Abstract
Purpose
To investigate the relationship between intraocular pressure (IOP) and GABA receptors within the arcuate nucleus (ARC).
Methods
In the chronic high IOP rat model, ibotenic acid (IBO) was injected to induce impairment of the ARC, and IOP was measured at the 0, 1, 2, 3, and 4 week time points with a Tono-Pen. To assess the expression of GABA-A/B receptors within the ARC under persistent high IOP, we performed immunofluorescence (IF) and immunohistochemical (IHC) staining at 2 weeks and 4 weeks. Furthermore, we treated the ARC with GABA-A/B receptor antagonists separately, and IOP was evaluated, as well as retinal ganglion cell apoptosis in the chronic high IOP rat model. In the following induced high IOP animal model, the expression of GABA-A/B receptors within the ARC was evaluated in DBA/2J mice which developed progressive eye abnormalities spontaneously that closely mimic human hereditary glaucoma.
Results
Compared with the control group, statistically significant downregulation of IOP was noted due to the IBO injection into the ARC at the 2, 3, and 4 week time points (p<0.05). Persistent high IOP elicited increased expression of the GABA-A/B receptors in the ARC compared with the control group (p<0.01). In addition, treatment with GABA-A/B receptor antagonists separately caused a decrease in the IOP, along with reduced retinal ganglion cell apoptosis (p<0.01). In the DBA/2J mice, the expression of the GABA receptors was statistically significantly increased (p<0.01).
Conclusions
GABA-A/B receptors in the ARC may be involved in regulation of IOP, and pathologically high IOP affects the expression of GABA-A/B receptors in the ARC.
Introduction
Glaucoma is a neurodegenerative disease involving apoptosis of retinal ganglion cells and irreversible vision loss [1]. Glaucoma is the second leading cause of blindness in the world [2]. Multicenter studies have verified that ocular hypertension is the most important risk factor for retinal ganglion cell apoptosis in glaucoma. However, treatment aimed at reducing high intraocular pressure (IOP) failed to reverse the loss of retina ganglion cells. For this reason, understanding the pathological mechanisms underlying high IOP and how they can be therapeutically modulated are of key importance.
Increasing clinical and experimental evidence supports that primary open-angle glaucoma (POAG) is more than an ocular disease as it also affects the structures and function of the central nervous system (CNS), including visual areas and non-visual areas in the brain [3,4]. Carlo et al. indicated that anterograde transynaptic central damage of the visual pathway might be triggered by ganglion cell death [5]. However, the exact mechanism remains unknown, and the relation between IOP and the CNS seems to be complicated. As we all know, IOP is not a constant value but follows a 24-h circadian rhythm [6]. The suprachiasmatic nucleus (SCN), which plays various roles in regulating circadian activities and receives direct projections from retinal ganglion cells, appears to participate in regulation of fluctuations in IOP [7]. Guzman-Ruiz et al. observed that neuronal activity of the hypothalamic arcuate nucleus (ARC) could be stimulated by the SCN [8]. Moreover, unilateral electrical stimulation of the ARC caused a decrease in IOP probably in an opioid peptides–mediated way [9]. Thus, we speculate that in addition to the SCN, the ARC of the hypothalamus is associated with IOP.
The ARC contains not only neuroendocrine neurons but also projecting neurons for mediating different regions within and outside the hypothalamus. The projecting neurons are mainly composed of two groups: POMC/CART neurons and neuropeptide Y (NPY)/AgRP neurons, both of which contain GABA, an important inhibitory neurotransmitter in the central nervous system [10-13]. There are two types of GABA receptors. GABA-A receptors are ligand-gated chloride channels that include an active binding site and allosteric binding sites that make it possible for different drugs to modulate the activity of the receptors [14]. GABA-B receptors, composed of GABA-B 1 and GABA-B 2 subunits, belong to the G protein-coupled family [15]. GABA receptors within the ARC are implicated in many critical homeostatic mechanisms, such as thermoregulation, foraging, as well as blood pressure regulation which is under circadian rhythms similar to IOP [16-19]. Samuels reported that microinjection of bicuculline methiodide, a GABA-A receptors antagonist, into the dorsomedial/perifornical hypothalamic leads to a significant increase in IOP [20]. Interestingly, the expression of GABA-A receptors in the primary visual cortex (V1) was found to be downregulated in the chronic high IOP primate model [21]. Nevertheless, no study has analyzed the relationship between IOP and GABA receptors within the ARC. The aim of the present study was to investigate whether GABA receptors within the ARC are related to IOP.
Methods
Animals
We obtained 10-month-old male DBA/2J mice (J000671) from Nanjing Biomedical Research Institute of Nanjing University (Nanjing, China). Additionally, 6- to 8-week-old male Sprague Dawley (SD) rats weighing 200±20.0 g, and 10-month-old male C57BL/6J mice were obtained from the Experiment Animal Center of the Tongji Medical College, Huazhong University of Science and Technology (HUST; Wuhan, China). All animal procedures were approved by the Institutional Animal Care and Use Committee of the Huazhong University of Science and Technology according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the U.S. National Institutes of Health.
Animal grouping
Chronic high IOP rats versus normal SD rats
The chronic high IOP rats were randomly divided into three groups, and the two chronic high IOP groups were euthanized at 2 weeks and 4 weeks separately, while the normal group was euthanized at 4 weeks (n=6 in each group). Non-treated SD rats served as control.
IBO-microinjected rats versus PBS-microinjected chronic high IOP rats
The chronic high IOP rats were randomly divided into two groups, and the IOP of each group was measured at the 0, 1, 2, 3, and 4 week time points (n=6 in each group). Ibotenic acid (IBO; I2765, a neurotoxin) microinjection was performed 30 min before the episcleral veins were cauterized. The chronic high IOP rats injected with PBS (1X; 136 mM NaCl, 2.6 mM KCL, 8 mM Na2HPO4, 2 mM KH2PO4, pH7.4) served as control.
Antagonist-microinjected rats versus DMSO-microinjected chronic high IOP rats
The chronic high IOP rats were randomly divided into four groups with different reagents, and all of the rats were euthanized at 4 weeks post-injection. The four groups were gabazine + chronic high IOP rats, CGP55845 + chronic high IOP rats, dimethyl sulfoxide (DMSO) + chronic high IOP rats, and chronic high IOP rats (n=6 in each group). The chronic high IOP rats with the DMSO microinjection served as the control.
Ten-month-old DBA/2J mice versus 10-month-old C57BL/6J mice
The DBA/2J mice group served as a spontaneous model of glaucoma [22]. The non-treated 10-month-old C57BL/6J mice group served as the control (n=6 in each group).
Animal models
Chronic high IOP rat model
The rats were anesthetized with an intraperitoneal injection of 10% chloral hydrate. Three episcleral veins of bilateral eyes were exposed by making an incision through the conjunctiva and Tenon’s capsule at the limbal periphery of the eye. Selected veins were precisely cauterized. The eyes were treated topically with tobramycin during recovery (0.3% tobramycin, 1–2 drops per time, 3 times per day) [23].
Microinjection in the ARC
The microinjector was introduced into the target areas for stereotaxic injection (Appendix 1). All microinjections into the ARC were unilateral (right side). The volume of each injection was 1 μl, and the injection rate was 0.1 μl/min. The needle was retained in place for 2 min after the completed injection.
Measurement of IOP
IOP was measured in the eyes of conscious rats (anesthetized with 0.5% proparacaine hydrochloride eye drops) using the Tono-Pen (Tono-Pen XL; Medtronic Inc., Jacksonville, FL). Measurements were taken before the surgery and once a week after surgery until the rats were euthanized. All data were collected during the light period of the day. Each measurement was repeated three times and then averaged.
Animals euthanasia
Animals were placed in a transparent euthanasia chamber (40*30*25cm). CO2 (>99.999%) was provided with flow rate at 8 l/min untill animals death (CO2 gas was maintanend for another 2 minutes after no obvious sigh of breath was observed).
Reagents and antibodies
Chloral hydrate was purchased from Sinopharm Chemical Reagent Co (Shanghai, China). IBO (dissolved in PBS, 5 μg/μl). Gabazine (S106, GABA-A receptor antagonist, diluted in DMSO, 1 M), and CGP55845 (SML0594, GABA-B receptor antagonist, diluted in DMSO, 1 M) were purchased from Sigma-Aldrich Co (St. Louis, MO). Anti-GABA-A receptor alpha 1 (ab94585, mouse-origin monoclonal antibody, 1:200), anti-GABA-B receptor 1 (ab55051, mouse-origin monoclonal antibody, 1:200), and anti-Brn3a antibody (ab81213, rabbit-origin monoclonal antibody, 1:200) were obtained from Abcam (Cambridge, MA).
Tissue preparation
For tissue collection, transcardial perfusion was performed under deep chloral hydrate anesthesia with 100 ml normal saline followed by 400 ml ice-cold 4% (w/v) formaldehyde for 15 min as previously described [24]. The brains were removed and fixed in 4% paraformaldehyde overnight. According to the stereotaxic coordinates of the rodent, paraffin-embedded brains were precisely sectioned to 5-μm thickness at the location of the ARC [25,26]. Then, the eyes were fixed in 1.2% picric acid. Paraffin-embedded eyes were sliced to 5 μm thickness, and all sections containing all layers of the retina were used for further analysis.
Immunofluorescence and immunohistochemical staining
The brain sections were gently washed twice with PBS, preheated to 37 °C. Then, the sections were blocked for 1 h at room temperature in PBS containing 10% normal donkey serum and 0.1% Triton X-100. The sections were incubated with the corresponding primary antibodies (mouse monoclonal anti-GABA-A receptor alpha 1 antibody and mouse monoclonal anti-GABA-B receptor 1 antibody, 1:200, diluted in blocking buffer) separately, at 4 °C overnight in a humidity chamber. Following three 10-min gentle washes with PBS, the sections were incubated with fluorescein-isothiocyanate (FITC) anti-mouse antibody (1:200; Sigma-Aldrich) for 2 h at room temperature in a humidity chamber. Then, the sections were observed under a fluorescence microscope (Zeiss 510 Meta, Zeiss, Jena, Germany) with an omnichrome air-cooled helium/neon laser set to produce emissions at 488 nm.
For immunohistochemical staining, the sections were incubated with biotinylated secondary antibody. 3′, 3′-Diaminobenzidine (DakoCytomation, Carpinteria, CA) was used as a peroxidase substrate to develop the color brown, and subsequently, hematoxylin (Merck Ltd., Taipei, Taiwan, ROC) was used as a counterstain.
BRN3A and TUNEL staining
The retinal cross sections were washed twice with PBS and blocked for 1 h at room temperature in blocking buffer. Then the sections were incubated with the corresponding primary antibody anti-BRN3A antibody (ab81213, rabbit-origin monoclonal antibody, 1: 200, diluted in blocking buffer) at 4 °C overnight in a humidity chamber. After washing with PBS, the sections were incubated with the secondary antibody for 2 h at room temperature in a humidity chamber. Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNE) staining was performed according to the manufacturer’s protocol (Sangon Biotech, Shanghai, Co., Ltd.).
Quantitative image analysis
The ARC lies around the third ventricle and contains five parts. In different levels, the size and shape of the ARC are slightly different except the medial part. There are almost no changes in the size, form, and even the location in the middle. For the analysis, an identical oval representing the medial part was used in all figures to make the data more accurate and convincing. The transverse diameter and the vertical diameter of the oval were 184 μm and 127 μm while the angle between the oval major axis and the horizontal axis was 26.4°.
The expression of the GABA-A/B receptors was visualized with immunohistochemical staining (IHC) or immunofluorescence (IF) staining in brain sections from six mice and rats in each group. Using the same microscope and camera settings, at least four digital images per section were taken to reflect the overall staining in the pons region of the brain. For the ARC, images at 20X were used for analysis using Image Pro Plus 4.0 (Media Cybernetics, Silver Spring, MD). Cells with a green or brown ring shown in the oval circle were identified as positive cells. The total immunoreactivity of the selected immunopositive area was divided by the area size, and the values relative to that of the control group are presented in the histograms. The proposed method allowed numerical analysis of the immunoreactivity. The sum immunoreactivity of the ARC was analyzed. For BRN3A and TUNEL staining, four sections were acquired per eye, and images from six random fields (40X) were taken for positive cells counting in the ganglion cell layer (GCL).
Statistical analysis
The data were analyzed using SPSS version 20.0 software (IBM Corporation, Armonk, NY). The differences between two groups were assessed using the Mann–Whitney U test. A p value of less than 0.05 was considered statistically significant in all cases. All data for cell counting was reported with mean ± standard error of the mean (SEM).
Results
Effect of elevated IOP on retina ganglion cells in the chronic high IOP rat model
To evaluate the effectiveness of the chronic high IOP rat model used in this study, the initial IOP of the control group and that of the two chronic high IOP groups were measured at 2 weeks and 4 weeks. IOP was statistically significantly elevated 2 weeks and 4 weeks after the episcleral vein cauterization surgery compared to the control (*p=0.037; **p=0.004; Figure 1A). BRN3A and TUNEL staining was performed to observe the morphologic changes in the retina (Figure 1B). Statistical analysis of the BRN3A or TUNEL staining positive cell numbers showed a statistically significant reduction induced by the persistent high IOP compared with the control (*p<0.01; Figure 1C,D). These data indicate that the animal models were successfully established.
Figure 1.
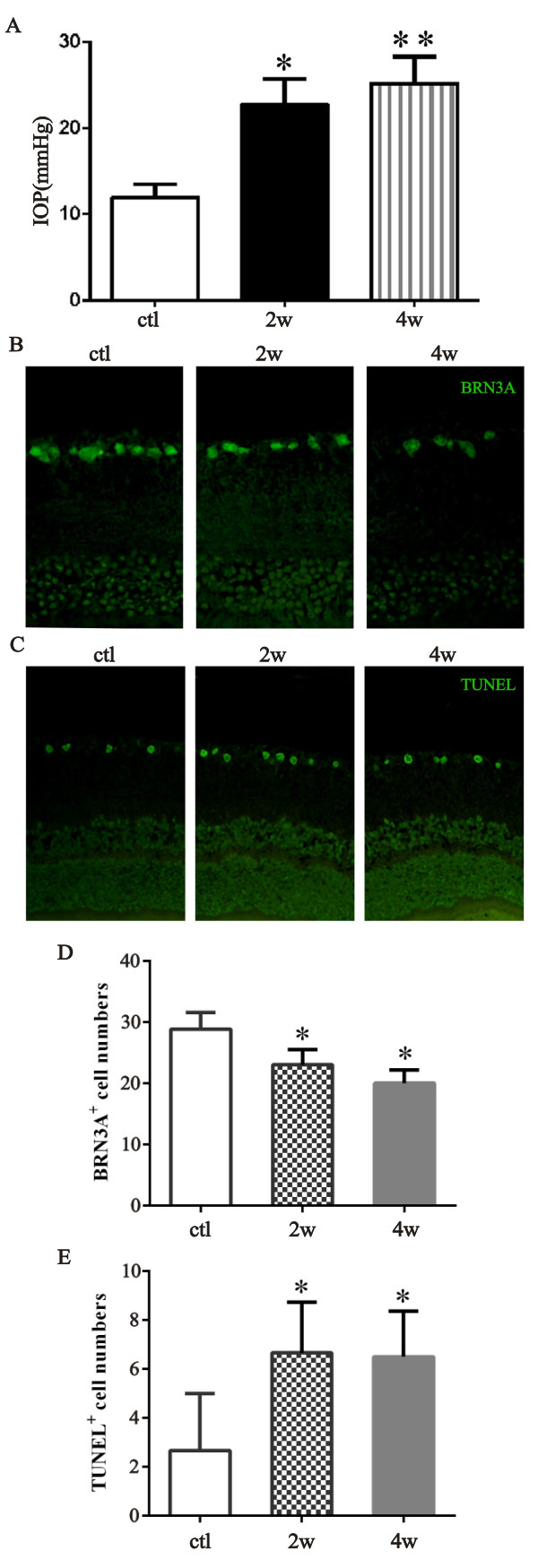
Effect of elevated IOP on retinal ganglion cells in the chronic high IOP rat model. A: The bar graph illustrates the intraocular pressure (IOP) of two chronic high IOP rat groups and the control group (ctl versus 2 weeks, *p=0.037; ctl versus 4 weeks, **p=0.004). B and C: The retinal cross sections were stained with BRN3A or terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL). D and E: The bar graph presents the number of BRN3A- or TUNEL-positive cells in the ganglion cell layer (GCL; ctl versus 2 weeks, *p<0.01; ctl versus 4 weeks, *p<0.01). The data are expressed as mean ± standard error of the mean (SEM), n=6 per group. ctl: normal untreated group, 2w: chronic high IOP rats group euthanized 2 weeks after the surgery, 4w: chronic high IOP rat group euthanized 4 weeks after the surgery.
Effect of IBO injection into the ARC on IOP of the ipsilateral eye in the chronic high IOP rat model
IBO is an excitatory neurotoxin that is widely used to cause neurochemical lesions to the central nerve system [27,28]. Here, IBO injection induced impairment of the ARC in the chronic high IOP rat model. Hematoxylin and eosin staining (H&E staining) was performed at 4 weeks after the microinjection indicating degeneration of cells around the injection site as shown in Appendix 1. Compared with the vehicle group, statistically significant downregulation of ipsilateral IOP was noted due to impairment of the ARC at the 2, 3, and 4 week time points (**p<0.01; Figure 2). No statistically significant difference was observed between the PBS-injected chronic group and the chronic group. The IOP of the contralateral eyes was statistically significantly decreased at 3 weeks and 4 weeks (p<0.05) post IBO injection, but to a slighter degree than the ipsilateral eye (Appendix 2).
Figure 2.
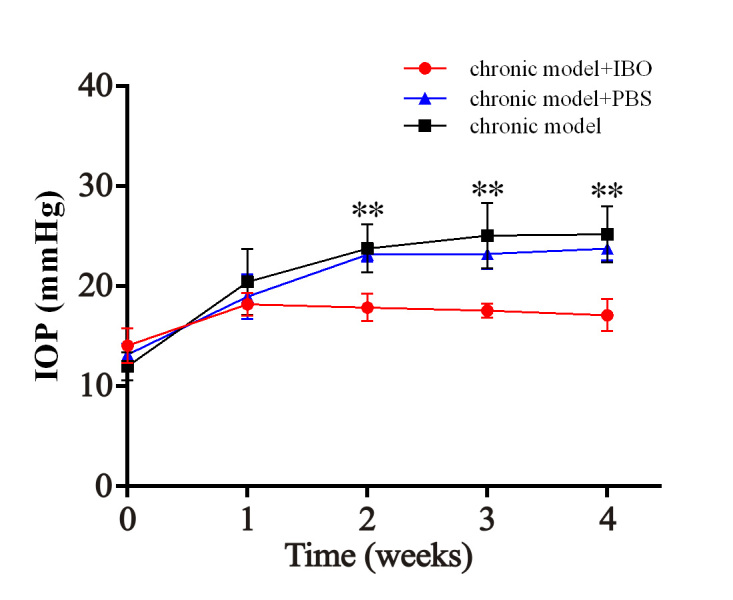
Effect of IBO injection into the ARC on IOP in chronic high IOP rat model. The intraocular pressure (IOP) of the ipsilateral eye in the ibotenic acid (IBO)-treated group and the control group at the 0, 1, 2, 3, and 4 week time points (chronic model group + PBS versus chronic model + IBO group, **p<0.01). The red line: chronic model + IBO group; the black line: chronic model group; the blue line: chronic model group + PBS group. The data are expressed as mean ± standard error of the mean (SEM), n=6 per group.
The expression of GABA receptors in the chronic high IOP rat model
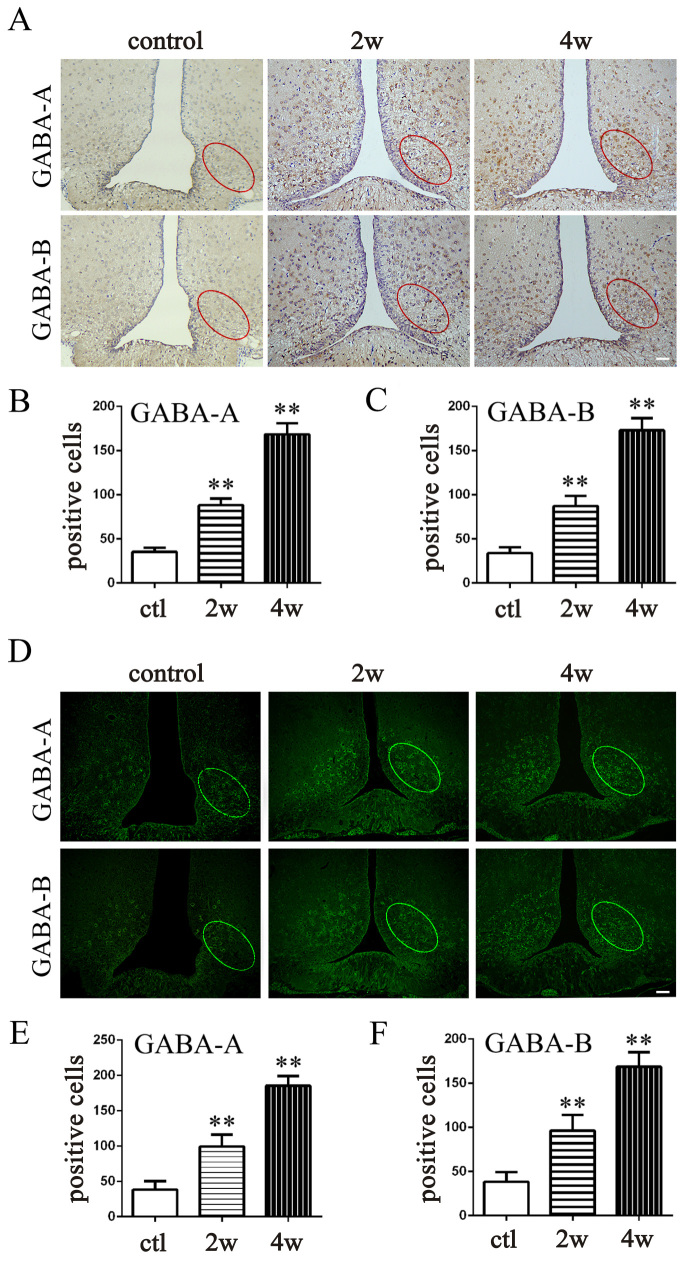
To validate the potential relationship between GABA receptors in the ARC and IOP, we performed IF and IHC (Figure 3A,D). In the chronic high IOP groups (at 2 weeks and 4 weeks), the number of positive cells in the selected oval area was statistically significantly increased (Figure 3B,C,E,F) compared with the control (p<0.01 for all). The expression of the GABA receptors in the ARC was upregulated in response to the persistent high IOP.
Figure 3.
The expression of GABA receptors in the ARC in the chronic high IOP rat model. A: Immunohistochemical (IHC) staining of GABA-A receptors and GABA-B receptors in brain sections at the location of the arcuate nucleus (ARC). B and C: Counting of positive cells in IHC-stained sections with Image J (ctl versus 2 weeks, **p<0.01; ctl versus 4 weeks, **p<0.01). D: Immunofluorescence (IF) staining of GABA-A receptors and GABA-B receptors in brain sections at the location of the ARC. E and F: Counting of positive cells in IF-stained sections with Image J (ctl versus 2 weeks, **p<0.01; ctl versus 4 weeks, **p<0.01). The data are expressed as mean ± standard error of the mean (SEM), n=6 per group. Bar=50 μm. ctl: normal untreated rat, 2w: chronic high IOP rat group euthanized 2 weeks after the surgery, 4w: chronic high IOP rats group euthanized 4 weeks after the surgery.
Effect of GABA-A/B receptors antagonist injection into the ARC on the IOP of the ipsilateral eye and retinal ganglion cells in the chronic high IOP rat model
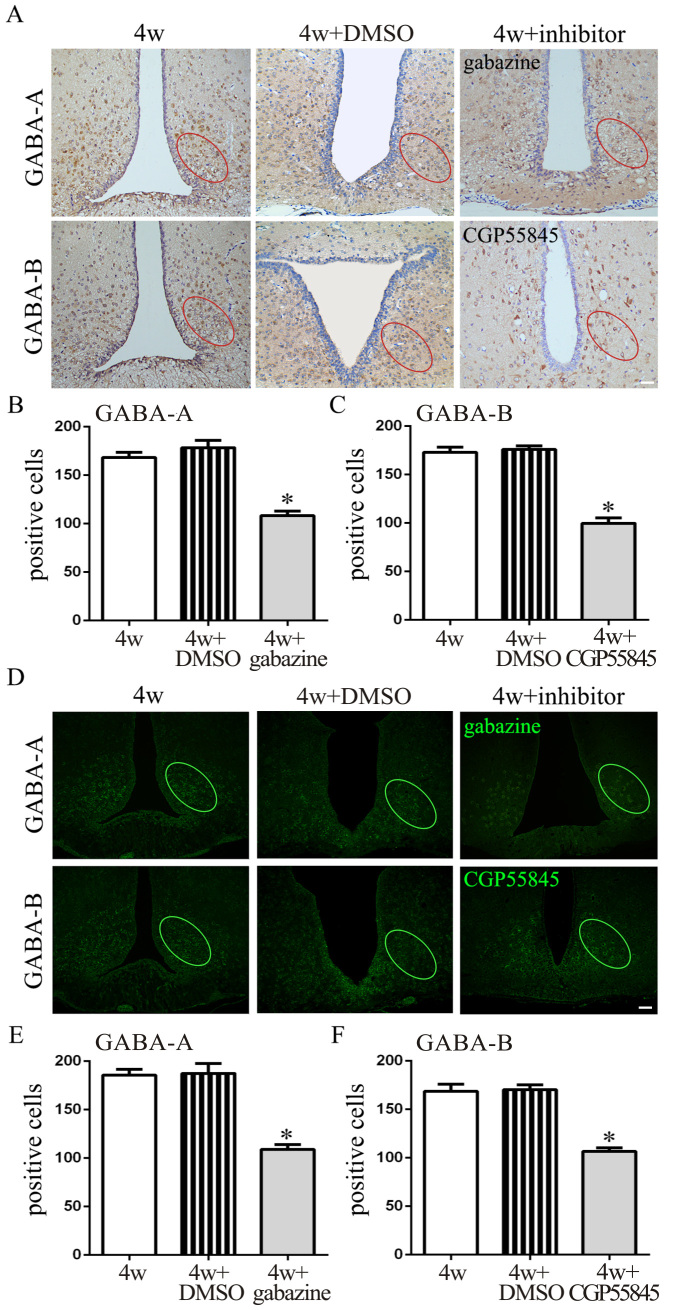
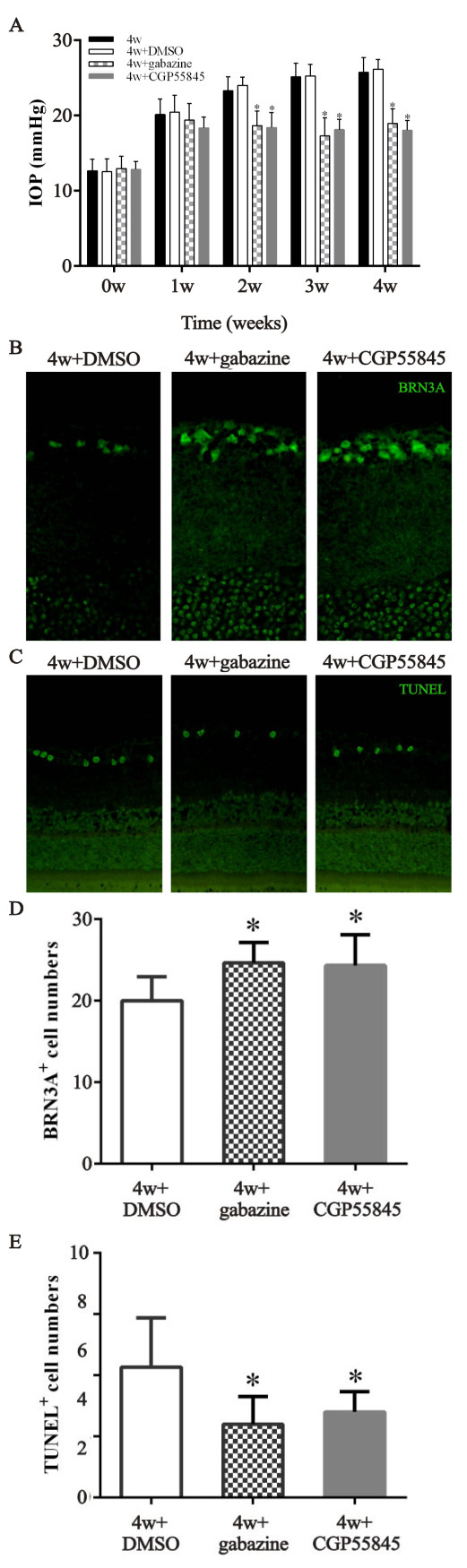
To further investigate the function of GABA receptors in modulating IOP, GABA-A and GABA-B receptor antagonists were injected into the ARC separately (30 min before the chronic high IOP rat model was generated). Gabazine serves as an allosteric inhibitor of GABA-A receptors resulting in channel opening and inhibition of chloride influx [29]. CGP55845 selectively blocks GABA-B receptors resulting in modulation of inwardly rectifying K+ channels (GIRKs) and the release of neurotransmitters [30]. The IF and IHC staining (Figure 4A,D) of the brain slices revealed that gabazine decreased the expression of GABA-A receptors in the ARC compared to the 4 week and 4 week + DMSO groups. The same decrease in the expression of the GABA-B receptors occurred in the rats injected with CGP55845 (**p<0.01; Figure 4B,C,E,F). As for IOP of the ipsilateral eye, a statistically significant decrease (p<0.01) was observed in the groups treated with gabazine or CGP55845 at the 2, 3, and 4 week time points compared to the control (Figure 5A). In addition, GABA antagonists were injected 2 weeks after high IOP was induced. As shown in Appendix 3, gabazine and CGP55845 statistically significantly decreased the IOP of the ipsilateral eyes compared to the 2 week + DMSO group at the 3 and 4 week time points (p<0.05; Appendix 3). The retinal cross sections stained with BRN3A and TUNEL (Figure 5B,C) showed increased cell numbers and less apoptosis of retinal ganglion cells in the two antagonist groups than in the control group (p<0.01; Figure 5D,E).
Figure 4.
Effect of antagonists on the expression of GABA receptors in the ARC in the chronic high IOP rat model. A: Immunofluorescence (IF) staining of GABA-A receptors and GABA-B receptors in brain slices at the location of the arcuate nucleus (ARC). B and C: Counting of positive cells in immunohistochemical (IHC)-stained sections with Image J (4 weeks + dimethyl sulfoxide (DMSO) versus 4 weeks + gabazine, *p<0.01; 4 weeks + DMSO versus 4 weeks + CGP55845, *p<0.01). D: IF staining of GABA-A receptors and GABA-B receptors in brain sections at the location of the ARC. E and F: Counting of positive cells in IF-stained sections with Image J (4 weeks + DMSO versus 4 weeks + gabazine, *p<0.01; 4 week + DMSO versus 4 weeks + CGP55845, *p<0.01). The data are expressed as mean ± standard error of the mean (SEM), n=6 per group. Bar=50 μm. 4w: chronic high intraocular pressure (IOP) rats without injection, 4w + DMSO: chronic high IOP rats with DMSO injection; 4 weeks + inhibitor: chronic high IOP rats with gabazine or CGP55845 injection.
Figure 5.
Effect of GABA receptor antagonists on the IOP and retinal ganglion cells of the ipsilateral eye in the chronic high IOP rat model. A: Intraocular pressure (IOP) was measured once a week after surgery (4 weeks + dimethyl sulfoxide (DMSO) versus 4 weeks + gabazine, *p<0.01; 4 weeks + DMSO versus 4 weeks + CGP55845, *p<0.01). B and C: The retinal cross sections were stained with BRN3A or terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL). D and E: The number of BRN3A- and TUNEL-positive cells (4 weeks + DMSO versus 4 weeks + gabazine, *p<0.01; 4 weeks versus 4 weeks + CGP55845, *p<0.01). The data are expressed as mean ± standard error of the mean (SEM), n=6 per group. 4w + gabazine group: with gabazine injection into the ARC, 4w + CGP55845 group: chronic high IOP rats with gabazine injection, 4w + DMSO: chronic high IOP rats with DMSO injection, 4w group: chronic high IOP rats without injection.
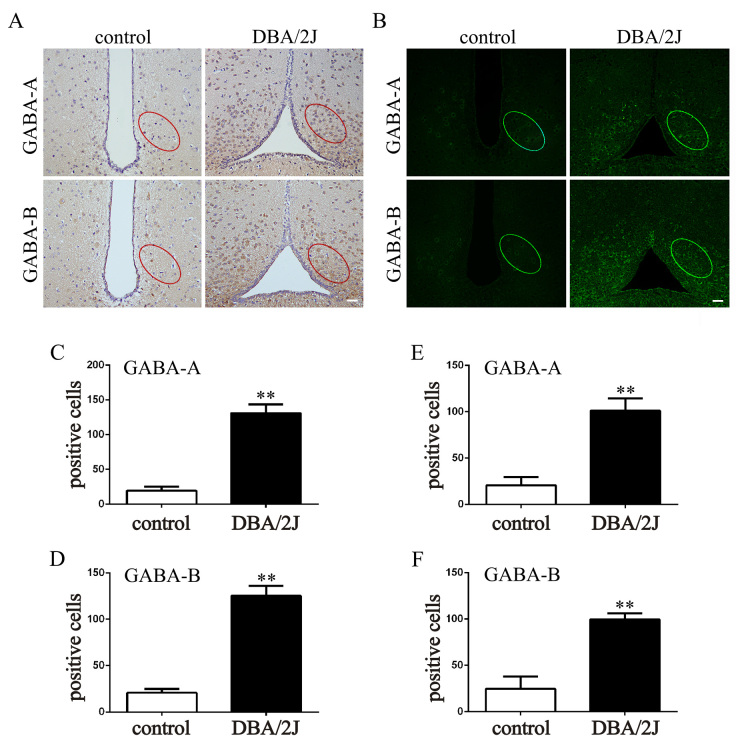
The expression of GABA receptors in DBA/2J mice
Following the induced high IOP animal model, attention was paid to the DBA/2J mice that developed progressive eye abnormalities spontaneously that closely mimic human hereditary glaucoma. The DBA/2J mice showed increased IOP from 6 to 16 months caused by blocking of the aqueous outflow pathway [31-33]. The IOP of the DBA/2J mice used in the present study was statistically significantly higher than that of the age-matched C57BL/6J mice (p<0.01; Appendix 4). In the normal control, the GABA receptors were expressed at low levels in the ARC. Compared to the control group, the expression of the GABA receptors was statistically significantly increased in the DBA/2J mice (p<0.01; Figure 6A–F). The effect of the GABA antagonists on the IOP of the ipsilateral eye was also tested. The IOP of the ipsilateral eye was statistically significantly decreased at 2, 3, and 4 weeks after treatment with gabazine or CGP55845 compared to that in the DMSO group (p<0.01; Appendix 5).
Figure 6.
The expression of GABA receptors in the ARC in DBA/2J mice. A: Immunohistochemical (IHC) staining of GABA-A receptors and GABA-B receptors in brain sections at the location of the arcuate nucleus (ARC). B: Immunofluorescence (IF) staining of GABA-A receptors and GABA-B receptors in brain sections at the location of the ARC. C and D: Counting of positive cells in IHC-stained sections with Image J (control versus DBA/2J mice: **p<0.01). E and F: Counting of positive cells in IHC-stained sections with Image J (control versus DBA/2J mice: **p<0.01). The data are expressed as mean ± standard error of the mean (SEM), n=6 per group. Bar=50 μm.
Discussion
The exact role of the CNS in the development of glaucoma has been the focus of research, but the role is still obscure. Functional magnetic resonance imaging (fMRI) provides a non-invasive method for assessing changes in the CNS induced by glaucoma [3]. However, MRI studies have failed to provide more detailed evidence for pathological alterations in the CNS. The present study first presented the increase in the expression of GABA receptors in the ARC in chronic high IOP models. We observed that the unilaterally impaired ARC (induced with ibotenic acid) reduced IOP in rats with chronic high IOP. Additionally, administration of GABA receptor antagonists in the ARC led to a decrease in IOP, along with less retinal ganglion cell apoptosis. Three different rodent strains (SD rats, C57BL/6J mice, and DBA /2J mice) and two pathologically high IOP models (the induced chronic high IOP model and the spontaneous high IOP model) were included. Although diversity existed in both strains and models, all the experimental animals exposed to persistent high IOP showed a statistically significant increase in expression of GABA receptors in the ARC. In sum, the ARC contributes to regulation of IOP in which GABA receptors might be involved, and the increase in the expression of GABA receptors in the ARC is found to be one of the alterations in the CNS evoked by pathologically elevated IOP.
IOP is determined by the balance between secretion and elimination of the aqueous humor (AH). In physiologic conditions, aqueous humor is secreted by the ciliary body and then outflows mainly through the conventional route (via the iridocorneal angle, the trabecular meshwork, Schlemm’s canal, and the episcleral venous) and the uveoscleral route (via the iris root, suprachoroidal spaces, and the sclera). Previous studies have revealed that AH dynamic relevant tissues, responsible for the fluctuations in IOP, are innervated by parasympathetic and sympathetic fibers [34]. A portion of the parasympathetic fibers arises from the Edinger-Westphal nucleus (EW) and then project to the ciliary ganglion before joining the ciliary nerves [35]. The ciliary muscles and sphincter pupillae are under the control of these cholinergic fibers. When the parasympathetic fibers are stimulated, acetylcholine is released which results in contraction of the ciliary muscle followed by an increase in AH outflow [36]. Regarding sympathetic innervation, fibers originating from the intermediolateral cell column (IML) of the spinal cord project to the superior cervical ganglion (SCG) accompanied by the sympathetic trunk, and terminate at the dilator pupillae, ciliary body blood vessels, the ciliary epithelium, and the trabecular meshwork [32].
However, little is known about the mechanisms for central control of IOP. The ARC is located in the middle of the hypothalamus and is generally accepted as the center of many physiologic homeostases. The ARC contains not only neuroendocrine neurons but also projecting neurons mainly for mediating different regions of the hypothalamus or other regions outside the hypothalamus. The projecting neurons contain different neuroactive peptides, such as oc-melanocyte-stimulating hormone or neuropeptide Y (NPY), as well as GABA [10,37,38]. GABA receptors within the ARC are involved in the regulation of body homeostasis, including thermoregulation, foraging, and blood pressure regulation [16-19]. Projections from the ARC to the EW and the IML have been observed via the retrograde trace method [39,40]. Building on this, we speculate that two pathways might make it possible for the ARC to contact with the eye (ARC-EW-ciliary ganglion-eye and ARC-IML-SCG-eye). Antagonism of GABA receptors in the ARC probably elicits alterations in sympathetic and parasympathetic activity, and further changes the level of neurotransmitters in the eye tissues to negatively modulate IOP. Although the reduction effect of GABA inhibitors on pathologically elevated IOP was notable, the IOP in the chronic glaucoma model was still beyond the normal range. That may be attributed to the aqueous humor outflow resistance which existed throughout the study. In addition, the potential regulation of the ARC on IOP is only a fraction of the CNS influences. Other nuclei, such as the SCN and the dorsomedial/perifornical hypothalamus (DMH/Pef), were previously shown to be related to IOP. Whether the ARC acts directly on IOP or through the SCN and the DMH/Pef requires further research.
Jin et al. reported that unilateral electrical stimulation and administration of an opioid peptide receptor agonist in the ARC caused a reduction in IOP in rabbits under physiologic conditions [9]. In the present study, we focused on the GABA receptors within the ARC, and two pathological animal models were performed. Although the ARC was treated with IBO, statistically significant alterations in IOP were observed, which appeared to be additional evidence that the ARC plays a role in regulating IOP. According to Jin et al.’s study, decrease in IOP evoked by electrical stimulation of the ARC could be eliminated through subconjunctival injection of naloxone (the antagonist of opioid peptide receptors), whereas the decrease caused by the injection of an ARC opioid peptide receptors agonist could not. These observations suggest that chemical and electrical stimulation of the ARC affect IOP in different mechanisms. As we did not interfere with the function of GABA in eye tissues, it remained difficult to distinguish exactly which peripheral neurotransmitter reacts to the injection of the GABA receptors antagonist in the ARC. In combination with the present study, either GABA or opioid peptide receptors in the ARC are involved in this regulatory function, which implies the participation of complicated multiple neurotransmitter mechanisms.
Similar to IOP, blood pressure shows circadian variations. Fluctuations in blood pressure have been attributed to the changes in sympathetic nerve activity (SNA) and circulating catecholamine levels [41]. Previous studies revealed that ARC neurons containing beta-endorphin, GABA, and NPY project to the ipsilateral paraventricular nucleus (PVN), and all three neurotransmitters elicit inhibitory effects on neurons. The ARC might regulate SNA and blood pressure via a paraventricular nucleus–mediated pathway [42]. As for IOP, it was found that the GABA antagonist (bicuculline) injection into the DMH/Pef produces increases in IOP and translaminar pressure (the pressure difference between IOP and intracranial pressure) in normal rats [20]. In contrast, the GABA antagonists injected into the ARC reduced IOP in the chronic high IOP rats in the present study. As the ARC is capable of inhibiting the SNA through NPY fibers projecting to the DMH [43], which contains parasympathetic neurons, there could be a new hypothesis different from the two direct pathways. The DMH/Pef may serve as a connection between the ARC and sympathetic innervations of the eye.
The limitation of this study is that the results were based on biochemical experiments. To investigate the exact role of GABA receptors within the ARC with respect to regulation of IOP, additional elaboration research approaches, such as radionuclide scanning [44,45] or the neural loop tracing technique [46,47], are needed. However, the nerve fiber networks in the brain are so complex that we might not be able to determine whether an even more intricate downstream pathway associated with other nuclei, such as the suprachiasmatic nucleus and the dorsomedial/perifornical hypothalamus, exists. In conclusion, this study showed that elevated IOP induces the expression of GABA receptors in the ARC, and in turn, blocking the GABA receptors within the ARC decreases IOP and prevents retinal ganglion cell apoptosis.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (grant numbers 81471744, 81770921), and grant from Huazhong University of Science and Technology (HUST grant number 2015TS142). Dr. Hong Zhang (tjyksys@163.com) and Yin Zhao (zhaoyin85@hotmail.com) are co-corresponding authors for this paper.
Appendix 1. The anatomy of ARC in mice and rats.
The anatomical schematic diagram in A and B separately showed the location of ARC in rats and mice. The elliptical red area indicated the scope of counting. A hematoxylin and eosin (H&E) staining of brain slice showed the injection site (pointed by the red arrow in C) in the ARC. Vε indicated the third ventricle of mice. To access the data, click or select the words “Appendix 1.”
Appendix 2. Effect of IBO injection into the ARC on IOP of contralateral eye in chronic high IOP rats model.
The IOP of the contralateral eye in the IBO-treated group, PBS-treated group and non-microinjection group at time points of 0, 1 , 2, 3 and 4w (chronic model group + PBS vs chronic model + IBO group, P < 0.05 *). The red line: chronic model + IBO group; the blue line: chronic model + PBS group; the black line: chronic model group. The data were expressed as mean±SEM, n=6 per group. To access the data, click or select the words “Appendix 2.”
Appendix 3. Effect of GABA receptor antagonists on IOP in rats 2 weeks post high IOP induction.
IOP of ipsilateral eye was recorded (gabazine vs DMSO, CGP55845 vs DMSO, P < 0.05 *) and expressed as mean±SEM, n=6 per group (P<0.05 *). To access the data, click or select the words “Appendix 3.”
Appendix 4. IOP of DBA/2J mice.
DBA/2J mice vs C57 mice, P < 0.01 *. The data were expressed as mean±SEM, n=6 per group. To access the data, click or select the words “Appendix 4.”
Appendix 5. Effect of GABA receptor antagonists on IOP of the ipsilateral eye in DBA/2J mice.
IOP was measured once a week after surgery (gabazine vs DMSO, CGP55845 vs DMSO, P < 0.01 *). The data were expressed as mean±SEM, n=6 per group. To access the data, click or select the words “Appendix 5.”
References
- 1.Quigley HA. Glaucoma. Lancet. 2011;377:1367–77. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- 2.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Song Y, Mu K, Wang J, Lin F, Chen Z, Yan X, Hao Y, Zhu W, Zhang H. Altered spontaneous brain activity in primary open angle glaucoma: a resting-state functional magnetic resonance imaging study. PLoS One. 2014;9:e89493. doi: 10.1371/journal.pone.0089493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Li T, Sabel BA, Chen Z, Wen H, Li J, Xie X, Yang D, Chen W, Wang N, Xian J, He H. Structural brain alterations in primary open angle glaucoma: a 3T MRI study. Sci Rep. 2016;6:18969. doi: 10.1038/srep18969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nucci C, Martucci A, Cesareo M, Mancino R, Russo R, Bagetta G, Cerulli L, Garaci FG. Brain involvement in glaucoma: advanced neuroimaging for understanding and monitoring a new target for therapy. Curr Opin Pharmacol. 2013;13:128–33. doi: 10.1016/j.coph.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Aptel F, Weinreb RN, Chiquet C, Mansouri K. 24-h monitoring devices and nyctohemeral rhythms of intraocular pressure. Prog Retin Eye Res. 2016;55:108–48. doi: 10.1016/j.preteyeres.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Liu JH, Shieh BE. Suprachiasmatic nucleus in the neural circuitry for the circadian elevation of intraocular pressure in rabbits. J Ocul Pharmacol Ther. 1995;11:379–88. doi: 10.1089/jop.1995.11.379. [DOI] [PubMed] [Google Scholar]
- 8.Guzman-Ruiz MA, Saderi N, Cazarez-Marquez F, Guerrero-Vargas NN, Basualdo MC, Acosta-Galvan G, Buijs RM. The suprachiasmatic nucleus changes the daily activity of the arcuate nucleus alpha-MSH neurons in male rats. Endocrinology. 2014;155:525–35. doi: 10.1210/en.2013-1604. [DOI] [PubMed] [Google Scholar]
- 9.Jin J, Xu GX, Yuan ZL. Influence of the hypothalamic arcuate nucleus on intraocular pressure and the role of opioid peptides. PLoS One. 2014;9:e82315. doi: 10.1371/journal.pone.0082315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everitt BJ, Hokfelt T, Wu JY, Goldstein M. Coexistence of tyrosine hydroxylase-like and gamma-aminobutyric acid-like immunoreactivities in neurons of the arcuate nucleus. Neuroendocrinology. 1984;39:189–91. doi: 10.1159/000123977. [DOI] [PubMed] [Google Scholar]
- 11.Horvath TL, Bechmann I, Naftolin F, Kalra SP, Leranth C. Heterogeneity in the neuropeptide Ycontaining neurons of the rat arcuate nucleus: GABAergic and non- GABAergic subpopulations. Brain Res. 1997;756:283–6. doi: 10.1016/s0006-8993(97)00184-4. [DOI] [PubMed] [Google Scholar]
- 12.Ovesjo ML, Gamstedt M, Collin M, Meister B. GABAergic nature of hypothalamic leptin target neurones in the ventromedial arcuate nucleus. J Neuroendocrinol. 2001;13:505–16. doi: 10.1046/j.1365-2826.2001.00662.x. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-Lasheras C, Konner AC, Bruning JC. Integrative neurobiology of energy homeostasisneurocircuits, signals and mediators. Front Neuroendocrinol. 2010;31:4–15. doi: 10.1016/j.yfrne.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Sieghart W. Allosteric modulation of GABAA receptors via multiple drug-binding sites. Adv Pharmacol. 2015;72:53–96. doi: 10.1016/bs.apha.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Falsafi SK, Ghafari M, Miklósi AG, Engidawork E, Gröger M, Höger H, Lubec G. Mouse hippocampal GABAB1 but not GABAB2 subunit-containing receptor complex levels are paralleling retrieval in the multiple-T-maze. Front Behav Neurosci. 2015;9:276. doi: 10.3389/fnbeh.2015.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guzman-Ruiz MA, Ramirez-Corona A, Guerrero-Vargas NN, Sabath E, Ramirez-Plascencia OD, Fuentes-Romero R, Leon-Mercado LA, Basualdo SM, Escobar C, Buijs RM. Role of the Suprachiasmatic and Arcuate Nuclei in Diurnal Temperature Regulation in the Rat. J Neurosci. 2015;35:15419–29. doi: 10.1523/JNEUROSCI.1449-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim YB, Kim YS, Kim WB, Shen FY, Lee SW, Chung HJ, Kim JS, Han HC, Colwell CS, Kim YI. GABAergic excitation of vasopressin neurons: possible mechanism underlying sodium-dependent hypertension. Circ Res. 2013;113:1296–307. doi: 10.1161/CIRCRESAHA.113.301814. [DOI] [PubMed] [Google Scholar]
- 18.Dietrich MO, Horvath TL. GABA keeps up an appetite for life. Cell. 2009;137:1177–9. doi: 10.1016/j.cell.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–34. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samuels BC, Hammes NM, Johnson PL. Dorsomedial/Perifornical Hypothalamic Stimulation Increases Intraocular Pressure, Intracranial Pressure, and the Translaminar Pressure Gradient. Invest Ophthalmol Vis Sci. 2012;53:7328–35. doi: 10.1167/iovs.12-10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dawn YL, Paul L. B’Ann TG, Eleanor CT, Joanne AM. Neurochemical Correlates of Cortical Plasticity after Unilateral Elevated Intraocular Pressure in a Primate Model of Glaucoma. Invest Ophthalmol Vis Sci. 2003;44:2573–81. doi: 10.1167/iovs.02-0779. [DOI] [PubMed] [Google Scholar]
- 22.John SW, Smith RS, Savinova OV, Hawes NL, Chang B, Turnbull D, Davisson M, Roderick TH, Heckenlively JR. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest Ophthalmol Vis Sci. 1998;39:951–62. [PubMed] [Google Scholar]
- 23.Yang H, Hirooka K, Fukuda K, Shiraga F. Neuroprotective effects of angiotensin II type 1 receptor blocker in a rat model of chronic glaucoma. Invest Ophthalmol Vis Sci. 2009;50:5800–4. doi: 10.1167/iovs.09-3678. [DOI] [PubMed] [Google Scholar]
- 24.Schulte T, Brecht S, Herdegen T, Illert M, Mehdorn HM, Hamel W. Induction of immediate early gene expression by high-frequency stimulation of the subthalamic nucleus in rats. Neuroscience. 2006;138:1377–85. doi: 10.1016/j.neuroscience.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 25.George P, Charles W. The Rat Brain in Stereotaxic Coordinates. Third edition, Academic Press, New York, 1998. [Google Scholar]
- 26.George P, Keith FBJ. The Mouse Brain in Stereotaxic Coordinates. Second edition, Academic Press, New York, 2001. [Google Scholar]
- 27.Freitas RL, Bassi GS, de Oliveira AM, Coimbra NC. Serotonergic neurotransmission in the dorsal raphe nucleus recruits in situ 5-HT(2A/2C) receptors to modulate the post-ictal antinociception. Exp Neurol. 2008;213:410–8. doi: 10.1016/j.expneurol.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Yu X, Wang LN, Ma L, You R, Cui R, Ji D, Wu Y, Zhang CF, Yang ZL, Ji H. Akebia saponin D attenuates ibotenic acid-induced cognitive deficits and pro-apoptotic response in rats: involvement of MAPK signal pathway. Pharmacol Biochem Behav. 2012;101:479–86. doi: 10.1016/j.pbb.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 29.Ueno S, Bracamontes J, Zorumski C, Weiss DS, Steinbach JH. Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABA-A receptor. J Neurosci. 1997;17:625–34. doi: 10.1523/JNEUROSCI.17-02-00625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yonggang G, Zhou J-J, Yun Z, Li W, Therese AK, Xiangjian Z, De-Pei L. Neuroadaptations of presynaptic and postsynaptic GABAB receptor function in the paraventricular nucleus in response to chronic unpredictable stress. Br J Pharmacol. 2017;174:2929–40. doi: 10.1111/bph.13924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawada K, Hiraoka M, Ohguro H. Effect of Antiglaucoma Medicine on Intraocular Pressure in DBA/2J Mice. Ophthalmic Res. 2016;55:205–11. doi: 10.1159/000444057. [DOI] [PubMed] [Google Scholar]
- 32.Moon JI, Kim IB, Gwon JS, Park MH, Kang TH, Lim EJ, Choi KR, Chun MH. Changes in retinal neuronal populations in the DBA/2J mouse. Cell Tissue Res. 2005;320:51–9. doi: 10.1007/s00441-004-1062-8. [DOI] [PubMed] [Google Scholar]
- 33.Libby RT, Anderson MG, Pang IH, Robinson ZH, Savinova OV, Cosma IM, Snow A, Wilson LA, Smith RS, Clark AF, John SW. Inherited glaucoma in DBA/2J mice: pertinent disease features for studying the neurodegeneration. Vis Neurosci. 2005;22:22637–48. doi: 10.1017/S0952523805225130. [DOI] [PubMed] [Google Scholar]
- 34.McDougal DH, Gamlin PD. Autonomic control of the eye. Compr Physiol. 2015;5:439–73. doi: 10.1002/cphy.c140014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westheimer G, Blair SM. The parasympathetic pathways to internal eye muscles. Invest Ophthalmol. 1973;12:193–7. [PubMed] [Google Scholar]
- 36.Erickson-Lamy K, Korbmacher C, Schuman JS, Nathanson JA. Effect of Endothelin on Outflow Facility and Accommodation in the Monkey Eye In Vivo. Invest Ophthalmol Vis Sci. 1991;32:492–5. [PubMed] [Google Scholar]
- 37.Chronwall BM. Anatomy and physiology of the neuroendocrine arcuate nucleus. Peptides. 1985;6(Suppl 2):1–11. doi: 10.1016/0196-9781(85)90128-7. [DOI] [PubMed] [Google Scholar]
- 38.Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 39.Da Silva AV, Torres KR, Haemmerle CA, Céspedes IC, Bittencourt JC. The Edinger–Westphal nucleus II: Hypothalamic afferents in the rat. J Chem Neuroanat. 2013;54:5–19. doi: 10.1016/j.jchemneu.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK. Leptin Activates Hypothalamic CART Neurons Projecting to the Spinal Cord. Neuron. 1998;21:1375–85. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- 41.Chung HJ, Hwang HB, Lee NY. The Association between Primary Open-Angle Glaucoma and Blood Pressure: Two Aspects of Hypertension and Hypotension. BioMed Res Int. 2015;2015:827516. doi: 10.1155/2015/827516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sapru HN. Role of the hypothalamic arcuate nucleus in cardiovascular regulation. Auton Neurosci. 2013;175:38–50. doi: 10.1016/j.autneu.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi Z, Madden CJ, Brooks VL. Arcuate neuropeptide Y inhibits sympathetic nerve activity via multiple neuropathways. J Clin Invest. 2017;127:2868–80. doi: 10.1172/JCI92008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Falsafi SK, Ghafari M, Miklósi AG, Engidawork E, Gröger M, Höger H, Lubec G. Test-retest reproducibility of quantitative binding measures of [(11)C]Ro15–4513, a PET ligand for GABAA receptors containing alpha5 subunits. Neuroimage. 2017;152:270–6. doi: 10.1016/j.neuroimage.2016.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naik R, Valentine H, Dannals RF, Wong DF, Horti AG. Horti Synthesis and Evaluation of a New 18F-Labeled Radiotracer for Studying the GABAB Receptor in the Mouse Brain. ACS Chem Neurosci. 2018;9 doi: 10.1021/acschemneuro.8b00038. [DOI] [PubMed] [Google Scholar]
- 46.Yang H, de Jong JW, Tak Y, Peck J, Bateup HS, Lammel S. Nucleus Accumbens Subnuclei Regulate Motivated Behavior via Direct Inhibition and Disinhibition of VTA Dopamine Subpopulations. Neuron. 2018;97:434–449.e4. doi: 10.1016/j.neuron.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vega-Quiroga I, Yarur HE, Gysling K. Lateral septum stimulation disinhibits dopaminergic neurons in the antero-ventral region of the ventral tegmental area: Role of GABA-A alpha 1 receptors. Neuropharmacology. 2018;128:76–85. doi: 10.1016/j.neuropharm.2017.09.034. [DOI] [PubMed] [Google Scholar]