Abstract
Background: The purpose of this study was to investigate the acute effects of a single oral administration of an essential amino acids enriched mixture (EAA) on myoelectric descriptors of fatigue and maximal force production after a resistance exercise protocol (REP).
Methods: Twenty adult males (age: 27 ± 6 years; body mass: 72.7 ± 7.50 kg; height: 1.76 ± 0.06 m) were enrolled in a double-blind crossover placebo-controlled study. Subjects were randomized to receive EAA mix (0.15 g/kg BM) or a placebo (PLA) in two successive trials 7 days apart. In both trials subjects completed a REP 2 h after the ingestion of the EAA mix or PLA. Before ingestion and after REP subjects performed isometric contractions of the dominant upper limb with the elbow joint at 120 degrees: (1) two maximal voluntary contractions (MVCs) for 2–3 s; (2) at 20% MVC for 90 s; (3) at 60% MVC until exhaustion. Mean values of MVC, conduction velocity initial values (CV), fractal dimension initial values (FD), their rates of change (CV slopes, FD slopes) and the Time to perform the Task (TtT) were obtained from a multichannel surface electromyography (sEMG) recording technique. Basal blood lactate (BL) and BL after REP were measured.
Results: Following REP a significant decrease of MVC was observed in PLA (P < 0.05), while no statistical differences were found in EAA between pre-REP and post-REP. After REP, although a significant increase in BL was found in both groups (P < 0.0001) a higher BL Δ% was observed in PLA compared to EAA (P < 0.05). After REP, at 60% MVC a significant increase of CV rate of change (P < 0.05) was observed in PLA but not in EAA. At the same force level TtT was longer in EAA compared to PLA, with a significant TtT Δ% between groups (P < 0.001).
Conclusion: Acute EAA enriched mix administration may prevent the loss of force-generating capacity during MVC following a REP. During isometric contraction at 60% MVC after REP the EAA mix may maintain CV rate of change values with a delay in the TtT failure.
Keywords: essential amino acids, amino acids supplementation, muscle fatigue, resistance exercise, time to task, maximal voluntary contraction
Introduction
The possibility that a nutritional supply could have an acute effect on resistance exercise (RE) performance has attracted a great deal of interest in recent decades, and several studies on this topic have been published (Kreider et al., 2010; Smith et al., 2010; Martinez et al., 2016). The market largely claims that amino acids (AA) consumption before RE may delay the appearance of fatigue and improve physical performance. Unfortunately, this interesting hypothesis is not adequately supported in literature which primarily deals with supplements containing rapid ATP replacement aids to increase strength and power (e.g., creatine) (Butts et al., 2018), stimulants able to increase muscle activation (e.g., caffeine) (Goldstein et al., 2010), or physiological buffers known to reduce lactate production (e.g., beta-alanine) (Hoffman et al., 2018).
Authors theorized that AA, mainly branched chain amino acids (BCAAs) and essential amino acids (EAAs), can increase exercise performance through various mechanisms, such as by improving secretion of anabolic hormones, modifying fuel use during exercise, preventing mental fatigue or stimulating muscle protein synthesis/hypertrophy responses (Williams, 2005; Blomstrand, 2006; Stepto et al., 2011; Jäger et al., 2017). However, a limited number of studies is available on the effects of these AA on central and peripheral aspects of fatigue during or after RE and, therefore, this subject has yet to be adequately addressed. References available indicate a possible role of BCAA in delaying central fatigue during sustained exercise through the regulation of brain’s uptake of tryptophan, as it was historically proposed by Blomstrand (2006); more recently, Gee and Deniel (2016) hypothesized that BCAA consumption before and after intense RE may lead to a reduction of subsequent damage-induced power loss due to increased muscle recovery; Eaton et al. (2016), based on prior reference using EAA to manipulate neurotransmitters (Pardridge, 1998), evaluated the possible effects of an acute EAA administration on central fatigue and muscle activation during high-intensity exercise protocol. Data on the possible effects of acute administration of BCAA or EAA on myoelectric descriptors of fatigue (peripheral and/or central) (De Luca, 1984; Enoka and Duchateau, 2008; Mesin et al., 2009; Gonzalez-Izal et al., 2012) measured before and after a RE bout are completely lacking and research in this direction needs further advancement.
Detection of central and peripheral components of fatigue (Bigland-Ritchie et al., 1978; Kent-Braun, 1999; Schillings et al., 2003) and their relative contribution during exercise are not particularly suitable for quantification (Merletti et al., 2004) and this explains why measurement of exertion in acute AAs administration protocols is mainly based on perceived fatigue scales, cognitive functions or specific reaction time tests (Blomstrand, 2006; Greer et al., 2011; Mikulski et al., 2015; Chen et al., 2016). However, accurate and continuous recording of local muscle fatigue during tasks is possible by evaluating myoelectric activity of a selected muscle by surface electromyography (sEMG). sEMG can analyze the myoelectric manifestations of fatigue, mainly linked to two physiological exercise-related phenomena: (1) the slowing of motor unit action potentials (MUAPs) as they travel along muscle fibers, that is the reduction of their conduction velocity (CV) (Komi and Tesch, 1979; Bouissou et al., 1989; Brody et al., 1991), and (2) the synchronization of motor unit (MU) by the central nervous system, which is described as a higher occurrence of simultaneous discharge of action potentials from various MUs to increase the force production when the whole MU pool is recruited (Holtermann et al., 2009), as observed in trained subjects (Semmler, 2002) and in presence of central lesions (Datta et al., 1991). Therefore, to assess peripheral components of fatigue, CV rate of change (i.e., slope) might be measured during isometric muscular tasks (Linssen et al., 1993; Bilodeau et al., 1994; Ng and Richardson, 1996; Merletti et al., 1998; Kollmitzer et al., 1999; Dedering et al., 2000; Rainoldi et al., 2001; Gonzalez-Izal et al., 2012), whereas to evaluate central components of fatigue, sEMG descriptors of MU synchronization may be used. An sEMG descriptor of the MU synchronization level is fractal dimension (FD), which shows high reliance on MU synchronization (Troiano et al., 2008; Mesin et al., 2009).
Based on the above, the purpose of the present trial was to evaluate the acute effects of a EAA rich mixture administration on force-generating capacity during maximal voluntary contraction (MVC) and peripheral and central myoelectric manifestations of fatigue (CV and FD rates of change, respectively) in the biceps brachii muscle, measured by multichannel sEMG after a RE fatiguing protocol.
Materials and Methods
Subjects
Twenty recreationally active males (age: 27 ± 6 years; body mass: 72.7 ± 7.50 kg; height: 1.76 ± 0.06 m) were recruited and completed the study. The recruitment phase took place within the month before the trial. Subjects were fully informed of the aims, risks, and discomfort associated with the investigation and provided their written informed consent to participate in this study, before completing a questionnaire to assess their health status. Inclusion criteria included physiological body weight and composition (adequate for sex, age, and physical activity level) and experience in RE training. Exclusion criteria included the presence of a medical condition (musculoskeletal, respiratory, or cardiac), more than 4 days per week of physical activity and the use of nutritional supplements in the month before the trial. The study was settled at the Sport Medicine Centre, University of Pavia, Voghera, Italy and it was approved by the local ethics committee of the Swiss Italian Health and Sociality Department, Switzerland. During tests the facility presented optimal conditions of temperature and humidity, 22°C and 50% respectively.
Study Design
This study was a double-blind randomized crossover placebo-controlled trial. Subjects received a EAA mix or an isocaloric placebo (PLA) in two successive trials separated by 7 days of washout.
Body Composition Assessment
Anthropometric characteristics were assessed during the recruitment phase and the day before the beginning of the first trial. The day before the trial, body weight and composition were re-assessed in order to verify that the anthropometric characteristics of all the participants had been maintained 24 h before the beginning of the procedures and to potentially exclude subjects that showed significant variations compared to the values measured during the recruitment phase. Body mass and height were measured using a mechanical scale with stadiometer (Seca Ltd., Hamburg, Germany) to the nearest 0.01 kg and 0.1 m, respectively. Body mass index (BMI) was calculated as body mass (kg) divided by stature (m) squared. Fat-free mass and fat mass were measured using a bioelectric impedance analysis (BIA EFG, Akern, Florence, Italy). Measurements were carried out in the fasted state within 30 min after urine voiding. Subjects lay in supine position with limbs extended away from the trunk and wore only light clothing but no shoes or socks. Active electrodes (BIATRODES, Akern, Italy) were placed on the right side on conventional metacarpal and metatarsal lines, whereas recording electrodes were placed in standard positions at the right wrist and ankle. The physical characteristics of the subjects are illustrated in Table 1.
Table 1.
Physical characteristics of subjects (n = 20).
| Anthropometrics | Mean ±SD | Range |
|---|---|---|
| Age (yrs) | 27 ± 6 | 21.0–39.0 |
| BM (kg) | 72.7 ± 7.50 | 58.0–82.1 |
| Height (m) | 1.76 ± 0.06 | 1.65–1.85 |
| BMI (kg⋅m-2) | 23.4 ± 2.03 | 20.1–25.2 |
| FM (kg) | 12.2 ± 3.11 | 8.10–19.4 |
| FFM (kg) | 56.5 ± 3.29 | 51.5–62.7 |
Values are mean ± SD. BM, body mass; BMI, body mass index; FM, fat mass; FFM, fat free mass.
Experimental Procedures
In both trials subjects refrained from strenuous physical activity and were discouraged from drinking alcohol, coffee, and beverages containing caffeine in the previous 24 h. The day of the experiment, 2 h before their arrival at the laboratory (fixed at 9:00 am), subjects had a standardized breakfast (rusks with jam; decaffeinated tea or juice). The trial consisted of the following steps: (1) basal blood lactate (BL1) determination; (2) first surface electromyography (sEMG-1) recording; (3) EAA or PLA ingestion; (4) after 2 h rest, a resistance exercise protocol (REP) was made; (5) post-REP blood lactate (BL2) determination; (6) second surface electromyography (sEMG-2) recording (Figure 1). An accurate simulation of the experimental procedures was performed 1 week before the first trial. This pre-trial session was conducted to allow the volunteers to familiarize themselves with all the procedures and to avoid an impairment of results caused by a “learning effect.”
FIGURE 1.
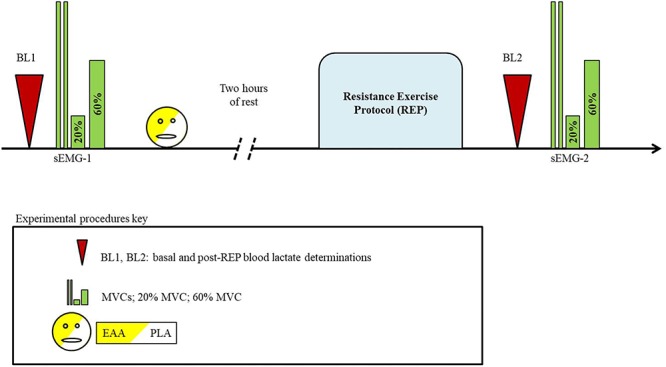
Schematic illustration of experimental procedures.
EAA Mix Administration Protocol
Participants ingested either a EAA mix, 0.15 g EAA powder/kg BM (Aminotrofic, Errekappa Euroterapici S.p.A., Milan, Italy), or a PLA (Aspartame based artificial sweetener powder with the same characteristics of appearance and flavor) both dissolved in 250 ml of water. EAA mix and PLA were administrated using an unmarked, coated sports drink bottle. Each administration was conducted in double blind fashion. Considering that a specific EAA dosage for improvement of fatigue is currently missing, we based our choice on the recent recommendations for EAA intake in athletes released by the International Society of Sports Nutrition (Jäger et al., 2017; Kerksick et al., 2017). We therefore investigated whether the dosage suggested for skeletal muscle adaptation could also be effective on RE performance and amelioration of fatigue. Artificial sweetener rather than a carbohydrate-based placebo was used to prevent a rise in insulin which could have altered the energetic metabolism. The EAA mix composition is described in Table 2.
Table 2.
Essential amino acids (EAA) mixture composition for a subject of 70 kg of body mass.
| Amino acids formula | Composition (g) | Composition (%) |
|---|---|---|
| Leucine | 3.30 | 31.4 |
| Lysine | 1.70 | 16.2 |
| Isoleucine | 1.64 | 15.6 |
| Valine | 1.64 | 15.6 |
| Threonine | 0.92 | 8.8 |
| Cysteine | 0.39 | 3.7 |
| Histidine | 0.39 | 3.7 |
| Phenylalanine | 0.26 | 2.5 |
| Methionine | 0.13 | 1.2 |
| Tyrosine | 0.08 | 0.8 |
| Tryptophan | 0.05 | 0.5 |
Surface Electromyography (sEMG) Recording Procedures
sEMG-1 and sEMG-2 recording procedures, before and after REP respectively, were carried out as follows: subjects’ dominant upper limb was fastened in a isometric-ergometer (MUC1, OT Bioelettronica, Turin, Italy) fitted with a load cell (CCT Transducer, linear, full scale 100 kg), in order to isolate the action of the biceps brachii. Participants were sitting, with the elbow at 120 degrees as shown in Figure 2.
FIGURE 2.
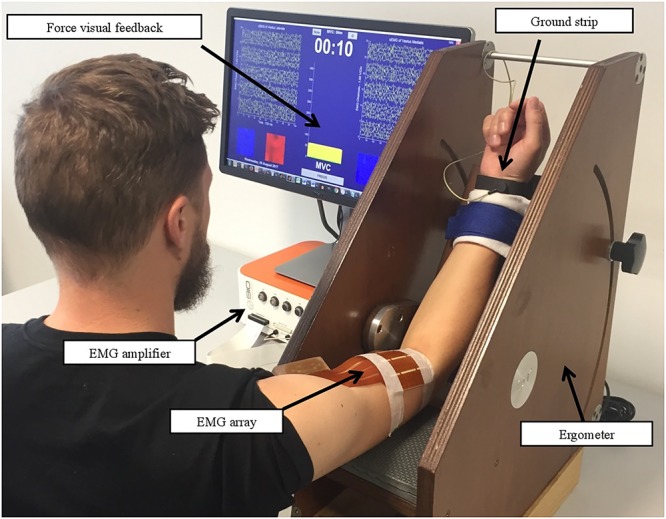
Setup of sEMG recording procedures. Written informed consent was obtained for the publication from the participant.
A 64-channel bidimensional array (10 mm IED, 8 lines, 8 columns) was positioned between the distal tendon and the innervation zone of the biceps brachii, with electrode columns parallel to the orientation of the muscle fibers in order to have a pure propagation of MUAPs. Biceps brachii was selected primarily to obtain high-quality sEMG signals due to the isolation of the muscle contraction, fluency of movement, and fiber orientation. The adhesive array was applied following muscle fiber leanings in correspondence to the muscle belly previously localized by ultrasound scan (Phillips CX-30). The sEMG signals were amplified (EMG-USB2+, OT Bioelettronica, Turin, Italy) and sampled at 2048 Hz.
Following 5 min rest, two isometric MVCs were completed, separated by 2 min rest. Two contractions were performed in order to consider the highest MVC value. Participants were instructed to increase the force as maximum as they can, and to hold it as steady as possible, for 2–3 s. Participants were given verbal stimulation.
Following 2 min rest, a low intensity sustained contraction (20% MVC) was performed for 90 s.
Following 4 min rest, subjects were asked to execute a high level sustained contraction (60% MVC) until exhaustion, during which they were verbally stimulated to keep the force level as long as possible, until the force value decreased to below 5% of the target (Merletti and Roy, 1996). At 60% of MVC the time to perform the task (TtT) was registered.
Signal Processing
Data were divided into 0.5 s periods and each variable was computed for each period. Exhaustion time was defined as the moment when force was below 5% of the target (Meduri et al., 2016). The regression line was computed for all the values from the beginning of contraction to exhaustion time. For each acquisition, the channels for the analysis were selected through visual inspection. The column showing the largest portion of propagating channels with the biggest amplitude was selected, and the channels between IZ and tendons were selected for CV computation. FD was computed for each selected channel and then averaged. FD initial value was estimated using the box counting method. Briefly, as expressed in Gitter and Czerniecki (1995), a grid of square boxes was used to cover the signal, and the number of boxes that the sEMG waveform passed through was counted. When decreasing the side of the boxes in a dichotomic process, the number of boxes required to cover the signal increased exponentially. However, by plotting the logarithm of the number of boxes counted (log N) vs. the logarithm of the inverse of the box size (log 1/S), the exponential relationship became linear. The slope of the interpolation line (estimated using the least mean squared procedure) is the FD. CV initial value (m/s) was estimated using a multichannel algorithm on double differential signals, based on the matching between signals filtered in the temporal and in the spatial domains (Farina and Merletti, 2003). CV values outside the physiological range (3–6 m/s) (Andreassen, 1987), were excluded from the analysis. CV and FD slopes were measured as rate of change (%) of CV and FD initial values.
Resistance Exercise Protocol (REP)
Resistance exercise protocol was developed to elicit fatigue in the biceps brachii and included a Wingate exercise (warm-up + 1 × 30 s) performed on an arms ergometer (Rehab Trainer 881E, Monark, Vansbro, Sweden), with a load corresponding to 5% of BM (Dotan and Bar-Or, 1983), followed by 3 sets of biceps curling with a dumbbell (Technogym, Gambettola, Italy) until exhaustion. Dumbbell load was calculated at 70% of the 1RM and a rest of 90 s between sets was held. Total time of REP was 362 ± 12.4 s. Indirect 1RM tests to establish the dumbbell load (with the use of Brzycki’s equation) were performed 1 week before the first trial. A week was considered appropriate to exclude any individual variability in relation to the time required for a complete muscle recovery and to resolve the Delayed Onset Muscle Soreness (DOMS). All subjects experienced fatigue in completing this protocol. REP specific outcomes measured include Wingate round per minute (WRPM) and biceps curls repetitions (BCRs).
Blood Lactate (BL) Determination
Blood sample was obtained from the earlobe and BL concentration was determined by a specific lactate detection device (Lactate Pro 2, Arkray, Kyoto, Japan).
Statistical Analysis
Shapiro–Wilk was used to test for normal distribution of all variables prior to analysis. A paired t-test was used in order to compare: (1) BCRs, WRPM, BL Δ%, TtT Δ% between EAA and PLA; (2) pre-REP and post-REP outcomes in EAA e PLA: BL, MVCs, TtT at 60% of MVC, CD and FD initial values and their rates of change (slopes). Significant mean differences of absolute values were used to calculate Cohen’s d as an effect size. Effect sizes were considered as follows: <0.2 = trivial; 0.2 to 0.49 = small; 0.5 to 0.79 = moderate; >0.8 = large.
Data were analyzed using Prism Graphpad (San Diego, CA, United States). Statistical significance was set at P < 0.05. Results are presented as mean ± standard deviation (SD).
Results
After REP a significant decrease of MVC value was observed in PLA (P < 0.05; effect size = 0.69), while no statistical differences were found in EAA (Table 3). REP outcomes showed no statistical differences for BCRs and WRPM between EAA and PLA, while statistical differences were found between BL1 (basal BL) and BL2 (post-REP) in both groups (P < 0.0001; EAA effect size = 3.38; PLA effect size = 4.12). A significant difference between EAA and PLA was found for BL Δ% (P < 0.05) (Table 4). Analysis of pre-REP and post-REP myoelectric manifestations of fatigue showed the following results: (1) no statistical differences in EAA and PLA for CV and FD initial values either at 20% MVC or 60% MVC; (2) at 60% MVC a significant increase of CV rate of change after REP was observed in PLA (P < 0.05; effect size = 0.80) but not in EAA; (3) no significant differences of FD slopes were found in either group either at 20% MVC or 60% MVC (Table 5).
Table 3.
pre-REP and post-REP values of MVC in EAA and PLA.
| EAA | PLA | ||
|---|---|---|---|
| MVC pre-REP | 19.6 ± 3.98 | MVC pre-REP | 19.5 ± 4.41 |
| MVC post-REP | 17.1 ± 5.27 | MVC post-REP | 16.3 ± 4.75∗ |
∗Statistically significant change of MVC between pre-REP and post-REP in PLA (P < 0.05). Values are mean ± SD. MVC, maximal voluntary contraction (kg); REP, resistance exercise protocol.
Table 4.
Resistance exercise protocol (REP) outcomes in EAA and PLA.
| REP Outcomes | EAA | PLA |
|---|---|---|
| BCRs | 7 ± 2.10 | 8 ± 3.20 |
| WRPM | 193 ± 12.7 | 191 ± 20.2 |
| BL1 (basal BL) | 1.17 ± 0.29 | 1.14 ± 0.14 |
| BL2 (post-REP) | 4.68 ± 1.44∗∗∗ | 5.01 ± 1.32∗∗∗ |
| BL Δ% | 242 ± 111 | 349 ± 138# |
∗∗∗Statistically significant change between BL1 and BL2 in EAA and PLA (P < 0.0001). #Statistically significant change of BLΔ% in PLA compared to EAA (P < 0.05). Values are mean ± SD. BCRs, biceps curls repetitions; WRPM, Wingate round per minute; BL, blood lactate concentration (mmol/L); BL Δ%, BL percentage difference between BL1 and BL2 in EAA and PLA (%).
Table 5.
pre-REP and post-REP values of sEMG derived myoelectric manifestations of fatigue (CV, FD initial values and their slopes).
| MVC % | CV initial value pre-REP EAA | CV initial value pre-REP PLA | FD initial value pre-REP EAA | FD initial value pre-REP PLA |
|---|---|---|---|---|
| 20% | 4.13 ± 0.42 | 4.13 ± 0.68 | 1.67 ± 0.02 | 1.67 ± 0.02 |
| 60% | 4.52 ± 0.36 | 4.48 ± 0.87 | 1.66 ± 0.03 | 1.68 ± 0.01 |
| MVC % | CV initial value post-REP EAA | CV initial value post-REP PLA | FD initial value post-REP EAA | FD initial value post-REP PLA |
| 20% | 4.32 ± 0.29 | 4.36 ± 0.72 | 1.66 ± 0.02 | 1.66 ± 0.01 |
| 60% | 4.87 ± 0.41 | 4.82 ± 0.32 | 1.67 ± 0.02 | 1.67 ± 0.01 |
| MVC % | CV slopes pre-REP EAA | CV slopes pre-REP PLA | FD slopes pre-REP EAA | FD slopes pre-REP PLA |
| 20% | −0.05 ± 0.04 | −0.08 ± 0.09 | −0.006 ± 0.009 | −0.008 ± 0.006 |
| 60% | −0.34 ± 0.31 | −0.36 ± 0.32 | −0.09 ± 0.06 | −0.09 ± 0.08 |
| MVC % | CV slopes post-REP EAA | CV slopes post-REP PLA | FD slopes post-REP EAA | FD slopes post-REP PLA |
| 20% | −0.08 ± 0.06 | −0.07 ± 0.05 | −0.01 ± 0.008 | −0.01 ± 0.01 |
| 60% | −0.34 ± 0.25 | −0.65 ± 0.40∗ | −0.088 ± 0.06 | −0.10 ± 0.076 |
∗Statistically significant change of CV slopes between pre-REP and post-REP in PLA (P < 0.05). Values are Mean ± SD. CV, conduction velocity (m/s); FD, fractal dimension. CV and FD slopes (%/s).
At 60% MVC, analysis of TtT between pre-REP and post-REP showed statistical decrease in EAA (P < 0.05; effect size = 0.72), but a significantly lower time was found in PLA (P < 0.001; effect size = 1.14). At 60% MVC, a significant difference for TtT Δ% between EAA and PLA was also found (P < 0.001) (Table 6).
Table 6.
pre-REP and post-REP values of TtT at 60% of MVC and TtT Δ%.
| EAA | PLA | ||
|---|---|---|---|
| TtT pre-REP | 49.4 ± 3.28 | TtT pre-REP | 47.4 ± 5.77 |
| TtT post-REP | 47.6 ± 1.26∗ | TtT post-REP | 40.4 ± 6.54∗∗ |
| TtT Δ% | −1.27 ± 11.8 | TtT Δ% | −13.9 ± 7.15# |
∗Statistically significant change between pre-REP and post-REP for TtT values in EAA (P < 0.05). ∗∗Statistically significant change between pre-REP and post-REP for TtT values in PLA (P < 0.001). #Statistically significant change for TtT Δ% in PLA compared to EAA (P < 0.001). Values are mean ± SD. TtT, time to perform the task (s) in pre-REP and post-REP; TtT Δ%, TtT difference percentage between pre-REP and post-REP in EAA and PLA (%).
Discussion
Change of Fatigue-Induced MVC
The initial aim of the present study was to examine the effects of EAA enriched mix consumption on MVC. MVC detection was used in order to sample the maximal force production during an isometric contraction and to establish how fatigue during and/or after REP could affect this muscle capacity. Our findings show that REP induced a significant decrease of MVC in PLA but not in EAA (Table 3). Previous works investigated the effects of acute amino acids supplementation on MVC showing positive effects (Shimomura et al., 2010; Howatson et al., 2012). However, it is important to note that these studies were designed to cause evident muscle damage rather than to evaluate acute demands in maintaining strength; in particular the capacity of nutritional intervention (BCAAs supplementation in both studies) to maintain MVC was detected several hours after RE bouts (24 h or more). As we known, strenuous RE bouts lead to a decrease of muscle function (strength and power) due to muscle damage that reaches a maximum about 24 h later and generally resolves in 48–72 h (Raastad and Hallén, 2000; Hoffman et al., 2010; Gee et al., 2012). In the studies from Shimomura et al. (2010) and Howatson et al. (2012), BCAAs supplementation showed positive effects on delayed post-exercise loss of MVC, probably due to protein synthesis stimulation, protein breakdown inhibition or both (Blomstrand et al., 2006; Zanchi et al., 2008; Nicastro et al., 2012). Conversely, in our experimental conditions, EAA mix consumption was able to reduce MVC loss measured within 15 min after REP, thus suggesting that this intervention may prevent early fatigue-induced MVC decrease.
TtT and Myoelectric Manifestations of Fatigue
We analyzed REP-induced changes of TtT failure, as a measure of endurance, and of myoelectric descriptors as indicators of peripheral (CV) and central fatigue (FD), during submaximal isometric contractions (60% MVC) to exhaustion.
Resistance exercise protocol was able to significantly change CV slope, unlike FD slope, thus suggesting that the adopted fatiguing protocol was able to determine the appearance of peripheral fatigue, unlike changes of MU synchronization associated with central fatigue.
A lower decrease of TtT and unchanged CV rate of change due to REP was observed in EAA compared to PLA (Tables 5, 6). In accordance with previous findings from our lab, the maintenance of CV slopes may relate to higher resistance to peripheral arising of fatigue that has a counterpart in TtT improvement (Meduri et al., 2016).
To the best of our knowledge, these data demonstrated, for the first time, the efficacy of acute EAA mix assumption in improving sEMG myoelectric descriptors of peripheral fatigue.
In fact, so far, only two studies from the same lab (Rahmani-Nia et al., 2013; Rahmani-Nia et al., 2014) have investigated the effects of prolonged (4 weeks) L-glutamine administration on sEMG parameters in order to correlate myoelectric outcomes (median frequency, MDF, and mean power frequency, MPF) to muscle damage (Zhou et al., 2011), but no measures of fatigue descriptors were carried out.
Potential Metabolic Role of EAA Mix Consumption
Even though EAA mix was unable to determine performance improvement in terms of number of BCRs and/or WRPM during REP, considering the effect on TtT and MVC a possible role on energy delivery during fatiguing task may be speculated. In our experimental conditions, intensity and duration of REP suggest that ATP/Creatine phosphate and glycolytic metabolisms were simultaneously involved in energy production to working muscles, as demonstrated by the observed subsequent moderate increase of blood lactate both in EAA and PLA (Table 4). Importantly, in EAA the increase of endurance was associated with less pronounced lactate percentage change, suggesting a potential contribution of nutritional intervention to muscle energetics, although we were not able to provide direct biochemical evidence of this assertion. At this stage, we can only underline that the role of EAA mix used in the present study in the regulation of ATP production/utilization is well established (Dioguardi, 2004; Pasini et al., 2004).
Apart from this possible mechanism, we cannot exclude that others relying on the presence of selected AA in the mixture may justify the effects here presented. In particular, as described in a previous study (Fernstrom, 2013), the ingestion of large neutral amino acids (LNAAs), notably tryptophan, tyrosine and BCAA, modifies tryptophan and tyrosine uptake into the brain and their conversion to serotonin and dopamine, respectively. This particular effect reflects the competitive nature of the transporter for LNAA at the blood–brain barrier. Typically, the administration of BCAA reduces not only tryptophan and serotonin in the brain, but also tyrosine and dopamine. This “dual effect” may have unwanted functional consequences: the positive effect on fatigue due to the serotonin reduction might be offset by the negative effects of dopamine reduction. Conversely, as reported by the same author (Fernstrom, 2013), BCAA administered in a mix with tyrosine could prevent the decline in dopamine, while still eliciting a drop in serotonin by reducing tryptophan brain uptake. However, since the concept of the ratio of serotonin to dopamine is closely related to central fatigue (Meeusen et al., 2006, 2007; Meeusen and Watson, 2007), and direct studies on the impact of this ratio on local fatigue in response to RE are lacking, it is only possible to speculate that an AA mixture containing LNAA could be effective in RE performance and improvement of fatigue based on neurotransmitters modulation.
Limitations
This study has limitations. In particular, we were unable to determine the blood profile changes of the EAA after their ingestion. However, other authors reported that an oral EAA administration, of a similar dose to that used in the present investigation, within the 3 h before an exercise bout, is followed by a significant increase of EAA blood concentration (Eaton et al., 2016). Furthermore we can only speculate on the exact biochemical role and the contribution to the observed effects of selected amino acids in the mixture. In addition, in support to the energy role of EAA, we were only able to detect lactate percentage change, as the variation of absolute values was not significant. This was caused by the high interindividual variability of lactate increase after the REP, probably due to differences in muscular adaptation to exercise in subjects. In fact, as shown in Table 4, the post-REP SD values were higher compared to basal values. Notwithstanding this, we believe that the lower rise of lactate percentage in EAA (compared to PLA) should be attributed to the effect of EAA on muscle energetics. Another limitation refers to FD here used as sEMG descriptor of central fatigue. Although FD is considered a reliable index that can be used in experimental studies focusing on fatigue, literature is currently lacking of studies on validity of this parameter in MU synchronization. Other procedures, such as twitch interpolation, based on electrical stimulation of nerve trunk or branches during MVC are widely recognized in assessing central failure during voluntary activation of skeletal muscle, but compared to sEMG are slightly more uncomfortable and less usable.
Conclusion
In order to delay fatigue and improve the overall quality of resistance training sessions, the use of pre-workout dietary supplements by both recreational and competitive athletes has been recently documented (Martinez et al., 2016; Jagim et al., 2016). This is the first study showing the efficacy of a pre-workout EAA mix administration on immediate post-RE decline of MVC, endurance capacity and peripheral muscle fatigue as evaluated with sEMG myoelectric descriptors. These data suggest that EAA-based compounds before power sport events or RE training sessions may be suitable to prevent muscle fatigue and to delay TtT failure.
Author Contributions
MN, VS, LCas, LCal performed the experiments. MN and GD’A conceived the original idea and wrote the paper. MB and CC analyzed the data.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by crowdfunding “#Sport4Therapy: together the mission is possible.”
References
- Andreassen S. (1987). Methods for computer-aided measurement of motor unit parameters. Electroencephalogr. Clin. Neurophysiol. Suppl. 39 13–20. [PubMed] [Google Scholar]
- Bigland-Ritchie B., Jones D. A., Hosking G. P., Edwards R. H. (1978). Central and peripheral fatigue in sustained maximum voluntary contractions of human quadriceps muscle. Clin. Sci. Mol. Med. 54 609–614. 10.1042/cs0540609 [DOI] [PubMed] [Google Scholar]
- Bilodeau M., Arsenault A. B., Gravel D., Bourbonnais D. (1994). EMG power spectrum of elbow extensors: a reliability study. Electromyogr. Clin. Neurophysiol. 34 149–158. [PubMed] [Google Scholar]
- Blomstrand E. (2006). A role for branched-chain amino acids in reducing central fatigue. J. Nutr. 136 544S–547S. 10.1093/jn/136.2.544S [DOI] [PubMed] [Google Scholar]
- Blomstrand E., Eliasson J., Karlsson H. K., Köhnke R. (2006). Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise. J. Nutr. 136 269S–273S. 10.1093/jn/136.1.269S [DOI] [PubMed] [Google Scholar]
- Bouissou P., Estrade P. Y., Goubel F., Guezennec C. Y., Serrurier B. (1989). Surface EMG power spectrum and intramuscular pH in human vastus lateralis muscle during dynamic exercise. J. Appl. Physiol. 67 1245–1249. 10.1152/jappl.1989.67.3.1245 [DOI] [PubMed] [Google Scholar]
- Brody L. R., Pollock M. T., Roy S. H., De Luca C. J., Celli B. (1991). pH-induced effects on median frequency and conduction velocity of the myoelectric signal. J. Appl. Physiol. 71 1878–1885. 10.1152/jappl.1991.71.5.1878 [DOI] [PubMed] [Google Scholar]
- Butts J., Jacobs B., Silvis M. (2018). Creatine use in sports. Sports Health 10 31–34. 10.1177/1941738117737248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I. F., Wu H. J., Chen C. Y., Chou K. M., Chang C. K. (2016). Branched-chain amino acids, arginine, citrulline alleviate central fatigue after 3 simulated matches in taekwondo athletes: a randomized controlled trial. J. Int. Soc. Sports Nutr. 13:28. 10.1186/s12970-016-0140-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A. K., Farmer S. F., Stephens J. A. (1991). Central nervous pathways underlying synchronization of human motor unit firing studied during voluntary contractions. J. Physiol. 432 401–425. 10.1113/jphysiol.1991.sp018391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca C. J. (1984). Myoelectrical manifestations of localized muscular fatigue in humans. Crit. Rev. Biomed. Eng. 11 251–279. [PubMed] [Google Scholar]
- Dedering A., Roos af Hjelmsater M., Elfving B., Harms-Ringdahl K., Nemeth G. (2000). Between-days reliability of subjective and objective assessments of back extensor muscle fatigue in subjects without lower-back pain. J. Electromyogr. Kinesiol. 10 151–158. 10.1016/S1050-6411(00)00009-2 [DOI] [PubMed] [Google Scholar]
- Dioguardi F. S. (2004). Wasting and the substrate-to-energy controlled pathway: a role for insulin resistance and amino acids. Am. J. Cardiol. 93(Suppl.) 6A–12A. 10.1016/j.amjcard.2003.11.002 [DOI] [PubMed] [Google Scholar]
- Dotan R., Bar-Or O. (1983). Load optimization for the Wingate Anaerobic Test. Eur J. Appl. Physiol. Occup. Physiol. 51 409–417. 10.1007/BF00429077 [DOI] [PubMed] [Google Scholar]
- Eaton T. R., Potter A., Billaut F., Panchuk D., Pyne D. B., Gore C. J., et al. (2016). A combination of amino acids and caffeine enhances sprint running capacity in a hot, 1 hypoxic environment. Int. J. Sport Nutr. Exerc. Metab. 26 33–45. 10.1123/ijsnem.2015-0108 [DOI] [PubMed] [Google Scholar]
- Enoka R. M., Duchateau J. (2008). Muscle fatigue: what, why and how it influences muscle function. J. Physiol. 586 11–23. 10.1113/jphysiol.2007.139477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina D., Merletti R. (2003). A novel approach for estimating muscle fiber conduction velocity by spatial and temporal filtering of surface EMG signals. IEEE Trans. Biomed. Eng. 50 1340–1351. 10.1109/TBME.2003.819847 [DOI] [PubMed] [Google Scholar]
- Fernstrom J. D. (2013). Large neutral amino acids: dietary effects on brain neurochemistry and function. Amino Acids 45 419–430. 10.1007/s00726-012-1330-y [DOI] [PubMed] [Google Scholar]
- Gee T. I., Deniel S. (2016). Branched-chain aminoacid supplementation attenuates a decrease in power-producing ability following acute strength training. J. Sports Med. Phys. Fit. 56 1511–1517. [PubMed] [Google Scholar]
- Gee T. I., Olsen P. D., Garland-Fritzdorf S. W., White D. G., Golby J., Kevin G., et al. (2012). Recovery of rowing sprint performance after high intensity strength training. Int. J. Sports Sci. Coach. 7 109–120. 10.3390/nu8080506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitter J. A., Czerniecki M. J. (1995). Fractal analysis of the electromyographic interference pattern. J. Neurosci. Methods 58 103–108. 10.1016/0165-0270(94)00164-C [DOI] [PubMed] [Google Scholar]
- Goldstein E. R., Ziegenfuss T., Kalman D., Kreider R., Campbell B., Wilborn C., et al. (2010). International society of sports nutrition position stand: caffeine and performance. J. ISSN 7:5. 10.1186/1550-2783-7-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Izal M., Malanda A., Gorostiaga E., Izquierdo M. (2012). Electromyographic models to assess muscle fatigue. J. Electromyogr. Kinesiol. 22 501–512. 10.1016/j.jelekin.2012.02.019 [DOI] [PubMed] [Google Scholar]
- Greer B. K., White J. P., Arguello E. M., Haymes E. M. (2011). Branched-chain amino acid supplementation lowers perceived exertion but does not affect performance in untrained males. J. Strength Cond. Res. 25 539–544. 10.1519/JSC.0b013e3181bf443a [DOI] [PubMed] [Google Scholar]
- Hoffman J. R., Ratamess N. A., Tranchina C. P., Rashti S. L., Kang J., Faigenbaum A. D. (2010). Effect of a proprietary protein supplement on recovery indices following resistance exercise in strength/power athletes. Amino Acids 38 771–778. 10.1007/s00726-009-0283-2 [DOI] [PubMed] [Google Scholar]
- Hoffman J. R., Varanoske A., Stout J. R. (2018). Effects of β-alanine supplementation on carnosine elevation and physiological performance. Adv. Food Nutr. Res. 84 183–206. 10.1016/bs.afnr.2017.12.003 [DOI] [PubMed] [Google Scholar]
- Holtermann A., Gronlund C., Karlsson J. S., Roeleveld K. (2009). Motor unit synchronization during fatigue: described with a novel sEMG method based on large motor unit samples. J. Electromyogr. Kinesiol. 19 232–241. 10.1016/j.jelekin.2007.08.008 [DOI] [PubMed] [Google Scholar]
- Howatson G., Hoad M., Goodall S., Tallent J., Bell P. G., French D. N. (2012). Exercise-induced muscle damage is reduced in resistance-trained males by branched chain amino acids: a randomized, double-blind, placebo controlled study. J. Int. Soc. Sports Nutr. 12 9–20. 10.1186/1550-2783-9-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäger R., Kerksick C. M., Campbell B. I., Cribb P. J., Wells S. D., Skwiat T. M., et al. (2017). International society of sports nutrition position stand: protein and exercise. J. Int. Soc. Sports Nutr. 14:20. 10.1186/s12970-017-0177-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagim A. R., Jones M. T., Wright G. A., St Antoine C., Kovacs A., Oliver J. M. (2016). The acute effects of multi-ingredient pre-workout ingestion on strength performance, lower body power, and anaerobic capacity. J. Int. Soc. Sports Nutr. 13:11. 10.1186/s12970-016-0122-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent-Braun J. A. (1999). Central and peripheral contributions to muscle fatigue in humans during sustained maximal effort. Eur. J. Appl. Physiol. Occup. Physiol. 80 57–63. 10.1007/s004210050558 [DOI] [PubMed] [Google Scholar]
- Kerksick C. M., Arent S., Schoenfeld B. J., Stout J. R., Campbell B., Wilborn C. D., et al. (2017). International society of sports nutrition position stand: nutrient timing. J. Int. Soc. Sports Nutr. 14:33. 10.1186/s12970-017-0189-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmitzer J., Ebenbichler G. R., Kopf A. (1999). Reliability of surface electromyographic measurements. Clin. Neurophysiol. 110 725–734. 10.1016/S1388-2457(98)00050-9 [DOI] [PubMed] [Google Scholar]
- Komi P. V., Tesch P. (1979). EMG frequency spectrum, muscle structure, and fatigue during dynamic contractions in man. Eur. J. Appl. Physiol. Occup. Physiol. 42 41–50. 10.1007/BF00421103 [DOI] [PubMed] [Google Scholar]
- Kreider R. B., Wilborn C. D., Taylor L., Campbell B., Almada A. L., Collins R., et al. (2010). ISSN exercise & sport nutrition review: research & recommendations. J. ISSN 7:7 10.1186/1550-2783-7-7 [DOI] [Google Scholar]
- Linssen W. H., Stegeman D. F., Joosten E. M., van’t Hof M. A., Binkhorst R. A., Notermans S. L. (1993). Variability and interrelationships of surface EMG parameters during local muscle fatigue. Muscle Nerve 16 849–856. 10.1002/mus.880160808 [DOI] [PubMed] [Google Scholar]
- Martinez N., Campbell B., Franek M., Buchanan L., Colquhoun R. (2016). The effect of acute pre-workout supplementation on power and strength performance. J. ISSN 13:29. 10.1186/s12970-016-0138-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meduri F., Beretta-Piccoli M., Calanni L., Segreto V., Giovanetti G., Barbero M., et al. (2016). Inter-gender sEMG evaluation of central and peripheral fatigue in biceps brachii of young healthy subjects. PLoS One 11:e0168443. 10.1371/journal.pone.0168443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeusen R., Watson P. (2007). Amino acid and the brain: do they play a role in “central fatigue”? Int. J. Sport Nutr. Exerc. Metab. 17 37–46. 10.1371/journal.pone.0168443 [DOI] [PubMed] [Google Scholar]
- Meeusen R., Watson P., Hasegawa H., Roelands B., Piacentini M. F. (2006). Central fatigue: the serotonin hypothesis and beyond. Sports Med. 36 881–909. 10.2165/00007256-200636100-00006 [DOI] [PubMed] [Google Scholar]
- Meeusen R., Watson P., Hasegawa H., Roelands B., Piacentini M. F. (2007). Brain neurotransmitters in fatigue and overtraining. Appl. Physiol. Nutr. Metab. 32 857–864. 10.1139/H07-080 [DOI] [PubMed] [Google Scholar]
- Merletti R., Bottin A., Cescon C., Farina D., Gazzoni M., Martina S., et al. (2004). Multichannel surface EMG for the non-invasive assessment of the anal sphincter muscle. Digestion 69 112–122. 10.1159/000077877 [DOI] [PubMed] [Google Scholar]
- Merletti R., Fiorito A., Lo Conte L. R., Cisari C. (1998). Repeatability of electrically evoked EMG signals in the human vastus medialis muscle. Muscle Nerve 21 184–193. [DOI] [PubMed] [Google Scholar]
- Merletti R., Roy S. (1996). Myoelectric and mechanical manifestations of muscle fatigue in voluntary contractions. J. Orthop. Sports Phys. Ther. 24 342–353. 10.2519/jospt.1996.24.6.342 [DOI] [PubMed] [Google Scholar]
- Mesin L., Cescon C., Gazzoni M., Merletti R., Rainoldi A. (2009). A bi-dimensional index for the selective assessment of myoelectric manifestations of peripheral and central muscle fatigue. J. Electromyogr. Kinesiol. 19 851–863. 10.1016/j.jelekin.2008.08.003 [DOI] [PubMed] [Google Scholar]
- Mikulski T., Dabrowski J., Hilgier W., Ziemba A., Krzeminsk K. (2015). Effects of supplementation with branched chain amino acids and ornithine aspartate on plasma ammonia and central fatigue during exercise in healthy men. Folia Neuropathol. 53 377–386. 10.5114/fn.2015.56552 [DOI] [PubMed] [Google Scholar]
- Ng J. K., Richardson C. A. (1996). Reliability of electromyographic power spectral analysis of back muscle endurance in healthy subjects. Arch. Phys. Med. Rehabil. 77 259–264. 10.1016/S0003-9993(96)90108-2 [DOI] [PubMed] [Google Scholar]
- Nicastro H., da Luz C. R., Chaves D. F., Bechara L. R., Voltarelli V. A., Rogero M. M., et al. (2012). Does branched-chain amino acids supplementation modulate skeletal muscle remodeling through inflammation modulation? Possible mechanisms of action. J. Nutr. Metab. 2012:136937. 10.1155/2012/136937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. M. (1998). Blood-brain barrier carrier-mediated transport and brain metabolism of amino acids. Neurochem. Res. 23 635–644. 10.1023/A:1022482604276 [DOI] [PubMed] [Google Scholar]
- Pasini E., Scarabelli T. M., D’Antona G., Dioguardi F. S. (2004). Effect of amino acid mixture on the isolated ischemic heart. Am. J. Cardiol. 93 30A–34A. 10.1016/j.amjcard.2003.11.007 [DOI] [PubMed] [Google Scholar]
- Raastad T., Hallén J. (2000). Recovery of skeletal muscle contractility after high- and moderate-intensity strength exercise. Eur. J. Appl. Physiol. 82 206–214. 10.1007/s004210050673 [DOI] [PubMed] [Google Scholar]
- Rahmani-Nia F., Farzaneh E., Damirchi A., Majlan A. S., Tadibi V. (2014). Surface electromyography assessments of the vastus medialis and rectus femoris muscles and creatine kinase after eccentric contraction following glutamine supplementation. Asian J. Sports Med. 5 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani-Nia F., Farzaneh E., Damirchi A., Shamsi-Majlan A. (2013). Effect of L-glutamine supplementation on electromyographic activity of the quadriceps muscle injured by eccentric exercise. Iran J. Basic Med. Sci. 16 808–812. [PMC free article] [PubMed] [Google Scholar]
- Rainoldi A., Bullock-Saxton J. E., Cavarretta F., Hogan N. (2001). Repeatability of maximal voluntary force and of surface EMG variables during voluntary isometric contraction of quadriceps muscles in healthy subjects. J. Electromyogr. Kinesiol. 11 425–438. 10.1016/S1050-6411(01)00022-0 [DOI] [PubMed] [Google Scholar]
- Schillings M. L., Hoefsloot W., Stegeman D. F., Zwarts M. J. (2003). Relative contributions of central and peripheral factors to fatigue during a maximal sustained effort. Eur. J. Appl. Physiol. 90 562–568. 10.1007/s00421-003-0913-4 [DOI] [PubMed] [Google Scholar]
- Semmler J. G. (2002). Motor unit synchronization and neuromuscular performance. Exerc. Sport Sci. Rev. 30 8–14. 10.1097/00003677-200201000-00003 [DOI] [PubMed] [Google Scholar]
- Shimomura Y., Inaguma A., Watanabe S., Yamamoto Y., Muramatsu Y., Bajotto G., et al. (2010). Branched-chain amino acid supplementation before squat exercise and delayed-onset muscle soreness. Int. J. Sport Nutr. Exerc. Metab. 20 236–244. 10.1123/ijsnem.20.3.236 [DOI] [PubMed] [Google Scholar]
- Smith A. E., Fukuda D. F., Kendall K. L., Stout J. R. (2010). The effects of a pre-workout supplement containing caffeine, creatine, and amino acids during three weeks of high-intensity exercise on aerobic and anaerobic performance. J. ISSN 7:10. 10.1186/1550-2783-7-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepto N. K., Shipperd B. B., Hyman G., McInerney B., Pyne D. B. (2011). Effects of high-dose large neutral amino acid supplementation on exercise, motor skill, and mental performance in Australian Rules Football players. Appl. Physiol. Nutr. Metab. 36 671–681. 10.1139/h11-073 [DOI] [PubMed] [Google Scholar]
- Troiano A., Naddeo F., Sosso E., Camarota G., Merletti R., Mesin L. (2008). Assessment of force and fatigue in isometric contractions of the upper trapezius muscle by surface EMG signal and perceived exertion scale. Gait Posture 28 179–186. 10.1016/j.gaitpost.2008.04.002 [DOI] [PubMed] [Google Scholar]
- Williams M. (2005). Dietary supplements and sports performance: amino acids. J. ISSN 2 63–67. 10.1186/1550-2783-2-2-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanchi N. E., Nicastro H., Lancha A. H., Jr. (2008). Potential antiproteolytic effects of L-leucine: observations of in vitro and in vivo studies. Nutr. Metab. 17 5–20. 10.1186/1743-7075-5-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Li Y., Wang R. (2011). Evaluation of exercise-induced muscle damage by surface electromyography. J. Electromyogr. Kinesiol. 21 356–362. 10.1016/j.jelekin.2010.09.009 [DOI] [PubMed] [Google Scholar]


