Abstract
Metabolic syndrome belongs to the most important risk factors of cardiovascular diseases. The aim of this study was to investigate changes in cardiovascular system induced by high cholesterol and high fat diet (HCHF) in HTG rats and their influence by a pyridoindole antioxidant – SMe1EC2 (S). The effects of S were compared with those of atorvastatin (A). Male HTG rats were fed HCHF (1% cholesterol + 7.5% lard) for 4 weeks. S and A were administered p.o., 50 mg/kg b.w. Following experimental groups were used: Wistar rats (W), hypertriglyceridemic rats (HTG), HTG rats fed HCHF (CHOL), HTG+S (S-HTG), CHOL+S (S-CHOL), and CHOL+A (A-CHOL). Values of blood pressure (BP) and selected ECG parameters were monitored in conscious animals, functions of the isolated heart and aorta were analyzed ex vivo. At the end of the experiment, systolic (sBP) and diastolic (dBP) blood pressure was increased in HTG and CHOL. S and A decreased BP in all treated groups. Accordingly with BP changes, the aortic endothelial function of CHOL was damaged. Both S and A administration ameliorated the endothelium-dependent relaxation to values of W. PQ and QTc intervals were prolonged in CHOL, while the treatment with S or A improved ECG findings. Prodysrhythmogenic threshold was decreased significantly in CHOL and both treatments returned it to the control values. In conclusion, HCHF increased BP, impaired endothelial relaxation of the aorta and potentiated susceptibility of myocardium to dysrhythmias. The effect of S on the changes induced by HCHF diet was more pronounced than that of A.
Keywords: metabolic syndrome, high-fat and high-cholesterol diet, SMe1EC2, atorvastatin, cardiovascular effects
Introduction
Metabolic syndrome (MetS) is the most important risk factor for cardiovascular diseases (CVD). Its prevalence in Slovakia is comparable with the prevalence in the European population and moves around 20.1% (National Cholesterol Education Program – NCEP/ATP III criteria) and 38.1% (International Diabetes Federation – IDF) (Galajda et al., 2007). MetS is characterized by a cluster of interrelated metabolic factors such as hypertension, dyslipidemia (elevated LDL-cholesterol, triglycerides, decreased HDL-cholesterol), insulin resistance, impaired glucose tolerance (DM2T), central (abdominal) obesity, proinflammatory and prothrombotic state (Alberti et al., 2009; Raal, 2009). Although the pathogenesis of the metabolic syndrome is not well understood, it is likely that it represents a complex of interplay between metabolic, genetic, and environmental factors. Inflammation and oxidative stress have been proposed as common ethiologic factors linking these processes (Watanabe et al., 2008).
All of the individual components of the MetS are important risk factors for CVD, e.g. of coronary heart disease, ischemic cerebrovascular disease, and peripheral vascular disease as well as for DM2T (Lew & Garfinkel, 1979; Grundy, 2007; Raal, 2009). Combination of these factors creates cardiometabolic syndrom.
Currently, the most studies have correlated the individual symptoms of MetS with the risk to develop cardiovascular disease, however specific cardiac alterations induced by MetS have not been reported. The results obtained from other models of obesity described increased left ventricular dysfunction after ischemia ex vivo (du Toit et al., 2005; Ooie et al., 2005; Nduhirabandi et al., 2011; Wensley et al., 2013) and in vivo (Huang et al., 2013). Our experimental model, using hereditary hypertriglyceridemic (HTG) rats, enables to study the consequences of metabolic and hemodynamic abnormalities separately. HTG rats have been developed as a genetic model of metabolic syndrome, manifested by hypertension, hypertriglyceridemia and hypercholesterolemia. Studies of Bezek et al. (2017) showed male HTG rats fed either standard or high fat and high cholesterol diet to have hypercholesterolemia and increased serum levels of total cholesterol (TC), triglyceride (TRG) and low-density lipoprotein cholesterol (LDL-C). Elevated plasma lipids belong to the major risk factors for atherosclerosis and coronary heart disease. Therefore, lipid lowering is one of the major approaches in prevention of cardiovascular diseases (Jain et al., 2010).
The hypolipidemic activity of SMe1EC2 – a novel pyridoindole antioxidant was documented in the study of Bezek et al. (2017). The results of this study showed that administration of SMe1EC2 to rats with genetic metabolic syndrome reduced hypertension, hypercholesterolemia and hyperlipidemia in groups of HTG rats fed a control diet as well as those fed with high cholesterol and high fat diet. As SMe1EC2 is a strong antioxidant (Štolc et al., 2006) its beneficial effects on cardiovascular system of HTG rats can be assumed, because oxidative stress is known to play very important role in cardiovascular disorders. Atorvastatin is a lipid lowering drug which moreover possesses several pleiotropic properties. Recent evidence suggests a pleiotropic mechanism of action including vasoprotective, antiinflammatory, and antidysrhythmic properties that imply an immediate role for statin medications (Dougherty & Arora, 2012).
The aim of the study is to investigate the changes in the blood pressure, electrical activity of the heart and vascular reactivity induced by CHOL diet in HTG rats. The cardiovascular effect of a novel pyridoindole derivative – SMe1EC2 in experimental MetS will be compared with the effect of clinical reference drug – atorvastatin – a competitive inhibitor of acyl-CoA cholesterol acyltransferase.
Material and methods
The experiments were performed in compliance with the Principles of Laboratory Animal Care published in the Colection of Laws of the Slovak Republic (Z.z. SR No. 436/2012). The experimental design was approved by the Ethical Committee of the Institute of Experimental Pharmacology and Toxicology, Slovak Academy of Sciences and by the State Veterinary and Food Administration of the Slovak Republic.
Animals
Male Wistar rats and male hereditary hypertriglyceridemic rats (HTG) rats, aged 3–4 months weight 373.6±18.5 g, obtained from the breeding station Dobrá Voda, Slovakia, were used. The animals were housed under standard experimental conditions (temperature 21±2°C, relative humidity 55±10%, 12/12 hr light-dark cycle, food and water provided ad libitum).
Treatment
The animals were randomly divided into 6 experimental groups consisting of 8 animals. Male Wistar rats (W) group fed standard diet served as healthy controls without metabolic disturbances present. Male HTG groups of rats were fed either standard diet (HTG) or high cholesterol and high fat diet (1% cholesterol and 7.5% lard fat, CHOL). The animals were treated with SMe1EC2 (S) or atorvastatin (A), both in a dose of 50 mg/kg/day p.o. for 4 weeks. Atorvastatin was dissolved in 0.5% methylcelulose. SMe1EC2 was administered to hereditary hypertriglyceridemic rats fed standard diet (S-HTG) or to hereditary hypertriglyceridemic rats fed high cholesterol and high fat diet (S-CHOL). Atorvastatin was added to hereditary hypertriglyceridemic rats fed high cholesterol and high fat diet (A-CHOL).
Experiments in vivo
After 7 days of adaptation, 5 days lasting careful handling before in vivo measurements to produce repeatable results was realized. We focused on the blood pressure and standard ECG recordings in conscious animals.
Blood pressure measurements
Blood pressure in rats was measured by non-invasive technique by Power Lab approach using tail-cuff (NIBP Controller, ADInstruments, Spechbach, Germany). Animals were placed into perspex restraint cage and preheated to 35 °C for 7–10 min (thermostat KBC G16/250, Zalimp, Warszawa, Poland). Warming of the tails by infrared lamp improved blood circulation in the tail during the measurements. The tail cuff was positioned at the proximal end of the tail and pulse transducer monitored caudal artery pulse. Blood pressure cycles were monitored and 5 consecutive spindles were used for offline analysis. Data analysis was performed by Chart 5 for Windows (ADInstruments, Spechbach, Germany).
ECG measurements
Standard lead ECG was recorded from conscious rats standing in the perspex restraint cage with plate electrodes (made at STU Bratislava, Slovakia) positioned on the bottom of the cage and connected to the ECG unit (EKG Praktik Veterinary ver. 6, Seiva, Prague, Czech Republic) and computer. Data collection and offline analysis were done by Seiva Database Veterinary program. The selected parameters PQ, QT intervals and QRS duration were analyzed from ECG recordings. To eliminate the effect of different heart frequencies, QT interval corrected to heart frequency (QTc) was evaluated. QTc interval (in ms) was calculated according to the formula:
| , |
where QT is duration of QT interval in ms, RR is interval between R amplitudes of 2 following QRS complexes in ms, and factor 200 is a minimal heart rate of rats. The mean values obtained from the 5 consecutive complexes analysis were taken for the next calculations.
Ventricular dysrhythmias were analyzed according to the Lambeth`s Convention and grouped into the simple dysrhythmias and life-threatening dysrhythmias (ventricular tachycardia VT, fibrillation VF). The incidence of individual episodes, as well as the duration of lifethreatening dysrhythmias were detected.
Experiments ex vivo
Isolated heart according to the Langendorff
The formation of life-treatening dysrhythmias tachycardia and fibrillation is closely related to the fibrillatory treshold and dysrhythmias persistence is related to the myocardial inability to spontaneously terminate previously induced disturbances. Detection of these disturbances is possible in spontaneously beating isolated hearts perfused according to the Langendorff. Isolated hearts were retrogradely perfused via aorta at constant pressure mode 80 mmHg. To assign basal diastolic pressure of the left ventricle, a latex balloon was inserted into the left ventricular cavity, filled with water and adjusted to the value of 8–10 mmHg. After 10 min lasting stabilization period, the fibrillation threshold was detected by steeply increased current intensity of stimulation by 5 mA/30 s from 10 mA to 50 mA. Myocardial susceptibility to persistent dysrhythmias was determined by induction of the 2 minute lasting sustained ventricular tachycardia (VT) or ventricular fibrillation (VF). Time to restore sinus rhythm by stop flow after sustained dysrhythmias was considered as the ability of myocardium to recovery electrical activity (Tribulova et al., 2002; Liptak et al., 2017).
Basic parameters of stimulation (Electrostimulator ST-3, Medicor, Budapest, Hungary) via a pair of stimulating electrodes attached to the epicardium of the right ventricle were set as current: 10 mA, train duration: 2 s, stimulation rate: 100 pps, delay: 0.1 ms, duration: 0.2 ms. The system BioLab F ver.1 (Institute of Measurement Science, Slovak Academy of Sciences, Bratislava, Slovakia) was used for data collection and offline analysis.
Isometric tension measurements in isolated thoracic aorta
The aorta was rapidly removed from sacrificed animal, immersed into the physiological solution (PSS) and carefully cleaned of all fat and connective tissue. Rings of the aorta (approximately 2 mm long) were mounted in a tissue chamber containing PSS, gassed and maintained at 37 °C, and attached to an isometric force transducer. Rings were passively stretched to optimal length by imposing an optimal initial tension of 10 mN found in previous studies. After stabilization period (60 min), the experimental protocol was as follows: Rings were precontracted with 1 μmol/l phenylephrine and relaxant responses of the preparations to acetylcholine (10 nmol/l – 10 μmol/l) were tested at the plateau of the contraction. Responses to acetylcholine are expressed as percentages of phenylephrineinduced contraction.
Solutions and drugs
Composition of the Krebs-Henseleit solution used for isolated heart perfusion (in mmol/1): NaCl, 118; KCl, 4.75; CaCl2 × 2H2O, 2.5; MgSO4 × 7H2O, 1.2; KH2PO4, 1.18; NaHCO3, 25.0; glucose, 11.1; saturated by the mixture of 95% O2 + 5% CO2, pH=7.4, temperature 37 °C.
Composition of the modified physiological solution (PSS) used for aortic rings (in mmol/1): NaCl, 122; KCl, 5.9; NaHCO3, 15; glucose, 10; MgCl2, 1.25; and CaCl2, 1.25, saturated by the mixture of 95% O2 + 5% CO2, pH=7.4, temperature 37 °C.
Used chemicals were from Centralchem (Bratislava, Slovakia) and mikroCHEM (Pezinok, Slovakia). Methylcelulose was a kind gift from VÚLM (Modra, Slovakia).
The pyridoindole derivative SMe1EC2 (S) SMe1EC2 (2-ethoxycarbonyl-8-methoxy-2,3,4,4a,5,9b-hexahydro1H-pyrido-[4,3b] indolinium chloride) was synthesized in the Institute of Experimental Pharmacology and Toxicology, Slovak Academy of Sciences, Slovakia. Atorvastatin was a generous gift from Saneca Pharmaceuticals Hlohovec, Slovakia.
Data calculation and statistic
Data are expressed as means ± S.E.M. and compared using Anova test with post hoc Bonferoni test. The difference was considered statistically significant at a level p≤0.05.
Results
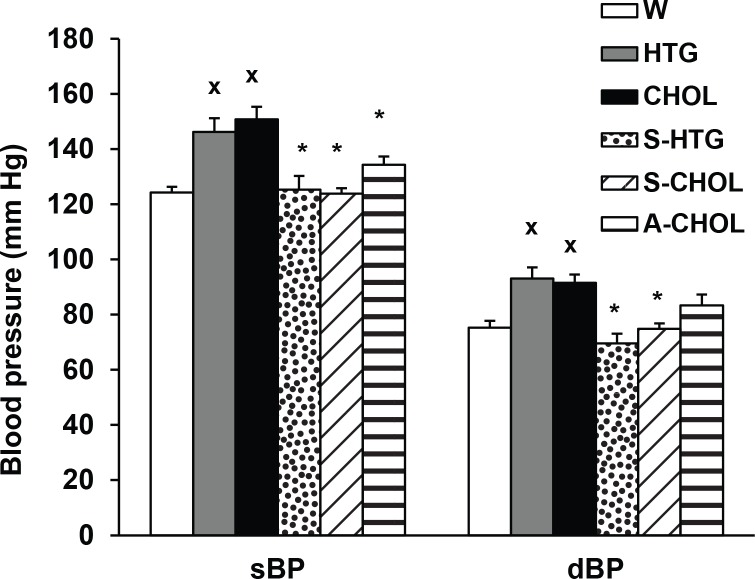
In our experiments we focused on the characterization of the cardiovascular effects of high cholesterol and high fat diet administered to the HTG rats as well as on the effects of novel pyridoindole antioxidant – SMe1EC2 and a competitive inhibitor of acyl-CoA cholesterol acyltransferase – atorvastatin. We found significantly higher systolic and diastolic blood pressure in HTG rats compared to control normotensive Wistar rats. Four weeks of HTG rat treatment with high cholesterol and high fat diet (CHOL) increased significantly systolic and diastolic blood pressure in comparison to HTG animals without diet. Repeated 4 weeks lasting administration of SMe1EC2 decreased both systolic (sBP) and diastolic (dBP) blood pressure in HTG and CHOL groups. Atorvastatin in the same dose significantly decreased sBP in CHOL group but did not influence dBP of CHOL animals without treatment (Figure 1).
Figure 1.
Systolic (sBP) and diastolic (dBP) blood pressure in conscious rats after 4 weeks lasting experiment. W – Wistar rats fed with standard diet; HTG – hereditary hypertriglyceridemic rats fed standard diet; CHOL – hereditary hypertriglyceridemic rats fed high cholesterol and high fat diet; S-HTG – hereditary hypertriglyceridemic rats fed standard diet administered SMe1EC2 50 mg/kg/day p.o.; S-CHOL – hereditary hypertriglyceridemic rats rats fed high cholesterol and high fat diet administered SMe1EC2 50 mg/kg/day p.o.; A-CHOL – hereditary hypertriglyceridemic rats fed high cholesterol and high fat diet administered atorvastatin 50 mg/kg/day p.o. x p<0.05 versus W, *p<0.05 versus HTG. Data are means ± S.E.M. of 8 experiments.
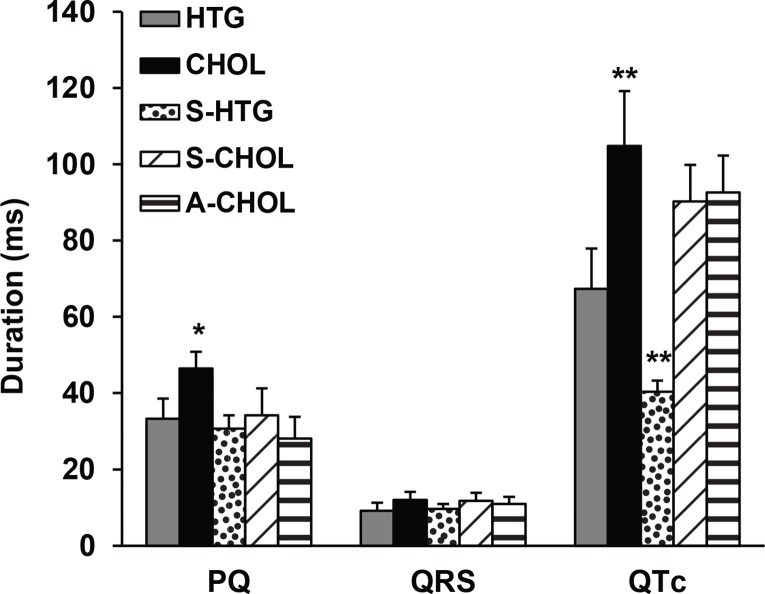
Analysis of selected ECG parameters showed a significant prolongation of both PQ and QTc intervals in CHOL rats compared to HTG rats (Figure 2). Both substances tested shortened duration of PQ interval in CHOL animals. We did not observe significant modification of the QRS complex duration. Administration of SMe1EC2 and atorvastatin had only tendency to shorten duration of QTc interval in CHOL rats, while SMe1EC2 significantly shortened the QTc interval duration in HTG rats.
Figure 2.
Selected ECG parameters recorded in conscious rats after 4 weeks lasting experiment – PQ interval, QRS interval and QTc interval in ms. HTG – hereditary hypertriglyceridemic rats fed standard diet (n=6); CHOL – hereditary hypertriglyceridemic rats fed high cholesterol and high fat diet (n=6); S-HTG – hereditary hypertriglyceridemic rats fed standard diet administered SMe1EC2 50 mg/kg/day p.o. (n=4); S-CHOL – hereditary hypertriglyceridemic rats fed high cholesterol and high fat diet administered SMe1EC2 50 mg/kg/day p.o. (n=4); A-CHOL – hereditary hypertriglyceridemic rats fed high cholesterol and high fat diet administered atorvastatin 50 mg/kg/day p.o. (n=5). *p<0.05 versus HTG, **p<0.01 versus HTG. Data are means ± S.E.M.
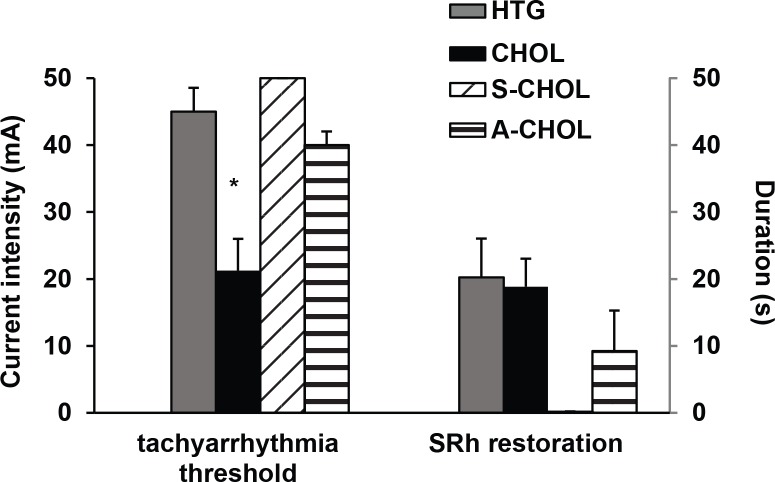
Experiments on isolated perfused rat hearts were designed to detect myocardial susceptibility to stimulation induced life-threatening tachyarrhythmias (ventricular tachycardia/ventricular fibrillation – VT/VF). The prodysrhythmogenic threshold was significantly decreased in hearts isolated from CHOL rats compared to Time to sinus rhythm appearance was getting shorter in the order HTG > CHOL > A-CHOL. Since we did not elicit VT/VF in S-CHOL animals there was no reason to monitor time for SRh in this group (Figure 3, part B).
Figure 3.
Tachyarrhythmia threshold (in mA) for persistent VT/VF induced by pacing of left ventricles at the end of experiments ex vivo (part A) and time to enforced restoration of sinus rhythm (SRh) by stop-flow (part B). HTG – hereditary hypertriglyceridemic rats fed standard diet; CHOL – hereditary hypertriglyceridemic rats fed high cholesterol and high fat diet; S-CHOL – hereditary hypertriglyceridemic rats fed high cholesterol and high fat diet administered SMe1EC2 50 mg/kg/day p.o.; A-CHOL – hereditary hypertriglyceridemic rats fed high cholesterol and high fat diet administered atorvastatin 50 mg/kg/day p.o. *p<0.05 versus HTG. Data are means ± S.E.M. of 8 experiments.
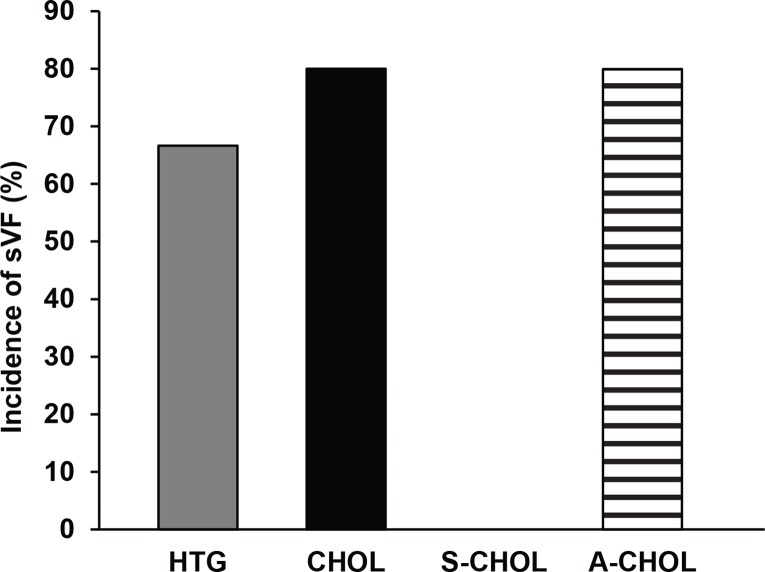
In hearts isolated from HTG rats the sustained tachyarrhythmias VT/VF were found in 67% of animals. The incidence of sustained VT/VF was 83% in CHOL rats and 80% in A-CHOL group, respectively. SMe1EC2 completely abolished the incidence of stimulation-induced sustained VT/ VF (Figure 4).
Figure 4.
Induced sustained ventricular fibrillation detected at the end of the experiment ex vivo (in percentage of hearts in which ventricular fibrillation lasted ≥2 min). HTG – hereditary hypertriglyceridemic rats fed standard diet; CHOL – hereditary hypertriglyceridemic rats fed high cholesterol and high fat diet; S-CHOL – hereditary hypertriglyceridemic rats fed high cholesterol and high fat diet administered SMe1EC2 50 mg/kg/day p.o.; A-CHOL – hereditary hypertriglyceridemic rats fed high cholesterol and high fat diet administered atorvastatin 50 mg/kg/day p.o. *p<0.05 versus HTG. Data are means ± S.E.M. of 8 experiments.
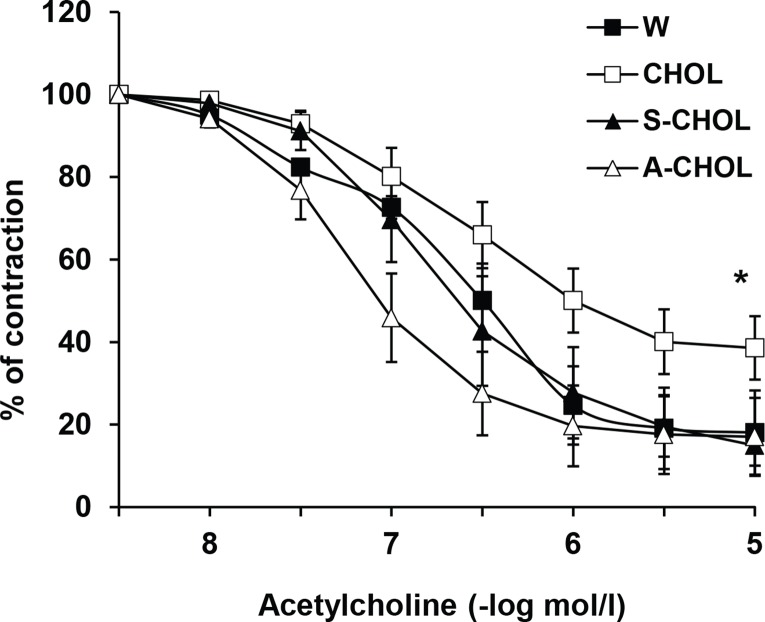
Further, changes in endothelium-dependent relaxation were studied in the aorta. Acetylcholine induced endothelium-dependent relaxation of the phenylephrineprecontracted aortic preparations which was significantly weaker in CHOL group in comparison to that in the control normotensive group. Administration of tested substances improved endothelium-dependent relaxation and reversed it to the control values. The effect of atorvastatin was more pronounced than the effect of SMe1EC2 (Figure 5.).
Figure 5.
Endotelium-dependent relaxation induced by acetylcholine of the aortic preparations precontracted with phenylephrine (1 μmol/l). W – Wistar rats fed standard diet; CHOL – hereditary hypertriglyceridemic rats fed high cholesterol and high fat diet; S-CHOL – hereditary hypertriglyceridemic rats fed high cholesterol and high fat diet administered SMe1EC2 50 mg/kg/day p.o.; A-CHOL – hereditary hypertriglyceridemic rats fed high cholesterol and high fat diet administered atorvastatin 50 mg/kg/day p.o.. *p<0.05 versus the other groups. Data are means ± S.E.M. of 8 experiments.
Discussion
HTG and mildly lowered in atorvastatin pretreatment of CHOL group. In SMe1EC2 treated group the stimulation exceeded maximal value (50 mA) without induction of dysrhythmias (Figure 3 part A). By application of stop-flow technique before termination of the experiment, time to enforced sinus rhythm (SRh) restoration was monitored. Our results showed that high cholesterol and high fat diet (HCHF) administered to HTG rats had impact on the cardiovascular system of animals – BP was increased, endothelial relaxation of the aorta was impaired and the myocardium had a potentiated susceptibility to lifethreatening dysrhythmias. The experimental model we used allow to study metabolic as well as cardiovascular consequences of fatty diet in animals with genetic predisposition to MetS One of the important components of MetS is hypertension (Galajda et al., 2007). In accordance with Klimes et al. (1995) we confirmed increase in blood pressure in hereditary hypertriglyceridemic animals which was even augmented in animals fed HCHF. Consistently with this finding the aortas displayed endothelial dysfunction. The involvement of vascular endothelium is of strategic importance in the regulation of vascular resistance and blood pressure. Due to its localization, endothelial cell layer is specially targeted in metabolic alterations. The endothelium is directly in contact with elevated concentration of glucose, insulin and triglycerides in blood suggesting that endothelial function alterations play an important role in cardiovascular diseases (Silva et al., 2015). Endothelial dysfunction is the first step leading from decreased vascular dilatation up to hypertension and other cardiovascular disorders. This fact was confirmed not only in experimental (Liu et al., 2017) but also in clinical studies (Reule et al., 2017) and is in accordance with our findings.
Our results demonstrated an important impact of HCHF diet on electrical parameters of the heart of HTG rats – longer duration of PQ interval, QRS complex and QTc interval. The myocardial electrical and mechanical dysfunction is a background for cardiovascular complications of MetS ( Yilmaz et al., 2015 ). Patients with MetS displayed significant changes in QRS complex indicating depolarization sequence deterioration ( Bacharova et al., 2012 ). The repolarization abnormalities in patients with uncomplicated metabolic syndrome involved wider dispersion of ventricular repolarization time as well as increased QTc-min and QTc-max, and prolongation of both corrected QT interval (QTc) and QT dispersion (QTd) on electrocardiogram (Soydinc et al., 2006). Prolongation of QT interval is a risk factor for development of cardiac rhythm disturbances.
Malignant dysrhythmias such as ventricular fibrillation and ventricular tachycardia are the most common dysrhythmias responsible for sudden cardiac death (Luu et al., 1989). Kurl et al. (2016) described the 2.2–2.6 times higher risk of sudden cardiac death in male with identified MetS. Cardiac dysrhythmias are in relation to dyslipidemia, obesity, diabetes mellitus 2 type (DM2T) and lifestyle (Duflou et al., 1995; Jouven et al., 2001; Luscher et al., 2003; Plourde et al., 2014). Both, atrial and ventricular fibrillation are considered to be induced by abnormal impulse formation and/or by circuit movement – re-entry (Allesie et al., 1984; Gray et al., 1998; Witkowski et al., 1998). However, the precise mechanisms through which MetS causes atrial fibrillation are not completely understood, but the syndrome has been associated with electrical remodeling of the atrium and sinoatrial nodes (Albarado-Ibañez et al., 2013).
To study myocardial susceptibility to dysrhythmias we used the ex vivo model of cardiac burst pacing for initiation and persistence of malignant re-entry dysrhythmias (Merrilat et al., 1990; Kihara & Morgan, 1991; Simor et al., 1997; Tribulová et al., 2002; Liptak et al., 2017). Using this model we demonstrated the decreased stimulating threshold and increased incidence of sustained ventricular dysrhythmias. Moreover, we found that in comparison with HTG animals, the fatty diet significantly potentiated the initiation of ventricular tachyarrhythmias without significant changes in the time for restoration of sinus rhythm. The increased vulnerability to VF in hypertriglyceridemic rats compared to normotensive Wistar rats was detected also by Tribulová et al. (2006 and 2008).
At the present, the only drugs approved for treatment of risk factors of metabolic syndrom are those drugs that target the individual risk factors: lipid-lowering drugs, antihypertensive agents, hypoglycemic drugs, and antiplatelet drugs. A lipid lowering mechanism is one of the major approaches in prevention of cardiovascular diseases. Though drugs of various categories acting through the different mechanisms are available in the antihyperlipidemic therapy, problems and side effects associated with the currently available lipid lowering drugs persist (Jain et al., 2010). Atorvastatin, 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor, is a lipid-lowering drug which in addition to this effect possesses several pleiotropic properties including vasoprotective, antiinflammatory, and antiarrhythmic properties that imply an immediate role for statin medications (Dougherty & Arora, 2012).
Our experiments with HTG rats fed HCHF diet demonstrated the potential of atorvastatin treatment to decrease the systolic blood pressure and to protect endothelial function. Our findings are in agreement with beneficial effects of atorvastatine on endothelium-dependent relaxation of the superior mesenteric artery (SMA) in aged HTG rats (Sotnikova et al., 2012). Among the other effects, atorvastatin improved endothelial function in aortas from diabetic rats (Simões et al., 2016), showed the positive impact on morphological and functional parameters of the vascular wall (Mikhin et al., 2016), and ameliorated oxidative stress and inflammatory reaction in rats with dyslipidemia (Zhang et al., 2014).
In our experiments atorvastatin restored the prolonged duration of selected ECG parameters i.e.. PQ, QRS and QTc intervals to values detected in HTG rats. However, it should be taken into account that coadministration of atorvastatin to patients treated with another additional QT-prolonging drug could induce life-threatening torsade de pointes arrhythmia (Niedrig et al., 2016).
Next we found out atorvastatin to increase the myocardial threshold for induction of tachyarrhythmias and to restore the sinus rhythm two-times faster than it was observed in non-treated HTG and HTG rats fed HCHF. However, atorvastatin only slightly reduced incidence of sustained tachyarrhythmias. Acute bolus administration of atorvastatin showed similar protective effects against arrhythmias induced by electrical pacing in HTG hearts (Benova et al., 2015).Cardiovascular protective effects of atorvastatin were also confirmed by clinical studies (Horwich & MacLellan, 2007; Xu et al., 2016).
Oxidative stress and inflammation are known to be involved in the pathogenesis of both metabolic syndrome and atrial and ventricular fibrillation (Tadic et al., 2013). Thus, drugs with antioxidant properties can be a good choice for cardiovascular protection in MetS. SMe1EC2 meets these criteria as it showed intensive antioxidant properties (Zúrová-Nedelčevová et al., 2006; Štolc et al., 2006; Broskova & Knezl, 2011). Indeed, in conditions of in vitro ischemia/reperfusion injury of the rat heart, this antioxidant showed beneficial effect during reperfusion. Administration of SMe1EC2 significantly increased the left ventricular developed pressure, decreased pathologically elevated left ventricular end-diastolic pressure and potentiated recovery from the serious reperfusion induced dysrhythmias such as ventricular tachycardia, ventricular fibrillation and stunned myocardium (Broskova & Knezl, 2011). In addition to antioxidant properties, the hypolipidemic activity of SMe1EC2 was documented in groups of HTG rats fed a standard diet as well as in rats fed high cholesterol and high fat diet. SMe1EC2 administration decreased serum levels of TC and TRG, and plasma levels of IL-1 (Bezek et al., 2017).
In the present experiments, administration of SMe1EC2 led to decrease of the elevated blood pressure of HTG animals fed HCHF diet. This beneficial effect was probably associated with amelioration of the aortic endothelium-dependent relaxation to acetylcholine to the control values of Wistar rats. The most important and original effects of SMe1EC2 were observed in the case of ECG parameters, where its administration led to adjustment of the increased values of PQ and QTc intervals to the control or even significantly lower values. Important effect of SMe1EC2 was found also in protection of the heart against induction of sustained VF/VT.
In summary, the present study shows for the first time cardioprotective effects of antioxidant SMe1EC2 in the model of HTG rats fed HCHF diet which are equal or even better that those of antilipidemic drug atorvastatin.
Acknowledgement
This study was supported by the Slovak Research Agency by realization of the project: „Transfer of Knowledge and Technologies from Research and Development in Toxicology on Evaluation of Environmental and Health Risks“ (ITMS 26240220005).
REFERENCES
- Albarado-Ibañez A, Avelino-Cruz JE, Velasco M, Torres-Jácome J, Hiriart M. Metabolic syndrome remodels electrical activity of the sinoatrial node and produces arrhythmias in rats. PLoS One. 2013;8:e76534. doi: 10.1371/journal.pone.0076534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti KG, Eckel RH, Grungy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr. Harmonizing the Metabolic Syndrome. A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- Allesie MA, Lammers WJEP, Bonke IM, Hollen J. Intra-atrial reentry as a mechanism for atrial flutter induced by acetylcholine and rapid pacing in dog. Circulation. 1984;70:123–135. doi: 10.1161/01.cir.70.1.123. [DOI] [PubMed] [Google Scholar]
- Bacharova L, Krivosikova Z, Wsolova L, Gajdos M. Alterations in the QRS complex in the offspring of patients with metabolic syndrome and diabetes mellitus: early evidence of cardiovascular pathology. J Electrocardiol. 2012;45:244–251. doi: 10.1016/j.jelectrocard.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Benova T, Knezl V, Viczenczova C, Bacova BS, Radosinska J, Tribulova N. Acute anti-fibrillating and defibrillating potential of atorvastatin, melatonin, eicosapentaenoic acid and docosahexaenoic acid demonstrated in isolated heart model. J Physiol Pharmacol. 2015;66:83–89. [PubMed] [Google Scholar]
- Bezek Š, Brnoliaková Z, Sotníková R, Knezl V, Paulovičová E, Navarová J, Bauer V. Monotherapy of experimental metabolic syndrome: I. Efficacy and safety. Interdiscip Toxicol. 2017;10(3):100–105. doi: 10.1515/intox-2017-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broskova Z, Knezl V. Protective effect of novel pyridoindole derivatives on ischemia/reperfusion injury of the isolated rat heart. Pharmacol Rep. 2011;63:967–974. doi: 10.1016/s1734-1140(11)70612-0. [DOI] [PubMed] [Google Scholar]
- Dougherty PJ, Arora RR. Utility of early high dose statins in acute coronary syndrome. Am J Ther. 2012;19:369–376. doi: 10.1097/MJT.0b013e31823735aa. [DOI] [PubMed] [Google Scholar]
- Duflou J, Virmani R, Rabi I, et al. Sudden death as a result of heart disease in morbid obese. Am Heart J. 1995;130:306–313. doi: 10.1016/0002-8703(95)90445-x. [DOI] [PubMed] [Google Scholar]
- Du Toit EF, Nabben M, Lochner A. A potential role of angiotensin II in obesity induced hypertrophy and ischaemic/reperfusion injury. Basic Res Cardiol. 2005;100:346–354. doi: 10.1007/s00395-005-0528-5. [DOI] [PubMed] [Google Scholar]
- Galajda P, Mokáň M, Prídavková D, Tomásková V, Sutarík L, Krucinská L, Bukovská A, Rusnáková G. Prevalencia metabolického syndrómu na Slovensku. (Prevalence of metabolic syndrome in Slovakia. In Slovak) Int med. 2007;7:325–331. [Google Scholar]
- Gray RA, Pertsov AM, Jalife J. Spatial and temporal organization during cardiac fibrillation. Nature. 1998;392:75–78. doi: 10.1038/32164. [DOI] [PubMed] [Google Scholar]
- Grundy SM. Controversy in Clinical Endocrinology. Metabolic Syndrome: A Multiplex Cardiovascular Risk Factor. JClin Endocr Met. 2007;92:399–404. doi: 10.1210/jc.2006-0513. [DOI] [PubMed] [Google Scholar]
- Horwich TB, MacLellan WR. Atorvastatin and statins in the treatment of heart failure. Expert Opin Pharmacother. 2007;17:3061–3068. doi: 10.1517/14656566.8.17.3061. [DOI] [PubMed] [Google Scholar]
- Huang JV, Lu L, Ye S, Bergman BC, Sparagna GC, Sarraf M, Reusch JE, Greyson CR, Schwartz GG. Impaired contractile recovery after low-flow myocardial ischemia in a porcine model of metabolic syndrome. Am J Physiol Heart C. 2013;304:H861–873. doi: 10.1152/ajpheart.00535.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain KS, Kulkarni RR, Jain DP. Current drug targets for antihyperlipidemic therapy. Mini Rev Med Chem. 2010;10:232–262. doi: 10.2174/138955710791185037. [DOI] [PubMed] [Google Scholar]
- Jouven X, Charles MA, Desnos M, Ducimetière P. Circulating nonesterified fatty acid levels as a predictive risk factor for sudden death in the population. Circulation. 2001;104:756–761. doi: 10.1161/hc3201.094151. [DOI] [PubMed] [Google Scholar]
- Liptak B, Knezl V, Gasparova Z. Metabolic disturbances induce malignant heart arrhythmias in rats. Bratisl Med J. 2017;118:539–543. doi: 10.4149/BLL_2017_103. [DOI] [PubMed] [Google Scholar]
- Liu C, Zhou M-S, Li Y, Wang A, Chadipiralla K, Tian R, Tian R, Raij L. Oral nicotine aggravates endothelial dysfunction and vascular inflammation in diet-induced obese rats: Role of macrophage TNFα. PLoS ONE. 2017;12:e0188439. doi: 10.1371/journal.pone.0188439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara Y, Morgan JP. Intracellular calcium and ventricular fibrillation. Circ Res. 1991;68:1378–1389. doi: 10.1161/01.res.68.5.1378. [DOI] [PubMed] [Google Scholar]
- Kurl S, Laaksonen DE, Jae SY, Mäkikallio TH, Zaccardi F, Kauhanen J, Ronkainen K, Laukkanen JA. Metabolic syndrome and the risk of sudden cardiac death in middle-aged men. Int J Cardiol. 2015;203:792–797. doi: 10.1016/j.ijcard.2015.10.218. [DOI] [PubMed] [Google Scholar]
- Lew EA, Garfinkel L. Variations in mortality by weight among 750 000 men and woman. J Chron Dis. 1979;32:563–576. doi: 10.1016/0021-9681(79)90119-x. [DOI] [PubMed] [Google Scholar]
- Luscher TF, Creager MA, Beckman JA, Cosentino F. Diabetes and vascular disease: patophysiology, clinical consequences, and medical therapy: part II. Circulation. 2003;108:1655–1661. doi: 10.1161/01.CIR.0000089189.70578.E2. [DOI] [PubMed] [Google Scholar]
- Luu M, Stevenson WG, Stevenson LW, Baron K, Walden J. Diverse mechanisms of unexpected cardiac arrest in advanced heart failure. Circulation. 1989;80:1675–80. doi: 10.1161/01.cir.80.6.1675. [DOI] [PubMed] [Google Scholar]
- Merrilat JC, Lakatta EG, Hano O, Guarnieri T. Role of calcium and the calcium channel in the initiation and maintenance of ventricular fibrillation. Circ Res. 1990;67:115–1123. doi: 10.1161/01.res.67.5.1115. [DOI] [PubMed] [Google Scholar]
- Mikhin VP, Zhilyaeva YA, Gromnaky NI. Pleotropic effects of atorvastatin in patients with chronic ischemic heart disease. Kardiologiia. 2016;56:42–46. doi: 10.18565/cardio.2016.5.42-46. [DOI] [PubMed] [Google Scholar]
- Nduhirabandi F, Du Toit EF, Blackhurst D, et al. Chronic melatonin consumption prevents obesity-related metabolic abnormalities and protects the heart against myocardial ischemia and reperfusion injury in a prediabetic model of diet-induced obesity. J Pineal Res. 2011;50:171–182. doi: 10.1111/j.1600-079X.2010.00826.x. [DOI] [PubMed] [Google Scholar]
- Niedrig D, Maechler S, Hoppe L, Corti N, Kovari H, Russmann S. Drug safety of macrolide and quinolone antibiotics in a tertiary care hospital: administration of interacting co-medication and QT prolongation. Eur J Clin Pharmacol. 2016;72:859–867. doi: 10.1007/s00228-016-2043-z. [DOI] [PubMed] [Google Scholar]
- Ooie T, Kajimoto M, Takahashi N, Shinohara T, Taniguchi Y, Kouno H, Wakisaka O, Yoshimatsu H, Saikawa T. Effects of insulin resistance on geranylgeranylacetone-induced expression of heart shock protein 72 and cardioprotection in high-fat diet rats. Life Sciences. 2005;77:869–881. doi: 10.1016/j.lfs.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Plourde B, Sarrazin J-F, Nault I, Poirier P. Sudden cardiac death and obesity. Expert Rev Cardiovasc Ther. 2014;12:1099–1110. doi: 10.1586/14779072.2014.952283. [DOI] [PubMed] [Google Scholar]
- Raal FJ. Pathogenesis and managenemt of the dyslipidaemia of the metabolic syndrome. Metab Syndr Relat Disord. 2009;7:83–88. doi: 10.1089/met.2008.0079. [DOI] [PubMed] [Google Scholar]
- Reule CA, Goyvaerts B, Schoen C. Effects of an L-arginine-based multi ingredient product on endothelial function in subjects with mild to moderate hypertension and hyperhomocysteinemia a randomized, doubleblind, placebo-controlled, cross-over trial. BMC Complement Altern Med. 2017;17:92. doi: 10.1186/s12906-017-1603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MT, Ribeiro FP, Medeiros MA, Sampaio PA, Silva YM, Silva MT, Quintans JS, Quintans-Júnior LJ, Ribeiro LA. The vasorelaxant effect of p-cymene in rat aorta involves potassium channels. Sci World J. 2015;2015:458080. doi: 10.1155/2015/458080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões FV, de Batista PR, Botelho T, Ribeiro-Júnior RF, Padilha AS, Vassallo DV. Treatment with high dose of atorvastatin reduces vascular injury in diabetic rats. Pharmacol Rep. 2016;68:865–873. doi: 10.1016/j.pharep.2016.04.022. [DOI] [PubMed] [Google Scholar]
- Simor T, Lorand T, Gaszner B, Elgavish GA. The modulation of pacinginduced changes in intracellular sodium levels by extracellular Ca in isolated perfused rat hearts. J Mol Cell Cardiol. 1997;29:1225–1235. doi: 10.1006/jmcc.1996.0359. [DOI] [PubMed] [Google Scholar]
- Sotnikova R, Bacova B, Vlkovicova J, Navarova J, Tribulova N. Sex differences in endothelial function of aged hypertriglyceridemic rats – effect of atorvastatin treatment. Interdiscip Toxicol. 2012;5:155–158. doi: 10.2478/v10102-012-0025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soydinc S, Davutoglu V, Akcay M. Uncomplicated metabolic syndrome is associated with prolonged electrocardiographic QTc interval and QTc dispersion. Ann Noninvasive Electrocardiol. 2006;11:313–317. doi: 10.1111/j.1542-474X.2006.00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Štolc S, Šnirc V, Májeková M, Gáspárová Z, Gajdošíková A, Štvrtina S. Development of the new group of indole-derived neuroprotective drugs affecting oxidative stress. Cell Mol Neurobiol. 2006;26:1493–1502. doi: 10.1007/s10571-006-9037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadic M, Ivanovic B, Cuspidi C. What do we currently know about metabolic syndrome and atrial fibrillation? Clin Cardiol. 2013;36:654–662. doi: 10.1002/clc.22163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribulová N, Okruhlicová Ľ, Nováková S, Pancza D, Bernátová I, Pechanová O, Weismann P, Manoach M, Seki S, Mochizuki M. Hypertensionrelated intermyocyte junction remodeling is associated with higher incidence of low K+induced lethal arrhythmias in isolated rat heart. Exp Physiol. 2002;87:195–205. doi: 10.1113/eph8702336. [DOI] [PubMed] [Google Scholar]
- Tribulová N, Fialová M, Dlugošová K, Knezl V, Okruhlicová L, Kristek F, Zicha J, Kuneš J. Myocardial gap junction remodelling in hypetriglyceridemic rat heart is associated with increased vulnerability to ventricular fibrillation. Cardiology. 2006;15:32S–33S. [Google Scholar]
- Tribulova N, Knezl V, Okruhlicova L, Slezak J. Myocardial gap junctions: targets for novel approaches in the prevention of life-threatening cardiac arrhythmias. Physiol Res. 2008;57:S1–S13. doi: 10.33549/physiolres.931546. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Tanabe N, Watanabe T, Roden D M, Sasaki S, Aizawa Y. Metabolic syndrome and risk of development of atrial fibrillation. Circulation. 2008;117:1255–1260. doi: 10.1161/CIRCULATIONAHA.107.744466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensley I, Salaveria K, Bulmer AC, Donner DG, du Toit EF. Myocardial structure, function, and ischaemic tolerance in a rodent model of obesity with insulin resistance. Exp Physiol. 2013;98:1552–1564. doi: 10.1113/expphysiol.2013.074948. [DOI] [PubMed] [Google Scholar]
- Witkowski FX, Leon LJ, Penkoske PA, Giles WR, Spano ML, Ditto WL, Wintre AT. Spatiotemporal evolution of ventricular fibrillation. Nature. 1998;392:78–82. doi: 10.1038/32170. [DOI] [PubMed] [Google Scholar]
- Xu XR, Li KB, Wang P, Xu L, Liu Y, Yang ZS, Yang XC. The impact of different doses of atorvastatin on plasma endothelin and platelet function in acute ST-segment elevation myocardial infarction after emergency percutaneous coronary intervention. Zhonghua Nei Ke Za Zhi. 2016;55:932–936. doi: 10.3760/cma.j.issn.0578-1426.2016.12.005. [DOI] [PubMed] [Google Scholar]
- Yilmaz H, Özcan KS, Sayar N, Kemaloglu T, Gungor B, Erer B, Yilmaz M, Gurkan U, Cakmak N, Oz D, Calik AN, Bolca O. Metabolic syndrome is associated with atrial electrical and mechanical dysfunction. Med Princ Pract. 2015;24:147–152. doi: 10.1159/000368754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WL, Yan WJ, Sun B, Zou ZP. Synergistic effects of atorvastatin and rosiglitazone on endothelium protection in rats with dyslipidemia. Lipids Health Dis. 2014;13:168. doi: 10.1186/1476-511X-13-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zúrová-Nedelčevová J, Navarová J, Drábiková K, Jančinová V, Petríková M, Bernátová I, Kristová V, Šnirc V, Nosáľová V, Sotníková R. Participation of reactive oxygen species in diabetes induced endothelial dysfunction. Neuroendocrinol Lett. 2006;27:168–171. [PubMed] [Google Scholar]