Abstract
The prevailing hypotheses for the evolution of cognition focus on either the demands associated with group living (the social intelligence hypothesis (SIH)) or ecological challenges such as finding food. Comparative studies testing these hypotheses have generated highly conflicting results; consequently, our understanding of the drivers of cognitive evolution remains limited. To understand how selection shapes cognition, research must incorporate an intraspecific approach, focusing on the causes and consequences of individual variation in cognition. Here, we review the findings of recent intraspecific cognitive research to investigate the predictions of the SIH. Extensive evidence from our own research on Australian magpies (Cracticus tibicen dorsalis), and a number of other taxa, suggests that individuals in larger social groups exhibit elevated cognitive performance and, in some cases, elevated reproductive fitness. Not only do these findings demonstrate how the social environment has the potential to shape cognitive evolution, but crucially, they demonstrate the importance of considering both genetic and developmental factors when attempting to explain the causes of cognitive variation.
This article is part of the theme issue ‘Causes and consequences of individual differences in cognitive abilities’.
Keywords: social intelligence hypothesis, individual variation, Australian magpies, cognition, intraspecific
1. Introduction: prevailing theories for the evolution of cognition
For over a century, scientists have investigated the factors governing cognitive evolution, yet the topic still remains intensely debated today. Hypotheses typically place the emphasis on either social or ecological challenges as predominant factors driving cognitive evolution, but studies addressing the potential role of these factors have produced highly conflicting results [1–4].
The cognitive buffer hypothesis (CBH), for instance, predicts that large brain size evolved to allow species to adjust their behaviour adaptively in response to variable environmental conditions [5]. Two environmental challenges in particular are hypothesized to be selective pressures influencing cognition: resource availability and seasonality [3]. If the availability of food is difficult to predict, then selection may favour the evolution of enhanced learning and memory to allow animals to maximize foraging intake [6]. For example, frugivorous spider monkeys (Ateles geoffroyi), whose primary food source is ephemeral and unpredictable, have larger relative brain size compared to the leaf-eating howler monkey (Alouatta palliate), whose food source is ubiquitous [7]. These findings are supported by a recent phylogenetic analysis by DeCasien et al. [2], which found a strong relationship between frugivory and brain size. Coupled with evidence of links between brain size and cognitive ability [8–12], this appears consistent with the CBH. Further support comes from behavioural studies. Field studies suggest that grey-cheeked mangabeys (Lophocebus albigena), for example, use integrated, episodic memory about the location and ripeness of fruit encountered on previous foraging trips and weather conditions over the intervening period when deciding whether to revisit particular fruiting trees [13]. Unpredictable resource availability may also favour more innovative individuals if novel foraging techniques allow the exploitation of a new food source. In this vein, there is evidence of a positive relationship between innovativeness and brain size in both primates and birds (reviewed in [14]). Larger-brained birds (relative to body mass) are also more successful than smaller-brained birds when establishing themselves in a novel environment [12].
Although not necessarily mutually exclusive from resource availability, seasonality has also been hypothesized to select for increased cognitive ability [15,16]. Seasonal changes in climatic conditions mean some species migrate while others endure the harsher environments [15,16]. Both scenarios may create situations that select for elevated cognitive performance [15,16]. Migratory birds have increased levels of long-term spatial memory compared to non-migratory birds [17–19] (although note Sayol et al. [3] found migratory birds have smaller relative brain size). Among non-migratory species, it has been discovered that subpopulations that cache food for the winter tend to have a larger hippocampus and elevated long-term spatial memory retention compared to non-caching subpopulations [20–22]. A comparative analysis of brain size in 1200 bird species found larger-brained birds were more likely to occur in areas with greater environmental variation, adding support to the idea of seasonality favouring increased information processing power [3]. However, a number of comparative studies suggest ecological factors alone cannot adequately explain interspecific differences in neuroanatomy and cognition; for example, Shultz & Dunbar [23] concluded that social factors were just as important as ecological factors in driving the evolution of ungulate brains.
The novel concept of social intelligence was first introduced over half a century ago in papers by Chance & Mead [24] and Jolly [25], although it is arguably Nick Humphrey's seminal paper, ‘The social function of intellect’ [26], that is recognized as giving rise to the social intelligence hypothesis (SIH) and the resulting research in this area. The SIH posits that group living can generate substantial challenges that favour selection for enhanced cognitive abilities [27]. Since the SIH was conceptualized, an abundance of literature has characterized some of the potential challenges of living in groups [28]. The need to maintain and coordinate multiple relationships, monitor other group members and recognize suitable cooperative partners are examples of factors unique to social animals that are hypothesized to be selective pressures requiring advanced cognition [28,29]. Byrne & Whiten [30] also highlighted the ‘Machiavellian’ nature of some animal societies, where the need to outwit others in competitive interactions may generate arms races of escalating cognitive abilities.
The majority of evidence supporting the SIH is derived from comparative studies on primates and birds [31,32], relating between-species or between-population differences in brain size or cognitive performance to differences in social organization or life history [1,32–36]. Several proxies of social complexity have been found to correlate with cognitive performance or measures of brain size or brain composition [1,32–34]. For instance, large brain size in birds has been linked to the establishment, maintenance and coordination of behaviour within long-term, monogamous pair bonds [32]. In anthropoid primates, positive correlations between neocortex size and group size are argued to stem from the greater need to remember, track and manage relationships in larger groups [1]. Brain size is also particularly large in species with low within-group kinship, where individuals must make regular strategic decisions to manage conflicts of interest [37]. In addition, comparative studies have also revealed links between social structure and performance in a number of cognitive tasks. For instance, primates experiencing fission–fusion dynamics outperform those with more stable groups in tests of inhibitory control [34], and the highly social pinyon jay (Gymnorhinus cyanocephalus) outperforms less social corvids in transitive inference and reversal learning tasks [33,35].
Nevertheless, a number of studies have reported findings inconsistent with the SIH (reviewed in Holekamp [38]). For example, Sayol et al.'s [3] comprehensive analysis found no relationship between mating system and brain size in birds, and one of the largest avian forebrains (relative to total brain size) is found in the non-social owl (Athene noctua) [39]. It is also worth noting that the majority of comparative studies investigating cognitive evolution use neuroanatomical measures as proxies for cognition. The relationship between cognition and neuroanatomy remains highly contentious [40]; for example, it has been argued that gross measures of brain size, or brain regions, do not explicitly quantify neural functioning, and therefore, more refined neurobiological measures are required, such as neuron counting. Conversely, there is also evidence for a link between brain size and cognitive performance within, as well as between species (e.g. [9,41]).
The conflicting evidence generated from comparative studies suggests the need for a novel approach to the study of cognitive evolution. Recent studies focusing on individual variation in cognition have produced exciting results (e.g. the role that cognition plays in mate choice [42]), indicating that an intraspecific approach to the study of cognitive evolution, may be a valuable addition to comparative studies as a means of furthering our understanding of cognitive evolution.
2. An intraspecific approach to the study of cognition
A focus on individual differences in cognitive performance within species allows the causes and consequences of variation in cognitive ability to be quantified [43–47]. This is in contrast with an interspecific approach, where variation in cognitive performance within species is often disregarded as ‘noise’ [44] and species-level estimates of cognitive performance or brain size are used. This is also true of explanatory terms in analyses; for example, comparative analyses of the relationship between group size and brain size typically use average group size per species, despite there often being considerable intraspecific variation in group size [45]. Moreover, while comparative analyses often attempt to control for ecological and phylogenetic confounds, these are difficult to remove altogether and analyses can yield very different results depending on which variables are included and how those variables are specified [48]. Thus, rather than focusing exclusively on species- or population-level averages, vital insights may be gained by focusing on the causes of individual variation and linking cognitive variation to fitness consequences.
3. Intraspecific evidence for the social intelligence hypothesis?
Over the past decade, studies investigating intraspecific variation in cognitive performance have started to accumulate evidence for profound effects of both ecological factors and social factors. For instance, several studies on birds and fish have shown cognitive differences between individuals exposed to different climatic variables, predation pressure and feeding regimes (see table 1 for studies investigating the effect of the non-social environment on cognition), while zoo and laboratory studies show that enrichment of the physical environment can promote cognitive performance (e.g. [65,66]). Intraspecific studies are also generating evidence consistent with the SIH, showing that social factors can influence cognitive development, and in some cases that this may have important fitness consequences as well (see table 2 for studies investigating the effect of the social environment on cognition).
Table 1.
Studies investigating the relationship between the non-social environment and individual cognitive performance/neuroanatomy in non-human animals (note this is an illustrative sample of studies, not a comprehensive list).
| study | study species | measure of cognition | environmental variable | effect of environmental variable on measure of cognition? | fitness consequences? |
|---|---|---|---|---|---|
| Pravosudov & Clayton [49] | black-capped chickadee, Poecile atricapillus | hippocampal volume and spatial memory | altitude | positive | high altitude more efficient at cache recovery |
| Roth & Pravosudov [22] | P. atricapillus | hippocampal volume and neuron number | altitude | positive | not tested |
| Chancellor et al. [50] | P. atricapillus | hippocampal neurogenesis | altitude | positive | not tested |
| Freas et al. [51] | mountain chickadee, P. gambelli | spatial memory, hippocampal volume and neuron number | altitude | positive | not tested |
| Roth et al. [52] | P. atricapillus | hippocampal volume | altitude | positive | not tested |
| Freas et al. [53] | P. gambelli; P. atricapillus | hippocampal neuron soma size (volume, neuron number, neuron soma area) | altitude | positive | not tested |
| Freas et al. [54] | P. gambelli | hippocampal neuron soma size, volume, neuron number | altitude | positive | not tested |
| Roth et al. [55] | P. atricapillus | hippocampal glial cells | altitude | positive | not tested |
| Croston et al. [20] | P. gambelli | spatial memory | altitude | positive | not tested |
| Kotrschal & Taborsky [56] | cichlid, Simochromis pleurospilus | associative learning | environmental unpredictability | positive | not tested |
| Tebbich & Teschke [57] | woodpecker finch, Cactospiza pallida | reversal learning | environmental unpredictability | positive | not tested |
| Odling-Smee et al. [58] | three-spine stickleback, Gasterosteus aculeatus | spatial learning | environmental complexity | positive | not tested |
| Spence et al. [59] | zebra fish, Danio rerio | maze learning | environmental complexity | positive | not tested |
| Brown & Braithwaite [60] | poeciliid, Brachyraphis episcopi | spatial learning | predation pressure | negative | not tested |
| Brydges et al. [61] | three-spine stickleback | spatial learning | predation pressure | negative | not tested |
| Burns & Rodd [62] | guppy, Poecilia reticulata | spatial memory, telencephalon size | predation pressure | no effect | not tested |
| Croston et al. [63] | P. gambelli | spatial memory, reversal learning | altitude | mixed (no effect and negative) | not tested |
| Hermer et al. [64] | great tit, Parus major | reversal learning | altitude | mixed (no effect and negative) | not tested |
Table 2.
Studies investigating the relationship between the social environment and individual cognitive performance/neuroanatomy in non-human animals (note this is an illustrative sample of studies, not a comprehensive list).
| study | study species | measure of cognition | measure of sociality | longitudinal testing? | effect of sociality on measure of cognition? | fitness consequences? |
|---|---|---|---|---|---|---|
| Ashton et al. [67] | Australian magpie (C. tibicen dorsalis) | behavioural inhibition, associative learning, reversal learning, spatial memory | group size | repeated testing of juveniles at 100, 200 and 300 days post-fledging | positive | positive relationship between cognitive performance and female reproductive success |
| Sallet et al. [68] | rhesus macaque, Macaca mulatta | size of various brain regions | social network size | not tested | positive | positive relationship between brain size and social dominance |
| Fischer et al. [69] | cichlid, Neolamprologus pulcher | size of various brain regions | rearing group size | not at the individual level, but treatment groups reared in isolation for different lengths of time | positive | not tested |
| Gonda et al. [70] | nine-spined stickleback, Pungitius pungitius | size of various brain regions | group size | not tested | positive | not tested |
| Fowler et al. [71] | prairie vole, Microtus ochrogaster | size of various brain regions | isolation versus male exposure | not at the individual level, but treatment groups reared in isolation for different lengths of time | positive | not tested |
| Lipkind et al. [72] | zebra finch, Taenogypia guttata | neuron number | group size | not tested | positive | not tested |
| Gonda et al. [73] | common frog, Rana temporaria | size of various brain regions | tadpole density | not tested | positive | not tested |
| Trokovic et al. [74] | R. temporaria | optic tecta | tadpole density | carry over effect from tadpole to froglet | positive | not tested |
| Ott & Rogers [75] | desert locust, Schistocerca gregaria | size of various brain regions | solitary versus gregarious | not tested | positive | not tested |
| Branchi et al. [76] | house mouse, Mus musculus | nerve growth factor | communal nest versus standard nest | not tested | positive | not tested |
| Dalesman [77] | pond snail, Lymnaea stagnalis | long-term memory | group living versus isolation | not tested | positive | not tested |
| Arnold & Taborsky [78] | cichlid, N. pulcher | social competence | parents and helpers versus no adults | not tested | positive | not tested |
| Taborsky et al. [79] | cichlid, N. pulcher | social competence | reared with older versus reared with same age conspecifics | not tested | positive | not tested |
| Seid & Junge [80] | ant, Camponotus floridanus | mushroom bodies | isolation versus groups | not at the individual level, but treatment groups reared in isolation for different lengths | positive | not tested |
| Smith et al. [81] | sweat bee, Megalopta genalis | mushroom bodies | social reproductives versus solitary reproductives | not tested | positive | not tested |
| Ehmer et al. [82] | paper wasp, Polistes dominulus | size of various brain regions | single foundress versus multiple foundress | not tested | positive | not tested |
| Amitai et al. [83] | rat, Rattus norvegicus | reversal learning | isolation reared versus socially reared | not tested | positive | not tested |
| Bianchi et al. [84] | rat, R. norvegicus | novel object recognition task | isolation reared versus socially reared | not tested | positive | not tested |
| Lu et al. [85] | rat, R. norvegicus | learning and spatial memory | group reared versus isolation reared | not at the individual level, but treatment groups reared in isolation for different lengths. | positive | not tested |
| Wongwitdecha & Marsden [86] | rat, R. norvegicus | place learning, reversal learning | isolation reared versus group reared | not tested | negative | not tested |
| Frisone et al. [87] | rat, R. norvegicus | spatial memory | isolation reared versus group reared | tested as juveniles and adults | mixed (negative and positive) | not tested |
| Riley et al. [88] | tree skink, Egernia striolata | motor, discrimination, and reversal learning | isolation reared versus group reared | not tested | no effect | not tested |
The majority of evidence supporting a social theory of intellect is derived from studies on humans (see Kwak et al. [89] and references therein). For example, social network size has been found to predict orbital prefrontal cortex size [90] and ventromedial prefrontal volume [91], and Kanai et al. [92] found that online (as well as real world) social networks predicted right superior temporal sulcus, left middle temporal gyrus and entorhinal cortex size.
A small but growing number of studies, encompassing a broad range of taxa, are accumulating evidence linking social factors with intraspecific cognitive variation in non-human animals. For example, Sallet et al. [68] found that social network size correlates with levels of grey matter in the brains of rhesus macaques (Mucaca mulatta), and social enrichment has a positive effect on neural development in prairie voles [71] (Microtus ochrogaster) and mice [76] (Mus musculus). There is also evidence for a relationship between social group size and the development of various brain regions in invertebrates [75,80–82], amphibians [73,74], fish [69,70] and birds [72]. Although these studies suggest the social environment may play an important role in cognitive evolution, none of them directly quantify cognitive traits, with the majority of studies using measures of brain size or structure as a proxy for cognitive abilities (e.g. [69]); see Healy & Rowe [93] for a critical review of correlational studies of brain size or structure.
Intraspecific evidence suggests there is a relationship between sociality and cognition (table 2). However, it is worth noting that a number of these studies [83,84] use social isolation as a treatment: as isolation is likely to be highly stressful for social animals, these results may reflect pathological impacts of developmental stress rather than the cognitive demands of group living. Furthermore, none of these studies were carried out on wild populations of animals (table 2). To quantify and analyse variation in cognitive traits in ecologically relevant contexts, particularly in larger animals whose natural conditions cannot be readily replicated in the laboratory, it is vital to carry out tests on wild populations of animals, as selective pressures may be substantially different in captive conditions compared to the wild [47]. In order to determine the potential for selection to act on cognitive traits, it is also vital to examine the fitness consequences of variation in cognition, something that has rarely been attempted (table 2). Although reliably quantifying cognitive traits and monitoring fitness, especially in the wild, presents a number of challenges, [43,45,47], it is crucial if we are to further our understanding of factors shaping cognitive evolution.
4. Social influences on cognitive development in Australian magpies
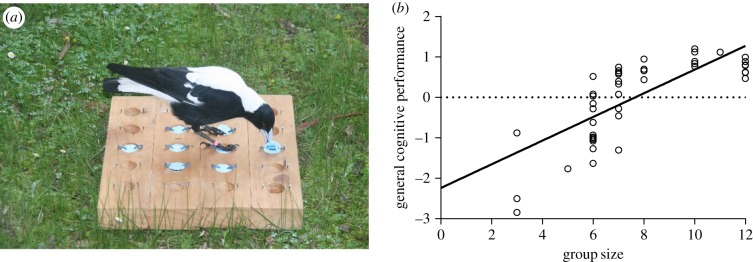
We investigated the causes and consequences of individual variation in cognition in a wild population of Australian magpies (Cracticus tibicen dorsalis) at our field site in Perth, Western Australia. Cognitive performance was quantified by presenting individuals in 14 groups (ranging in size from three to 12 individuals) with a battery of four psychometric tests designed to measure inhibitory control, associative learning, reversal learning and spatial memory. We found individual performance was significantly positively correlated across all four tasks, and a principal component analysis (PCA) revealed evidence of a general cognitive factor (referred to as general cognitive performance hereafter) underlying cognitive performance [67]. Although there is evidence of general cognitive performance in a wide range of taxa [94–99], there are few examples of it being recorded in wild populations (although see [94,95]). Crucially, we found a strong positive association between group size and general cognitive performance ([67], figure 1). This relationship could not be explained by food intake (recorded during focal follows carried out outside of cognitive testing), body size, neophobia or time spent interacting with the task, suggesting that adults in large groups do well on tasks not because they are better fed or better able to focus on tasks, but rather because living in larger groups involves informational demands that affect cognitive development.
Figure 1.
(a) An Australian magpie interacting with a cognitive task, and (b) the relationship between group size and general cognitive performance. Reproduced with permission from Ashton et al. [67]. (Online version in colour.)
An important strength of the individual-based approach to the study of cognition is that it allows individual cognitive performance to be recorded over time [45]. Obtaining such longitudinal data allows us to determine factors affecting cognitive development. Few of the limited number of studies investigating the causes of intraspecific cognitive variation have attempted this (table 2), but there is growing evidence that the early social environment can affect brain development [69,74,76] and adult social learning ability [100]. In our research, we presented our cognitive test battery to juvenile Australian magpies at 100, 200 and 300 days post-fledging, finding that the relationship between group size and cognitive performance emerges as birds get older [67], adding to the weight of evidence that social factors can drive the development of domain-general cognitive abilities. One important, but as yet relatively unexplored issue, is whether this relationship may in fact be bi-directional: that is, while social factors may influence cognitive development, an individual's cognitive phenotype may also influence their social interactions with others [101].
5. Cognitive plasticity and evolution
Thus far, we have presented evidence that differences in the social environment experienced by different individuals may influence the development of their cognitive abilities. However, the SIH, as originally formulated, is an evolutionary, not a developmental hypothesis. To begin to address evolutionary questions, it is therefore necessary to ask whether elevated cognitive performance provides selective benefits. Previous studies using psychologically grounded psychometric tests have found both positive [102–106], negative [107] and no relationships [94,105] between individual cognitive performance and measures of fitness. In Australian magpies, we found that females that performed well in cognitive tasks had more successful nesting attempts, fledged more chicks and had more offspring that survived to independence [67]. Furthermore, cognitive performance was a stronger predictor of individual reproductive success than both foraging efficiency and body mass, indicating that variation in fitness was a direct consequence of cognitive performance, rather than nutritional intake [67]. Thus, it seems that in this species, the size of the group an individual grows up in influences the development of its cognitive performance, and cognitive performance in turn may have important consequences for reproductive fitness. As we were unable to manipulate group size experimentally, this proposed causal pathway cannot be demonstrated unequivocally, but these findings raise important questions. How does selection act upon a trait that is, at least in part, shaped by the social environment during early development? Is cognitive plasticity itself adaptive? Can developmental reaction norms themselves be shaped by selection? The answers to these questions have far reaching implications, not only in terms of understanding cognitive evolution, but also how we approach the study of evolution in general. Our results are consistent with the view that the proximate/ultimate distinction may be blurrier than is often suggested [108], as developmental processes may often be vital in shaping phenotypes that serve an ultimate function. Further work is needed to understand the interplay between development, inheritance and selection in shaping cognitive phenotypes.
6. Future directions
Although there is evidence for a link between sociality, cognition and fitness, the underlying mechanisms driving these associations are unclear. First, to unequivocally determine causality in the group size–cognition relationship, experimental manipulations of group size would be required. Cross-fostering experiments present the best opportunity to do this, but in the wild, they may only be feasible in species that breed synchronously and will accept eggs or young introduced from other groups. Another priority for future research is to determine precisely how and why group size affects cognitive development. Although we have argued that our findings cannot be explained by greater foraging intake for individuals in large groups, it is possible that nutrient quality, rather than total amount of food captured/received, drives cognitive development. Stable isotope analyses [109] may reveal if diet significantly differs between individuals from larger and smaller groups, and between individuals that exhibit differing cognitive performance. It is also important to characterize the social demands of living in larger groups, and relate these to cognitive development. Even within a group of a given size, different group members may well experience different information-processing demands, depending on the pattern of their agonistic and affiliative interactions and the strength and number of their relationships [110]. Social network analyses can help to quantify these relationships and characterize each individual's position within the wider social network (e.g. [111]), allowing us to relate each individual's cognitive profile to the specific social challenges it has faced during development.
A related point is the need to identify informational challenges more broadly. Studies of cognitive evolution have often tended to adopt a dichotomous approach: the key selection pressures acting on cognitive traits are either ecological or social. However, in reality, this distinction is not clear-cut: social animals, after all, solve ecological problems in a social context (see also [34]). Western-scrub jays (Aphelocoma californica), for example, use episodic memory to solve an ecological problem: remembering and retrieving food they have cached for the winter [112], but if there are other scrub jays present, they also face the need to outwit conspecifics so as to avoid having their caches stolen [113]. Thus, the problem is both ecological and social. Moreover, while some proponents of the SIH have argued that the demands of group living should specifically drive the evolution of socio-cognitive traits, there is increasing evidence that social behaviour often relies on the same, domain-general cognitive processes that are used to solve ecological problems [114,115]. Our research [67] speaks to this issue, in that social factors (specifically group size) appear to influence the development of basic cognitive processes (learning, memory and inhibitory control) that are not specifically social. The routes through which sociality affects cognitive development are still unknown, but could involve both explicitly social challenges (e.g. having to learn the characteristics of multiple different individuals) and ecological challenges that happen to be played out in a social context. For instance, if adults within a group show differing foraging niches [116], then dependent young in large groups may be exposed to a greater range of foraging locations, strategies and food types, driving elevated cognitive performance compared to youngsters in small groups. Thus, future studies may benefit from abandoning the explicit social/ecological dichotomy and focus instead on characterizing the full range of informational challenges that animals must solve.
Finally, while several studies have now identified a relationship between cognition and measures of fitness [102–106], our understanding of why cognition confers fitness benefits is limited. To resolve this, studies need to investigate how cognitive performance may influence the specific aspect of fitness being measured; for example, in the Australian magpie, an important next step will be to investigate if females with greater general cognitive performance provide offspring with improved parental care (perhaps through provisioning food of greater nutritional quality), and/or whether they are better at protecting their fledglings from threats from predators and conspecifics. Such research would have the potential to reveal why smarter females are capable of rearing offspring more successfully.
7. Conclusion
Understanding the factors driving cognitive evolution is one of the greatest challenges in biology today. Here, we highlight how several recent studies, using the relatively novel approach of focusing on the causes and consequences of individual variation in cognition, provide evidence of a link between sociality and cognition. While these results are broadly consistent with the SIH, we suggest that the distinction between social and ecological influences may, to a large extent, be artificial. Adopting an individual-based approach to the study of cognition will be important in revealing the information-processing challenges animals face in their physical and social environments, and elucidating the role of developmental processes in cognitive evolution.
Acknowledgements
We thank E. Russell and the late I. Rowley for access to their life-history records, and for allowing us to continue work on their Guildford magpie population.
Data accessibility
This article has no additional data.
Authors' contributions
B.J.A. wrote the original draft. A.T. and A.R.R. provided substantial contributions to the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by an ARC Discovery grant awarded to A.R.R., A.T. and M.B.V. Bell, and an Endeavour Research Fellowship awarded to B.J.A. A.T. received additional support from a BBSRC David Phillips Fellowship (BB/H021817/2) and the Human Frontiers Research Program (RGP00049).
References
- 1.Dunbar RIM, Shultz S. 2007. Evolution in the social brain. Science 317, 1344–1347. ( 10.1126/science.1145463) [DOI] [PubMed] [Google Scholar]
- 2.DeCasien AR, Williams SA, Higham JP. 2017. Primate brain size is predicted by diet but not sociality. Nat. Ecol. Evol. 1, 0112 ( 10.1038/s41559-017-0112) [DOI] [PubMed] [Google Scholar]
- 3.Sayol F, Maspons J, Lapiedra O, Iwaniuk AN, Székely T, Sol D. 2016. Environmental variation and the evolution of large brains in birds. Nat. Commun. 7, 13 971 ( 10.1038/ncomms13971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosati AG. 2017. Foraging cognition: reviving the ecological intelligence hypothesis. Trends Cogn. Sci. 21, 691–702. ( 10.1016/j.tics.2017.05.011) [DOI] [PubMed] [Google Scholar]
- 5.Sol D. 2009. The cognitive buffer hypothesis for the evolution of large brains. In Cognitive ecology II (eds Dukas R, Ratcliffe JM), pp. 111–136. Chicago, IL: Chicago University Press. [Google Scholar]
- 6.Shettleworth SJ. 2010. Cognition, evolution and behaviour, 2nd edn. Oxford, UK: Oxford University Press. [Google Scholar]
- 7.Milton K. 1988. Foraging behaviour and the evolution of primate intelligence. In Machiavellian intelligence (eds Byrne RW, Whiten A), pp. 285–305. Oxford, UK: Clarendon Press. [Google Scholar]
- 8.Kotrschal A, Corral-Lopez A, Amcoff M, Kolm N. 2014. A larger brain confers a benefit in a spatial mate search learning task in male guppies. Behav. Ecol. 26, 527–532. ( 10.1093/beheco/aru227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotrschal A, Rogell B, Bundsen A, Svensson B, Zajitschek S, Brännström I, Immler S, Maklakov AA, Kolm N. 2013. The benefit of evolving a larger brain: big-brained guppies perform better in a cognitive task. Anim. Behav. 86, e4 ( 10.1016/j.anbehav.2013.07.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotrschal A, Rogell B, Bundsen A, Svensson B, Zajitschek S, Brännström I, Immler S, Maklakov AA, Kolm N. 2013. Artificial selection on relative brain size in the guppy reveals costs and benefits of evolving a larger brain. Curr. Biol. 23, 168–171. ( 10.1016/j.cub.2012.11.058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reader SM, Laland KN. 2002. Social intelligence, innovation, and enhanced brain size in primates. Proc. Natl Acad. Sci. USA 99, 4436–4441. ( 10.1073/pnas.062041299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sol D, Duncan RP, Blackburn TM, Cassey P, Lefebvre L. 2005. Big brains, enhanced cognition, and response of birds to novel environments. Proc. Natl Acad. Sci. USA 102, 5460–5465. ( 10.1073/pnas.0408145102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janmaat KRL, Byrne RW, Zuberbühler K. 2006. Primates take weather into account when searching for fruits. Curr. Biol. 16, 1232–1237. ( 10.1016/j.cub.2006.04.031) [DOI] [PubMed] [Google Scholar]
- 14.Lefebvre L, Reader SM, Sol D. 2004. Brains, innovations and evolution in birds and primates. Brain. Behav. Evol. 63, 233–246. ( 10.1159/000076784) [DOI] [PubMed] [Google Scholar]
- 15.Sol D, Lefebvre L, Rodríguez-Teijeiro JD. 2005. Brain size, innovative propensity and migratory behaviour in temperate Palaearctic birds. Proc. R. Soc. B 272, 1433–1441. ( 10.1098/rspb.2005.3099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Woerden JT, Willems EP, van Schaik CP, Isler K. 2012. Large brains buffer energetic effects of seasonal habitats in catarrhine primates. Evolution 66, 191–199. ( 10.1111/j.1558-5646.2011.01434.x) [DOI] [PubMed] [Google Scholar]
- 17.Pravosudov VV, Kitaysky AS, Omanska A. 2006. The relationship between migratory behaviour, memory and the hippocampus: an intraspecific comparison. Proc. R. Soc. B 273, 2641–2649. ( 10.1098/rspb.2006.3624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cristol DA, Reynolds EB, Leclerc JE, Donner AH, Farabaugh CS, Ziegenfus CWS. 2003. Migratory dark-eyed juncos, Junco hyemalis, have better spatial memory and denser hippocampal neurons than nonmigratory conspecifics. Anim. Behav. 66, 317–328. ( 10.1006/anbe.2003.2194) [DOI] [Google Scholar]
- 19.Mettke-Hofmann C, Gwinner E. 2003. Long-term memory for a life on the move. Proc. Natl Acad. Sci. USA 100, 5863–5866. ( 10.1073/pnas.1037505100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Croston R, Kozlovsky DY, Branch CL, Parchman TL, Bridge ES, Pravosudov VV. 2016. Individual variation in spatial memory performance in wild mountain chickadees from different elevations. Anim. Behav. 111, 225–234. ( 10.1016/j.anbehav.2015.10.015) [DOI] [Google Scholar]
- 21.Croston R, Branch CL, Kozlovsky DY, Roth TC, LaDage LD, Freas CA, Pravosudov VV. 2015. Potential mechanisms driving population variation in spatial memory and the hippocampus in food-caching chickadees. Integr. Comp. Biol. 55, 354–371. ( 10.1093/icb/icv029) [DOI] [PubMed] [Google Scholar]
- 22.Roth TC, Pravosudov VV. 2009. Hippocampal volumes and neuron numbers increase along a gradient of environmental harshness: a large-scale comparison. Proc. R. Soc. B 276, 401–405. ( 10.1098/rspb.2008.1184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shultz S, Dunbar RI. 2006. Both social and ecological factors predict ungulate brain size. Proc. R. Soc. B 273, 207–215. ( 10.1098/rspb.2005.3283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chance MRA, Mead AP. 1953. Social behaviour and primate evolution. Symp. Soc. Exp. Biol. 7, 395–439. [Google Scholar]
- 25.Jolly A. 1966. Lemur social behavior and primate intelligence. Science 153, 501–506. ( 10.1126/science.153.3735.501) [DOI] [PubMed] [Google Scholar]
- 26.Humphrey N. 1976. The social function of intellect. In Growing points in ethology (eds Bateson PPG, Hinde RA), pp. 303–317. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 27.Dunbar RIM. 1998. The social brain hypothesis. Evol. Anthropol. Issues News Rev. 6, 178–190. ( 10.1002/(SICI)1520-6505(1998)6:5%3C178::AID-EVAN5%3E3.3.CO;2-P) [DOI] [Google Scholar]
- 28.Brosnan SF, Salwiczek L, Bshary R. 2010. The interplay of cognition and cooperation. Phil. Trans. R. Soc. B 365, 2699–2710. ( 10.1098/rstb.2010.0154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massen JJM, Szipl G, Spreafico M, Bugnyar T. 2014. Ravens intervene in others' bonding attempts. Curr. Biol. 24, 2733–2736. ( 10.1016/j.cub.2014.09.073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Byrne RW, Whiten A (eds). 1988. Machiavellian intelligence: social expertise and the evolution of intellect in monkeys, apes, and humans, 1st edn. Oxford, UK: Clarendon Press. [Google Scholar]
- 31.Silk JB. 2007. Social components of fitness in primate groups. Science 317, 1347–1351. ( 10.1126/science.1140734) [DOI] [PubMed] [Google Scholar]
- 32.Emery NJ, Seed AM, von Bayern AMP, Clayton NS. 2007. Cognitive adaptations of social bonding in birds. Phil. Trans. R. Soc. B 362, 489–505. ( 10.1098/rstb.2006.1991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bond AB, Kamil AC, Balda RP. 2003. Social complexity and transitive inference in corvids. Anim. Behav. 65, 479–487. ( 10.1006/anbe.2003.2101) [DOI] [Google Scholar]
- 34.Amici F, Aureli F, Call J. 2008. Fission–fusion dynamics, behavioral flexibility, and inhibitory control in primates. Curr. Biol. 18, 1415–1419. ( 10.1016/j.cub.2008.08.020) [DOI] [PubMed] [Google Scholar]
- 35.Bond AB, Kamil AC, Balda RP. 2007. Serial reversal learning and the evolution of behavioral flexibility in three species of North American corvids (Gymnorhinus cyanocephalus, Nucifraga columbiana, Aphelocoma californica). J. Comp. Psychol. 121, 372–379. ( 10.1037/0735-7036.121.4.372) [DOI] [PubMed] [Google Scholar]
- 36.Silk JB. 2007. The adaptive value of sociality in mammalian groups. Phil. Trans. R. Soc. B 362, 539–559. ( 10.1098/rstb.2006.1994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lukas D, Clutton-brock T. 2018. Social complexity and kinship in animal societies. Ecol. Lett. ( 10.5063/F1FB513K) [DOI] [PubMed] [Google Scholar]
- 38.Holekamp KE. 2007. Questioning the social intelligence hypothesis. Trends Cogn. Sci. 11, 65–69. ( 10.1016/j.tics.2006.11.003) [DOI] [PubMed] [Google Scholar]
- 39.Burish MJ, Kueh HY, Wang SS-H. 2004. Brain architecture and social complexity in modern and ancient birds. Brain. Behav. Evol. 63, 107–124. ( 10.1159/000075674) [DOI] [PubMed] [Google Scholar]
- 40.Healy SD, Rowe C. 2013. Costs and benefits of evolving a larger brain: doubts over the evidence that large brains lead to better cognition. Anim. Behav. 86, e1–e3. ( 10.1016/j.anbehav.2013.05.017) [DOI] [Google Scholar]
- 41.MacLean EL, et al. 2014. The evolution of self-control. Proc. Natl Acad. Sci. USA 111, E2140–E2148. ( 10.1073/pnas.1323533111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Templeton CN, Laland KN, Boogert NJ. 2014. Does song complexity correlate with problem-solving performance in flocks of zebra finches? Anim. Behav. 92, 63–71. ( 10.1016/j.anbehav.2014.03.019) [DOI] [Google Scholar]
- 43.Rowe C, Healy SD. 2014. Measuring variation in cognition. Behav. Ecol. 25, 1287–1292. ( 10.1093/beheco/aru090) [DOI] [Google Scholar]
- 44.Thornton A, Isden J, Madden JR. 2014. Toward wild psychometrics: linking individual cognitive differences to fitness. Behav. Ecol. 25, 1299–1301. ( 10.1093/beheco/aru095) [DOI] [Google Scholar]
- 45.Thornton A, Lukas D. 2012. Individual variation in cognitive performance: developmental and evolutionary perspectives. Phil. Trans. R. Soc. B 367, 2773–2783. ( 10.1098/rstb.2012.0214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Croston R, Branch CL, Kozlovsky DY, Dukas R, Pravosudov VV. 2015. Heritability and the evolution of cognitive traits. Behav. Ecol. 26, 1447–1459. ( 10.1093/beheco/arv088) [DOI] [Google Scholar]
- 47.Morand-Ferron J, Cole EF, Quinn JL. 2015. Studying the evolutionary ecology of cognition in the wild: a review of practical and conceptual challenges. Biol. Rev. 91, 367–389. ( 10.1111/brv.12174) [DOI] [PubMed] [Google Scholar]
- 48.Lukas D, Clutton-Brock T. 2017. Comparative studies need to rely both on sound natural history data and on excellent statistical analysis. R. Soc. open sci. 4, 171211 ( 10.1098/rsos.170346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pravosudov VV, Clayton NS. 2002. A test of the adaptive specialization hypothesis: population differences in caching, memory, and the hippocampus in black-capped chickadees (Poecile atricapilla). Behav. Neurosci. 116, 515–522. ( 10.1037/0735-7044.116.4.515) [DOI] [PubMed] [Google Scholar]
- 50.Chancellor LV, Roth TC, LaDage LD, Pravosudov VV. 2011. The effect of environmental harshness on neurogenesis: a large-scale comparison. Dev. Neurobiol. 71, 246–252. ( 10.1002/dneu.20847) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Freas CA, LaDage LD, Roth TC, Pravosudov VV. 2012. Elevation-related differences in memory and the hippocampus in mountain chickadees, Poecile gambeli. Anim. Behav. 84, 121–127. ( 10.1016/j.anbehav.2012.04.018) [DOI] [Google Scholar]
- 52.Roth TC, LaDage LD, Freas CA, Pravosudov VV. 2012. Variation in memory and the hippocampus across populations from different climates: a common garden approach. Proc. R. Soc. B 279, 402–410. ( 10.1098/rspb.2011.1020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freas CA, Roth TC, Ladage LD, Pravosudov VV. 2013. Hippocampal neuron soma size is associated with population differences in winter climate severity in food-caching chickadees. Funct. Ecol. 27, 1341–1349. ( 10.1111/1365-2435.12125) [DOI] [Google Scholar]
- 54.Freas CA, Bingman K, Ladage LD, Pravosudov VV. 2013. Untangling elevation-related differences in the hippocampus in food-caching mountain chickadees: the effect of a uniform captive environment. Brain. Behav. Evol. 82, 199–209. ( 10.1159/000355503) [DOI] [PubMed] [Google Scholar]
- 55.Roth TC, Chevalier DM, Ladage LD, Pravosudov VV. 2013. Variation in hippocampal glial cell numbers in food-caching birds from different climates. Dev. Neurobiol. 73, 480–485. ( 10.1002/dneu.22074) [DOI] [PubMed] [Google Scholar]
- 56.Kotrschal A, Taborsky B. 2010. Environmental change enhances cognitive abilities in fish. PLoS Biol. 8, e1000351 ( 10.1371/journal.pbio.1000351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tebbich S, Teschke I. 2014. Coping with uncertainty: woodpecker finches (Cactospiza pallida) from an unpredictable habitat are more flexible than birds from a stable habitat. PLoS ONE 9, e91718 ( 10.1371/journal.pone.0091718) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Odling-Smee LC, Boughman JW, Braithwaite VA. 2008. Sympatric species of threespine stickleback differ in their performance in a spatial learning task. Behav. Ecol. Sociobiol. 62, 1935–1945. ( 10.1007/s00265-008-0625-1) [DOI] [Google Scholar]
- 59.Spence R, Magurran AE, Smith C. 2011. Spatial cognition in zebrafish: the role of strain and rearing environment. Anim. Cogn. 14, 607–612. ( 10.1007/s10071-011-0391-8) [DOI] [PubMed] [Google Scholar]
- 60.Brown C, Braithwaite VA. 2005. Effects of predation pressure on the cognitive ability of the poeciliid Brachyraphis episcopi. Behav. Ecol. 16, 482–487. ( 10.1093/beheco/ari016) [DOI] [Google Scholar]
- 61.Brydges NM, Heathcote RJP, Braithwaite VA. 2008. Habitat stability and predation pressure influence learning and memory in populations of three-spined sticklebacks. Anim. Behav. 75, 935–942. ( 10.1016/j.anbehav.2007.08.005) [DOI] [PubMed] [Google Scholar]
- 62.Burns JG, Rodd FH. 2008. Hastiness, brain size and predation regime affect the performance of wild guppies in a spatial memory task. Anim. Behav. 76, 911–922. ( 10.1016/j.anbehav.2008.02.017) [DOI] [Google Scholar]
- 63.Croston R, Branch CL, Pitera AM, Kozlovsky DY, Bridge ES, Parchman TL, Pravosudov VV. 2017. Predictably harsh environment is associated with reduced cognitive flexibility in wild food-caching mountain chickadees. Anim. Behav. 123, 139–149. ( 10.1016/j.anbehav.2016.10.004) [DOI] [Google Scholar]
- 64.Hermer E, Cauchoix M, Chaine AS, Morand-Ferron J. 2018. Elevation related difference in serial reversal learning ability in a non-scatter hoarding passerine. Behav. Ecol. 1–8. ( 10.1093/beheco/ary067) [DOI] [Google Scholar]
- 65.Krech D, Rosenzweig MR, Bennett EL. 1960. Effects of environmental complexity and training on brain chemistry. J. Comp. Physiol. Psychol. 53, 509–519. ( 10.1037/h0045402) [DOI] [PubMed] [Google Scholar]
- 66.Meyer S, Puppe B, Langbein J. 2010. Cognitive enrichment in zoo and farm animals—implications for animal behaviour and welfare. Berl. Munch. Tierarztl. Wochenschr. 123, 446–456. [PubMed] [Google Scholar]
- 67.Ashton BJ, Ridley AR, Edwards EK, Thornton A. 2018. Cognitive performance is linked to group size and affects fitness in Australian magpies. Nature 554, 364–367. ( 10.1038/nature25503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sallet J, et al. 2011. Social network size affects neural circuits in macaques. Science 334, 697–700. ( 10.1126/science.1210027) [DOI] [PubMed] [Google Scholar]
- 69.Fischer S, Bessert-Nettelbeck M, Kotrschal A, Taborsky B. 2015. Rearing-group size determines social competence and brain structure in a cooperatively breeding cichlid. Am. Nat. 186, 123–140. ( 10.1086/681636) [DOI] [PubMed] [Google Scholar]
- 70.Gonda A, Herczeg G, Merila J. 2009. Habitat-dependent and -independent plastic responses to social environment in the nine-spined stickleback (Pungitius pungitius) brain. Proc. R. Soc. B 276, 2085–2092. ( 10.1098/rspb.2009.0026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fowler CD, Liu Y, Ouimet C, Wang Z. 2002. The effects of social environment on adult neurogenesis in the female prairie vole. J. Neurobiol. 51, 115–128. ( 10.1002/neu.10042) [DOI] [PubMed] [Google Scholar]
- 72.Lipkind D, Nottebohm F, Rado R, Barnea A. 2002. Social change affects the survival of new neurons in the forebrain of adult songbirds. Behav. Brain Res. 133, 31–43. ( 10.1016/S0166-4328(01)00416-8) [DOI] [PubMed] [Google Scholar]
- 73.Gonda A, Trokovic N, Herczeg G, Laurila A, Merilä J. 2010. Predation- and competition-mediated brain plasticity in Rana temporaria tadpoles. J. Evol. Biol. 23, 2300–2308. ( 10.1111/j.1420-9101.2010.02066.x) [DOI] [PubMed] [Google Scholar]
- 74.Trokovic N, Gonda A, Herczeg G, Laurila A, Merilä J. 2011. Brain plasticity over the metamorphic boundary: carry-over effect of larval environment on froglet brain development. J. Evol. Biol. 24, 1380–1385. ( 10.1111/j.1420-9101.2011.02275.x) [DOI] [PubMed] [Google Scholar]
- 75.Ott SR, Rogers SM. 2010. Gregarious desert locusts have substantially larger brains with altered proportions compared with the solitarious phase. Proc. R. Soc. B 277, 3087–3096. ( 10.1098/rspb.2010.0694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Branchi I, D'Andrea I, Fiore M, Di Fausto V, Aloe L, Alleva E. 2006. Early social enrichment shapes social behavior and nerve growth factor and brain-derived neurotrophic factor levels in the adult mouse brain. Biol. Psychiatry 60, 690–696. ( 10.1016/j.biopsych.2006.01.005) [DOI] [PubMed] [Google Scholar]
- 77.Dalesman S. 2018. Habitat and social context affect memory phenotype, exploration and covariance among these traits. Phil. Trans. R. Soc. B 373, 20170291 ( 10.1098/rstb.2017.0291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arnold C, Taborsky B. 2010. Social experience in early ontogeny has lasting effects on social skills in cooperatively breeding cichlids. Anim. Behav. 79, 621–630. ( 10.1016/j.anbehav.2009.12.008) [DOI] [Google Scholar]
- 79.Taborsky B, Arnold C, Junker J, Tschopp A. 2012. The early social environment affects social competence in a cooperative breeder. Anim. Behav. 83, 1067–1074. ( 10.1016/j.anbehav.2012.01.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Seid MA, Junge E. 2016. Social isolation and brain development in the ant Camponotus floridanus. Naturwissenschaften 103, 42 ( 10.1007/s00114-016-1364-1) [DOI] [PubMed] [Google Scholar]
- 81.Smith AR, Seid MA, Jimenez LC, Wcislo WT. 2010. Socially induced brain development in a facultatively eusocial sweat bee Megalopta genalis (Halictidae). Proc. R. Soc. B 277, 2157–2163. ( 10.1098/rspb.2010.0269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ehmer B, Reeve HK, Hoy RR. 2001. Comparison of brain volumes between single and multiple foundresses in the paper wasp Polistes dominulus. Brain Behav. Evol. 57, 161–168. ( 10.1159/000047234) [DOI] [PubMed] [Google Scholar]
- 83.Amitai N, Young JW, Higa K, Sharp RF, Geyer MA, Powell SB. 2014. Isolation rearing effects on probabilistic learning and cognitive flexibility in rats. Cogn. Affect. Behav. Neurosci. 14, 388–406. ( 10.3758/s13415-013-0204-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bianchi M, Fone KFC, Azmi N, Heidbreder CA, Hagan JJ, Marsden CA. 2006. Isolation rearing induces recognition memory deficits accompanied by cytoskeletal alterations in rat hippocampus. Eur. J. Neurosci. 24, 2894–2902. ( 10.1111/j.1460-9568.2006.05170.x) [DOI] [PubMed] [Google Scholar]
- 85.Lu L, Bao G, Chen H, Xia P, Fan X, Zhang J, Pei G, Ma L. 2003. Modification of hippocampal neurogenesis and neuroplasticity by social environments. Exp. Neurol. 183, 600–609. ( 10.1016/S0014-4886(03)00248-6) [DOI] [PubMed] [Google Scholar]
- 86.Wongwitdecha N, Marsden CA. 1996. Effects of social isolation rearing on learning in the Morris water maze. Brain Res. 715, 119–124. ( 10.1016/0006-8993(95)01578-7) [DOI] [PubMed] [Google Scholar]
- 87.Frisone DF, Frye CA, Zimmerberg B. 2002. Social isolation stress during the third week of life has age-dependent effects on spatial learning in rats. Behav. Brain Res. 128, 153–160. ( 10.1016/S0166-4328(01)00315-1) [DOI] [PubMed] [Google Scholar]
- 88.Riley JL, Küchler A, Damasio T, Noble DWA, Byrne RW, Whiting MJ. 2018. Learning ability is unaffected by isolation rearing in a family-living lizard. Behav. Ecol. Sociobiol. 72, 20 ( 10.1007/s00265-017-2435-9) [DOI] [Google Scholar]
- 89.Kwak S, Joo W, Youm Y, Chey J. 2018. Social brain volume is associated with in-degree social network size among older adults. Proc. R. Soc. B 285, 20172708 ( 10.1098/rspb.2017.2708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Powell J, Lewis PA, Roberts N, Garcia-Finana M, Dunbar RIM. 2012. Orbital prefrontal cortex volume predicts social network size: an imaging study of individual differences in humans. Proc. R. Soc. B 279, 2157–2162. ( 10.1098/rspb.2011.2574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lewis PA, Rezaie R, Brown R, Roberts N, Dunbar RIM. 2011. Ventromedial prefrontal volume predicts understanding of others and social network size. Neuroimage 57, 1624–1629. ( 10.1016/j.neuroimage.2011.05.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kanai R, Bahrami B, Roylance R, Rees G. 2011. Online social network size is reflected in human brain structure. Proc. R. Soc. B 279, 1327–1334. ( 10.1098/rspb.2011.1959) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Healy SD, Rowe C. 2007. A critique of comparative studies of brain size. Proc. R. Soc. B 274, 453–464. ( 10.1098/rspb.2006.3748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Isden J, Panayi C, Dingle C, Madden J. 2013. Performance in cognitive and problem-solving tasks in male spotted bowerbirds does not correlate with mating success. Anim. Behav. 86, 829–838. ( 10.1016/j.anbehav.2013.07.024) [DOI] [Google Scholar]
- 95.Shaw RC, Boogert NJ, Clayton NS, Burns KC. 2015. Wild psychometrics: evidence for ‘general’ cognitive performance in wild New Zealand robins, Petroica longipes. Anim. Behav. 109, 101–111. ( 10.1016/j.anbehav.2015.08.001) [DOI] [Google Scholar]
- 96.Galsworthy MJ, Paya-Cano JL, Liu L, Monleón S, Gregoryan G, Fernandes C, Schalkwyk LC, Plomin R. 2005. Assessing reliability, heritability and general cognitive ability in a battery of cognitive tasks for laboratory mice. Behav. Genet. 35, 675–692. ( 10.1007/s10519-005-3423-9) [DOI] [PubMed] [Google Scholar]
- 97.Hopkins WD, Russell JL, Schaeffer J. 2014. Chimpanzee intelligence is heritable. Curr. Biol. 24, 1649–1652. ( 10.1016/j.cub.2014.05.076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chandra SB, Hosler JS, Smith BH. 2000. Heritable variation for latent inhibition and its correlation with reversal learning in honeybees (Apis mellifera). J. Comp. Psychol. 114, 86–97. ( 10.1037/0735-7036.114.1.86) [DOI] [PubMed] [Google Scholar]
- 99.Burkart JM. 2017. The evolution of general intelligence. Behav. Brain Sci. 40, e195 ( 10.1017/S0140525X16000959) [DOI] [PubMed] [Google Scholar]
- 100.Lévy F, Melo AI, Galef BG, Madden M, Fleming AS. 2003. Complete maternal deprivation affects social, but not spatial, learning in adult rats. Dev. Psychobiol. 43, 177–191. ( 10.1002/dev.10131) [DOI] [PubMed] [Google Scholar]
- 101.Wascher CAF, Kulahci IG, Langley EJG, Shaw RC. 2018. How does cognition shape social relationships? Phil. Trans. R. Soc. B 373, 20170293 ( 10.1098/rstb.2017.0293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Plomin R, Deary I. 2014. Genetics and intelligence differences: five special findings. Mol. Psychiatry 20, 98–108. ( 10.1038/mp.2014.105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Raine NE, Chittka L. 2008. The correlation of learning speed and natural foraging success in bumble-bees. Proc. R. Soc. B 275, 803–808. ( 10.1098/rspb.2007.1652) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maille A, Schradin C. 2016. Survival is linked with reaction time and spatial memory in African striped mice. Biol. Lett. 12, 277–286. ( 10.1098/rsbl.2016.0346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boogert NJ, Anderson RC, Peters S, Searcy WA, Nowicki S. 2011. Song repertoire size in male song sparrows correlates with detour reaching, but not with other cognitive measures. Anim. Behav. 81, 1209–1216. ( 10.1016/j.anbehav.2011.03.004) [DOI] [Google Scholar]
- 106.Dukas R. 2008. Evolutionary biology of insect learning. Annu. Rev. Entomol. 53, 145–160. ( 10.1146/annurev.ento.53.103106.093343) [DOI] [PubMed] [Google Scholar]
- 107.Mery F, Kawecki TJ. 2003. A fitness cost of learning ability in Drosophila melanogaster. Proc. R. Soc. Lond. B 270, 2465–2469. ( 10.1098/rspb.2003.2548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.West-Eberhard MJ. 2003. Developmental plasticity and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 109.Inger R, Bearhop S. 2008. Applications of stable isotope analyses to avian ecology. Ibis 150, 447–461. ( 10.1111/j.1474-919X.2008.00839.x) [DOI] [Google Scholar]
- 110.Barrett L, Henzi SP, Lusseau D. 2012. Taking sociality seriously: the structure of multi-dimensional social networks as a source of information for individuals. Phil. Trans. R. Soc. B 367, 2108–2118. ( 10.1098/rstb.2012.0113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Blumstein DT, Williams DM, Lim AN, Kroeger S, Martin JGA. 2018. Strong social relationships are associated with decreased longevity in a facultatively social mammal. Proc. R. Soc. B 285, 20171934 ( 10.1098/rspb.2017.1934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Clayton NS, Dickinson A. 1998. Episodic-like memory during cache recovery by scrub jays. Nature 395, 272–274. ( 10.1038/26216) [DOI] [PubMed] [Google Scholar]
- 113.Clayton NS, Dally JM, Emery NJ. 2007. Social cognition by food-caching corvids. The western scrub-jay as a natural psychologist. Phil. Trans. R. Soc. B 362, 507–522. ( 10.1098/rstb.2006.1992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leadbeater E. 2015. What evolves in the evolution of social learning? J. Zool. 295, 4–11. ( 10.1111/jzo.12197) [DOI] [Google Scholar]
- 115.Heyes C. 2012. Simple minds: a qualified defence of associative learning. Phil. Trans. R. Soc. B 367, 2695–2703. ( 10.1098/rstb.2012.0217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bolnick DII, Svanbäck R, Fordyce JAA, Yang LHH, Davis JMM, Hulsey CDD, Forister MLL. 2003. The ecology of individuals: incidence and implications of individual specialization. Am. Nat. 161, 1–28. ( 10.1086/343878) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.