl) 229, 515–525. ( 10.1007/s00213-013-3135-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Loos M, Mueller T, Gouwenberg Y, Wijnands R, Van Der Loo RJ, Birchmeier C, Smit AB, Spijker S. 2014. Neuregulin-3 in the mouse medial prefrontal cortex regulates impulsive action. Biol. Psychiatry 76, 648–655. ( 10.1016/j.biopsych.2014.02.011) [DOI] [PubMed] [Google Scholar]
- 79.Loos M, Staal J, Schoffelmeer ANM, Smit AB, Spijker S, Pattij T. 2010. Inhibitory control and response latency differences between C57BL/6 J and DBA/2 J mice in a Go/No-Go and 5-choice serial reaction time task and strain-specific responsivity to amphetamine. Behav. Brain Res. 214, 216–224. ( 10.1016/j.bbr.2010.05.027) [DOI] [PubMed] [Google Scholar]
- 80.Thornton A, Wilson AJ. 2015. In search of the Darwinian holy trinity in cognitive evolution: a comment on Croston et al. Behav. Ecol. 26, 1460–1461. ( 10.1093/beheco/arv119) [DOI] [Google Scholar]
- 81.Walsh B, Blows MW. 2009. Abundant genetic variation + strong selection = multivariate genetic constraints: a geometric view of adaptation. Annu. Rev. Ecol. Evol. Syst. 40, 41–59. ( 10.1146/annurev.ecolsys.110308.120232) [DOI] [Google Scholar]
- 82.Gonzalez-Voyer A, Winberg S, Kolm N. 2009. Brain structure evolution in a basal vertebrate clade: evidence from phylogenetic comparative analysis of cichlid fishes. BMC Evol. Biol. 9, 238 ( 10.1186/1471-2148-9-238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gygax L. 2014. The A to Z of statistics for testing cognitive judgement bias. Anim. Behav. 95, 59–69. ( 10.1016/j.anbehav.2014.06.013) [DOI] [Google Scholar]
- 84.Murphy E, Nordquist RE, van der Staay FJ. 2013. Responses of conventional pigs and Göttingen miniature pigs in an active choice judgement bias task. Appl. Anim. Behav. Sci. 148, 64–76. ( 10.1016/j.applanim.2013.07.011) [DOI] [Google Scholar]
- 85.Koolhaas JM, de Boer SF, Coppens CM, Buwalda B. 2010. Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front. Neuroendocrinol . 31, 307–321. ( 10.1016/j.yfrne.2010.04.001) [DOI] [PubMed] [Google Scholar]
- 86.Iyasere OS, Beard AP, Guy JH, Bateson M. 2017. Elevated levels of the stress hormone, corticosterone, cause ‘pessimistic’ judgment bias in broiler chickens. Sci. Rep. 7, 6860 ( 10.1038/s41598-017-07040-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brilot BO, Asher L, Bateson M. 2010. Stereotyping starlings are more ‘pessimistic’. Anim. Cogn. 13, 721–731. ( 10.1007/s10071-010-0323-z) [DOI] [PubMed] [Google Scholar]
- 88.Destrez A, Deiss V, Lévy F, Calandreau L, Lee C, Chaillou-Sagon E, Boissy A. 2013. Chronic stress induces pessimistic-like judgment and learning deficits in sheep. Appl. Anim. Behav. Sci. 148, 28–36. ( 10.1016/j.applanim.2013.07.016) [DOI] [Google Scholar]
- 89.Sperling SE. 1965. Reversal learning and resistance to extinction: a review of the rat literature. Psychol. Bull. 63, 281–297. ( 10.1037/h0021838) [DOI] [PubMed] [Google Scholar]
- 90.Aspinwall LG, Richter L. 1999. Optimism and self-mastery predict more rapid disengagement from unsolvable tasks in the presence of alternatives. Motiv. Emot . 23, 221–246. ( 10.1023/A:1021367331817) [DOI] [Google Scholar]
- 91.Sauce B, Wass C, Lewis M, Matzel LD. 2017. A broader phenotype of persistence emerges from individual differences in response to extinction. Psychon. Bull. Rev. 1–9. ( 10.3758/s13423-017-1402-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marzouki Y, Gullstrand J, Goujon A, Fagot J. 2014. Baboons' response speed is biased by their moods. PLoS ONE 9, e102562 ( 10.1371/journal.pone.0102562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schwarz N, Clore GL. 2003. Mood as information: 20 years later. Psychol. Inq. 14, 296–303. ( 10.1080/1047840X.2003.9682896) [DOI] [Google Scholar]
- 94.Madden JR, Langley EJG, Whiteside MA, Beardsworth CE, van Horik JO. 2018. The quick are the dead: pheasants that are slow to reverse a learned association survive for longer in the wild. Phil. Trans. R. Soc. B 373, 20170297 ( 10.1098/rstb.2017.0297) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available as electronic supplementary material.