Abstract
Background
The impact of HIV pre-exposure prophylaxis (PrEP) depends on uptake, adherence, and sexual practices.
Methods
Men and transgender women who have sex with men (MSM/TGW) previously enrolled in PrEP trials were enrolled in a 72 week open label extension (iPrEx OLE). Drug concentrations were measured in plasma and dried blood spots (DBS) in seroconverters and a random sample of seronegatives.
Findings
1603 HIV uninfected persons were enrolled, of whom 76% received PrEP. PrEP uptake was higher among those reporting condomless receptive anal intercourse (ncRAI; P=0.003) and having serological evidence of herpes (P=0.03). Among those receiving PrEP, HIV incidence was 1.8/100PY, which was 49% (95% CI: −1 to 74%) lower than among those who concurrently did not choose PrEP after adjusting for sexual behavior, and 53% (95% CI: 26 to 70%) lower than in the placebo arm of the prior randomized phase (3.9/100PY). Among those receiving PrEP, HIV incidence was 4.7/100PY if drug was not detected in DBS, 2.3/100PY if drug concentrations indicated use of less than 2 tablets per week, 0.6/100PY for use of 2 to 3 tablets per week, and 0/100PY for use of 4 or more tablets per week (P<0.0001). PrEP drug concentrations were higher among people with older age, more schooling, ncRAI, more sexual partners, trans-identification, and a history of syphilis or herpes.
Interpretation
PrEP uptake was high when made available free of charge by experienced providers. PrEP impact is increased by greater uptake and adherence during periods of higher risk; disengagement after initial use is common. DBS drug concentrations are strongly correlated with PrEP’s protective benefit.
Introduction
Pre-exposure prophylaxis (PrEP) with oral emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) is effective for preventing the acquisition of HIV infection among men and transgender women who have sex with men (MSM/TGW), (1) heterosexual couples, (2) and heterosexual men and women. (3) The impact of PrEP depends on its high biological efficacy when used, (4, 5) and multiple social interactions and behaviors related to PrEP use.
In randomized placebo controlled trials, PrEP adherence indicated by drug detection in blood was a strong correlate of PrEP efficacy.(1, 2) Two trials in African women showed no evidence of efficacy on an intention to treat basis; despite high reported adherence, less than a third of trial participants receiving active drug had detectable drug in their blood.(6, 7)
The theory of risk compensation predicts that sexual practices could become more risky with the advent of biomedical prevention strategies, including medical male circumcision, antiretroviral treatment for HIV infection, and PrEP. (8) In PrEP trials of MSM/TGW and heterosexual couples, self-reported sexual practices became safer, (2, 9, 10) including among those who thought they were receiving the active treatment and that it would be effective. (9) Self-reported safer behavior was corroborated by decreases in syphilis incidence and acute HIV-1 infection prevalence. (9)
There could be changes in PrEP use (11) and sexual practices (12) as PrEP transitions from clinical trials to clinical practice. Participants in blinded and placebo controlled efficacy trials are informed that they may be receiving a placebo, or a drug having no benefit, and that product safety requires further confirmation. Such messages provided during trials could undermine adherence and limit risk compensation. As information about PrEP safety and efficacy becomes available from trials, adherence could increase and condom use may decrease. Open label access could also alter PrEP uptake; as people focus more on their personal goals rather than research goals, intentions to use PrEP may be higher when HIV exposure is greatest or PrEP uptake in clinical practice may occur primarily among the “worried well” who are already protecting themselves in other ways. The overall impact of PrEP in practice depends on these behaviors.
The open label extension of the iPrEx study (iPrEx OLE) provided an opportunity to investigate PrEP uptake, adherence and sexual practices in a way that more closely resembles clinical practice. As social desireability can bias self-reported adherence, we use a novel biomarker of long-term PrEP use: tenofovir diphosphate (TFV-DP) measured in dried blood spots (DBS).
Methods
Population
All participants in iPrEx OLE were assigned male sex at birth, reported anal intercourse with men, were over the age of 18 and had previously participated in a randomized blinded placebo-controlled trial of once daily oral PrEP using FTC/TDF (iPrEx(1) or ATN 082(13)) or TDF (US Safety Study(14)). Participants who were diagnosed with HIV infection during the randomized phases of prior trials were followed, although they were not eligible for PrEP and are not included in this report.
Clinical Procedures
Participants were unblinded to their randomized assignment prior to iPrEx OLE enrollment. Computer assisted structured interviews conducted after informed consent and before HIV-1 testing were used to assess desire to use PrEP, reasons for declining PrEP (selected from a list as all that apply), self-identification as “trans” (selected a list of identities as all that apply, translated according to local custom), education, alcohol use (in the past 6 months), and substance use (in the past 6 months). At the enrollment visit, all participants were offered daily oral FTC/TDF PrEP if they were HIV-1 antibody negative and there were no symptoms that might indicate an acute HIV infection. Among those with an acute viral syndrome, PrEP was deferred until HIV-1 RNA testing was negative or anti-HIV antibody testing continued to be negative after resolution of symptoms. All benefits of study participation were provided regardless of whether the participant chose to take PrEP; such benefits varied by study site according to local standards and ethical committee requirements. Visits were conducted at enrollment and weeks 4, 8, 12, 24, 36, 48, 60, and 72 weeks. Participants could start PrEP on any visit during the first 48 weeks of follow-up, and were followed at weeks 4, 8 and 12 after starting PrEP then every 12 weeks until completing a total of 72 weeks on study (off or on PrEP). Counseling support included integrated next step counseling, (15, 16) which involves counseling for sexual health for all participants and PrEP adherence assessment and counseling for those receiving PrEP. All participants were informed that the results of PrEP drug testing would be shared with them; results were provided by a medical officer. Results from drug testing conducted during prior randomized trials were not provided to the study sites nor to the study participants. The OLE protocol was approved by ethical committees governing each study site and by national regulatory authorities in each country, including registration with the US FDA under IND#71,859.
Drug Concentration Cohort
Drug concentrations in blood plasma were performed on all participants at one of their study visits during the first 12 weeks after receiving PrEP. Drug concentrations in DBS were measured among participants who opted to receive PrEP using a case-cohort design. (17) This design tested all time points after PrEP dispensation among those with confirmed HIV infection and a site-stratified random sample of seronegative participants. Analysis of drug concentrations used the design weights to acknowledge the case cohort sampling. Only results from DBS were used in the analysis of correlates of drug detection.
Laboratory Procedures
HIV antibody testing was conducted at all visits and testing for STIs (syphilis, herpes, urethritis) was conducted every 24 weeks or if there were symptoms. HIV testing using 2 rapid tests, with Western Blot testing to confirm any reactive test, was performed at all scheduled study visits as previously described. (1) PrEP was discontinued at the time of any reactive test, and resumed if confirmatory tests were negative. Blood plasma (with EDTA) was drawn and DBS were prepared at enrollment and all 12 week follow-up visits regardless of receipt of PrEP. Plasma and DBS were also collected 4 and 8 weeks after initiating PrEP. DBS were stored at −20°C within 24 hours of collection and shipped on dry ice to the laboratory where they were analyzed for TFV-DP by liquid chromatography and tandem mass-spectroscopy, as previously described.(18, 19) Creatinine clearance (eCrCl) was estimated using the Cockcroft-Gault equation.
Dosingestimation
Dosing was estimated from the TFV-DP concentration in DBS using pharmacokinetic modeling from observations of drug accumulation and decay after one month of daily dosing.(18) TFV-DP in DBS has a 17 day half-life corresponding with a 25-fold accumulation with daily dosing. The lower limit of quantitation of the assay was 2.5 fmol/punch.(19) Dosing categories were below LLOQ, LLOQ to 350 fmol/punch (<2 tablets per week), 350 to 699 fmol/punch (2 to 3 tablets per week), 700 to 1250 fmol/punch (4 to 6 tablets per week), and >1250 fmol/punch (daily dosing).
Statistical methods
HIV incidence comparing on and off PrEP periods used a Poisson model (with a robust standard error), facilitating the comparison across the randomized and open label periods. HIV incidence by TFV-DP concentration measured in DBS used profile likelihood confidence intervals due to the small number of infections in each dosing category. The concentration of drug in DBS associated with 90% protection (EC90) was estimated as a relative hazard of 0.10 compared with the concurrent off PrEP arm, after adjustment for ncRAI, age, number of partners, history of syphilis and enrolling site as previously described. (4) Predictors of TFV-DP concentrations in DBS, by dosing category, used all results over time available from the drug concentration cohort using an ordinal logistic regression model (20) with a robust standard error, and adjusting for study site and time on study.
Role of the funding source
The study was sponsored by the US NIH, which had input into the study design and the analysis of the data. Study drug was donated by Gilead Sciences, which did not have input into the study design nor the analysis of the data. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Cohort characteristics
Participants were enrolled between June 2011 and June 2012. All participants were previously enrolled in randomized placebo controlled trials of TDF-containing PrEP regimens (Fig. 1). The majority came from the randomized phase of the iPrEx study that completed treatment visits in November 2010; former ATN082 participants completed treatment visits in November 2010 and were enrolled at the Chicago site and were all men of color between the ages of 18 and 25; former US Safety Study participants ended treatment visits in 2009 and enrolled in San Francisco and Boston. Those enrolling were 17% white (265/1603), 8% black (125/1603), 5% Asian (73/1603), and 70% mixed or other race (1063/1603); 72% were Latino (1094/1603). Among those eligible, persons enrolling in OLE, vs. eligible persons who did not enroll, were older (mean age 28 vs. 26, P<0.01), more likely to report non-condom receptive anal intercourse (ncRAI) at their original screening visit (911/1526, 60% vs.447/814, 55%; P=0.03), more likely to have a history of syphilis (222/1526, 15% vs.80/814, 10%, P<0.01) or herpes (578/1526, 38% vs.246/814, 30%, P<0.01), and were comparable in schooling, race, ethnicity, transactional sex, trans-identification, and prior randomization group (active vs placebo). Among 446 people previously in the active arm of iPrEx, and for whom drug testing had been blindly performed at week 8, those with detectable drug were more likely to enroll in OLE (191/277, 69% vs.93/169, 57% after sample weighting, P=0.02). Of 814 eligible iPrEx participants who did not enroll in OLE, 344 (42%) were last seen before the end of the randomized treatment phase of iPrEx, 192 (24%) were last seen during the post-treatment phase of the randomized study, and 278 (34%) were last seen at the unblinding visit that occurred shortly before OLE began enrollment at each site.
Figure 1.
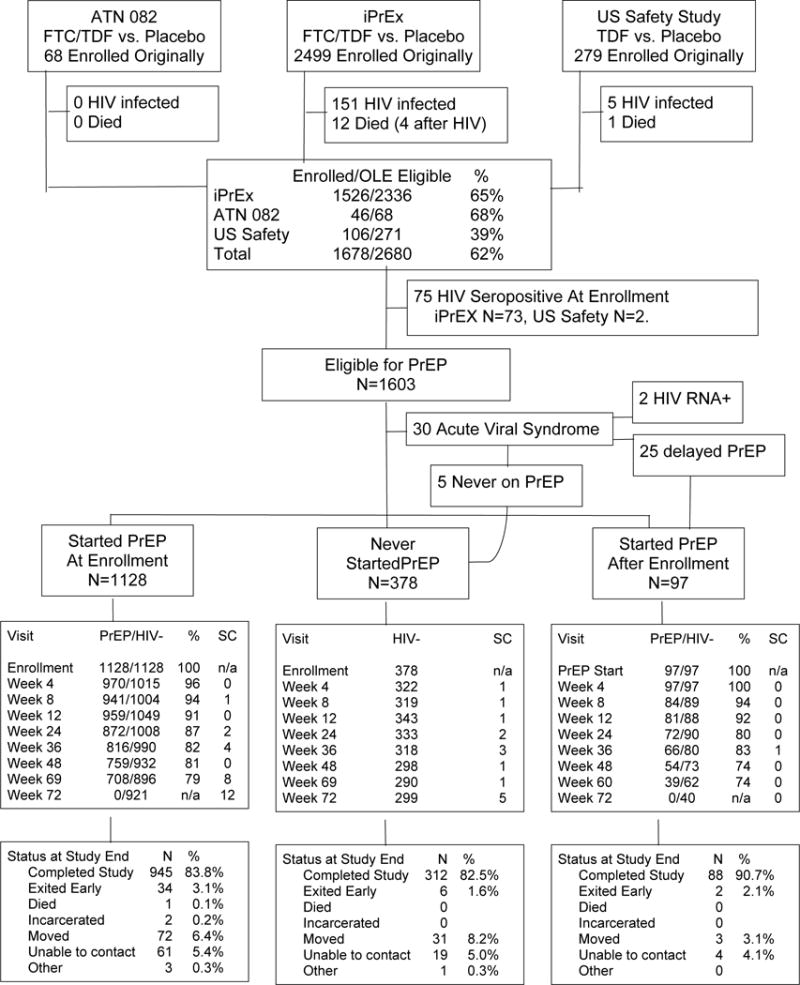
Consort Diagram of the HIV Uninfected iPrEx OLE Cohort. The sources of participants, HIV status, PrEP eligibility, and PrEP use is tracked. All HIV infected persons were offered participation in the study. Numbers of visits and seroconversions at week 72 are higher due to participants who had been out of follow up who returned for a final visit.
PrEP Uptake
Desire to receive PrEP was expressed by 1263/1761 (72%) during the CASI at OLE enrollment before HIV testing or clinical evaluation. Of these, 42/1263 (3.3%) were found to be HIV seropositive. Acute HIV-1 infection was suspected clinically in an additional 30 of whom 2 (7%) were subsequently found to have detectable HIV-1 RNA. HIV-1 RNA testing was negative among the other 28, of whom 25 started PrEP after an average delay of 44 days (range 9 to 136 days). PrEP was dispensed to 1125/1603 (76%), including 72% of the asymptomatic seronegatives (1128/1573) who received PrEP at the enrollment visit, and 6% of all seronegatives (97/1603) who received PrEP at a later visit. Receipt of PrEP was higher among those who reported ncRAI and had serological evidence of HSV-2 infection at OLE enrollment (Table 1). PrEP uptake was not associated with prior randomization, age, education, alcohol or substance use. Among people choosing not to receive PrEP, the reasons for not requesting PrEP were concern about side effects (188/384, 49%), not wanting to take a pill every day (58/384, 15%), not liking taking pills (46/384, 12%), preference for other prevention methods (54/384, 14%), fear that people will think they have HIV (27/384, 7%), and fear that people will know they have sex with men or trans people (11/384, 3%); the reasons for declining PrEP did not differ by prior randomization group.
Table 1.
Characteristics of the Enrolled Cohort and Those Choosing to Receive PrEP
| Characteristic | % of cohort | Total Eligible (N) | Received PrEP (% of row) | P-Value |
|---|---|---|---|---|
|
| ||||
| Region N=1603 | ||||
| USA | 18% | 287 | 224 (78%) | <0.0001 |
| Brazil | 13% | 208 | 192 (92%) | |
| Peru | 52% | 838 | 562 (67%) | |
| Ecuador | 10% | 161 | 153 (95%) | |
| South Africa | 3% | 48 | 40 (83%) | |
| Thailand | 4% | 61 | 54 (89%) | |
|
| ||||
| Age at Entry N=1603 | ||||
| 18–24 | 20% | 317 | 247 (78%) | 0.15ˆ |
| 25–29 | 27% | 437 | 315 (72%) | |
| 30–39 | 31% | 502 | 394 (78%) | |
| ≥40 | 22% | 347 | 269 (78%) | |
|
| ||||
| Education N=1590 | ||||
| < Secondary | 21% | 327 | 264 (81%) | 0.98ˆ |
| Secondary | 34% | 547 | 387 (71%) | |
| > Secondary | 45% | 716 | 566 (79%) | |
|
| ||||
| Alcohol Use (on days when drank) N=1603 | ||||
| None/< once a month | 9% | 144 | 103 (72%) | 0.45ˆ |
| 1–4 per day | 32% | 508 | 403 (79%) | |
| •5 per day | 20% | 324 | 250 (77%) | |
| Refused/Don’t know | 39% | 627 | 469 (75%) | |
|
| ||||
| Methamphetamine N=1603 | ||||
| No | 98% | 1572 | 1190 (76%) | 0.83ˆ |
| Yes | 2% | 31 | 26 (84%) | |
|
| ||||
| Cocaine N=1603 | ||||
| No | 91% | 1470 | 1070 (73%) | 0.64ˆ |
| Yes | 9% | 133 | 101 (76%) | |
|
| ||||
| ncRAI at OLE entry N=1603 | ||||
| No | 68% | 1084 | 809 (75%) | 0.003ˆ |
| Yes | 32% | 519 | 416 (81%) | |
|
| ||||
| Trans identified N=1603 | ||||
| No | 96% | 1533 | 1167 (76%) | 0.12ˆ |
| Yes | 4% | 70 | 58 (83%) | |
|
| ||||
| Known HIV+ partner N=1603 | ||||
| No | 89% | 1431 | 1083 (76%) | 0.36ˆ |
| Yes | 11% | 172 | 142 (83%) | |
|
| ||||
| Syphilis RPR at entry N=1603 | ||||
| No | 84% | 1350 | 1028 (76%) | 0.39ˆ |
| Yes | 16% | 253 | 197 (78%) | |
|
| ||||
| HSV-2 N=1603 | ||||
| No | 87% | 812 | 613 (75%) | 0.03ˆ |
| Yes | 13% | 791 | 612 (77%) | |
|
| ||||
| Gonorrhea by urine PCR N=1187 | ||||
| No | 98% | 1156 | 1186 (76%) | 0.95ˆ |
| Yes | 2% | 31 | 25 (81%) | |
|
| ||||
| Randomized Experience N=996 | ||||
| Placebo | 72% | 720 | 550 (76%) | 0.64ˆ |
| Active: No drug week 8 | 9% | 91 | 65 (71%) | |
| Active: Yes Drug week 8 | 19% | 185 | 155 (84%) | |
P value adjusted for site.
Initial drug detection in blood plasma among PrEP recipients
Tenofovir was tested using blood plasma from week 8 (N=851), week 4 (N=305), or 12 (N=33). Among all people who received PrEP, drug was detected in blood plasma in 847 (71%) of participants, and varied by study region: United States (185/222, 83%), Brazil (142/185, 77%), Peru (357/538, 63%), Ecuador (93/150, 62%), South Africa (27/40, 68%), Thailand (43/54, 80%). The overall drug detection rate in plasma was similar in OLE compared with during the first 8 weeks of previous randomized phase of the iPrEx trial (149/213 which is 70%, or 60% after weighting for sampling fraction, P=0.09). Among 63 participants who were tested during both phases of the iPrEx study, drug detection rates increased in Peru from 44% (28/63) in the randomized phase to 63% (40/63) in OLE (P=0.02), and were comparable at other sites.
Sensitivity and specificity of DBS analysis
As adherence to PrEP is the important determinant of PrEP efficacy, sensitive indicators of long-term PrEP use are needed. TFV-DP concentrations were detected in DBS among 77% of people (70/92, the percentage is weighted for sampling), who had no detectable drug in blood plasma at week 8, indicating the higher sensitivity of DBS analysis. Drug was detected in DBS in one (2%) of 60 persons who never received PrEP in OLE; the participant had previously been randomized to the active arm of iPrEx and had not returned all pill bottles.
HIV incidence
There were 2 RNA positive infections at the time of enrollment; both were suspected clinically and PrEP was not initiated. In addition, there were 41 on study HIV infections; 13 among those not receiving PrEP (2.61/100PY, 95% CI: 1.5 to 4.5) and 28 among those receiving PrEP (1.83/100PY, 95% CI: 1.3 to 2.6). The clinic had stopped dispensing PrEP in 7 people more than 2 months before seroconversion due to toxicity perceived by the provider (N=2; hypersensitivity in one, gastritis in the other), side effects perceived by the user (N=2, dizziness, nausea, and flatulence in one, weight gain in the other), loss to follow up (N=1) and client preference (N=2). Among those receiving PrEP, HIV incidence was 49% (95% CI: −1 to 74%) lower than among those who did not choose PrEP after adjusting for the higher risk sexual practices at baseline among PrEP users, and 36% lower before adjustment (95% CI: −24 to 67%). Considering only participants from iPrEx, the HIV incidence on PrEP was 53% (95% CI: 26 to 70%) lower than in the placebo arm of the randomized phase (3.93/100PY) and 51% (95% CI: 23% to 69%) lower than during the gap between the randomized phase and OLE (3.81/100PY).
Drug Concentrations in DBS and HIV Incidence
Drug concentrations in DBS were strongly associated with HIV incidence among those receiving PrEP (Fig. 2; P<0.0001). There were no infections at visits where TFV-DP was •700 fmol/punch, indicating use of 4 to 7 tablets per week (95% CI 0 to 0.4 per 100 PY). Such protective drug concentrations were evident during 33% of visits among those receiving PrEP. The hazard ratio was zero corresponding to a 100% reduction in incidence (95% CI: 83% to 100% if compared with the previous placebo arm; 95% CI: 86% to 100% if compared to concurrent off-PrEP group). The DBS concentration associated with 90% reduced risk of HIV acquisition relative to the off-PrEP arm was 611 fmol/punch (95% CI: 216 to 1006), consistent with use of 2 to 3 tablets per week.
Figure 2.
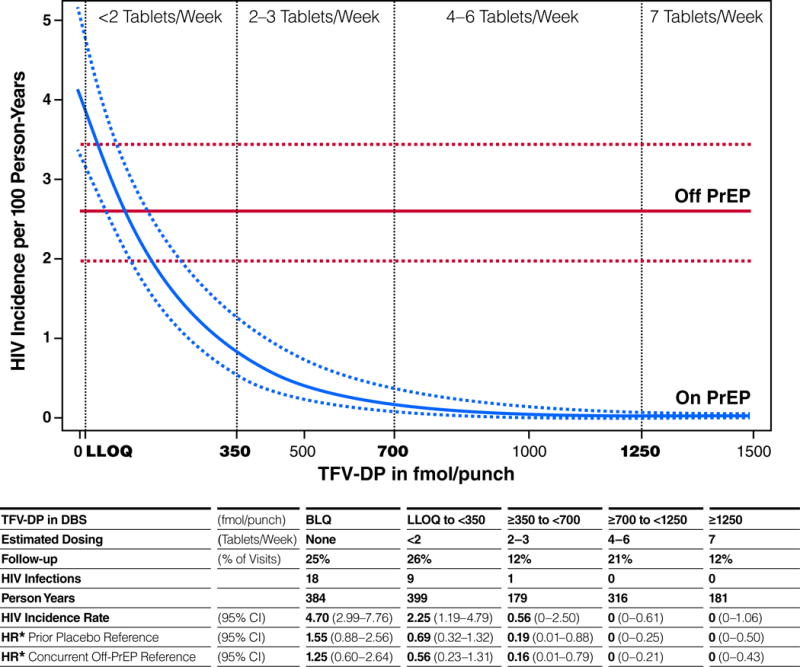
HIV-1 Incidence and TFV-DP in DBS. For those visits on PrEP, the incidence of HIV is estimated by exponential regression by TFV-DP in DBS. The incidence for the prior placebo group is depicted as a constant for reference. The dotted lines represent the estimate bounded by one standard error. Dosing for each interval is estimated by pharmacokinetic modeling. The hazard ratios were adjusted for study site, age, ncRAI at entry and syphilis. TFV-DP measurements were not available for 5% of visits in the cohort.
Correlates of PrEP Use indicated by Drug Concentrations in DBS
Drug concentrations were higher among participants with older age, more years of schooling, ncRAI, larger numbers of sexual partners, a history of syphilis or herpes, having any HIV+ sexual partner, or being trans-identified (Table 2). The effect of age was not explained by differences in eCrCl and was distributed across the range of ages. Drug concentration in DBS was not associated with alcohol use, methamphetamine use, or cocaine use. DBS drug detection at week 4 was associated with sustained use of PrEP over time (Fig. 3A). Examining individual patterns of PrEP use, the most common pattern was clinically significant use (>350 fmol/punch) followed by discontinuation over time; intermittent use (with periods of starting and stopping) was not a common pattern (Fig. 3B). HIV infection occurred during gaps in PrEP use: At the time of the first laboratory evidence of HIV infection, drug concentrations were •350 fmol/punch in 4% (1/28) of seroconverters and 33% (442/1338) of controls (Fig 3C, P<0.001). The proportion having such drug concentrations were comparable among HIV serconverters and seronegative controls earlier during PrEP use, decreased over time in both groups (P<0.001), and decreased more rapidly among seroconverters (P=0.02).
Table 2.
Predictors of Drug Concentration in DBS in the Drug Level Cohort.
| Factor | Adjusted OR | 95% CI | P-Value |
|---|---|---|---|
|
| |||
| Non-condom Intercourse at Entry | |||
| No condomless intercourse | ref | ||
| ncIAI | 1.22 | 0.82 to 1.81 | 0.3 |
| ncRAI | 1.69 | 1.40 to 2.04 | <0.0001 |
|
| |||
| Male Sex Partners in 6 monthsprior to entry | |||
| None to 1 | ref | ||
| 2 to 4 | 1.23 | 1.00 to 1.57 | 0.05 |
| 5+ | 1.57 | 1.22 to 2.01 | 0.0004 |
|
| |||
| Known HIV+ Partner | 1.40 | 1.02 to 1.92 | 0.03 |
|
| |||
| Any sexually transmitted infection at enrollment in OLE | 0.99 | 0.80 to 1.22 | 0.91 |
|
| |||
| Trans Identified | 2.03 | 1.27 to 3.25 | <0.0001 |
|
| |||
| Age at OLE | |||
| 18–24 | ref | ||
| 25–29 | 1.08 | 0.84 to 1.38 | 0.54 |
| 30–40 | 2.02 | 1.55 to 2.63 | <0.001 |
| •40 | 3.16 | 2.29 to 4.38 | <0.0001 |
|
| |||
| Education | |||
| < secondary | ref | ||
| secondary | 1.89 | 1.54 to 2.32 | <0.0001 |
| > secondary | 2.40 | 1.82 to 3.14 | <0.0001 |
|
| |||
| Alcohol drinks per day (on days when drank) | |||
| <5 | ref | ||
| >=5 | 0.84 | 0.67 to 1.05 | 0.13 |
|
| |||
| Methamphetamine use | 0.79 | 0.44 to 1.40 | 0.42 |
|
| |||
| Cocaine use | 1.02 | 0.79 to 1.30 | 0.89 |
|
| |||
| Body Mass Index (per kg/m2) | 0.99 | 0.98 to 1.01 | 0.66 |
|
| |||
| Entry estimated creatinine clearance (ml/min) | 0.984 | 0.976 to 0.988 | <0.0001 |
Figure 3.
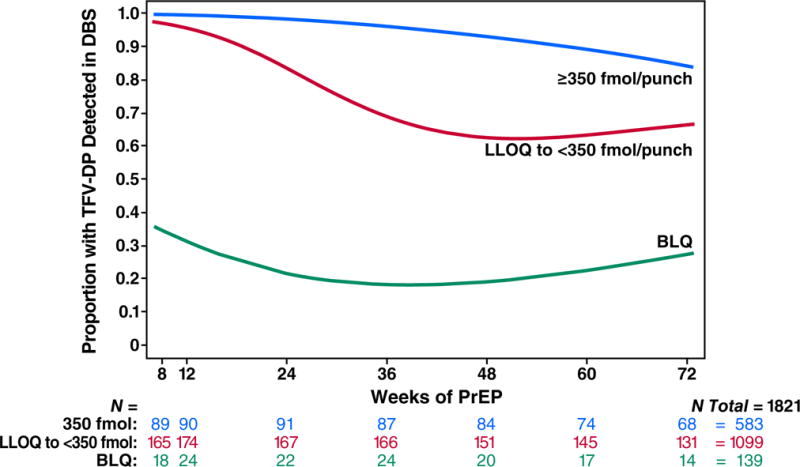
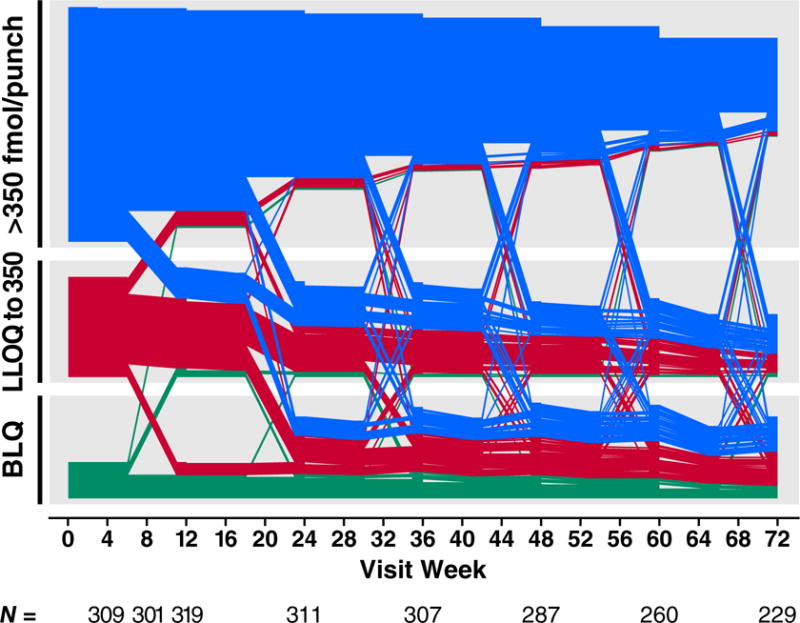
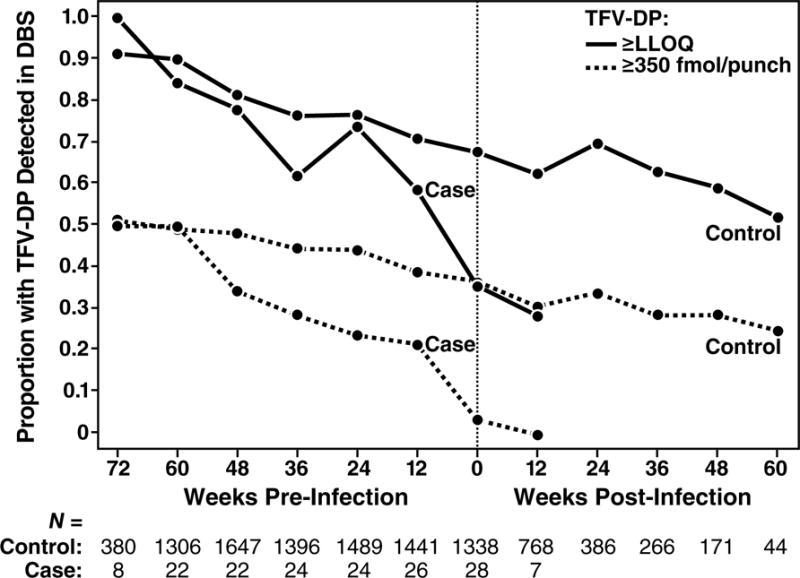
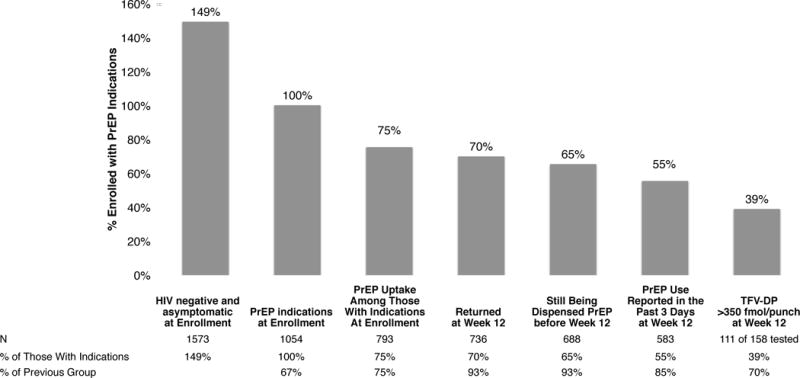
PrEP Drug Detection Over Time. Panel A is the proportion of study participants who had TFV-DP detected in DBS over the course of the study, stratified by the level of detection in DBS at week 4. Week 4 concentrations greater than 350 fmol/punch were associated with sustained PrEP use over time. PrEP discontinuation occurs mainly in the first 24 weeks. Panel B depicts patterns of drug concentrations in DBS for each seronegative participant who received PrEP at enrollment. Each line reflects one participant. Panel C depicts the proportion of seronegative controls and seronconverters having drug concentrations that were detectable or clinically significant (>350/punch) over time. The x axis plots time from the first laboratory evidence of HIV-1 infection, which could have been seroconversion, or detection of HIV RNA, or both in seroconverters. Seronegatives were frequency matched to cases by site on this time scale. Panel D depicts a cascade of PrEP uptake and treatment as a percentage of people with indications for PrEP at enrollment. Indications for PrEP use included more than one anal intercourse partner in the past 6 months, ncRAI, syphilis, GC, or CT infection at enrollment. DBS levels of TFV-DP of >350 fmol/punch are associated with substantial reductions in HIV incidence.
The PrEP Cascade: HIV Risk, PrEP Uptake, Visit Retention, and Adherence (Fig 3D)
PrEP impact requires uptake during periods of risk for HIV acquisition, PrEP adherence, and retention in care during periods of risk. At enrollment, 67% (1054/1573) of participants eligible for PrEP had indications for PrEP as defined as ncRAI, more than one anal intercourse partner, or a recent sexually transmitted infection (syphilis, gonorrhea or chlamydia diagnosed at the visit). Of that group, 75% (793/1054) chose to use PrEP. Of those at risk who started PrEP, 93% (736/793) returned at week 12, of whom 93% (688/736) were still being dispensed PrEP. Of those still being dispensed PrEP, 85% (583/688) reported taking PrEP within the past 3 days, of whom 70% (111/158) had clinically significant drug concentrations (TFV-DP >350 fmol/punch) in DBS. Overall, 39% of those who had HIV risk at baseline had clinically significant PrEP use through week 12 (Fig. 3D). Retention in the study was not associated with receipt of PrEP (945/1128, 84% vs 312/378, 82.5%, P=0.42). Older people were less likely to miss a visit (defined as more than 4 months between visits regardless of drug detection) (P=0.006). Retention was not related to ncRAI (P=0.95), having 5 or more sex partners in the past 6 months (P=0.92), or being trans-identified (P=0.82).
Safety and Interruptions in PrEP Dispensation
PrEP treatment was interrupted 380 times among 365 participants for reasons other than loss to follow-up, end of the study, or HIV infection. These interruptions represented 15.6% of the observation period. The reasons for interruptions were client preference (151/380 times, 6.6% of follow-up), side effects (93/380 times, 3.7% of follow-up), safety during treatment for a significant but unrelated comorbidity (38/380 times, 1.1% of follow-up), relocation or travel (52/380 times, 2.4% of follow-up), and other (53/380 times, 1.8% of follow-up). Other causes included suspected acute HIV infection (8/380 times, 0.2% of follow-up) and recent sexual exposure deemed to warrant a 3-drug regimen for post-exposure prophylaxis (15/380 times, 0.1% of follow-up). Gastrointestinal symptoms (such as nausea or abdominal pain) were the most common symptom leading to interrupting PrEP treatment. There were 3 confirmed elevations in serum creatinine, all grade 1, all returned to baseline after stopping PrEP, and none recurred after restarting PrEP. One seroconverter on PrEP had the RT M184V mutation associated with FTC resistance.
Sexual practices
Self-reported total numbers of sexual partners, ncRAI, and non-condom insertive anal intercourse (ncIAI), all decreased during follow-up, in the group receiving PrEP and in the group not receiving PrEP. The proportion reporting ncRAI decreased from 34% (377/1115) to 25% (232/926) among PrEP recipients (P =0.006) and from 27% (101/369) to 20% (61/304) among non-recipients (P=0.03); the rates of decrease in ncRAI, ncIAI, and numbers of sexual partners were comparable in the two groups (P=0.95, P=0.56, P=0.64 respectively). Syphilis incidence was comparable among PrEP recipients and non-recipients (7.2 vs. 5.4/100 PY respectively, HR 1.35, CI: 0.83–2.19).
Discussion
PrEP uptake was high across a range of demographic subgroups of MSM/TGW who were previously enrolled in blinded placebo-controlled trials, and had access to PrEP at no charge from experienced health care providers. Such high uptake was also observed among heterosexual couples finishing the placebo-controlled phase of the Partner’s PrEP trial.(21) These findings contrast with population surveys of MSM indicating that utilization of PrEP is still low overall, (22, 23) with barriers including low awareness of PrEP, (23) suboptimal knowledge and experience among health care providers, (24) and ambiguity about whether PrEP should be provided by HIV specialists or general practitioners.(25) Minimizing these barriers to access, as occurred in this study and other settings, (26) revealed substantial demand for PrEP. Concerns about the safety of antiretroviral medications emerged as the most common reason for declining PrEP among those having ready access. While most public discussion of PrEP has focused on efficacy and adherence, information about safety that was confirmed in trials is important for all considering PrEP.
People having higher risk sexual practices and STIs were more likely to join the study, more likely to choose PrEP, and more likely to have sustained protective levels of PrEP use. Such preferential use of PrEP during times of greater risk is expected to increase the impact and cost-effectiveness of PrEP services, and reflects people’s capacity to recognize and respond appropriately to risks when given attractive options. Access to PrEP was associated with an approximately 50% reduction in HIV incidence compared with concurrent and historical controls. The higher concentrations of drug among trans-identified women in this study supports their addition to PrEP recommendations for heterosexuals and MSM. (27)
The percentage of PrEP users with detectable drug concentrations increased substantially among Peruvian men after the release of information about efficacy and safety from randomized trials. Such men are young and racially diverse, and comprised the majority of participants in this study.
Sustained engagement is a significant challenge for PrEP services. In this open label extension, in contrast with the randomized phase of the study, we inquired about desire to start or stop PrEP at every visit and explored current perceptions of risks and preferences. Disengagement from PrEP services was substantial and HIV infection rates during gaps in PrEP use were high. Among those who stop PrEP, disengagement typically occurs early after a brief period of experimentation with PrEP as revealed using sensitive analysis of DBS. The causes for disengagement were not identified for most clients, as reported side effects and toxicity were rare. Substance and alcohol use were not associated with disengagement. While some younger people sustained effective PrEP use in this young cohort having an average age of 28, drug concentrations were lower with younger age, across a broad range of ages, and retention was lower in youth. While low adherence among youth is influenced by developing neurocognitive capacities (28) and social development in emerging adulthood, (29) age-related social and structural characteristics likely contributed to this finding, possibly involving concomitant use of other daily medications, age parity with clinic staff, income, employment, housing, and stigma. Novel ways to attract and engage younger MSM/TGW are needed, especially given their higher HIV incidence.
The overall protection conferred by PrEP was strongly associated with a long-term measure of cumulative PrEP dosing, TFV-DP in red blood cells measured with DBS. No infections occurred during periods when drug concentrations were commensurate with use of 4 or more tablets per week. The concentration of drug in DBS associated with a 90% reduction in HIV-1 incidence corresponded to use of 2 to 3 tablets per week; this estimate is consistent with dose-effect relationships observed during the randomized phase of iPrEx. (4) While oral FTC/TDF PrEP is recommended for daily use, which helps foster dosing habits, the drug concentrations achieved with daily dosing (TFV-DP >1250 fmol/punch) are substantially higher than the protective threshold for MSM/TGW, providing some forgiveness for occasional missed doses. These relationships between drug concentrations in blood and protection from HIV apply to this cohort for whom rectal intercourse was the primary risk factor; the minimum required adherence to PrEP and the relationship between blood drug concentrations and protection from vaginal or other viral exposure may be different.
We found that reporting plasma drug testing results to PrEP users was accepted well: those informed of positive results appreciated the validation of their adherence efforts, and those informed of negative results were not surprised. (30) Participants frequently asked for quantitative measurements of drug concentrations, so sharing information from DBS analysis may prove to be an attractive way to reinforce and troubleshoot adherence, especially now that levels of protection associated with different drug concentrations in DBS have been determined for MSM/TGW. Analysis of drug concentrations in hair could have the same advantages. (31)
There was no evidence of risk compensation during open label access to PrEP. Sexual practices became safer by self-report and syphilis incidence was not greater among PrEP users. PrEP use among heterosexual couples in Africa also showed no change in sexual practices with HIV-1 infected partners.(32) While PrEP may serve as a daily reminder of imminent risk, we observed comparable trends toward safer reported behavior among PrEP users and non-users, suggesting that cohort participation and access to comprehensive prevention services were stronger drivers of these behavioral trends. Making PrEP available provided multiple “fringe” benefits, including engagement of people at risk, HIV testing, identification of HIV infections, including some acute infections, diagnosis and treatment of sexually transmitted infections, and longer-term counseling. The direct benefits of providing PrEP included a substantial reduction in HIV transmission among MSM/TGW, including high-level protection among active users.
Panel: Research in Context.
Systematic Review
PubMed was searched on June 30, 2014 using the search terms “preexposure prophylaxis and HIV” or “tenofovir, HIV, and prevention,” yielding 630 publications. There were primary reports of randomized clinical trials of preexposure HIV prophylaxis (9 publications and 1 conference abstract), attitudinal surveys of interest in using PrEP if it were available, and behavioral surveys of self-reported PrEP use. There were no longitudinal studies of open-label PrEP uptake and adherence. Recently released US CDC guidelines recommend daily oral FTC/TDF PrEP for HIV uninfected heterosexuals and men who have sex with men who are not in mutually monogamous relationships and who are not using condoms consistently, who have a HIV-infected partner, or who have a recent diagnosis of a sexually transmitted infection. (27) Transgender women are not mentioned in these guidelines. Despite FDA-approval for PrEP and these broad recommendations by the CDC, overall self-reported PrEP use has been uncommon in surveys (less than 5%). Barriers to PrEP uptake include low levels of awareness among people at risk for HIV, suboptimal information about PrEP among potential providers, ambiguities as to whether PrEP should be provided by HIV specialists or general practitioners, concerns about insurance coverage and co-payments, and provider concerns about risk compensation.
Interpretation
This study shows PrEP uptake in the majority men and transgender women who have sex with men who are offered PrEP free of charge by experienced providers, showing that the main barriers to PrEP uptake are on the supply side, related to access and provider characteristics. PrEP uptake and adherence was higher among people reporting higher sexual risk of acquiring HIV infection rather than among “the worried well.” The PrEP cascade shows substantial discontinuation of PrEP after initiation, despite the paucity of side effects, especially among younger people. A substantial portion of people having no detectable drug in blood plasma had previously experimented with PrEP as indicated by the advent of more sensitive methods for drug analysis using dried blood spots. Drug concentrations in dried blood spots strongly correlated with PrEP protection, with no HIV infections occurring if drug concentrations suggested use of 4 or more tablets per week over long time periods. Transgender women had higher drug concentrations supporting their inclusion in PrEP recommendations currently published for gay and bisexual men and heterosexual men and women. Self-reported sexual practices became safer in the cohort, regardless of whether PrEP was used or not; the lack of risk compensation is corroborated by comparable syphilis incidence among PrEP users and non-users. Making PrEP available had a substantial impact on HIV transmission in populations suffering a disproportionate burden of the epidemic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions of authors:
RMG designed and led the study and wrote the first draft of the MS. DVG designed the analysis. DVG and MM analyzed the data. VM coordinated the study and oversaw data management. KRA designed the counseling interventions. SH and KRA reviewed literature regarding adherence and youth. PLA and LB developed and conducted pharmacological assays. OM, SB, MC, CM, VGV, KM, SC, LGB, EGK, MS, and JG were investigators who conducted the study at their sites. All authors critically reviewed and approved the MS.
Conflicts of Interest:
SH and KRA received an unrestricted educational grant from Gilead Sciences. PLA receives study drug and contract work from Gilead Sciences. MS received honoraria from Gilead for lecturing. JR is an employee of Gilead Sciences.
References Cited
- 1.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure Chemoprophylaxis for HIV Prevention in Men Who Have Sex with Men. N Engl J Med. 2010 Nov 23; doi: 10.1056/NEJMoa1011205. Epub 2010/11/26. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012 Aug 2;367(5):399–410. doi: 10.1056/NEJMoa1108524. Epub 2012/07/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012 Aug 2;367(5):423–34. doi: 10.1056/NEJMoa1110711. Epub 2012/07/13. eng. [DOI] [PubMed] [Google Scholar]
- 4.Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, et al. Emtricitabine-Tenofovir Concentrations and Pre-Exposure Prophylaxis Efficacy in Men Who Have Sex with Men. Sci Transl Med. 2012 Sep 12;4(151):151ra25. doi: 10.1126/scitranslmed.3004006. Epub 2012/09/14. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donnell D, Baeten JM, Bumpus NN, Brantley J, Bangsberg DR, Haberer JE, et al. HIV Protective Efficacy and Correlates of Tenofovir Blood Concentrations in a Clinical Trial of PrEP for HIV Prevention. J Acquir Immune Defic Syndr. 2014 Apr 29; doi: 10.1097/QAI.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marrazzo J, Ramjee G, Nair G, Palanee T, Mkhize B, Nakabiito C, et al. Pre-exposure prophylaxis for HIV in women: daily oral tenofovir, oral tenofovir-emtricitabine, or vaginal tenofovir gel in the VOICE Study (MTN 003). Conference on Retroviruses and Opportunistic Infections; Atlanta, GA. 2013. [Google Scholar]
- 7.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012 Aug 2;367(5):411–22. doi: 10.1056/NEJMoa1202614. Epub 2012/07/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassell MM, Halperin DT, Shelton JD, Stanton D. Risk compensation: the Achilles’ heel of innovations in HIV prevention? Bmj. 2006 Mar 11;332(7541):605–7. doi: 10.1136/bmj.332.7541.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcus JL, Glidden DV, Mayer KH, Liu AY, Buchbinder SP, Amico KR, et al. No evidence of sexual risk compensation in the iPrEx trial of daily oral HIV preexposure prophylaxis. PLoS One. 2013;8(12):e81997. doi: 10.1371/journal.pone.0081997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu AY, Vittinghoff E, Chillag K, Mayer K, Thompson M, Grohskopf L, et al. Sexual risk behavior among HIV-uninfected men who have sex with men participating in a tenofovir preexposure prophylaxis randomized trial in the United States. J Acquir Immune Defic Syndr. 2013 Sep 1;64(1):87–94. doi: 10.1097/QAI.0b013e31828f097a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amico KR, Stirratt MJ. Adherence to preexposure prophylaxis: current, emerging, and anticipated bases of evidence. Clin Infect Dis. 2014 Jul 1;59(Suppl 1):S55–60. doi: 10.1093/cid/ciu266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Underhill K. Study designs for identifying risk compensation behavior among users of biomedical HIV prevention technologies: balancing methodological rigor and research ethics. Soc Sci Med. 2013 Oct;94:115–23. doi: 10.1016/j.socscimed.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosek SG, Siberry G, Bell M, Lally M, Kapogiannis B, Green K, et al. The acceptability and feasibility of an HIV preexposure prophylaxis (PrEP) trial with young men who have sex with men. J Acquir Immune Defic Syndr. 2013 Apr 1;62(4):447–56. doi: 10.1097/QAI.0b013e3182801081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grohskopf LA, Chillag KL, Gvetadze R, Liu AY, Thompson M, Mayer KH, et al. Randomized Trial of Clinical Safety of Daily Oral Tenofovir Disoproxil Fumarate (TDF) Among HIV-uninfected Men Who Have Sex With Men (MSM) in the United States. J Acquir Immune Defic Syndr. 2013 Mar 5; doi: 10.1097/QAI.0b013e31828ece33. [DOI] [PubMed] [Google Scholar]
- 15.Amico R, McMahan V, Goicochea P, Vargas L, Marcus JL, Grant RM, et al. Supporting study product use and accuracy in self-report in the iPrEx study: next step counseling and neutral assessment. AIDS Behav. 2012 Jul;16(5):1243–59. doi: 10.1007/s10461-012-0182-5. [DOI] [PubMed] [Google Scholar]
- 16.Amico K, McMahan V, Marcus J, Goicochea P, Vargas L, Grant R, et al., editors. Integrated Next Step Counseling (iNSC): A discussion based sexual health promotion conversation to support men who have sex with men using PrEP in the iPrEx open label extension. 7th Annual HIV Treatment and Prevention Adherence Conference of the International Association of Providers in AIDS Care; Miami, FL. 2012. [Google Scholar]
- 17.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73(1):1–11. [Google Scholar]
- 18.Castillo-Mancilla JR, Zheng JH, Rower JE, Meditz A, Gardner EM, Predhomme J, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses. 2013 Feb;29(2):384–90. doi: 10.1089/aid.2012.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bushman LR, Kiser JJ, Rower JE, Klein B, Zheng JH, Ray ML, et al. Determination of nucleoside analog mono-, di-, and tri-phosphates in cellular matrix by solid phase extraction and ultra-sensitive LC-MS/MS detection. Journal of pharmaceutical and biomedical analysis. 2011 Sep 10;56(2):390–401. doi: 10.1016/j.jpba.2011.05.039. Epub 2011/07/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenland S. An application of logistic models to the analysis of ordinal responses. Biometrical Journal. 1985;27(2):189–97. [Google Scholar]
- 21.Ndase P, Celum C, Campbell J, Bukusi E, Kiarie J, Katabira E, et al. Successful Discontinuation of the Placebo Arm and Provision of an Effective HIV Prevention Product After a Positive Interim Efficacy Result: The Partners PrEP Study Experience. J Acquir Immune Defic Syndr. 2014 Jun 1;66(2):206–12. doi: 10.1097/QAI.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 22.Zablotska IB, Prestage G, de Wit J, Grulich AE, Mao L, Holt M. The informal use of antiretrovirals for preexposure prophylaxis of HIV infection among gay men in Australia. J Acquir Immune Defic Syndr. 2013 Mar 1;62(3):334–8. doi: 10.1097/QAI.0b013e31827e854a. [DOI] [PubMed] [Google Scholar]
- 23.Krakower DS, Mimiaga MJ, Rosenberger JG, Novak DS, Mitty JA, White JM, et al. Limited Awareness and Low Immediate Uptake of Pre-Exposure Prophylaxis among Men Who Have Sex with Men Using an Internet Social Networking Site. PLoS One. 2012;7(3):e33119. doi: 10.1371/journal.pone.0033119. Epub 2012/04/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mimiaga MJ, White JM, Krakower DS, Biello KB, Mayer KH. Suboptimal awareness and comprehension of published preexposure prophylaxis efficacy results among physicians in Massachusetts. AIDS Care. 2014;26(6):684–93. doi: 10.1080/09540121.2013.845289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krakower D, Ware N, Mitty JA, Maloney K, Mayer KH. HIV Providers’ Perceived Barriers and Facilitators to Implementing Pre-exposure Prophylaxis in Care Settings: A Qualitative Study. AIDS Behav. 2014 Jun 26; doi: 10.1007/s10461-014-0839-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu A, Cohen S, Follansbee S, Cohan D, Weber S, Sachdev D, et al. Early experiences implementing pre-exposure prophylaxis (PrEP) for HIV prevention in San Francisco. PLoS Med. 2014 Mar;11(3):e1001613. doi: 10.1371/journal.pmed.1001613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.CDC U. PREEXPOSURE PROPHYLAXIS FOR THE PREVENTION OF HIV INFECTION IN THE UNITED STATES - 2014: A CLINICAL PRACTICE GUIDELIN. http://www.cdc.gov/hiv/pdf/PrEPguidelines2014.pdf2014.
- 28.Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008 Mar;1124:111–26. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnett JJ. Adolescence and emerging adulthood: a cultural approach. 2nd. xxi. Upper Saddle River, NJ: Pearson Prentice Hall; 2004. p. 538. [Google Scholar]
- 30.Liu A, Koester K, Eden C, Amico K, McMahan V, Goicochea P, et al., editors. IAPAC. Miami: 2013. Providing Drug-level Feedback to US Men Who Have Sex with Men (MSM) using Pre-exposure Prophylaxis in the iPrEx Open Label Extension (OLE): A Qualitative Evaluation of Participant Perspectives. [Google Scholar]
- 31.Liu AY, Yang Q, Huang Y, Bacchetti P, Anderson PL, Jin C, et al. Strong relationship between oral dose and tenofovir hair levels in a randomized trial: hair as a potential adherence measure for pre-exposure prophylaxis (PrEP) PLoS One. 2014;9(1):e83736. doi: 10.1371/journal.pone.0083736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mugwanya KK, Donnell D, Celum C, Thomas KK, Ndase P, Mugo N, et al. Sexual behaviour of heterosexual men and women receiving antiretroviral pre-exposure prophylaxis for HIV prevention: a longitudinal analysis. Lancet Infect Dis. 2013 Dec;13(12):1021–8. doi: 10.1016/S1473-3099(13)70226-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


