Blood monocytes from patients with visceral leishmaniasis (VL) are skewed toward a disease-promoting antiinflammatory phenotype. They have a reduced phagocytic capacity and limited ability to generate microbicidal molecules. These finding may help improve therapeutic options for VL treatment.
Keywords: Visceral leishmaniasis, monocytes, antiinflammatory, proinflammatory, phagocytosis, blood
Abstract
Background
Monocytes are important effector cells during Leishmania infection, and changes in their functions may impact development of immunity. However, functional characteristics of monocytes in patients with visceral leishmaniasis (VL) remains poorly understood.
Methods
Peripheral blood monocytes from patients with VL and healthy endemic controls from Muzaffarpur, India, were isolated and compared in an ex vivo setting, using cell-culture techniques, flow cytometry, and reverse transcription quantitative polymerase chain reaction analysis.
Results
A blood monocyte population with a gene signature comprising upregulated expression of TGM2, CTLRs, VDR, PKM, SOCS1, and CAMP1 and downregulated expression of NOS2 and HIF1A was observed in patients with VL but not in controls. Monocytes from patients with VL also had impaired expression of chemokine receptors and adhesion molecules and decreased frequencies of interleukin 1β– and interleukin 6–producing cells. Importantly, monocytes from patients with VL had a markedly reduced capacity for phagocytosis of amastigotes, p47phox and p67phox expression, and reactive oxygen species production.
Conclusions
Monocytes from patients with VL express antiinflammatory molecules and lack a classically activated phenotype. They have reduced expression of molecules related to activation and antiparasitic effector functions, indicating that monocytes are skewed toward an antiinflammatory phenotype. These findings provide insights into the functional status of monocytes during VL and advise that therapeutic manipulation of this important cell population may result in favorable patient outcomes.
Visceral leishmaniasis (VL) is a protozoan parasitic disease with approximately 0.2–0.4 million cases each year [1]. It constitutes a major public health issue in the Indian subcontinent, where it is caused by Leishmania donovani. Parasites infect and reside within tissue macrophages, although dendritic cells, monocytes, and neutrophils can all be infected, albeit for only a short time [2]. The outcome of L. donovani infection depends upon various host and parasite factors and can range from asymptomatic infection to clinical syndromes, including life-threatening visceral disease and post–kala azar dermal leishmaniasis (PKDL). However, only a minority of infected individuals develop symptoms of VL [3]. The production of interferon γ and tumor necrosis factor α (TNF-α) by T-bet+CD4+ T helper type 1 (Th1) cells is critical for production of reactive oxygen species (ROS) and reactive nitrogen species by infected cells, which is crucial for control of parasite growth [4].
Monocytes develop in the bone marrow and migrate to peripheral tissues in response to chemotactic signals initiated by tissue damage caused by infection and inflammation [5]. Once monocytes have migrated into tissues, they can adjust their phenotype in response to microenvironmental cues, thereby displaying plasticity and heterogeneity under various pathological situations [6, 7]. Monocyte recognition of parasites is mediated through pathogen-recognition receptors [8, 9], leading to a cascade of events that result in the production of oxygen radicals capable of killing internalized parasites [10]. As a consequence, they are critical for defense and also play an important role in tissue repair after an infection has been controlled. However, Leishmania parasites have evolved various mechanisms for subversion or evasion of host immune mechanisms, allowing for their intracellular survival [11]. Under the influence of Th1-cell cytokines, monocytes display a proinflammatory phenotype with antiparasitic functions. Conversely, in a milieu of Th2 cytokines, monocytes acquire an antiinflammatory phenotype that can impede protective immunity and promote persistent infection [12]. Interestingly, the polarization of monocytes toward an antiinflammatory phenotype has been reported in patients with PKDL [13].
The aim of the present study was to define the functional and phenotypic characteristics of monocytes in patients with VL before and after antiparasitic drug administration and to compare the characteristics to those of monocytes from ECs. Previous work with in vitro experimental models and human studies has identified various activation markers for monocyte-macrophages [12, 14–16]. However, monocytes display a range of phenotypes in vivo, and assessment of only a particular set of markers is often not sufficient to establish the activation status [17]. Therefore, we assessed monocytes by using a combination of established proinflammatory and antiinflammatory markers at transcriptional and functional levels to better understand their role in VL.
Our results show that blood monocytes from patients with VL display a dominant antiinflammatory phenotype that is associated with a reduced phagocytic capacity and a decreased ability to generate microbicidal molecules. These data identify monocytes as potential targets for immune modulation to improve clinical outcomes in patients with VL.
METHODS
Recruitment and Characteristics of Human Subjects
All patients presented with clinical symptoms of VL at the Kala-Azar Medical Research Center (Muzaffarpur, India), and their diagnosis was confirmed by detection of amastigotes in splenic smears or by a positive result of a rK39 dipstick test. In total, 147 patients with VL and 134 endemic controls (hereafter, “controls”) belonging to the households of patients were enrolled in the study. Pregnant females, individuals aged <14 years, and patients with human immunodeficiency virus coinfection, hepatitis, or tuberculosis were excluded from the study. Aggregate clinical features of all subjects are listed in Table 1.
Table 1.
Aggregate Clinical Characteristics of Patients With Visceral Leishmaniasis (VL) and Healthy Endemic Controls (ECs)
| Variables | VL Group (n = 147) | EC Group (n = 134) |
|---|---|---|
| Age, y | ||
| Mean ± SD | 31.60 ± 15.16 | 34.97 ± 11.87 |
| Median | 32 | 33.5 |
| Sex, no. | ||
| Male | 47 | 51 |
| Female | 100 | 83 |
| Illness duration, d | ||
| Mean ± SD | 50.75 ± 56.58 | NA |
| Median | 30 | NA |
| Hemoglobin level, mg/mL | ||
| Before treatment | ||
| Mean ± SD | 8.19 ± 1.72 | ND |
| Median | 8.1 | ND |
| After treatment | ||
| Mean ± SD | 10.30 ± 1.41 | ND |
| Median | 10.2 | ND |
| WBC, ×10 3 cells/mm 3 | ||
| D-0 | ||
| Mean ± SD | 4281.63 ± 1671.23 | ND |
| Median | 4100 | ND |
| D-Dis | ||
| Mean ± SD | 11 094.56 ± 3800.23 | ND |
| Median | 10 500 | ND |
| Splenic enlargement, cm | ||
| D-0 | ||
| Mean ± SD | 4.33 ± 3.25 | NA |
| Median | 3 | NA |
| D-Dis | ||
| Mean ± SD | 0.41 ± 1.09 | NA |
| Median | 0 | NA |
Abbreviations: D-Dis, 28 days after treatment and prior to discharge; D-0, before treatment; NA, not applicable; ND, not done.
Ethics Statement
The use of human subjects followed the recommendations outlined in the Declaration of Helsinki. All subjects were enrolled in this study and previous receipt of their consent, and written informed assent was obtained from all participants and/or the legal guardian of those aged <18 years. This study was approved by ethics review board of the Institute of Medical Sciences, Banaras Hindu University (Varanasi, India; Dean/2008–09/314 and Dean/2012–2013/89).
Isolation of Peripheral Blood Mononuclear Cells (PBMCs) and Enrichment of CD14+ Cells
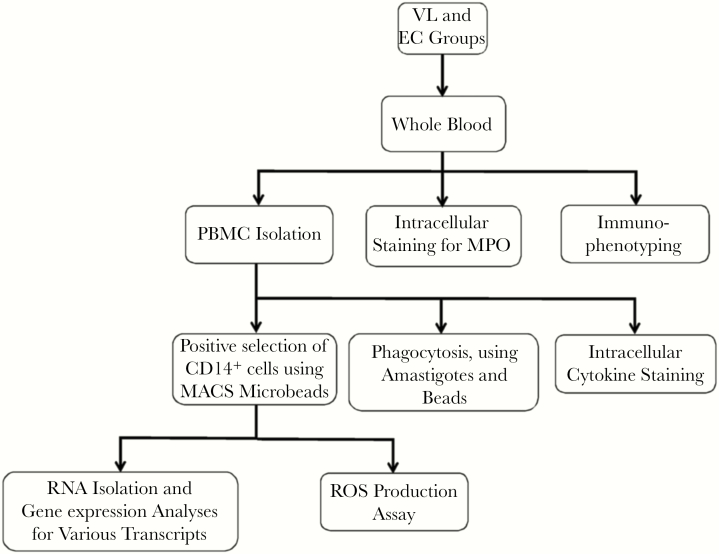
A heparinized venous blood specimen (volume, 8–10 mL) was collected from controls and patients with VL before the start of drug treatment (D-0). At the time of discharge (D-Dis), all patients began 30-day treatment with amphotericin B [18]. A diagram showing the work flow for processing and analyzing samples is shown in Figure 1. PBMCs were isolated using Lymphoprep (StemCell Technologies, Oslo, Norway) as described elsewhere [19], and cell viability was assessed using 0.4% (w/v) Trypan blue (Lonza, Walkersville, MD) followed by microscopy and found to be >97%. CD14+ cells were positively selected from PBMCs by using magnetic-activated cell-sorting MicroBeads (catalog number 130 050 201; Miltenyi Biotech, Bergisch Gladbach, Germany) according to manufacturer’s instructions.
Figure 1.
Schematic diagram showing sample processing and various immunological assays performed in the study on blood samples from patients with visceral leishmaniasis (VL) and healthy endemic controls (ECs). MACS, magnetic-activated cell sorting; MPO, myeloperoxidase; PBMC, peripheral blood mononuclear cell; ROS, reactive oxygen species.
RNA Isolation, Complementary DNA (cDNA) Synthesis, and Real-Time Polymerase Chain Reaction (PCR) Analysis
Total RNA was isolated from enriched CD14+ cells, using an RNeasy mini kit (catalog number 74106; Qiagen, Hilden, Germany) and QIAshredder homogenizers (catalog number 79656; Qiagen) according to the manufacturer’s instructions. RNA (500–1000 ng) was reverse transcribed in a 20-μL reaction volume with random primers and a multiscribe MuLV reverse transcriptase, using a high-capacity cDNA reverse transcription kit (catalog number 4368813; Applied Biosystem, Foster City, CA) as per the manufacturer’s instructions. Real-time PCR analysis was performed on an ABI Prism 7500 real-time PCR system (Applied Biosystems), using cDNA-specific FAM-MGB–labeled probes and primer sets for messenger RNAs (mRNAs) of interest (Supplementary Table 1) and VIC-MGB–labeled eukaryotic 18S ribosomal RNA (rRNA) as an endogenous control. Relative gene expression was calculated as the number of cycles for the mRNA of interest over 18S rRNA.
Fluorescence-Activated Cell-Sorting (FACS) Analysis
For staining of cell surface molecules, whole blood cells from patients with VL and controls were stained for surface markers, using combinations of fluorescently conjugated antibodies (Supplementary Table 2), for 30 minutes at room temperature. Erythrocytes were then lysed using FACS lysing solution (catalog number 349202; BD Biosciences), and cells were washed using FACS buffer (phosphate-buffered saline [PBS] supplemented with 1% [w/v] bovine serum albumin). For intracellular cytokine staining, PBMCs from patients with VL and controls were cultured at 1 × 106 cells/mL in round-bottomed polypropylene tubes (BD Biosciences) for 4 hours at 37°C in 5% (v/v) CO2, in complete Roswell Park Memorial Institute (cRPMI) medium (RPMI supplemented with 10% [v/v] heat-inactivated fetal calf serum, 100 μg/mL streptomycin, and 100 U/mL penicillin; Invitrogen). Cells were stimulated with soluble Leishmania antigen (SLA; 10 µg/mL) or lipopolysaccharide (LPS; 5 µg/mL) in the presence of Golgi plug (1 μL/mL; BD Biosciences). Surface-stained cells were fixed and permeabilized using Cytofix/Cytoperm, as per the manufacture’s instruction (BD Biosciences); washed in permeabilization buffer (BD Biosciences); and stained for intracellular cytokines with the fluorescent conjugated antibodies listed in Supplementary Table 2.
The expression of myeloperoxidase (MPO) was assessed by ex vivo intracellular whole-blood staining by using an anti-human MPO flow kit (Biolegend) according to the manufacturer’s instructions. For expression of p47phox and p67phox, PBMCs (1 × 106) were incubated in cRPMI medium alone or stimulated with L. donovani promastigotes (5 × 106) or phorbol myristate acetate (PMA; 10 ng/mL) for 3 hours at 37°C in 5% CO2. After surface staining, cells were fixed and permeabilized as described above and then underwent intracellular staining for detection of purified anti-p47phox (clone 1/p47Phox; BD Biosciences) or anti-p67phox (clone D-6; Santa Cruz Biotechnology) for 20 minutes. Cells were stained for another 20 minutes with anti–immunoglobulin G1 (clone A851; BD) conjugated with PE. Following staining, samples were fixed in 200 μL of phosphate-buffered saline/1% (w/v) paraformaldehyde, acquired on a FACSort machine (BD Biosciences), and analyzed using FlowJo, version 10.2 (Tree Star). Analysis was performed on cells gated according to their forward- and side-scatter properties, followed by gating on CD14+ cells.
Parasite
L. donovani promastigotes (1 × 108) originally isolated from a splenic aspirate culture from a patient with VL were used for intracardial inoculation and generation of amastigotes in Syrian golden hamsters [20]. Amastigotes were isolated aseptically from hamster spleens 40–60 days after infection, as described elsewhere [21, 22].
Phagocytosis Assay
L. donovani amastigotes (107) were labeled with 2.5 μM carboxyfluorescein succinimidyl ester (CFSE; catalog number 423801; Biolegend) for 10 minutes at 37°C. PBMCs (3.5 × 106 cells/100 μL) were incubated with equal numbers of Fluoresbrite 641 carboxylate microspheres (diameter, 0.50 μm; catalog number 21116-1; Polysciences) or CFSE-labeled amastigotes suspended in 100 μL of phagocytosis buffer (a 1:1 ratio of RPMI and Dulbecco’s modified Eagle’s medium supplemented with 1% [w/v] bovine serum albumin and HEPES) in a round-bottomed tube. Control PBMCs were treated with 10 μg/mL of cytochalasin D (Sigma-Aldrich) for 30 minutes, prior to incubation with parasite or beads. Samples were then centrifuged at low speed to synchronize phagocytosis and incubated for 30 minutes at 37°C in 5% CO2. Cells were kept on ice in chilled phosphate-buffered saline containing 2.5 mM ethylenediaminetetraacetic acid, to terminate phagocytosis. Cells were then stained with anti-human CD14-FITC/APC (Biolegend). The proportion of monocytes ingesting parasites or beads was quantified by flow cytometry.
ROS Detection
To measure ROS production, the CellROX Green flow cytometry kit (catalog number C10492; Invitrogen) was used according to the manufacturer’s instructions. Following staining, cells were placed on ice and immediately acquired by flow cytometry.
Statistical Analysis
Data sets were analyzed using Graph Pad Prism7 software and are presented as mean values ± standard errors of the mean, unless otherwise stated. The statistical significance of differences between groups was determined using the Wilcoxon signed rank test and the Mann-Whitney test, as appropriate. A P value of < .05 was considered statistically significant.
RESULTS
Changes in the Frequency and Phenotype of Circulating Monocytes in Patients With VL
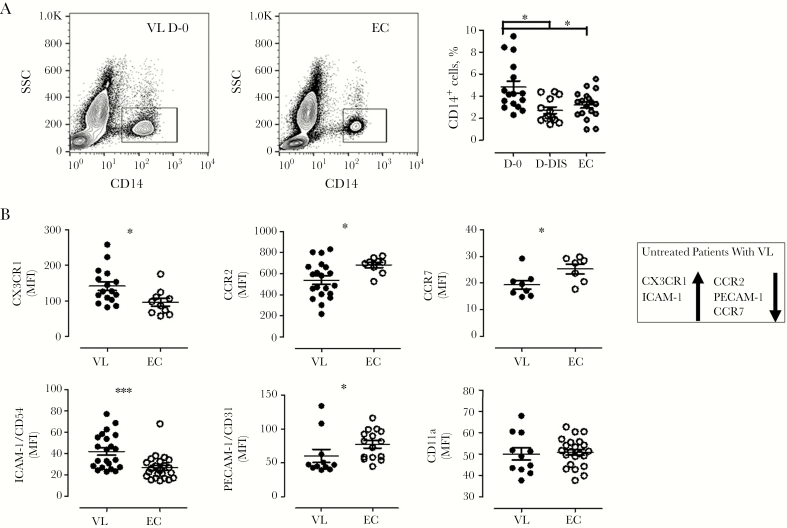
Flow cytometry of specimens from patients with VL revealed an increased frequency of CD14+ cells at disease presentation, compared with specimens collected on D-Dis and those from controls (P < .05; Figure 2A). Since monocyte migration is critical to their immune functions, we next examined the surface expression of chemokine receptors and cell adhesion molecules on CD14+ cells (Figure 2B). We found that monocytes observed at the time of VL presentation displayed significantly enhanced expression of CX3CR1 (P < .05) and ICAM-1 (P < .001) but decreased expression of CCR2 (P < .05), CCR7 (P < .05), and PECAM-1 (P < .05), compared with controls. We found no disease-associated change in the expression of CD11a (Figure 2B). Overall, these data show an increased frequency of monocytes at disease presentation that was associated with differential expression of several chemokine receptors and cell adhesion molecules.
Figure 2.
Patients with visceral leishmaniasis (VL) have altered monocyte frequencies accompanied by impaired expression of chemokine receptors and adhesion molecules on CD14+ cells. Peripheral blood specimens collected from patients with VL before or after treatment and from healthy endemic controls (ECs) during the same period were stained for flow cytometry. A, Representative contour plots (left panel) and monocyte frequencies (16 paired samples from the VL group and 18 samples from the EC group). B, Mean fluorescence intensities (MFIs) of CX3CR1 (16 specimens from the VL group and 10 from the EC group), CCR2 (20 and 9, respectively), CCR7 (8 and 7, respectively), ICAM-1 (22 and 23, respectively), PECAM-1 (11 and 15, respectively), and CD11a (11 and 22, respectively) were evaluated on CD14+ cells from patients with VL and ECs. Each circle on the vertical scatter plot indicates an individual sample, and lines represent mean values ± standard errors of the mean. *P < .05, **P < .01, and ***P < .001 by Wilcoxon signed rank or Mann-Whitney tests, as appropriate. D-Dis, specimens collected after 28 days of treatment and prior to discharge from subjects with VL; D-0, specimens collected from subjects with VL before treatment; SSC, side scatter.
Monocytes From Patients With VL Have an Antiinflammatory Gene Signature
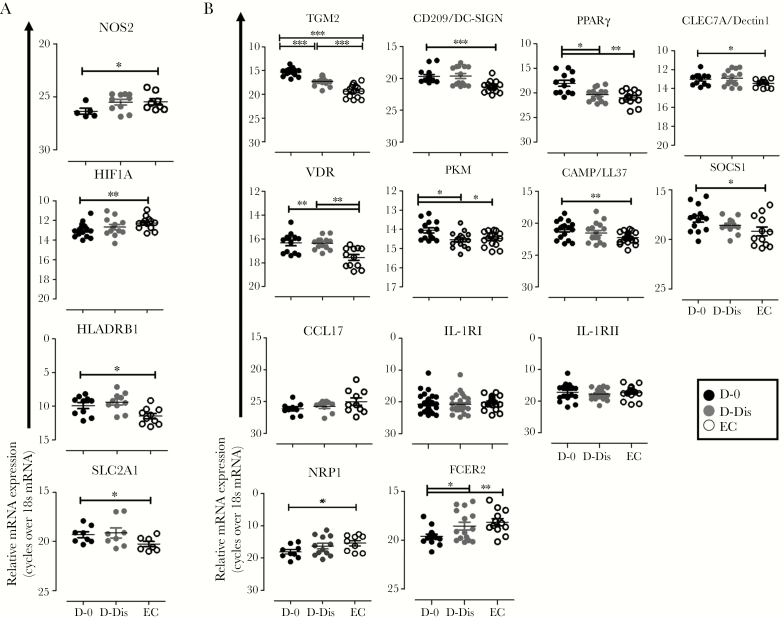
We next performed ex vivo gene expression analysis on CD14+ cells, purified by magnetic-activated cell sorting, from patients with VL and controls. We found decreased accumulation of mRNA encoding inflammation-associated nitric oxide synthase 2 (NOS2; P < .05) and hypoxia-inducible factor 1A (HIF1A; P < .01) but increased accumulation of mRNA encoding major histocompatibility complex class II DR β1 (HLA-DRB1; P < .05) and solute carrier family 2 member 1 (SLC2A1; P < .05) in patients with VL, compared with controls (Figure 3A). Among the genes usually associated with antiinflammatory responses, we found enhanced expression of mRNA encoding transglutaminase 2 (TGM2; P < .001), dendritic cell–specific intracellular adhesion molecule 3–grabbing nonintegrin (DC-SIGN; P < .001), c-type lectin domain family 7 member A (CLEC7A; P < .05), peroxisome proliferator-activated receptor γ (PPARγ; P < .01), vitamin D signaling receptor (VDR; P < .01), pyruvate kinase M (PKM; P < .05), suppressor of cytokine signaling 1 (SOCS-1; P < .05), and cathelicidin antimicrobial peptide (CAMP; P < .01) in patients with VL, compared with controls (Figure 3B). We found no changes in expression of mRNA encoding the antiinflammatory genes CCL17, IL-1R1, and IL-1R2, while neuropilin 1 precursor (NRP1) and Fc fragment of IgE receptor 2 (FCER2) mRNA was downmodulated in patients with VL, compared with controls (P < .01; Figure 3B). Expression of arginase 1 mRNA was not detectable in CD14+ cells in any group (data not shown). Treatment reduced levels of most transcripts, although levels were not always comparable with those in controls. Taken together, these data indicate that VL is associated with expression of a number of antiinflammatory gene transcripts by monocytes, but this was not a universal observation.
Figure 3.
Expression of messenger RNA (mRNA) associated with inflammatory and antiinflammatory phenotype of blood monocytes in patients with visceral leishmaniasis (VL) and healthy endemic controls (ECs). A and B, Representative vertical scatterplots show the differential expression of inflammatory (A) and antiinflammatory (B) gene transcripts in peripheral blood specimens collected from subjects with VL before treatment (D-0; solid black dots) and after 28 days of treatment and prior to discharge (D-Dis; gray black dots; 17 pairs) and from 15 ECs (clear black dots). Specimens were assessed by real-time polymerase chain reaction analysis. Data are mean gene expression ± standard errors of the mean over 18S ribosomal RNA and are representative of 3 independent experiments performed for each gene. Overall, we evaluated 34 mRNA pairs from the VL group and 30 from the EC group. *P < .05, **P < .01, and ***P < .001. CAMP, cathelicidin antimicrobial peptide; CCL17, chemokine ligand 17; CLEC7A, C-type lectin domain family 7 member A; DC-SIGN, dendritic cell–specific intercellular adhesion molecule-3-grabbing nonintegrin; FCER2, Fc fragment of immunoglobulin E receptor II; HIF1A, hypoxia-inducible factor 1-alpha; HLA-DRB1, major histocompatibility complex class II DR β1; IL1R1, interleukin 1 receptor type 1; IL1R2, interleukin 1 receptor type 2; NRP-1, neuropilin 1 precursor; NOS2, nitric oxide synthase 2; PKM, pyruvate kinase M; PPARγ, peroxisome proliferator-activated receptor γ; SLC2A1, solute carrier family 2, member 1; SOCS1, suppressor of cytokine signaling 1; TGM2, transglutaminase 2; VDR, vitamin D receptor.
Impaired Monocyte Cytokine Production in Patients With VL
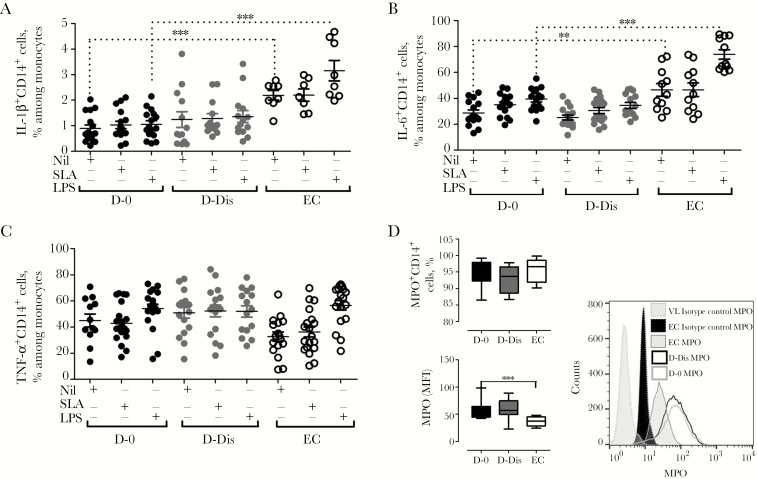
VL is generally associated with a mixed proinflammatory and antiinflammatory cytokine response, but the contributions made by monocytes to this milieu are not clear. Therefore, to understand the impact of VL on monocyte effector functions, we assessed the cytokine-producing ability of monocytes in response to stimulus with SLA or LPS. Intracellular interleukin 1β (IL-1β), interleukin 6 (IL-6), and TNF-α expression was examined in CD14+ cells from patients with VL and controls (Figure 4). We found a reduced frequency of IL-1β–expressing CD14+ cells (P < .001) and IL-6–expressing CD14+ cells (P < .001) at VL presentation following no stimulation or stimulation with LPS, compared with controls (Figure 4A and 4B). Interestingly, there was no change in production of these cytokines or TNF-α in response to SLA stimulation, suggesting some selectivity in the effects of disease on pattern-recognition pathways (Figure 4C). IL-1β and IL-6 are involved in host protection, so their modulation during VL may be associated with disease outcome. We also measured ex vivo MPO expression by CD14+ cells and found no change in the frequency of cells expressing this molecule, although we found increased expression of MPO on a per-cell basis, as indicated by an increased mean fluorescence intensity, in CD14+ cells at VL presentation, compared with controls (Figure 4D).
Figure 4.
Analyses of intracellular production of inflammatory cytokines and myeloperoxidase (MPO) during visceral leishmaniasis (VL). A–C, Soluble Leishmania antigen (SLA)– and lipopolysaccharide (LPS)–induced production of interleukin 1β (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor α (TNF-α) by CD14+ cells in cultures of peripheral blood mononuclear cells from patients with VL and healthy endemic controls (ECs) was measured to assess the cytokine-producing ability of monocytes. Analysis was performed on CD14+ cells, and the frequency of CD14+ cells with a particular phenotype was expressed as a percentage of the total number of CD14+ cells. Representative plots indicate the percentage of CD14+ cells expressing IL-1β (A), IL-6 (B), and TNF-α (C) in samples from patients with VL before treatment (D-0; solid black circles) and after 28 days of treatment and prior to discharge (D-Dis; solid gray circles) and in samples from ECs (clear black circles) following stimulation with SLA and LPS. Graphs depict data from 16 paired specimens from the VL group and 11 specimens from the EC group (A and B) and 21 specimens from D-0, 17 from D-Dis, and 20 from the EC group (C). D, Ex vivo intracellular whole-blood staining for MPO showed no changes in the frequency of MPO-expressing CD14+ cells but increased expression of MPO on monocytes in 8 paired specimens from patients with VL, compared with specimens from 10 ECs. Representative histograms displaying MPO mean fluorescence intensities in 1 sample from D-0, 1 sample from D-Dis, and 1 EC sample are shown.
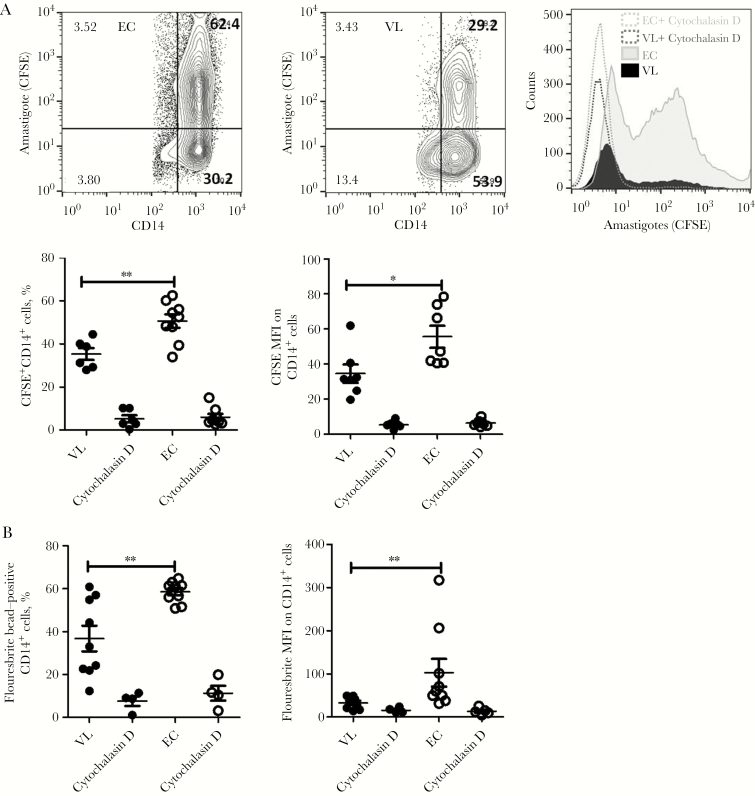
Monocytes From Patients With VL Have Diminished Phagocytic Capacity
Given our finding that monocytes from patients with VL expressed a relatively dominant antiinflammatory gene signature and that this signature has been associated with reduced phagocytic capacity, we next examined this function. We again defined monocytes as CD14+ cells in our assay and found that they had a reduced ability to phagocytose CFSE-labeled amastigotes, compared with monocytes from controls. This was indicated by both a reduced frequency of cells taking up amastigotes (P < .01) and a reduced number of parasites being phagocytosed, as indicated by a reduced mean fluorescence intensity (P < .05; Figure 5A). The diminished phagocytic capacity of monocytes from patients with VL was also confirmed using Fluoresbrite beads (Figure 5B). In both assays, cytochalasin D inhibited phagocytosis, indicating that cells from patients with VL and controls were capable of phagocytic activity. These results indicate that the phagocytic potential of monocytes during VL is impaired and that disruption of a cellular function capable of monocyte activation could possibly favor parasite spread to neighboring cells.
Figure 5.
Monocytes from patients with visceral leishmaniasis (VL) exhibit a marked reduction in phagocytic ability. A, A quantitative analysis of CD14+ cell phagocytosis, using carboxyfluorescein succinimidyl ester (CFSE)–labeled amastigotes and Fluoresbrite beads, was performed. Monocytes interacted with amastigotes or beads, and flow cytometry was performed as described in Materials and Methods. Representative contour plots from healthy endemic controls (ECs) and patients with VL, indicating the frequency of CD14+ cells that could take up amastigotes (right panel), and a histogram plot, showing the mean fluorescence intensity (MFI) of CFSE within monocytes (left panel), are shown. Vertical scatterplots indicate frequencies of monocytes that could take up amastigotes (middle right) and MFIs of CFSE (middle left) within monocytes. Data are from 7 patients with VL and 9 ECs and are representative of 2 independent experiments. B, Frequency of monocytes that phagocytosed Fluoresbrite bead and MFIs within CD14+ cells. Data are from 9 patients with VL (black solid circles) and 9 ECs (clear circles) and are representative of 2 independent experiments.
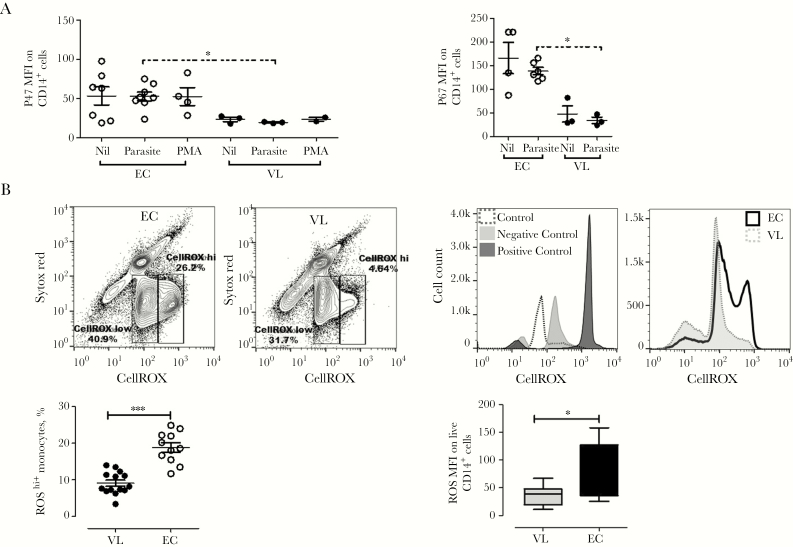
Decreased Generation of ROS by Monocytes From Patients With VL
Phagocytosis leads to NADPH oxidase activation and generation of ROS. To understand the impact of diminished phagocytosis on ROS production, we examined the expression of p47phox and p67phox, cytosolic components of NADPH oxidase, in monocytes following short-term culture with L. donovani promastigotes or PMA. We found significantly reduced expression of p47phox (P < .05) and p67phox (P < .05) in monocytes from patients with VL, compared with controls (Figure 6A). Finally, we examined the ability of monocytes to produce intracellular ROS and, in support of our p47phox and p67phox data, found significantly lower levels of ROShi-producing monocytes (P < .001) in patients with VL, compared with controls (Figure 6B). Together, these data indicates that monocytes from patients with VL have a suppressed ability to generate ROS and that attenuation of oxidative molecules capable of killing parasites could favor parasite survival.
Figure 6.
Monocytes from patients with visceral leishmaniasis (VL) have reduced respiratory burst after interaction with Leishmania donovani parasites. A, Representative data showing mean fluorescent intensities (MFIs) of p47phox and p67phox within CD14+ cells from patients with VL and healthy endemic controls (ECs), measured by flow cytometry following culture for 3 hours in medium alone or with promastigotes or phorbol myristate acetate (PMA). Data are from 3 patients with VL and 8 ECs. B, L. donovani promastigotes induce reduced reactive oxygen species (ROS) production in CD14+ cells from patients with VL. Human monocytes were loaded with the CellROX probe for 1 hour following exposure to promastigotes. Representative contour plots (top right panel) and histograms (top left panel) of ROS expression are shown. Plots show ROS expression by CD14+ cells and ROS MFIs within CD14+ cells. Results are from 14 patients with VL before treatment and 11 ECs and are representative of 3 independently performed experiments.
DISCUSSION
Monocytes play an essential role in many infectious diseases, including experimental VL [23–25]. A recent study with samples from patients with PKDL reported that blood monocytes had an antiinflammatory phenotype that the authors proposed may contribute to disease chronicity [13]. However, limited studies have been conducted to establish the phenotypic and functional characteristics of monocytes in humans with VL. We attempted to address this shortcoming in the present study and showed that blood monocytes appeared to acquire a disease-promoting antiinflammatory phenotype during VL, with reduced phagocytic potential and diminished ability to generate microbicidal molecules.
We found an enhanced frequency of monocytes at disease presentation. An increased frequency of CD16+ monocytes has been shown to be associated with disease pathology in patients with cutaneous leishmaniasis [26]. In addition, reduced recruitment and activation of inflammatory monocytes to skin lesions and draining lymph nodes has been associated with suboptimal priming of Th1 responses and consequent development of chronic infection [27], thus supporting the idea that these cells can play a disease-promoting role during VL. Impaired expression of chemokines and chemokine receptors has been reported in patients with VL [28], and CCR2-deficient mice have dysregulated monocyte migration to infected tissue that is associated with disease progression [29]. We found reduced expression of CCR2 and CCR7 but increased expression of CX3CR1 on CD14+ cells in patients with VL. CCR7 is required for migration of monocytes into inflamed tissues in experimental cutaneous leishmaniasis [30]. Additionally, CCR7 is needed for migration of monocytes that ultimately develop into lymphatic trafficking dendritic cells [31]. Therefore, the reduced CCR7 expression we report likely influences several monocyte functions. The altered CCR2 and CX3CR1 expression may affect monocyte recruitment to inflamed tissues and possibly indicates a parasitic strategy to limit monocyte migration. Monocytes also use various cell adhesion molecules to migrate into infected tissues from blood via cell-cell and endothelial cell–surface interactions [32]. An earlier study with Leishmania chagasi reported no change in expression of ICAM-1 and downregulated CD11b expression on CD14+ cells [33]. However, we found enhanced ICAM-1 expression, reduced PECAM-1 expression, and no change in CD11b expression on CD14+ cells in the VL group, suggesting that changes in these molecules are disease specific. Of note, ICAM-1–dependent recruitment of monocytes to the liver and hepatic granuloma assembly has been shown to be important for host responsiveness to conventional antiparasitic chemotherapy in experimental VL [34]. Furthermore, enhanced ICAM-1 and decreased PECAM-1 expression on monocytes could avoid simultaneous expression of these molecules, which may enhance cell adhesion to vascular endothelium and thereby promote parasite escape mechanisms. Thus, changes in expression of these molecules may not just influence cell migration but may also determine responses to antiparasitic drugs.
We observed elevated mRNA expression for HLADRB1 and SLC2A1, which encode proteins promoting acute inflammation and inflammatory activation of monocytes [35]. However, NOS2 and HIF1A gene expression were downregulated in CD14+ cells from patients with VL, indicating a selective impact of L. donovani infection on inflammatory gene expression by monocytes. Interestingly, in an experimental model of VL, HIF-1α promoted inhibitory functions in myeloid cells and rendered them more susceptible to L. donovani infection [36]. Thus, it appears that this mechanism of parasite persistence is not operating in human cells. Further, TGM-2 was identified as an antiinflammatory gene [37] involved in parasite growth and survival, cross-linking of cellular proteins, and extracellular matrix generation [38]. The enhanced expression of TGM-2 in CD14+ cells from patients with VL provides support for the skewing of these cells toward an antiinflammatory phenotype. We also observed enhanced expression of DC-SIGN, dectin-1, and PPARγ, which have also been associated with antiinflammatory phenotypes [39]. Cell responses against L. infantum are characterized by a C-type lectin receptor gene signature [39]. Upregulated PPARγ expression and activation has also been reported in experimental VL model and associated with parasite survival and disease chronicity [40]. Similarly, enhanced PPARγ is associated with an antiinflammatory monocyte phenotype in patients with PKDL [13]. VDR-dependent signaling inhibits proinflammatory cytokine and NO production [41] and contributes to susceptibility to L. major infection [42]. We observed enhanced VDR and CAMP transcripts in CD14+ cells at D-0, again supporting the presence of CD14+ cells with an antiinflammatory phenotype in patients with VL. Importantly, metabolic manipulation of host cell by parasites to enhance the access to bioenergy and essential nutrients is recognized to play an important role in the pathology of leishmaniasis [43], and an observed increase in transcription of glycolytic enzyme PKM suggests a shift in metabolism of CD14+ cells toward aerobic glycolysis. Activation of transcripts typical for noninflammatory pathways indicate that VL induces the molecular switch required for skewing of monocyte toward antiinflammatory phenotype.
The inflammatory monocyte phenotype primarily produces proinflammatory cytokines, but a reduced population of monocytes expressed IL-1β and IL-6 in the VL group. Significant differences in the capacity of CD14+ cells from the VL and control groups to respond to LPS and produce IL-1β and IL-6 indicate a capacity of parasites to infect monocytes without triggering the production of potentially host protective cytokines. Our results are in agreement with those from a previous study demonstrating that Leishmania organisms are capable of infecting monocytes without inducing IL-1β production [44]. Interestingly, CD14+ cells from patients with VL had a reduced capacity to produce IL-1β and IL-6 but not TNF-α in response to activation with SLA or LPS. This finding suggests that CD14+ cells are not fully suppressed to produce proinflammatory molecules, a finding further supported by the ability of these cells to upregulate expression of MPO, which is believed to augment cellular cytotoxic activity by catalyzing the generation of hypochlorous acid [45]. However, functional defects in the ability of these cells to phagocytose parasites or beads were found. This diminished phagocytosis was in concordance with findings from a study of canine VL [46]. We also showed reduced production of ROS, accompanied by diminished expression of p47phox and p67phox, in CD14+ cells. ROS are important effector molecules for killing of Leishmania organisms [47], and our data showed marked attenuation in ROS production by CD14+ cells in patients with VL, similar to a previous study [48], suggesting that the variation in redox status is attributable to VL. Decreased generation of ROS is suggestive of antiinflammatory activation [49], and alterations in ROS production influence the redox balance and thereby monocyte host defense functions.
Our findings support the notion that encounters between monocytes and Leishmania primarily stimulate the transcription of antiinflammatory genes, but some inflammatory capacity is also retained. Monocytes from patients with VL did not effectively respond to classical activation signals and were poor effector cells, as indicated by their diminished capacity to produce cytokines, perform phagocytosis, and generate ROS. During VL, monocytes are not fully directed toward an inflammatory phenotype but are skewed toward a disease-promoting antiinflammatory phenotype. Further, impaired monocyte functions improved after drug treatment, suggesting that reorientation of monocyte activation status can restore the proinflammatory and antiinflammatory balance needed to be effective for parasite control in VL. Knowledge about the dynamic changes in monocyte phenotypes during VL may instruct the interpretation of future mRNA and protein analysis of these cells, which may reveal more-selective therapeutic manipulation. Our study identified genes, molecules, and pathways associated with monocyte activation status in VL that can potentially be modulated and provides a guide for future functional reprogramming of monocytes to enhance their proinflammatory properties while limiting antiinflammatory properties.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank hospital staff at the Kala-Azar Medical Research Center for their assistance in the collection of patient samples.
Disclaimer. The findings and views expressed are those of author(s), and funders had no role in study design, data collection, analysis, decision to publish, and preparation of the manuscript.
Financial support. This work was supported by the Extramural Research Program, Indo-Australian Department of Biotechnology (project BT/Indo-Aus/06/22/2011); the Tropical Medicine Research Centers, National Institute of Allergy and Infectious Diseases, National Institutes of Health (grants P50 AI-074321 and U19AI-074321); the University Grants Committee, Council of Scientific and Industrial Research (to N. S.); and the Sitaram Memorial Trust, Kala-Azar Medical Research Center.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 6th World Congress on Leishmaniasis, Toledo, Spain, 16–20 May 2017.
References
- 1. Alvar J, Vélez ID, Bern C, et al. ; WHO Leishmaniasis Control Team Leishmaniasis worldwide and global estimates of its incidence. PLoS One 2012; 7:e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaye P, Scott P. Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol 2011; 9:604–15. [DOI] [PubMed] [Google Scholar]
- 3. Sacks DL, Lal SL, Shrivastava SN, Blackwell J, Neva FA. An analysis of T cell responsiveness in Indian kala-azar. J Immunol 1987; 138:908–13. [PubMed] [Google Scholar]
- 4. Murray HW. Cell-mediated immune response in experimental visceral leishmaniasis. II. Oxygen-dependent killing of intracellular Leishmania donovani amastigotes. J Immunol 1982; 129:351–7. [PubMed] [Google Scholar]
- 5. van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med 1968; 128:415–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol 2009; 27:451–83. [DOI] [PubMed] [Google Scholar]
- 7. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8:958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gazzinelli RT, Denkers EY. Protozoan encounters with Toll-like receptor signalling pathways: implications for host parasitism. Nat Rev Immunol 2006; 6:895–906. [DOI] [PubMed] [Google Scholar]
- 9. Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010; 140:805–20. [DOI] [PubMed] [Google Scholar]
- 10. Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol 2008; 26:421–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sacks D, Sher A. Evasion of innate immunity by parasitic protozoa. Nat Immunol 2002; 3:1041–7. [DOI] [PubMed] [Google Scholar]
- 12. Gordon S. Alternative activation of macrophages. Nat Rev Immunol 2003; 3:23–35. [DOI] [PubMed] [Google Scholar]
- 13. Mukhopadhyay D, Mukherjee S, Roy S, et al. . M2 polarization of monocytes-macrophages is a hallmark of Indian post kala-azar dermal leishmaniasis. PLoS Negl Trop Dis 2015; 9:e0004145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 2006; 177:7303–11. [DOI] [PubMed] [Google Scholar]
- 15. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002; 23:549–55. [DOI] [PubMed] [Google Scholar]
- 16. Gensel JC, Kopper TJ, Zhang B, Orr MB, Bailey WM. Predictive screening of M1 and M2 macrophages reveals the immunomodulatory effectiveness of post spinal cord injury azithromycin treatment. Sci Rep 2017; 7:40144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murray PJ, Allen JE, Biswas SK, et al. . Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014; 41:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sundar S, Chakravarty J. Leishmaniasis: an update of current pharmacotherapy. Expert Opin Pharmacother 2013; 14:53–63. [DOI] [PubMed] [Google Scholar]
- 19. Higdon LE, Lee K, Tang Q, Maltzman JS. Virtual global transplant laboratory standard operating procedures for blood collection, PBMC isolation, and storage. Transplant Direct 2016; 2:e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sacks DL, Melby PC. Animal models for the analysis of immune responses to leishmaniasis. Curr Protoc Immunol 2001; Chapter 19:Unit 19.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pearson RD, Steigbigel RT. Mechanism of lethal effect of human serum upon Leishmania donovani. J Immunol 1980; 125:2195–201. [PubMed] [Google Scholar]
- 22. Hart DT, Vickerman K, Coombs GH. A quick, simple method for purifying Leishmania mexicana amastigotes in large numbers. Parasitology 1981; 82:345–55. [DOI] [PubMed] [Google Scholar]
- 23. Weinberg JB, Volkheimer AD, Rubach MP, et al. . Monocyte polarization in children with falciparum malaria: relationship to nitric oxide insufficiency and disease severity. Sci Rep 2016; 6:29151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Antonelli LR, Leoratti FM, Costa PA, et al. . The CD14+CD16+ inflammatory monocyte subset displays increased mitochondrial activity and effector function during acute Plasmodium vivax malaria. PLoS Pathog 2014; 10:e1004393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cervia JS, Rosen H, Murray HW. Effector role of blood monocytes in experimental visceral leishmaniasis. Infect Immun 1993; 61:1330–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Passos S, Carvalho LP, Costa RS, et al. . Intermediate monocytes contribute to pathologic immune response in Leishmania braziliensis infections. J Infect Dis 2015; 211:274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Petritus PM, Manzoni-de-Almeida D, Gimblet C, Gonzalez Lombana C, Scott P. Leishmania mexicana induces limited recruitment and activation of monocytes and monocyte-derived dendritic cells early during infection. PLoS Negl Trop Dis 2012; 6:e1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singh N, Sundar S. Inflammatory chemokines and their receptors in human visceral leishmaniasis: gene expression profile in peripheral blood, splenic cellular sources and their impact on trafficking of inflammatory cells. Mol Immunol 2017; 85:111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boring L, Gosling J, Chensue SW, et al. . Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest 1997; 100:2552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kling JC, Mack M, Körner H. The absence of CCR7 results in dysregulated monocyte migration and immunosuppression facilitating chronic cutaneous leishmaniasis. PLoS One 2013; 8:e79098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qu C, Edwards EW, Tacke F, et al. . Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J Exp Med 2004; 200:1231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Imhof BA, Aurrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol 2004; 4:432–44. [DOI] [PubMed] [Google Scholar]
- 33. De Almeida MC, Cardoso SA, Barral-Netto M. Leishmania (Leishmania) chagasi infection alters the expression of cell adhesion and costimulatory molecules on human monocyte and macrophage. Int J Parasitol 2003; 33:153–62. [DOI] [PubMed] [Google Scholar]
- 34. Murray HW. Mononuclear cell recruitment, granuloma assembly, and response to treatment in experimental visceral leishmaniasis: intracellular adhesion molecule 1-dependent and -independent regulation. Infect Immun 2000; 68:6294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Palmer CS, Anzinger JJ, Zhou J, et al. . Glucose transporter 1-expressing proinflammatory monocytes are elevated in combination antiretroviral therapy-treated and untreated HIV+ subjects. J Immunol 2014; 193:5595–603. [DOI] [PubMed] [Google Scholar]
- 36. Hammami A, Abidin BM, Charpentier T, et al. . HIF-1α is a key regulator in potentiating suppressor activity and limiting the microbicidal capacity of MDSC-like cells during visceral leishmaniasis. PLoS Pathog 2017; 13:e1006616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martinez FO, Helming L, Milde R, et al. . Genetic programs expressed in resting and IL-4 alternatively activated mouse and human macrophages: similarities and differences. Blood 2013; 121:e57–69. [DOI] [PubMed] [Google Scholar]
- 38. Mehta K, Kumar A, Kim HI. Transglutaminase 2: a multi-tasking protein in the complex circuitry of inflammation and cancer. Biochem Pharmacol 2010; 80:1921–9. [DOI] [PubMed] [Google Scholar]
- 39. Lefèvre L, Lugo-Villarino G, Meunier E, et al. . The C-type lectin receptors dectin-1, MR, and SIGNR3 contribute both positively and negatively to the macrophage response to Leishmania infantum. Immunity 2013; 38:1038–49. [DOI] [PubMed] [Google Scholar]
- 40. Chan MM, Adapala N, Chen C. Peroxisome proliferator-activated receptor-γ-mediated polarization of macrophages in leishmania infection. PPAR Res 2012; 2012:796235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang Y, Leung DY, Richers BN, et al. . Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol 2012; 188:2127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ehrchen J, Helming L, Varga G, et al. . Vitamin D receptor signaling contributes to susceptibility to infection with Leishmania major. FASEB J 2007; 21:3208–18. [DOI] [PubMed] [Google Scholar]
- 43. Moreira D, Rodrigues V, Abengozar M, et al. . Leishmania infantum modulates host macrophage mitochondrial metabolism by hijacking the SIRT1-AMPK axis. PLoS Pathog 2015; 11:e1004684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reiner NE, Ng W, Wilson CB, McMaster WR, Burchett SK. Modulation of in vitro monocyte cytokine responses to Leishmania donovani. Interferon-gamma prevents parasite-induced inhibition of interleukin 1 production and primes monocytes to respond to Leishmania by producing both tumor necrosis factor-alpha and interleukin 1. J Clin Invest 1990; 85:1914–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Klebanoff SJ. Myeloperoxidase: contribution to the microbicidal activity of intact leukocytes. Science 1970; 169:1095–7. [DOI] [PubMed] [Google Scholar]
- 46. Brandonisio O, Ceci L, Cedola MC, Caretto G, Antonaci S, Jirillo E. Phagocytosis of Leishmania infantum promastigotes by monocytes isolated from Leishmania-infected dogs. Microbiologica 1986; 9:173–8. [PubMed] [Google Scholar]
- 47. Novais FO, Nguyen BT, Beiting DP, et al. . Human classical monocytes control the intracellular stage of Leishmania braziliensis by reactive oxygen species. J Infect Dis 2014; 209:1288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Roy S, Mukhopadhyay D, Mukherjee S, et al. . A defective oxidative burst and impaired antigen presentation are hallmarks of human visceral leishmaniasis. J Clin Immunol 2015; 35:56–67. [DOI] [PubMed] [Google Scholar]
- 49. Carneiro PP, Conceição J, Macedo M, Magalhães V, Carvalho EM, Bacellar O. The role of nitric oxide and reactive oxygen species in the killing of Leishmania braziliensis by monocytes from patients with cutaneous leishmaniasis. PLoS One 2016; 11:e0148084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.