Abstract
Background
Perinatal morbidity and mortality are significant public health issues with an enduring impact on the health and well-being of women and their families. Millions of pregnant women now download and use mobile applications to access, store, and share health information. However, little is known about the consequences. An investigation of their impact on perinatal health outcomes is particularly topical.
Objective
To determine the effects of mobile app interventions during pregnancy on influencing healthy maternal behavior and improving perinatal health outcomes.
Methods
Searches of PubMed, Embase, the Cochrane Library, CINAHL, WHO Global Health Library, POPLINE, and CABI Global Health were conducted with no date or language restrictions. Randomized and non-randomized studies were included if they reported perinatal health outcomes of interventions targeting pregnant women, using mobile apps compared with other communication modalities or with standard care. The primary outcome measure was the change in maternal behaviors (as defined by trial authors), by intervention goals. Two reviewers independently extracted data using standardized forms.
Results
Four randomized controlled trials (RCTs) involving 456 participants were included. All studies targeted participants in early pregnancy; however, wide variation was evident in participant characteristics, intervention, and study outcomes measures. Three trials were based in hospital settings, comparing women using mobile apps with routine antenatal care. One community-based trial gave all participants a device to promote physical activity; the intervention arm was also given a mobile app. All studies reported data for the primary outcome measure, describing some benefit from the intervention compared with controls. However, few statistically significant primary or secondary outcomes were reported. Due to insufficient data, the planned meta-analysis and subgroup analyses were not performed.
Conclusions
Due to limited numbers, heterogeneity of interventions, comparators, and outcome measures, no firm conclusions can be drawn on the effects of mobile application interventions during pregnancy on maternal knowledge, behavior change, and perinatal health outcomes. As millions of women utilize mobile apps during pregnancy, rigorous studies are essential for health care and maternity care providers to optimally design, implement, and evaluate interventions.
Keywords: apps, pregnancy, perinatal, maternal, infant, mobile, systematic review, behavior change, intervention
Introduction
Adoption, practice, and maintenance of healthy behaviors during pregnancy can potentially improve maternal and child health. Adverse perinatal health outcomes such as emergency cesarean section, preterm birth, low birthweight, and stillbirth [1] are associated with maternal risk factors that may be modifiable through changes in maternal behavior [2-4]. During pregnancy and in preparation for motherhood, many women seek information and try to adopt a healthy lifestyle [5]. Pregnant women are increasingly using digital resources such as mobile apps—computer programs designed to run on mobile devices such as mobile phones and tablet computers—to access information, monitor fetal development, track individual health indicators, and provide reassurance [6-10]. Collectively, pregnancy apps have been downloaded hundreds of millions of times and are an integral source of information for many pregnant women [11]. Pregnant women may feel heightened support for informed decision-making and a sense of control by using a familiar device to access, store, and share information [9].
In 2017, over 325,000 health and fitness and medical apps were available [12]; apps directed at pregnancy constitute a major genre [13]. These apps can include health information, motivational messages, monitoring, and behavior change tools. The content of apps can be tailored by demographics such as gestational age, maternal age, language or risk factors. App developers may employ methods to engage users, such as “push communication,” including notifications designed to encourage users to follow a prompt (eg, read, listen, view content or perform an activity). Pregnancy apps may also link to a device such as a camera, glucometer, fitness activity tracker, Kegel “exerciser,” fetal heartbeat “listener,” or other monitoring equipment. Some devices associated with an app are marketed directly to consumers and avoid regulatory scrutiny, while a woman’s health care provider may provide other devices as part of clinical care.
From an institutional perspective, health systems and maternity care facilities are investigating whether and how to integrate digital patient support modalities into care and are seeking evidence to support decision making. It has been hypothesized that mobile apps may improve perinatal outcomes by encouraging access to health information, modifying demand for services, and enabling provision of targeted care [14]. This systematic review aims to determine the effects of mobile app interventions during pregnancy on influencing healthy maternal behavior and improving perinatal health outcomes, compared to interventions using other communication modalities or standard care.
Methods
Criteria for Considering Studies for this Review
Study Design
All randomized controlled trials (RCTs) and non-randomized studies including controlled before-after studies, interrupted time-series studies, and prospective comparative cohort studies were considered for inclusion. Case-control studies and cross-sectional studies were excluded due to their retrospective design. Crossover trials were also excluded as they are considered most suitable for temporary effects and chronic conditions [15]. Women at any stage of pregnancy or labor were considered for participation.
Interventions
Studies assessing the effects of mobile app-based interventions designed to influence maternal knowledge or behavior during pregnancy were considered for inclusion if they provided general information for pregnant women or focused on a specific maternal risk factor or perinatal outcome. There was no restriction on who sponsored the intervention or type of setting.
Studies were excluded if the intervention (1) did not utilize a mobile app, (2) used a mobile phone solely for telephone conversations or SMS (short message service) text messaging, (3) did not report on maternal or infant health outcomes, (4) did not target pregnant women (focus on clinicians, partners), and (5) focused on physical effects of mobile phone usage (such as radiation) during pregnancy.
Comparators
The following comparisons were planned:
Mobile health apps versus paper-based or SMS text messaging-based communications.
Mobile health apps versus interpersonal communication modes (ie, face-to-face or telephone conversation).
Mobile health apps versus standard care or no specified intervention.
Outcome Measures
The primary outcome measure was a change in maternal behaviors (as defined by trial authors), by intervention goals. Secondary outcomes addressed maternal and infant health outcomes, maternal experience with the intervention, and health service utilization measures (Table 1).
Table 1.
Primary and secondary outcome measures.
| Outcome | Outcome characteristics | |
| Primary outcomes |
|
|
|
|
Maternal |
|
| Secondary outcomes |
|
|
|
|
Maternal |
|
|
|
Infant |
|
Search Methods for Identification of Studies
Sources
Systematic searches were performed using seven electronic bibliographic databases: PubMed, Embase, The Cochrane Library, CINAHL, World Health Organization Global Health Library, POPLINE, and CABI Global Health. Handsearching of journals and conference proceedings from the reference lists of retrieved studies were also conducted. No language or date restrictions were applied. Abstracts and full-length articles were obtained for each citation, where available.
Search Strategy
The specific search strategies were developed by the primary author (LMD) and an experienced clinical research librarian, with input from all authors. Electronic searches using subject headings and all fields for keywords were conducted to avoid missing non-indexed concepts. Search terms and returns by the database and handsearching are presented in Multimedia Appendix 1. Articles published in non-peer reviewed publications were excluded, as per the review protocol. Remaining citations and abstracts were uploaded to the Web-based software platform Covidence [16]. Throughout the review process, authors were not blinded to journal titles, study authors or institutions. If it was unclear whether studies met inclusion criteria, study authors were contacted up to two times by email to request further information.
Study Selection
Abstracts of articles retrieved through the search strategy were independently screened by 2 review authors to determine if inclusion and exclusion criteria were met. A third author addressed any concerns about inclusion. If necessary, additional information was sought from study authors to resolve eligibility queries. For selected studies, full-text articles were obtained and read by 2 authors to confirm that they met inclusion criteria.
Bias Assessment
Studies were assessed for quality by 2 reviewers independently (LMD, VF), according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions [17], by 6 domains: (1) selection bias, (2) performance bias, (3) detection bias, (4) attrition bias, (5) reporting bias, and (6) any other possible bias. All included studies were assessed on the risk of bias, likely magnitude, and potential impact on findings.
Data Collection and Analysis
Data Extraction and Management
Once the studies were selected, using standardized forms, 2 reviewers (LMD, VF) independently extracted data including study objective, study design, inclusion and exclusion criteria, data sources, study period, methodology, sample size, intervention details and effects, and outcomes. Due to the complexity of the interventions found, a post-hoc decision was taken to collect data on the interventions based on the Template for Intervention Description and Replication (TIDieR) checklist [18,19].
Synthesis of Results
As described in the review protocol [20], we set out to synthesize data and present measures of treatment effects including summary risk ratios for dichotomous outcomes and mean difference for continuous data and subgroup analysis. Due to the considerable heterogeneity of participant characteristics, intervention features, and reported outcomes, it was decided that meta-analysis could not be performed, and results were summarized in a narrative synthesis.
Results
Description of Studies
Included Studies
The search strategy for this review has been consolidated into a Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) diagram (Figure 1), summarizing inclusion and exclusion of studies [21]. For database screening, one author (LMD) searched the databases on 15-16 February 2017, with a yield of 5,089 articles. After initial screening to remove duplicates and articles from non-peer reviewed journals, the titles and abstracts of 2,241 articles were reviewed by 2 authors independently (among LMD, VF, DH, PFM, and FMB). Title and abstract screening of 1,091 additional articles identified through handsearching was performed by 2 reviewers (LMD and VF); however, no additional studies were identified. Full-text screening of 69 articles was performed by 2 review authors (among LMD, VF, PFM, and DH). At all stages, disagreements between authors were resolved by consultation with a third reviewer. Reasons were recorded for excluding studies (Multimedia Appendix 2). Several articles had multiple reasons for exclusion, although each was allocated to a single category. A total of 4 trials met the inclusion criteria.
Figure 1.
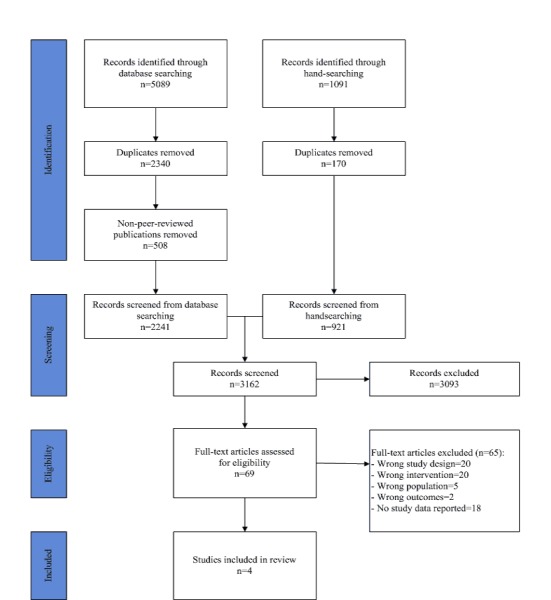
Preferred Reporting Items for Systematic Reviews and Meta-Analysis diagram of included and excluded studies.
The characteristics of included studies are presented in Table 2. Though not specified as a requirement for inclusion, all studies that met inclusion criteria used RCT designs and involved pregnant women in high-income countries. Three trials were based in hospital settings, comparing women using mobile apps with standard antenatal care. One community-based trial gave all participants a device to promote physical activity; participants in the intervention arm were also given a mobile app.
Table 2.
The characteristics of included studies.
| Study characteristics | Ainscough et al [22] | Choi et al [23] | Ledford et al [24] | Zairina et al [25] |
| Country | Ireland | United States | United States | Australia |
| Year | 2016 | 2016 | 2016 | 2016 |
| Design | Randomized controlled trial | Randomized controlled trial | Randomized controlled trial | Randomized controlled trial |
| Aim | To investigate the influence of a mobile phone app-supported antenatal healthy lifestyle intervention on the behavioral stage of change among overweight and obese pregnant women | To examine the feasibility of subject recruitment, randomization, intervention, and efficacy of an mHealth physical activity program for physically inactive pregnant women | To compare the effectiveness of a mobile application with a spiral-notebook guide in prenatal care | To evaluate the efficacy of a telehealth program supported by a handheld respiratory device in improving asthma control during pregnancy |
| Participants (risk category) | 204 pregnant women with body mass index≥25 and <40 kg/m2 (Moderate) | 30 pregnant women with a sedentary lifestyle and intent to be physically active (Low) | 150 low-risk obstetrics participants following standard care pathway (Low) | 72 pregnant women with asthma (Moderate) |
| Control group (n) | Standard care: no consistent diet or lifestyle advice offered (98) | Recommendations for gestational weight gain and safety instruction for promoting physical activity during pregnancy, and an accelerometer (15) | Standard care: a spiral notebook designed to educate participants about pregnancy and enable recording of pregnancy experiences (78) | Standard care through a participant information brochure (36) |
| Intervention group (n) | A “healthy lifestyle package” of individualized nutrition counseling and exercise advice, supported by a mobile phone app (106) | The same information and an accelerometer as women in the control group plus a mobile phone application, a daily message, activity diary, and feedback graphs of personal data (15) | The standard care spiral notebook replaced with a mobile app with identical content. (72) | In addition to standard care, participants were given a mobile app to record data, a proprietary medical device intended for measuring lung function (COPD-6) and a written asthma action plan (36) |
Bias Assessment of Included Studies
Objective assessments and validated data collection tools were employed in all included studies. Studies performed generally well regarding the risk of bias in random sequence generation; 3 studies were low risk and 1 study was unclear, as it was not described. High risk of performance bias was shared across all studies. Due to the nature of mobile app interventions, blinding participants is difficult, and did not occur in any of the included studies. Blinding health care providers can also be difficult and occurred in only 1 study. Most studies had a low risk of attrition bias, with low rates of withdrawal, drop-out or loss to follow-up. Reporting biases were also low, with all studies reporting results for their respective primary outcomes. The overall risk assessment is presented in Multimedia Appendix 3.
Description of Participants
Overall, 456 pregnant women participated in the 4 included trials, with 180 women categorized as low risk in 2 trials and 276 as moderate risk in 2 trials. Of these, 1 trial included pregnant women with asthma and another recruited pregnant women classified as overweight or obese. All trials recruited women prior to 20 weeks gestation. Table 3 shows the participant characteristics reported by each study.
Table 3.
Participant characteristics.
| Study characteristics | Ainscough et al [22] | Choi et al [23] | Ledford et al [24] | Zairina et al [25] | |
| Participant risk category | Moderate | Low | Low | Moderate | |
| Total number of participants, N | 204 | 30 | 150 | 72 | |
|
|
Control, n | 98 | 15 | 78 | 36 |
|
|
Intervention, n | 106 | 15 | 72 | 36 |
| Inclusion characteristic among pregnant women | Body mass index≥25 and <40 kg/m2 | Desire to increase physical activity | Low-risk | Asthma | |
| Group differences | No | No | No | No | |
| Age (years), mean (SD) | —a | 33.7 (2.6) | 28.91 (5.03) | 31.45 (4.5) | |
| Gestation age at recruitment (weeks); mean (SD) | 15b | 17.2 (3.4) | 10-12b | 16.35 (2.9) | |
| Marriedc, n (%) | — | 29 (97) | 133 (92.4) | 56 (78) | |
| Body mass index (kg/m2)d, mean (SD) | — | 27.7 (3.7) | — | 28.5 (5.7) | |
| Race/ethnicitye n (%) |
|
|
|
|
|
|
|
White | — | 13 (43) | 100 (69.4) | 60 (83) |
|
|
Asian | — | 12 (40) | 8 (5.6) | 6 (8) |
|
|
Black/African-American | — | 2 (7) | 14 (9.7) | — |
|
|
Hispanic/Latina | — | 3 (10) | 15 (10.4) | — |
|
|
Other | — | — | 7 (4.7) | 6 (8) |
| Education, n (%) |
|
|
|
|
|
|
|
High school graduate | — | 6 (20) | 51 (34.0) | 9 (13) |
|
|
University graduate | — | 24 (80) | 92 (61.3) | 45 (63) |
| Ability to communicate in English | — | Yes | — | Yes | |
aDashes indicate unreported values.
bStandard deviation was not reported.
cStudies reporting data used the term “married,” except the Choi et al study, with response category “married/cohabitating.”
dChoi et al reported prepregnancy body mass index. Zairina et al reported study baseline.
eResponse categories as described by study authors.
Description of Interventions
All interventions used mobile apps specifically designed for the study, rather than apps available through commercial “app stores.” None of the included studies reported if modification of the intervention occurred during the trial or described a cost-benefit analysis or compared cost of the app to other communication modalities. To motivate users to engage with the content, 2 studies developed apps utilizing “push” communication strategies. These same interventions also included a device available through a “plug-in” [23,25]. Three studies allowed users to record and track personal data within the app and provided individualized information [22,23,25]. None of the studies reported that their apps included shared participant “chat” spaces. Intervention features are described using the TIDieR checklist [18,19] presented in Table 4. Additional information about intervention characteristics—including user experience, content, patient-provider communication, functionality, and data tracking—was also collected by one reviewer (LMD) using a customized form (Multimedia Appendix 4).
Table 4.
Intervention features by the Template for Intervention Description and Replication (TIDieR) checklist.
| Study characteristics | Ainscough et al [22] | Choi et al [23] | Ledford et al [24] | Zairina et al [25] |
| Brief name (trial registration) | Pears (Pregnancy, exercise, and nutrition research study with mobile phone app support) study (ICTRN29316280) | MOTHER (Mobile Technologies to Help Enhancing Regular Physical Activity) Trial for Pregnant Women (NCT 01461707) | Mobile app as a prenatal education and engagement tool (No registration provided) | MASTERY (Management of Asthma with Supportive Telehealth of Respiratory function in Pregnancy Telehealth to improve asthma control in pregnancy) study (ACTRN 12613000800729) |
| Why | Investigate the influence of mobile app-supported antenatal healthy lifestyle intervention on the behavioral stage of change | Examine the feasibility of an mHealth physical activity program | Compare the effectiveness of a mobile app with a spiral-notebook guide in prenatal care | Evaluate the efficacy of a telehealth program supported by a handheld respiratory device in improving asthma control during pregnancy |
| What (materials) | Mobile app | Mobile app, accelerometer | Mobile app | Mobile app, a proprietary medical device intended for measuring lung function (COPD-6), individualized written asthma action plan (WAAP) |
| What (procedures) | Participants received individualized nutrition counseling and exercise advice, and mobile app. Behavioral stage-of-change score measured at baseline and late pregnancy | Participants provided with a mobile app, accelerometer, and goal-setting session at baseline. Data remotely monitored and extracted | Participants provided with a mobile app. Paper-based surveys completed at each prenatal appointment. App use assessed. Outcomes reported from health record at delivery | Participants received a COPD-6, mobile app, and individualized WAAP. Data transmitted automatically to a server accessed by researchers, participants, and clinicians. Data collected at 3 and 6 months from baseline, and after delivery |
| Who provided | Not described | Research staff | Antenatal care providers, researchers | Antenatal care providers, researchers |
| How | Mobile app | Mobile app | Mobile app | Mobile app |
| Where | Not described. Study authors based in Dublin, Ireland | Not described. Participants recruited by obstetricians, prenatal clinics, and communities (San Francisco, United States) | Community hospital in women’s health and family medicine departments (Maryland, United States) | Antenatal clinics of two large maternity hospitals (Melbourne, Australia) |
| When and how much | From (mean of) 15 weeks’ gestation to 28 weeks’ gestation | From 10-20 weeks’ gestation, continuing for 12 weeks | From 8-10 weeks’ gestation, continuing throughout pregnancy | From (mean of) 16.7 weeks’ gestation, continuing throughout pregnancy |
| Tailoring | Individualized nutrition and exercise advice | Individualized prescheduled weekly step goals | Not described | Individualized WAAP and weekly feedback messages |
| Modification of intervention during trial | Not described | Not described | Not described | Not described |
| Strategies to improve or maintain intervention fidelity | Not described | Feedback offered on user progress, based on uploaded activity diaries | Not described | Feedback based on individualized WAAP, lung function and asthma symptoms |
| Extent of intervention fidelity | Retention and adherence rates not reported | 97% (29/30) participants retained during the intervention. Adherence by intervention group waned over the study period | 73% (127/173) participants retained during the intervention. Change mainly due to miscarriage and elevation to high-risk care | 96% (69/72) participants retained during the intervention |
Effects of Interventions
The included trials reported different maternal and infant health outcomes such that meta-analysis or subgroup analysis was not possible.
Primary Outcomes
The primary outcome of interest was a change in maternal behaviors (as defined by trial authors), by intervention goals. All studies reported some type of behavior change and better results for the intervention group than controls for their respective primary outcomes (Table 5). Inventories and data collection tools used in the included studies are listed in Multimedia Appendix 5. The Ainscough et al study [22] concluded that a significantly higher proportion of the intervention group had transitioned to a “maintenance stage” of healthy lifestyle behaviors by 28 weeks’ gestation, compared to the control group (52.8% versus 32.7%, P=.004). The primary outcome measure for the Choi et al study [23] of physical activity was weekly mean steps, and intervention participants had a greater increase in daily steps at 12 weeks with 1096 (SD 1898) steps, compared with 259 (SD 1604) steps among control participants (P=.13). The change between groups reported across the 12-week study period was not statistically significant (P=.38). The Ledford et al study [24] found that by 32 weeks’ gestation, participants using a mobile app recorded information more frequently than the control group, and that they had developed greater “patient activation” than the control group (F [1127]=4.99, P ≥.05, n2=.04, marginal mean of 79.88 versus 74.81). The Zairina et al study [25] reported that the intervention group had a higher proportion of participants with well-controlled asthma than the control group (82% versus 58%, P=.03) at 6 months from baseline.
Table 5.
Primary maternal outcomes: change in maternal behaviors by intervention goals.
| Study | Study results | |
|
|
Primary maternal outcome | Results |
| Ainscough et al [22] |
|
|
| Choi et al [23] |
|
|
| Ledford et al [24] |
|
|
| Zairina et al [25] |
|
|
Secondary Outcomes
Of the 4 studies in this review, 2 studies [23,25] report maternal secondary outcomes relevant to this review, as further detailed in Multimedia Appendix 6. One study of asthma control reports a clinically significant improvement in the asthma-related quality of life among the intervention group compared with usual care at 6 months from baseline, but the mean change was not statistically significant [25]. Another study of physical activity during pregnancy reported reduced barriers such as lack of energy, time, and willpower among the intervention group, and decreased severity of pregnancy symptoms [23]. No studies reported data on major adverse maternal outcome, maternal knowledge about the targeted health topic, maternal evaluation of the intervention, or successful initiation of breastfeeding. Furthermore, none of the trials report statistically significant differences in neonatal outcomes between intervention and control groups.
Discussion
Principal Findings
Despite the broad search criteria used, this systematic review identified only 4 studies for inclusion. This was an unexpected outcome of the review given the expanding use of mobile applications in maternity care. All studies included in the review reported on the primary outcome, “change in maternal behaviors by intervention goals,” but the specific outcomes reported varied by intervention. None of the studies included in this review reported statistically significant differences between intervention and control groups for neonatal outcomes, delivery or pregnancy complications. As advocated through the Core Outcomes in Women’s and Newborn Health initiative, a standard set of perinatal outcome measures, reported alongside those appropriate to specific health conditions or interventions, would enhance comparability [26-27]. A standardized approach using reliable and valid methods to analyze participant usage, navigation, adherence, and satisfaction would also improve comparability further and inform the design of future interventions.
Further areas for research include investigation of which intervention features yield the desired results, for example, to establish if it is an individualised clinical care plan or the advice supported by a mobile app that makes a difference. Future studies could also explore how technology can support individualized patient care plans, and if technology can be used for data tracking or streamlined reporting of symptoms to automatically prompt closer clinical scrutiny. A more in-depth exploration of the theories of behavior change underpinning study results could also add an important dimension to understand how mobile interventions influence behavior and improved perinatal outcomes.
None of the studies were designed to gauge the longitudinal benefit of mobile app interventions commenced during the perinatal period. This would be another important avenue to understand longer-term benefits, potential diminishing effects, data tracking and patient engagement opportunities offered by interventions commenced during pregnancy. Qualitative research or follow-up surveys of interventions could provide insight into users’ experiences of these interventions, including how such apps are used, and if they augment or affect perceptions of care.
Hundreds of pregnancy apps are available to the public, yet all the studies in this review developed their own mobile apps. Researchers may find it easier to guide content, facilitate communication, track data and assure user privacy with their own apps. The potential commercialization of successful interventions that could generate income for research programs could also be a consideration. However, bespoke models are likely to require more investment in development, testing, maintenance, and marketing than existing apps.
Despite creating their own apps, reviewed studies did not report extensively on their development, testing or architecture, or whether modifications were required, which would assist replication efforts. Further, none of the included studies reported an economic analysis, comparing the cost of the intervention with standard care or comparators. Policymakers and those guiding educational or clinical interventions during pregnancy would likely require such information to gauge investment alongside projected perinatal health benefits.
Strengths and Limitations
There are several important strengths and limitations in our review. To the best of our knowledge, this systematic review is the first to assess the effects of mobile app interventions during pregnancy on influencing healthy maternal behavior and improving perinatal outcomes. This review followed an established methodology for the conduct of systematic reviews [17,19] and used a highly sensitive search strategy with no language or date restrictions to identify as many relevant studies as possible. Two authors completed the review process and extracted data independently at all stages based on prespecified criteria, and a third author participated when required to achieve consensus. Included studies were limited to those which provided comparators between mobile applications and any other intervention, including standard care, so that the role of the communication modality on intervention effects could be analyzed.
This review may have some methodological limitations. Findings are limited by the few studies that met inclusion criteria, and the small sample sizes involved in each study. Although search criteria and the databases searched were comprehensive, it is possible that relevant articles were missed. Only articles published in peer-reviewed journals were included, which may have left out some studies. Over 3,000 published study abstracts were assessed, and it was unexpected that only 4 studies would meet inclusion criteria. The heterogeneity of outcome measures further hampered the ability to meta-analyse data as originally intended, or to explore impact. Future updates of this review could search additional databases and expand the inclusion criteria to enable the analyses originally intended by the authors.
Conclusions
As an increasing number of pregnant women use mobile apps, further research on intervention components, usage, and associated perinatal health outcomes should influence content, features, and quality of interventions. The effect of mobile app interventions on maternal knowledge, behaviour, and perinatal outcomes is still largely underreported, as evidenced by the few studies that met inclusion criteria for this review, and the minimal significant impact reported on perinatal health outcomes. Results of this systematic review may contribute to decision making by health systems, hospitals, and clinicians about the integration of mobile applications into clinical care. Emerging evidence from future trials should help to make firmer conclusions about the effectiveness of mobile app interventions during pregnancy on primary and secondary outcomes, compared to other communication modes.
Acknowledgments
The research reported in this publication is part of the Centre of Research Excellence in Stillbirth (Stillbirth CRE). Core funding to support the Stillbirth CRE is provided by the National Health and Medical Research Council (NHMRC). This systematic review is part of the corresponding author’s doctoral thesis, which is funded through an Australian Government Research Training Program (RTP) scholarship facilitated through The University of Queensland, a Frank Clair Scholarship from Mater Research Institute, and a Top-up Scholarship from the Stillbirth CRE. The following institutions have provided support to review authors to allow them to undertake this review: (1) Mater Research Institute, The University of Queensland, Australia, (2) South Australian Health and Medical Research Institute (SAHMRI), Adelaide, Australia, and (3) School of Psychology and Public Health, La Trobe University, Australia. Supporting institutions are not involved in aspects of the project such as the design of the protocol, and analysis plan, data collection and analyses, data interpretation or publication of study results.
Abbreviations
- COPD-6
a proprietary medical device intended for measuring lung function
- RCT
randomized controlled trial
- SMS
short message service
- TIDieR
Template for Intervention Description and Replication
- WAAP
written asthma action plan
Search terms and returns.
Excluded studies.
Bias assessment.
Intervention characteristics.
Inventories used in included studies.
Secondary outcomes.
Footnotes
Conflicts of Interest: None declared
Authors' Contributions: LMD and VF conceptualized the review in consultation with the coreviewers. LMD performed the literature search with a clinical librarian. All authors (LMD, FMB, DH, PFM, VF) screened studies to determine inclusion. LMD and VF performed data collection, data extraction, and bias assessment. LMD wrote the manuscript, with review and input from all authors. The corresponding author is the guarantor of this review.
References
- 1.Lawn JE, Blencowe H, Waiswa P, Amouzou A, Mathers C, Hogan D, et al with the Lancet Ending Preventable Stillbirths Series study group and the Lancet Stillbirth Epidemiology investigator group Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet. 2016 Feb 06;387(10018):587–603. doi: 10.1016/S0140-6736(15)00837-5.S0140-6736(15)00837-5 [DOI] [PubMed] [Google Scholar]
- 2.Aune D, Saugstad OD, Henriksen T, Tonstad S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA. 2014 Apr 16;311(15):1536–46. doi: 10.1001/jama.2014.2269.1860462 [DOI] [PubMed] [Google Scholar]
- 3.Marufu TC, Ahankari A, Coleman T, Lewis S. Maternal smoking and the risk of still birth: systematic review and meta-analysis. BMC Public Health. 2015 Mar 13;15:239. doi: 10.1186/s12889-015-1552-5. https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-015-1552-5 .10.1186/s12889-015-1552-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flenady V, Middleton P, Smith GC, Duke W, Erwich JJ, Khong TY, et al for the Lancet's Stillbirths Series steering committee Stillbirths: the way forward in high-income countries. Lancet. 2011 May 14;377(9778):1703–17. doi: 10.1016/S0140-6736(11)60064-0.S0140-6736(11)60064-0 [DOI] [PubMed] [Google Scholar]
- 5.Deave T, Johnson D, Ingram J. Transition to parenthood: the needs of parents in pregnancy and early parenthood. BMC Pregnancy Childbirth. 2008 Jul 29;8:30. doi: 10.1186/1471-2393-8-30. https://bmcpregnancychildbirth.biomedcentral.com/articles/10.1186/1471-2393-8-30 .1471-2393-8-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lupton D, Pedersen S. An Australian survey of women's use of pregnancy and parenting apps. Women Birth. 2016 Aug;29(4):368–75. doi: 10.1016/j.wombi.2016.01.008.S1871-5192(16)00032-9 [DOI] [PubMed] [Google Scholar]
- 7.O'Higgins A, Murphy OC, Egan A, Mullaney L, Sheehan S, Turner MJ. The use of digital media by women using the maternity services in a developed country. Ir Med J. 2014;107(10):313–5. [PubMed] [Google Scholar]
- 8.Lupton D. The use and value of digital media for information about pregnancy and early motherhood: a focus group study. BMC Pregnancy Childbirth. 2016 Jul 19;16(1):171. doi: 10.1186/s12884-016-0971-3. https://bmcpregnancychildbirth.biomedcentral.com/articles/10.1186/s12884-016-0971-3 .10.1186/s12884-016-0971-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hearn L, Miller M, Fletcher A. Online healthy lifestyle support in the perinatal period: what do women want and do they use it? Aust J Prim Health. 2013;19(4):313–8. doi: 10.1071/PY13039.PY13039 [DOI] [PubMed] [Google Scholar]
- 10.Wellde PT, Miller LA. There's an App for That!: New Directions Using Social Media in Patient Education and Support. J Perinat Neonatal Nurs. 2016;30(3):198–203. doi: 10.1097/JPN.0000000000000177.00005237-201607000-00010 [DOI] [PubMed] [Google Scholar]
- 11.Daly LM. Apps and opps: mobile applications and opportunities for longitudinal data collection across the reproductive lifespan. ALSWH Scientific Meeting 2016 - Abstracts; Australian Longitudinal Study of Women's Health Scientific Meeting; 4-5 May 2016; Newcastle. 2016. https://www.alswh.org.au/images/content/scientificmeeting/ALSWH_Scientific_Meeting_Abstracts.pdf . [Google Scholar]
- 12.research2guidance . mHealth App Economics 2017: Current Status and Future Trends in Mobile Health. Berlin, Germany: research2guidance; 2017. pp. 1–25. [Google Scholar]
- 13.Thomas GM, Lupton D. Threats and thrills: pregnancy apps, risk and consumption. Health, Risk & Society. 2015 Dec 24;17(7-8):495–509. doi: 10.1080/13698575.2015.1127333. [DOI] [Google Scholar]
- 14.Agarwal S, Labrique A. Newborn health on the line: the potential mHealth applications. JAMA. 2014 Jul 16;312(3):229–30. doi: 10.1001/jama.2014.6371.1883978 [DOI] [PubMed] [Google Scholar]
- 15.Gates S Cochrane Pregnancy and Childbirth Group Methodological Guidelines. Cochrane Pregnancy and Childbirth Group, Cochrane Collaboration; 2012. http://pregnancy.cochrane.org/sites/pregnancy.cochrane.org/files/public/uploads/MethodologicalGuidelines%20%28July%202012%29_0.pdf .
- 16.Veritas Health Innovation . Covidence systematic review software. Melbourne, Australia: 2016. [2018-07-24]. https://www.covidence.org/home . [Google Scholar]
- 17.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. http://training.cochrane.org/handbook .
- 18.Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M, Lamb SE, Dixon-Woods M, McCulloch P, Wyatt JC, Chan A, Michie S. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. doi: 10.1136/bmj.g1687. http://www.bmj.com/cgi/pmidlookup?view=long&pmid=24609605 . [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann TC, Oxman AD, Ioannidis JP, Moher D, Lasserson TJ, Tovey DI, Stein K, Sutcliffe K, Ravaud P, Altman DG, Perera R, Glasziou P. Enhancing the usability of systematic reviews by improving the consideration and description of interventions. BMJ. 2017 Dec 20;358:j2998. doi: 10.1136/bmj.j2998. [DOI] [PubMed] [Google Scholar]
- 20.Daly LM, Horey D, Middleton PF, Boyle FM, Flenady V. The effect of mobile application interventions on influencing healthy maternal behaviour and improving perinatal health outcomes: a systematic review protocol. Syst Rev. 2017 Feb 08;6(1):26. doi: 10.1186/s13643-017-0424-8. https://systematicreviewsjournal.biomedcentral.com/articles/10.1186/s13643-017-0424-8 .10.1186/s13643-017-0424-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009 Jul 21;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. http://dx.plos.org/10.1371/journal.pmed.1000097 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ainscough K, Kennelly M, Lindsay K, O'Sullivan E, McAuliffe F. Impact of an mHealth supported healthy lifestyle intervention on behavioural stage of change in overweight and obese pregnancy. Proc. Nutr. Soc. 2016 Nov 24;75(OCE3) doi: 10.1017/S0029665116001002. [DOI] [Google Scholar]
- 23.Choi J, Lee JH, Vittinghoff E, Fukuoka Y. mHealth Physical Activity Intervention: A Randomized Pilot Study in Physically Inactive Pregnant Women. Matern Child Health J. 2016 May;20(5):1091–101. doi: 10.1007/s10995-015-1895-7.10.1007/s10995-015-1895-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ledford CJW, Canzona MR, Cafferty LA, Hodge JA. Mobile application as a prenatal education and engagement tool: A randomized controlled pilot. Patient Educ Couns. 2016 Apr;99(4):578–82. doi: 10.1016/j.pec.2015.11.006.S0738-3991(15)30119-1 [DOI] [PubMed] [Google Scholar]
- 25.Zairina E, Abramson MJ, McDonald CF, Li J, Dharmasiri T, Stewart K, Walker SP, Paul E, George J. Telehealth to improve asthma control in pregnancy: A randomized controlled trial. Respirology. 2016 Jul;21(5):867–74. doi: 10.1111/resp.12773. [DOI] [PubMed] [Google Scholar]
- 26.van 't Hooft J, Alfirevic Z, Asztalos EV, Biggio JR, Dugoff L, Hoffman M, Lee G, Mol BW, Pacagnella RC, Pajkrt E, Saade GR, Shennan AH, Vayssière C, Khan KS. CROWN initiative and preterm birth prevention: researchers and editors commit to implement core outcome sets. BJOG. 2018 Jan;125(1):8–11. doi: 10.1111/1471-0528.14987. [DOI] [PubMed] [Google Scholar]
- 27.Khan K on behalf of Chief Editors of Journals participating in The CROWN Initiative (Appendix 1) The CROWN Initiative: journal editors invite researchers to develop core outcomes in women's health. BJOG. 2016 Sep;123 Suppl 3:103–4. doi: 10.1111/1471-0528.14363. doi: 10.1111/1471-0528.14363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search terms and returns.
Excluded studies.
Bias assessment.
Intervention characteristics.
Inventories used in included studies.
Secondary outcomes.


