Abstract
Artemisinin, an effective anti-malarial drug is synthesized in the specialized 10-celled biseriate glandular trichomes of some Artemisia species. In order to have an insight into artemisinin biosynthesis in species other than A. annua, five species with different artemisinin contents were investigated for the expression of key genes that influence artemisinin content. The least relative expression of the examined terpene synthase genes accompanied with very low glandular trichome density (4 No. mm−2) and absence of artemisinin content in A. khorassanica (S2) underscored the vast metabolic capacity of glandular trichomes. A. deserti (S4) with artemisinin content of 5.13 mg g−1 DW had a very high expression of Aa-ALDH1 and Aa-CYP71AV1 and low expression of Aa-DBR2. It is possible to develop plants with high artemisinin synthesis ability by downregulating Aa-ORA in S4, which may result in the reduction of Aa-ALDH1 and Aa-CYP71AV1 genes expression and effectively change the metabolic flux to favor more of artemisinin production than artemisinic acid. Based on the results, the Aa-ABCG6 transporter may be involved in trichome development. S4 had high transcript levels and larger glandular trichomes (3.46 fold) than A. annua found in Iran (S1), which may be due to the presence of more 2C-DNA (3.48 fold) in S4 than S1.
Introduction
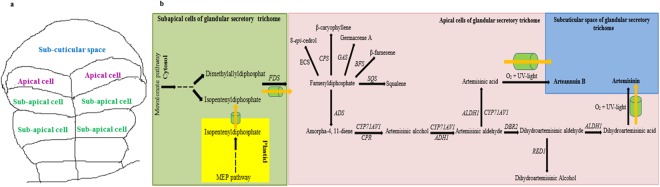
The specialized 10-celled biseriate glandular trichomes (Fig. 1a) of some Artemisia species are the sites of artemisinin synthesis1–6. Artemisinin is a sesquiterpene lactone, an efficacious anti-malarial drug against a number of cancers and viral diseases7. Artemisia genus of Asteraceae family possesses over 500 species, which are mainly found in Asia, Europe and North America8. 35 of these species are found in Iran9. All Artemisia species produce less artemisinin contents than A. annua1–6. The main source of artemisinin is A. annua. Artemisinin yield by the wide type of A. annua is very low and insufficient to cover the need of all patients10.
Figure 1.
The specialized 10-celled biseriate glandular secretory trichome of Artemisia annua (a) and Summary of terpene metabolism and Transporters (ABCG6, ABCG7) involved in artemisinin biosynthesis and cuticle development (illustrated as green cylinders, (b)). ECS: epi-cedrol synthase, CPS: β-caryophyllene synthase, GAS: germacrene A synthase, BFS: β-farnesene synthase, SQS: Squalene synthase, FDS: farnesyl diphosphate synthase, ADS: amorpha-4,11-diene synthase, CYP71AV1: amorphadiene-12-hydroxylase, CPR: cytochrome P450 reductase, ADH1: alcohol dehydrogenase 1, ALDH1: aldehyde dehydrogenase 1, DBR2: artemisinic aldehyde Δ11(13) reductase, RED1: dihydroartemisinic aldehyde reductase.
Hitherto, metabolic engineering for high artemisinin production has failed due to lack of genetic evidence for the biosynthesis pathway11. The knowledge of factors influencing the entire biosynthetic pathway and mechanisms regulating the onset and flux of the pathway in other Artemisia species can lead to favorable metabolic engineering when compared to A. annua.
Artemisinin biosynthetic pathway in eight Artemisia species was studied. It was reported that A. absinthium had a higher expression level of both Aa-ALDH1 and Aa-CYP71AV1 genes when compared to A. annua during the developmental stages5. Salehi et al.6 investigated artemisinin biosynthetic pathway and two trichome formation genes in five Artemisia species. In addition to the genes that regulate trichome formation and the artemisinin pathway, artemisinin yield was affected by genes of branching pathways (Fig. 1b), transcription factors and transporters (Fig. 1b) involved in artemisinin biosynthesis. To the best of our knowledge, there are no published studies on sesquiterpene synthases (Fig. 1b) that compete for the same substrate, farnesyl diphosphate (FDP), transcription factors (TFs) and transporters (Fig. 1b) that are involved in artemisinin production in any other Artemisia species when compared to A. annua.
The basic C5 precursors for terpenoid biosynthesis (isopentenyl diphosphate (IDP) and dimethylallyl diphosphate (DMADP)) are synthesized via two distinct pathways: the mevalonate (MVA) pathway in the cytosol and the methylerythritol phosphate (MEP) pathway in plastid12. One molecule of IDP and one molecule of DMADP are condensed to produce geranyl diphosphate (GDP). GDP condenses with one unit of IDP to produce FDP. FDP is a significant product at the branching point of terpenoid metabolism. It is converted to β-farnesene, β-caryophyllene, germacrene A, epi-cedrol, squalene and amorphadiene by β-farnesene synthase (Aa-BFS), β-caryophyllene synthase (Aa-CPS), germacrene A synthase (Aa-GAS), epi-cedrol synthase (Aa-ECS), squalene synthase (Aa-SQS) and amorpha-4, 11-diene synthase (Aa-ADS), respectively13–18 (Fig. 1b). These products are produced in T-shaped trichomes or glandular trichomes or in both types of trichomes. Amorphadiene is first converted into dihydroartemisinic acid (DHAA) by a series of enzymes19–22 (Fig. 1b), and thereafter, DHAA is converted into artemisinin by an enzyme-independent reaction23. The expression of several sesquiterpene synthase genes and Aa-SQS may have a negative impact on artemisinin production in plants by competing for the same substrate, FDP24. Blocking of the branch pathways in A. annua is a useful technique for obtaining a high artemisinin producing plant25.
In plants, spatial-temporal regulation of secondary metabolites production and storage is usually regulated by TFs26. Overexpression of these factors have been proposed as an auspicious approach for increasing secondary metabolism in plants more efficiently since the plant transcription factors regulate a series of genes in one specific pathway27.
Artemisinin intermediates, especially the aldehydes are toxic to cells28. Some transporters exist between plastid and cytosol (transport of isopentenyl diphosphate from plastid to cytosol), subapical and apical cells (transport of FDP from subapical cells to apical cells), apical cells and subcuticular space (transport of artemisinin and arteannuin B from apical cells to subcuticular space) of glandular trichomes (Fig. 1b). These transporters carry the precursors to the sites where artemisinin is produced and accumulated29. Moreover, some transporters, which are involved in trichome development could affect artemisinin yield29.
Genome size (i.e. the DNA content of the unreplicated nucleus, 2C30; which is expressed in picograms or in millions base pairs, 1 pg = 978 Mbp31) as an important character in biodiversity correlates with many different kinds of biological parameters32. ‘C value’ (holoploid genome size) shows the DNA content of the unreplicated haploid complement irrespective of the degree of generative polyploidy, aneuploidies etc. ‘Monoploid genome size’ (1Cx) is the DNA content in a basic chromosome set (x) of a somatic cell30.
In the current study, five Artemisia species with different artemisinin contents were assessed in terms of expression of six terpene synthase genes (Fig. 1b) competing for the same substrate, FDP, three transcription factor genes (Aa-ORA, Aa-ERF1, Aa-WIRKY1) and two transporter genes (Fig. 1b) influencing artemisinin production. A. deserti (S4) had high transcript levels and was reported to have larger trichomes than A. annua found in Iran (S1, 3.46 fold)6. Consequently, the chromosome number and nuclear DNA content were also determined to identify the relationship of high transcript levels and gland size of S4 with its genome size.
Results and Discussion
Artemisinin content and glandular trichome density
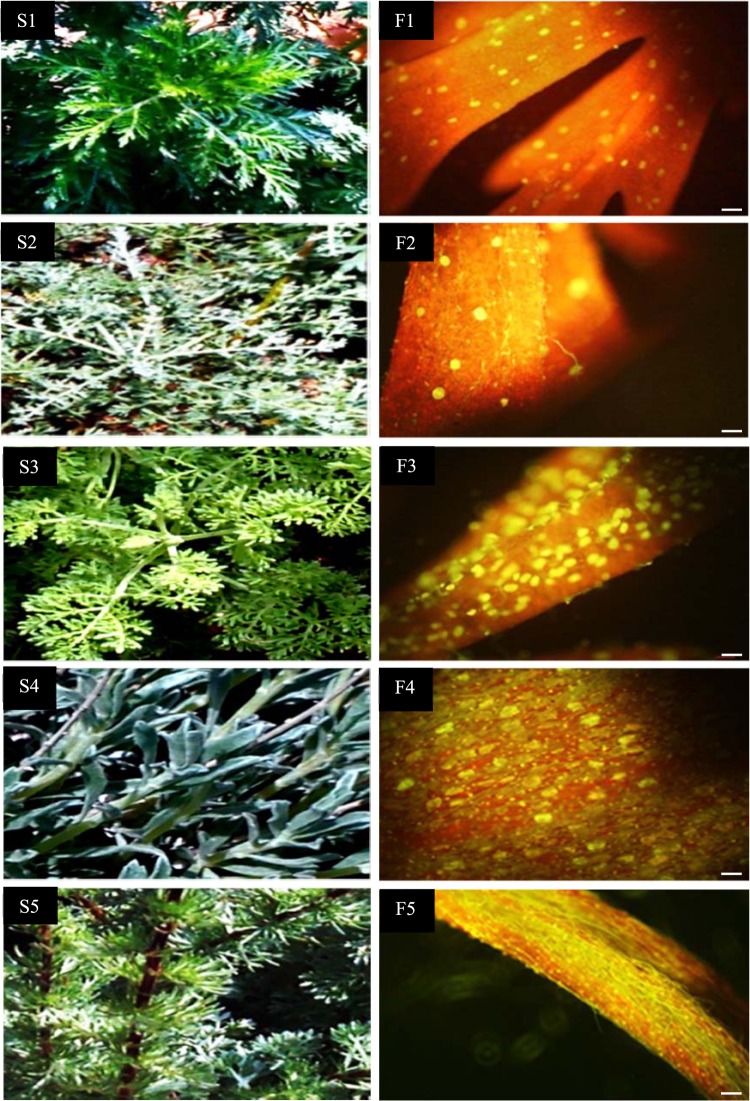
S1 had the highest amount of artemisinin (6.60 mg g−1 DW) followed by S4 (5.13 mg g−1 DW), S5 (3.50 mg g−1 DW) and S3 (0.96 mg g−1 DW). No artemisinin content was observed in S2 (Fig. 2). All previous studies revealed that other Artemisia species produced less artemisinin compared to A. annua1–6. A. annua L. found in Iran has been reported to be a low artemisinin producing plant6,33. In the present study, S4 had slightly less artemisinin content than S1 (Fig. 2). The glandular trichome densities of five Artemisia species were determined employing fluorescence microscopy images (Fig. 3). The highest glandular trichome density was observed in S5 (121 No. mm−2) followed by S4 (100 No. mm−2), S3 (58 No. mm−2), S1 (19 No. mm−2) and S2 (4 No. mm−2, Fig. 2). Artemisinin content had no significant correlation (r = 0.25ns) with glandular trichome density.
Figure 2.
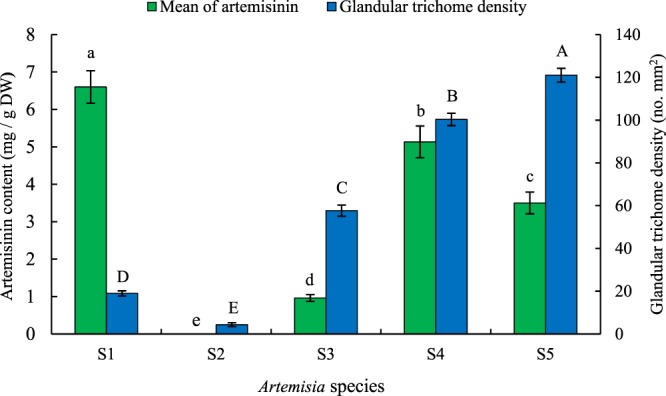
Artemisinin content and glandular trichome density of five Artemisia species including S1 (A. annua found in Iran), S2 (A. khorassanica), S3 (A. persica), S4 (A. deserti), and S5 (A. marschalliana). Error bars are shown as SE (n = 3). Means followed by the same letter are not significantly different according to the LSD at 0.01 probability level.
Figure 3.
Glandular trichomes showing the content of autofluorescing aromatic oils (F1, F2, F3, F4, F5), Scale bar 100 μm, 10× objective, 10× on ocular of five Artemisia species including A. annua found in Iran (S1, F1), A. khorassanica (S2, F2), A. persica (S3, F3), A. deserti (S4, F4) and A. marshalliana (S5, F5).
Gene expression
The qPCR technique was applied to ascertain the relationship of artemisinin content with the expression pattern of key genes influencing artemisinin content.
Relative expression analysis of six terpene synthase genes
The sesquiterpene synthases including Aa-ADS (amorpha-4, 11-diene synthase), Aa-ECS (epi-cedrol synthase), Aa-CPS (β-caryophyllene synthase), Aa-GAS (germacrene A synthase), and Aa-BFS (β-farnesene synthase) compete for the same substrate, FDP (Fig. 1-b). In addition, FDP is used for the biosynthesis of sterols and triterpenes by squalene synthase (Aa-SQS, Fig. 1-b). Therefore, they may influence artemisinin production in a plant. S4 had higher expression of Aa-ADS (3.33 fold), Aa-CPS (77.81 fold) and Aa-GAS (75.29 fold) than S1 (Fig. 4). It can be concluded that the blocking of two genes (Aa-CPS and Aa-GAS) at the branching points in S4 may be an efficacious technique for inducing plants to produce higher levels of artemisinin. The high transcript levels of S4 may result in high metabolic capacity. However, the impact of post-transcriptional and translational regulation must also be considered. S5 had higher expression of Aa-ADS (1.39 fold), Aa-BFS (77.36 fold) and Aa-SQS (6.70 fold) than S1 (Fig. 4). S3 had relatively high glandular trichome density and very low artemisinin content (Fig. 2) due to higher expression of Aa-CPS (1.35 fold), Aa-GAS (9.87 fold) and Aa-BFS (11.78 fold, Fig. 4). S3 also had less expression of artemisinin biosynthesis genes compared to S1.
Figure 4.
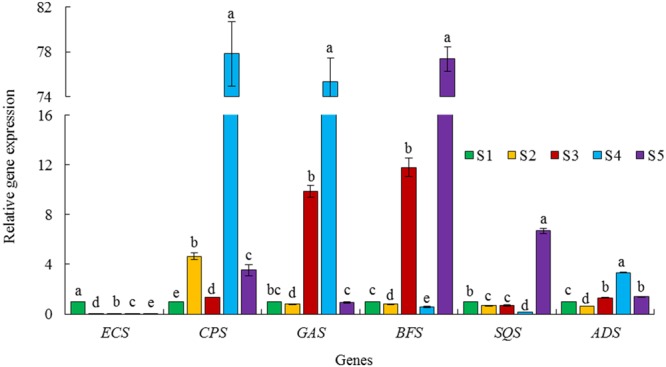
Relative expression of six terpene synthase genes in five Artemisia species including S1 (A. annua found in Iran), S2 (A. khorassanica), S3 (A. persica), S4 (A. deserti), and S5 (A. marschalliana). Error bars are shown as SE (n = 3). Means within a gene followed by the same letter are not significantly different according to the LSD at 0.01 probability level.
Artemisinin is synthesized in the specialized 10-celled glandular trichomes34. β-caryophyllene, a volatile metabolite is located in non-glandular trichomes35. β-farnesene, which plays an important role as an alarm pheromone36,37 is located in both glandular trichomes and non-glandular trichomes35. Aa-GAS is probably located in the glandular trichomes since it was cloned from glandular trichomes expressed sequence tag (EST) library18. Among six studied terpene synthase genes, only S2 had higher expression of CPS gene (4.64 fold) compared to S1 (Fig. 4). This is understandable because S2 had very low glandular trichome density (Fig. 2) and high non-glandular trichome density6. The least relative expression of the examined terpene synthase genes (Fig. 4) accompanied with very low glandular trichome density and absence of artemisinin content in S2 (Fig. 2) underscored the vast metabolic capacities of glandular trichomes.
While trichomes of many species produce a high content of one or a few specialized metabolites, it is possible that many others do not38. Trichomes function as a closed biochemical system with a simple input and little highly active biochemical pathways of both primary metabolism (for generating energy and precursors) and secondary (specialized) metabolism (for generating final products)39. Since the biosynthetic capacity of trichomes is limited by the amount and type of carbon source imported into them, it can be observed that the total output is limited when a given type of trichome is allowed to generate various classes of compounds38. Therefore, it can be concluded that blocking of active branch pathways in artemisinin producing plants is an effective technique for generating high yield artemisinin. The hairpin RNA-mediated gene silencing of Aa-SQS in A. annua resulted in downregulaton of Aa-SQS and 3-fold increase in artemisinin synthesis40. In addition, the blocking of branch pathways in A. annua was reported to be an efficacious method for generating high yield artemisinin25.
Relative expression analysis of three transcription factors
Transcription factors regulate the activity of genes involved in the biosynthesis of secondary metabolites in plants by binding to the cis-acting regulatory elements of the promoters. Aa-WRKY1 had the ability to bind to the W-box in Aa-ADS promoter and activate Aa-ADS gene expression in transgenic tobacco plants and transient expression of A. annua leaf system41. In another study, overexpression of Aa-WRKY improved the transcription level of Aa-CYP71AV1, while the transcription levels of Aa-ADS and Aa-DBR2 did not change significantly in transgenic plants42. The expression of Aa-ORA, Aa-ADS, Aa-CYP71AV1 and Aa-DBR2 were promoted in Aa-ORA overexpressing transgenic plants43. Aa-ERF1 was a positive regulator of Aa-ADS and Aa-CYP71AV144. In this study, we monitored the relative expression of three transcription factors including Aa-ORA, Aa-ERF1 and Aa-WIRKY1 (Fig. 5). Gene expression of Aa-ORA, Aa-ERF1 and Aa-WIRKY1 in S4 were 71.90, 2.38 and 0.49 fold, respectively compared to S1 (Fig. 5). S3 and S5 had a little higher expression of Aa-ERF1 (1.46 fold) and Aa-ORA (1.2 fold), respectively than S1 (Fig. 5). Since Aa-ORA (Fig. 5), Aa-ALDH1 and Aa-CYP (data not shown) in the studied species were found to have a similar expression pattern, we suggest that Aa-ORA is a transcription factor that regulates the promoters of Aa-ALDH1 and Aa-CYP genes. It may be concluded that S4 produced more artemisinic acid/arteannuin B than artemisinin because Aa-DBR2 and Aa-ALDH1 acted on the same pool of intermediates. The relative turnover of Aa-ALDH1 was much higher than Aa-DBR2 in S46. Hence, the low artemisinin content of S4 was probably due to the flux of intermediate through the two branches (the oxidation of the artemisinic aldehyde to artemisinic acid or the reduction of the artemisinic aldehyde to dihydroartemisinic aldehyde, Fig. 1-b) of the pathway6. It is possible to develop high artemisinin producing plant by downregulating Aa-ORA, which may decrease the expression of Aa-ALDH1 and Aa-CYP and change the metabolic flux more efficiently to artemisinin production than artemisinic acid.
Figure 5.
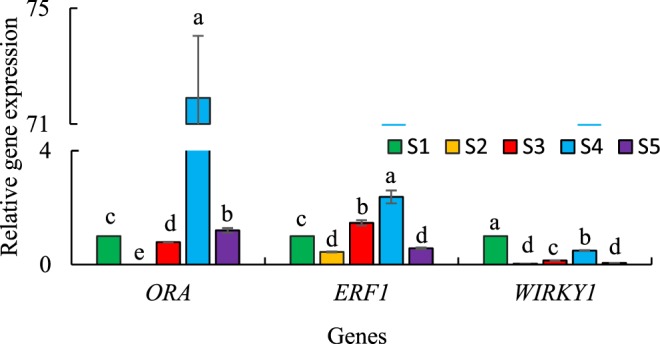
Relative expression of three transcription factor in five Artemisia species including S1 (A. annua found in Iran), S2 (A. khorassanica), S3 (A. persica), S4 (A. deserti), and S5 (A. marschalliana). Error bars are shown as SE (n = 3). Means within a gene followed by the same letter are not significantly different according to the LSD at 0.01 probability level.
Relative expression analysis of ABCG6 and ABCG7 transporter unigenes
Two ABC (ATP-binding cassette) transporter, Aa-ABCG6 and Aa-ABCG7 (Fig. 1-b) showed parallel expression pattern as two artemisinin biosynthesis specific genes (Aa-ADS and Aa-CYP)29. It was concluded that the two transporters were involved in A. annua glandular trichome cuticle formation and/or played roles in transportation that were related to artemisinin production and accumulation. We investigated genes expression of Aa-ABCG6 and Aa-ABCG7 as effective transporters in this study (Fig. 6). S3, S4 and S5 had higher glandular trichome density than S1 (Fig. 2), and these species also had higher expression of Aa-ABCG6 gene (Fig. 6). The ABCG6 transporter most likely is not part of any transportation related to artemisinin production and accumulation because S3 with low artemisinin content had high expression of ABCG6. S2 had very low glandular trichome density (4 No. mm−2, Fig. 2) and less expression of Aa-ABCG6 gene (0.57 fold, Fig. 6) than S1. Therefore, it can be speculated that the ABCG6 transporter might be involved in glandular trichome cuticle development. Some transporters involved in glandular trichome cuticle development could be relevant to artemisinin yield as previously reported29.
Figure 6.
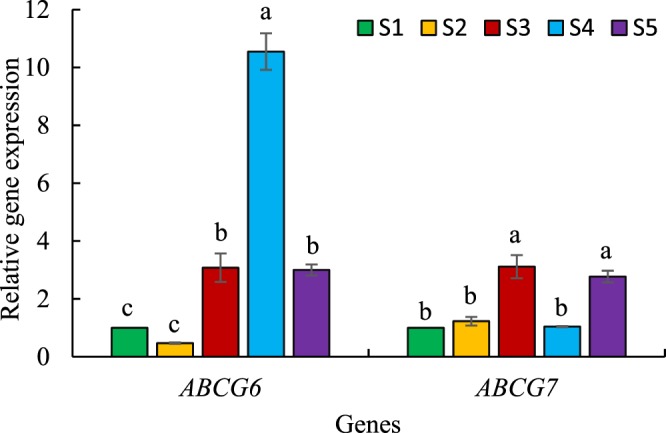
Relative expression of ABCG6 and ABCG7 transporter unigenes in five Artemisia species including S1 (A. annua found in Iran), S2 (A. khorassanica), S3 (A. persica), S4 (A. deserti), and S5 (A. marschalliana). Error bars are shown as SE (n = 3). Means within a gene followed by the same letter are not significantly different according to the LSD at 0.01 probability level.
Cytogenetic studies
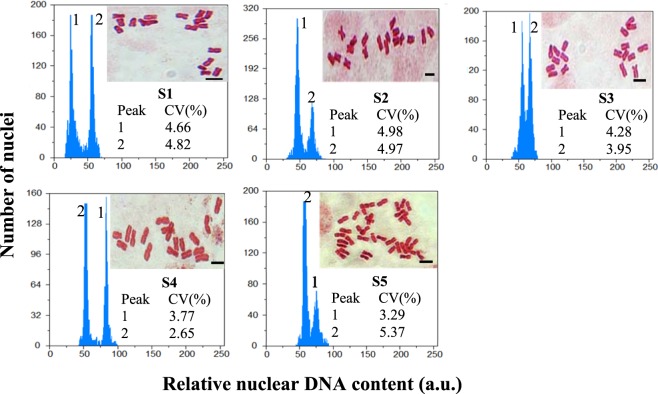
Among five studied Artemisia species, four species (S1–S4) were diploid (2n = 2x = 18 m), while S5 was tetraploid (2n = 4x = 36 m, Fig. 7 and Table 1). In the diploid species, the mean CL was 4.52 μm, varying from 3.37 μm (S1) to 6.11 μm (S4, Supplementary Table S1) and the mean TCV was 7.04 μm3, ranging from 4.60 μm3 (S1) to 11.76 μm3 (S4, Supplementary Table S1). In the tetraploid species (S5), the mean CL and TCV were 4.35 μm and 5.92 μm3, respectively (Supplementary Table S1). The histograms used for analysis of the nuclear DNA content contained two peaks: peak 1 refers to the G1 of unknown Artemisia species samples and peak 2 represents the G1 of the known Pisum sativum cv. Ctirad (2C DNA = 9.09 pg) internal reference standard (Fig. 7). The variation coefficients (CV) of G1 peaks were less than 5% for Artemisia species and P. sativum samples. ANOVA showed significant differences between diploid (p < 0.01) species for nuclear 2C DNA amount. Interestingly, among the four diploid species, a difference of 9.97 pg in 2C value [4.02 (S1)–13.99 (S4)] was observed despite the four species having the same chromosome numbers of 18 (Table 1). Among the five studied Artemisia species, S1, which is an annual plant (4.05 pg) had the least 2C DNA content; S2-S5 are perennial plants. This is in consonance with the idea that a bigger genome implies a longer cell cycle; thus, they are prevented from the short life cycle that is typical of annual plants45. The diploid S4 with the most genome size, 2C DNA = 13.99 pg had the highest transcript levels.
Figure 7.
Histograms of flow cytometric 2C DNA content and somatic chromosomes of five Artemisia species including S1 (A. annua found in Iran), S2 (A. khorassanica), S3 (A. persica), S4 (A. deserti), and S5 (A. marschalliana).
Table 1.
Mean (n = 3) 2C-DNA of five Artemisia species including S1 (A. annua found in Iran), S2 (A. khorassanica), S3 (A. persica), S4 (A. deserti), and S5 (A. marschalliana).
| Species | Ploidy level | 2n | 2C DNA mean (pg) ± SE | Holoploid | Monoploid | ||
|---|---|---|---|---|---|---|---|
| (1C DNA) genome size (pg) | (1C DNA) genome size (Mbp) | (1Cx DNA) genome size (pg) | (1Cx DNA) genome size (Mbp) | ||||
| S1 | 2x | 18 | 4.02d ± 0.02 | 2.010 | 1965.78 | 2.010 | 1965.78 |
| S2 | 2x | 18 | 6.12c ± 0.05 | 3.060 | 2992.68 | 3.060 | 2992.68 |
| S3 | 2x | 18 | 7.14b ± 0.08 | 3.570 | 3491.46 | 3.570 | 3491.46 |
| S4 | 2x | 18 | 13.99a ± 0.06 | 6.995 | 6841.11 | 6.995 | 6841.11 |
| S5 | 4x | 36 | 11.50 ± 0.01 | 5.750 | 5623.50 | 2.875 | 2811.75 |
Means within a column followed by the same letter are not significantly different according to the LSD at the 0.01 probability level.
Conclusion
Several Artemisia species produce less artemisinin than A. annua. The aim of our project was to comprehend the cause of the low artemisinin content of Artemisia species other than A. annua in order to have a better insight into artemisinin biosynthesis. Our previous study6 showed that biseriate, capitate glandular trichomes were prevalent in the genus of Artemisia and the density of glandular trichome and gland size had no significant relationship with artemisinin content. In addition, in our previous study6, the expression of artemisinin biosynthesis and trichome formation genes in five Artemisia species with different artemisinin content was reported.
In the current study, in order to have a better insight into artemisinin biosynthesis in Artemisia species other than A. annua, these five species with different artemisinin content were assessed in terms of key genes expression that affects artemisinin production including six terpene synthase genes (Aa-ECS, Aa-CPS, Aa-GAS, Aa-BFS, Aa-ADS, and Aa-SQS, Fig. 1-b), three transcription factor genes (Aa-ORA, Aa-ERF1, Aa-WIRKY1) and two transporter genes (Aa-ABCG6 and Aa-ABCG7, Fig. 1-b).
S4 (A. deserti) had the highest expression of Aa-ADS (3.33 fold), Aa-CPS (77.81 fold) and Aa-GAS (75.29 fold, Fig. 4). Blocking of two genes at branching points (Aa-CPS and Aa-GAS) in S4 may be an efficacious method for generating high artemisinin producing plant.
Aa-ORA, Aa-ALDH1 and Aa-CYP in the studied species had a similar expression pattern; we therefore suggested that Aa-ORA is a transcription factor that regulates the promoters of Aa-ALDH1 and Aa-CYP genes. Our previous study6 showed that the relative turnover potential of ALDH1 was 112 folds higher than DBR2 in S4 (A. deserti) and converted more metabolic flux into artemisinic acid than artemisinin. It is possible to develop high artemisinin producing plant by the downregulation of Aa-ORA, which may decrease the expression of Aa-ALDH1 and Aa-CYP and efficiently change more metabolic flux to favor artemisinin production compared to artemisinic acid.
The relationship between expression patterns of the two studied transporter genes (Fig. 6), artemisinin content and glandular trichome density (Fig. 2) in five Artemisia species suggested that Aa-ABCG6 transporter plays a major role in glandular trichome development while it does not play a role in the transportation of artemisinin precursors.
There was a positive and significant correlation coefficient between individual data of monoploid genome size and area of glandular trichomes (r = 0.87**, Supplementary Fig. S1). The diploid S4 having the most genome size; 2C DNA = 13.99 pg (Table 1) had higher transcript levels and larger trichomes (3.46 fold, Supplementary Fig. S1) than A. annua found in Iran (S1). The amount of DNA control the cell size and cell cycle length such that the correlation of genome size and cell size was much stronger, more constant and direct than it was for cell cycle length46. It was reported that cell size correlated significantly with the transcription rate and that genome expression can be regulated genetically to induce changes in cell size47. The larger cells could sustain their larger biomass by owning more gene copies that can produce more RNAs and proteins accordingly48. Therfore, the high transcript levels of S4 might be due to the presence of more 2C value (3.48 times) in S4 than S1.
Materials and Methods
Seeds of 17 Artemisia species were gathered from different parts of Iran6. Also, A. annua cv. Anamed which is regarded as a high artemisinin cultivar was included in the experiment6. To eliminate the environmental effects, the plants were propagated under the same conditions and their seeds were gathered and planted in the Iranian Biological Resource Center6. Based on their artemisinin content and morphology of the glandular secretory trichome (assessing density and area of glandular trichome using fluorescent microscopy and scanning electron microscopy, respectively) at the flowering stage, five Artemisia species, including A. annua L. found in Iran (S1) as a control species, A. khorassanica Podlech (S2, no artemisinin content and very low glandular trichome density), A. persica Boiss (S3, low artemisinin content, medium density and low area of glandular trichome), A. deserti Krasch (S4, high artemisinin content, high density and area of glandular trichome) and A. marschalliana Sprengel (S5, medium artemisinin content, high density and low area of glandular trichome)6 were selected. In our previous study, these five species were evaluated in terms of the expression of artemisinin biosynthesis genes and two other genes (Aa-TTG1 and Aa-TFAR1) that are involved in trichome formation6. It is noteworthy that based on scanning electron micrograph, S2 with very low glandular trichome density and very high non-glandular trichome density and S1 with high glandular trichome density and very low non-glandular trichome density (Supplementary Fig. S2) were included in the experiment. In the current study, in order to have a better insight into artemisinin biosynthesis in species other than A. annua, these five species (Fig. 3) were evaluated in terms of six terpene synthase (Aa-ECS, Aa-CPS, Aa-GAS, Aa-BFS, Aa-ADS, and Aa-SQS, Fig. 1-b), three transcription factor (Aa-ORA, Aa-ERF1, Aa-WIRKY1) and two transporter (Aa-ABCG6 and Aa-ABCG7, Fig. 1-b) genes expression. In addition, to determine the relationship between ABCG transporter gene expression with artemisinin content and glandular trichome density, these traits were re-evaluated. The transcription levels of the above-mentioned genes of four species including S2, S3, S4 and S5 were relatively compared to S1, which was chosen as a reference species. Half of each leaf (upper branches) was cut and mixed for RNA extraction and expression analysis, and another half was considered for artemisinin measurement at flowering stage. Artemisinin and RNA extraction were performed with three replications and each replication was a mixture of three sampled plants (upper branches). Since the glandular trichome area of these five species varied, chromosome number and nuclear DNA content were also determined employing flow cytometry in order to distinguish the ploidy level of species, as well as find out any possible relationship between gland size and monoploid genome size.
Fluorescence microscopy
Glandular trichome density of the abaxial leaf epidermis (upper branches) was evaluated. Leaf samples were analyzed under the Olympus IX-71 Inverted Fluorescence Microscope (Olympus, Tokyo, Japan) for assessment of glandular trichome density. All tissue images were taken using the same magnification (10x objective, 10x ocular, Fig. 3). Each replication was the average of three samples.
Artemisinin extraction
For HPLC (high performance liquid chromatography) analysis, the leaves were sampled from the upper branches and dried in the dark. Artemisinin was extracted from the leaves employing the procedure described by Peng et al.49. The artemisinin content of the extracts was evaluated by an HPLC system (Waters, USA) equipped with a C18 column (NUCLEODUR 100-5 C18 ec, 250 mm × 4.6 mm, China) and detection was conducted at 210 nm wavelength. The acetonitrile: water 65: 35% (v/v) was used as a mobile phase with 1 ml/min flow rate50. The retention time of artemisinin reference standard and artemisinin of Artemisia species was 8.35 ± 0.05 min. Artemisinin production in A. persica (S3), A. deserti (S4) and A. marschalliana (S5) was verified using spike artemisinin standard in extraction of these species. The calibration curve was constructed by plotting the peak area (y) against concentration (150, 300, 600, 1200, and 2400 ppm) of standard solutions (x). The determination coefficient (R2) was 0.9984. The contents of artemisinin (mg g−1 DW) were determined employing calibration curves.
Real-time RT-PCR
Total RNA was extracted using RiboEx Total RNA reagent (GeneAll Biotechnology Co., Ltd., Songpa-gu, South Korea) according to the manufacturer’s instruction. In addition, the extracted RNA was treated with Qiagen RNase-free DNase (Qiagen, 79254, Qiagen Inc., Midland, ON, Canada) according to the manufacture’s instruction to remove any genomic DNA contamination. To ensure non-amplification of possible contaminated genomic DNA, two strategies were used: (1) Conduction of PCR with RNA template for each primer pairs, (2) SQS primer pairs was designed for spanning of an exon-exon junction. The quality and quantity of RNA were evaluated using agarose gel electrophoresis (Supplementary Fig. S3) and Nanodrop (Thermo Scientific, Germany) spectrophotometer analyses, respectively. cDNA was synthesized with 1 µg total RNA using Thermo Scientific Revert-Aid™ First-Strand cDNA Synthesis Kit (Fermentas, K1622, Thermo Fisher Scientific, Hudson, NH, USA) according to the manufacturer’s protocol in order to obtain a 20 µl cDNA solution. The qPCR primers were designed employing Oligo 7 primer analysis software and then checked with Oligoanalyzer tool (eu.idtdna.com/calc/analyzer) and NCBI/Primer-BLAST (www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi?LINK_LOC=BlastHome). Based on the studies of Olofsson et al.24 and Salehi et al.6 Aa-β-actin and Aa-CPR were selected as reference genes (Table 2). In the first step, mRNA, complete cds of five artemisinin biosynthesis genes of A. deserti (S4, species with high artemisinin content) were isolated and sequenced. The sequences of these genes were identical to mRNA of artemisinin biosynthesis genes in A. annua (Supplementary Fig. S4). Also, PCR products of CPR, GAS, CPS, ORA, ABCG6 and ABCG7 primer pairs and mRNA, partial cds of Actin of four studied species were isolated and sequenced. The sequencing showed that these sequences in the four species were identical to those in A. annua (Supplementary Fig. S5). The qPCR was performed using specific primers (Table 2) on a BioRad MiniOption real-time PCR detection system (Applied Biosystems, Foster City, CA, USA) with the fluorescent dye SYBR®Green Master Mix 2X (Ampliqon, A323402, Denmark) according to the manufacturer’s instructions. 1 μL of the first strand cDNA was used as a template in 20 μL reactions, including 10 μL SYBR®Green PCR Master Mix and three pmol of each primer. The qPCR was run at 95 °C (15 min); 40 cycles at 95 °C (20 s), 57 °C (30 s), 72 °C (30 s) followed by gradient, 60–95 °C (5 s). The dissociation stage was completed to detect possible primer dimers or non-specific products. The qPCR was carried out with three biological replications for each sample and three technical replications for each biological sample. The negative control of the Master Mix in addition to the primers was performed in all qPCR running. The fluorescence data showed good specificity of PCR products [the amplification curve of each primer pairs was sigmoidal in shape and the melting curve showed only one peak that is related to the specific product (after conducting the PCR, specific identity of each amplicon was verified by gel electrophoretic analysis) and there were no primer dimer and non-specific products, Supplementary Fig. S6]. It was remarkable that ct (cycle threshold) of Actin and CPR in the five Artemisia species in this experiment ranged between 21 and 23, and this range was stable in the vegetative and flowering stages. The melting curves of amplicons (Supplementary Fig. S7) and gel electrophoretic analysis (Supplementary Fig. S8) verified specific amplifications of Actin and CPS primers pairs in the five Artemisia species. Efficiencies of all primer pairs were computed with cDNA serial dilutions using this formula: E = 10−1/slope − 1. The efficiency of all primer pairs ranged between 0.973 and 0.995. Relative expression levels were calculated using the 2−∆∆CT method51,52.
Table 2.
Primer nucleotide sequences used in qPCR.
| Genes | accession number (Gene Bank) |
Forward and Reverse Primer Sequences | Fragment size (bp) |
|---|---|---|---|
| Aa-β-actin | EU531837 | F: 5′-CCCCTGCTATGTATGTTGCCA-3′ | 202 |
| R: 5′-CGCTCGGTAAGGATCTTCATCA-3′ | |||
| Aa-CPR | EF197890 | F: 5′-CGGAACAGCCATCTTATTCTTCG-3′ | 149 |
| R: 5′-GTTGCACGTACTCCTTAGTGG-3′ | |||
| Aa-ECS | AJ001539 | F: 5′-GCAACAAGCCTACGAATCACTCAA-3′ | 126 |
| R: 5′-CGTGAAAAATTAAGGACCCTCATAGC-3 | |||
| Aa-CPS | AF472361 | F: 5′-GAGGCGACATATTTGAGAGTGC-3′ | 116 |
| R: 5′-GATAGTGTTGGGTTGGTGTGA-3′ | |||
| Aa-GAS | DQ447636 | F: 5′-CAAAGTGGTGGAAGGATATGAGGT-3′ | 202 |
| R: 5′-AGGCGAATCTCTTCAATGGTAGC-3′ | |||
| Aa-BFS | AY835398 | F: 5′-CAAGGAGGAACAAGAGAGAGG-3′ | 176 |
| R: 5′-GCATAAGTAGAGGAAATGGGACA-3′ | |||
| Aa-SQS | AY445505 | F: 5′-TGAGGTTTTCAGGGGTGTAGTC-3′ | 166 |
| R: 5′-CCTAGTGATGGTCGTTTGGGCA-3′ | |||
| Aa-ADS | HQ315833 | F: 5′-CCGAGCAAGAAAGAAAACATAG-3′ | 203 |
| R: 5′-AACTTCAAGAAACTGGCACA-3′ | |||
| Aa-ORA | JQ797708 | F: 5′-GGCGAGATTATGGCTTGGTACG-3′ | 184 |
| R: 5′-CGATGGTTGATGTGGTTCTTGTG-3′ | |||
| Aa-ERF1 | JN162091 | F: 5′-TGAACTTCCCACATAGAATCGG-3′ | 148 |
| R: 5′-TCAACTACCTCAGCCAATGATAC-3′ | |||
| Aa-WIRKY | FJ390842 | F: 5′-CAAGAACTACCAAGACCGAATCC-3′ | 210 |
| R: 5′-GGAGATAACAGGTGGCGAATAGAC-3′ | |||
| Aa-ABCG6 | Aan.67737 | F: 5′-CGATAGCCAATAGCCATAAGTG-3′ | 195 |
| R: 5′-ATCCTACATTGCTTTCCATACG-3′ | |||
| Aa-ABCG7 | Aan.68336 | F: 5′-GGTATCTGTAAATGGGGCAAAGTC-3′ | 173 |
| R: 5′-ACAATGGCATCCTCAACAACAC-3′ |
Cytogenetic studies
Chromosome analysis
Growing roots were used for cytogenetic studies. The best technique for mitosis study is the use of root tip meristem tissues for preparation of karyotype. The appropriate root length and time (when the largest number of cells are in metaphase) were chosen to cut the roots. For the cytological preparations, about 1 cm long growing roots were isolated and pretreated in 0.02% (w/v) colchicine for 3.5 h at room temperature (RT) in the dark to induce metaphase arrest, followed by washing (each for 5 min) in dsH2O three times (each for 5 min) and immersion in freshly prepared 3: 1 (v/v) absolute ethanol: glacial acetic acid for 24 h at 4 °C. The fixed roots were hydrolyzed in 5 M HC1 for 10 min at RT, then washed (each for 5 min) in dsH2O three times and stained in 2% (w/v) aceto-orcein for 3 h at RT. The five well-spread monolayer metaphase plates from different individuals were analyzed for each Artemisia species. High resolution microscopic digital photographs were taken employing an Olympus BX50 microscope (Olympus Optical Co., Tokyo, Japan) equipped with an Olympus DP12 digital camera (Olympus Optical Co., Tokyo, Japan). Six chromosomal parameters including long arm length (L), short arm length (S), chromosome length (CL), arm ratio (AR; L/S), total chromosome volume (TCV = πr2 CL) where r = mean chromosome radius and centromeric index (CI = S/CL) were estimated (Supplementary Table S1). Stebbins asymmetry categories53 were also identified (Supplementary Table S1).
Flow cytometric (FCM) analysis
FCM studies were conducted using propidium iodide (PI) staining technique and Pisum sativum cv. Ctirad (2C DNA = 9.09 pg54) as an internal reference standard plant. 1 cm2 of plant materials (leaves of Artemisia species and Pisum sativum cv. Ctirad) were chopped in a petri dish with a sharp razor blade in 1 ml of woody plant buffer (WPB55) followed by filtering of the nuclei suspension using a Partec (Partec, Münster, Germany) 30 µm nylon mesh. Then, 50 µl of PI and 50 µl of RNase were added to the nuclei suspension. To determine the amount of genomic 2C DNA, the nuclei suspension was analyzed by BD FACSCantoTM-KE flow cytometer (BD Biosciences, Bedford, MA, USA) equipped with an argon ion laser (488 nm) using BD FACSDivaTM software. Three replications were considered for genome size measurements. Histograms were gated employing Partec (Partec, Münster, Germany) FloMax ver. 2.4e. The measurements of relative fluorescence intensity of stained nuclei were performed on a linear scale. The amount of absolute DNA of a sample was computed based on the values of the G1 peak means31,54 as follows: Sample 2C DNA (pg) content = (sample G1 peak mean/standard G1 peak mean) × standard 2C DNA amount (pg). 1Cx-DNA value was calculated based on a conversion formula proposed by Doležel et al.31; where 1 pg of DNA represents 978 mega base pairs (Mbp).
Statistical analysis
The experiment was carried out using a completely randomized design (CRD) with five replications for karyological data and three replications for nuclear DNA content, artemisinin content and gene expression. After initially testing the normal distribution of the data, analyses of variances were conducted using PROC GLM of SAS56. Mean comparisons were done by Fisher’s least significant differences (LSD) at 0.01 probability level. In addition, the standard error (SE) was computed.
Electronic supplementary material
Acknowledgements
Authors gratefully acknowledge the support provided for this survey by the Tarbiat Modares University, Tehran, Iran. We greatly acknowledge Dr. Naser Safaie, head of the Plant Pathology Department, Faculty of Agriculture, Tarbiat Modares University, Tehran for his review of the manuscript and helpful comments. Also, Prof. M.R. Naghavi is grateful to Iran National Science Foundation, Deputy of Science and Technology, Presidential Office for financial support of the part of this research project.
Author Contributions
M. Salehi carried out the experiments and prepared the manuscript under the joint supervision of Assoc. Prof. G. Karimzadeh and Prof. M.R. Naghavi, and the advisory of Assoc. Prof. H. Naghdi Badi and Assist. Prof. S. Rashidi Monfared. All authors read and approved the final manuscript. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
10/15/2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-31079-0.
References
- 1.Arab HA, Rahbari S, Rassouli A, Moslemi MH, Khosravirad F. Determination of artemisinin in Artemisia sieberi and anticoccidial effects of the plant extract in broiler chickens. Trop. Anim. Health Prod. 2006;38:497–503. doi: 10.1007/s11250-006-4390-8. [DOI] [PubMed] [Google Scholar]
- 2.Hsu E. The history of qing hao in the Chinese materia medica. Trans. R. Soc. Trop. Med. Hyg. 2006;100:505–508. doi: 10.1016/j.trstmh.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Zia M, Mannan A, Chaudhary MF. Effect of growth regulators and amino acids on artemisinin production in the callus of Artemisia absinthium. Pakistan J. Bot. 2007;39:799–805. [Google Scholar]
- 4.Mannan A, et al. Survey of artemisinin production by diverse Artemisia species in northern Pakistan. Malar. J. 2010;9:310. doi: 10.1186/1475-2875-9-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranjbar M, Naghavi MR, Alizadeh H, Soltanloo H. Expression of artemisinin biosynthesis genes in eight Artemisia species at three developmental stages. Ind. Crops. Prod. 2015;76:836–843. doi: 10.1016/j.indcrop.2015.07.077. [DOI] [Google Scholar]
- 6.Salehi M, Karimzadeh G, Naghavi MR, Naghdi Badi H, Rashidi Monfared S. Expression of artemisinin biosynthesis and trichome formation genes in five Artemisia species. Ind. Crops Prod. 2018;112:130–140. doi: 10.1016/j.indcrop.2017.11.002. [DOI] [Google Scholar]
- 7.Ramawat K.G., editor. Herbal Drugs: Ethnomedicine to Modern Medicine. Berlin, Heidelberg: Springer Berlin Heidelberg; 2009. [Google Scholar]
- 8.Bora KS, Sharma A. The genus Artemisia: a comprehensive review. Pharm. Biol. 2011;49:101–109. doi: 10.3109/13880209.2010.497815. [DOI] [PubMed] [Google Scholar]
- 9.Abad MJ, Bedoya LM, Apaza L, Bermejo P. The Artemisia L. genus: a review of bioactive essential oils. Molecules. 2012;17:2542–2566. doi: 10.3390/molecules17032542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao L, Tan H, Zhang L. Artemisia annua glandular secretory trichomes: the biofactory of antimalarial agent artemisinin. Sci. Bull. 2016;61:26–36. doi: 10.1007/s11434-015-0980-z. [DOI] [Google Scholar]
- 11.Xie DY, Ma DM, Judd R, Jones AL. Artemisinin biosynthesis in Artemisia annua and metabolic engineering: questions, challenges, and perspectives. Phytochem. Rev. 2016;15:1093–1114. doi: 10.1007/s11101-016-9480-2. [DOI] [Google Scholar]
- 12.Vranová E, Coman D, Gruissem W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 2013;64:665–700. doi: 10.1146/annurev-arplant-050312-120116. [DOI] [PubMed] [Google Scholar]
- 13.Bouwmeester HJ, et al. Amorpha-4, 11-diene synthase catalyses the first probable step in artemisinin biosynthesis. Phytochem. 1999;52:843–854. doi: 10.1016/S0031-9422(99)00206-X. [DOI] [PubMed] [Google Scholar]
- 14.Mercke P, Crock J, Croteau R, Brodelius PE. Cloning, expression, and characterization of epi-cedrol synthase, a sesquiterpene cyclase from Artemisia annua L. Arch. Biochem. Biophys. 1999;369:213–222. doi: 10.1006/abbi.1999.1358. [DOI] [PubMed] [Google Scholar]
- 15.Mercke P, Bengtsson M, Bouwmeester HJ, Posthumus MA, Brodelius PE. Molecular cloning, expression, and characterization of amorpha-4, 11-diene synthase, a key enzyme of artemisinin biosynthesis in Artemisia annua L. Arch. Biochem. Biophys. 2000;381:173–180. doi: 10.1006/abbi.2000.1962. [DOI] [PubMed] [Google Scholar]
- 16.Cai Y, et al. A cDNA clone for [beta]-caryophyllene synthase from Artemisia annua. Phytochem. 2002;61:523–529. doi: 10.1016/S0031-9422(02)00265-0. [DOI] [PubMed] [Google Scholar]
- 17.Picaud S, Brodelius M, Brodelius PE. Expression, purification and characterization of recombinant (E)-[beta]-farnesene synthase from Artemisia annua. Phytochem. 2005;66:961–967. doi: 10.1016/j.phytochem.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 18.Bertea CM, et al. Isoprenoid biosynthesis in Artemisia annua: cloning and heterologous expression of a germacrene A synthase from a glandular trichome cDNA library. Arch. Biochem. Biophys. 2006;448:3–12. doi: 10.1016/j.abb.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 19.Teoh KH, Polichuk DR, Reed DW, Nowak G, Covello PS. Artemisia annua L. (Asteraceae) trichome-specific cDNAs reveal CYP71AV1, a cytochrome P450 with a key role in the biosynthesis of the antimalarial sesquiterpene lactone artemisinin. FEBS Lett. 2006;580:1411–1416. doi: 10.1016/j.febslet.2006.01.065. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, et al. The molecular cloning of artemisinic aldehyde 11 (13) reductase and its role in glandular trichome-dependent biosynthesis of artemisinin in Artemisia annua. J. Biol. Chem. 2008;283:21501. doi: 10.1074/jbc.M803090200. [DOI] [PubMed] [Google Scholar]
- 21.Teoh KH, Polichuk DR, Reed DW, Covello PS. Molecular cloning of an aldehyde dehydrogenase implicated in artemisinin biosynthesis in Artemisia annua. Botany. 2009;87:635–642. doi: 10.1139/B09-032. [DOI] [Google Scholar]
- 22.Rydén AM, et al. The molecular cloning of dihydroartemisinic aldehyde reductase and its implication in artemisinin biosynthesis in Artemisia annua. Planta Med. 2010;76:1778–1783. doi: 10.1055/s-0030-1249930. [DOI] [PubMed] [Google Scholar]
- 23.Brown GD. The biosynthesis of artemisinin (Qinghaosu) and the phytochemistry of Artemisia annua L. (Qinghao) Molecules. 2010;15:7603–7698. doi: 10.3390/molecules15117603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olofsson L, Engström A, Lundgren A, Brodelius PE. Relative expression of genes of terpene metabolism in different tissues of Artemisia annua L. BMC Plant Biol. 2011;11:45. doi: 10.1186/1471-2229-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lv Z, et al. Branch pathway blocking in Artemisia annua is a useful method for obtaining high yield artemisinin. Plant Cell Physiol. 2016;57:588–602. doi: 10.1093/pcp/pcw014. [DOI] [PubMed] [Google Scholar]
- 26.Yang CQ, et al. Transcriptional regulation of plant secondary metabolism. J. Integr. Plant. Biol. 2012;54:703–712. doi: 10.1111/j.1744-7909.2012.01161.x. [DOI] [PubMed] [Google Scholar]
- 27.Verpoorte R, Memelink J. Engineering secondary metabolite production in plants. Curr. Opin. Biotechnol. 2002;13:181–87. doi: 10.1016/S0958-1669(02)00308-7. [DOI] [PubMed] [Google Scholar]
- 28.Benedetti A, Comporti M. Formation, reactions and toxicity of aldehydes produced in the course of lipid-peroxidation in cellular membranes. Bioelectrochem. Bioenerg. 1987;18:187–202. doi: 10.1016/0302-4598(87)85021-3. [DOI] [Google Scholar]
- 29.Zhang L, et al. Identification of putative Artemisia annua ABCG transporter unigenes related to artemisinin yield following expression analysis in different plant tissues and in response to methyl jasmonate and abscisic acid treatments. Plant Mol. Biol. Report. 2012;30:838–847. doi: 10.1007/s11105-011-0400-8. [DOI] [Google Scholar]
- 30.Greilhuber J, Doležel J, Lysák MA, Bennett MD. The origin, evolution and proposed stabilization of the terms “genome size” and “C-value” to describe nuclear DNA contents. Ann. Bot. 2005;95:255–260. doi: 10.1093/aob/mci019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doležel J, Bartoš J, Voglmayr H, Greilhuber J. Nuclear DNA content and genome size of trout and human. Cytometry. 2003;51:127–128. doi: 10.1002/cyto.a.10013. [DOI] [PubMed] [Google Scholar]
- 32.Bennett MD, Leitch IJ. Nuclear DNA amounts in angiosperms: Targets, trends and tomorrow. Ann. Bot. 2011;107:467–590. doi: 10.1093/aob/mcq258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang K, Rashidi Monfared S, Wang H, Lundgren A, Brodelius PE. The activity of the artemisinic aldehyde Δ11 (13) reductase promoter is important for artemisinin yield in different chemotypes of Artemisia annua. Plant Mol. Biol. 2015;88:325–340. doi: 10.1007/s11103-015-0284-3. [DOI] [PubMed] [Google Scholar]
- 34.Duke MV, Paul RN, Elsohly HN, Sturtz G, Duke SO. Localization of artemisinin and artemisitene in foliar tissues of glanded and glandless biotypes of Artemisia annua L. Int. J. Plant Sci. 1994;155:365–372. doi: 10.1086/297173. [DOI] [Google Scholar]
- 35.Wang H, Han J, Kanagarajan S, Lundgren A, Brodelius PE. Studies on the expression of sesquiterpene synthases using promoter β-glucuronidase fusions in transgenic Artemisia annua L. Plos One. 2013;8:e80643. doi: 10.1371/journal.pone.0080643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Francis F, Vandermoten S, Verheggen F, Lognay G, Haubruge. E. Is the (E)-b-farnesene only volatile terpenoid in aphids? J. Appl. Entomol. 2005;129:6–11. doi: 10.1111/j.1439-0418.2005.00925.x. [DOI] [Google Scholar]
- 37.Köllner TG, et al. A maize (E)-β-caryophyllene synthase implicated in indirect defense responses against herbivores is not expressed in most American maize varieties. Plant Cell. 2008;20:482–494. doi: 10.1105/tpc.107.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schilmiller AL, Last RL, Pichersky E. Harnessing plant trichome biochemistry for the production of useful compounds. Plant J. 2008;54:702–711. doi: 10.1111/j.1365-313X.2008.03432.x. [DOI] [PubMed] [Google Scholar]
- 39.Gang DR, et al. An investigation of the storage and biosynthesis of phenylpropenes in sweet basil. Plant Physiol. 2001;125:539–555. doi: 10.1104/pp.125.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L, et al. Development of transgenic Artemisia annua (Chinese wormwood) plants with an enhanced content of artemisinin, an effective anti-malarial drug, by hairpin-RNA-mediated gene silencing. Biotechnol. Appl. Biochem. 2009;52:199–207. doi: 10.1042/BA20080068. [DOI] [PubMed] [Google Scholar]
- 41.Ma D, et al. Isolation and characterization of AaWRKY1, an Artemisia annua transcription factor that regulates the amorpha-4, 11-diene synthase gene, a key gene of artemisinin biosynthesis. Plant Cell Physiol. 2009;50:2146–2161. doi: 10.1093/pcp/pcp149. [DOI] [PubMed] [Google Scholar]
- 42.Han J, Wang H, Lundgren A, Brodelius PE. Effects of overexpression of AaWRKY1 on artemisinin biosynthesis in transgenic Artemisia annua plants. Phytochem. 2014;102:89–96. doi: 10.1016/j.phytochem.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 43.Lu X, et al. AaORA, a trichome specific AP2/ERF transcription factor of Artemisia annua, is a positive regulator in the artemisinin biosynthetic pathway and in disease resistance to Botrytis cinerea. New Phytol. 2013;198:1191–1202. doi: 10.1111/nph.12207. [DOI] [PubMed] [Google Scholar]
- 44.Yu ZX, et al. The jasmonate-responsive AP2/ERF transcription factors AaERF1 and AaERF2 positively regulate artemisinin biosynthesis in Artemisia annua L. Mol. Plant. 2012;5:353–365. doi: 10.1093/mp/ssr087. [DOI] [PubMed] [Google Scholar]
- 45.Rees H, Narayan RKJ. Chromosomal DNA in higher plants. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 1981;292:569–578. doi: 10.1098/rstb.1981.0051. [DOI] [Google Scholar]
- 46.Cavalier-Smith T. Economy, speed and size matter: evolutionary forces driving nuclear genome miniaturization and expansion. Ann. Bot. 2005;95:147–175. doi: 10.1093/aob/mci010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marguerat S, Bähler J. Coordinating genome expression with cell size. Trends Genet. 2012;28:560–565. doi: 10.1016/j.tig.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Kobayashi T. Regulation of ribosomal RNA gene copy number and its role in modulating genome integrity and evolutionary adaptability in yeast. Cell Mol. Life Sci. 2011;68:1395–1403. doi: 10.1007/s00018-010-0613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng CA, Ferreira JFS, Wood AJ. Direct analysis of artemisinin from Artemisia annua L. using high-performance liquid chromatography with evaporative light scattering detector, and gas chromatography with flame ionization detector. J. Chromatogr. A. 2006;1133:254–258. doi: 10.1016/j.chroma.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 50.Lapkin AA, et al. Development of HPLC analytical protocols for quantification of artemisinin in biomass and extracts. J. Pharm. Biomed. Anal. 2009;4:908–915. doi: 10.1016/j.jpba.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 51.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 52.Sehringer B, et al. Evaluation of different strategies for real-time RT-PCR expression analysis of corticotropin-releasing hormone and related proteins in human gestational tissues. Anal Bioanal. Chem. 2005;383:768–775. doi: 10.1007/s00216-005-0067-9. [DOI] [PubMed] [Google Scholar]
- 53.Stebbins, G. L. Chromosomal evolution in higher plants. Edward Arnold, London, UK (1971).
- 54.Doležel J, Greilhuber J, Suda J. Estimation of nuclear DNA content in plants using flow cytometry. Nature Prot. 2007;2:2233–2244. doi: 10.1038/nprot.2007.310. [DOI] [PubMed] [Google Scholar]
- 55.Loureiro J, Rodriguez E, Doležel J, Santos C. Two new nuclear isolation buffers for plant DNA flow cytometry: a test with 37 species. Ann. Bot. 2007;100:875–888. doi: 10.1093/aob/mcm152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.SAS Institute. SAS/STAT User’s Guide. SAS Institute, Inc, Cary (2002).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.