Abstract
Elevated plasma cholesterol, especially low density lipoprotein (LDL) cholesterol, is one of the major risk factors for atherosclerosis and coronary heart disease. Hereditary hypertriglyceridemic rats (hHTG) were developed as a new inbred model for the study of relationships between blood pressure and metabolic abnormalities. The aim of this work was to determine the cholesterol-lowering and antioxidant effects of the novel pyridoindol derivative SMe1EC2, compared to the cholesterol-lowering drug atorvastatin, in rats fed either standard or high-fat and high-cholesterol diet (HFC; 1% cholesterol and 7.5% lard fat). Male hHTG rats fed HFC (HTG+HFC) were administered with SMe1EC2 or atorvastatin (both 50 mg/kg/day p.o.) for 4 weeks. Physiological status of animals was monitored by the measurement of preprandial glucose levels and blood pressure. Lipid profile was characterized by the serum levels of total cholesterol (TC), HDL-, LDL-cholesterol and triglycerides (TRG). The concentration of thiobarbituric acid reactive substances (TBARS) was evaluated in the kidney, liver and serum. Further, the assessment of pro-inflammatory cytokines TNF-α, IL-1 and IL-6 in the serum was completed. Feeding the animals with HFC diet resulted in increased serum levels of TC, LDL and TRG. SMe1EC2 ameliorated serum levels of LDL in hHTG rats, both on standard and HFC diet. These effects were comparable with those of the standard hypolipidemicum atorvastatin. SMe1EC2 lowered blood pressure, tissue TBARS concentrations and serum IL-1 levels of HTG+HFC rats. Beneficial effects together with very good toxicity profile predestinate SMe1EC2 to be promising agent for further surveys related to metabolic syndrome features.
Keywords: metabolic syndrome, high-fat and high-cholesterol diet, SMe1EC2, atorvastatin
Introduction
The metabolic syndrome is characterized by a cluster of interrelated metabolic factors such as insulin resistance, hyperinsulinemia, abdominal obesity, impaired glucose tolerance, dyslipidemia, hypertension, and a proinflammatory and prothrombotic state, leading to coronary heart disease and peripheral vascular disease. The main components of dyslipidemia of the metabolic syndrome, which most likely initiate atherosclerosis, are the „lipid triad“ of high plasma triglycerides, low levels of high-density lipoprotein cholesterol (HDL), and a preponderance of small, dense low-density lipoprotein (LDL) particles (Raal, 2009; Kwasny et al., 2017) At present, the only drugs approved for treatment of risk factors of metabolic syndrom are those that target the individual risk factors: lipid-lowering drugs, antihypertensive agents, hypoglycemic drugs, and anti-platelet drugs. There is recognition of the importance of treating all components of the atherogenic dyslipidemic profile associated with low high-density lipoprotein cholesterol and elevated triglyceride levels, in addition to lowering LDL cholesterol (Davidson, 2008). The new drugs under development should promise to better treat the syndrome as a whole. Drugs might be developed that will simultaneously modify all of the risk factors, but such drugs are not currently available. The challenge for developing a new drug that would substantially reduce multiple risk factors is attractive (Vakhrushev & Lyapina, 2017).
SMe1EC2 is a novel derivative of hexahydropyridoindole stobadine – the compound which was found to have beneficial effects on cardiovascular system. Moreover, essential metabolic studies provided the evidence that a low dose of stobadin was able to lower blood glucose, cholesterol and triacylglycerol levels and to prevent lipid peroxidation and protein glycation (Stefek et al., 2000; Pekiner et al., 2002).
The stobadine derivative SMe1EC2 showed many beneficial effects at in vitro conditions (Štolc et al., 2006; Gáspárová et al., 2011; Balcerczyk et al., 2014) and in experimental models of civilization diseases (Drimal et al., 2008). Although antioxidant properties of SMe1EC2 play very important role in its effects, metabolic activity is also suggested (Mézešová et al., 2012). It is expected that the potential drug under investigation will simultaneously influence multiple risk factors of experimental metabolic syndrome and reduce hypertension, hyperglycemia, hypercholesterolemia and hypertriglyceridemia.
The hereditary hypertriglyceridemic (hHTG) rats were developed as a genetic model for the study of relationships between blood pressure and metabolic abnormalities. It was demonstrated that these rats are not obese, they are hypertensive and insulin resistant and they have some disturbances in glucose metabolism (Zicha et al., 2006). hHTG rats display hypertriglyceridemia, impaired glucose tolerance, hyperinsulinemia, insulin resistance and increased blood pressure even without nutritional stimuli. High sucrose feeding further aggravates these symptoms (Klimes et al., 1995).
The purpose of this work was to study the effect of long-term oral administration of pyridoindol derivative SMe1EC2 on development of particular risk factors of metabolic syndrome in hHTG rats, and to compare SMe1EC2 actions with clinically used reference drug atorvastatin.
Methods
Animals
Male Wistar rats and male hHTG rats, aged 3–4 months, weighing 240–260 g, obtained from the breeding station Dobrá Voda, Slovakia, were used. The animals were housed under standard experimental conditions (temperature 21±2 °C, relative humidity 55±10%, 12/12 hr light-dark cycle, food and water provided ad libitum).
Drugs
The pyridoindole derivative SMe1EC2 (2-ethoxycarbonyl-8-methoxy-2,3,4,4a,5,9b-hexahydro-1H-pyrido-[4,3b] indolinium chloride) (S) was synthesized in the Institute of Experimental Pharmacology and Toxicology, Slovak Academy of Sciences, Slovakia.
Atorvastatin (A), clinical reference, was generous gift from Saneca Pharmaceuticals a.s. Hlohovec (Slovakia). Diagnostic kits were purchased from Randox Laboratories Ltd., (UK) and the other chemicals and kits were from commercial sources.
Treatment
The experiments were performed in compliance with the Principles of Laboratory Animal Care issued by the Ethical Committee of the Institute of Experimental Pharmacology and Toxicology, Slovak Academy of Sciences. The experimental design was approved by the State Veterinary and Food Administration of the Slovak Republic. Animals were acclimated one week prior to the experiments.
Male Wistar rats (W) fed standard diet were used within the experiment as the pattern of healthy population with no metabolic disturbances present. Male hHTG groups of rats fed either standard (HTG) or high cholesterol diet (HFC – 1% cholesterol and 7.5% lard fat) (HTG+HFC) were administered with SMe1EC2 or atorvastatin, both in dosage 50 mg/kg/day p.o. for 4 weeks in vehiculum (0.5% methylcelulose).
On the day preceding the blood sampling (at the day 1 and 28 of the experiment), food was removed from the cages. Blood was withdrawn from retroorbital venous plexus. The physiological status of the experimental animals was regularly monitored by the evaluation of preprandial glucose levels and finally also by the plethysmografic measurement of systolic blood pressure on the tail.
Lipid profil was characterized by serum levels of total cholesterol (TC), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL) and triglycerides (TRG). TC levels were measured by CHOD-POD method (Rai et al., 2013) and another biochemical parameters were assayed by commercially available diagnostic kits (RANDOX Laboratories Ltd., UK). Markers of lipid peroxidation and oxidative stress involvement were evaluated as the concentration of thiobarbituric acid reactive substances (TBARS) in serum, the kidney and the liver. Serum pro-inflammatory cytokinine levels (TNFα, IL-1, IL-6) were measured by microplate ELISA method using a commercial kit (Affimetrics USA).
Results
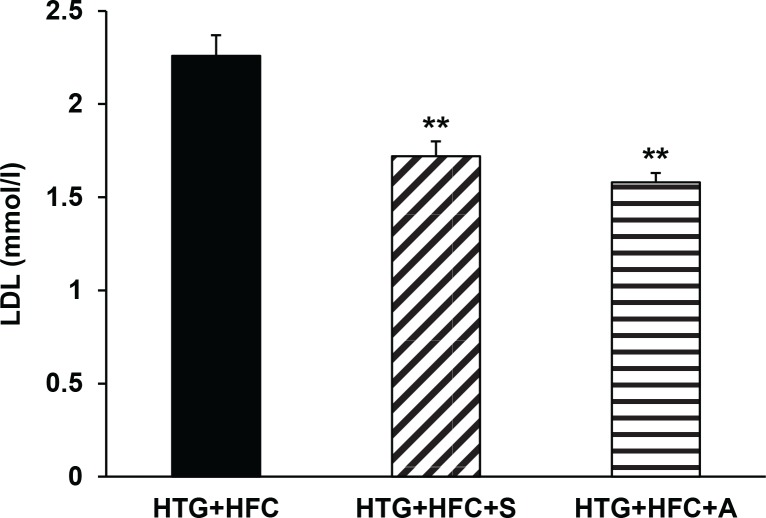
The hereditary hypertriglyceridemic rat strain has been developed as a genetic model of metabolic syndrome, which in our experiments was manifested by hypertension, hypertriglyceridemia and hypercholesterolemia. The results of the experiments showed a different lipid profile of Wistar rats and HTG. Dyslipidemia and pro-inflammatory state were further highlighted in HTG+HFC rats. The most notable changes were found in the case of serum TRG, TC, HDL, IL-6 and TNFα (Table 1). Longterm administration of the test substance SMe1EC2 to HTG+HFC significantly reduced blood serum TRG (p<0.01), TC (p<0.01) and LDL (p<0.001) levels. This effect was comparable to that of the clinically used reference atorvastatin (Figure 1). Moreover, the hypolipidemic activity of SMe1EC2 was demonstrated also in the group of rats fed standard diet. Thus, the results of this study showed that SMe1EC2 administration to rats with genetic metabolic syndrome was able to reduce hypercholesterolemia and hyperlipidemia.
Table 1.
Main characteristics of animals of the individual experimental groups.
| W | HTG | HTG+HFC | |
|---|---|---|---|
| Blood glucose (mmol/l) | 4.98±0.35 | 5.24±0.99 | 5.12±0.28 |
| Serum TRG (mmol/l) | 1.485±0.13 | 2.767±0.09*** | 3.000±0.15*** |
| Serum TC (mmol/l) | 0.964±0.11 | 1.158±0.04 | 1.477±0.06***## |
| Serum LDL (mmol/l) | 0.95±0.05 | 1.676±0.21 | 2.259±0.11 |
| Serum HDL (mmol/l) | 0.652±0.04 | 0.602±0.03 | 0.509±0.02* |
| Blood pressure (mm/Hg) | 113.21±2.81 | 118.76±5.26 | 121.90±3.49 |
| Serum IL-1 (pg/ml) | 98.23±27.57 | 89.63±18.50 | 67.51±18.40 |
| Serum IL-6 (pg/ml) | 4.65±1.68 | 12.82±2.04* | 14.22±3.02* |
| Serum TNFα (pg/ml) | 45.72±19.37 | 167.11±22.37** | 181.83±35.06** |
W – Wistar rats, HTG – hereditary hypertriglyceridemic rats fed standard diet, HTG+HFC – hereditary hypertriglyceridemic rats fed high cholesterol diet. TRG – triglycerides, TC – total cholesterol, LDL – low-density-lipoprotein cholesterol fraction, HDL – high-density-lipoprotein cholesterol fraction. Data are means ± SEM of 8 experiments.
p<0.05 vs W
p<0.01 vs W
p<0.001 vs W
p<0.01 vs HTG
Figure 1.
The effect of SMe1EC2 (S) and atorvastatin (A) on serum LDL levels of hereditary hypertriglyceridemic rats fed high-fat and high-cholesterol diet (HTG+HFC). Data are means ± SEM of 8 experiments. *p<0.05 versus HTG+HFC.
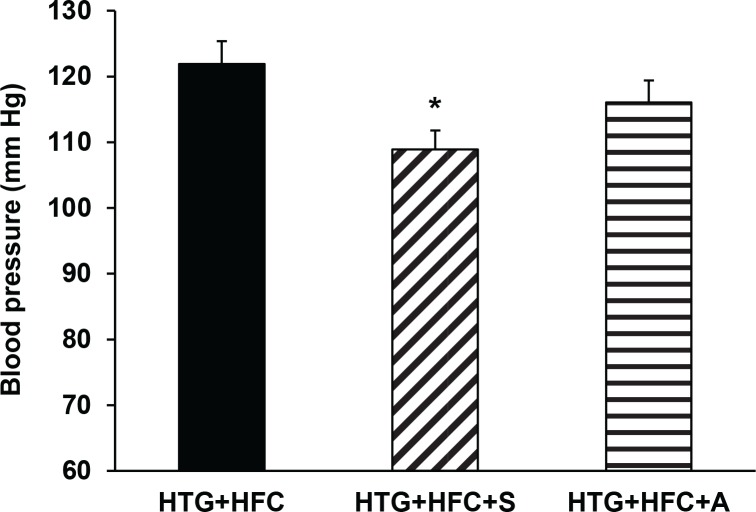
Systolic blood pressure of HTG and HTG+HFC rats continually increased during experiment such that differences between values at the beginning and the end of the experiment were statistically significant (p<0.05). Final blood pressure had tendency to be higher in HTG and HTG+HFC groups compared to W (Table 1). The tested compound SMe1EC2 normalized these values (Figure 2).
Figure 2.
The effect of SMe1EC2 (S) and atorvastatin (A) on blood pressure of hereditary hypertriglyceridemic rats fed high-fat and high-cholesterol diet (HTG+HFC). Data are means ± SEM of 8 experiments. *p<0.05 versus HTG+HFC.
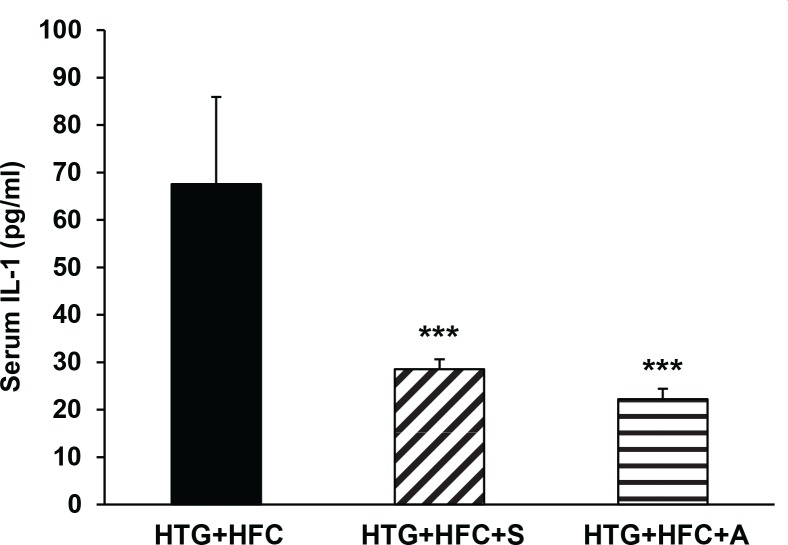
To investigate whether inflammatory processes play the role in pathogenesis of hypercholesterolemia and in hypolipidemic protective effects of compounds tested, the assessment of pro-inflammatory cytokines TNFα, IL-1 and IL-6 in the serum was completed. At the end of the administration period, HTG+HFC animals administered SMe1EC2 and atorvastatin had significantly reduced plasma levels of IL-1 compared to the group HTG+HFC without treatment (Figure 3), but failed to show any reduction of plasma TNFα and IL-6 levels.
Figure 3.
The effect of SMe1EC2 (S) and atorvastatin (A) on serum IL-1 levels of hereditary hypertriglyceridemic rats fed high-fat and high-cholesterol diet (HTG+HFC). Data are means ± SEM of 8 experiments. ***p<0.001 versus HTG+HFC.
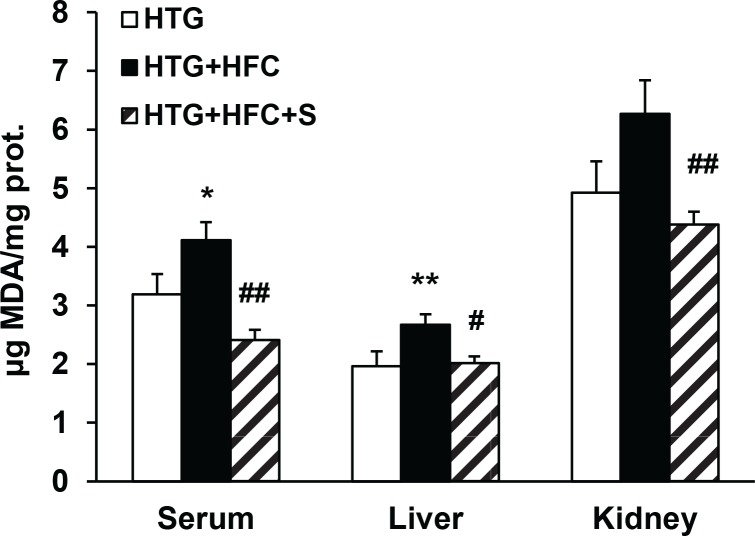
Safety parameters providing an index of lipid peroxidation and of oxidative stress involvement –TBARS – were also evaluated in serum, the kidney and the liver. As seen in Figure 4, administration of SMe1EC2 to HTG+HFC significantly depressed TBARS concentrations in all tissues tested.
Figure 4.
The effect of SMe1EC2 (S) on TBARs levels in serum, liver and kidney of hereditary hypertriglyceridemic rats fed standard diet (HTG) and hereditary hypertriglyceridemic rats fed high-fat and high-cholesterol diet (HTG+HFC). Data are means ± SEM of 8 experiments. *p<0.05 versus HTG, **p<0.01 versus HTG, #p<0.05 versus HTG+HFC, ##p<0.01 versus HTG+HFC.
Discussion
Results of our experiments showed that the model of hereditary triglyceridemic rats fed high-fat and highcholesterol diet sickens for some signs of metabolic syndrome – dyslipidemia, i.e. increased serum levels of TC, TRG, LDL, decreased levels of HDL, and increased blood pressure. Ascertained elevated serum levels of pro-inflammatory cytokine IL-1 together with enhanced TBARS concentrations in the liver, kidney and serum agree with participation in pathogenesis of experimental metabolic syndrome. This model enabled us to study separately the consequences of metabolic and hemodynamic abnormalities in metabolic syndrome. Repeated administration of a pyridoindole derivative SMe1EC2 in rats with hereditary hypercholesterolemia showed its beneficial effect on several risk factors of experimental metabolic syndrome as hypercholesterolemia, hypertriglyceridemia and hypertension.
Dyslipidemia is known as a major cause of atherosclerosis and atherosclerosis-associated diseases such as coronary heart disease, ischemic cerebrovascular disease, and metabolic syndrome. It is manifested by elevated triglyceride levels and by reduced HDL, which contributes to increased inflammatory activity (Mendrick et al., 2017). The other mechanism participating in the etiopathogenesis of metabolic syndrome and associated pathologies such as hypertension, dyslipoproteinemia, insulin resistance and glucose intolerance, is oxidative stress (Isomaa, 2003). Elevated inflammatory activity together with increased formation of reactive oxygen species causes atherogenic endothelial dysfunction (Meerarani et al., 2006) which consequently leads to hypertension (Paravicini & Touyz, 2006). In our experiments, metabolic syndrome was characterized by increased production of proinflammatory cytokines TNF-α, IL-1 and IL-6. Moreover, the production of reactive oxygen species was increased, which was demonstrated by elevated levels of TBARS in several tissues. These findings suggest the important role of inflammation and oxidative stress in pathogenesis of signs of metabolic syndrome in our experimental model. It is also known that oxidative stress plays a critical role in the pathogenesis of atherosclerosis and hypertension by directly affecting vascular wall cells (Wihastuti & Heriansyah, 2017). Therefore, drugs with anti-inflammatory and anti-oxidative properties could be a right choice for treating cardiovascular complications associated with metabolic syndrome. This occurred in results of Knezl et al. (2017) who found SMe1EC2 to improve functional state of cardiovascular system of HTG rats fed with high-fat and high-cholesterol diet. In our experiments, administration of SMe1EC2 and atorvastatin significantly reduced serum levels of IL-1 and serum concentrations of TBARS. Thus, the anti-oxidative and anti-inflammatory mechanism could also contribute to lowering blood pressure in HTG+HFC animals administered SMe1EC2.
Beneficial effects found in this work provide SMe1EC2 to be a promising candidate in the treatment of metabolic syndrome. A big advantage of this compound is its very good toxicity profile. The results of the toxicity study and reproductive toxicity study in rats indicated a high safety of SMe1EC2 – no toxicity or prenatal toxicity. In the prenatal developmental toxicity study in rats, SMe1EC2 exerted neither embryotoxic nor teratogenic effects on rat fetuses and their postnatal development. Further, any signs of maternal toxicity were found (Ujhazy et al., 2008, 2011).
In our study we compared effects of SMe1EC2 with the most frequently used statin – atorvastatin. Statins are the most effective agents for treating dyslipidemia and they are recognized as first-line therapy for lowering of cholesterol levels (American Diabetes Association, 2002; De Backer et al., 2003). Moreover, statins have ‘pleiotropic’ effects, such as reducing oxidative stress and modulating inflammatory responses (Liao, 2002; Yamada et al., 2017; Zamani et al., 2017) and these effects may improve other risk factors associated with the metabolic syndrome (Kabaklić & Fras, 2017). However, statins showed increased risk of adverse effects (myopathy and hepatotoxicity), thus it is important to search for new therapies or combination therapies with improved efficacy and safety.
Our findings showed SMe1EC2 to affect specific parameters of lipid profile and blood pressure. These effects together with its very good toxicity profile predestinate SMe1EC2 to be promising agent for further surveys related to metabolic syndrome features.
Acknowledgement
This study was supported by the Slovak Research Agency by realization of the project: „Transfer of Knowledge and Technologies from Research and Development in Toxicology on Evaluation of Environmental and Health Risks“ (ITMS 26240220005).
REFERENCES
- American Diabetes Association Management of dyslipidemia in adults with diabetes. Diabetes Care. 2002;25:74–77. doi: 10.2337/diacare.21.1.179. [DOI] [PubMed] [Google Scholar]
- Balcerczyk A, Bartosz G, Drzewinska J, Piotrowski Ł, Pulaski Ł, Stefek M. Antioxidant action of SMe1EC2, the low-basicity derivative of the pyridoindole stobadine, in cell free chemical models and at cellular level. Interdiscip Toxicol. 2014;7:27–32. doi: 10.2478/intox-2014-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M. Review of the current status of the management of mixed dyslipidemia associated with diabetes mellitus and metabolic syndrome. Am J Cardiol. 2008;102:19L–27L. doi: 10.1016/j.amjcard.2008.09.071. [DOI] [PubMed] [Google Scholar]
- De Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova R, Dallongeville J, et al. European guidelines on cardiovascular disease prevention in clinical practice: Third joint task force of European and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of eight societies and by invited experts) Eur J Cardiovasc Prev Rehabil. 2003;10:S1–10. doi: 10.1097/01.hjr.0000087913.96265.e2. [DOI] [PubMed] [Google Scholar]
- Drimal J, Knezl V, Navarova J, Nedelcevova J, Paulovicova E, Sotnikova R, Snirc V, Drimal D. Role of inflammatory cytokines and chemoattractants in the rat model of streptozotocin-induced diabetic heart failure. Endocr Regul. 2008;42:129–135. [PubMed] [Google Scholar]
- Gáspárová Z, Snirc V, Stolc S. The new pyridoindole antioxidant SMe1EC2 and its intervention in hypoxia/hypoglycemia-induced impairment of longterm potentiation in rat hippocampus. Interdiscip Toxicol. 2011;4:56–61. doi: 10.2478/v10102-011-0011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomaa B. A major health hazard: the metabolic syndrome. Life Sci. 2003;73:2395–2411. doi: 10.1016/s0024-3205(03)00646-5. [DOI] [PubMed] [Google Scholar]
- Kabaklić A, Fras Z. Moderate-dose atorvastatin improves arterial endothelial function in patients with angina pectoris and normal coronary angiogram: a pilot study. Arch Med Sci. 2017;13:827–836. doi: 10.5114/aoms.2017.68238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimes I, Vrána A, Kunes J, Seböková E, Dobesová Z, Stolba P, Zicha J. Hereditary hypertriglyceridemic rat: a new animal model of metabolic alterations in hypertension. Blood Press. 1995;4:137–142. doi: 10.3109/08037059509077585. [DOI] [PubMed] [Google Scholar]
- Knezl V, Sotníková R, Brnoliaková Z, Bauer V, Bezek Š. Monotherapy of experimental metabolic syndrome II. Study of cardiovascular effects. Interdiscip Toxicol. 2017;10(2) doi: 10.1515/intox-2017-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwasny C, Manuwald U, Kugler J, Horm U. Systematic review of the epidemiology and natural history of the metabolic vascular syndrome and its coincidence with Type 2 diabetes mellitus and cardiovascular diseases in different european countries. Metab Res. 2017 doi: 10.1055/s-0043-122395. [Epub ahead of print]PMID:29183091) ] [DOI] [PubMed] [Google Scholar]
- Liao JK. Beyond lipid lowering: The role of statins in vascular protection. Int J Cardiol. 2002;86:5–18. doi: 10.1016/s0167-5273(02)00195-x. [DOI] [PubMed] [Google Scholar]
- Mendrick DL, Diehl AM, Topor LS, Dietert RR, Will Y, La Merrill MA, Bouret S, Varma V, Hastings KL, Schug TT, Emeigh Hart SG, Burleson FG. Metabolic syndrome and associated diseases: From the bench to the clinic. Toxicol Sci. 2017 doi: 10.1093/toxsci/kfx233. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerarani P, Badimon JJ, Zias E, Fuster V, Moreno PR. Metabolic syndrome and diabetic atherothrombosis: implications in vascular complications. Curr Mol Med. 2006;6:501–514. doi: 10.2174/156652406778018680. [DOI] [PubMed] [Google Scholar]
- Mézešová L, Jendruchová-Javorková V, Vlkovičová J, Kyselova Z, Navarová J, Bezek S, Vrbjar N. Antioxidant SMe1EC2 may attenuate the disbalance of sodium homeostasis in the organism induced by higher intake of cholesterol. Mol Cell Biochem. 2012;366:41–48. doi: 10.1007/s11010-012-1281-3. [DOI] [PubMed] [Google Scholar]
- Paravicini TM, Touyz RM. Redox signaling in hypertension. Cardiovasc Res. 2006;71:247–258. doi: 10.1016/j.cardiores.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Pekiner B, Ulusu NN, Das-Evcimen N, Sahilli M, Aktan F, Stefek M, Stolc S, Karasu I. In vivotreatment with stobadine prevents lipid peroxidation, protein glycation and calcium overload but does not ameliorate Ca2+ATPase activity in heart and liver of streptozotocin-diabetic rats: comparison with vitamin E. Biochim Biophys Acta. 2002;1588:71–78. doi: 10.1016/s0925-4439(02)00141-2. [DOI] [PubMed] [Google Scholar]
- Raal FJ. Pathogenesis and management of the dyslipidemia of the metabolic syndrome. Metab Syndr Relat Disord. 2009;7:83–88. doi: 10.1089/met.2008.0079. [DOI] [PubMed] [Google Scholar]
- Rai KN, Kumari NS, Gowda Km D, Kr S. The evaluation of micronutrients and oxidative stress and their relationship with the lipid profile in healthy adults. J Clin Diagn Res. 2013;7:1314–1318. doi: 10.7860/JCDR/2013/6127.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefek M, Sotnikova R, Okruhlicova L, Volkovova K, Kucharska J. Effect of dietary supplementation with the pyridoindole antioxidant stobadine on antioxidant state and ultrastructure of diabetic rat myocardium. Acta Diabetol. 2000;37:111–117. doi: 10.1007/s005920070012. [DOI] [PubMed] [Google Scholar]
- Štolc S, Šnirc V, Májeková M, Gáspárová Z. A, Gajdošíková A, Štvrtina S. Development of the new group of indole-derived neuroprotective drugs affecting oxidative stress. Cell Mol Neurobiol. 2006;26:1493–1502. doi: 10.1007/s10571-006-9037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujhazy E, Dubovicky M, Ponechalova V, Navarova J, Brucknerova I, Snirc V, Mach M. Prenatal developmental toxicity study of the pyridoindole antioxidant SMe1EC2 in rats. Neuro Endocrinol Lett. 2008;29:639–643. [PubMed] [Google Scholar]
- Ujhazy E, Mach M, Navarova J, Brucknerova I, Dubovicky M. Safety assessment of the pyridoindole derivative SMe1EC2: developmental neurotoxicity study in rats. Interdiscip Toxicol. 2011;4:47–51. doi: 10.2478/v10102-011-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakhrushev YM, Lyapina MV. Enteral failure and metabolic syndrome: Common neurohormonal mechanisms of development, possibilities of their rational therapy. Ter Arkh. 2017;89:95–101. doi: 10.17116/terarkh2017891095-101. [DOI] [PubMed] [Google Scholar]
- Wihastuti TA, Heriansyah T. The inhibitory effects of polysaccharide peptides (PsP) of Ganoderma lucidum against atherosclerosis in rats with dyslipidemia. Heart Int. 2017;12:e1–e7. doi: 10.5301/heartint.5000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Takeuchi S, Yoneda M, Ito S, Sano Y, Nagasawa K, Matsuura N, Uchinaka A, Murohara T, Nagata K. Atorvastatin reduces cardiac and adipose tissue inflammation in rats with metabolic syndrome. Int J Cardiol. 2017;240:332–338. doi: 10.1016/j.ijcard.2017.04.103. [DOI] [PubMed] [Google Scholar]
- Zamani E, Mohammadbagheri M, Fallah M, Shaki F. Atorvastatin attenuates ethanol-induced hepatotoxicity via antioxidant and anti-inflammatory mechanisms. Res Pharm Sci. 2017;12:315–321. doi: 10.4103/1735-5362.212049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zicha J, Pechanova O, Cacanyiova S, Cebova M, Kristek F, Torok J, Simko F, Dobesova Z, Kunes J. Hereditary hypertriglyceridemic rat: a suitable model of cardiovascular disease and metabolic syndrome? Physiol Res. 2006;55:S49–S63. doi: 10.33549/physiolres.930000.55.S1.49. [DOI] [PubMed] [Google Scholar]