Abstract
Back ground
The secondary alveolar grafting is an integral part in the management of alveolar cleft defect. Particulate cancellous bone and marrow (PCBM) graft obtained from iliac crest are considered as the gold standard.
Aim
Aim of the study was to evaluate the graft uptake clinically and radiologically using the Bergland’s radiographic scale.
Material and methods
A longitudinal descriptive study was conducted on twenty patients of unilateral CLP in the age group of 6–13 years, presenting with residual/secondary alveolar cleft defect with unerupted maxillary lateral incisor/canine adjacent to the defect. Autologous PCBM graft obtained from iliac crest was used in all cases. Post operative clinical and radiological evaluation was carried out using the Bergland’s radiographic scale at intervals of 1 week, 1 month and 6 months.
Data collection and result
Clinical evaluation consisted of assessment for infection, exposure of graft, rejection of graft, wound dehiscence and status of oronasal communication. A four-point Bergland’s radiographic scale was used to compare the interdental height of the bone graft with unaffected side and categorized from grade I to IV. After six months, 6 cases were graded as grade I, 11 cases as grade II and 2 cases were grade III. Only one case deteriorated to grade IV which is considered as failure.
Summary and conclusion
Satisfactory results were obtained in 95% cases. Bergland’s radiographic assessment scale is a valuable, easily available and inexpensive diagnostic tool to assess the condition of the grafted bone in SABG.
Keywords: Secondary alveolar grafting, Iliac crest cortico cancellous, Berglands scale
1. Introduction
The secondary alveolar grafting has been established as the gold standard for alveolar cleft defect reconstruction and has provided a foundational support in contemporary cleft management. There is general agreement that particulate bone grafts from whatever source are preferred over block cortico cancellous grafts. Particulate grafts are more readily incorporated and remodeled with the adjacent alveolus. Boyne and Sands suggested that particulate cancellous bone and marrow (PCBM) obtained from iliac crest are the donor material of choice.1 They emphasized that this viable graft material once incorporated into the recipient site, not only stabilizes the maxillary arch segment but also responds to physiologic and functional demands such as tooth movement and arch expansion. To assess the success of the bone graft, several criteria have been suggested such as eruption of teeth in the cleft, periodontal status, alveolar bone height, alar base support and radiographic assessment of the grafted bone. The Bergland radiographic criteria of measuring bone graft success is a simple, practical, readily available and accurate method that is most often used.2 With this system, the occlusal level of the interdental bone is compared with that of the normal side and a four point scale is used to categorise each graft. In our study, we carried out secondary alveolar grafting in twenty patients using particulate cortico-cancellous graft from the anterior iliac crest. Aim of the study was to evaluate graft uptake clinically and radiologically using the Bergland’s scale.
2. Material and methods
The study consisted of individuals selected from the outpatient department and those referred from peripheral hospitals. A longitudinal descriptive study design was utilized after obtaining due permission from the IEC. Twenty patients were randomly selected in the age group of 6–13 years with early/late mixed dentition, who had been previously operated for unilateral CLP, presenting with residual/secondary alveolar cleft defect with unerupted maxillary lateral incisor/canine adjacent to the defect, requiring bone grafting. Medically compromised patients, patients less than 6 years age and older than 13 years, those with insufficient volume of anterior iliac bone and unwilling to participate in the study were excluded.
Signed informed consent form was taken from the parents. All the cases were evaluated clinically for nasal regurgitation of liquids, clarity of speech, and articulation of sounds. Evaluation of dentition included number of teeth, supernumerary or ectopically erupted teeth, caries and periodontal status. Alveolar cleft site was assessed for size and presence of oronasal fistula, position of the unerupted teeth, vestibular depth, lip length and dehiscence. Radiographic examination included occlusal radiograph and A-P view of pelvis.
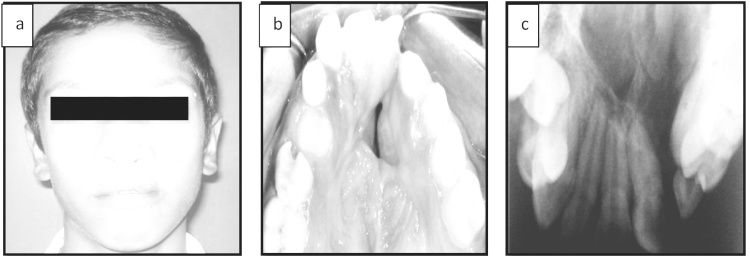
Pre surgical orthodontic arch expansion was carried in required cases. Pre operative laboratory investigations were carried out and anaesthetic clearance obtained. Pre operative extra oral, intra oral photographs and radiographs (Fig. 1) were taken.
Fig. 1.
Pre operative extra oral and intraoral photographs and radiograph.
2.1. Surgical Procedure
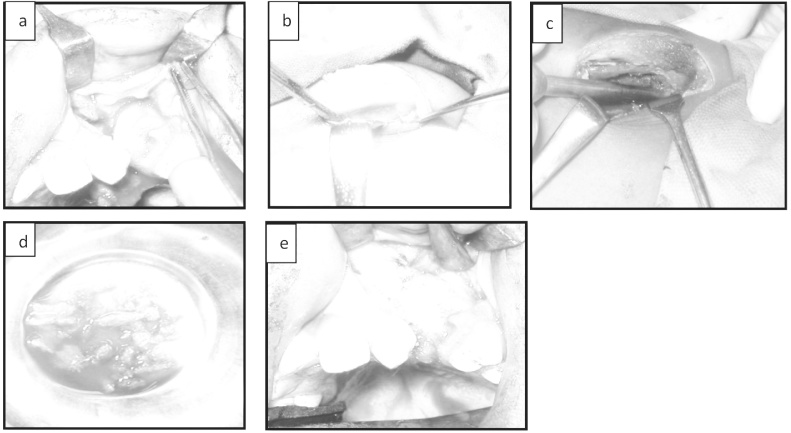
Under general anesthesia peri-fistular incision was made through the mucosa overlying the cleft down to the bony margins to allow the vertical portion of the cleft to be used for the closure of the nasal floor. Full thickness mucoperiosteal flaps were elevated and rotated palatally. The labial soft tissue was reflected completely exposing the alveolar defect. The flaps were sutured, sealing off the nasal cavity and palatal floor from the alveolar cleft and the graft bed prepared.
The donor iliac crest site was prepared and draped. The incision line was marked and 2% Lignocaine with Adrenaline (1:80,000) infiltrated. An incision was made through the skin and periosteum 2 cm posterior to the anterior iliac spine extending posteriorly for about 5 cm. The medial aspect of the Ilium was exposed subperiosteally after stripping the iliacus muscle. A bone trephine was used to gouge out required amount of cortico cancellous graft and preserved in normal saline. Hemostasis achieved and a closed circuit suction drain was fixed and wound sutured in layers. 2 ml of 0.5% Bupivacaine was infiltrated and pressure dressing applied.
The alveolar defect was packed with the bone graft and primary closure was done with 3-0 vicryl and extraoral pressure dressing applied (Fig. 2).
Fig. 2.
Graft bed, harvested graft and obturated defect.
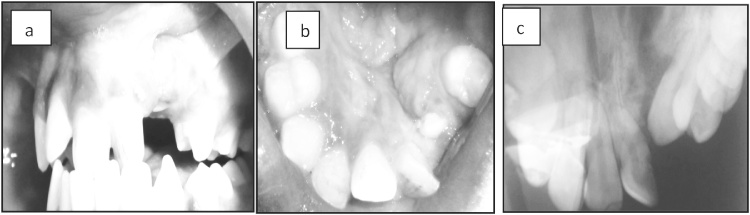
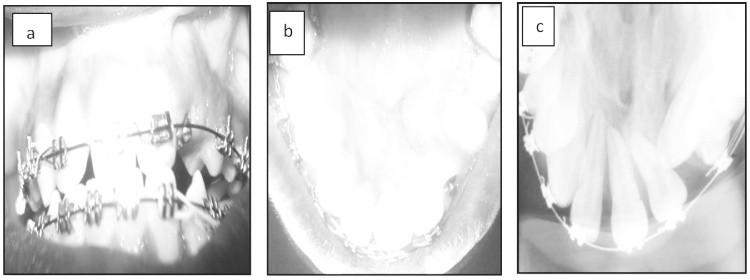
Post operatively, the patients were placed on parenteral antibiotics and analgesics and discharged at the end of one week. Clinical and radiological evaluation was done at intervals of 1 week (Fig. 3a–c), 1 month (Fig. 4a–c) and 6 months (Fig. 5a–c). Clinical evaluation consisted of assessment for infection, exposure of graft, rejection of graft, wound dehiscence and status of oro nasal communication. Radiographic assessment using Bergland’s scale was carried out to evaluate the graft consolidation and alveolar bone height. A four-point scale was used to compare the interdental height of the bone graft with normal/unaffected side and categorized as:
Fig. 3.
a, b & c - 1 week post op clinical photograph and radiograph.
Fig. 4.
a, b & c - 1 month post op photographs and radiograph.
Fig. 5.
a, b & c – 6 months post op photographs and radiograph.
Grade I − Bone septum of approximate normal height, up to the cemento − enamel junction
Grade II − Septum height at least 3/4 of normal height
Grade III − Septum height less than 3/4 of the normal height
Grade IV − No continuous bony bridge across the cleft, which is failure.
3. Results
Twenty patients from 6 to 13 years of age were included in the study. The average age was 10.4 year. There were 10 males and 10 females. Average age of male and female patients was 10.8 and 10 year respectively. The data base of the patients showing postoperative Bregland’s score is given in Table 1.
Table 1.
Data base of patients with radiographic grades during follow up.
| S. No. | Age (years) | Sex | Bergland’s Grading (Post OP) |
GRADE CHANGE | ||
|---|---|---|---|---|---|---|
| 1 WEEK | 1 MONTH | 6 MONTHS | ||||
| 1 | 11 | M | GRADE II | GRADE II | GRADE II | II-II-II |
| 2 | 11 | M | GRADE I | GRADE I | GRADE II | I-I-II |
| 3 | 9 | F | GRADE II | GRADE II | GRADE II | II-II-II |
| 4 | 12 | M | GRADE I | GRADE I | GRADE I | I-I-I |
| 5 | 10 | F | GRADE I | GRADE I | GRADE I | I-I-I |
| 6 | 10 | F | GRADE II | GRADE II | GRADE II | II-II-II |
| 7 | 11 | M | GRADE II | GRADE II | GRADE IV | II-II-IV |
| 8 | 9 | M | GRADE II | GRADE II | GRADE II | II-II-II |
| 9 | 10 | F | GRADE I | GRADE II | GRADE II | I-II-II |
| 10 | 10 | F | GRADE II | GRADE II | GRADE II | II-II-II |
| 11 | 11 | F | GRADE I | GRADE I | GRADE I | I-I-I |
| 12 | 12 | M | GRADE I | GRADE II | GRADE II | I-II-II |
| 13 | 9 | M | GRADE II | GRADE III | GRADE III | II-III-III |
| 14 | 9 | F | GRADE I | GRADE I | GRADE I | I-I-I |
| 15 | 12 | M | GRADE I | GRADE I | GRADE I | I-I-I |
| 16 | 11 | F | GRADE II | GRADE II | GRADE II | II-II-II |
| 17 | 10 | F | GRADE I | GRADE II | GRADE III | I-II-III |
| 18 | 10 | F | GRADE I | GRADE I | GRADE I | I-I-I |
| 19 | 9 | M | GRADE II | GRADE II | GRADE II | II-II-II |
| 20 | 12 | M | GRADE II | GRADE II | GRADE II | II-II-II |
At the end of six months follow up 6 cases were graded as grade I, 11 cases as grade II and 2 cases were grade III. Only one case deteriorated to grade IV which is considered as failure.Sequential changes in the radiographic grades during the observation period are shown in Table 2 and Fig. 6. There were 6 cases (30%) maintained as grade I and 8 cases (40%) as grade II. One case (5%) was graded as I, one week and one month post op, deteriorated to grade II at the end of 6 months. Two cases (10%) were graded as I; one week post op eventually remained as grade II at the end of 1 month 6 months. One case (5%) was graded as I at the end of 1 week, deteriorated to grade-II at 1 month and grade-III at the 6 months follow up period. One case (5%) was graded as grade II one week post op, grade III at 1 month and reduced to grade III at the end of 6 months. One case (5%) which failed completely was graded as Grade II one week post op, grade II at the end of 1 month but eventually deteriorated to grade IV at the end of 6 months.
Table 2.
Alveolar defects: post operative radiographic changes in grades over the periods- 1 week, 1 month, 6 months.
| Change | Number | Percent |
|---|---|---|
| I-I-I | 6 | 30 |
| II-II-II | 8 | 40 |
| I-I-II | 1 | 5 |
| I-II-II | 2 | 10 |
| I-II-III | 1 | 5 |
| II-III-III | 1 | 5 |
| II-II-IV | 1 | 5 |
| Total | 20 | 100 |
Fig. 6.
Radiographic changes in grades.
Only 2 patients (5%) presented with hypertrophied scar. Since, the number was too small and there were no other complications, no significant statistical analysis could be made for this.
Radiological comparison of graft changes at one week and six months post op is presented in Table 3. The changes in the grades are statistically significant (T- value = 2.63 and P – value = 0.017).
Table 3.
Paired t-test and CI: 1 week vs 6 month.
| N | Mean | St Dev | SE Mean | |
|---|---|---|---|---|
| 1 WEEK | 20 | 1.500 | 0.513 | 0.115 |
| 6 MONTH | 20 | 1.900 | 0.788 | 0.176 |
| Difference | 20 | −0.400 | 0.681 | 0.152 |
95% CI for mean difference: (−0.719, −0.081).
T-Test of mean difference = 0 (vs not = 0): T-Value = -2.63* P-Value = 0.017.
4. Discussion
CLP patients require prolonged treatment by a multidisciplinary team to achieve desired functional and esthetic outcome. Grafting of alveolar cleft defect is an integral part of the treatment protocol.
Significant advantages are documented in achieving continuity between the cleft alveolar segments, regardless of how and when this is accomplished. The advantages of grafting are stability of dental arch, prevention of alveolar collapse, provision of room for eruption of lateral incisors and cuspid, bony support to the adjacent teeth and preservation of dental and oral health.3 Grafting restores continuity of the alveolus and the maxilla particularly at the piriform rim. This supports the ala and provides enhanced support and stability for the nose. This not only has a direct esthetic benefit also proves to be of long-term benefit when formal rhinoplasty procedures are performed.4 Grafting of the alveolar defect provides an opportunity for the surgeon to address the residual oronasal fistula. This has potential benefit for both hygiene and speech. Many cleft patients present with history of chronic upper respiratory tract and sinus disease, which may be related to reflux into the nasal cavity and sinus. There is evidence that closure of the fistula and grafting the cleft defect can improve nasal emission and nasality.5
In our study sample of 20, pre operatively all the patients had a continuity defect of the alveolar process, alar base slumping and oro nasal communication. At the end of 6 months following grafting, the continuity defect of the alveolus was restored, alar base support improved (Fig. 7) and oro nasal communication eliminated in 19 out of 20 patients (95%). Except in one case of graft failure, successful uptake of the graft was seen, thereby achieving the aims of grafting of the residual alveolar cleft.
Fig. 7.
Extra oral post op photos depicting improved alar base support.
Perhaps the most controversial topic in managing the alveolar cleft is the time of grafting. Outcome measures for various approaches are also defined inconsistently, which makes comparison difficult. According to the chronological age grafting it can be classified as Primary grafting which is carried out before 2 years of age after lip repair but before palate repair. Secondary grafting is carried out after 2 years of age. The secondary grafting can be further divided as early secondary grafting, early mixed dentition, late mixed dentition and late secondary grafting. The cases operated by us were form early mixed dentition and late mixed dentition group. The mixed dentition is arguably the most accepted time frame for performing alveolar grafting. Rationale of grafting during this time are (a) minimal maxillary growth after the age of 6–7 years, and the effect of grafting at this time does not pose restriction to facial growth,6, 7 (b) predictable orthodontic intervention like arch expansion, (c) availability of adequate autogenous bone graft, (d) bone volume may improve by eruption of the tooth into the grafted site,8 (e) and enhancement the health of teeth in and adjacent to the alveolar cleft.
Boyne and Sands landmark papers established that grafting in the mixed dentition achieves many of the goals of reconstruction of the cleft alveolus.1 The ideal patient is between the ages of 8 and 12 years with a maxillary canine root displaying one-half to two-thirds development. This timing is supported by several well-documented studies.1, 9, 10 However, they also suggested that earlier grafting should be considered as a means of preserving the lateral incisor as well.11
Autogenous bone from the iliac crest is the gold standard by which other types of alveolar grafts should be compared.12, 13 It is easy to access and can supply adequate volume of cancellous bone with pluripotent or osteogenic precursor cell that support osteogenesis. Because of its higher content of osteogenic cells, cancellous bone is thought to be superior to corticocancellous bone.
Bone from the iliac crest can be harvested by an open approach or with a trephine. We used bone trephine which reduces the post operative donor site morbidity as compared to open approach. Local infiltration of 0.5% Bupivacaine along the donor site at the time of closure of surgical wound further minimizes early postoperative pain and discomfort. The average hospital stay was 5–7 days. All patients had a satisfactory scar at the donor site except for two cases which developed a hypertrophied scar.
Radiographic assessment is equally important, in fact more so than clinical evaluation. Radiographic assessment objectively indicates the changes in the structural pattern of the grafted bone, the response of the graft to spontaneous tooth eruption or to orthodontic movement and the height of the interdental septum formed.14 It also permits determination of the distribution of bone tissue in the grafted area. Hence the objective of the present study was to assess the presence of bone matter which had consolidated well in the cleft site using the Bergland scale.
At the end of 6 months follow up period there were 6 cases (30%) in Grade I, 11(55%) in Grade II, 2 (10%) in Grade III and only 1 (5%) case in grade IV which is considered as failure. Since we have considered Grades I, II and III as measures of surgical success, according to the Bergland scale, the results of our investigation demonstrated a total of 95% of cases with surgical success. The unerupted teeth adjacent to the defect can be safely guided to erupt into the grafted area. It was only 1 case (5%), an 11 years old male patient who deteriorated to Grade-IV at the end of 6 months, which was considered a surgical failure. Possible reason for degradation of graft from Grade I to Grade II/III/IV is resorption of cancellous particulate bone matter due to decreased vascular supply.
5. Conclusion
The outcome of 20 cases of residual alveolar cleft defect treated by bone grafting using autogenous cancellous bone from the anterior iliac crest was evaluated over a period of 6 months clinically and radiographically using the Bergland’s radiographic assessment scale which is a valuable, easily available and inexpensive diagnostic tool to assess the condition of the grafted bone. Satisfactory results were obtained in 95% cases.
Conflict of interest
The authors have none to declare.
Ethical approval
Institutional ethical committee clearance obtained.
Funding source
None.
Acknowledgements
None.
References
- 1.Boyne P.J., Sands N.R. Secondary bone grafting of residual alveolar and palatal clefts. J Oral Surg. 1972;30:87–95. [PubMed] [Google Scholar]
- 2.Rawashdeh M.A. Morbidity of iliac crest donor site following open bone harvesting in cleft lip and palate patients. Int J Oral Maxillofac Surg. 2008;37:223–227. doi: 10.1016/j.ijom.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Horswell B.B., Henderson J.M. Secondary osteoplasty of the alveolar cleft defect. J Oral Maxillofac Surg. 2003;61:1082–1090. doi: 10.1016/s0278-2391(03)00322-7. [DOI] [PubMed] [Google Scholar]
- 4.Kalaaji A., Lilja J., Friede H. Bone grafting at the stage of mixed and permanent dentition in patients with clefts of the lip and primary palate. Plast Reconst Surg. 1994;93:690–696. doi: 10.1097/00006534-199404000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Bureau S., Penko M., McFadden L. Speech outcome after closure of oronasal fistulas with bone grafts. J Oral Maxillofac Surg. 2001;59:1408–1413. doi: 10.1053/joms.2001.28270. [DOI] [PubMed] [Google Scholar]
- 6.Witsenburg B. The reconstruction of anterior residual bone defects in patients with cleft lip, alveolus and palate: a review. J Maxillofac Surg. 1985;13:197–208. doi: 10.1016/s0301-0503(85)80048-5. [DOI] [PubMed] [Google Scholar]
- 7.Daskalogiannakis J., Ross R.B. Effect of alveolar bone grafting in the mixed dentition on maxillary growth in complete unilateral cleft lip and palate patients. Cleft Palate Craniofacial J. 1997;34:455–458. doi: 10.1597/1545-1569_1997_034_0455_eoabgi_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 8.Dempf R., Teltzrow T., Kramer F.J., Hausamen J.E. Alveolar bone grafting in patients with complete clefts: a comparative study between secondary and tertiary bone grafting. Cleft Palate Craniofac J. 2002;39:18–25. doi: 10.1597/1545-1569_2002_039_0018_abgipw_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 9.Bergland O., Abyholm F., Semb G. Secondary bone grafting of alveolar clefts. J Plast Reconstr Surg. 1981;15:127–134. doi: 10.3109/02844318109103425. [DOI] [PubMed] [Google Scholar]
- 10.Boyne P.J., Sands N.R. Combined orthodontics surgical management of residual palatoalveolar cleft defects. Am J Orthod. 1976;70:20–37. doi: 10.1016/0002-9416(76)90258-x. [DOI] [PubMed] [Google Scholar]
- 11.Boyne P.J. Bone grafting in the osseous reconstruction of alveolar and palatal clefts. Oral Maxillofac Clin North Am. 1991;3:589–597. [Google Scholar]
- 12.Bosker Koole R., Vander Dumen F.N. Late secondary autogenous bone grafting in cleft patients comparing Mandibular (ectomesenchymal) and Iliac crest (Mesenchymal) grafts. J Craniomaxillofac Surg. 1989;17:28–36. doi: 10.1016/s1010-5182(89)80036-8. [DOI] [PubMed] [Google Scholar]
- 13.Sindet Pedersen S., Enemark H. Reconstruction of alveolar clefts with mandibular or iliac crest bone grafts: a comparative study. J Oral Maxillofac Surg. 1990;48(6):554–558. doi: 10.1016/s0278-2391(10)80466-5. [DOI] [PubMed] [Google Scholar]
- 14.KiemleTrindade Ivy, Mazzottini Reinaldo. Long-term radiographic assessment of secondary alveolar bone grafting outcomes in patients with alveolar clefts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:271–277. doi: 10.1016/j.tripleo.2005.03.012. [DOI] [PubMed] [Google Scholar]