Highlights
-
•
Treatment of relapsed/metastatic head and neck cancer is unclear.
-
•
Cyclophosphamide, avelumab, and radiotherapy may be effective for R/M-HNC.
-
•
Combined treatment may improve avelumab activity without increasing its toxicity.
-
•
Ongoing trials will clarify the potential of immunotherapy in RM-HNC patients.
Keywords: Head and neck cancer, Avelumab, Cyclophosphamide, Radiotherapy, Clinical trial, Immunotherapy
Abstract
Introduction and background
Second-line treatment of platinum-resistant relapsed/metastatic (R/M) head and neck cancer (HNC) is a currently unmet clinical need. Clinical trials showed improvement in overall survival and quality of life of R/M-HNC patients treated with anti-PD-1 regardless of the number of prior chemotherapy lines; however, the percentage of long-term survivors remains limited.
This study aims to test the hypothesis that attacking the tumor microenvironment at multiple levels can increase immunogenicity of R/M-HNC without worsening the safety profile of immune checkpoint inhibitors.
Methods/design
In this open label, multi-center, single-arm, Phase Ib/II, R/M-HNC patients pretreated with at least one line of therapy containing platinum, fluorouracil, and cetuximab will receive a daily metronomic dose of 50 mg cyclophosphamide without a drug-free break, 10 mg/kg avelumab on day 1 and every other week until progression, and a single fraction of 8 Gy radiotherapy on day 8.
Discussion
The treatment protocol aims to reverse immune evasion of the tumor through a radiotherapy-induced self-vaccination effect, suppression of CD4+ CD25+ FoxP3+ regulatory T-cell function by metronomic cyclophosphamide, and effector T-cell reactivation owing to the inhibition of the PD-1–PD-L1 axis by avelumab.
The immunologic interplay induced by the proposed combined treatment may theoretically improve the activity of avelumab without increasing its toxicity profile.
Finally, an ancillary translational study will be extended to all the patients’ population.
Trial registration
EudraCT n. 2017-000353-39.
Introduction
Platinum-resistant relapsed/metastatic (R/M) head and neck cancer (HNC) has a poor prognosis, and immunotherapy is the only approved second-line treatment, although most patients do not respond to it and its long-term survival remains poor [1].
The EGFR-targeting monoclonal antibody cetuximab (plus platinum-based chemotherapy) was approved in the US and Europe [2] as a first-line therapy for R/M disease. However, the FDA approved cetuximab only as second line due to the limited benefit observed. However, no study has shown any improvement in overall survival (OS). New advances in immunology and improved understanding of the immunologic effects of both chemotherapy and radiotherapy initiate a novel approach to cancer treatment based on strengthening the host’s natural defenses rather than directly targeting the tumor. Clinical trials showed improvement in OS and quality of life (QoL) of patients treated with anti-PD-1 regardless of the number of prior chemotherapy lines. Results also showed a limited but constant number of long-term survivors. However, most patients do not achieve an objective response or relapse within one year from treatment initiation. This outcome reflects the limitation of immune checkpoint inhibitor (ICI) monotherapy in treating R/M-HNC patients [3], [4].
R/M-HNC is an immune suppressive malignancy with few tumor-infiltrating T lymphocytes, impaired natural killer (NK) cell activity, poor antigen-presenting function, and recruited regulatory T-cells (Tregs) [5]. Clinical studies of HNC have reported that PD-L1 is expressed in 66–77% of patients [4], [6], leading to exhaustion of PD-1-positive T cells.
The PD-1–PD-L1 axis is common among immune cell populations, including CTLs, Tregs, NK cells, NKT cells, and antigen-presenting cells (APCs) [7]. Opposite effects of PD-1–PD-L1 interactions are elicited in different cells, e.g., inhibitory cell signals in CD8+ CTL [8] and activating signals in CD4+ CD25+ Foxp3+ Treg [9].
Avelumab is a fully human anti-PD-L1 IgG1 monoclonal antibody that selectively binds to PD-L1 and competitively blocks its interaction with PD-1, enabling the activation of T-cells and the adaptive immune system by inhibiting the PD-1–PD-L1 axis [10]. Moreover, IgG1 monoclonal antibodies, such as avelumab, trigger antibody-dependent cell-mediated cytotoxicity and engage the innate immune system as an additional mechanism of tumor control.
A mechanism employed by HNC to escape antitumor immunity is through the immunosuppressive action of Tregs. Various studies have demonstrated an increased number of Tregs in the tumor microenvironment (TME) in HNC patients [11]. Depletion of Treg results in tumor regression in experimental models [12], [13]. The effect is dependent on the extent of Treg suppression [14]. Long-term, low-dose (metronomic) cyclophosphamide treatment selectively reduces Treg populations both in experimental models and in humans, but does not affect effector T-cells [15]. The PD-1–PD-L1 axis enhances and sustains the expression of Foxp3 and the suppressive function of inducible Treg cells (iTreg) [9]. The contemporary use of two independent mechanisms of Treg control (avelumab inhibiting Treg clonal expansion and function, and mCTX reducing Treg populations) may result in the profound inhibition of Treg populations.
If immune-suppressive mechanisms are weakened, the release of high levels of tumor-specific antigens or stress-related antigens (EpCAM, HSPs, HMBG-1, Calreticulin, ATP) through the induction of immunogenic cell death (ICD) [16] may result in an effective immune response. Radiotherapy (RT) can also induce ICD [17], even in patients previously treated with heavy doses of chemotherapy in whom the HNC developed resistance to cytotoxic chemotherapy. This radiation-induced immune effect is considered the basis of the abscopal effect: the regression of metastatic tumors outside the irradiated field. The optimal dose of RT to generate an in situ tumor vaccine has yet to be established, but new insights into the molecular mechanism responsible for RT-induced abscopal responses outline the importance of the size of the single RT dose, not of the total dose delivered [18]. Thus, irradiation with a non-ablative dose, which preserves the immune cells, can elicit an abscopal response.
This study aimed to test pharmacologic and physical interventions that cooperate to activate the immune system rather than a single pathway, thereby restoring immune competence toward the tumor.
Materials and methods
Study design and patient population
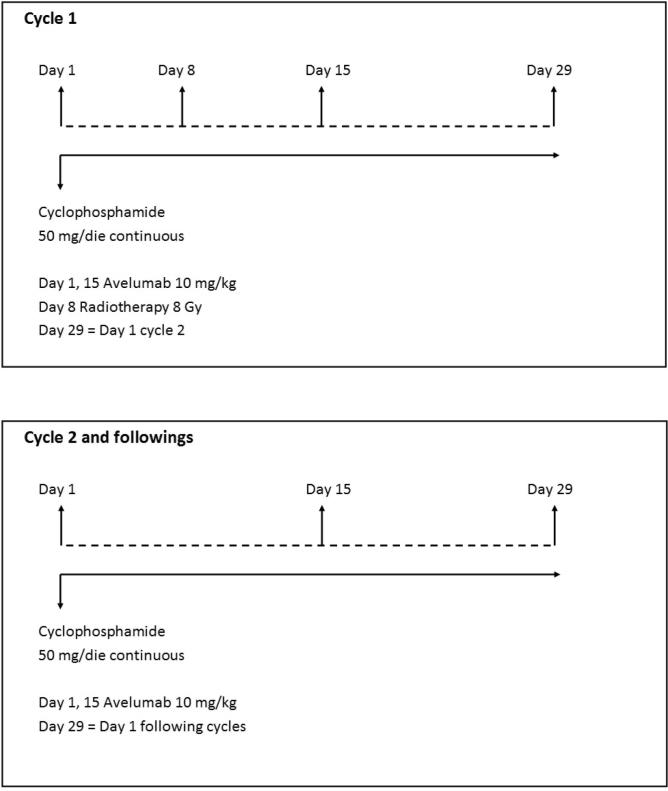
In this multicentric phase I-II study, patients with HNC who received at least one prior line of systemic therapy for relapsed or metastatic disease will receive avelumab (PD-L1 inhibitor) 10 mg/kg i.v. on day 1 and repeated every 14 days until progression, unacceptable toxicity, or informed consent withdrawal; CTX 50/mg tab daily continuously until progression or unacceptable toxicity; and radiotherapy on day 8 targeting one macroscopic lesion with highly conformal, hypofractionated RT at a dose of 8 Gy in one fraction (flow-chart is shown in Fig. 1). This study is designed as a phase Ib–II trial because the combination under investigation has not been previously tested. Phase II will be performed only after demonstration of tolerability of the schedule within the phase I study.
Fig. 1.
Study schema.
Inclusion criteria
The main inclusion criteria are listed below:
-
•
ECOG Performance Status 0–2;
-
•
Histologically or cytologically confirmed recurrent or metastatic (disseminated) head and neck squamous cell carcinoma;
-
•
Disease progression after treatment with at least one line of therapy including at least Cisplatin, Fluorouracil, and Cetuximab for recurrent (disease not amenable to curative treatment)/metastatic disease;
-
•
Measurable disease by RECIST criteria;
-
•
At least one metastatic site suitable for irradiation;
-
•
Adequate bone marrow, liver, and renal function.
Exclusion criteria
Patients will be excluded if any of the following conditions are present:
-
•
History of malignant disease (except for non-melanoma skin tumors and/or in situ cervical cancer) in the preceding five years;
-
•
Brain metastases;
-
•
Autoimmune or allergic disorders; patients with diabetes type I, vitiligo, psoriasis, or hypo- or hyperthyroid diseases not requiring immunosuppressive treatment are eligible.
-
•
Prior treatment with inhibitors of the PD-1–PD-L1 axis or inhibitors of CTLA-4 (immune checkpoint inhibitors);
-
•
Previous HBV or HCV infections;
-
•
Current use of immunosuppressive medication;
-
•
Radiotherapy within 6 weeks before enrolment;
-
•
Clinically significant (i.e., active) cardiovascular disease.
Study objectives
The primary endpoint of the phase Ib trial is the absence of unacceptable toxicity. Assessment of the safety profile of the association of avelumab and metronomic cyclophosphamide (mCTX) will be graded using the common toxicity criteria and adverse events (NCI CTC-AE v 4.0).
The primary activity endpoint (phase II) is the achievement of an objective response. Objective response is the sum of the complete responses and the partial responses defined as per RECIST evaluation criteria v1.1 (RECIST 1.1). The rates of objective responses will be reported.
The secondary endpoints are: assessment of the safety profile of the association of avelumab and mCTX; progression-free survival (PFS), defined as the time from study treatment initiation to the first occurrence of disease progression or death for any cause, whichever occurs first; OS, defined as the time from treatment initiation to death for any cause; QoL will be assessed using EORTC QLQ-30 and EORTC QLQ–H&N35.
The exploratory objectives of the study are the effects of the immunological treatment (RT + avelumab + cyclophosphamide) on both cell and humoral homeostasis developed by the tumor, and to identify variations among the major circulating immunological factors during the study and the impact of observed changes (if any) on patient outcomes (Table 1). The expression of PD-1/PD-L1 in tumor tissues will not be investigated due to the difficulty in obtaining repeated biopsies for analyzing the dynamic changes induced by the treatment itself.
Table 1.
Translational research overview.
| Evaluation of the main circulating T cells, MDSC and of circulating dendritic cells (DCs) | Evaluation of cell subpopulations-specific interleukins | Evaluation of the major cytokines (as VEGF, TGF-beta, TNF-alpha, IFN-γ and IDO) | Evaluation of the main tumor associated antigens (TAA) inducible by ICD (as HSP70, HSP90, HMGB1, EpCAM) |
|---|---|---|---|
| Baseline | Baseline | Baseline | Baseline |
| 1 day after RT | 1 day after RT | 1 day after RT | 1 day after RT |
| At beginning of cycle 2 | At beginning of cycle 2 | At beginning of cycle 2 | |
| At disease progression | At disease progression | At disease progression |
Abbreviations: MDSC = myeloid-derived suppressor cell, DCs = dendritic cells, VEGF = vascular endothelial growth factor, TGF-beta = transforming growth factor-beta, TNF-alpha = tumor necrosis factor-alpha, IFN-γ = interferon-gamma, IDO = indoleamine 2,3-DiOxygenase, ICD = immunogenic cell death, HSP70 = heat shock protein 70, HSP90 = heat shock protein 90, HMGB1 = high-mobility group box 1, EpCAM = epithelial cell adhesion molecule.
Statistical analysis
The objective response rate can be recognized as the activity endpoint for the second-line treatment of relapsed/metastatic HNC.
The historical response rate with chemotherapy or target therapy is 6–10% and the proportion of non-responders to immunotherapy is still 80–85%.
We hypothesize a 40% response rate as the level to justify further investigations of the proposed experimental approach.
Even though a low toxicity profile can be anticipated for the combination of treatments, the study is designed as a phase Ib–II trial. The phase II trial will be conducted only after the demonstration of tolerability in patients enrolled in the phase Ib part. The phase II trial will be conducted according to Simon's two-stage optimal design [19]. The null hypothesis is that the true response rate is 25% (considered the best response rate reported with other PD-1 inhibitors), and will be tested against a one-sided alternative. In the first stage, we will accrue 20 patients. If there are 5 or fewer objective responses in these 20 patients, the study will be stopped. Otherwise, 51 additional patients will be accrued for a total of 71. The null hypothesis will be rejected if 24 or more responses are observed in 71 patients. This design yields a type I error rate of 0.05 and power of 80% when the true response rate is 40%.
The first six patients will be treated with CTX 50 mg daily without a drug-free interval, avelumab 10 mg/kg every two weeks, and radiotherapy at 8 Gy in one fraction. The maximum follow-up for evaluation of unacceptable toxicity for patients enrolled in phase I will be 120 days. If two or more unacceptable toxicities are observed, the study will be closed (unacceptable toxicities are detailed in Appendix I). If no more than one unacceptable toxicity is observed in the first six patients, the study will continue with the enrolment of 14 patients up to the completion of stage I of Simon's two-stage design. Patients enrolled in phase Ib will also be evaluated for activity and will correspond to the number of subjects planned for the first stage of phase II. All patients who will be enrolled in the study and who started the treatment will be considered for toxicity evaluation. All patients who completed at least one course of treatment will be considered evaluable for response. All patients recruited for the study will be considered evaluable for PFS and OS. All patients recruited into the study who will complete at least two QoL questionnaires will be considered for QoL analysis.
Translational study objectives and methods
The goal of this translational study is to identify variations among the major circulating immunological factors during treatment and link them to patient outcome.
We will collect 15-ml blood samples at baseline, on the day after RT, at the beginning of cycle 2, and at disease progression. Plasma samples will be obtained by centrifugation. We will repeat the following determinations at each point:
-
•
Evaluation of the major circulating T-cells, myeloid-derived suppressor cells and dendritic cells;
-
•
Evaluation of cell subpopulation-specific interleukins;
-
•
Evaluation of the major cytokines (such as VEGF, TGFβ, TNFα, IFNγ, and IDO).
We will evaluate ICD-associated antigens (such as HSP70, HSP90, HMGB1, EpCAM) the day after radiation therapy. Cell populations will be studied by flow cytometry. VEGF, TGFβ, and the other cytokines and interleukins will be analyzed with commercially available ELISA kits. The statistical analysis of translational data, considering the number of comparisons planned, will be adjusted for multiple testing using the “false discovery rate method” which is the adequate standard procedure of adjustment for multiple testing. The correlation between the levels of circulating immunological factors at baseline and during treatment, and the objective clinical response (responders vs. non-responders) will be descriptive and does not apply formal statistical texts.
Discussion
Locally advanced squamous cell carcinoma of the head and neck recurs in up to 50% of cases even after aggressive combined-modality therapy [20]. A small number of patients with localized recurrence can be treated with curative intent, but the vast majority receives palliative systemic therapy. In the first-line treatment of R/M-HNC, the “EXTREME” regimen has shown the best results so far in terms of overall response rate (ORR), PFS, and OS without significant difference in PFS between those who had received prior systemic therapy and those who had not [21]. For patients who failed to respond or relapsed less than six months after their initial treatment and for patients who progress after the “EXTREME” regimen, no second-line therapy has been established. Immunotherapies using is the PD-1-directed ICIs can be proposed according to the result of two recently published phase III trials (CheckMate 141 [22] and KEYNOTE-040 [23]). Taken together, these trials show that nivolumab and pembrolizumab are well tolerated, but produce a modest overall response rate of approximately 15% in second-line treatment. The therapeutic efficacy of ICIs in monotherapy may be limited because immune dysfunction in the TME of R/M-HNC is mediated by tumor-driven mechanisms other than immune checkpoint overexpression.
Multiple immunotherapeutic agent combinations, addressing different additional escape mechanism, are under evaluation in ongoing trials (NCT02551159, NCT02369874). Toxicity of ICIs in combination is an actual concern and should be carefully considered when treating patients with R/M-HNC. For example, in the CheckMate 067 phase III trial on melanoma patients, the incidence of grade 3 or 4 toxicity with the combination of nivolumab and ipilimumab was increased compared with either single agent (59% versus 21% and 28% for nivolumab and ipilimumab, respectively) [24]. Because ICIs elicit delayed clinical effects, safety analysis, including QoL analysis, remains an essential objective of immunotherapy in R/M-HNC patients.
The CONFRONT trial is a proof-of-concept study combining two low-toxicity treatments (mCTX and non-ablative radiotherapy) with a PD-L1 inhibitor. Each one of these treatments drives positive immunological effects, and their combination may, in theory, favor a reversal of immune suppression in the TME. The expected immune-related toxicity, compared with anti-PD-1 antibodies that target T-cells, avelumab is specific for PD-L1 and mainly targets tumor cells; therefore, avelumab has fewer side effects, including a lower risk of immune-related adverse events, because the blockade of PD-L1 leaves the PD-1–PD-L2 pathway intact, which promotes peripheral self-tolerance. Moreover, data published very recently added new insight into the safety profile of the proposed combination. In a phase I study [25], avelumab showed acceptable toxicity up to 20 mg/kg, and the maximum tolerated dose was not reached. Many clinical trials investigated mCTX in combination with other metronomic chemotherapeutic drugs and/or with target agents or immunotherapy (inhibitors of the PD-1–PD-L1 axis) resulting in a well-tolerated treatment with no significant side effects [26], [27], [28], [29], [30]. Concurrent delivery of PD-1–PD-L1 axis inhibitors and hypofractionated, non-ablative RT is well tolerated, with manageable immune-related adverse events that did not seem to be associated with the particular site irradiated [31], [32]. All the above mentioned studies suggest that the combination planned for this study should not add toxicity in excess to that expected for avelumab alone.
Multiple preclinical studies have investigated different dose and fractionation regimens in combination with immunotherapies [33], [34], [35], [36], [37], [38], [39], showing different results for fractionated or single-dose RT depending on the associated strategy and the investigated tumor model. Most immune-stimulatory effects of RT occur at 5–20 Gy; however, a preclinical study showed that radiation doses above 12–18 Gy attenuate tumor cell immunogenicity by upregulating the exonuclease Trex1, which causes degradation of interferon-stimulating cytosolic double-stranded DNA. Conversely, lower RT doses instead stimulate IFN-β secretion, thereby activating dendritic cells that are critical for CD8+ T-cell priming [40].
The optimal range of dose and fractionation, below which immune stimulation may be suboptimal and above which immunosuppression prevails, have not been established so far in clinical studies. The dose of 8 Gy, which we use in this trial, can be considered safe when treating R/M-HNC patients because the majority of failures are in-field in reported intensity-modulated radiation therapy series [41], [42]. This dose level can also induce ICD and subsequent dendritic cell activation, which facilitate the presentation of tumor antigens. Alternatively, surviving cancer cells can undergo immunogenic modulation, which makes them more susceptible to cytotoxic T-lymphocyte-mediated lysis.
Two ongoing trials (NCT02318771, NCT02684253) in R/M-HNC combine RT at doses of 8 Gy, 4 Gy in five fractions, or 27 Gy in three fractions with anti-PD-1 ICIs. The results of ongoing trials will better clarify the potential of immunotherapy in R/M-HNC patients. Complementary multimodal immunotherapy has a strong rationale to counterbalance the established immunosuppression of R/M-HNC, and theoretically, the proposed combined treatment could improve the activity of avelumab without increasing its toxic profile.
Declaration of interest
MM served on ad boards for Merck KGaA, Darmstadt, Germany, Merck Sharp and Dohme, and Bristol-Myers Squibb and had a speaker role in Merck- promoting meetings.
LL reports grants from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eisai, Merck Serono, Merck Sharpe & Dohme, Novartis, and Roche; personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Debiopharm, Eisai, Merck Serono, Merck Sharpe & Dohme, Novartis, and Roche; travel arrangements from Bayer, Debiopharm, Merck Serono, and Sobi.
MDM acted as a consultant for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Bayer, Merck Sharp & Dohme and Bristol Myers Squibb.
Acknowledgments
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Funding
The Gruppo Oncologico Nord-Ovest (GONO) sponsors the study and Merck KGaA (Dorstmund, Germany) partially supports this study and provides Avelumab®.
Appendix I
Unacceptable toxicities:
Unacceptable toxicities are defined as it follows:
grade 4 neutropenia persisting ≥7 days or requiring treatment with granulocyte colony- stimulating factor,
febrile neutropenia,
grade IV thrombocytopenia
grade III decreased platelet count requiring platelet transfusion
grade IV anemia requiring a red blood cell transfusion
grade ≥ 3 non hematological toxicity, excluding controllable grade III nausea, vomiting, or diarrhea that recovered to grade ≤ 1 as a result of treatment prior to infusion in the next cycle
References
- 1.Gillison M.L., Blumenschein G., Fayette J., Guigay J., Colevas A.D., Licitra L. CheckMate 141: 1-year update and subgroup analysis of nivolumab as first-line therapy in patients with recurrent/metastatic head and neck cancer. Oncologist. 2018 doi: 10.1634/theoncologist.2017-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfister D.G., Foote R.L., Gilbert J., Gillison M.L., Ridge J.A., Rocco J. NCCN clinical practice guidelines in oncology (NCCN Guidelines®) Head Neck Cancers. 2018 https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed July 30, 2018) [Google Scholar]
- 3.Ferris R.L., Blumenschein G., Fayette J., Guigay J., Colevas A.D., Licitra L. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seiwert T.Y., Burtness B., Mehra R., Weiss J., Berger R., Eder J.P. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956–965. doi: 10.1016/S1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 5.Ferris R.L. Immunology and immunotherapy of head and neck cancer. J Clin Oncol. 2015;33:3293–3304. doi: 10.1200/JCO.2015.61.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim H.S., Lee J.Y., Lim S.H., Park K., Sun J.-M., Ko Y.H. Association between PD-L1 and HPV status and the prognostic value of PD-L1 in oropharyngeal squamous cell carcinoma. Cancer Res Treat Off J Korean Cancer Assoc. 2016;48:527–536. doi: 10.4143/crt.2015.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keir M.E., Butte M.J., Freeman G.J., Sharpe A.H. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Intlekofer A.M., Thompson C.B. At the bench: preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J Leukoc Biol. 2013;94:25–39. doi: 10.1189/jlb.1212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francisco L.M., Salinas V.H., Brown K.E., Vanguri V.K., Freeman G.J., Kuchroo V.K. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu K., Tan S., Chai Y., Chen D., Song H., Zhang C.W.-H. Structural basis of anti-PD-L1 monoclonal antibody avelumab for tumor therapy. Cell Res. 2017;27:151–153. doi: 10.1038/cr.2016.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duray A., Demoulin S., Hubert P., Delvenne P., Saussez S. Immune suppression in head and neck cancers: a review. Clin Dev Immunol. 2010;2010:701657. doi: 10.1155/2010/701657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marabelle A., Kohrt H., Sagiv-Barfi I., Ajami B., Axtell R.C., Zhou G. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J Clin Invest. 2013;123:2447–2463. doi: 10.1172/JCI64859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ladoire S., Arnould L., Apetoh L., Coudert B., Martin F., Chauffert B. Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating foxp3+ regulatory T cells. Clin Cancer Res Off J Am Assoc Cancer Res. 2008;14:2413–2420. doi: 10.1158/1078-0432.CCR-07-4491. [DOI] [PubMed] [Google Scholar]
- 14.Li X., Kostareli E., Suffner J., Garbi N., Hämmerling G.J. Efficient Treg depletion induces T-cell infiltration and rejection of large tumors. Eur J Immunol. 2010;40:3325–3335. doi: 10.1002/eji.201041093. [DOI] [PubMed] [Google Scholar]
- 15.Ghiringhelli F., Menard C., Puig P.E., Ladoire S., Roux S., Martin F. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother CII. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kroemer G., Galluzzi L., Kepp O., Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 17.Adkins I., Fucikova J., Garg A.D., Agostinis P., Špíšek R. Physical modalities inducing immunogenic tumor cell death for cancer immunotherapy. Oncoimmunology. 2014;3:e968434. doi: 10.4161/21624011.2014.968434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodríguez-Ruiz M.E., Vanpouille-Box C., Melero I., Formenti S.C., Demaria S. Immunological mechanisms responsible for radiation-induced abscopal effect. Trends Immunol. 2018;39:644–655. doi: 10.1016/j.it.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 20.Argiris A., Harrington K.J., Tahara M., Schulten J., Chomette P., Ferreira Castro A. Evidence-based treatment options in recurrent and/or metastatic squamous cell carcinoma of the head and neck. Front Oncol. 2017;7:72. doi: 10.3389/fonc.2017.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vermorken J.B., Mesia R., Rivera F., Remenar E., Kawecki A., Rottey S. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 22.Ferris R.L., Blumenschein G.R., Fayette J., Guigay J., Colevas A.D., Licitra L. Two-year update from CheckMate 141: outcomes with Nivolumab (Nivo) vs investigator’s choice (IC) in recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN) in the overall population and PD-L1 subgroups. Int J Radiat Oncol Biol Phys. 2018;100:1317. [Google Scholar]
- 23.Cohen E.E., Harrington K.J., Le Tourneau C., Dinis J., Licitra L., Ahn M.-J. LBA45_PR Pembrolizumab (pembro) vs standard of care (SOC) for recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC): Phase 3 KEYNOTE-040 trial. Ann Oncol. 2017;28 [Google Scholar]
- 24.Wolchok J.D., Chiarion-Sileni V., Gonzalez R., Rutkowski P., Grob J.-J., Cowey C.L. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heery C.R., O’Sullivan-Coyne G., Madan R.A., Cordes L., Rajan A., Rauckhorst M. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol. 2017;18:587–598. doi: 10.1016/S1470-2045(17)30239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kummar S., Ji J., Morgan R., Lenz H.-J., Puhalla S.L., Belani C.P. A phase I study of veliparib in combination with metronomic cyclophosphamide in adults with refractory solid tumors and lymphomas. Clin Cancer Res Off J Am Assoc Cancer Res. 2012;18:1726–1734. doi: 10.1158/1078-0432.CCR-11-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayer E.L., Isakoff S.J., Klement G., Downing S.R., Chen W.Y., Hannagan K. Combination antiangiogenic therapy in advanced breast cancer: a phase 1 trial of vandetanib, a VEGFR inhibitor, and metronomic chemotherapy, with correlative platelet proteomics. Breast Cancer Res Treat. 2012;136:169–178. doi: 10.1007/s10549-012-2256-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orlando L., Cardillo A., Rocca A., Balduzzi A., Ghisini R., Peruzzotti G. Prolonged clinical benefit with metronomic chemotherapy in patients with metastatic breast cancer. Anticancer Drugs. 2006;17:961–967. doi: 10.1097/01.cad.0000224454.46824.fc. [DOI] [PubMed] [Google Scholar]
- 29.Garcia A.A., Hirte H., Fleming G., Yang D., Tsao-Wei D.D., Roman L. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26:76–82. doi: 10.1200/JCO.2007.12.1939. [DOI] [PubMed] [Google Scholar]
- 30.Toulmonde M., Penel N., Adam J., Chevreau C., Blay J.-Y., Le Cesne A. Use of PD-1 targeting, macrophage infiltration, and IDO pathway activation in sarcomas: A phase 2 clinical trial. JAMA Oncol. 2018;4:93–97. doi: 10.1001/jamaoncol.2017.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aboudaram A., Modesto A., Chaltiel L., Gomez-Roca C., Boulinguez S., Sibaud V. Concurrent radiotherapy for patients with metastatic melanoma and receiving anti-programmed-death 1 therapy: a safe and effective combination. Melanoma Res. 2017;27:485–491. doi: 10.1097/CMR.0000000000000386. [DOI] [PubMed] [Google Scholar]
- 32.Bang A., Wilhite T.J., Pike L.R.G., Cagney D.N., Aizer A.A., Taylor A. Multicenter evaluation of the tolerability of combined treatment with PD-1 and CTLA-4 immune checkpoint inhibitors and palliative radiation therapy. Int J Radiat Oncol Biol Phys. 2017;98:344–351. doi: 10.1016/j.ijrobp.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Milas L., Mason K.A., Ariga H., Hunter N., Neal R., Valdecanas D. CpG oligodeoxynucleotide enhances tumor response to radiation. Cancer Res. 2004;64:5074–5077. doi: 10.1158/0008-5472.CAN-04-0926. [DOI] [PubMed] [Google Scholar]
- 34.Deng L., Liang H., Burnette B., Beckett M., Darga T., Weichselbaum R.R. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dewan M.Z., Galloway A.E., Kawashima N., Dewyngaert J.K., Babb J.S., Formenti S.C. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res Off J Am Assoc Cancer Res. 2009;15:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verbrugge I., Hagekyriakou J., Sharp L.L., Galli M., West A., McLaughlin N.M. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. 2012;72:3163–3174. doi: 10.1158/0008-5472.CAN-12-0210. [DOI] [PubMed] [Google Scholar]
- 37.Demaria S., Kawashima N., Yang A.M., Devitt M.L., Babb J.S., Allison J.P. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2005;11:728–734. [PubMed] [Google Scholar]
- 38.Gandhi S.J., Minn A.J., Vonderheide R.H., Wherry E.J., Hahn S.M., Maity A. Awakening the immune system with radiation: Optimal dose and fractionation. Cancer Lett. 2015;368:185–190. doi: 10.1016/j.canlet.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 39.Demaria S., Ng B., Devitt M.L., Babb J.S., Kawashima N., Liebes L. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Vanpouille-Box C., Formenti S.C., Demaria S. TREX1 dictates the immune fate of irradiated cancer cells. Oncoimmunology. 2017;6:e1339857. doi: 10.1080/2162402X.2017.1339857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Studer G., Luetolf U.M., Glanzmann C. Locoregional failure analysis in head-and-neck cancer patients treated with IMRT. Strahlenther Onkol Organ Dtsch Rontgengesellschaft Al. 2007;183:417–423. doi: 10.1007/s00066-007-1663-8. discussion 424–425. [DOI] [PubMed] [Google Scholar]
- 42.Eisbruch A., Marsh L.H., Dawson L.A., Bradford C.R., Teknos T.N., Chepeha D.B. Recurrences near base of skull after IMRT for head-and-neck cancer: implications for target delineation in high neck and for parotid gland sparing. Int J Radiat Oncol Biol Phys. 2004;59:28–42. doi: 10.1016/j.ijrobp.2003.10.032. [DOI] [PubMed] [Google Scholar]