Abstract
Intestinal fatty acid binding protein (iFABP) levels did not differ between human immunodeficiency virus type 1 (HIV-1)– infected infants and uninfected infants exposed to HIV-1, but those who breastfed had substantially lower levels. Zonulin levels increased from 3 to 5.3 months of age with perinatal acquisition of HIV-1 despite early antiretroviral treatment. Biomarkers of intestinal integrity (ie, iFABP and zonulin) were compared in 56 HIV-1–positive African infants who received early antiretroviral treatment and 53 HIV-1–exposed but uninfected (HEU) controls. Despite heightened inflammation and immune activation in HIV-positive infants, iFABP and zonulin levels at 3 months of age were not different from those in HEU infants and largely were not correlated with inflammatory and immune activation biomarkers. However, zonulin levels increased and became significantly higher in HIV-positive infants as compared to HEU infants by 5 months of age, despite viral suppression due to antiretroviral treatment. These findings have implications for intestinal integrity biomarker profiling in perinatal HIV-1 infection.
Keywords: Perinatal HIV-1 infection, intestinal integrity, inflammation, immune activation, zonulin, intestinal fatty acid binding protein
Increased inflammation and immune activation are characteristic of human immunodeficiency virus type 1 (HIV-1) infection and persist with effective antiretroviral treatment (ART) [1, 2]. Bacterial translocation from loss of gut-associated CD4+ T cells is implicated in HIV-1–infected adults [3] and supported by significant correlations between plasma concentrations of biomarkers of inflammation (ie, interleukin 6 [IL-6]), monocyte activation (ie, soluble CD14 [sCD14]), and intestinal damage (ie, intestinal fatty acid binding protein [iFABP]) [4, 5].
The interaction between intestinal integrity, inflammation, and immune activation during perinatal HIV-1 infection among those who receive early treatment is unknown. In Zimbabwe, untreated perinatally HIV-1–infected infants had significantly higher levels of iFABP, IL-6, and sCD14 than uninfected infants who were exposed to HIV-1 [6]. The associations between iFABP, inflammation, and immune activation biomarkers were not reported, nor was the effect of early ART evaluated [6]. In this study, we examined intestinal integrity biomarkers in early treated, perinatally HIV-1–infected infants from Botswana, Zambia, and Zimbabwe who were enrolled in a randomized, double-blinded, placebo-controlled clinical trial (International Maternal Pediatric Adolescent AIDS Clinical Trial [IMPAACT] P1072) of the safety and immunogenicity of a live, attenuated rotavirus vaccine (RotaTeq), in whom we previously characterized inflammation and immune activation profiles [7, 8].
METHODS
Study Design
P1072 randomly assigned 202 infants to receive either 3 doses of RotaTeq or placebo, with the first dose administered between 4 and <15 weeks of age and subsequent doses administered at 4–10-week intervals, with the last dose administered by 32 weeks of age. Participants for this analysis were chosen from those with available plasma samples (ie, 56 of 76 perinatally HIV-1–infected infants [the HIV-positive group] and 97 of 126 uninfected infants who were exposed to HIV-1 [the HEU group]). All 56 HIV-positive infants were included. A total of 53 HEU infants were randomly selected to have similar numbers as the HIV-positive group with respect to age (ie, ≤93 days, the median age of study population) and breastfeeding status at study entry. Plasma concentrations of iFABP and zonulin (markers of impaired intestinal tight junctions [9]) were measured before vaccination and 14–42 days after receipt of the third vaccine dose. Plasma concentrations of markers of inflammation (tumor necrosis factor α [TNF-α], interleukin 8 [IL-8], interleukin 6 [IL-6], interleukin 4 [IL-4], interleukin 2 [IL-2], interleukin 13 [IL-13], interleukin 12 [IL-12], interleukin 10 [IL-10], interleukin 1β [IL-1β], and interferon γ [IFN-γ]) were measured before vaccination, and plasma concentrations of a marker of monocyte activation (sCD14) were measured before and after vaccination [7]. This was an exploratory analysis, and results should be viewed as hypothesis generating.
Quantitation of Markers
Plasma concentrations of iFABP (Hycult Biotech; the Netherlands) and zonulin (Immundiagnostik; Germany) were measured in duplicate, using commercially available enzyme-linked immunosorbent assays. Plasma was diluted at 1:10 and 1:20 for measurement of iFABP and zonulin levels, respectively, using the manufacturer’s protocol. ODs of analytes were measured using a microtiter plate reader (VersaMax Plus ROM, version 1.21; SoftMax, Sunnyvale, CA), and values were quantified in GraphPad Prism, version 5 (GraphPad Software, La Jolla, CA). To minimize variation between runs, the same reagent lot was used to test all samples. To minimize bias, all samples were processed in a blinded fashion. If duplicates differed by ≥15% from the average concentration, testing was repeated (16% of samples). Methods for analyzing biomarkers of inflammation, monocyte activation, and serum antirotavirus immunoglobulin A (IgA) levels have been previously described [7, 8].
Statistical Methods
Analyses by vaccine group were reported according to the study product (RotaTeq or placebo) actually received (2 participants did not receive the treatment to which they had been randomly assigned). Postvaccination data were excluded for 1 participant who only received the first vaccine dose. Since relatively few iFABP levels measured (12%) were below the level of detection, these values were set to the detection level. No zonulin levels were below the level of detection. Categorical variables were compared using the Fisher exact test, and continuous variables were measured by Wilcoxon rank sum tests. Associations between biomarkers and other outcome measures were assessed using Spearman correlations. Multivariable linear regression (log10 scale) was used to compare changes in iFABP and zonulin levels from entry to after vaccination, according to HIV-1 infection, breastfeeding, and vaccine status. A P value of < .05 was considered statistically significant. Analyses were done using SAS software, version 9.4 (SAS Institute).
RESULTS
Study Population
Supplementary Table 1 summarizes demographic, virologic, and immunologic characteristics, trimethoprim-sulfamethoxazole use, and antiretroviral histories for the study participants, by HIV-1 status. The median age at study entry was similar (93 days in the HIV-positive group and 92 days in the HEU group), as were proportions who were breastfed (66% and 68%, respectively), received RotaTeq (52% and 49%, respectively), and received the first dose of oral polio vaccine (79% and 81%, respectively) on the same day as the first dose of the study vaccine. The median CD4+ T-cell percentage at study entry was significantly lower in HIV-positive infants (30%; interquartile range [IQR], 23%–37%), compared with HEU infants (36%; IQR, 32%–41%; P < .001). HIV-positive infants had significantly lower World Health Organization weight-for-age z scores than HEU infants (-1.4 vs -0.6; P = .005). Among HIV-positive infants, 91% had initiated ART before receipt of the first vaccine dose. The 5 infants who were not receiving ART at study entry initiated ART within 28 days of the first vaccine dose. The median plasma viral load at study entry in the HIV-positive group was 4.7 log10 copies/mL, and decreased to 2.95 log10 copies/mL by the end of study follow-up (data not shown). The median age at the time of the third vaccine dose was 5.3 months.
Biomarker Profiles at Study Entry
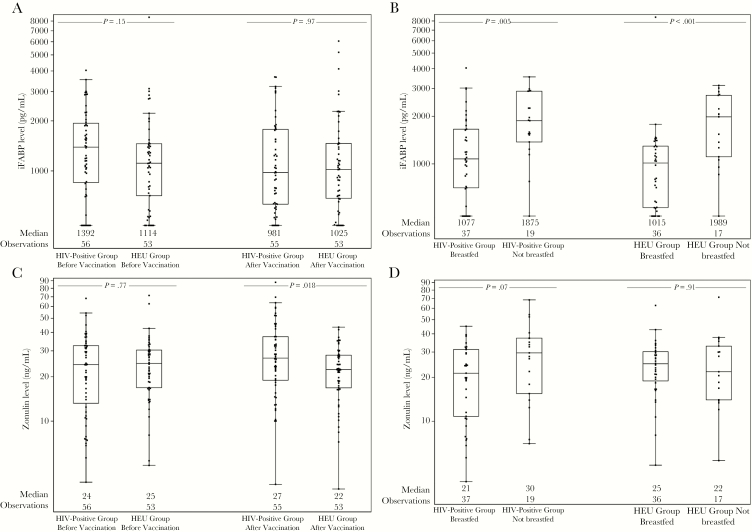
iFABP and zonulin levels at study entry, by HIV-1 and breastfeeding status, are shown in Figure 1. Neither marker was correlated with HIV-1 status. iFABP levels were significantly lower in breastfed infants in both the HEU group (P < .001) and the HIV-positive group (P = .005). Zonulin levels did not differ according to breastfeeding status. Breastfeeding status was confounded by country, with 89% of infants from Botswana not breastfed, compared with 6% in Zimbabwe and 0% in Zambia.
Figure 1.
A, Intestinal fatty acid binding protein (iFABP) levels at entry and after vaccination, by human immunodeficiency virus type 1 (HIV) status. B, iFABP levels at entry, by HIV-1 and breastfeeding status. C, Zonulin levels at entry and after vaccination, by HIV-1 status. D, Zonulin levels at entry, by HIV-1 and breastfeeding status. Data are for HIV-1–infected infants (HIV-positive group) and uninfected infants exposed to HIV-1 (HEU group).
Pearson correlations (with associated P values) of the intestinal biomarkers to CD4+ T-cell percentages and HIV-1 RNA levels (in HIV-positive infants only) and weight-for-age z scores and inflammatory markers (for HEU infants and HIV-positive infants separately) are shown in Table 1. No significant correlations were found between iFABP levels and CD4+ T-cell percentages or HIV-1 RNA levels in HIV-positive infants or between iFABP levels and weight-for-age z scores in HIV-positive or HEU infants. iFABP levels were positively correlated with IL-6 levels in the HIV-positive group (r = 0.30, P = .03) and negatively correlated with IL-13 levels (r = -0.48, P < .001) in the HEU group.
Table 1.
Spearman Correlation Analysis of Factors Potentially Associated With Zonulin and Intestinal Fatty Acid Binding Protein (iFABP) Levels at Study Entry Among Human Immunodeficiency Virus Type 1 (HIV)–Infected Infants (HIV Positive) and Uninfected Infants Exposed to HIV-1 (HEU)
| Factor | HEU Group | HIV-Positive Group | ||||||
|---|---|---|---|---|---|---|---|---|
| Zonulin Level | P | iFABP Level | P | Zonulin Level | P | iFABP Level | P | |
| CD4+ T-cell percentage | … | … | -0.09 | .51 | 0.23 | .09 | ||
| HIV RNA load | … | … | -0.28 | .045 | -0.19 | .17 | ||
| Weight-for-age z score | -0.16 | .26 | -0.20 | .15 | 0.16 | .24 | -0.14 | .31 |
| IFN-γ level | 0.11 | .44 | -0.02 | .92 | -0.24 | .08 | 0.13 | .35 |
| IL-1β level | 0.08 | .56 | -0.03 | .83 | -0.18 | .19 | 0.10 | .45 |
| IL-10 level | 0.18 | .19 | -0.08 | .57 | -0.32 | .020 | 0.11 | .44 |
| IL-12p70 level | 0.01 | .95 | -0.05 | .75 | 0.11 | .41 | -0.24 | .08 |
| IL-13 level | -0.19 | .18 | -0.48 | <.001 | -0.23 | .09 | -0.02 | .87 |
| IL-2 level | 0.30 | .030 | 0.19 | .18 | -0.04 | .77 | 0.09 | .51 |
| IL-4 level | 0.32 | .022 | 0.14 | .33 | -0.20 | .14 | 0.08 | .55 |
| IL-6 level | 0.16 | .27 | 0.05 | .70 | -0.08 | .55 | 0.30 | .030 |
| IL-8 level | -0.26 | .06 | 0.05 | .73 | -0.25 | .07 | 0.25 | .07 |
| TNF-α level | 0.15 | .29 | -0.18 | .21 | -0.16 | .24 | 0.14 | .32 |
| sCD14 level | 0.20 | .15 | -0.01 | .97 | -0.19 | .18 | -0.12 | .39 |
| iFABP level | 0.30 | .026 | 1 | 0.03 | .81 | 1 | ||
P values of <.05 are considered statistically significant and are highlighted in bold.
Abbreviations: IFN-γ, interferon γ; IL-1β, interleukin 1β; IL-2, interleukin 2; IL-4, interleukin 4; IL-6, interleukin 6; IL-8, interleukin 8; IL-10, interleukin 10; IL-12p70, interleukin 12p70; IL-13, interleukin 13; sCD14, soluble CD14; TNF-α, tumor necrosis factor α.
In the HIV-positive group, zonulin levels were negatively correlated with HIV-1 RNA loads (r = -0.28, P = .05) but not with CD4+ T-cell percentages. Weight-for-age z scores were not associated with zonulin levels in HIV-positive or HEU infants. Zonulin levels were negatively correlated with IL-10 levels (r = -0.32, P = .02) in the HIV-positive group and were positively correlated with IL-4 levels (r = 0.32, P = .02) and IL-2 levels (r = 0.30, r = 0.03) in the HEU group. Zonulin and iFABP levels were significantly associated in HEU infants (r = 0.30, P = .03) but not in HIV-positive infants.
Changes in Biomarker Profiles After Vaccination
Changes in biomarker levels between study entry and the postvaccination period were assessed using linear regression on the changes adjusted for HIV-1 status, breastfeeding at entry, and vaccine receipt. There were no significant changes in iFABP levels in either the HIV-positive group or the HEU group (Figure 1). However, zonulin levels changed in HEU infants by a median of -2 ng/mL (IQR, -14–8 ng/mL) and changed in HIV-positive infants by a median of 5 ng/mL (IQR, -6–17 ng/mL; P = .010). After vaccination, absolute zonulin levels were significantly higher in the HIV-positive group (P = .018), and zonulin and iFABP levels were not associated in either study group.
Intestinal Integrity and RotaTeq Vaccine Response, Based on Serum Antirotavirus IgA Levels
No significant correlations were found between postvaccination iFABP levels and serum antirotavirus IgA levels in 28 HIV-positive infants (r = 0.11, P = .58) or 25 HEU infants (r = 0.23, P = .28; data not shown). In the HIV-positive group, zonulin levels after vaccination were not significantly associated with serum antirotavirus IgA levels (r = -0.23, P = .24), but they were significantly positively correlated in HEU infants (r = 0.48, P = .014; data not shown).
DISCUSSION
In HIV-1–infected adults, inflammation and immune activation correlate with a higher risk of HIV-associated mortality and cardiovascular and metabolic diseases [2]. The long-term effects of inflammation and immune activation during perinatal HIV-1 infection are unknown, but in contrast to adults, inflammation and immune activation were not correlated with increased mortality in HIV-1–infected infants [6]. In our previous report, inflammation and immune activation did not impact RotaTeq responses [7].
In this study, where distinct differences in inflammation and immune activation were found between HIV-positive infants and HEU infants at a median age of 3 months [7], HIV-positive infants were indistinguishable from HEU infants, based on 2 plasma biomarkers of intestinal integrity, despite differences in CD4+ T-cell percentages and weight-for-age z scores. Unlike HIV-1–infected adults [5], no association between iFABP and sCD14 levels was observed in the HIV-positive group, but as in adults [5], iFABP levels positively correlated with IL-6 levels, suggesting some association between intestinal integrity and inflammation during perinatal HIV-1 infection. This is further supported by our findings of significant changes in zonulin levels between study entry and the postvaccination period, by HIV-1 status, which suggest an ongoing effect of HIV-1 infection on the intestinal epithelial tight junctions during perinatal infection, even with suppressive ART.
The lack of difference in iFABP levels between the HIV-positive and HEU groups may reflect potential differences in expression of this biomarker in early infancy or consequences of in utero HIV-1 exposure in HEU infants [10] that may also affect the gut. Age-related changes in iFABP levels have been reported in the first 18 months of life in African infants [11]. Furthermore, in untreated HIV-1–infected African infants, iFABP levels were higher at age 6 months, compared with levels in HEU infants [6]. In our study, iFABP levels remained stable and did not change differentially in the HIV-positive and HEU groups between a median of 3 and 5.3 months of age, which may reflect the effect of early ART. Additionally, our finding of lower iFABP concentrations in breastfed infants, irrespective of HIV-1 status, provides credence for this biomarker to assess infant intestinal integrity.
This is the first study, to our knowledge, to examine zonulin levels in perinatal HIV-1 infection. We demonstrated that, while zonulin levels did not differ between HIV-positive and HEU infants at age 3 months, levels increased significantly in HIV-positive infants by a median age of 5.3 months despite early effective ART. Several unexplained correlations between zonulin levels and HIV-1 infection status were identified in this exploratory study, including the negative correlation between zonulin levels and plasma viral loads at study entry and the negative correlation between zonulin levels and IL-10 levels. In HIV-1–infected adults, negative correlations between zonulin levels and advanced HIV-1 disease were reported [5]. While none of the HIV-positive infants in this study had advanced HIV-1 disease at study entry, CD4+ T-cell percentages were significantly lower than those in HEU infants, although zonulin levels were not correlated with CD4+ T-cell percentages.
Our findings indicate that zonulin may identify intestinal disruption in perinatal HIV-1 infection, even when early ART is implemented. Importantly, serum antirotavirus IgA levels were comparable in HIV-positive infants and HEU infants in the parent study [8]. We found a positive correlation between zonulin levels and antirotavirus IgA levels in HEU infants. Indeed, a recent study found elevated zonulin and iFABP levels to be positively associated with rotavirus vaccine seroconversion in Zambian infants with environmental enteric dysfunction and impaired intestinal integrity [12]. Increased intestinal permeability was postulated to increase dendritic cell exposure to luminal antigens and, hence, enhanced immunogenicity [12]. Understanding the relationship between intestinal integrity and vaccine responses in infancy may provide important insight into aspects of infant mucosal immunity and perinatal HIV-1 infection.
This study has several limitations, which include relatively small sample sizes, lack of HIV-1–unexposed, uninfected infant controls, lack of microbiome data, and unexplained correlations due to the exploratory nature of the study. Nevertheless, this is the first study to evaluate biomarkers of intestinal integrity, inflammation, and immune activation in perinatal HIV-1 infection treated early and in the context of an oral live virus vaccine. We observed no differences in iFABP levels between the HIV-positive and HEU groups, but iFABP levels were substantially lower in breastfeeding infants. iFABP levels were positively correlated with IL-6 levels in the HIV-positive group. Zonulin levels increased from 3 to 5.3 months of age with perinatal acquisition of HIV-1 infection despite ART suppression but without consequences altering serum IgA rotavirus vaccine responses, likely related to antigen access to dendritic cells through impaired tight junctions. Additional studies are needed to identify whether HEU infants have altered intestinal integrity, compared with infants not exposed to HIV-1. The long-term consequences of increased zonulin levels despite early effective ART in perinatal HIV-1 infection are not known.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments We thank the site investigators and site staff who conducted the P1072 study, for their contributions.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Institutes of Health (grant R01 HD080474 to D. P. and T32 training grant AI052071 to W. L. A. K.), the Johns Hopkins University School of Medicine Physician Scientist Training Program (microgrant to W. L. A. K.). Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network was provided by the National Institute of Allergy and Infectious Diseases (awards UM1AI068632, UM1AI068616, and UM1AI106716), the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Mental Health.
Potential conflicts of interest. M. J. L. is an advisor for, shares intellectual property with, and receives grant support from Merck, Sharp, and Dohme and is an advisor for and receives grant support from GlaxoSmithKline. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Persaud D, Patel K, Karalius B, et al. ; Pediatric HIV/AIDS Cohort Study Influence of age at virologic control on peripheral blood human immunodeficiency virus reservoir size and serostatus in perinatally infected adolescents. JAMA Pediatr 2014; 168:1138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hart BB, Nordell AD, Okulicz JF, et al. ; INSIGHT SMART and ESPRIT Groups Inflammation-related morbidity and mortality among HIV-positive adults: how extensive is it?J Acquir Immune Defic Syndr 2018; 77:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brenchley JM, Schacker TW, Ruff LE, et al. . CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med 2004; 200:749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jenabian MA, El-Far M, Vyboh K, et al. ; Montreal Primary infection and Slow Progressor Study Groups Immunosuppressive tryptophan catabolism and gut mucosal dysfunction following early HIV infection. J Infect Dis 2015; 212:355–66. [DOI] [PubMed] [Google Scholar]
- 5. Hunt PW, Sinclair E, Rodriguez B, et al. . Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 2014; 210:1228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prendergast AJ, Chasekwa B, Rukobo S, et al. . Intestinal damage and inflammatory biomarkers in human immunodeficiency virus (HIV)-exposed and HIV-infected Zimbabwean infants. J Infect Dis 2017; 216:651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uprety P, Lindsey JC, Levin MJ, et al. . Inflammation and immune activation in antiretroviral-treated human immunodeficiency virus type 1-infected African infants and rotavirus vaccine responses. J Infect Dis 2017; 215:928–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levin MJ, Lindsey JC, Kaplan SS, et al. . Safety and immunogenicity of a live attenuated pentavalent rotavirus vaccine in HIV-exposed infants with or without HIV infection in Africa. AIDS 2017; 31:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fasano A. Physiological, pathological, and therapeutic implications of zonulin-mediated intestinal barrier modulation: living life on the edge of the wall. Am J Pathol 2008; 173:1243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Afran L, Garcia Knight M, Nduati E, Urban BC, Heyderman RS, Rowland-Jones SL. HIV-exposed uninfected children: a growing population with a vulnerable immune system?Clin Exp Immunol 2014; 176:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prendergast AJ, Rukobo S, Chasekwa B, et al. . Stunting is characterized by chronic inflammation in Zimbabwean infants. PLoS One 2014; 9:e86928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mwape I, Bosomprah S, Mwaba J, et al. . Immunogenicity of rotavirus vaccine (RotarixTM) in infants with environmental enteric dysfunction. PLoS One 2017; 12:e0187761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.