Abstract
Agronomic biofortification is the deliberate use of mineral fertilizers to increase the concentration of a target mineral in edible portions of crops to increase dietary intake of the target mineral. Globally, increased dietary intake of potassium (K) is becoming a part of the strategy to address hidden hunger and related non-communicable diseases such as hypertension and cardiac disorders. This study aimed at demonstrating the efficacy of increasing the concentration of K in the edible portions of three commonly consumed but underutilized solanacea vegetables (Solanum aethiopicum, S. macrocarpon and S. torvum) in Ghana. The effects of different types and rates of K fertilizer application on the leaf- and fruit-K contents of the vegetables, as well as the K loss between the raw and cooked fruits were investigated. Five levels of each of three types of K fertilizer (liquid drench of potassium chloride, granular Muriate of potash and Sulphate of potash) were applied to each of the three field-grown vegetables. Yield data were collected and the fruits and leaves were analysed for the content of K, N, P, Ca, Fe, Zn and Cu. The results showed the rate of fertilizer application had significant effect on the yields of S. aethiopicum and macrocarpon but the yield of S. torvum was significantly affected by type, rate and interactive effect of type and rate of fertilizer application. Fruit K concentrations were greatest for S. aethiopicum (2130 mg K kg−1 DW) and S. torvum (1883 mg K kg−1 DW) with liquid KCl but with Sulphate of Potash for S. macrocarpon (1801 mg K kg−1 DW). There were higher K concentrations in leaves than in fruits of all the vegetables. Household cooking of the fruits resulted in the retention of over 70% of the K content in the raw fruits. Potassium fertilization increased the Ca, Fe, and Zn contents of S. aethiopicum and S. torvum. It is concluded that agronomic biofortification may be a useful strategy to increase K intakes and other important elements (e.g. Fe and Zn) in the vegetables studied.
Keyword: Agriculture
1. Introduction
The Sustainable Development Goal 2 (SDG 2) aims to “end hunger, achieve food security and improved nutrition, and promote sustainable agriculture” (https://sustainabledevelopment.un.org/, accessed 24/01/2018). Potassium (K), a major intracellular cation, plays crucial roles in the nutrition and health of humans, as well as animals and plants (Institute of Medicine, 2005). In humans, K is derived primarily from dietary sources, and functions in the maintenance of water balance, osmotic pressure and acid-base balance (Bhaskarachary, 2011; He and MacGregor, 2008). Potassium is also involved in the activation of enzymes, protein metabolism and in the regulation of neuromuscular activity and heartbeat (Bhaskarachary, 2011; He and MacGregor, 2008). Suboptimal dietary K intake can contribute to poor tissue health, skeletal muscle contraction and gastrointestinal dysfunction (Institute of Medicine, 2005). Severe K deficiency, or hypokalemia (a serum K concentration of <3.5 mmol L−1), could result in cardiac arrhythmias, muscle weakness and glucose intolerance (Institute of Medicine, 2005). The health benefits of adequate dietary intake of K have been reported in several studies (He and MacGregor, 2003, 2008; Whelton et al., 1997; WHO, 2003, 2012). Adequate dietary intake of K has, therefore, been shown to be instrumental in combating non-communicable diseases, which are major contributors to global mortality and morbidity (He and MacGregor, 2003, 2008; WHO, 2003, 2012).
For adults, a daily adequate intake (AI; i.e.: the average daily level of intake assumed to ensure nutritional adequacy) of 3510 mg (or 90 mmol) of K is recommended (WHO, 2012); and this can be obtained from K-rich fruits and vegetables such as spinach and oranges (Institute of Medicine, 2005). In rural sub-Saharan Africa (SSA), cereal crops (such as maize) and root and tuber crops (such as cassava and yam) are the dominant staple foods. Given that roots and tubers are heavy feeders of K, (i.e.: requires and uses more soil K than other crops) (Yawson et al., 2011) and tend to have good K content, the dietary K needs of these rural consumers should be theoretically met. However, shifts to western diets and reductions in the consumption of fruits and vegetables (Amo-Adjei and Kumi-Kyereme, 2015; Darfour-Oduro et al., 2018), as well as narrowing dietary diversity for especially the rural populations in SSA might be leading to reduced dietary intake of K (Montoya et al., 2013; Nyadanu and Lowor, 2015; Sibhatu and Qaim, 2017). In fact, the per capita intake of fruits and vegetables in Ghana is still far below the 400 g per capita per day suggested by WHO and FAO (Amo-Adjei and Kumi-Kyereme, 2015). Despite the lack of data on K deficiency in Ghana, Darkwa et al. (2017) reported lower serum K concentrations among preeclamptic women than normotensive women, although serum K concentrations were not associated with mean arterial blood pressure among preeclamptics. Thus, the mechanism remains unclear. In addition, excretory losses of K can be high in the tropics through extensive electrolyte losses in sweat, further reducing the K concentration even in people who get sufficient dietary K intake (Malhotra et al., 1981). There is, therefore, a potential risk of inadequate dietary K supply at both individual and population levels in SSA.
Dietary deficiencies of nutrients can be addressed through a number of approaches including dietary diversification, food fortification, supplementation, or by genetic and agronomic biofortification (Broadley et al., 2010). Dietary diversification, for example, is an attractive option in terms of general protein, mineral and vitamin intake but access to diverse diets is not possible in many socio-economic contexts (Chilimba et al., 2012; Nair et al., 2016). For socioeconomic groups with limited access to expensive and commercially-marketed fortified foods, agronomic biofortification is likely to be a cost-effective strategy to address zinc and selenium deficiencies in certain contexts (Nestel et al., 2006; Wang et al., 2016; Zhang et al., 2018), whereas the cost-effectiveness for K has not been quantified. In general, biofortification is defined as increasing the bio-available concentrations of essential elements in the edible portions of crop plants (Graham et al., 2001). Agronomic biofortification of crops is achieved through the application of mineral fertilizers to increase the concentrations of minerals in edible crops, but it may have important extra effects for increasing yield on marginal or infertile soils (Cakmak, 2008). Whilst the biofortification of staple crops would be ideal, due to the wider consumption of staples by the majority of the population, biofortifying and promoting potentially beneficial but underutilised crops also offer an alternative approach of promoting dietary diversification.
Solanaceae vegetables are major constituents of many Ghanaian dishes for both the urban and rural populations. Solanaceae vegetables are widely eaten in stews, soups, and sauces, or as part of medicinal preparations by all family members, including rural dwellers, women and children, who are most at risk of macro/micronutrient malnutrition. Hence, solanaceae vegetables can play vital roles in providing dietary diversity, food and nutrition security and income generation for many households in Ghana (Nyadanu and Lowor, 2015). Solanaceae vegetables such as Solanum aethiopicum (garden eggs), S. macrocarpon (Gboma) and S. torvum (turkey berry {‘abedru’ or ‘Kwahu nsusua’}) are three of the well-known and commonly consumed but neglected and underutilized crop species (NUS) in Ghana (Nyadanu and Lowor, 2015). The fruits of S. aethiopicum L. are commonly eaten grilled, fried or steamed or stewed with other vegetables, meat or fish, and in soups (Nyadanu and Lowor, 2015). Similarly, the fruits and or the leaves of S. torvum are a major constituent of soups and sauces in Ghana and are believed to have hematinic and haemopoietic effects (Balachandran et al., 2012; Nyadanu and Lowor, 2015; Yousaf et al., 2013). Solanum macrocarpon is mostly an indigenous leafy vegetable but its fruits are also cooked and consumed as a vegetable (Nyadanu and Lowor, 2015). These solanaceae vegetables have been known locally to have both nutritional and medicinal value (Nyadanu and Lowor, 2015).
In this study, we take advantage of the wide consumption and the unexplored potentials of these underutilised solanaceae vegetables to demonstrate agronomic biofortification strategy to increase dietary K intake through the consumption of important but underutilized vegetables in Ghana. Specifically, the aims were to (i) to determine response of field-grown Solanum aethiopicum, S. macrocarpon and S. torvum to K fertilization; (ii) determine the effect of different sources of K and application rates on yield and K concentration in fruits and leaves of the target vegetables; and (iii) determine the effect of cooking on K concentration in cooked fruits of the vegetables grown in the field under the different K forms and rates and under typical Ghanaian rain-fed conditions.
2. Material and methods
2.1. Overview of soil and environmental conditions
A field experiment was conducted at the Teaching and Research Farm of the School of Agriculture, University of Cape Coast (UCC; 5.1155°N, 1.2909°W) between February and December, 2017, under rain-fed conditions. The study site experiences two seasons of rainfall with a peak in May to June and the minor in October, with dry periods (harmattan) experienced between November and February. Rainfall pattern at the study site is characterised by low spatial variation. The soil was a haplic acrisol sandy loam, with a 16.2, 76.3 and 7.24% clay, sand and silt, respectively and was typical of arable soils of the coastal savannah agro-ecological zone. The soil had a pH of 6.1, 0.84% organic carbon, 0.1% total nitrogen (N), and 51.1 μg phosphorus (P) g−1. In addition, the soil had a cation exchange capacity (CEC) of 6.23 cmolc kg−1, 3.33 cmol kg−1 calcium (Ca), 0.89 cmol kg−1 magnesium (Mg), and 0.5 cmol kg−1 K. Soil pH, organic carbon and total nitrogen were determined by pH meter, colorimetric method and micro Kjeldahl method, respectively (FAO, 2008). The Bray No. 1 method using a spectrophotometer was used in determining available phosphorus, whilst determination of Ca and Mg were by wet digestion and spectrophotometrically using an atomic absorption spectrophotometer (AAS; model 210 VGP, Buck scientific), as described by FAO (2008). The CEC was determined by displacement of cations using sodium acetate and ammonium acetate (FAO, 2008). The site had been previously cropped with soybean, but under fallow for 3 years before the current experiment and had not received inorganic fertilizer application for over 5 years. Day length at the experimental site ranges from approximately 11.30 to 12.40 hours while solar radiation ranges from 3151 kJ cm−2 day−1 to 3804 kJ cm−2 day−1, respectively (Adu et al., 2017).
2.2. Plant material, cultivation, and experimental design
Seeds of S. torvum and macrocarpon were unavailable in certified seed stores or in germplasm repositories, and were sourced from farmers' fields and/or backyards. A number of accessions of these genetic materials were collected but the accession for which there were sufficient seeds from the same plant was used in the current study. A variety of Solanum aethiopicum (Ntrowa pa) was purchased from a local certified seed store in Cape Coast and used for the study. Prior to transplanting to the experimental field, seeds of the three vegetables were nursed and kept in a nursery for approximately 5 weeks when they were at the 5–6 leaf stage. Preliminary trials suggested that S. torvum seeds are characterised by some sort of dormancy and do not germinate well when nursed under normal conditions. Seeds of S. torvum were therefore treated with 500 ppm of gibberellic acid overnight before nursing. All nursery beds received no fertiliser application but water was applied daily to seedlings at recommended irrigation rates (approximately 50 mm). Seedlings were transplanted in March 2017 to a non-ridged experimental field ploughed and harrowed to a depth of about 30 cm. A randomised block design with two blocks was adopted. Within each block were two replicate plots per treatment and within each plot were twelve replicate plants. For S. aethiopicum and S. macrocarpon, the spacing between plants was 0.9 × 0.9 m, giving a plot size of 1.8 × 2.7 m. The spacing for S. torvum was 1.2 × 1.2 m, giving plot size of 2.4 × 3.6 m. Outer plants of each plot served as guard rows. There were 1.5 m row paths between experimental plots and 1.5 m row path between blocks. Recommended irrigation levels of approximately 400 mm of water every 10 days (https://mofa.gov.gh/site/; accessed on 20/01/2017) in the absence of rainfall and good agronomic practices, involving control of weeds, pests and diseases, were adopted to care for the plants. Plots were weeded on three occasions prior to achieving total crop cover to smother weeds but no major pests and diseases were encountered.
2.3. Fertiliser treatments and applications
The field experiment was conducted to determine the response of Solanum aethiopicum, S. macrocarpon and S. torvum to three different forms of K fertilisers. The K fertilizers were (i) liquid drench of potassium chloride (KCl) containing 50 to 52% K (60–63% K2O) and 45 to 47% Cl−; (ii) Granular Muriate of potash (MoP) or potassium chloride (KCl), containing 60 to 63% K2O; and (iii) Granular Sulphate of potash (SoP) or potassium sulphate, containing 50% K2O (Chemico Limited, Tema, Ghana). In the liquid KCl experiment, five levels of K, comprising of control with no K fertiliser [K0], 5 mM KCl [K1], 10 mM KCl [K2], 15 mM KCl [K3] and 20 mM KCl [K4], were applied to each of the vegetables. A 50:50 split application was adopted for the liquid KCl application. The first application was applied at 2 weeks after transplanting when seedlings have established and the second at anthesis of each crop. To ensure even application to the crop, the KCl was applied as a high-volume drench to leaves using a knapsack sprayer, with the operator wearing personal protective equipment of overalls, boots, and face-shield and nitrile gloves. Ergonomically acceptable drench rates were identified and calibrated so that all the replicates plants of the three vegetables were treated from a tank at an appropriate walking distance (Broadley et al., 2010; Chilimba et al., 2012). Care was taken to avoid aerosol drift to other plots. Plots were treated in ascending order of target K application rates and no water was applied to control plots at the time of liquid fertiliser application to minimise any risk of K-contamination (Chilimba et al., 2012).
There were five K treatment levels each for the granular MoP and SoP fertilizers to each crop. The treatments for MoP consisted of a control with no K fertiliser (K0), 100 (K1), 200 (K2), 300 (K3) and 450 (K4) kg KCl ha−1. Similarly, the treatments for SoP consisted of a control with no K fertiliser [K0], 100 (K1), 200 (K2), 300 (K3) and 450 (K4) kg K2O ha−1. For each granular fertilizer type, granules were applied via calibrated cups to the base of individual plants using a hand-placement method. A 50:50 split application was adopted for all fertilizers. The first application was applied at 2 weeks after transplanting when seedlings have established and the second at anthesis of each crop. These treatments were applied to each of the plots. Thus, in this field experiment, there were 15 fertiliser treatments and these were tested on 3 crops. Each crop × fertilizer treatment was replicated twice per block. There were 2 blocks, giving effectively 4 replicates and a total of 180 plots. Recommended basal application of 210 kg ha−1 nitrogen (N) from urea (46% N) and 50 kg phosphorus (P2O5) ha−1 from triple super phosphate (45% P2O5, 15% Ca) were administered to all plots (https://mofa.gov.gh/site/; accessed on 20/01/2017). Basal applications were given shortly after transplanting when seedlings have established.
2.4. Yield, fruit and leaf tissue analysis for K and other minerals
Yield data was collected from all trials including the non-fertilized controls and the four K fertilizer application rates for each K source and vegetable. Harvesting begun when, approximately, 50% of fruits were at harvesting maturity stage and continued for 5 to 8 weeks, depending on the crop. Fruits from six plants in the centre of each plot were harvested weekly, immediately counted and weighed. Yield data included overall fruit number, overall fruits fresh weight, fruit number per plant and fresh weight per plant. Fruits from second harvest of each crop were used for whole fruit K analysis. Fruits from the six middle plants of each plot were harvested and composited into one replicate, giving two replicates per block for all treatments. The fruits were placed into paper bags, sent to the lab, cleaned and oven-dried at 70 °C to constant weight before elemental tissue analysis.
For leaf tissue analysis, the most-recently-matured-leaf (MRML) was considered to provide most sensitive indicator of the nutritional status of the plant. The MRMLs are the matured, fully open, full-sized physiologically active leaves that has turned light-green juvenile colour to darker-green colour (Hochmuth et al., 2012). Five each of MRML (petiole + leaflet blade) were sampled between 0900 and 1100 hour from the six middle plants of each treatment plot just before fruit-set (Hochmuth et al., 2012). Leaves from the six plants were composited into one, giving two replications per block for all treatments. To ensure integrity of samples, the plants from each composite were immediately placed in paper bags and carted to the lab for decontamination and subsequent oven-drying and elemental leaf tissue analysis. Both fruit and leaf samples were decontaminated from potential contaminants by quickly rinsing in dilute (2%) phosphate free detergent and rinsed twice under distilled water before slicing into pieces and dried at 65 °C in a forced-air oven for 72 hours, and homogenized into powdery form using a heavy duty stainless steel Waring commercial laboratory blender (Waring Products, Inc., USA).
To estimate fruit K bioavailability, K concentration was determined in the raw fruits as well as in processed (cooked) fruits but leaf K was determined only in raw samples. In order to obtain cooked fruits, fruits from six plants composited into two replicates of each treatment were boiled for 10 min in 100 mL of tap water in Pyrex flasks previously cleaned with nitric acid (ultrapure, 10% v/v). Fruits of S. aethiopicum and S. macrocarpon were diced before cooking and the boiling time of 10 min was informed by the typical cooking time for these vegetables in Ghanaian homes. Here, fruits of S. torvum were not diced before cooking because these fruits are small in size and are typically not diced before cooking in Ghanaian homes. Cooked fruits were dried at 65 °C in a forced-air oven for 72 hours and ground using a heavy duty stainless steel Waring laboratory blender (Waring Products, Inc., USA) to fine powder. The milled samples (∼0.4 g DW) were digested at 360 °C for two hours using the sulphuric acid-hydrogen peroxide digestion method (Allen et al., 1974). Digested samples were made up to volume and stored at room temperature pending elemental analysis. Flame photometry (model PFP7 Jenway Ltd, UK) was used for the detection of K using standard K solutions for calibration and deionised water as reference and the K concentration was calculated in percent K DW (Deal, 1954; Allen et al., 1974). Supplementary figure 1 shows an example of the standard curves from the flame photometer, generated from analytical standards during the tissue K analysis in the laboratory. In addition to K, analysis of tissue concentration of nitrogen (N), phosphorus (P), calcium (Ca), iron (Fe), copper (Cu) and zinc (Zn) was carried out. The micro-Kjedahl procedure (Coombs et al., 1985) was used for the determination of tissue N concentration. The P content was determined with the molybdate ascorbic acid blue method (John, 1970) after digestion with HClO4 and H2SO4 acid. The digested samples were analysed for Ca, Fe, Cu and Zn using atomic absorption spectrophotometer (AAS; model 210 VGP, Buck scientific), following aspiration into and the calibration for respective minerals on the AAS.
2.5. Data analysis
Data were analysed by analysis of variance (ANOVA) using the GenStat Twelfth Edition, Procedure Library Release PL20.1, 2009 (VSN International Limited, Rothamsted Experimental Station, Hemel Hempstead, UK). Graphical data representations were produced either in GenStat, R (R Core Team, 2012) or Microsoft Excel. Yield was calculated as number of fruits per plant and fruit fresh weight per plant. Yield data were analysed independently for each vegetable and all data are presented as the mean of the two blocks and aggregate weekly harvests. The ANOVA was used to estimate mean differences across treatments with the treatment means compared by least significant difference (l.s.d.) (P = 0.05). Factors for the general ANOVA were K fertilizer type and rate of application and the interaction of K fertilizer type and rate of application. Analysis of concentration of K and other minerals were performed independently for leaf and fruits. A multifactor ANOVA model was used for treatment comparisons at P = 0.05, with separation of the means by the l.s.d. method. Factors for the tissue K ANOVA were crop, K fertilizer type and rate of application, as well as the two-way and three-way interactions of all the factors. Data exploration suggested that assumptions of data normality were acceptable. Sample residual plots shown in Supplementary figure 2 suggest that residuals were, to a large extent, randomly distributed with constant variance.
3. Results
3.1. Yield
Yield analysis of S. aethiopicum showed no statistically significant effect of K fertilizer type on both yield parameters measured in this study (i.e. number of fruits plant−1 [NoF; P = 0.840] and fruits fresh weight plant−1 [FFW; P = 0.655]; data not shown). There was however significant gain (P < 0.001) in both NoF and FFW with increasing K fertiliser application rate, with the highest FFW of 328 g plant−1 observed for treatment K2 (i.e. 200 kg/ha or 10 mM K; Fig. 1D) with a gradual decline in this parameter as the rate of K application increased. According to the two-way analysis, with respect to FFW, it appears that yield obtained depended on the type of K fertiliser and the rate of application (P < 0.001). The fertilizer type × application rate interaction indicated that highest FFW is obtained at K2 (10 mM liquid KCl), K3 (300 kg ha−1 granular KCl) and at K1 (100 kg ha−1 granular K2O) for liquid KCl, MoP and SoP, respectively (Fig. 1A). Analogous trends were observed in the analysis of NoF (data not shown). Of the mathematical models tested (linear, logarithmic, polynomial and power), a quadratic model best described the K rate relationship to yield (R2 = 0.82; Fig. 1D).
Fig. 1.
Effect of type and rate of application of K fertilizer on fruit yield of (A) Solanum aethiopicum; (B) S. macrocarpon; (C) S. torvum. The error bars are standard errors. Differences in fruit fresh weight between fertilizers and rates of application were established using ANOVA and are shown by l.s.d. (P < 0.05), with ‘Fert’ representing type of K fertilizer, ‘Rate’ representing rate of fertilizer application, and ‘Fert × Rate’ representing the interaction of type of K fertilizer and rate of fertilizer application. (D) Relationship between rate of fertilizer application and overall fruit fresh weight for all the three crops, with trend models fitted. Control (no K fertiliser); K100/5 (100 kg granular K2O ha−1 or 100 granular kg KCl ha−1 or 5 mM KCl); K200/10 (200 kg granular K2O ha−1 or 200 granular kg KCl ha−1 or 10 mM KCl); K300/15 (300 kg granular K2O ha−1 or 300 granular kg KCl ha−1 or 15 mM KCl); K450/20 (450 kg granular K2O ha−1 or 450 granular kg KCl ha−1 or 20 mM KCl).
Similarly, yield analysis of S. macrocarpon showed no statistically significant effect of type of K fertilizer on both NoF (P = 0.383) and FFW (P = 0.661, data not shown), albeit the FFW of 407 g plant−1 recorded by plants grown under SoP was higher than the 378 and 362 g plant−1 recorded by plants grown under liquid drench of KCl and MoP, respectively. Although there appeared to be an increasing FFW to increasing K application rate, with a linear K rate relationship to yield (R2 = 0.75; Fig. 1D) and about 1.2-fold increase in FFW between K0 (control; 338 g plant−1) and K4 (439 g plant−1), there was no significant effect (P = 0.663) of the rate of application on yield (Fig. 1B and D). There was also no significant interaction effect (P = 0.092) of K fertilizer type and the rate of application on FFW (Fig. 1B) with parallel trends observed for NoF (P = 0.426).
Yield analysis of S. torvum showed statistically significant effect (P < 0.001) of type of K fertilizer, rate of application and the interaction of fertilizer type and rate of application on FFW (Fig. 1C and D). Equally there was statistically significant effect of type of K fertilizer (P < 0.024), rate of application (P = 0.016) and the interaction of fertilizer type and rate of application (P < 0.004) on NoF (data not shown). The yield of plants grown under SoP (i.e. FFW of 154.3 g plant−1 and NoF of 15 trusses of fruits plant−1) was lower than that of plants grown under liquid KCl (FFW of 233.3 g plant−1 and NoF of 19 trusses of fruits plant−1) and MoP (FFW of 228.1 g plant−1 and NoF of 18 trusses of fruits plant−1). In comparison to the control, the NoF of K1 increased by approximately 1.5 fold, decreased slightly thereafter and then plateaued (Ko = 13 K1 = 20, K2 = 18 trusses of fruits plant−1; data not shown). In comparison to the control, the FFW of K1 increased by approximately 2.2 fold, decreased slightly thereafter and then plateaued (Ko = 118.6 K1 = 257.8, K2 = 251.5 g plant−1; Fig. 1D). The fertilizer type × application rate interaction indicated that highest FFW of 315.6, 357.6 and 210.1 g plant−1 is obtained at K2 (10 mM liquid KCl), K1 (100 kg ha−1 granular KCl) and at K4 (450 kg ha−1 granular K2SO4) for liquid KCl, MoP and SoP, respectively (Fig. 1C). Similar trends were observed in the analysis of NoF (data not shown). Of the mathematical models tested, a quadratic model best described the K rate relationship to yield (R2 = 0.67; Fig. 1D).
3.2. K concentration in raw leaves and fruits
3.2.1. Main treatment effects
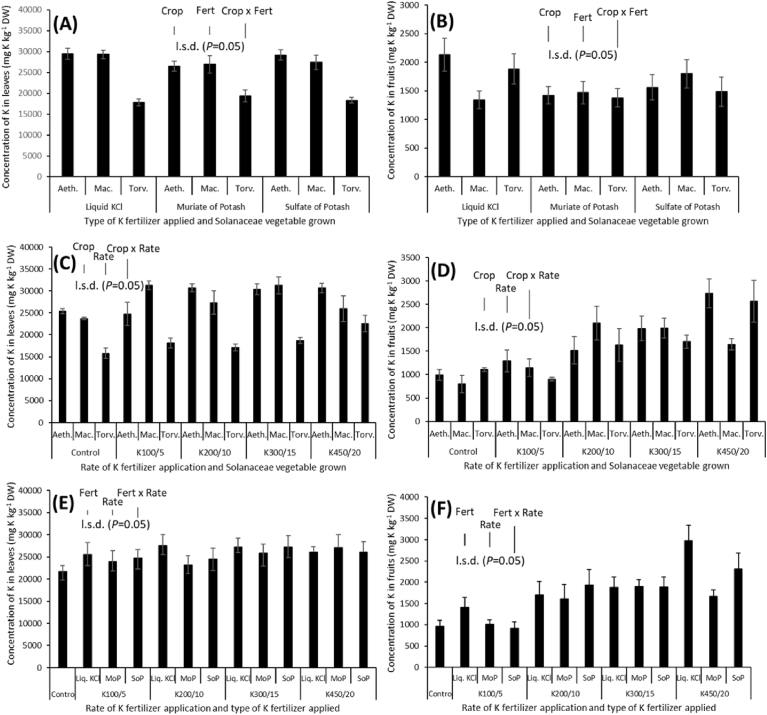
The overall K content in the leaves of the solanaceae vegetables ranged between 2003 to 41272 mg K kg−1 DW, with a grand mean of approximately 25000 ± 561 mg K kg−1 DW (mean ± s.e.m). Potassium concentration in the leaves of all the three crops varied significantly (P < 0.001), with S. torvum leaves recording the lowest concentration of 18477 ± 598 mg K kg−1 DW (Figs. 2A and 4A). With respective values of 25527 ± 938, 24254 ± 1024 and 24977 ± 963 mg K kg−1 DW for liquid drench of KCl, granular MoP and SoP, no statistically significant effect of the type of K fertilizer was observed on the leaf K concentration across all the three crops (Fig. 2C). Across all the three crops, the application of different K fertilizer rates had a significant effect (P < 0.001) on leaf K concentration (Fig. 2E). The relationship between leaf K concentration and the fertilisation rate was approximately linear (R2 = 0.81; Fig. 2E). The highest leaf K concentration of 26781 ± 1239 mg K kg−1 DW was recorded by plants grown under treatment K3, and in comparison to the control, this was about 24 % increase or 1.24-fold increase (Fig. 2E).
Fig. 2.
Concentration of K in leaves (A, C) and fruits (B, D), depending on type of solanaceae vegetable (A, B) and type of K fertilizer applied (C, D) (n = 4). (E) Influence of fertilization with K on leaf and fruit K concentration in solanaceae vegetables grown in a field under rain-fed conditions (±s.e.m).
Fig. 4.
Concentration of K in leaves (A) and fruits (B), depending on the interaction of the crop, the type of K fertilizer applied and rate of K fertiliser application. Data are the mean of 4 plants, with error bars representing the s.e.m. Differences between crop, K fertilizer type and rate of application were established using ANOVA and are shown by l.s.d. (P < 0.05), with ‘Crop’ representing type of solanaceae vegetable, ‘Fert’ representing type of K fertilizer, ‘Rate’ representing rate of K fertilizer application, and their respective interactions indicated by ‘x’.
The overall K content in the fruits of the solanaceae vegetables ranged between 118.0 to 4674 mg K kg−1 DW, with a grand mean of 1607 ± 75 mg K kg−1 DW. The effect of crop was not significant (P = 0.469; Fig. 2B). The effect of K fertiliser type was significant (P = 0.04) with average fruit K concentration dropping from 1784 + 145 mg K kg−1 DW for plants grown under drench of liquid KCl to 1616 ± 97 mg K kg−1 DW for plants fertilised with SoP and then to 1423 ± 139 mg K kg−1 DW for plants grown under MoP fertilization (Fig. 2D). Across all the three crops, the application of different K fertilizer rates had a significant effect (P < 0.001) on fruit K concentration (Fig. 2E). The relationship between fruit K concentration and the fertilisation rate was approximately linear (R2 = 0.96; Fig. 2E). The highest fruit K concentration of 2316 ± 197 mg K kg−1 DW was recorded by plants grown under treatment K4, and in comparison to the control, this was about 140 % increase (2.40-fold increase; Fig. 2E).
3.2.2. Interaction effects on leaf and fruit K concentration
There was no significant crop × type of K fertilizer interaction for concentration of K in leaves (P = 0.353; Fig. 3A). There was however significant interaction effect for crop × rate of K fertilizer application for K concentration in leaves (P = 0.009) (Fig. 3C). The nature of the response of leaf K concentration to rate of K fertilizer application was inconsistent among the three crops. The leaf K concentration of the control treatment (25382 ± 561 mg K kg−1 DW) and treatment K1 (approximately 25000 ± 2650 mg K kg−1 DW) for S. aethiopicum appear to be similar, but increased to 30693 ± 900 mg K kg−1 DW for the treatment K2 and then plateaued (Fig. 3C). The trend was inexplicably inconsistent for S. macrocarpon, increasing from K0 (23723 ± 256 mg K kg−1 DW) to K1 (31292 ± 1038 mg K kg−1 DW) and then falling to 27361 ± 2682 mg K kg−1 DW at K2 before appearing to rise again to 31280 ± 1930 mg K kg−1 DW at treatment K3 (Fig. 3C). For S. torvum, there was largely an increasing trend with increased rate of K fertilizer application (Fig. 3C). The K contained in leaves of the three crops was not significantly (P = 0.871) affected by the interaction of the type of K fertilizer and the rate of its application (Fig. 3E). There was no significant interaction for crop × type of K fertilizer × rate of fertilizer application on leaf K concentration (Fig. 4A).
Fig. 3.
Concentration of K in leaves (A, C, E) and fruits (B, D, F), depending on (A, B) the interaction of the crop and the type of K fertilizer applied; (C, D) the interaction of crop and rate of K fertiliser applied; (E, F) the interaction of the type of K fertilizer applied and rate of application. Aeth.: S. aethiopicum; Marc.: S. macrocarpon; Torv.: S. torvum. Data are the mean of 4 plants, with error bars representing the s.e.m. Differences between crop, K fertilizer type and rate of application were established using ANOVA and are shown by l.s.d. (P < 0.05), with ‘Crop’ representing type of solanaceae vegetable, ‘Fert’ representing type of K fertilizer, ‘Rate’ representing rate of K fertilizer application, and their respective interactions indicated by ‘x’.
There was significant crop × type of K fertilizer interaction for concentration of K in fruits (P = 0.027; Fig. 3B). Whilst the highest fruit K concentration for S. aethiopicum (2130 ± 288 mg K kg−1 DW) and that for S. torvum (1883 ± 267 mg K kg−1 DW) were recorded by plants fertilised with liquid KCl, the highest fruit K concentration for S. macrocarpon (1801 ± 247 mg K kg−1 DW) was obtained by plants to which SoP was applied (Fig. 3B). There was also significant interaction effect for crop × rate of K fertilizer application for fruits (P = 0.016) K concentration (Fig. 3D). The K concentration in fruits of S. aethiopicum appear to increase linearly with increasing rate of K fertilizer application from treatment Ko (992 ± 120 mg K kg−1 DW) to K4 (2736 ± 309 mg K kg−1 DW), but that of S. macrocarpon increased up to treatment K2 and subsequently declined (Fig. 3D). Interestingly, the K concentration in fruits of S. torvum at Ko (1109 ± 36 mg K kg−1 DW) was close to that of K1 (approximately 1000 ± 38 mg K kg−1 DW) but subsequently showed an increasing trend to K4 (2567 ± 445 mg K kg−1 DW) (Fig. 3D). The K contained in fruits of the three crops was not significantly (P = 0.073) affected by the interaction of the type of K fertilizer and its rate of application (Fig. 3F). There was significant (P = 0.007) interaction for crop × type of K fertilizer × rate of fertilizer application on fruit K concentration (Fig. 4B). For example, fruit K concentration for S. aethiopicum seem to be increasing with increasing rate of liquid KCl application but such a consistent trend is evident for other crops and K fertiliser types (Fig. 4B). Again, it also seems that at higher rates of K fertilizer application, fruit K concentration of S. aethiopicum was largest, followed by that of S. torvum (Fig. 4B).
3.2.3. Effect of K fertilizer treatments on K concentration in cooked solanaceae fruits
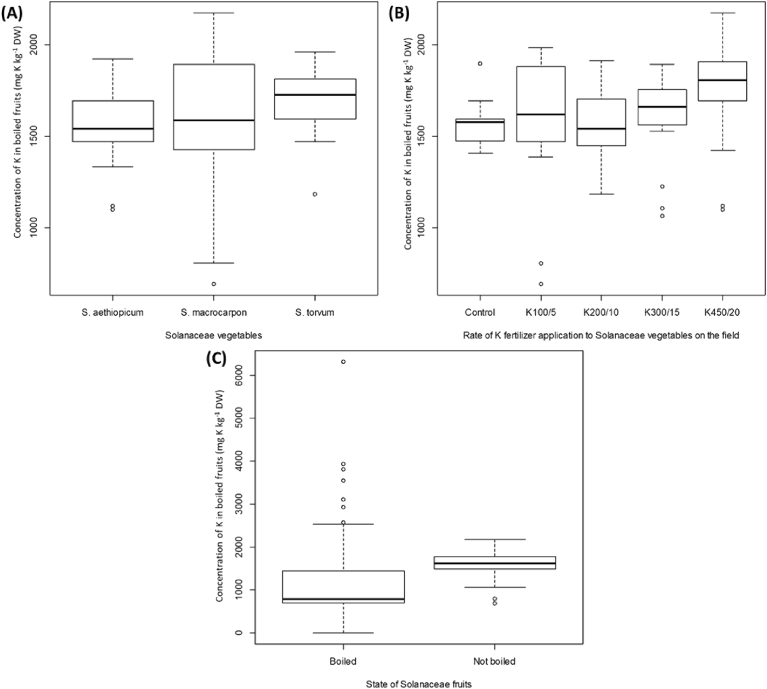
The K concentration in cooked fruits was significantly influenced by the crop (P = 0.003; Fig. 5B), the rate of K fertilizer application (P = 0.008; Fig. 5B), the interaction of crop × rate of soil or foliar K fertilizer application (P < 0.001; data not shown), the interaction of type of K fertilizer application × rate of soil or foliar K fertilizer application (P < 0.001; data not shown) and the interaction of the crop × the type of K fertilizer application × rate of soil or foliar K fertilizer application (P = 0.024; data not shown). There was no significant effect of K fertilizer type (P = 0.084) and the interaction of crop × and type of K fertilizer (P = 0.085) on K concentration in cooked fruits. Among the three crops, the highest K concentration in cooked fruits was found in S. torvum (1710 ± 29 mg K kg−1 DW; Fig. 5A). To a large extent, progressively higher K concentrations were found in cooked fruits with increasing foliar or soil K fertiliser applications (Fig. 5B). Compared to the control treatment (K0; 1579 ± 33 mg K kg−1 DW), there was 1.1 fold (11.37%) increase in the K concentration of cooked fruits of treatment K4 (1757 ± 70 mg K kg−1 DW; Fig. 5B). An analysis of variance between K concentration of raw versus cooked fruits revealed a significant difference (P < 0.001), with about 26 percent (or 1.35 fold) decrease in the K concentration of the cooked samples relative to the raw samples (Fig. 5C).
Fig. 5.
The K concentration in cooked fruits as affected by (A) type of solanaceae vegetable, (B) the K soil or foliar fertilizer treatments; (C) K concentration of cooked and raw fruits of three solanaceae vegetables averaged across all treatments.
3.2.4. Leaf and fruit K concentration of other minerals
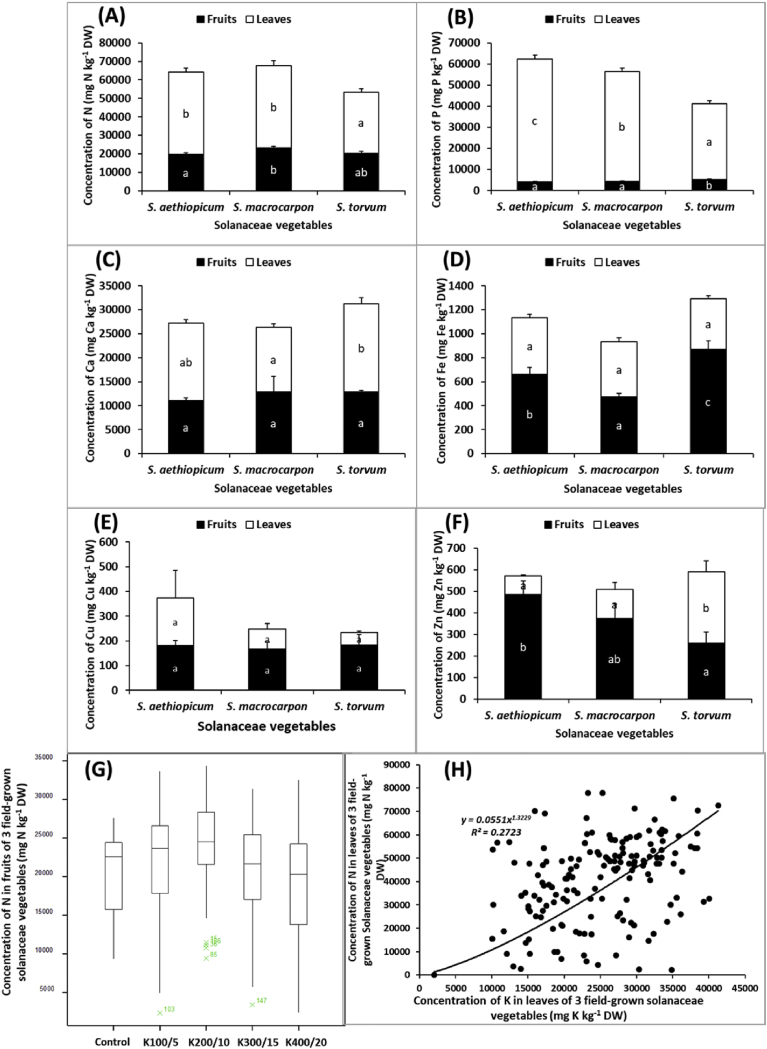
Across all K fertilizer types and application rates, the type of vegetable affected leaf (P < 0.001) and fruit (P = 0.018) N concentration (Fig. 6A: Table 1); leaf (P < 0.001) and fruit (P < 0.001) P concentration (Fig. 6B; Table 1); leaf (P = 0.001) but not fruit (P = 0.765) Ca concentration (Fig. 6C; Table 1) as well as leaf (P < 0.001) and fruit (P = 0.038) Zn concentration (Fig. 6F; Table 1). The type of crop also affected leaf (P < 0.001) and fruit (P < 0.001) Fe concentration (Fig. 6D); and fruit (P < 0.001) but not leaf (P = 0.927) Cu concentration (Fig. 6E; Table 1). The leaf N concentration of S. torvum (33110 ± 1813 mg N kg−1 DW) was significantly lower than the other two crops (Fig. 6A), for which N concentrations were similar. Similar trend was observed for fruit N concentration, although S. aethiopicum in this case was comparable to S. torvum (Fig. 6A). Whilst most tissue P was partitioned into the leaves than fruits, the concentration in S. torvum was found to be lower (Fig. 6B). Concentration of Ca and Fe in leaves and fruits respectively, was greater in S. torvum (Fig. 6C and D).
Fig. 6.
Effect of crop on fruit and leaf (A) N, (B), P, (C), Ca, (D) Fe, (E) Cu, and (F) Zn concentration of field-grown vegetables. Differences between means were compared by Duncan's Multiple Range Test. Bars with the same letter do not differ significantly (n = 4; +SEM). (G) Effect of K fertilizer rate on fruit N concentration of 3 field-grown vegetables; (H) Relationship between leaf K and N concentration of 3 field-grown vegetables.
Table 1.
Effect of crop, K fertilizers and application rates on N, P, Ca, Fe, Cu and Zn in (A) fruits and (B) leaves of 3 Solanaceae crops grown under field conditions. Means of 4 replicates (in mg [mineral] kg−1 DW) followed by the same letter (established using Tukey's 95% confidence intervals) do not differ significantly; Bold: significant main or interaction effect.
| Crop | Rate | N | P | Ca | Fe | Cu | Zn |
|---|---|---|---|---|---|---|---|
| A: Fruits (mean ± s.e.m. mg kg−1) | |||||||
| S. aethiopicum | Control | 18933a ± 2004 | 4433ab ± 327 | 14793a ± 862 | 645abc ± 50 | 220a ± 36 | 547a ± 154 |
| K100/5 | 18659a ± 2560 | 3412a ± 435 | 10002a ± 997 | 612abc ± 113 | 153a ± 55 | 411a ± 101 | |
| K200/10 | 21904a ± 1879 | 3743ab ± 509 | 12174a ± 1435 | 935bc ± 232 | 202a ± 60 | 321a ± 98 | |
| K300/15 | 20684a ± 1974 | 4300ab ± 379 | 9290a ± 920 | 518abc ± 61 | 149a ± 51 | 549a ± 153 | |
| K450/20 | 18266a ± 2134 | 4555ab ± 974 | 9222a ± 1265 | 593abc ± 104 | 177a ± 36 | 595a ± 191 | |
| S. macrocarpon | Control | 24386a ± 445 | 3583ab ± 454 | 10513a ± 1326 | 545abc ± 60 | 94a ± 29 | 111a ± 21 |
| K100/5 | 24509a ± 2410 | 4731ab ± 433 | 25543a ± 1239 | 339a ± 41 | 168a ± 85 | 284a ± 128 | |
| K200/10 | 24779a ± 1940 | 4115ab ± 475 | 9089a ± 1166 | 396ab ± 76 | 235a ± 80 | 429a ± 136 | |
| K300/15 | 24323a ± 1553 | 4339ab ± 314 | 9053a ± 1014 | 586abc ± 64 | 155a ± 68 | 614a ± 242 | |
| K450/20 | 17659a ± 2352 | 4820ab ± 314 | 9873a ± 822 | 489ab ± 94 | 176a ± 45 | 430a ± 136 | |
| S. torvum | Control | 17919a ± 1686 | 4838ab ± 163 | 11280a ± 412 | 646abc ± 89 | 107a ± 22 | 70a ± 11 |
| K100/5 | 21389a ± 1800 | 5551ab ± 162 | 15025a ± 884 | 775abc ± 127 | 115a ± 23 | 235a ± 122 | |
| K200/10 | 25256a ± 1691 | 4968ab ± 669 | 12756a ± 981 | 919abc ± 129 | 147a ± 23 | 155a ± 58 | |
| K300/15 | 16962a ± 2116 | 5535ab ± 359 | 13054a ± 852 | 1098c ± 182 | 284a ± 132 | 328a ± 119 | |
| K450/20 | 20101a ± 2430 | 5851b ± 445 | 12102a ± 656 | 917abc ± 215 | 258a ± 175 | 507a ± 172 | |
| Crop |
F2,135 = 4.1; P = 0.019 |
F2,135 = 10.16 P < 0.001 |
F2,135 = 0.26; P = 0.768 | F2,135 = 13.94; P < 0.001 | F2,135 = 0.08; P = 0.924 |
F2,135 = 3.2; P = 0.044 |
|
| Type of K fertilizer. |
F2,135 = 0.27; P = 0.767 |
F2,135 = 0.91; P = 0.405 |
F2,135 = 0.93; P = 0.396 |
F2,135 = 1.17; P = 0.313 |
F2,135 = 1.97; P = 0.144 |
F2,135 = 0.35; P = 0.707 |
|
| Rate of fertilizer application |
F4,135 = 2.75; P = 0.031 |
F4,135 = 1.51; P = 0.202 |
F4,135 = 1.14; P = 0.343 |
F4,135 = 1.19; P = 0.316 |
F4,135 = 0.5; P = 0.7 |
F4,135 = 2.28; P = 0.064 |
|
| Crop × Type of K Fertilizer |
F4,135 = 0.68; P = 0.606 |
F4,135 = 0.47; P = 0.759 |
F4,135 = 0.69; P = 0.599 |
F4,135 = 3.58; P = 0.008 |
F4,135 = 2.8; P = 0.055 |
F4,135 = 0.43; P = 0.788 |
|
| Crop × Rate of application |
F8,135 = 1.37; P = 0.216 |
F8,135 = 0.77; P = 0.634 |
F8,135 = 0.98; P = 0.454 |
F8,135 = 1.67; P = 0.11 |
F8,135 = 0.74; P = 0.658 |
F8,135 = 0.79; P = 0.611 |
|
| Type of K fertilizer × Rate |
F8,135 = 0.70; P = 0.694 |
F8,135 = 0.69; P = 0.700 |
F8,135 = 0.81; P = 0.593 |
F8,135 = 1.46; P = 0.177 |
F8,135 = 0.80; P = 0.603 |
F8,135 = 0.98; P = 0.454 |
|
| Crop × K fertilizer × Rate |
F16,135 = 0.72; P = 0.772 |
F16,135 = 1.20; P = 0.275 |
F16,135 = 1.0; P = 0.451 |
F16,135 = 0.69; P = 0.802 |
F16,135 = 1.38; P = 0.158 |
F16,135 = 0.58; P = 0.893 | |
| B: Leaves (mean ± s.e.m. mg kg−1) | |||||||
| S. aethiopicum | Control | 51457a ± 5913 | 59081ef ± 3731 | 13984a ± 2098 | 410ab ± 47 | 130a ± 53 | 108a ± 11 |
| K100/5 | 43816a ± 5710 | 57283def ± 1980 | 17936a ± 1438 | 355a ± 60 | 51a ± 9 | 69a ± 7 | |
| K200/10 | 45216a ± 4317 | 49930bcdef ± 3787 | 17329a ± 1545 | 539abc ± 39 | 631a ± 548 | 88a ± 13 | |
| K300/15 | 45504a ± 3000 | 58623ef ± 4855 | 16782a ± 1564 | 491abc ± 77 | 83a ± 23 | 70a ± 8 | |
| K450/20 | 37075a ± 4755 | 66278f ± 4652 | 14453a ± 1588 | 572bc ± 91 | 74a ± 12 | 99a ± 15 | |
| S. macrocarpon | Control | 40789a ± 6283 | 58351def ± 3052 | 12277a ± 1197 | 420ab ± 29 | 132a ± 46 | 324ab ± 133 |
| K100/5 | 43180a ± 6947 | 51288cdef ± 3599 | 12376a ± 968 | 501abc ± 86 | 41a ± 12 | 137a ± 62 | |
| K200/10 | 48674a ± 4154 | 55845def ± 2887 | 12987a ± 1082 | 375ab ± 53 | 34a ± 9 | 68a ± 7 | |
| K300/15 | 47908a ± 5852 | 50190bcdef ± 4509 | 18033a ± 2136 | 640c ± 90 | 47a ± 8 | 67a ± 12 | |
| K450/20 | 42748a ± 5811 | 44320bcde ± 5172 | 12091a ± 1842 | 371ab ± 76 | 158a ± 99 | 91a ± 24 | |
| S. torvum | Control | 34052a ± 6217 | 34380abc ± 1777 | 15000a ± 1645 | 422ab ± 44 | 27a ± 5 | 306ab ± 70 |
| K100/5 | 27939a ± 2588 | 26235a ± 1727 | 19982a ± 3858 | 425ab ± 71 | 74a ± 10 | 569b ± 152 | |
| K200/10 | 34737a ± 3517 | 41311abcd ± 3372 | 15962a ± 2327 | 497abc ± 62 | 60a ± 15 | 212ab ± 57 | |
| K300/15 | 34423a ± 3398 | 33344ab ± 1749 | 19887a ± 2501 | 374ab ± 45 | 69a ± 17 | 158a ± 63 | |
| K450/20 | 34399a ± 3982 | 43231abcde ± 4939 | 21307a ± 2904 | 393ab ± 62 | 28a ± 6 | 410ab ± 169 | |
| Crop | F2,135 = 9.6; P < 0.001 | F2,135 = 55.03; P < 0.001 | F2,135 = 6.86; P = 0.001 |
F2,135 = 0.92; P = 0.399 |
F2,135 = 1.3; P = 0.276 |
F2,135 = 14.6; P < 0.001 |
|
| Type of K fertilizer. | F2,135 = 0.95; P = 0.39 |
F2,135 = 0.55; P = 0.576 |
F2,135 = 0.26; P = 0.77 | F2,135 = 0.57; P = 0.564 | F2,135 = 0.75; P = 0.474 |
F2,135 = 2.6; P = 0.078 |
|
| Rate of K fertilizer application | F4,135 = 0.4; P = 0.566 |
F4,135 = 1.6; P = 0.178 |
F4,135 = 1.9; P = 0.115 | F4,135 = 0.9; P = 0.465 | F4,135 = 0.8; P = 0.528 |
F4,135 = 2.66; P = 0.035 |
|
| Crop × Type of K fertilizer | F4,135 = 0.1; P = 0.935 |
F4,135 = 2.07; P = 0.088 |
F4,135 = 0.31; P = 0.873 | F4,135 = 2.11; P = 0.083 | F4,135 = 1.06; P = 0.377 |
F4,135 = 1.22; P = 0.306 |
|
| Crop × Rate of application |
F8,135 = 0.4; P = 0.746 |
F8,135 = 3.65; P < 0.001 |
F8,135 = 1.04; P = 0.413 | F8,135 = 2.5; P = 0.014 | F8,135 = 1.1; P = 0.368 |
F8,135 = 2; P = 0.051 |
|
| Type of K fertilizer × Rate | F8,135 = 2.32; P = 0.023 |
F8,135 = 1.14; P = 0.342 |
F8,135 = 0.31; P = 0.963 | F8,135 = 1.17; P = 0.324 | F8,135 = 1; P = 0.439; |
F8,135 = 0.8; P = 0.601 |
|
| Crop × K fertilizer × Rate | F16,135 = 1.38; P = 0.158 | F16,135 = 1.58; P = 0.081 | F16,135 = 1.30; P = 0.204 | F16,135 = 1.58; P = 0.082 | F16,135 = 0.83; P = 0.646; | F16,135 = 0.73; P = 0.761 | |
Tissue N concentration was particularly interesting because the rate of K fertilizer application had an effect on fruit N concentration. Although the significance of this effect was marginal (P = 0.031), fruit N concentration increased with increasing K fertilizer application rate up to treatment K2 with an overall difference of approximately 10% compared to the control (Fig. 6G). Averaged across all the treatments, there was a significantly strong positive correlation between leaf K concentration and leaf N concentration (r = 0.35; P < 0.001; Fig. 6H). But for the significant effect (P = 0.031) on fruit N concentration, the type and rate of K fertiliser applied did not have significant influence on fruit or leaf tissue concentration of any of the other element (Table 1). Only a few interaction effects were observed on fruit and leaf tissue concentration of the other elements, including type of K fertilizer × rate on leaf N (P = 0.023), crop × type of K Fertilizer on fruit Fe concentration (P = 0.008), crop × rate of application on leaf P (P < 0.001) and Fe (P = 0.014) concentration (Table 1). For example, averaged across all rates of K fertilizer application, S. torvum recorded the highest fruit Fe concentration, with values of 828 ± 79, 666 ± 84 and 1119 ± 163 mg Fe kg−1 DW for liquid KCl, MoP and SoP, respectively. However, S. macrocarpon recorded the lowest fruit Fe concentration, with values of 441 ± 41, 523 ± 73 and 450 ± 49 mg Fe kg−1 DW for liquid KCl, MoP and SoP, respectively.
4. Discussion
4.1. Tissue K concentration
4.1.1. Effect of type of crop
Potassium concentration in the leaves of all the three crops varied significantly (P < 0.001), and the ranking followed: S. aethiopicum (28371 mg K kg−1 DW) > S. macrocarpon (27911 mg K kg−1 DW) > S. torvum (18477 mg K kg−1; Figs. 2A and 4A). Given that the leaves of S. macrocarpon are commonly eaten, unlike S. aethiopicum and torvum, it is interesting to note that agronomic biofortification could raise the average K content of the leaves of macrocarpon close to that of aethiopicum in the current study. Although the effect of crop was not significant for fruit-K (Fig. 2B), the relatively higher fruit-K content of S. aethiopicum and torvum than macrocarpon is interesting since the fruits of S. aethiopicum and S. torvum are commonly eaten. Considerably, the relatively high fruit-K content of S. aethiopicum agrees with Nyadanu and Lowor (2015) who reported higher K composition in fruits of S. aethiopicum, compared to S. macrocarpon and S. torvum. Genotype-dependent variation of K+ concentrations in shoots has been reported in other crops (Harada and Leigh, 2006) including tomatoes (Taber, 2006), wild and domesticated watermelons (Fan et al., 2013) and maize (Harada and Leigh, 2006). The variation observed here could be explained by a number of factors including, size and soil volume exploration by root system, uptake and efflux by roots, storage in root cell vacuoles, loading into the xylem, uptake and storage in leaf cells, among others (Harada and Leigh, 2006).
4.1.2. Effect of edible plant part (leaf versus fruits)
It was also evident from the study that grand mean leaf K-concentration of 24919 mg K kg−1 in the selected crops was significantly greater (P < 0.001) than the grand mean fruit K-concentration of 1607 mg K kg−1 DW. There is paucity of comparative evidence of leaf-versus fruit-K concentration of the selected crops in the grey literature but in a proximate analysis, Nyadanu and Lowor (2015) reported that the leaf and fruit K-composition of S. macrocarpon collected from farmers' field were 326.54 and 329.52 mg, respectively, suggesting that there was not much of a difference. The report of Bar-Tal and Pressman (1996) however suggests that in greenhouse-grown tomatoes, leaf-K concentrations were higher than that of fruit K-concentrations but K concentration in plant organs was a function of plant age and leaf position on the plant. In sunflower (Helianthus annuus L.), compared to seeds and roots, leaves had the highest proportion of the total plant K at anthesis, but the amount of K decreased markedly during seed filling (Hocking and Steer, 1983). It has been reported that many arable crops often reach their maximal K content at anthesis and because K is very mobile, once in the plant, it is transported and redistributed from senescing leaves to young and growing tissues (Hochmuth et al., 2012; White, 2013). Whilst the greater leaf K-content recorded here could be attributable to our choice of relatively younger leaves (i.e.: MRML, which were at the receiving end of K-redistribution) for leaf-K analysis, the aforementioned reports also suggests that dietary K content is dependent on the plant organ that is consumed and the time of harvest. Interestingly, for S. macrocarpon, whose young leaves are the main parts cooked and consumed as a vegetable, the increased leaf-K composition recorded in the present study is a welcome news.
4.1.3. Effect of type of K fertilizer
In the present study, we sought to examine the effect of the K source on tissue K-composition of our selected vegetables. Various studies have indicated that forms of K fertilizers used in growing crops could influence yield and quality, due to the presence of accompanying anions, such as Cl−, SO42− and NO3− (Michałojć and Buczkowska, 2009). For example, Akhtar et al. (2010) reported that the effect of K on reducing sugar content was more pronounced in fruits fertilised with SoP than those fertilised with MoP. On the other hand, vitamin C contents in tomato fruits increased but incidence of leaf blight disease and insect pest attack reduced with K application in the form of MoP as compared to SOP and control treatments (Akhtar et al., 2010). For Solanaceae vegetables, the use of SoP as a preferred K source has been suggested because SoP is said to enhance dry matter, firmness and pigments, and as well, plays beneficial roles in the formation of sugars and organoleptic components (Tessenderlo Group, 2014). Most vegetables are also sensitive to chloride, making SoP the chloride-free option in delivering K and sulphur in a concentrated form (Tessenderlo Group, 2014). In the current study, we found no significant effect of type of K fertilizer (Fig. 2C) or crop × K source interaction (Fig. 3A) on the leaf K concentration, but significant K source effect (Fig. 2D) and crop × K source interaction were observed on fruit K concentration (Fig. 3B). This result seems to suggest that the source of K has some effect on the translocation or redistribution of K within the plant and for K biofortification programs, the desired plant part should be among the critical considerations in the choice of K fertiliser. This must however be indexed with the potential effect of the type of K fertilizer on other quality parameters of the crop. In addition, we investigated whether K application from granular forms or foliar application using liquid drench of the fertiliser was the most effective. The variance analysis indicated that applying K in liquid via foliar sprays might be advantageous than side placement using granular forms of K (Figs. 2C, and 4). Perhaps, certain soil conditions constrained K availability for uptake by the roots. It is possible that the K applied to the soil was rendered unavailable due to leaching or reduction in soil pH as the soil used was a haplic acrisol.
4.1.4. Effect of rate of K fertilizer application
Although tissue-K concentration of all three vegetables increased significantly with the addition of K (Figs. 2E and 4), the mean ambient tissue K concentration from control plots (967 mg K kg−1 DW for fruits and 21635 mg K kg−1 DW for leaves) does not suggest that control plants were K deficient. Given that leaves of K deficient plants typically contain less than 1.5% K (Hochmuth et al., 2012) and tissue concentrations of 5–40 mg K g dry matter (DM)−1 are generally considered adequate for most crops (White, 2013), the control plants had adequate K to mask deficiency symptoms, but as has been alluded to previously, there were still yield penalties. This suggests that our experimental field is responsive to K fertilization but even so, unfertilized plots have some capacity to retain or supply K to plants, probably due to a high potential buffering capacity (Yawson et al., 2011). Averaged across all treatments, there were 1.24 and 2.40 fold increases in leaf and fruit K concentrations, respectively, over the control (Fig. 1E). The mean leaf and fruit K-concentration of all crops increased significantly with the addition of K (Fig. 2E), but the response curve did not reach an asymptote and was described by a linear equation (y = a + bx). Using the equation, it could be calculated that an application of 100 kg K ha−1 in the form of granular SoP or MoP or 5 mM drench of liquid KCl would increase the leaf and fruit K concentration of the selected Solanaceae vegetables by 11.52 and 3.48%, respectively from its ambient levels. Thus, for soils with similar properties to that used in this study, a reduced rate must be applied or great care must be taken to monitor end products of crops grown under such high rates of K to ensure that dietary K intakes do not exceed the RDA of 3510 mg day−1 person−1 (WHO, 2012).
4.1.5. Effect of cooking on K retention
Loss of K from cooking of high-K foods can be significant (Kimura and Itokawa, 1990). It was therefore important to estimate the retention of K in the cooked edible portion of the biofortified plants. Here, retention of K was tested for fruits only and for a 10-minute cooking, there was a 26% drop in K concentration. Thus, household cooking of the fruits of the selected solanaceaes resulted in the retention of over 70% of the K concentration in the raw fruits. This is comparable to the average cooking losses of 24% reported by Kimura and Itokawa (1990). This drop in K+ has been attributed to the high solubility of K ions in water, leading to leaching or outflow of the mineral into the cooking water when K-rich foods are cooked (Kimura and Itokawa, 1990). Measures to prevent cooking loss have been suggested and may include (i) cooking with as little water as possible or steaming; (ii) cooking for a short duration or avoidance of too much boiling; (iii) eating the boiled food with the soup or incorporating the water in which the food was cooked into the diet; (iv) addition of small amount of salt (about 1% NaCl) in boiling or (v) selection of a cooking method that causes less mineral loss (Kimura and Itokawa, 1990). Meanwhile, in order to optimize the efficiency of K biofortification of the solanaceae vegetables studied here, the fate of K in the food-chain and in the soil must be determined.
The highest K concentration was found in cooked fruits of S. torvum (Fig. 5A). This observation is interesting with the knowledge that the effect of crop on raw fruit K concentration was not significant and even so, K concentration in S. torvum appeared lower than the other two crops in the raw fruits (Fig. 2B). Perhaps, what is worth noting and could offer some explanation is the fact that the fruits of S. aethiopicum and S. macrocarpon were diced into chunks before cooking but S. torvum fruits were not cut into pieces before cooking, due to its smaller fruit size and in accordance with local cooking methods. Increasing the surface area of S. aethiopicum and S. macrocarpon may have therefore contributed to higher leaching of K ions, suggesting that cooking the fruits as a whole may reduce, as much as possible, the loss of K. Moreover, there was a significant effect of the rate of K fertilizer application on K concentration in cooked fruits (Fig. 5B). Comparing the K in the boiled fruits from the Ko plots with those from the K4 plots, the results suggested that approximately 11% increase in K intake could be achieved with biofortification.
4.2. Effect of K fertilization on tissue concentration of other minerals
Some additional minerals were analysed in this study. Across all fertiliser treatments, S. aethiopicum leaves recorded greatest concentrations of N, P, Ca and Cu and its fruits also recorded greater concentration of Zn but comparable Fe concentration as torvum (Fig. 6). The concentration of N in the leaves and fruit of S. macrocarpon were comparable to that of S. aethiopicum and the Zn of the fruits of S. macrocarpon and S. aethiopicum were also not different (Fig. 6). The leaves of S. torvum obtained greater concentrations of Zn and its Ca content was greater but comparable to that of S. aethiopicum (Fig. 6). Fruits of S. torvum obtained the greatest Fe, albeit not significantly different from that of S. aethiopicum (Fig. 6). Akoto et al. (2015) have also reported of high Fe content in S. torvum. This gives some credence to the assertion in Ghana that S. torvum could be used by anaemics as a natural remedy to reduce the symptoms of iron deficiency anaemia (Akoto et al., 2015; Otu et al., 2017). The results here however suggest that S. aethiopicum obtained the greatest tissue concentration of most of the minerals examined. Nyadanu and Lowor (2015) similarly observed significant variation among indigenous leafy and fruit vegetables in their proximate composition and noted that S. aethiopicum was significantly higher in Fe and K, whilst S. torvum was significantly higher in P and Zn. Additionally, K+ often interacts with many other nutrients (Better Crops, 1998; Dibb and Thompson, 1985). In the present study, some interaction effects were observed on fruit and leaf tissue concentration of other elements (Table 1). Tissue N concentration was particularly interesting because the historically well documented synergistic interaction between N and K was observed (Malvi, 2011). The rate of K fertilizer application had a positive effect on fruit N concentration with fruit N concentration increasing with increasing K fertilizer application rate (Fig. 6G and H). This is consistent with the report that K affects nitrate (NO3) uptake and that rapid NO3 uptake depends on adequate K in the soil solution (Better Crops, 1998). This could be an economically and environmentally important interaction to exploit to improve yields of crops. In all, the results in the current study suggest that a combination of S. aethiopicum and torvum (which is a common practice) could be nutritious and healthy and should be a basis for promoting the wider cultivation and use of these vegetables.
4.3. Effect of K on yield
In the present study, there were gains in yield with increasing rate of K fertiliser application for all three crops (Fig. 1). These observations are consistent with other studies on field-grown Solanaceous crops (El-Bassiony et al., 2010; Taber, 2006; Wuzhong, 2002). The role of K in many physiological and biochemical processes needed to sustain and promote growth and yield has been established (Marschner, 1995). Growth or yield stimulation observed here might be related to increased water absorption, photosynthesis and accelerated flow of assimilates, as well as improved nitrogen (N) use efficiency by plants supplied with high K (Ivanova, 2005; Mengel and Kirkby, 1978; Wuzhong, 2002). Crops must maintain tissue K concentration of above 5–40 mg K (g DM)−1 in order to avoid losses in yield (White, 2013). Compared to other crops such as cereals, legumes and brassicas, Solanaceous vegetables require higher K fertiliser for maximum growth and yield (White, 2013). Our results thus indicate that without additional K supply, there could have been notable yield penalty in the current study. Except for S. macrocarpon, in which the relationship was linear, there was a quadratic relationship between K application rate and yield, with quadratic equations maximised at treatment K2 (i.e.: 200 kg ha−1 granular SoP/MoP or 10 mM liquid KCl; Fig. 1D). This is consistent with the observations of Hochmuth et al. (1993) and Taber (2006) and also agrees with report of White (2013) that plant growth and yield generally follow the law of diminishing returns with increasing K phytoavailability. It does appear that, to maximise yields of the solanaceae vegetables studied, recommendations for K would be around 200 kg K ha−1 SoP/MoP or 10 mM foliar application of KCl under growth conditions similar to the current study. Recommended nutrient requirements for S. aethiopicum in Ghana by the Ministry of Food and Agriculture (MoFA) is 207 kg ha−1 of N, 46 kg ha−1 of P2O5 and 340 kg ha−1 of K2O (http://mofa.gov.gh/site/?page_id=14167; accessed on 20/01/2017). Given that yields were maximised at 200 kg K ha−1 in this study, it does appear that the quantity of K fertilizer required to achieve maximum yields of solanaceae vegetables in Ghana might be lower than currently recommended by MoFA. On the other hand, K fertiliser source or form did not have significant effect on yield, except for S. torvum, agreeing with the report of Michałojć and Buczkowska (2009) that the type of K fertilizer had insignificant effect on total, marketable yields, and the number of quality fruits of plastic-tunnel-grown eggplants. Experimenting on silty loam soils, Ni et al. (2001) however reported that SoP was more effective than MoP in increasing the yield of eggplant fruits at the high fertilization rate. Interactions of environmental and soil factors with type of K fertiliser might explain the inconsistencies regarding the effect of type of K fertilizer on yield. This suggests that soil conditions and other environmental factors are critical in determining which form of K+ fertilizer is best suited to obtain high yields.
5. Conclusion
Agronomic biofortification provides an economical and speedy path to addressing dietary deficiencies of some nutrients. The current study demonstrates the twin benefits of improving the yields and K content of solanaceous vegetables cherished and consumed by both the poor and the rich people in Ghana. The results of the study show that agronomic biofortication with K can raise the nutritional value of the solanaceae vegetables studied by increasing the concentrations of K, Zn, Fe and Ca in the edible portions. This provides an opportunity for addressing some nutritional and health challenges in Ghana and elsewhere, as well as improving livelihoods and incomes. Further, the study demonstrates that the quantity of fertilizer required to achieve these benefits might be lower than currently recommended.
Declarations
Author contribution statement
Michael O. Adu: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Paul A. Asare: Performed the experiments; Analyzed and interpreted the data.
David O. Yawson: Conceived and designed the experiments; Wrote the paper.
Mishael A. Nyarko: Performed the experiments.
Kwabena Osei-Agyeman: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the University of Cape Coast Directorate of Research, Innovation and Consultancy (UCC DRIC) (2016–2017 Research Support Grant – RSG).
Competing interest statement
The authors declare no conflict of interest.
Additional information
Datasets associated with this study are available from the corresponding author on reasonable request.
Acknowledgements
We would like to thank the Research Assistants of the School of Agriculture, University of Cape Coast (UCC), including Gyebi Daniel, Michael Prescott Johnson, Azinaho Ebenezer and Hilda Etornam Zigah, for their immense support during the field and laboratory work of this study. We would also like to thank the reviewers for their feedback and also thank the internal monitoring team of UCC Directorate of Research, Innovation and Consultancy (DRIC) for their valuable comments during the development of this work.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Adu M.O., Asare P.A., Yawson D.O., Ackah F.K., Amoah K.K., Nyarko M.A., Andoh D.A. Quantifying variations in rhizosheath and root system phenotypes of landraces and improved varieties of juvenile maize. Rhizosphere. 2017;3:29–39. [Google Scholar]
- Akhtar M.E., Khan M.Z., Rashid M.T., Ahsan Z., Ahmad S. Effect of potash application on yield and quality of tomato (Lycopersicon esculentum Mill.) Pak. J. Bot. 2010;42:1695–1702. [Google Scholar]
- Akoto O., Borquaye L.S., Howard A.S., Konwuruk N. Nutritional and mineral composition of the fruits of Solanum torvum from Ghana. Int. J. Mol. Sci. 2015;4:222–226. [Google Scholar]
- Allen S.E., Grimshaw H.M., Parkinson J.A., Quarmby C. Blackwell Scientific Publications; London: 1974. Chemical Analysis of Ecological Materials. [Google Scholar]
- Amo-Adjei J., Kumi-Kyereme A. Fruit and vegetable consumption by ecological zone and socioeconomic status in Ghana. J. Biosoc. Sci. 2015;47:613–631. doi: 10.1017/S002193201400025X. [DOI] [PubMed] [Google Scholar]
- Balachandran C., Duraipandiyan V., Al-Dhabi N.A., Balakrishna K., Kalia N.P., Rajput V.S., Khan I.A., Ignacimuthu S. Antimicrobial and antimycobacterial activities of methyl caffeate isolated from Solanum torvum Swartz fruit. Indian J. Microbiol. 2012;52:676–681. doi: 10.1007/s12088-012-0313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Tal A., Pressman E. Root restriction and potassium and calcium solution concentrations affect dry-matter production, cation uptake, and blossom-end rot in greenhouse tomato. J. Am. Soc. Hortic. Sci. 1996;121:649–655. [Google Scholar]
- Better Crops Potassium interactions with other nutrients. Better Crops. 1998;82(3):13–14. http://www.ipni.net/publication/bettercrops.nsf/0/DD30AA083C1F782A8525798000820361/$FILE/Better%20Crops%201998-3%20p12.pdf Retrieved on 11 November, 2017 from: [Google Scholar]
- Bhaskarachary K. Potassium and human nutrition: the soil-plant-human continuum. Karnataka J. Agric. Sci. 2011;24:39–44. [Google Scholar]
- Broadley M.R., Alcock J., Alford J., Cartwright P., Foot I., Fairweather-Tait S.J., Hart D.J., Hurst R., Knott P., McGrath S.P., Meacham M.C. Selenium biofortification of high-yielding winter wheat (Triticum aestivum L.) by liquid or granular Se fertilisation. Plant Soil. 2010;332:5–18. [Google Scholar]
- Cakmak I. Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant Soil. 2008;302:1–7. [Google Scholar]
- Chilimba A.D., Young S.D., Black C.R., Meacham M.C., Lammel J., Broadley M.R. Agronomic biofortification of maize with selenium (Se) in Malawi. Field Crop. Res. 2012;125:118–128. [Google Scholar]
- Coombs J., Hind G., Leegood R.C., Tieszen L.L., Vonshak A. Analytical techniques. In: Coombs J., Hall D.O., Long S.P., Scurlock J.M.O., editors. Techniques in Bioproductivity and Photosynthesis. second ed. Pergamon Press; Oxford, UK: 1985. pp. 219–228. [Google Scholar]
- Darfour-Oduro S.A., Buchner D.M., Andrade J.E., Grigsby-Toussaint D.S. A comparative study of fruit and vegetable consumption and physical activity among adolescents in 49 Low-and-Middle-Income Countries. Sci. Rep. 2018;8:1623. doi: 10.1038/s41598-018-19956-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darkwa E.O., Djagbletey R., Antwi-Boasiako C., Aryee G., Sottie D., Akowuah A. Serum sodium and potassium levels in preeclampsia: a case-control study in a large tertiary hospital in Ghana. Cogent Med. 2017;4:1376898. [Google Scholar]
- Deal S.B. Flame photometric determination of sodium and potassium. Anal. Chem. 1954;26:598–599. [Google Scholar]
- Dibb D.W., Thompson W.R. Interaction of potassium with other nutrients. In: Munson R.D., editor. Potassium in Agriculture. (Potassiuminagri) American Society of Agronomy; Madison, WI: 1985. pp. 515–533. [Google Scholar]
- El-Bassiony A.M., Fawzy Z.F., El-Samad E.A., Riad G.S. Growth, yield and fruit quality of sweet pepper plants (Capsicum annuum L.) as affected by potassium fertilization. J. Am. Sci. 2010;6:722–729. [Google Scholar]
- Fan M., Bie Z., Xie H., Zhang F., Zhao S., Zhang H. Genotypic variation for potassium efficiency in wild and domesticated watermelons under ample and limited potassium supply. J. Plant Nutr. Soil Sci. 2013;176:466–473. [Google Scholar]
- FAO . FAO Communications Division; Rome: 2008. Guide for Fertilizer and Plant Nutrient Analysis. [Google Scholar]
- Graham R.D., Welch R.M., Bouis H.E. Addressing micronutrient malnutrition through enhancing the nutritional quality of staple foods: principles, perspectives and knowledge gaps. Adv. Agron. 2001;70:77–142. [Google Scholar]
- Harada H., Leigh R.A. Genetic mapping of natural variation in potassium concentrations in shoots of Arabidopsis thaliana. J. Exp. Bot. 2006;57:953–960. doi: 10.1093/jxb/erj081. [DOI] [PubMed] [Google Scholar]
- He F.J., MacGregor G.A. Potassium: more beneficial effects. Climacteric. 2003;(Suppl 3):36–48. [PubMed] [Google Scholar]
- He F.J., MacGregor G.A. Beneficial effects of potassium on human health. Physiol. Plant. 2008;133:725–735. doi: 10.1111/j.1399-3054.2007.01033.x. [DOI] [PubMed] [Google Scholar]
- Hochmuth G.J., Hochmuth R.C., Donley M.E., Hanlon E.A. Eggplant yield in response to potassium fertilization on sandy soil. Hortscience. 1993;28:1002–1005. [Google Scholar]
- Hochmuth G.J., Maynard D., Vavrina C., Hanlon E., Simonne E. 2012. Plant Tissue Analysis and Interpretation for Vegetable Crops in Florida.http://edis.ifas.ufl.edu/ep081 Retrieved on 14 November 2017 from: [Google Scholar]
- Hocking P.J., Steer B.T. Uptake and partitioning of selected mineral elements in sunflower (Helianthus annuus L.) during growth. Field Crop. Res. 1983;6:93–107. [Google Scholar]
- Institute of Medicine and Food and Nutrition Board . National Academies Press; Washington, DC: 2005. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. [Google Scholar]
- Ivanova S. 2005. The Effect of Potassium on Yield and Quality of Selected Solanaceae.https://www.ipipotash.org/udocs/The%20effect%20on%20K%20on%20Solanaceae.pdf Presented at the First International Symposium on Sustainable Agriculture for Subtropical Regions (ISSASR-1) Changsha, People’s Republic of China November 23-25, 2005. Retrieved 17 September from: [Google Scholar]
- John M.K. Colorimetric determination of phosphorus in soil and plant materials with ascorbic acid. Soil Sci. 1970;109:214–220. [Google Scholar]
- Kimura M., Itokawa Y. Cooking losses of minerals in foods and its nutritional significance. J. Nutr. Sci. Vitaminol. 1990;36(Supplement I):25–33. [PubMed] [Google Scholar]
- Malhotra M.S., Sridharan K., Venkataswamy Y., Rai R.M., Pichan G., Radhakrishnan U., Grover S.K. Effect of restricted potassium intake on its excretion and on physiological responses during heat stress. Eur. J. Appl. Physiol. Occup. Physiol. 1981;47:169–179. doi: 10.1007/BF00421669. [DOI] [PubMed] [Google Scholar]
- Malvi U.R. Interaction of micronutrients with major nutrients with special reference to potassium. Karnataka J. Agric. Sci. 2011;11:24. [Google Scholar]
- Marschner H. Functions of mineral nutrients: macronutrients. In: Marschner H., editor. Mineral Nutrition of Higher Plants. third ed. Academic Press; London: 1995. [Google Scholar]
- Mengel K., Kirkby E.A. International Potash Institute. Bern; Switzerland: 1978. Principles of Plant Nutrition; pp. 436–437. [Google Scholar]
- Michałojć Z., Buczkowska H. Influence of varied potassium fertilization on eggplant yield and fruit quality in plastic tunnel. Folia Hortic. 2009;21:17–26. [Google Scholar]
- Montoya S., Ortega E., Navarro E., Lorenzo M.L. Cucumber biofortification with potassium. Eur. Sci. J. 2013;30:9. [Google Scholar]
- Nair M.K., Augustine L.F., Konapur A. Food-based interventions to modify diet quality and diversity to address multiple micronutrient deficiency. Front. Public Health. 2016;3:277. doi: 10.3389/fpubh.2015.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestel P., Bouis H.E., Meenakshi J.V., Pfeiffer W. Biofortification of staple food crops. J. Nutr. 2006;136:1064–1067. doi: 10.1093/jn/136.4.1064. [DOI] [PubMed] [Google Scholar]
- Ni W.Z., Liang J.S., Hardter R. Yield and quality responses of selected solanaceous vegetable crops to potassium fertilization. Pedosphere. 2001;11:251–255. [Google Scholar]
- Nyadanu D., Lowor S.T. Promoting competitiveness of neglected and underutilized crop species: comparative analysis of nutritional composition of indigenous and exotic leafy and fruit vegetables in Ghana. Genet. Resour. Crop Evol. 2015;62:131–140. [Google Scholar]
- Otu P.N., Sarpong F., Gidah J.E., Labanan A.M., Anim D. Characterization of Turkey berry (Solanum torvum)-fresh, dry and powder. Afr. J. Food Integr. Agric. 2017;1:9–14. [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2012. R: a Language and Environment for Statistical Computing.http://www.Rproject.org/ [Google Scholar]
- Sibhatu K.T., Qaim M. Rural food security, subsistence agriculture, and seasonality. PLoS One. 2017;12(10) doi: 10.1371/journal.pone.0186406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber H.G. Potassium application and leaf sufficiency level for fresh-market tomatoes grown on a Midwestern United States fine-textured soil. HortTechnology. 2006;16:247–252. [Google Scholar]
- Tessenderlo Group . 2014. SOP Plant Nutrition: Sulphate of Potash and Solanaceae (Tomato, Eggplant, Bell Pepper and Chilli)http://www.cropvitality.com/en/solid-soluble-fertilizers/documents/downloads#k=Sulphate%20of%20Potash%20and%20Solanaceae Retrieved 17 December, 2017 from: [Google Scholar]
- Wang Y.H., Zou C.Q., Mirza Z., Li H., Zhang Z.Z., Li D.P., Xu C.L., Zhou X.B., Shi X.J., Xie D.T., He X.H. Cost of agronomic biofortification of wheat with zinc in China. Agron. Sustain. Dev. 2016;36:44. [Google Scholar]
- Whelton P.K., He J., Cutler J.A., Brancati F.L., Appel L.J., Follmann D., Klag M.J. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. J. Am. Med. Assoc. 1997;277:1624–1632. doi: 10.1001/jama.1997.03540440058033. [DOI] [PubMed] [Google Scholar]
- White P.J. Improving potassium acquisition and utilisation by crop plants. J. Plant Nutr. Soil Sci. 2013;176:305–316. [Google Scholar]
- WHO . World Health Organization (WHO); Geneva: 2003. Prevention of Recurrent Heart Attacks and Strokes in Low and Middle Income Populations: Evidence-based Recommendations for Policy Makers and Health Professionals.http://www.who.int/cardiovascular_diseases/resources/pub0402/en/ Retrieved 18 November 2017 from: [Google Scholar]
- WHO . World Health Organization (WHO); Geneva: 2012. Guideline: Potassium Intake for Adults and Children. [PubMed] [Google Scholar]
- Wuzhong N. Yield and quality of fruits of solanaceous crops as affected by potassium fertilization. Better Crops Int. 2002;16:6. [Google Scholar]
- Yawson D.O., Kwakye P.K., Armah F.A., Frimpong K.A. The dynamics of potassium (K) in representative soil series of Ghana. ARPN J. Agric. Biol. Sci. 2011;6:48–55. [Google Scholar]
- Yousaf Z., Wang Y., Baydoun E. Phytochemistry and pharmacological studies on Solanum torvum Swartz. J. Appl. Pharmaceut. Sci. 2013;3:152–160. [Google Scholar]
- Zhang C.M., Zhao W.Y., Gao A.X., Su T.T., Wang Y.K., Zhang Y.Q., Zhou X.B., He X.H. How could agronomic biofortification of rice be an alternative strategy with higher cost-effectiveness for human iron and zinc deficiency in China? Food Nutr. Bull. 2018;39:246–259. doi: 10.1177/0379572117745661. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.