Abstract
Objectives
Traumatic iatrogenic pneumothorax occurs most often after a transthoracic needle biopsy. Since this procedure has become a common outpatient intervention, emergency department admissions of post-biopsy pneumothorax patients have increased. The aim of this study was to determine the factors that predict the need for tube thoracostomy in patients with post-biopsy pneumothorax in the emergency department.
Methods
A retrospective cross-sectional study was conducted on 191 patients with post-biopsy pneumothorax who were admitted to the emergency department between 2010 and 2017. Patient characteristics, clinical findings at the emergency department presentation, and procedural and radiological features were reviewed. A multivariate logistic regression model was constructed using the variables from univariate comparisons to determine the need for tube thoracostomy in patients with iatrogenic pneumothorax, and the effect sizes were demonstrated with odds ratios.
Results
Tube thoracostomies were performed on 69 out of 191 patients (36.1%). A total of 122 patients (63.9%) were treated with supplemental oxygen therapy without any other intervention, and 126 patients (66.0%) were hospitalized. In the multivariate model, the variables predicting the need for a tube thoracostomy were decreased breath sounds, dyspnea, decreased systolic blood pressure, decreased oxygen saturation and increased pleura–lesion distance. A distance of 19.7 mm predicted the need with a sensitivity of 69.6% and a specificity of 62.3%.
Conclusion
Decreased breath sounds, dyspnea, decreased systolic blood pressure, decreased oxygen saturation, and increased pleura-lesion distance may predict the need for a tube thoracostomy in patients with post-biopsy pneumothorax.
Keywords: Pneumothorax, Iatrogenic disease, Needle biopsy, Chest tubes, Tube thoracostomy
1. Introduction
Pneumothorax is classified as either spontaneous or traumatic depending on its etiology.1,2 The term “iatrogenic pneumothorax” (IP) is used when the pneumothorax results from an interventional procedure,3 including such common procedures such as transthoracic needle biopsy (TTNB; 24% of all IP's), subclavian vein catheterization (22%), and thoracentesis (20%).4 Therefore, the incidence of IP is even higher than that of spontaneous pneumothorax.5 As the number of diagnostic or therapeutic procedures increases, the rate of IP increases. In order to better manage this condition, there is a need for intervention methods based on the clinical signs of IP.
TTNB is usually performed as an outpatient procedure, which is a safe procedure after which the patient can be discharged, following a short observation period.6 However, large surveys have shown that the most common complication of TTNB is pneumothorax,7,8 for which patients may require follow-up in an observation unit or ward. The patients may also be admitted to the emergency department (ED) if any symptoms present after discharge. Although most pneumothoraces are resolved without any intervention other than supplemental oxygen therapy, a small number of pneumothoraces can expand and may result in a tension pneumothorax,9 in which case immediate intervention is necessary before a cardiovascular collapse develops.10 Therefore, the early recognition of an expanding pneumothorax is of critical importance in order to be prepared for the necessary intervention.
The American College of Chest Physicians in 2001 and the British Thoracic Society in 2010 published guidelines for the management of spontaneous pneumothorax.5,11 Though the guidelines offer diverse recommendations, none of the guidelines address the management of IP. Consequently, the management of IP is still being debated. Therefore, to define the patient population who undergoes post-TTNB tube thoracostomy, we aimed to determine the predictive factors for tube thoracostomy in patients with postbiopsy pneumothorax.
2. Methods
2.1. Study design
This retrospective cross-sectional study was conducted in an academic ED serving approximately 45,000 patients per year between January 2010 and January 2017. The institution is also an interventional radiology center that performs about 4000 procedures per year. Institutional review board approval was obtained for the study and informed consent was waived.
2.2. Study setting and population
The study population included all patients older than 18 years who presented to the ED with post-TTNB pneumothorax. At our institution, patients with post-TTNB pneumothorax are referred to the ED for further evaluation and treatment. The exclusion criteria were patients who had an inpatient TTNB, patients who were not referred to the ED after TTNB, and patients who had missing data in their archive records.
According to our ED management, tube thoracostomy decision is made based on patient's clinical and radiological findings in conjunction with thoracic surgery department. All the patients who had a tube thoracostomy performed were admitted to the thoracic surgery ward. All the patients who had a small pneumothorax without symptoms were observed in the ED. After 6 h of observation, if no pneumothorax was seen on the control chest x-ray, the patients were discharged from the ED with instructions. Patients were admitted to the wards if the pneumothorax was enlarged or no improvement was seen during the ED observation.
2.3. Study protocol
Patient characteristics including age; gender; medical history; tobacco use; presence of COPD or malignity; clinical findings which were documented by an emergency physician at the initial ED presentation including vital signs, symptoms, and physical examination features; ED observation duration or length of stay (LOS) at the hospital; procedural features including fine-needle or core biopsy; and radiological features including lesion size, cavitation of the lesion, pleura–lesion distance, fissure or atelectasis in the affected lung, and lesion location (central or peripheral) were reviewed from the hospital's archive records using international classification of disease 9 (ICD-9) codes in conjunction with the hospital's imaging database. Computed tomography (CT) images were evaluated by a radiologist who was blind to the patient outcomes. All the variables that were available at the initial ED presentation were evaluated to predict the need for tube thoracostomy.
2.4. Outcome measures
The primary outcome measure was the definition of the patient population that may require tube thoracostomy after TTNB according to demographic parameters such as age, gender and past medical history; clinical parameters at the initial ED presentation such as symptoms, vital signs and physical examination findings and radiological parameters such as pleura-lesion distance or lesion characteristics. The secondary outcome measures were defined as the hospitalization rate and the hospital LOS.
2.5. Data analysis
All statistical analyses were performed with SPSS for Windows (version. 15.0, SPSS Inc., Chicago, IL, USA). The Kolmogorov-Smirnov test was used to test for a normal distribution of the variables. A logistic regression model was constructed to assess the factors predicting the need for tube thoracostomy in patients with IP. The fitness of the multivariable regression model was evaluated with the Hosmer- Lemeshow test. The univariate model included demographic characteristics, radiographic records, and the variables that were available upon ED presentation. Each variable was tested in the univariate model, and those that were significant at an alpha level of 0.2 were then tested in the multivariate model. Odds ratios (ORs) were presented with 95% confidence intervals (CIs). An ROC curve analysis was also performed to determine which pleura–lesion distance could predict the need for tube thoracostomy.
3. Results
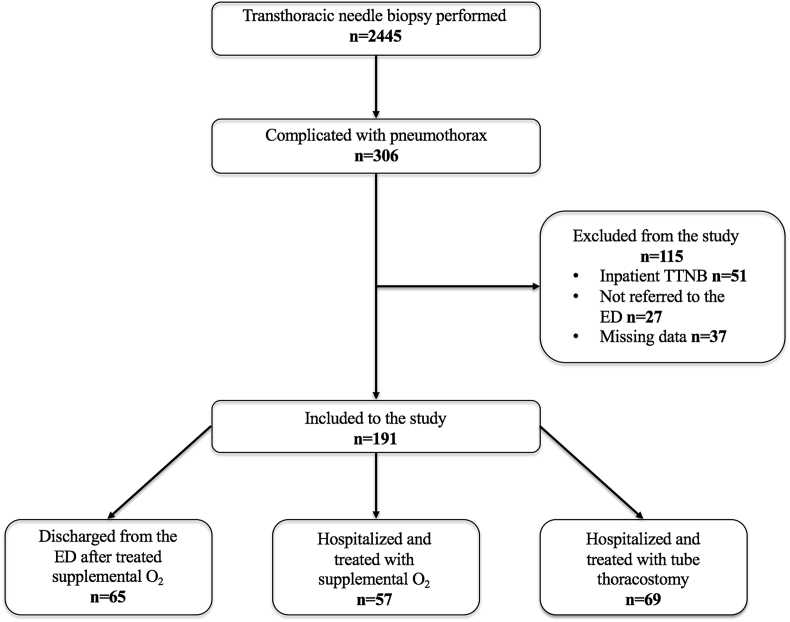
Between 2010 and 2017, 306 patients had complications of pneumothorax, and 115 patients were excluded (Fig. 1). A total of 191 patients were included in the study. Tube thoracostomy was performed on 69 out of 191 patients (36.1%). A total of 122 patients (63.9%) were treated with supplemental oxygen therapy without any other intervention, and 126 patients (66.0%) were hospitalized. A total of 69 patients underwent tube thoracostomy; fıne needle aspiration (FNA) was performed in 47 patients, core biopsies were performed in 10 patients, and both FNA and core biopsies were performed in 12 patients.
Fig. 1.
Patient flowchart.
The overall incidence of pneumothorax was 12% (306/2445), and the tube thoracostomy rate was 3% (69/2330) of all the TTNB cases in our study after excluding the 115 patients.
Demographic features of the patients with post-TTNB pneumothorax and the univariate analyses of the variables are shown in Table 1. The demographic and radiological variables that were determined to be significant predictors of the need for tube thoracostomy by univariate analysis were included in the multivariate logistic regression.
Table 1.
Demographic features and univariate comparisons of the patients with pneumothorax after TTNB.
| All patients (n = 191) | Treated with tube thoracostomy (n = 69) | Treated with conservative strategies (n = 122) | p value | |
|---|---|---|---|---|
| Age (median, IQR) | 64 (57–70) | 63 (55–69) | 65 (57–70) | 0.298 |
| Male gender (n, %) | 157 (82.2%) | 60 (87.0%) | 97 (79.5%) | 0.196 |
| Chest pain (n, %) | 60 (31.4%) | 21 (30.4%) | 39 (32.0%) | 0.827 |
| Dyspnea (n, %) | 96 (50.3%) | 46 (66.7%) | 50 (41.0%) | <0.001 |
| Decreased breath sounds (n, %) | 96 (50.3%) | 56 (81.2%) | 40 (32.8%) | <0.001 |
| Hemoptysis (n, %) | 4 (2.1%) | 0 (0.0%) | 4 (3.3%) | 0.299 |
| Diabetes Mellitus (n, %) | 29 (15.2%) | 6 (8.7%) | 23 (18.9%) | 0.060 |
| Hypertension (n, %) | 59 (30.9%) | 15 (21.7%) | 44 (36.1%) | 0.040 |
| Coronary Artery Disease (n, %) | 31 (16.2%) | 8 (11.6%) | 23 (18.9%) | 0.191 |
| COPD (n, %) | 31 (16.2%) | 9 (13.0%) | 22 (18.0%) | 0.369 |
| Malignancy (n, %) | 80 (41.9%) | 35 (50.7%) | 45 (36.9%) | 0.063 |
| Previous tuberculosis (n, %) | 7 (3.7%) | 4 (5.8%) | 3 (2.5%) | 0.256 |
| Smoking (n, %) | 125 (65.4%) | 54 (78.3%) | 71 (58.2%) | 0.005 |
| Pack/year (median, IQR) | 20 (0–40) | 30 (10–40) | 20 (0–40) | 0.125 |
| Pulse,/min (median, IQR) | 82 (71–91) | 86 (76–95) | 78 (70–90) | 0.006 |
| Respiratory rate,/min (median, IQR) | 24 (21–27) | 24 (22–29) | 24 (21–26) | 0.269 |
| Systolic blood pressure, mmHg (median, IQR) | 138 (121–152) | 137 (120–145) | 141 (127–160) | 0.007 |
| O2 saturation, % (median, IQR) | 97 (94–98) | 96 (93–98) | 97 (96–99) | <0.001 |
TTNB: Transthoracic needle biopsy.
IQR: Interquartile range.
COPD: Chronic obstructive lung disease.
The results of the multivariate logistic regression analysis to determine the factors predicting the need for tube thoracostomy are shown in Table 2. The Hosmer-Lemeshow test revealed that our regression results were fit (p = 0.128). According to the multivariate regression, decreased breath sounds (OR = 5.6, 95% CI = 2.4–12.8, p < 0.001), dyspnea (OR = 2.7, 95% CI = 1.2–6.2, p = 0.015), decreased SBP (OR = 0.9, 95% CI = 0.9–0.9, p = 0.04), decreased oxygen saturation (OR = 0.9, 95% CI = 0.7–0.9, p = 0.011), and increased pleura–lesion distance (OR = 1.1, 95% CI = 1.1–1.1; p = 0.002) predict the need for tube thoracostomy in patients with post-TTNB pneumothorax.
Table 2.
Results of multivariate logistic regression analysis to determine the factors predicting tube thoracostomy need.
| Wald | p value | Odds ratio (95% CI) | |
|---|---|---|---|
| Male gender | 0.4 | 0.518 | 1.5 (0.5–4.9) |
| Decreased breath sounds | 16.5 | <0.001 | 5.6 (2.4–12.8) |
| Dyspnea | 5.9 | 0.015 | 2.7 (1.2–6.2) |
| Smoking | 0.6 | 0.424 | 1.5 (0.6–3.8) |
| Systolic blood pressure | 4.2 | 0.040 | 0.9 (0.9–0.9) |
| Pulse | 0.7 | 0.411 | 1.0 (1.0–1.0) |
| SaO2 | 6.5 | 0.011 | 0.9 (0.7–0.9) |
| Diabetes Mellitus | 1.6 | 0.201 | 0.4 (0.1–1.6) |
| Hypertension | 0.1 | 0.872 | 0.9 (0.3–2.6) |
| Coronary arterial disease | 0.2 | 0.694 | 0.8 (0.3–2.5) |
| Malignancy | 0.5 | 0.504 | 1.3 (0.6–3.0) |
| Lesion pleura distance | 10.0 | 0.002 | 1.1 (1.1–1.1) |
| Fissure - athelectasis | 0.3 | 0.585 | 1.3 (0.5–3.3) |
CI: confidence interval.
SaO2: Oxygen Saturation
Statistically significant variables were showed as bold.
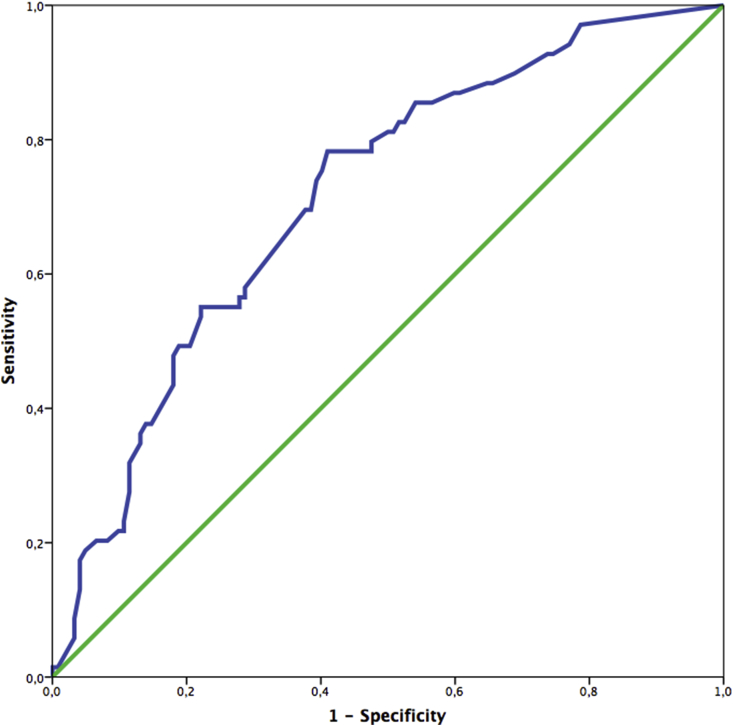
A radiological evaluation of the patients with post-TTNB pneumothorax is shown in Table 3. A ROC curve was generated to determine which pleura–lesion distance can predict the need for tube thoracostomy (Fig. 2). The area under the curve (AUC) was 0.715 (95% CI = 0.641–0.790, p < 0.001). The sensitivity was 69.6%, and the specificity was 62.3% for determining the need for tube thoracostomy for a pleura–lesion distance of 19.7 mm.
Table 3.
Radiological evaluation of the patients with pneumothorax after TTNB.
| All patients (n = 191) | Patients treated with tube thoracostomy (n = 69) | Patients treated with conservative strategies (n = 122) | p value | |
|---|---|---|---|---|
| Lesion size (median, IQR) | 26 (20–40) | 25.8 (20.0–33.2) | 28.5 (20.0–40.0) | 0.392 |
| Pleura-lesion distance, mm (median, IQR) | 19 (6–30) | 25.0 (17.5–35.0) | 12.8 (3.0–24.0) | <0.001 |
| Cavitation appearence (n, %) | 6 (3.1%) | 1 (1.4%) | 5 (4.1%) | 0.421 |
| Fissure or athelectasis on the needle tract (n, %) | 46 (24.1%) | 21 (30.4%) | 25 (20.5%) | 0.123 |
| Peripheral lesion (n, %) | 171 (89.5%) | 64 (92.8%) | 107 (87.7%) | 0.274 |
IQR: Interquartile range.
Fig. 2.
ROC curve for the determination of the pneumothorax requiring tube thoracostomy.
A total of 126 patients required hospitalization. The median LOS of the patients who underwent tube thoracostomy was 5 days (IQR = 3–10), and the median LOS of the patients treated with supplemental oxygen therapy was 0 days (IQR = 0–2).
4. Discussion
Iatrogenic pneumothorax should always be considered as a differential diagnosis in any patients who have pneumothorax symptoms such as pain, dyspnea, or coughing during an interventional chest procedure.12 A physical examination and vital signs may reveal decreased breath sounds, subcutaneous emphysema, tachypnea, tachycardia, decreased blood pressure, or decreased oxygen saturation.12,13 Our study results revealed that dyspnea, decreased breath sounds, decreased SBP, and decreased oxygen saturation upon the initial presentation to the ED and an increased pleura–lesion distance are related to the need for tube thoracostomy in patients with post-TTNB pneumothorax. To our knowledge, no prior study has included clinical findings associated with the need for tube thoracostomy in patients who have post-TTNB pneumothorax.
The overall incidence of pneumothorax was 12% (306/2445), and the tube thoracostomy rate was 3% (69/2330) of all the TTNB cases in our study. The incidence of pneumothorax after TTNB is reported between 9% and 54% with an average of 20%.14 Therefore, the incidences of pneumothorax and tube thoracostomy in our study were comparable to those in previous studies.15, 16, 17, 18, 19, 20, 21, 22, 23, 24
The risk factors of pneumothorax and the complication rates of TTNB have been previously studied.14, 15, 16, 17, 18, 19, 20, 21, 22 In a few studies, the need for tube thoracostomy in patients with post-TTNB pneumothorax was evaluated.19,21, 22, 23, 24 However, the risk factors are usually limited to patient demographics or radiological findings. In our study, in addition to some of those risk factors, clinical findings including symptoms and physical examination signs were evaluated in order to predict the need for tube thoracostomy in an ED setting. According to previous studies, a history of smoking,25 the presence of pulmonary emphysema,19,21,23 a history of COPD,23,25 the lesion depth,19,23 the needle size21,24 and the patient position during the TTNB22,24 have been found to be associated with pneumothorax requiring tube thoracostomy.
Several previous studies reported that an increased pleura-lesion distance may lead to an increased rate of pneumothorax.17,19,20,23, 24, 25, 26, 27, 28, 29 However, few studies have evaluated the relationship between the pleura-lesion distance and pneumothorax requiring tube thoracostomy.19,21,22,25 Nakamura et al.22 and Malone et al.21 reported no significant relationship between the pleura–lesion-distance and pneumothorax requiring tube thoracostomy. However, Hiraki et al.19 showed a significant correlation between an increased pleura–lesion distance and pneumothorax requiring tube thoracostomy. The results of our study suggest that an increased pleura-lesion distance may lead to pneumothorax requiring tube thoracostomy.
Laurent et al.23 classified the pleura-lesion distance into three categories: depth = 0 mm (lesion in contact with the pleura); depth between 0 and 49 mm; and a depth >50 mm. Of 61 patients, 6 who had pneumothorax required tube thoracostomy and had a mean pleura-lesion distance of 48.3 mm (range = 20–100 mm).21 Hiraki et al.19 reported a mean pleura-lesion distance of 31.2 mm (range = 14.7–47.7 mm) in 54 patients who had pneumothorax requiring tube thoracostomy. Our study showed a mean pleura-lesion distance of 25.0 mm (range = 17.5–35.0 mm) in 69 pneumothorax patients who underwent tube thoracostomy. We generated a ROC curve to determine a cutoff value for the pleura-lesion distance of 19.7 mm in patients with pneumothorax requiring tube thoracostomy; it showed an AUC of 0.715. Although this value probably fails to reach sufficient diagnostic accuracy, there is no preexisting cutoff value for the prediction of the need of tube thoracostomy in patients with post-TTNB pneumothorax.
Previous studies showed a strong correlation between emphysema and post-TTNB pneumothorax requiring tube thoracostomy.19,21,23 Our study did not include pulmonary emphysema. However, we deemed pulmonary emphysema to be a component of COPD. Kazerooni et al.25 and Laurent et al.23 reported that the severity of obstructive lung disease is correlated with post-TTNB pneumothorax requiring tube thoracostomy. However, our results showed no significant relation between COPD and tube thoracostomy.
Covey et al.24 reported that an extensive history of smoking is predictive of pneumothorax requiring intervention. The results of our univariate analysis determined that the severity of smoking may increase the risk of pneumothorax requiring tube thoracostomy; however, our multivariate analysis did not confirm this finding.
5. Limitation
There are many limitations of this study. First, this was a retrospective study; therefore, certain possibly significant variables could not be evaluated. Second, we excluded approximately one-third of the patient population, which could have had an effect on the study results. Third, the variables such as the sensation of dyspnea and decreased breath sound may be more subjective than the other variables. This may lead to differences in the patient statements and the treating physician perceptions. Fourth, no patient was treated with needle aspiration in this study. Needle aspiration would probably decrease the number of tube thoracostomies in our study population. Fifth, this is a single-center study, so the generalizability of our findings is limited.
6. Conclusion
Decreased breath sounds, dyspnea, decreased SBP, decreased oxygen saturation and increased pleura-lesion distance may predict the need for tube thoracostomy in patients with post-TTNB pneumothorax. Larger prospective studies are needed in future research to consider all the possible predictors.
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Peer review under responsibility of The Emergency Medicine Association of Turkey.
Contributor Information
İbrahim Ulaş Özturan, Email: ozturan.iu@gmail.com, @dr_ozturanMD.
Nurettin Özgür Doğan, Email: nurettinozgurdogan@gmail.com.
Cansu Alyeşil, Email: drcansualyesil@gmail.com.
Murat Pekdemir, Email: mpekdemir@yahoo.com.
Serkan Yılmaz, Email: syilmazmd@gmail.com.
Hüseyin Fatih Sezer, Email: hfs.hfs@gmail.com.
References
- 1.Noppen Marc. Tom de keukeleire: pneumothorax. Respiration. 2008;76:121–127. doi: 10.1159/000135932. [DOI] [PubMed] [Google Scholar]
- 2.Haynes D., Baumann M.H. Management of pneumothorax. Semin Respir Crit Care Med. 2010;31(6):769–780. doi: 10.1055/s-0030-1269837. [DOI] [PubMed] [Google Scholar]
- 3.Haynes D., Baumann M.H. Pleural controversy: aetiology of pneumothorax. Respirology. 2011;16(4):604–610. doi: 10.1111/j.1440-1843.2011.01968.x. [DOI] [PubMed] [Google Scholar]
- 4.Sassoon C.S.H., Light R.W., O'Hara V.S. Iatrogenic pneumothorax: etiology and morbidity. Results a department veterans affairs cooperative study. Respiration. 1992;59:215–220. doi: 10.1159/000196061. [DOI] [PubMed] [Google Scholar]
- 5.MacDuff A., Arnold A., Harvey J. Management of spontaneous pneumothorax: British Thoracic Society pleural disease guideline 2010. Thorax. 2010;65(suppl 2) doi: 10.1136/thx.2010.136986. ii18-ii31. [DOI] [PubMed] [Google Scholar]
- 6.Dennie C.J., Matzinger F.R., Marriner J.R. Transthoracic needle biopsy of the lung: results of early discharge in 506 outpatients. Radiology. 2001;219(1):247–251. doi: 10.1148/radiology.219.1.r01ap11247. [DOI] [PubMed] [Google Scholar]
- 7.Tomiyama N., Yasuhara Y., Nakajima Y. CT- guided needle biopsy of lung lesions: a survey of severe complication based on 9783 biopsies in Japan. Eur J Radiol. 2006;59:60–64. doi: 10.1016/j.ejrad.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Richardson C.M., Pointon K.S., Manhire A.R. Percutaneous lung biopsies: a survey of UK practice based on 5444 biopsies. Br J Radiol. 2002;75:731–735. doi: 10.1259/bjr.75.897.750731. [DOI] [PubMed] [Google Scholar]
- 9.Loiselle A., Parish J.M., Wilkens J.A. Managing iatrogenic pneumothorax and chest tubes. J Hosp Med. 2013;8(7):402–408. doi: 10.1002/jhm.2053. [DOI] [PubMed] [Google Scholar]
- 10.Fullerton D.A., Grover F.L., McKneally M.F. Chest trauma: pathophysiology and initial management. In: Pearson F.G., Deslauriers J., Ginsberg R.J., Hiebert C.A., McKneally M.F., Urschel H.C. Jr., editors. Thoracic Surgery. Churchill Livingstone; New York: 1995. pp. 1523–1534. [Google Scholar]
- 11.Baumann M.H., Strange C., Heffner J.E. Management of spontaneous pneumothorax. Chest. 2001;119(2):590–602. doi: 10.1378/chest.119.2.590. [DOI] [PubMed] [Google Scholar]
- 12.Weissberg D., Refaely Y. Pneumothorax: experience with 1,199 patients. Chest. 2000;117(5):1279–1285. doi: 10.1378/chest.117.5.1279. [DOI] [PubMed] [Google Scholar]
- 13.Yarmus L., Feller-Kopman D. Pneumothorax in the critically ill patient. Chest. 2012;141(4):1098–1105. doi: 10.1378/chest.11-1691. [DOI] [PubMed] [Google Scholar]
- 14.Boskovic T., Stanic J., Pena-Karan S. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J Thorac Dis. 2014;6(Suppl 1):S99–S107. doi: 10.3978/j.issn.2072-1439.2013.12.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi C.M., Um S.W., Yoo C.G. Incidence and risk factors of delayed pneumothorax after transthoracic needle biopsy of the lung. Chest. 2004;126(5):1516–1521. doi: 10.1378/chest.126.5.1516. [DOI] [PubMed] [Google Scholar]
- 16.Heerink W.J., de Bock G.H., de Jonge G.J. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol. 2017;27(1):138–148. doi: 10.1007/s00330-016-4357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Topal U., Ediz B. Transthoracic needle biopsy: factors effecting risk of pneumothorax. Eur J Radiol. 2003;48(3):263–267. doi: 10.1016/s0720-048x(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 18.Lendeckel D., Kromrey M.-L., Ittermann T. Pulmonary emphysema is a predictor of pneumothorax after CT-guided transthoracic pulmonary biopsies of pulmonary nodules. PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0178078. e0178078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiraki T., Mimura H., Gobara H. Incidence of and risk factors for pneumothorax and chest tube placement after CT fluoroscopy-guided percutaneous lung biopsy: retrospective analysis of the procedures conducted over a 9-year period. Am J Roentgenol. 2010;194(3):809–814. doi: 10.2214/AJR.09.3224. [DOI] [PubMed] [Google Scholar]
- 20.Lee H.Y., Lee I.J. Assessment of independent risk factors of developing pneumothorax during percutaneous core needle lung biopsy: focus on lesion depth. Iran J Radiol. 2016;13(4):2–6. doi: 10.5812/iranjradiol.30929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malone L.J., Stanfill R.M., Wang H. Effect of intraparenchymal blood patch on rates of pneumothorax and pneumothorax requiring chest tube placement after percutaneous lung biopsy. Am J Roentgenol. 2013;200(6):1238–1243. doi: 10.2214/AJR.12.8980. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura M., Yoshizako T., Koyama S. Risk factors influencing chest tube placement among patients with pneumothorax because of CT-guided needle biopsy of the lung. J Med Imaging Radiat Oncol. 2011;55(5):474–478. doi: 10.1111/j.1754-9485.2011.02283.x. [DOI] [PubMed] [Google Scholar]
- 23.Laurent F., Michel P., Latrabe V. Pneumothoraces and chest tube placement after CT-guided transthoracic lung biopsy using a coaxial technique: incidence and risk factors. AJR (Am J Roentgenol) 1999;172:1049–1053. doi: 10.2214/ajr.172.4.10587145. [DOI] [PubMed] [Google Scholar]
- 24.Covey A.M., Gandhi R., Brody L.A. Factors associated with pneumothorax and pneumothorax requiring treatment after percutaneous lung biopsy in 443 consecutive patients. J Vasc Intervent Radiol. 2004;15:479–483. doi: 10.1097/01.rvi.0000124951.24134.50. [DOI] [PubMed] [Google Scholar]
- 25.Kazerooni E.A., Tim F.T., Mikhail A. Risk of pneumothorax in CT-guided transthoracic needle aspiration biopsy of the lung. Radiology. 1996;198(2):371–375. doi: 10.1148/radiology.198.2.8596834. [DOI] [PubMed] [Google Scholar]
- 26.Miller K.S., Fish G.B., Stanley J.H. Prediction of pneumothorax rate in percutaneous needle aspiration of the lung. Chest. 1988;93(4):742–745. doi: 10.1378/chest.93.4.742. [DOI] [PubMed] [Google Scholar]
- 27.Cox J.E., Chiles C., McManus C.M. Transthoracic needle aspiration biopsy: variables that affect risk of pneumothorax. Radiology. 1999;212(1):165–168. doi: 10.1148/radiology.212.1.r99jl33165. [DOI] [PubMed] [Google Scholar]
- 28.vanSonnenberg E., Casola G., Ho M. Difficult thoracic lesions: CT- guided biopsy experience in 150 cases. Radiology. 1988;167:457–461. doi: 10.1148/radiology.167.2.3357956. [DOI] [PubMed] [Google Scholar]
- 29.Poe R.H., Kallay M.C., Wicks C.M. Predicting risk of pneumothorax in needle biopsy of the lung. Chest. 1984;85:232–235. doi: 10.1378/chest.85.2.232. [DOI] [PubMed] [Google Scholar]