Weekly iron supplementation, given to young nulliparous women living in a malaria-endemic area, neither improved iron status nor increased malaria risk, suggesting that current iron recommendations may need revisiting for these women.
Keywords: iron, malaria, adolescents, nonpregnant, pregnant
Abstract
Background
The safety of iron supplementation for young women is uncertain in malaria-endemic settings.
Methods
This was a double-blind, randomized controlled noninferiority trial in rural Burkina Faso.
Results
A total of 1959 nulliparae were assigned to weekly supplementation (60 mg iron and 2.8 mg folic acid) (n = 980) or 2.8 mg folic acid (n = 979) until first antenatal visit (ANC1), or 18 months if remaining nonpregnant. Three hundred fifteen women attended ANC1, and 916 remained nonpregnant. There was no difference at ANC1 in parasitemia prevalence (iron, 53.4% [95% confidence interval {CI}, 45.7%–61.0%]; control, 55.3% [95% CI, 47.3%–62.9%]; prevalence ratio, 0.97 [95% CI, .79–1.18]; P = .82), anemia (adjusted effect, 0.96 [95% CI, .83–1.10]; P = .52), iron deficiency (adjusted risk ratio [aRR], 0.84 [95% CI, .46–1.54]; P = .58), or plasma iron biomarkers. Outcomes in nonpregnant women were parasitemia (iron, 42.9% [95% CI, 38.3%–47.5%]; control, 39.2% [95% CI, 34.9%–43.7%]; prevalence ratio, 1.09 [95% CI, .93–1.28]; P = .282); anemia (aRR, 0.90 [95% CI, .78–1.05]; P = .17), and iron deficiency (aRR, 0.99 [95% CI, .77–1.28]; P = .96), with no iron biomarker differences.
Conclusions
Weekly iron supplementation did not increase malaria risk, improve iron status, or reduce anemia in young, mostly adolescent menstruating women, nor in early pregnancy. World Health Organization Guidelines for universal supplementation for young nulliparous women may need reassessment.
Clinical Trials Registration
Iron deficiency debilitates millions of young women in malaria-endemic areas [1]. Iron supplementation is recommended where anemia is prevalent, but its safety and efficacy in malarious areas is uncertain. Reviews of daily or intermittent iron supplementation of nonpregnant women include few studies from malaria-endemic settings [2–4], and in iron supplementation trials in pregnancy, malaria outcomes are mostly not reported [5]. Two trials showed no increased malaria risk at delivery with daily antenatal iron supplementation, but malaria exposure was very low in one trial [6]. The other recruited mainly multigravidae [7], although in sub-Saharan Africa the highest prevalence of Plasmodium falciparum malaria occurs during early pregnancy in primigravidae [8]. World Health Organization (WHO) guidelines on iron supplementation are applicable to both all-age pregnant women and all-age menstruating women. Safety of iron–folic acid supplementation in young nulliparous women before and during their first pregnancy in malaria-endemic areas has not been evaluated [9], and primigravidae are a higher malaria risk group than multigravidae.
As there had been no periconceptional trials of the safety and efficacy of iron supplementation in young nulliparous women living in malaria-endemic areas, we carried out a double-blind, randomized controlled noninferiority trial of malaria risk prior to and during early pregnancy in nulliparous women receiving weekly iron and folic acid supplementation over a period of up to 18 months. The primary outcome was malaria parasitemia at first antenatal visit (ANC1). Secondary outcomes assessed malaria in women who remained nonpregnant, and supplement efficacy in reducing iron deficiency. Malaria endemicity in this study area is typical of many settings in sub-Saharan Africa with perennial malaria transmission.
METHODS
This periconceptional trial compared 2 cohorts supplemented with iron and folic acid vs folic acid alone: nonpregnant women and women who experienced pregnancy (additional details in Supplementary Data 1). The study received ethical approval in Burkina Faso (Comité d’Ethique pour la Recherche en Santé), the United Kingdom (Liverpool School of Tropical Medicine), and Belgium (University Hospital, Antwerp). All women in the trial provided written informed consent.
The study was conducted (April 2011–January 2014) in the Nanoro Health and Demographic Surveillance System area (DHSS) where malaria is hyperendemic, with highest transmission between June and December [10]. The iron regimen used followed WHO guidelines updated in 2016 [3, 11] and satisfied recommended nutrient intakes for nonpregnant women assuming 10%–25% iron absorption, with potential for good compliance and reduced side effects compared to daily use.
Sample
Eligible participants were nulliparous, nonpregnant residents in 30 villages within the DHSS area. Potential participants aged 15–24 years identified through the DHSS database were approached by female field assistants (FFAs) for prescreening (additional details on informed consent procedures are shown in Supplementary Data 1). We excluded women with possible or confirmed pregnancy, chronic disease (eg, sickle cell), or illness requiring hospitalization. In this area, human immunodeficiency virus prevalence was 1.2% among women 15–49 years of age and 0.76% among pregnant women [12]. At enrollment demographic data, history of illness, obstetric history, last menstrual period, age at menarche, and sexual activity were recorded. A study clinician performed a general clinical examination, duplicate anthropometric measurements, and axillary temperature. We collected venous blood for plasma ferritin, serum transferrin receptor (sTfR), hepcidin, and C-reactive protein (CRP) measurements. Plasma was stored at –80°C (additional laboratory details are shown in Supplementary Data 1). All participants received a long-lasting insecticidal net (LLIN) and single doses of albendazole (400 mg) and praziquantel.
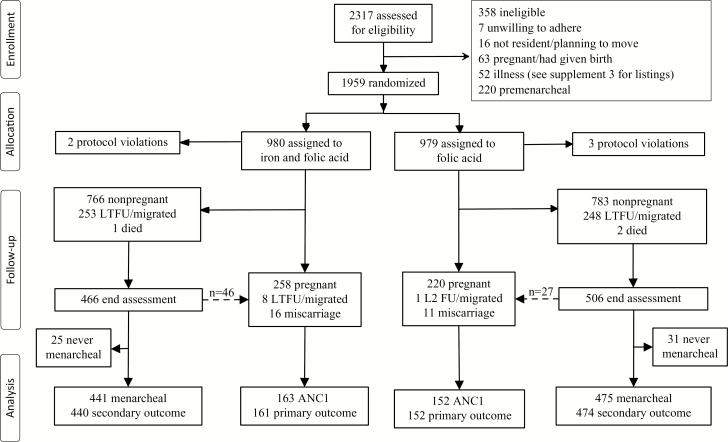
Recruitment continued until the target number was reached. We anticipated 34% malaria prevalence in controls at ANC1 [13]. Three hundred ninety women in the intervention and control groups would provide 90% power to detect a noninferiority margin of 10% of malaria prevalence between trial arms. The number of nonpregnant nulliparous women aged 15–24 years necessary to reach the required number of pregnant women was estimated from the proportion of nulliparous women (0.5) of childbearing age (0.23), aged 15–24 years (0.33), which provides 1973 potentially eligible women for a population of 52000 in the DHSS area (Figure 1).
Figure 1.
Trial profile. Stippled arrow indicates the number of women identified in early pregnancy at or within 2 months of the end assessment survey. Secondary outcome in nonpregnant women is malaria parasitemia. Abbreviations: ANC1, first antenatal visit; LTFU, lost to follow-up.
Randomization and Blinding
Participants were individually randomized to receive weekly one of identical red colored vegetable cellulose (hypromellose) capsules (disintegration time <30 minutes; mean, 9.5 minutes), containing either ferrous gluconate (60 mg elemental iron, 479 mg gluconate) and folic acid 2.8 mg, or folic acid alone. A block allocation sequence was used with randomly determined block lengths. Four containers (4 × 20 capsules) were assigned the same randomization code and were allocated to each participant. The FFA kept one container per participant, obtaining a replacement as required. Supplements were not kept by participants. Supplement codes, unknown to investigators and maintained independently by the sponsor, were revealed only after database lock and completion of data analysis. Women received a card with a unique study number corresponding to the randomization list. Supplements were stored at 20°C–25°C and at ambient temperature while with FFAs.
Follow-up
FFAs provided supplements during weekly home visits and ingestion was directly observed. Participants not located for 2 consecutive attempts but then recontacted, were reported as temporarily absent. In cases of fever (temperature ≥37.5°C) or history of fever in the previous 48 hours, the FFA performed a malaria Rapid Diagnostic Test (RDT; Bioline SD, Malaria Antigen P.f) and if positive, collected a blood sample for a thick film. RDT positives were treated with artesunate-amodiaquine following national guidelines. If no menses were reported at 5 consecutive weekly visits, a urine pregnancy test was done for human chorionic gonadotropin, immediately or at the next weekly visit. Supplement safety and tolerability were evaluated by recording and grading adverse events (AEs). There were 2 levels of recording: weekly FFA symptom using a checklist, and AEs through passive follow-up at health facilities. Serious AEs (SAEs), including deaths, were collected by active (weekly) and passive surveillance and reported according to available information from FFAs and health center staff. SAE classification used all available clinical evidence and was not bound by stringent laboratory or diagnostic criteria. If a woman died outside hospital, verbal autopsies were done by the DHSS team following a standardized protocol.
At the end of the first malaria transmission season, finger-prick samples were taken from all nonpregnant participants for an interim safety analysis of malaria risk (microscopy and RDT). Positive RDTs were treated following national guidelines (additional details in procedures, Supplementary Data 1).
Weekly supplements continued for 18 months, when women who remained nonpregnant were referred for end assessment. This included medical history, clinical examination, anthropometry, and axillary temperature. A venous blood sample was collected for malaria smear, hemoglobin (Hemocue AB), plasma ferritin, TfR, hepcidin, and CRP measurements, and for storage on filter paper. If febrile (temperature ≥37.5°C) or experiencing fever in the previous 48 hours, an RDT was performed and women with positive results were treated. Urine pregnancy tests were repeated when indicated. On trial completion, anemic nonpregnant women (hemoglobin [Hb] level <12 g/dL) received iron and folic acid tablets daily for 1 month and, if severely anemic (Hb <8 g/dL), were referred to hospital.
Women becoming pregnant within the follow-up period were referred to Nanoro hospital for ANC1 with a study nurse/doctor at 13–16 weeks’ gestation according to the last menstrual period. The weekly supplement was withdrawn and hematinics provided according to national policy (60 mg iron, 400 µg folic acid daily), although weekly follow-up continued. All women received the first dose of intermittent preventive treatment with sulfadoxine-pyrimethamine (IPTp-SP) if gestational age was >13 weeks. Women ≤13 weeks’ gestation, if RDT positive, were treated with oral quinine. At ANC1, procedures included routine antenatal care, venous blood collection for RDT, malaria film, hemoglobin, plasma ferritin, TfR, hepcidin, zinc protoporphyrin, and CRP measurements. Ultrasound was performed to confirm pregnancy and to determine fetal location, number and viability, and gestational age. Following ANC1, women with moderate anemia (Hb 7.0–9.9 g/dL) received twice the recommended daily supplement during 1 month and, if severely anemic (Hb <7 g/dL), were referred to Nanoro hospital. Women followed routine ANC visits where they received a second IPTp dose. Routine ANC and unscheduled health center visits were recorded on study questionnaires by study clinicians/nurses who collected data in health centers regularly (additional details in “Methods” in Supplementary Data 1). Procedures were similar at the second study visit (33–36 weeks’ gestation) when women were encouraged to deliver at Nanoro Hospital or the nearest health center, where free obstetric care was provided by the study. Study nurses examined babies for congenital malformations within 24–48 hours of delivery and information on infant survival was obtained by FFAs who visited or telephoned families.
Laboratory Measurements
Blood samples were transported to the laboratory within 3 hours, centrifuged, and aliquoted. Blood films were Giemsa stained and read for malaria and parasite density (additional details on laboratory procedures are shown in Supplementary Data 1). Hemoglobin was measured (Sysmex automated analyzer) on fresh whole blood, and zinc protoporphyrin by fluorometry (Aviv Biomedical). Plasma ferritin and sTfR were measured using mean values from duplicate enzyme-linked immunosorbent assay (ELISA) samples (Spectro Ferritin S-22 and TFC 94 TfR, RAMCO Inc). CRP was measured by ELISA (EU59131IBL, GmbH). Intra-assay coefficients of variation were all <10%. Plasma hepcidin was measured by competitive ELISA at an international reference laboratory (see laboratory procedures in Supplementary Data 1).
Outcomes
The primary outcome was Plasmodium parasitemia prevalence at ANC1. Prespecified secondary outcomes at ANC1 were prevalence of the following: RDT positivity with or without fever (temperature ≥37.5°C), clinical malaria (fever or history of fever in previous 48 hours with parasitemia), iron deficiency (definitions in Supplementary Data 1), anemia, adverse pregnancy outcomes (ie, miscarriage, stillbirth, perinatal or neonatal death), and congenital abnormality. In nonpregnant women at the end assessment, the proportion infected in the first year was assessed, along with the same prespecified outcomes as at ANC1. Adverse events captured incidence of prespecified gastrointestinal events (definitions and MedRA classification, grading and severity; see Supplementary Data 1 and 2 and Supplementary Table 3). Malaria parasitemia prevalence was measured in nonpregnant women during the first rainy season after at least 6 months of supplementation. Adherence to supplementation is reported.
Statistical Analysis
A statistical analysis plan was approved before database lock and release of the data and analyses follow that plan with only minor variation. Analyses followed CONSORT (Consolidated Standards of Reporting Trials) guidelines for noninferiority trials [14]. Investigators were blinded to allocation (coded as A/B) until completion of the primary outcome analysis. Prevalence analyses based on data at specific time points utilized risk-ratio binomial models unadjusted and adjusted for season of transmission (low or high), LLIN use (proportion of weekly visits with reported use prior to visit date), antimalarial use in 4 weeks prior to visit, and whether menarcheal at baseline. Parasite density and iron biomarkers were analyzed using analogous ordinary regression models following logarithmic transformation. Results are expressed as risk ratios (or density/biomarker level ratios) for iron treatment with 95% confidence interval (CI). Analyses of outcomes by iron deficiency utilized the same methodology, but with no menarche covariate. Mean hepcidin levels were compared between malaria parasitemia-positive and -negative women at ANC1 and the end assessment using Mann–Whitney U tests. Differences in proportions with elevated hepcidin were estimated using Fisher exact test and based on the 95th percentile value for a healthy Dutch female population of comparable age [15]. Statistical significance was one-sided at α = .05.
Malaria incidence was calculated using Poisson regression models for the number of malaria episodes per woman with an offset of the logarithm of the period under observation (ie, until final assessment, ANC1, or loss to follow-up) with adjustment for LLIN use and menarche. Results are presented as incidence ratios for iron treatment with 95% CI. Time to first episode of malaria was analyzed using Cox regression models with participants censored at ANC1, final assessment, or loss to follow-up with adjustment for season (baseline hazard stratified by enrollment month to allow for timing of rainy season), LLIN use, menarche, and iron deficiency at baseline (using adjusted ferritin definition). Results are expressed as hazard ratios for iron treatment with 95% CI. All analyses were performed using R statistical environment version 3.3 [16].
RESULTS
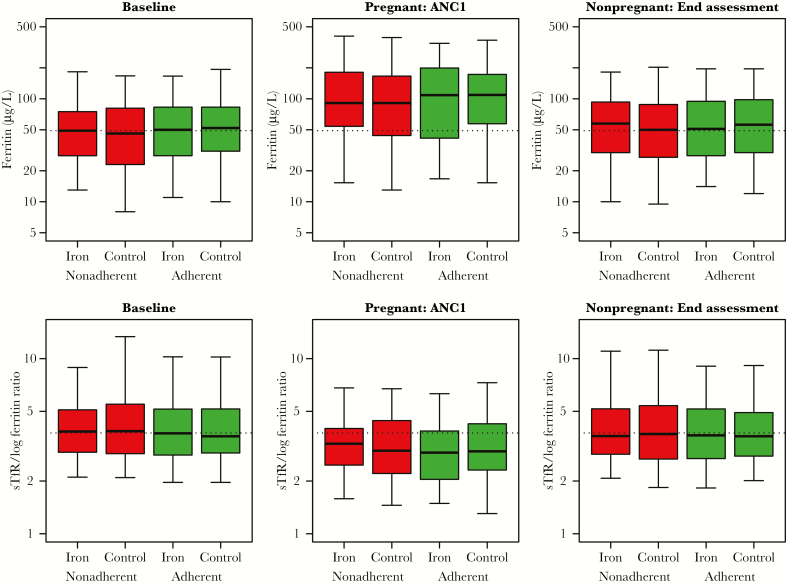
Of 2317 women invited for screening, 1959 were randomly assigned weekly supplements (n = 980, iron and folic acid; n = 979, folic acid); 1954 (99%) comprised the intention-to-treat population for the primary outcome. Four hundred five pregnancies (21%) occurred during follow-up, with another 73 (4%) early pregnancies identified at or shortly after the end assessment survey (giving 478 pregnancies in total) (Figure 1). Median weekly supplement adherence at ANC1 was 79% (95% CI, 65%–90%, iron) and 80% (95% CI, 59%–91%, folic acid) and at end assessment for nonpregnant women 83% (95% CI, 72%–91%, iron) and 84% (95% CI, 70%–92%, folic acid) (Supplementary Table 1 and Supplementary Data 2). Mean plasma ferritin and sTfR/log ferritin ratios were not improved in women with weekly adherence ≥80% (Figure 2). Median weekly LLIN use to ANC1 was 48.8%, and to end assessment was 46.7%.
Figure 2.
Mean ferritin concentration and serum transferrin receptor (sTfR)/log ferritin ratio in nonadherent and adherent women, by trial arm. Boxplots (median, interquartile range, and 90% range) for iron biomarkers in controls and iron-supplemented women at the 3 assessment points, subdivided by adherence to treatment. Adherence defined as receiving ≥80% weekly supplement intake up to first antenatal visit (ANC1), or end assessment in nonpregnant women. Broken reference lines are median baseline levels.
Baseline
Characteristics were similar between groups (Table 1). Adolescents (<20 years) comprised 93%. Women who remained nonpregnant were younger with lower body mass index. Women not attending were more illiterate and sexually active (Supplementary Table 2 and Supplementary Data 2). Iron deficiency prevalence at baseline did not differ in women lost to follow-up before ANC1 (9 of 478 [2%]), or for nonpregnant women lost to follow-up before end assessment (501 of 1549 [32%]) compared to those followed successfully. Iron deficiency prevalence was almost 2-fold higher using the sTfR/log ferritin ratio (22%) as biomarker than adjusted ferritin (12%) (Table 1). Elevated hepcidin concentration (>10.5 nmol/L) occurred in a quarter of women.
Table 1.
Baseline Characteristics of the Intention-to-Treat Dataset
| Characteristic | All Iron (n = 978) |
All Control (n = 976) |
Nonpregnant Iron (n = 766) |
Nonpregnant Control (n = 783) |
Pregnant Iron (n = 258) |
Pregnant Control (n = 220) |
|
|---|---|---|---|---|---|---|---|
| Sociodemographics | |||||||
| Mean age, y (SD) | 16.8 (1.7) | 16.8 (1.8) | 16.7 (1.7) | 16.7 (1.8) | 17.1 (1.7) | 17.1 (1.7) | |
| Age <20 y | 914/978 (93.5) | 910/976 (93.2) | 719/766 (93.9) | 737/783 (94.1) | 239/258 (92.6) | 199/220 (90.5) | |
| Ethnicity | Mossi | 936/978 (95.7) | 946/975 (97.0) | 730/766 (95.3) | 757/782 (96.8) | 250/258 (96.9) | 214/220 (97.3) |
| Missing | 0/978 (0) | 1/976 (0.1) | 0/766 (0) | 1/783 (0.1) | 0/258 (0) | 0/220 (0) | |
| Religion | Catholic | 414/978 (42.3) | 405/975 (41.5) | 317/766 (41.4) | 327/782 (41.8) | 118/258 (45.7) | 86/219 (39.3) |
| Protestant | 126/978 (12.9) | 120/975 (12.3) | 113/766 (14.8) | 106/782 (13.6) | 18/258 (7.0) | 19/219 (8.7) | |
| Muslim | 251/978 (25.7) | 250/975 (25.6) | 187/766 (24.4) | 203/782 (26) | 75/258 (29.1) | 52/219 (23.7) | |
| Traditional | 187/978 (19.1) | 200/975 (20.5) | 149/766 (19.4) | 146/782 (18.7) | 47/258 (18.2) | 62/219 (28.3) | |
| Missing | 0/978 (0) | 1/976 (0.1) | 0/766 (0) | 1/783 (0.1) | 0/258 (0) | 1/220 (0.5) | |
| Education | None | 595/975 (61.0) | 578/974 (59.3) | 447/763 (58.6) | 450/781 (57.6) | 176/257 (68.5) | 142/220 (64.5) |
| Primary | 212/975 (21.7) | 201/974 (20.6) | 168/763 (22.0) | 169/781 (21.6) | 54/257 (21) | 38/220 (17.3) | |
| Secondary | 168/975 (17.2) | 195/974 (20.0) | 148/763 (19.4) | 162/781 (20.7) | 27/257 (10.5) | 40/220 (18.2) | |
| Missing | 3/978 (0.3) | 2/976 (0.2) | 3/766 (0.4) | 2/783 (0.3) | 1/258 (0.4) | 0/220 (0) | |
| Literacy | Literate | 329/966 (34.1) | 347/966 (35.9) | 278/756 (36.8) | 297/774 (38.4) | 68/255 (26.7) | 62/218 (28.4) |
| Missing | 12/978 (1.2) | 10/976 (1.0) | 10/766 (1.3) | 9/783 (1.1) | 3/258 (1.2) | 2/220 (0.9) | |
| Occupationa | Student | 300/978 (30.7) | 317/976 (32.5) | 255/766 (33.3) | 274/783 (35) | 60/258 (23.3) | 51/220 (23.2) |
| Trading | 32/978 (3.3) | 31/976 (3.2) | 24/766 (3.1) | 21/783 (2.7) | 10/258 (3.9) | 10/220 (4.5) | |
| Domestic | 534/978 (54.6) | 515/976 (52.8) | 401/766 (52.3) | 407/783 (52) | 154/258 (59.7) | 120/220 (54.5) | |
| Farmer | 375/978 (38.3) | 380/976 (38.9) | 269/766 (35.1) | 290/783 (37) | 130/258 (50.4) | 101/220 (45.9) | |
| Other | 5/978 (0.5) | 4/976 (0.4) | 3/766 (0.4) | 2/783 (0.3) | 3/258 (1.2) | 3/220 (1.4) | |
| Clinical characteristics | |||||||
| Menarcheal | 844/978 (86.3) | 829/976 (84.9) | 639/766 (83.4) | 645/783 (82.4) | 241/258 (93.4) | 203/220 (92.3) | |
| Sexually active | 249/978 (25.5) | 241/975 (24.7) | 159/766 (20.8) | 164/782 (21.0) | 98/258 (38) | 82/220 (37.3) | |
| Height, cm (SD) | 159.0 (6.0) | 159.2 (6.0) | 158.6 (6.0) | 159.1 (6.1) | 160.0 (5.7) | 159.4 (5.6) | |
| Weight, kg (SD) | 50.2 (6.8) | 50.2 (7.2) | 49.6 (6.9) | 49.7 (7.2) | 51.6 (6.0) | 51.6 (6.7) | |
| BMI, kg/m2 (SD) | 19.8 (2.1) | 19.7 (2.2) | 19.7 (2.1) | 19.6 (2.2) | 20.1 (1.8) | 20.3 (2.1) | |
| BMI <18.5 kg/m2 | 257/978 (26.3) | 277/976 (28.4) | 222/766 (29.0) | 246/783 (31.4) | 46/258 (17.8) | 39/220 (17.7) | |
| MUAC, cm (SD) | 23.7 (2.1) | 23.7 (2.2) | 23.6 (2.1) | 23.6 (2.2) | 24.1 (1.8) | 24.3 (2.2) | |
| Serum iron biomarkers | |||||||
| Median CRP, mg/L (IQR) | 0.59 (0.23–1.47) | 0.51 (0.20–1.35) | 0.58 (0.23–1.43) | 0.50 (0.18–1.29) | 0.72 (0.27–1.64) | 0.70 (0.22–1.66) | |
| Missing | 13/978 (1.3) | 13/976 (1.3) | 6/766 (0.8) | 12/783 (1.5) | 7/258 (2.7) | 2/220 (0.9) | |
| CRP ≥10 mg/L | 38/965 (3.9) | 41/963 (4.3) | 30/760 (3.9) | 31/771 (4.0) | 9/251 (3.6) | 10/218 (4.6) | |
| Median ferritin, µg/L (IQR) | 49.0 (28.0–78.0) | 49.0 (26.0–82.0) | 49.0 (28.0–79.8) | 50.0 (27.0–81.0) | 48.0 (28.0–74.5) | 44.0 (26.0–81.0) | |
| Missing | 12/978 (1.2) | 14/976 (1.4) | 8/766 (1.0) | 12/783 (1.5) | 4/258 (1.6) | 3/220 (1.4) | |
| Median sTfR, mg/L (IQR) | 6.32 (5.08–7.87) | 6.28 (5.14–7.90) | 6.32 (5.10–7.95) | 6.26 (5.09–7.92) | 6.20 (5.02–7.67) | 6.34 (5.31–7.80) | |
| Missing | 11/978 (1.1) | 18/976 (1.8) | 7/766 (0.9) | 16/783 (2.0) | 4/258 (1.6) | 3/220 (1.4) | |
| Median sTfR/log10 ferritin ratio (IQR) | 3.80 (2.88–5.13) | 3.74 (2.88–5.36) | 3.80 (2.88–5.13) | 3.69 (2.86–5.25) | 3.77 (2.88–5.13) | 3.87 (2.94–5.61) | |
| Missing | 14/978 (1.4) | 18/976 (1.8) | 9/766 (1.2) | 16/783 (2.0) | 5/258 (1.9) | 3/220 (1.4) | |
| Median hepcidin, nmol/L (IQR) | 4.80 (2.00–10.75) | 4.30 (1.90–10.10) | 4 .80 (1.90–10.75) | 4.40 (1.90–10.00) | 4.90 (2.32–10.67) | 4.10 (1.80–10.70) | |
| Missing | 11/978 (1.1) | 16/976 (1.6) | 7/766 (0.9) | 14/783 (1.8) | 4/258 (1.6) | 3/220 (1.4) | |
| Elevated hepcidin >10.5 nM/Lb | 251/967 (26.0) | 222/960 (23.1) | 196/759 (25.8) | 172/769 (22.4) | 65/254 (25.6) | 55/217 (25.3) | |
| Missing | 11/978 (1.1) | 16/976 (1.6) | 7/766 (0.9) | 14/783 (1.8) | 4/258 (1.6) | 3/220 (1.4) | |
| Iron deficiencyc (adjusted ferritin) | 105/962 (10.9) | 125/961 (13) | 84/758 (11.1) | 100/770 (13) | 25/250 (10) | 26/217 (12) | |
| Missing | 16/978 (1.6) | 15/976 (1.5) | 8/766 (1.0) | 13/783 (1.7) | 8/258 (3.1) | 3/220 (1.4) | |
| Iron deficiencyd (sTfR/log ferritin ratio >5.6) | 205/964 (21.3) | 218/958 (22.8) | 162/757 (21.4) | 168/767 (21.9) | 52/253 (20.6) | 55/217 (25.3) | |
| Missing | 14/978 (1.4) | 18/976 (1.8) | 9/766 (1.2) | 16/783 (2.0) | 5/258 (1.9) | 3/220 (1.4) | |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; CRP, C-reactive protein; IQR, interquartile range; MUAC, mid-upper arm circumference; SD, standard deviation; sTfR, serum transferrin receptor.
aMultiple answers possible for subsistence activities (ie, trading, farming, and domestic work).
bHepcidin 95% range for women aged 18–24 years from reference Dutch population was median 2.6 nM; 2.5th percentile 0.7 nM; and 97.5th percentile 10.5 nM [15].
cFerritin <15 µg/L if CRP <10 mg/L or ferritin <70 µg/L if CRP ≥10 mg/L.
dRatio: sTfR (µg/L) to log10 ferritin (µg/L) >5.6.
Prevalence of P. falciparum parasitemia in nonpregnant women during the first rainy season was 36.4% (95% CI, 33.7%–39.3%; n = 1167), with no difference by trial arm.
ANC1 Outcomes
Mean gestational age was 18.5 (standard deviation [SD], 5.5) weeks (iron and folic acid) and 18.0 (SD, 6.0) weeks (folic acid). Plasmodium parasitemia prevalence was 54.3%. This did not differ by trial arm (adjusted ratio, 1.0 [95% CI, .97–1.03]), nor when associated with fever. Incidence and time to the first symptomatic episode (fever plus parasitemia or RDT positivity) were not different between arms (Table 2).
Table 2.
Malaria at First Antenatal Visit and at End Assessment in Nonpregnant Nulliparae
| Endpoint | Iron | Control | Ratio | P Value | Adjusted Ratio | Adjusted P Valuea |
|---|---|---|---|---|---|---|
| Prevalence: ANC1 | ||||||
| No. | 163 | 152 | ||||
| RDT positive, No. (%) | 95/161 (59.0) [51.3–66.3] | 89/152 (58.6) [50.6–66.1] | 1.01 [.84–1.21] | 1.000 | 1.00 [.98–1.03] | .718 |
| RDT positive and fever, No. (%) | 15/163 (9.2) [5.7–14.6] | 6/152 (3.9) [1.8–8.3] | 2.33 [.93–5.85] | .072 | 2.30 [.91–5.79] | .075 |
| Microscopy positiveb, No. (%) | 86/161 (53.4) [45.7–61.0] | 84/152 (55.3) [47.3–62.9] | 0.97 [.79–1.18] | .820 | 1.00 [.97–1.03] | .975 |
| Clinical malariac, No. (%) | 9/161 (5.6) [3.0–10.3] | 4/152 (2.6) [1.0–6.6] | 2.12 [.67–6.75] | .259 | 2.14 [.68–6.76] | .194 |
| Parasite density, parasites/mm3, GM | 2000 [1483–2696] | 2244 [1674–3008] | 0.89 [.59–1.35] | 0.585 | 0.89 [.58–1.36] | .584 |
| Prevalence: end assessmenta | ||||||
| No. | 441 | 475 | ||||
| RDT positive & fever, No. (%) | 19/432 (4.4) [2.8–6.8] | 30/464 (6.5) [4.6–9.1] | 0.68 [.39–1.19] | .188 | 0.69 [.39–1.21] | .193 |
| Microscopy positiveb, No. (%) | 189/441 (42.9) [38.3–47.5] | 186/474 (39.2) [34.9–43.7] | 1.09 [.93–1.28] | .282 | 1.10 [.95–1.28] | .180 |
| Clinical malariac, No. (%) | 13/440 (3.0) [1.7–5.0] | 22/474 (4.6) [3.1–6.9] | 0.64 [.32–1.25] | .228 | 0.67 [.34–1.31] | .237 |
| Parasite density, parasites/mm3, GM | 302 [248–368] | 316 [259–386] | 0.96 [.72–1.27] | .754 | 0.96 [.73–1.27] | .793 |
| Incidence to end assessment in nonpregnantd | ||||||
| Person-years of follow-up (No.) | 1114.9 (936) | 1121.1 (942) | ||||
| RDT positive (no./No.) | 0.40 (445/1115) | 0.40 (444/1121) | 1.01 [.88–1.15] | .908 | 1.01 [.89–1.15] | .867 |
| RDT positive and fever (no./No.) | 0.16 (174/1115) | 0.17 (188/1121) | 0.93 [.76–1.14] | .494 | 0.94 [.76–1.15] | .525 |
| Microscopy positive (no./No.) | 0.25 (282/1115) | 0.26 (286/1121) | 0.99 [.84–1.17] | .919 | 1.00 [.85–1.18] | .971 |
| Clinical malariac (no./No.) | 0.07 (79/1115) | 0.08 (87/1121) | 0.91 [.67–1.24] | .558 | 0.92 [.68–1.25] | .582 |
| Proportion infected in first yeare | ||||||
| No. at risk | 978 | 976 | ||||
| No. censored at 1 y (%) | 294 (30.1) | 294 (30.1) | ||||
| RDT positive and fever, % | 13 [11–15] | 14 [12–16] | 0.89 [.72–1.11] | .295 | 0.91 [.73–1.13] | .386 |
| Clinical malariac, % | 6 [5–8] | 7 [5–8] | 0.92 [.67–1.25] | .588 | 0.92 [.68–1.26] | .617 |
Values in square brackets indicate the 95% confidence interval.
Abbreviations: ANC1, first antenatal visit; GM, geometric mean; RDT, rapid diagnostic test.
aPrevalence adjusted for season at visit date, bed net use up to visit date, antimalarial use in month prior to visit, and menarcheal status at baseline. Ratio is risk ratio for iron treatment.
bParasite positive on blood smear.
c Plasmodium falciparum blood smear positive and fever ≥37.5°C.
dIncidence per person-year adjusted for weekly bed net adherence and menarcheal status at baseline; ratio is incidence ratio.
eCox regression model of time to first malaria episode, adjusted for baseline iron deficiency (ferritin), bed net use over observation time, visit, and menarcheal status at baseline, and stratified by enrollment month; ratio is hazard ratio.
There was no difference between study arms in anemia or iron deficiency prevalence, or mean concentrations of iron biomarkers (Table 3). Median hepcidin concentration was 3.9 nmol/L (interquartile range [IQR], 1.7–9.0) with, and 2.9 nmol/L (IQR, 0.7–7.0) without, malaria parasitemia (P = .005). Elevated hepcidin was more frequent in parasitemic (22%) compared to nonparasitemic women (11%) (P = .015).
Table 3.
Anemia and Iron Biomarkers at First Antenatal Visit and at End Assessment in Nonpregnant Nulliparae
| Endpoint | No. | Iron | Controla | Effectb | P Value | Adjusted Effectc | Adjusted P Value |
|---|---|---|---|---|---|---|---|
| ANC1 | |||||||
| Mean hemoglobin, g/dL | 314 | 10.17 [9.96–10.38] (1 missing) |
10.22 [9.99–10.46] | –0.06 [–.36 to .25] | .722 | –0.01 [–.32 to .3] | .944 |
| Anemiad | 314 | 112/162 (69.1) [61.6–75.7] | 107/152 (70.4) [62.7–77.1] | 0.98 [.85–1.14] | .902 | 0.96 [.83–1.10] | .524 |
| Severe anemiae | 314 | 2/162 (1.2) [.3–4.4] | 4/152 (2.6) [1.0–6.6] | 0.469 [.087–2.524] | .435 | 0.443 [.082–2.401] | .343 |
| Iron deficiency (%) (adjusted ferritin)f | 310 | 11/160 (6.9) [3.9–11.9] | 19/150 (12.7) [8.3–18.9] | 0.54 (.27–1.10) | .123 | 0.53 (.26–1.09) | .083 |
| Iron deficiency (%) (sTfR/log ferritin ratio)g | 312 | 18/162 (11.1%) [7.1–16.9] | 19/150 (12.7%) [8.3–18.9] | 0.88 (.48–1.61) | .728 | 0.84 (.46–1.54) | .578 |
| Mean ferritin, µg/L | 313 | 91 [78–107] (1 missing) | 87 [74–103] (1 missing) | 1.05 [.84–1.31] | .694 | 1.07 [.86–1.33] | .563 |
| Mean sTfR, mg/L | 313 | 6.0 [5.6–6.4] (1 missing) | 5.9 [5.6–6.3] (1 missing) | 1 [.91–1.1] | .949 | 1 [.91–1.1] | .962 |
| Mean ZPP, µmol/mol heme | 285 | 105 [100–111] (14 missing) | 106 [101–112] (16 missing) | 0.99 [.92–1.07] | .841 | 0.99 [.92–1.07] | .857 |
| Mean hepcidin, nmol/L | 311 | 3.01 [2.5–3.7] (3 missing) | 2.86 [2.3–3.5] (1 missing) | 1.05 [.79–1.41] | .729 | 1.07 [.80–1.43] | .664 |
| Elevated hepcidin, >10.5 nM/Lh | 311 | 29/160 (18.1) [12.9–24.8] | 25/151 (16.6) [11.5–23.3] | 1.09 (.67–1.78) | .766 | 1.12 (.69–1.83) | .649 |
| Nonpregnant | |||||||
| Mean hemoglobin, g/dL | 913 | 12.02 [11.91–12.12] (1 missing) | 11.93 [11.82–12.04] (2 missing) |
0.09 [–.07 to .24] | .261 | 0.06 [–.09 to .22] | .420 |
| Anemiai | 913 | 179/440 (40.7) [36.2–45.3] | 217/473 (45.9) [41.4–50.4] | 0.89 [.76–1.03] | .124 | 0.90 [.78–1.05] | .168 |
| Severe anemiaj | 913 | 0/440 (0.0) [0.0–0.9] | 3/473 (0.6) [0.2–1.8] | NA | .250 | NA | .996 |
| Iron deficiency (%) (adjusted ferritin)f | 910 | 38/439 (8.7) [6.4–11.7] | 50/471 (10.6) [8.1–13.7] | 0.82 [.55–1.22] | .369 | 0.82 [.55–1.23] | .336 |
| Iron deficiency (sTfR/log ferritin ratio)g | 909 | 89/437 (20.4) [16.9–24.4] | 97/472 (20.6) [17.2–24.4] | 0.99 [.77–1.28] | 1.000 | 0.99 [.77–1.28] | .960 |
| Mean ferritin, µg/L | 912 | 49 [46–53] (2 missing) | 51 [47–55] (2 missing) | 0.97 [.87–1.09] | .632 | 0.97 [.86–1.08] | .564 |
| Mean sTfR, mg/L | 909 | 6.4 [6.1–6.6] (4 missing) | 6.5 [6.2–6.7] (3 missing) | 0.98 [.94–1.04] | .558 | 0.98 [.93–1.04] | .534 |
| Mean ZPP, µmol/mol heme | 914 | 101 [97–104] (1 missing) | 101 [98–105] (1 missing) | 0.99 [.94–1.04] | .783 | 0.99 [.94–1.04] | .784 |
| Mean hepcidin, nmol/L | 909 | 2.85 [2.5–3.2] (3 missing) | 2.83 [2.5–3.2] (4 missing) | 1.01 [.86–1.17] | .939 | 1.0 [.86–1.17] | .960 |
| Elevated hepcidin, >10.5 nM/Lh | 909 | 70/438 (16.0) [12.8–19.7] | 72/471 (15.3) [12.3–18.8] | 1.05 [.77–1.41] | .785 | 1.05 [.78–1.43] | .736 |
Values in square brackets indicate the 95% confidence interval (CI).
Abbreviations: ANC1, first antenatal visit; NA, not applicable; sTfR, serum transferrin receptor; ZPP, zinc protoporphyrin.
aData are presented as No. (%) for binary variables, mean [95% CI] for hemoglobin; geometric mean [95% CI] for iron biomarkers.
bRisk ratio for binary outcomes; difference between arms for hemoglobin; ratio between arms for iron biomarker levels.
cAnemia measures adjusted for baseline menarche, season at assessment, and use of antimalarials in the previous month. Iron measures adjusted for baseline menarche.
dHemoglobin <11 g/dL.
eHemoglobin <8 g/dL.
fFerritin <15 µg/L if C-reactive protein (CRP) <10 mg/L or ferritin <70 µg/L if CRP ≥10 mg/L.
gRatio: sTfR µg/L to log10 ferritin >5.6.
hHepcidin 95% reference range for women aged 18–24 years from reference Dutch population was median 2.6 nM; 2.5th percentile 0.7 nm; and 97.5th percentile 10.5 nM [15].
iHemoglobin <12 g/dL.
jHemoglobin <7 g/d.
End Assessment Outcomes
Plasmodium parasitemia prevalence was 49.0% and did not differ by trial arm (adjusted ratio, 1.1 [95% CI, .95–1.28]), or if associated with fever. There was no difference between study arms in prevalence of anemia or iron deficiency, or mean concentrations of iron biomarkers (Table 3). Median hepcidin concentrations in women with and without malaria parasitemia were 3.4 (95% CI 1.7–6.5) nmol/L and 2.9 (95% CI 1.1–6.2) nmol/L, respectively (P = .011). Elevated hepcidin was similar in parasitemic (15%) compared with nonparasitemic women (16%) (P = .85).
Adverse Events
Supplements were well tolerated but with more frequent gastrointestinal events with iron supplementation (Relative Risk, 1.29 [95% CI, .93–1.79]; P = .12). Adverse events for all categories did not differ by trial arm (Supplementary Table 3 and Supplementary Data 2). We recorded 106 adult and 81 infant SAEs with almost equal frequency between groups (Table 4). Six adult deaths occurred (3 at delivery), unrelated to the intervention or malaria. All congenital abnormalities (1.4%) occurred in controls. There were 17 infant/perinatal deaths, 12 to mothers who received iron.
Table 4.
Serious Adverse Events
| Adverse Event | Iron | Control |
|---|---|---|
| Adult SAE | (n = 978) | (n = 976) |
| Accidental deatha | 0 | 1 |
| Obstetric death | 2 | 1 |
| Other obstetric SAEb | 12 | 7 |
| Severe malaria | 27 | 20 |
| Other deathc | 1 | 1 |
| Otherd | 12 | 22 |
| Total SAE | 54 | 52 |
| Infant SAE | (n = 231) | (n = 206) |
| Miscarriage | 16 | 11 |
| Stillbirth | 13 | 17 |
| Perinatal death* | 6 | 0 |
| Neonatal death | 4 | 3 |
| Infant death | 1 | 1 |
| Death from congenital abnormalitye | 0 | 1 |
| Other congenital abnormalitiesf, ** | 0 | 5 |
| Other obstetric SAEb | 2 | 0 |
| Other deathg | 1 | 0 |
| Total SAE | 43 | 38 |
Abbreviation: SAE, serious adverse event.
aOne death from drowning.
bSystems Order Classification = Pregnancy, puerperium and perinatal conditions, excluding deaths.
cNeoplasms: benign, malignant, and unspecified (liposarcoma and thoracic pain).
dSee Supplementary Data for summary of adverse events.
eSpina bifida.
fClub foot; congenital anomaly; polydactyly (all classified as unlikely or definitely not treatment related). Excludes abnormalities associated with stillbirth/miscarriage (one microcephaly).
gParalytic ileus.
*P = .021 (Fisher exact test).
**P = .011 for all abnormalities.
DISCUSSION
We found that weekly iron and folic acid supplementation for up to 18 months did not affect significantly prevalence of Plasmodium infection, iron deficiency, or anemia, compared with folic acid supplements alone in either the pregnant or nonpregnant cohort. Women receiving iron experienced nonsignificantly increased gastrointestinal effects. Asymptomatic falciparum malaria infection was highly prevalent despite weekly active surveillance, access to free treatments, and treatment of all RDT positives at the interim safety survey.
Our trial was double blind, tablet consumption was directly observed with balanced adherence covering wet and dry seasons, and active surveillance identified malaria episodes. Inadequate supplementation is unlikely as women absent for a number of weeks were tracked and resumed supplements, allowing them to reestablish their iron levels. Untreated chronic asymptomatic malaria may lead to tissue pathology within the gut [17] as well as elevated hepcidin concentrations [18], which would be expected to reduce iron absorption. In relation to a safety trial, an effect of iron supplementation on malaria risk becomes difficult to establish if there is limited iron absorption. Baseline prevalence of iron deficiency was comparable to estimates in a recent meta-analysis for low-income countries [19]. In the only in vivo iron absorption study to date in young women, conducted in Benin, markedly reduced iron absorption with asymptomatic malaria infection was demonstrated, and those participants had lower median parasite densities than we report at ANC1 [20]. Increasing iron dosage may be inadequate to overcome this limitation. Because participant diets also influence iron absorption due to high phytate content or other micronutrient deficiencies, effects of iron supplements may also depend on food habits and bioavailability. Better malaria control for adolescents could potentially both lower infection rates and improve food iron absorption, helping to meet growth requirements.
In 2016, WHO revised its guidelines on iron supplementation in menstruating women and adolescents girls, distinguishing settings where anemia prevalence is ≥40% from >20%. It recommended daily use (30–60 mg elemental iron) provided for 3 consecutive months per year where prevalence is ≥40% [11], and weekly intermittent supplementation in 3 monthly annual cycles where anemia prevalence is 20%–40% [3]. Among nonpregnant controls in our study, anemia prevalence was 45% at the end assessment but ≤20% were iron deficient. As we saw no improved iron status at the higher dosage of 60 mg with up to 18 months of supplementation (maximum of 78 doses), it is questionable whether daily dosage, given over 3 months, would be more beneficial (maximum 90 doses). This population has low iron deficiency prevalence despite high anemia prevalence. Without evidence that iron supplementation is effective in young nulliparous women living in malaria-endemic areas, the basis for the WHO recommendation is weak and possibly unsafe in this group.
Adverse events were evenly balanced across trial arms, including miscarriages, which could result from early pregnancy malaria. Gastrointestinal adverse events were nonsignificantly increased in iron-supplemented women. We have showed a 2-fold increased use of antibiotics for treatment of gastrointestinal infections in these women, with increased use of antifungals for lower genital infection in the nonpregnant iron-supplemented cohort [21]. Iron supplementation is reported to increase diarrhea frequency in menstruating women from non-malaria-endemic areas [2].
Our study has some limitations. Nonattendance was greater than expected, with 25% of nonpregnant women not attending end assessment and 30% of pregnant women at ANC1, although contact with their families was retained to monitor AEs up to birth. Attrition was equivalent between trial arms. Domestic labor, early sexual activity, pregnancy before marriage, and migration outside the study area at marriage were contributory factors in failure to attend [22], and would be higher than in a nonadolescent population. Six percent of women were identified as nonmenarcheal at end assessment, possibly related to intentional misreporting to gain free treatment [23]. Bias due to different premenarcheal iron requirements would be unlikely as there were equal numbers across study arms, but premenarcheal status reduced the conception rate.
CONCLUSIONS
In a high-malaria-transmission area, weekly iron supplementation did not increase malaria risk, improve iron status, or reduce anemia in young, mostly adolescent menstruating women, nor in early pregnancy. Iron absorption studies are required to clarify whether chronic malaria influences iron uptake. Baseline characteristics were typical for adolescents in low-income, rural sub-Saharan Africa; thus, our results should apply to similar malaria-endemic areas with high rates of asymptomatic adolescent malaria. Studies are warranted to improve malaria prevention and control in adolescent populations and to shape relevant interfacing iron deficiency reduction strategies. Iron supplementation, as routinely given to populations such as this, is not helpful and is potentially harmful. WHO guidelines for universal supplementation in young nulliparous women may need to be reassessed.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We gratefully acknowledge the contribution and support of participating women, local communities, His late Majesty Naaba Tigré of Nanoro, study teams, female field assistants, nurses, midwives and supervisors, doctors, and staff of St Camille Hospital, Nanoro Health District, and peripheral Health Centers; laboratory assistance of Greg Harper and Marc Christian Tahita; quality control staff at G&G Food Supplies Ltd, East Grinstead, West Sussex, United Kingdom; members of the data safety and monitoring board, Chris Roberts, Chair, Centre for Biostatistics, Faculty of Biology, Medicine and Health, University of Manchester, United Kingdom, Patrick van Rheenen, Department of Pediatrics, University of Groningen, Department of Public Health, the Netherlands, Marleen Boelaert and Veerle Vanlerberghe, Institute of Tropical Medicine, Antwerp, Belgium; Siem Klaver and Rian Roelofs of HepcidinAnalysis.com, Nijmegan, the Netherlands; Carl Henry from the Clinical Monitoring Department at the Liverpool School of Tropical Medicine and Gibby Koshy and Ray Brown for technical support; Raffaella Ravinetto and Celine Schurmans of the Clinical Trials Unit, Institute of Tropical Medicine, Antwerp, for external safety monitoring, and Isidore Traoré for local safety monitoring; and staff at the Liverpool School of Tropical Medicine for assistance with the statistical analysis plan and data preparation.
Author contributions. S. A. R., B. J. B., and S. G. had full access to all study data and take responsibility for the integrity of the data and accuracy of the data analysis. Study concept and design: B. J. B., S. G., L. B., and U. D. Acquisition, analysis, or interpretation of data: S. G., S. D., A. K., S. O., D. W. S., A. J. G.-M., Y. C., H. T., B. F., B. J. B. Drafting of the manuscript: B. J. B., S. A. R., L. B. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: S. A. R., M. P., B. F. Obtaining funding: B. J. B. Administrative, technical, or material support: S. G., S. D., A. K., S. O., Y. C., H. T., U. D., B. J. B. Study supervision: S. G., S. D., B. J. B.. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclaimer. The funder or study sponsor had no role in study design, data collection, analysis, interpretation or report writing, or approval of the manuscript and decision to submit the manuscript for publication.
Financial support. The study was funded by the National Institute of Child Health and Human Development/Bill & Melinda Gates Foundation (grant number NIH-1U01HD061234-01A1), and the National Institutes of Health Office of Dietary Supplements to the Liverpool School of Tropical Medicine. G&G Food Supplies Ltd, West Sussex, United Kingdom, prepared supplement capsules with financial compensation.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kyu HH, Pinho C, Wagner JA, et al. Global Burden of Disease Pediatrics Collaboration Global and national burden of diseases and injuries among children and adolescents between 1990 and 2013: findings from the Global Burden of Disease 2013 study. JAMA Pediatr 2016; 170:267–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Low MSY, Speedy J, Styles CE, De-Regil LM, Pasricha SR. Daily iron supplementation for improving anaemia, iron status and health in menstruating women. Cochrane Database Syst Rev 2016; 4:CD009747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Guideline: intermittent iron and folic acid supplementation in menstruating women. Geneva, Switzerland: WHO, 2011. [PubMed] [Google Scholar]
- 4. Fernández-Gaxiola AC, De-Regil LM. Intermittent iron supplementation for reducing anaemia and its associated impairments in menstruating women. Cochrane Database Syst Rev 2011; CD009218. [DOI] [PubMed] [Google Scholar]
- 5. Peña-Rosas JP, De-Regil LM, Garcia-Casal MN, Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database Syst Rev 2015; 7:CD4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Etheredge AJ, Premji Z, Gunaratna NS, et al. Iron supplementation in iron-replete and nonanemic pregnant women in Tanzania: a randomized clinical trial. JAMA Pediatr 2015; 169:947–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mwangi MN, Roth JM, Smit MR, et al. Effect of daily antenatal iron supplementation on Plasmodium infection in Kenyan women: a randomized clinical trial. JAMA 2015; 314:1009–20. [DOI] [PubMed] [Google Scholar]
- 8. Brabin BJ. An analysis of malaria in pregnancy in Africa. Bull World Health Organ 1983; 61:1005–16. [PMC free article] [PubMed] [Google Scholar]
- 9. Christian P, Black RE. Antenatal iron use in malaria endemic settings: evidence of safety?JAMA 2015; 314:1003–5. [DOI] [PubMed] [Google Scholar]
- 10. Derra K, Rouamba E, Kazienga A, et al. Profile: Nanoro health and demographic surveillance system. Int J Epidemiol 2012; 41:1293–301. [DOI] [PubMed] [Google Scholar]
- 11. World Health Organization. Guideline: daily iron supplementation in adult women and adolescent girls. Geneva, Switzerland: WHO, 2016. [PubMed] [Google Scholar]
- 12. Institut National de la Statistique et de la Démographie (INSD)/ICF International. Enquète Demographique et de Santé et Indicateurs Multiples du Burkina Faso 2010. Calverton, MD: INSD/ICF International, 2012. [Google Scholar]
- 13. Gies S, Coulibaly SO, Ouattara FT, D’Alessandro U. Individual efficacy of intermittent preventive treatment with sulfadoxine-pyrimethamine in primi- and secundigravidae in rural Burkina Faso: impact on parasitaemia, anaemia and birth weight. Trop Med Int Health 2009; 14:174–82. [DOI] [PubMed] [Google Scholar]
- 14. Piaggio G, Elbourne DR, Pocock SJ, Evans SJW, Altman DG; CONSORT Group Reporting of non-inferiority and equivalence randomized trials. Extension of the CONSORT 2010 statement. JAMA 2012; 308:2594–604. [DOI] [PubMed] [Google Scholar]
- 15. Galesloot TE, Vermeulen SH, Geurts-Moespot AJ, et al. Serum hepcidin: reference ranges and biochemical correlates in the general population. Blood 2011; 117:e218–25. [DOI] [PubMed] [Google Scholar]
- 16. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2016. [Google Scholar]
- 17. Coban C, Lee MSJ, Ishii KJ. Tissue-specific immunopathology during malaria infection. Nat Rev Immunol 2018; 18:266–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spottiswoode N, Duffy PE, Drakesmith H. Iron, anemia and hepcidin in malaria. Front Pharmacol 2014; 5:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petry N, Olofin I, Hurrell RF, et al. The proportion of anemia associated with iron deficiency in low, medium, and high human development index countries: a systematic analysis of national surveys. Nutrients 2016; 8:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cercamondi CI, Egli IM, Ahouandjinou E, et al. Afebrile Plasmodium falciparum parasitemia decreases absorption of fortification iron but does not affect systemic iron utilization: a double stable-isotope study in young Beninese women. Am J Clin Nutr 2010; 92:1385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brabin L, Roberts SA, Gies S, et al. Effects of long-term weekly iron and folic acid supplementation on lower genital tract infection—a double blind, randomised controlled trial in Burkina Faso. BMC Med 2017; 15:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Campaoré A, Gies S, Brabin B, Tinto H, Brabin L. Community approval required for periconceptional adolescent adherence to weekly iron and/orfolic acid supplementation: a qualitative study in rural Burkina Faso. Reprod Health 2018; 15:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paré Toe L, Ravinetto RM, Dierickx S, et al. Could the decision of trial participation precede the informed consent process? Evidence from Burkina Faso. PLoS One 2013; 8:e80800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.