Version Changes
Revised. Amendments from Version 1
The revised version of this manuscript incorporates alterations and additions as suggested by the reviewers. Most significantly, the Introduction has been extended so as to emphasise the importance of both biological and social factors in explaining the disparity of TB incidence between migrants and ethnic minorities in the UK compared with the general population.
Abstract
Migrants and ethnic minorities in the UK have higher rates of tuberculosis (TB) compared with the general population. Historically, much of the disparity in incidence between UK-born and migrant populations has been attributed to differential pathogen exposure, due to migration from high-incidence regions and the transnational connections maintained with TB endemic countries of birth or ethnic origin. However, focusing solely on exposure fails to address the relatively high rates of progression to active disease observed in some populations of latently infected individuals. A range of factors that disproportionately affect migrants and ethnic minorities, including genetic susceptibility, vitamin D deficiency and co-morbidities such as diabetes mellitus and HIV, also increase vulnerability to infection with Mycobacterium tuberculosis (M.tb) or reactivation of latent infection. Furthermore, ethnic socio-economic disparities and the experience of migration itself may contribute to differences in TB incidence, as well as cultural and structural barriers to accessing healthcare. In this review, we discuss both biological and anthropological influences relating to risk of pathogen exposure, vulnerability to infection or development of active disease, and access to treatment for migrant and ethnic minorities in the UK.
Keywords: Tuberculosis, UK, Migrants, Ethnic minorities, Socio-economic inequality, Stigma
Introduction
Tuberculosis (TB) is a bacterial disease caused by Mycobacterium tuberculosis ( M.tb), which most commonly affects the lungs 1. M.tb infection is acquired by inhalation of infectious particles released from close contacts 2. While 10% of those infected develop active disease, the majority of individuals mount an effective immune response leading to successful containment of M.tb growth; a condition known as latent M.tb infection or LTBI 3. Active TB disease results in systemic symptoms including fever, weakness and weight loss, as well as site-specific symptoms. For active pulmonary TB disease, the most common symptoms are persistent coughing (sometimes producing blood) and chest pain 3. In 15–20% of active cases, and usually in those with immunosuppression, the infection spreads outside the lungs causing extrapulmonary TB. The symptoms of extrapulmonary TB vary considerably depending on the site of infection, which may include lymph nodes, pleura and osteoarticular areas 4. Untreated, the 10-year case fatality rate of active disease is between 54 and 86% in HIV-negative individuals 5. LTBI, which is asymptomatic, ordinarily has a 5–10% lifetime risk of reactivation 2. LTBI may be diagnosed through cutaneous tuberculin skin test (TST) or interferon-γ release assays (IGRA), while clinically suspected TB disease is evaluated through chest radiograph and diagnostic microbiology for acid-fast bacilli. Effective antibiotic treatment is available, but involves long and complex regimens. Furthermore, rates of multi-drug-resistant TB (MDR-TB) and extensively-drug-resistant TB (XDR-TB) are increasing 6.
Globalisation, conflict and financial reasons have become increasingly important drivers of migration flows, leading to more permanent migrants moving from low/middle income to high-income countries 7. Studies of the incidence of TB in migrant communities in several high-income countries including Western Europe, the United States and Australia provide important evidence of disparity between subpopulations 8– 12. In the UK, a substantial proportion of foreign-born migrants arrive from former colonies in sub-Saharan Africa and the Indian Subcontinent (ISC) 13. Incidence of TB disease is higher among all migrant and ethnic minority groups living in the UK compared with the UK-born population. In 2016, 73.6% of individuals with diagnosed TB disease were foreign-born, with India and Pakistan the most frequent countries of birth among such cases 14. While TB rates have been falling slowly across all UK populations since 2011, they remain 15 times higher in the foreign-born than the UK-born population. Furthermore, within the UK-born population, non-white ethnic groups had TB rates 3 to 14 times higher than the white ethnic group 14. There is much heterogeneity in both absolute number of cases and incidence rates (per 100,000 of population group) among migrants from different countries and among different ethnic groups. While number of cases is confounded by size of population group, variation in incidence rate reflects varying levels of risk for different migrant and ethnic groups. Migrants from the ISC (India, Pakistan and Bangladesh) and black ethnic groups demonstrate particularly high incidence 15.
In explaining this disparity, the media have claimed that migrants and ethnic minorities ‘import’ TB into the UK. For example, according to the Daily Express, a TB outbreak in Leicester in 2001 indicated that “we are still vulnerable to infection from other countries” because “immigrants and visitors from less medically-advanced countries can carry the infection into Britain” 16. In this way, migrants and ethnic minorities are labelled as carriers of infectious agents 17. This popular view attributes the disparity in TB rates solely to differential pathogen exposure. Although exposure to M.tb in country of origin plays an important role, such an attitude obscures the mix of factors that increase the vulnerability of migrants and ethnic minorities to development of active TB disease from latent infection, and the influence of differential treatment-seeking behaviour in promoting the spread and therefore incidence of TB within these communities. As King highlights, TB is a complex disease and “the disproportionate burden of TB on certain populations is seldom the result of a single obvious cause” 18. In particular, it is difficult to disentangle socio-economic disadvantage from migrant/ethnic cultural identity in analyses of how reactivation of latent infection as well as transmission contribute towards TB incidence in migrant and ethnic minority populations; especially given that their relative importance differs between and within these groups. Nevertheless, it is clear that each factor - from the biological to the social 19 - has a role to play and should be considered in strategies to tackle this inequality.
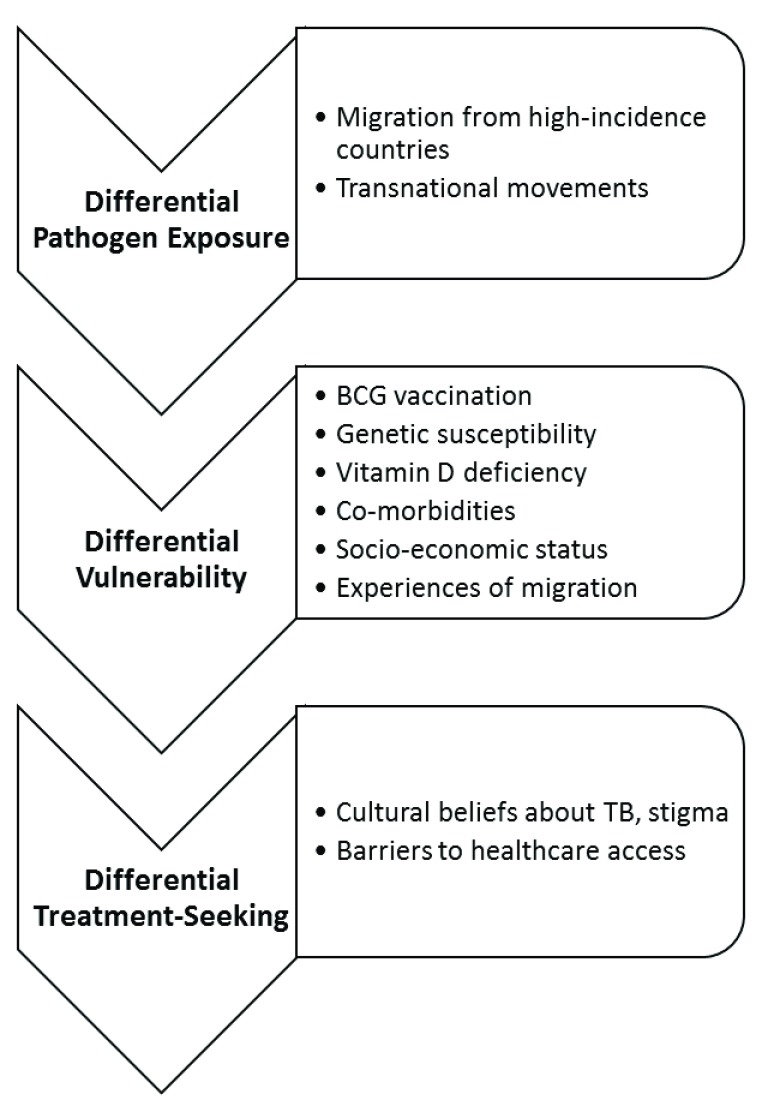
We discuss the biological, social and cultural factors relating to risk of pathogen exposure, vulnerability to infection or development of active disease, and access to treatment which contribute to the increased incidence of TB in migrant and ethnic minorities in the UK ( Figure 1).
Figure 1. Summary of factors contributing to the increased incidence of TB in migrant and ethnic minorities in the UK.
Epidemiology
The higher burden of TB observed among foreign-born individuals in the UK could be due to arrival of migrants with active TB, reactivation of remotely-acquired LTBI post-arrival, or local transmission 20. Meta-analyses of screens for active TB at entry have indicated that only a small proportion (~0.35%) of immigrants have active TB at time of arrival in the EU/EEA 21, 22. Since 2012, the UK Home Office has required pre-arrival screening for active pulmonary TB disease for all long-term visa applicants from endemic countries; those diagnosed with active disease are denied a medical clearance certificate 23. Thus arrival of migrants with active TB is not thought to contribute significantly to the overall burden of disease among foreign-born individuals in the UK; rather several studies suggest a more prominent role for the reactivation of remotely-acquired LTBI post-arrival 20, 24, 25. In the initial years following arrival in a lower incidence setting, migrants with LTBI have a higher risk of reactivation than the host population 8, 26, 27.
Local transmission within immigrant communities in the UK may also contribute to the higher incidence of TB cases observed in migrants and ethnic minorities. Such groups are more likely to live in densely-populated areas with a high concentration of their ethnic community, which may foster spread of TB, particularly given the mode of transmission 28. Moreover, Bakhshi suggests that larger household size among migrants and ethnic minorities - perhaps due to cultural factors favouring a multi-generational rather than nuclear family structure - increases M.tb transmission, as approximately one-third of household contacts will become infected in a household with an active TB case 29.
In order to establish the relative importance of local transmission versus reactivation of LTBI, molecular fingerprinting and other strain typing have been used since the 1990s. Genetic clusters have been assumed to represent epidemiologically linked chains of recent transmission, whereas unique isolates have been taken to represent reactivational disease 30. Early findings were conflicting, likely due to the inability of such techniques to reliably distinguish past and recent transmission 31. The advent of whole-genome sequencing of M.tb offered additional resolution, and in one such study of Oxfordshire TB cases in 2007–2012, those born in a high-incidence country were less likely to be part of a recent transmission cluster than those born in a low-incidence country (especially the UK), even when adjusting for social risk factors 32. Similarly, Aldridge et al. identified only 35 of over 300,000 migrants screened prior to entry into England, Wales and Northern Ireland as assumed index cases for transmission clusters 33. In addition, a Canadian study found no evidence to support concerns about transmission from immigrants born in high-incidence countries. These findings suggest that reactivation of LTBI is more important in explaining the higher incidence of TB among migrants than exogenous infection due to local transmission.
Differential exposure
Rates of active TB disease diagnosed after arrival in the UK correlate with TB incidence in the country of origin 34, indicating that differential exposure among migrants is a key factor influencing TB incidence in foreign-born populations. 13% of foreign nationals in the UK are from a country where TB incidence is ≥250 cases per 100,000 13. After the Second World War, substantial numbers of migrants arrived from the Commonwealth and former British Empire; particularly from the ISC. These movements were driven by factors such as Britain’s labour shortages for post-war reconstruction and political turbulence after decolonisation, for example following the creation of Pakistan 35. Many Commonwealth countries have high TB incidences: the highest incidences globally are found in Africa (275 per 100,000 population in 2015) and South East Asia (246 per 100,000) 6. Migrants from these countries are at a greater risk of having been exposed to M.tb and contracting LTBI. Indeed, of the 11 countries that were each the source of more than 2% of foreign-born cases in 2001–2003 (collectively accounting for 73% of foreign-born cases), all were in South Asia or sub-Saharan Africa 36.
It is useful to consider migration to the UK from the perspective of transnationalism, defined as “the process by which immigrants forge and sustain multi-stranded social relations that link together their societies of origin and settlement” 37. From the 1920s until recently, migration research has tended to focus on the incorporation of migrants in their destination country rather than continued ties with their country of origin. However, since the 1990s, “the transnational turn” has provided “a new analytic optic” 38. From this perspective, given that migrants maintain ties across the borders of nation-states, return visits to their country of origin and overseas visitors to the UK may result in increased exposure to M.tb. Where data was available, 17.2% of TB cases in 2016 in England had travelled outside the UK (excluding Western Europe, US, Canada, New Zealand and Australia) in the two years before diagnosis, and 7.3% had received an overseas visitor. 23.3% of non-UK born cases had travelled outside the UK in 2016 compared with only 4.7% of UK born cases 14. Such movements are increasing as globalisation leads to the intensification of international interconnectedness: in the UK, travel to visit family and friends abroad increased by 67% between 1998 and 2007 39. In 2007, UK residents made nearly 900,000 trips to the ISC for the purpose of visiting friends and family 39.
There is some evidence to suggest that travel to countries with high TB incidence increases the risk of acquiring LTBI, with greater risk associated with more prolonged travel and higher TB burden in the destination country 40. Such individuals are then at risk of developing active disease after returning to the UK. A study in Blackburn, Hyndburn and Ribble Valley found that 12.8% of active cases among Indian, Pakistani and Bangladeshi ethnic groups occurred within 3 years of revisiting the ISC 41. Furthermore, a case-control study in Liverpool found that TB cases were 7.4 times more likely to have recently received visitors from abroad 42. A case-control study of patients of ISC ethnic origin in North West England found a weak association between revisiting the ISC and TB cases within the following 3 years 43. However, there is currently limited data in this area and further research is needed to determine the proportion of TB acquired through travel 20. In particular, there is a need to elucidate whether certain types of travel are associated with a greater risk of acquiring LTBI, for example health professionals or volunteers 44.
Second generation immigrants (born in the UK but with parents born overseas) are at lower risk of TB than first generation immigrants (born overseas and migrated to the UK) but still remain at increased risk compared with the general population 15. This suggests a role for exposure to M.tb in the country of origin, but the higher incidence of TB among ethnic minorities compared with the general population is likely to be partially attributable to transnational connections, transmission within migrant populations, and other factors discussed in this review. However, quantification of the TB burden in this group is challenging as second generation migration is insufficiently captured by the prevailing European surveillance variables, and prospective studies on the risk of TB in successive migrant generations are lacking 45.
A further dimension of differential exposure is variation in pathogen factors such as global genomic diversity in the M.tb complex. There are six major genetic lineages of M.tb found in distinct geographical regions 46. Lineages can induce different host immune responses 47– 49, and it is possible that M.tb strain may contribute to variation in clinical TB phenotype between ethnic groups, for example the higher prevalence of extrapulmonary disease among non-white ethnic than UK-born cases 50. However, a study in an ethnically heterogeneous UK population found that ethnicity was strongly associated with clinical TB phenotype independent of M.tb lineage, suggesting that host factors may be more important in determining the clinical manifestation of TB 51.
BCG vaccination
Mycobacterium bovis Bacille Calmette-Guerin (BCG) is the only currently available vaccine against TB. BCG confers reliable protection against disseminated forms of TB such as miliary disease and meningitis in infants 52, 53. However, protection against pulmonary TB (the most common form of disease) varies considerably by geographical region 54. While the UK has one of the highest levels of BCG efficacy (~80%), a low level, or complete lack of, protection has been reported in many migrant countries of origin such as India 54– 56. The problem may be further confounded by limited access to vaccines and other healthcare in low-income country settings, but prevalence of M.tb infection in endemic countries remains high even where there is good BCG coverage 57, 58.
It has been hypothesised that exposure to non-tuberculous environmental mycobacteria (NTM), which increases with proximity to the equator, plays a central role in limiting BCG efficacy 59. Individuals may develop an immune response to NTM that either ‘masks’ or ‘blocks’ the ability of BCG to induce a protective response 59– 62. In a trial in Chingleput, India, 95% of individuals were PPD positive by 15–20 years of age 63. In a trial in Malawi where there is high NTM exposure and poor BCG efficacy, individuals with lower immune responses to NTM showed greater IFN-γ responses to BCG 64. Furthermore, in mice sensitised with NTMs, the protective effect of BCG (but not a TB subunit vaccine) was considerably reduced 65. The low levels of BCG protection found in countries with high TB incidence likely contribute to the prevalence of LTBI among migrant populations.
Genetic susceptibility
The idea of a heritable component to TB was suggested as early as 1886 by Hirsch: “That phthisis [TB] propagates itself in many families from generation to generation is so much a matter of daily experience, that the severest sceptic [sic] can hardly venture to deny a hereditary element in the case” 66. There are several lines of evidence suggesting that host genetic factors may contribute to TB susceptibility and resistance 67. Early studies demonstrated that monozygotic twins have a higher risk of developing active TB compared with dizygotic twins 68, 69, and several relevant loci have since been identified using candidate gene studies and genome-wide association studies 70– 75. Variation in susceptibility to M.tb infection and progression to active disease has been observed in different ethnic and geographic populations 76, 77. A study of >25,000 residents in racially-integrated nursing homes in Arkansas, USA, found that 13.8% of African-American compared with 7.2% of Caucasian residents had evidence of a new M.tb infection 78. Furthermore, in a study of three TB outbreaks in two prisons, African-Americans had approximately twice the relative risk compared with Caucasians of becoming infected with M.tb 78. However, although these studies largely controlled for environmental factors, confounders such as differing vitamin D levels cannot be ruled out.
More recently, genotyping technologies have supported a role for genetic ancestry in TB susceptibility. A case-control study genotyped a panel of ancestry informative markers to estimate the ancestry proportions in a South African Coloured [sic] population. African ancestry (particularly San ancestry) was higher in TB cases than controls, and European and Asian ancestries were lower in TB cases than controls 76. However, a limitation is that the study did not adjust for socioeconomic confounders. Differences in alleles that encode components of the immune response provide a possible mechanism for ethnic variation in TB susceptibility. Indeed, a study of African and Eurasian pulmonary TB patients in London indicated ethnic differences in the host inflammatory profile at presentation including lower neutrophil counts, lower serum concentrations of CCL2, CCL11 and DBP, and higher serum concentrations of CCL5 in those of African ancestry 79. These differences became more marked following initiation of antimicrobial therapy, and were associated with ethnic variation in host genotype but not M.tb strain 79.
Attributing variation in TB incidence between populations to genetic differences has been criticised, particularly due to fears that a discussion of ‘innate’ differences will perpetuate the naturalisation of inequalities in TB incidence 80. Moreover, ‘race’ is a problematic concept, acting only as a proxy for genetic variation that is continuously distributed according to ancestry 81. However, to recognise that there is some genetic variation in TB susceptibility that broadly aligns with ethnic groups is not to deny that there are other factors contributing to differences in TB incidence, as discussed in this review.
Host-pathogen co-evolution is a likely driver of variation in TB susceptibility in different human populations 82. M.tb has been co-evolving with humans for millennia, with evidence that humans were exposed before the Neolithic transition 83. The differential susceptibility of particular populations may be based on M.tb exposure history, with long-term exposure resulting in strong positive selection for resistance-related alleles. There is evidence from European colonialism that previously underexposed populations are more susceptible to TB, which played a large part in the deaths of many Qu’Appelle Indian and Inuit populations in Canada 84, 85. Similarly, in contrast to Europeans, Southern African populations have only been exposed to modern M.tb strains relatively recently 76. It has been suggested that selection pressure for resistance would have been strongest in areas of high population density. Accordingly, duration of urban settlement is correlated with the frequency of the SLC11A1 1729 + 55del4 allele which plays a role in natural resistance to intracellular pathogens including M.tb 86. Lower rates of TB among those of European ancestry could be due to centuries of exposure in densely populated settlements driving the evolution of increased resistance.
Vitamin D deficiency
It has long been recognised that low vitamin D levels are associated with active TB, with sunlight exposure in sanatoria and direct administration of vitamin D commonly used as treatments prior to the advent of antibiotics 87. Today, evidence supporting a link between TB and vitamin D deficiency is accumulating, although the association is complex 88– 91. A meta-analysis indicated a 70% probability that, when chosen at random from a population, an individual with TB disease would have a lower serum vitamin D level than a healthy individual 92, although the direction of causality is not clear. It has been demonstrated that 1,25(OH) 2D, the active metabolite of vitamin D, promotes the ability of macrophages to phagocytose M.tb and enhances the production of cathelicidin LL-37, an antimicrobial peptide that has direct bactericidal activity and attracts other immune cells to the site of infection 93. There is evidence that a drop in serum vitamin D compromises the immune response and can lead to reactivation of LTBI 94. Vitamin D deficiency may also be a risk factor for extrapulmonary dissemination of TB, which is more common among migrants and those of non-white ethnic groups 95, 96.
Vitamin D can be acquired from the diet or endogenously synthesised in the skin by the photolytic action of solar UV light on the precursor molecule 7-dehydrocholesterol 97, 98. Certain migrant and ethnic minority groups are at a greater risk of vitamin D deficiency 99– 101. Indeed, vitamin D levels have been shown to be lower among Asian children living in England compared with children of the same age in the general population 102. Vegetarians are at increased risk of vitamin D deficiency since oily fish are a major dietary source 103, and Hindu Asians are more frequently vegetarian than the general population due to socio-religious factors 104. A study of Asian immigrants with TB disease in Wandsworth found that Hindus were at higher risk of contracting TB than Muslims 105. Darker skin pigmentation also increases risk of deficiency, as melanin reduces the efficiency of vitamin D synthesis from UVR 101, 106. Hindu women in particular have been found to be at high risk, as in a 1976 study by Hunt et al. they spent on average only 2.5 hours a week outside for cultural reasons, whereas men were exposed to sunlight travelling to work 107.
1,25(OH) 2D mediates its immune activity through binding to the vitamin D receptor (VDR) on target cells; thus receptor abnormalities as well as vitamin D deficiencies may impair host immunity to M.tb 108. Some polymorphisms in the VDR gene increase susceptibility to TB, while others increase resistance 109, 110. In a systematic review of seven studies comparing the prevalence of VDR polymorphisms in TB patients and healthy controls, BsmI and FokI VDR polymorphisms were found to increase TB susceptibility 111. The VDR gene shows striking genetic variation in allele frequency between populations 112. Moreover, certain polymorphisms play different roles in different populations 111, although further research is required to elucidate how this translates into variation in patterns of susceptibility and resistance to TB in different ethnic groups. Epigenetic variation in the VDR gene in different ethnic groups, arising from differential exposure to environmental factors, may influence gene regulation and therefore contribute to differential TB susceptibility. Indeed, methylation variable positions at the 3' end of VDR have been identified that are significantly correlated with ethnicity and TB status 113.
Co-morbidities
Risk of progression to active TB disease is increased in those with conditions that impair immunity, such as diabetes mellitus (DM), human immunodeficiency virus (HIV) and chronic kidney disease (CKD) 114. Certain migrant and ethnic groups are at a higher risk of these conditions; diabetes mellitus disproportionately affects South Asians whereas HIV is more prevalent among those of African origin, and chronic kidney disease affects both groups.
a) Diabetes mellitus
Clinicians have noted a possible association between TB and DM since the early 20 th century 115. More recently, a meta-analysis of 13 cohort studies found that DM increases the risk of active TB 3.11-fold 116. A causal relationship between DM and impaired immunity to TB is supported by studies of diabetic mice, which have higher bacterial loads when infected with M.tb than non-diabetic mice 117. DM-TB comorbidity increases both the risk of new and reactivational TB 118, although diabetes as an independent risk factor was associated with only a modest overall increased risk of TB in a UK General Practice cohort 119. Various mechanisms have been suggested including impaired immune function due to DM, complications of DM, and deficiencies in vitamins A, C and D associated with both TB and DM risk 120.
Type 2 DM (T2DM) and associated risk factors, especially obesity, show marked associations with ethnicity 121. In the UK, obesity and T2DM risk is significantly higher among South Asians (including those of ISC origin), and moderately higher among black African-Caribbeans compared with white Europeans 122. The prevalence of DM among South Asians in England was 14% in 2010; approximately double the 6.9% prevalence in the general population 123. Some studies have suggested that ethnic differences in T2DM can be explained by differences in socio-economic status 124, while others do not support this 121. It is clear there are complex genetic and environmental explanations for ethnic differences in T2DM prevalence, which are beyond the scope of this review (for example, see 125).
b) Human immunodeficiency virus
Infection with HIV is the strongest known risk factor for the development of TB disease 126. TB-HIV co-infection synergistically worsens both conditions, leading it to be termed ‘the cursed duet’ 127. HIV increases both the risk of rapid progression to active disease following infection and reactivation of LTBI, with an increased risk of TB throughout the course of HIV-1 disease 128, 129 and incidence rate ratios >5 when averaged across all levels of immunodeficiency 130. The depletion of CD4+ T cells associated with HIV-1 infection is thought to play a major role in the increased risk of TB and its extra-pulmonary dissemination in infected individuals, as M.tb infected macrophages require CD4+ T cells to augment intracellular clearance 131. Furthermore, peripheral blood lymphocytes of HIV-positive patients produce less interferon-γ when exposed to M.tb in vitro than those of HIV-negative patients 132. These and other possible immune mechanisms such as chronic inflammation promoting an immunoregulatory phenotype and attenuation of phagocytosis have been recently reviewed 133.
A systematic review on the prevalence, incidence and mortality of HIV-TB co-infection in Europe observed a disproportionate vulnerability of migrants to co-infection across studies 134. Given that only 3.8% of TB cases in England in 2015 involved co-infection with HIV 14, TB-HIV co-infection cannot be considered a major driver of higher TB incidence among migrants and ethnic minorities in the UK; although this group represents an important target in the prevention of M.tb infection and progression to active disease. TB-HIV coinfection undoubtedly plays a role in explaining the higher TB incidence rates among those of African origin. In 2015, 81.8% of TB-HIV co-infected cases in England were foreign-born, of which 68.7% were born in sub-Saharan Africa 14 This reflects the global distribution of TB-HIV co-infection. In the WHO African region, 38% of new TB cases were co-infected 130. In turn, the global pattern of TB-HIV co-infection reflects the global distribution of HIV: 69.5% of all people living with HIV are in the WHO African region 135.
c) Chronic kidney disease
The association between CKD and TB was first reported in 1974 136, and has been subsequently confirmed by several studies 137– 139. The mechanism is thought to be impaired immunity: CKD is associated with functional abnormalities in various immune cells, such as B and T cells, monocytes, neutrophils, and natural killer cells 140. This increases the risk of both newly acquired and reactivated TB. Furthermore, immunosuppressive medications in kidney transplant patients are aimed at T cell-mediated immunity, which is central to maintaining TB latency in LTBI individuals 141. Patients with CKD are 10–25 times more likely to develop active TB 142.
Ethnic minorities in the UK are at a 3–5 times higher risk of developing CKD 143. A study in London from 1994–1997 found that the incidence rate among white Caucasians was 58/million adult population per year, 221 among South Asians, and 163 among African-Caribbeans 144. More recently, in a study of CKD patients with TB in South East London, 74% were born outside of the UK 145. CKD also interacts with other risk factors that contribute to higher incidence of TB among migrants and ethnic minorities. DM patients are 4–5 times more likely to have CKD 146, CKD patients are more likely to have low vitamin D levels 147, and CKD is a complication associated with HIV 148. Furthermore, CKD disproportionately affects economically disadvantaged groups, possibly due to the direct impact of poverty or malnutrition, or indirect effects of poverty-associated co-morbidities including DM and HIV 149. Given the complex interactions between multiple risk factors, it is difficult to establish the direction of causality.
Socio-economic status
The association between deprivation and TB has long been recognised, leading it to be dubbed a “social disease” 150 and “poverty’s penalty” 151. There is a strong socio-economic gradient in TB burden between and within countries and communities, with economically disadvantaged groups having the highest risk 152. The importance of social factors in TB risk is supported by McKeown’s observation that a considerable proportion of the decline in TB-associated mortality occurred before the advent of antibiotics and the BCG vaccine, implicating improved living standards and nutrition as the main drivers 153. Szreter contests the McKeown thesis, emphasising the key role of public health measures in regulating the urban environment 154. Either way, it is clear that TB disproportionately affects the socially and economically marginalised, with a recognised role for poverty, homelessness, and overcrowding in both the spread of infection and number of active cases 18. In the UK in 2009, TB cases among the homeless were 20 times higher than the general population at 300 cases per 100,000 155. In a study of London districts, the TB notification rate increased by 12% for every 1% rise in the number of people living in overcrowded conditions 156.
The wider social determinants of health are entwined with ethnicity, meaning that ethnic socio-economic disparities throughout the life course often lead to health inequalities 157. There are marked economic inequalities between ethnic groups in the UK, with both Asian and black ethnic groups having lower employment probability than the population average 158. Alongside economic issues of unemployment, low income and poor working conditions, migrants and ethnic minorities are also more likely to face problems of homelessness, poor housing, and overcrowding 159. Foreign nationals accounted for 13% of the general UK population in 2015 160, but 20% of the homeless population 161. In 2011, dwellings with a Household Reference Person (HRP) from a minority ethnic group represented 16.1% of all households in England and Wales, but 47.9% of overcrowded households. The most commonly overcrowded households were those with a Bangladeshi HRP (30.2% overcrowded), followed by Pakistani (22.3%) and black-African (21.8%) 162.
King rejects what he terms ‘essentialist’ explanations for the higher TB incidence of migrants and ethnic minorities, which claim that TB disproportionately affects certain groups due to intrinsic differences, which may be biological, genetic, physiological or cultural. Instead he suggests that “Disparities in health that may at first seem to arise from essential racial or ethnic differences are often in fact the result of contingent socioeconomic differences” 18. Similarly, Farmer rejects psychological or cultural explanations, emphasising that “tuberculosis is inextricably tied to poverty and inequality”. He criticises studies that neglect to address the political-economic forces that shape TB distribution, and calls for anthropologists to pay more attention to structural violence (the systematic ways in which social structures disadvantage individuals) and social inequality 163.
King and Farmer claim that, given that socio-economic status affects TB risk, biological and genetic approaches are largely irrelevant 18, 163. Similarly, the medical anthropologist Singer criticised the use of adaptation as a conceptual tool on the basis that such explanations ignore how the political economy shapes the environment that humans adapt to. She argues that differential mortality between socio-economic groups is “unnaturally selected” by the conditions created to further the interests of the dominant class 164. However, as discussed, there is evidence to suggest that adaptation resulting from host-pathogen co-evolution influences direct and indirect genetic susceptibility to TB infection and progression to active disease. Perhaps as Mason et al. suggest, a more constructive approach is required, recognising that “the social model is an important complement to the biomedical model” 165.
Given the complex association between ethnicity and socio-economic status, it is hard to disentangle the extent to which socio-economic disadvantage influences TB incidence in migrant and ethnic minority populations 166. One study in children from Leeds found that overall, ethnicity explained a high proportion of TB incidence independently of deprivation and population density, although for non-South Asian children, the strongest risk factor was deprivation 167. Similarly, a study in Liverpool suggested an association between ethnicity and TB incidence that was independent of deprivation level 168, and a study investigating TB trends in England in 1999–2003 indicated that affluent ethnic minority groups are still at greater risk 166. It has been suggested that the absence of a strong correlation between deprivation and M.tb infection in the South Asian community may be due to the smaller relative differences in deprivation within this group than across the general population 169. Indeed, a study in Newham found an association between the proportion of non-white residents and TB diagnosis in each ward, but no association with deprivation as the borough as a whole was deprived 170.
There is significant heterogeneity in the role that social risk factors play in increasing TB risk in different migrant and ethnic groups. Among UK-born cases notified in 2010–2016, 33.1 % of those in the black-Caribbean ethnic group had at least one social risk factor (homelessness, imprisonment, drug or alcohol misuse), higher than any other ethnic group 14. 19.4 % of black-Caribbean cases were drug users, and 18.6% had a history of imprisonment. The countries of origin with the highest number of homeless TB cases were Somalia, at 95 cases, and Eritrea, at 91 cases 14. This suggests that socio-economic disadvantage may play a particularly important role in explaining higher TB incidence among the black-African and black-Caribbean ethnic groups.
Experiences of migration
The difficulties faced during and shortly after migration may increase risk of progression to active disease by compromising immunity, including poor nutrition, concurrent poor health, socioeconomic marginalisation, and the stress of relocation 18. In an anthropological study of illegal Chinese immigrants with TB in New York, it was found that migrants often experience shortages of food and water during long migratory journeys. Upon arrival, temporary residence in detention centres or illegal refuges is associated with overcrowding and malnutrition 171. Migrants then face additional challenges including loss of a social support network, communication issues, discrimination, and acculturation 172. Ho calls for a focus on the macro-level structural forces that shape TB risk on migratory journeys, such as a lack of government regulation and exploitation by human traffickers 171.
Psychological effects include higher rates of anxiety among refugees and asylum seekers compared with the general population or other migrant groups 173, and poorer mental health in forced compared with voluntary migrants 174. Furthermore, Africans in Britain are at a higher risk of mental illness than non-Africans 175, and survey data suggests that immigration is a primary cause of mental distress in about 40% of Africans in the UK 176. It has been suggested that the psychological stress and depression associated with migration may play a role in increasing risk of progression to active disease, potentially via neuroendocrine pathways or a negative effect on the cell-mediated immune system 177.
Importantly, rather than transporting active cases of TB across national borders, the majority of immigrant cases of active TB disease develop following arrival in the UK 20. This supports King’s assertion that “The higher rate of TB among immigrants owes as much to the hardships they face during and shortly after migration, as it does to their country of origin” 18. Further research is required to establish the extent to which stress and adverse migratory journeys affect specific migrant groups. However, experiences of migration are likely to contribute at least in part to the higher rates of active TB among migrants compared with UK-born ethnic minorities and the general population, especially for those who are marginalised or are travelling illegally.
Treatment-seeking
Knowledge about TB among migrants and ethnic minorities is shaped by cultural beliefs, often arising from experiences in the country of origin 178. Certain ideas, including misconceptions about TB causation, transmission and risk, can act as barriers to clinical treatment. Gerrish et al. suggest that “TB is not just a medical disease to be treated with antibiotic therapy but an entity with historical and cultural roots” 179. Several studies have identified widespread misconceptions about TB causation and transmission among migrant communities, and a limited understanding of LTBI in particular 172. The disease has been variously attributed to climate conditions 180, poisoning, pneumonia 181, exposure to chemical products 182, and witchcraft 183. Several members of a focus group of Somali women believed TB to be a punishment for past ill deeds 178.
Migrants may feel a false sense of having ‘left behind’ the high risk of TB in their country of origin 172, and TB may be considered by migrants to be a different, more severe, disease in their country of origin 184. In some cases, TB may be thought of as incurable due to poor health services in low-income countries 185. Moreover, immigrants may favour traditional systems of care and healing over Western medicine upon arrival 186, making them more likely to turn to traditional folk healers, self-diagnosis or self-medication before accessing public healthcare facilities 159. Cultural beliefs that lead to delays in treatment-seeking and reduced adherence to treatment may increase the risk of TB transmission within such communities.
Conversely, some have suggested that the cultural beliefs held by migrants are not barriers to treatment-seeking, but rather promote such behaviour. The higher prevalence of TB in migrants’ countries of origin could lead to greater awareness; as Bakhshi argues, “people born in developing countries are too familiar with the disease to neglect it” 29. Moreover, Ho describes how traditional Chinese medical beliefs are often complementary to clinical TB treatment in New York, such as through the use of traditional Chinese medicine to reduce the side effects of anti-TB drugs 171.
TB-related stigmatisation of immigrants has been reported in multiple studies (reviewed in 172). Stigma is defined as “the situation of the individual who is disqualified from full social acceptance”, and is therefore “reduced in our minds from a whole and usual person to a tainted, discounted one” 187. Some cultures consider TB to be sinful and dirty 178. The feelings of guilt and shame 188 and risk of rejection and discrimination 189 that may result from stigmatisation affect attitudes towards diagnosis, treatment and prevention, and therefore hinder control of TB and facilitate its transmission within certain migrant and ethnic groups 190. Sufferers may hide their illness to avoid stigma and discrimination and protect personal or family dignity 183. One study indicated that stigma prevented some immigrants from sharing information with their doctors, even TB-related symptoms 191. Furthermore, patients may be less likely to identify contacts due to concerns about social repercussions, meaning that subsequent preventable TB cases may occur 179. Feelings of stigma produced by attitudes in the country of origin are likely to be exacerbated by the negative stereotyping of migrant groups as ‘dirty’ or ‘diseased’ due to the association of TB with immigrants, which may lead to xenophobia and discrimination of sufferers 185, termed ‘sociomedical racism’ by McBride (1991) 192.
The Somali community in the UK provides an informative case study of the socio-cultural meaning and perceived consequences of TB. In Somalia, TB is associated with extreme stigma and social isolation. In a focused ethnography of Somali-born UK residents, Gerrish et al. found that interviewees tended to base their attitudes towards TB on those prevalent in Somalia 179. The stigma associated with TB led to expectations of social isolation, shame and loss of self-worth, sometimes extending to the whole family. Although most had an understanding that TB is contagious, it was also commonly believed that people remain infectious after treatment, as TB was often thought to be hereditary and therefore impossible to eradicate. This led to fears that friends would not resume normal social interactions after treatment, and that a diagnosis would jeopardise marriage prospects. Therefore, sufferers tended to isolate themselves or conceal their illness. In reality, anticipated consequences tended to be worse than actual experiences of discrimination, but felt stigma was nonetheless a powerful deterrent to disclosing illness, leading to delays in diagnosis and treatment 179.
Access to healthcare
Migrants may have difficulties establishing ‘entitlement’ to good healthcare 193. For example, a study in the UK found that only 32.5% of new migrants who were instructed to register with a GP had done so, and the migrant groups with the smallest proportion registered were likely to have greatest need 194. This is consistent with the Inverse Care Law, that those with the greatest need are least able to access healthcare services 195. Various studies have found that migrants face barriers in accessing healthcare services for TB diagnosis or treatment. These include lack of awareness of the local health system, including availability of free services 196, language barriers 197, and fears about loss of privacy due to the use of interpreters 184. Therefore, even in cases where there are minimal geographic or economic barriers to accessing health facilities, there are often racial, linguistic and cultural barriers to using these facilities effectively and adhering to treatment regimens 198. Worryingly, institutional racism within the NHS may pose a further barrier to healthcare access for both migrants and ethnic minorities. There is evidence that, when commissioning services, Trusts often fail to meet the needs of black and ethnic minority populations, which may reflect a lack of diversity among the NHS senior workforce 199– 201.
Studies have found that migrants face various structural barriers to accessing healthcare services, such as transport difficulties associated with poor services in deprived areas 178, and rigid opening hours for medication that do not fit with the working hours and lifestyles of patients 202. Moreover, economic barriers include not only direct costs associated with illness, such as the costs of repeated journeys to clinics for treatment, but also indirect costs including losing a job or being evicted by a landlord 203. Farmer criticises anthropological investigations for conflating structural violence with cultural difference, tending to exaggerate the role of patient agency and minimise the role of poverty and the barriers that it creates to accessing adequate care and completing treatment 163. Nevertheless, whether structural or cultural, barriers to healthcare access among migrant and ethnic groups can lead to delays in diagnosis and treatment, resulting in increased transmission and incidence.
Access to healthcare services varies across different migrant populations. Although treatment of TB is free for all in the UK, refugees and asylum seekers have poorer access to health services 204. Irregular residence status is likely to lead to significant delays in seeking medical assistance, due to uncertainties surrounding entitlement to services and fears of deportation, since TB patients can be legally deported while receiving ongoing treatment 205. Furthermore, irregular migrants may face difficulties in completing long-term TB treatment, which involves repeated consultations, if they do not have housing or employment and are short-term residents. Irregular migrants are also less likely to be willing to provide details of their migratory route 184 and provide information about contacts 183. Contact tracing is further compromised given the high mobility of migrants, and the fact that many do not reside at their official address, but with family and friends 184.
Conversely, there is evidence to suggest that UK-born cases experience longer delays from symptom onset to commencement of treatment than foreign-born cases 15. Moreover, TB treatment completion is actually marginally higher in migrants (85%) than the UK-born (81%) 50. However, these observations are problematic in that UK-born TB cases are often drawn from homeless individuals, problem drug users and prisoners, and so are frequently lost to follow-up and poorly adherent 206; they are not representative of the UK-born population as a whole. Furthermore, migrants are at a higher risk of contracting TB due to the various unique factors discussed (including genetics, vitamin D deficiency, co-morbidities, and experiences of migration), which do not apply to the UK-born, making socio-economic issues the key driver of TB incidence in this group. Indeed, in 2016, 2.4 times as many UK-born cases (20%) as foreign-born cases (8.2%) had at least one social risk factor (drug misuse, alcohol misuse, homelessness, or imprisonment) 14, which is incongruent with the higher overall rates of deprivation in foreign-born compared with UK-born populations 32.
Conclusions
It is a common misconception that migrants have a higher incidence of TB disease compared with the general population simply because they ‘import’ it from abroad. Bakhshi suggests that they “present a tuberculosis picture from the country of origin and not the United Kingdom where the disease eventually manifests” 29. Indeed, differential pathogen exposure can explain much of the higher incidence of TB among migrants and ethnic minorities, due to both pre-migration residence in high-incidence countries and maintenance of transnational links with the country of birth or ethnic origin. However, positing this as the sole driver fails to address the complex interplay of factors driving the vulnerability of particular migrant and ethnic groups to infection and progression to active disease. These include genetic susceptibility, vitamin D deficiency due to climatic and dietary factors, co-morbidities including DM, HIV and CKD, socio-economic deprivation, and factors linked to the experience of migration itself. Furthermore, certain migrant and ethnic groups face barriers to accessing treatment including cultural differences in treatment-seeking behaviours, stigmatisation of sufferers, and barriers to healthcare access. As stated by Offer et al., “TB in ethnic minorities does not occur in isolation but against a backdrop of socioeconomic, political and cultural context that affects their knowledge, attitudes and behaviours” 169. The resultant delays in diagnosis and treatment lead to increased transmission and incidence in these communities.
In this way, there are factors disadvantaging migrants and ethnic minorities at each stage of the disease, relating to risk of pathogen exposure, vulnerability to infection, development of active disease, and access to treatment. Although heterogeneity between and within broad migrant and ethnic groups leads to variation in risk at each of these stages, there is a net effect of higher incidence among migrants and ethnic minorities compared with the general UK population. It is important to understand the complex and multifactorial drivers of this disparity in order to implement effective policies for tackling TB in these vulnerable groups. Currently, migrants from countries with high TB incidences are screened for active TB before entry to the UK. However, to complement such measures, which only consider the driver of differential pathogen exposure, more consideration is needed regarding policies that address the factors making migrants and ethnic minorities more vulnerable to reactivation of LTBI following their arrival in the UK. This might include vitamin D supplementation, measures targeting co-morbidities, and policies that promote socio-economic equity and migrant rights. In order to reduce delays in diagnosis and treatment, and thereby minimise transmission within migrant and ethnic minority communities, increased health education on TB causation, risk and transmission is required, as well as tackling stigmatisation of vulnerable groups. It is also important to raise awareness of migrants’ entitlement to diagnosis and treatment through the NHS, alongside reducing cultural and economic barriers to its access.
Data availability
The data referenced by this article are under copyright with the following copyright statement: Copyright: © 2018 Hayward S et al.
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication). http://creativecommons.org/publicdomain/zero/1.0/
No data are associated with this article.
Acknowledgments
McShane H and Tanner R are members of the VALIDATE Network.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 2; referees: 2 approved]
References
- 1. Tuberculosis Fact Sheet 2016. WHO (World Health Organisation).2016. [Google Scholar]
- 2. Cruz-Knight W, Blake-Gumbs L: Tuberculosis: an overview. Prim Care. 2013;40(3):743–56. 10.1016/j.pop.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 3. Fogel N: Tuberculosis: a disease without boundaries. Tuberculosis (Edinb). 2015;95(5):527–31. 10.1016/j.tube.2015.05.017 [DOI] [PubMed] [Google Scholar]
- 4. Golden MP, Vikram HR: Extrapulmonary tuberculosis: an overview. Am Fam Physician. 2005;72(9):1761–8. [PubMed] [Google Scholar]
- 5. Tiemersma EW, van der Werf MJ, Borgdorff MW, et al. : Natural history of tuberculosis: duration and fatality of untreated pulmonary tuberculosis in HIV negative patients: a systematic review. PLoS One. 2011;6(4):e17601. 10.1371/journal.pone.0017601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. WHO: Global Tuberculosis Report 2016.Geneva: World Health Organisation;2016. Reference Source [Google Scholar]
- 7. International Organisation for Migration: World Migration Report 2018.Geneva: IOM.2017. Reference Source [Google Scholar]
- 8. Lillebaek T, Andersen AB, Dirksen A, et al. : Persistent high incidence of tuberculosis in immigrants in a low-incidence country. Emerg Infect Dis. 2002;8(7):679–84. 10.3201/eid0807.010482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vos AM, Meima A, Verver S, et al. : High incidence of pulmonary tuberculosis persists a decade after immigration, The Netherlands. Emerg Infect Dis. 2004;10(4):736–9. 10.3201/eid1004.030530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zuber PL, McKenna MT, Binkin NJ, et al. : Long-term risk of tuberculosis among foreign-born persons in the United States. JAMA. 1997;278(4):304–7. 10.1001/jama.1997.03550040060038 [DOI] [PubMed] [Google Scholar]
- 11. Talbot EA, Moore M, McCray E, et al. : Tuberculosis among foreign-born persons in the United States, 1993-1998. JAMA. 2000;284(22):2894–900. 10.1001/jama.284.22.2894 [DOI] [PubMed] [Google Scholar]
- 12. Watkins RE, Plant AJ, Gushulak BD: Tuberculosis rates among migrants in Australia and Canada. Int J Tuberc Lung Dis. 2002;6(7):641–4. [PubMed] [Google Scholar]
- 13. Gilbert RL, Antoine D, French CE, et al. : The impact of immigration on tuberculosis rates in the United Kingdom compared with other European countries. Int J Tuberc Lung Dis. 2009;13(5):645–51. [PubMed] [Google Scholar]
- 14. PHE: Tuberculosis in England: 2017 report.2017. Reference Source [Google Scholar]
- 15. PHE: Tuberculosis in England: 2016.London: Public Health England.2016. Reference Source [Google Scholar]
- 16. Pearson E: Breakthroughs in medical technology are announced every day but still the risks to the nation's youngsters continue to take their toll; diseases that pose a threat to our children's future. The Daily Express. 2001. [Google Scholar]
- 17. Bell M, Brown T, Faire L: Germs, genes and postcolonial geographies: reading the return of tuberculosis to Leicester, UK, 2001. Cult Geogr. 2006;13(4):577–99. 10.1191/1474474006cgj376oa [DOI] [Google Scholar]
- 18. King NB: Immigration, Race and Geographies of Difference in the Tuberculosis Pandemic.In: M. Gandy and A. Zumla, A., eds., The Return of the White Plague: Global Poverty and the ‘New’ Tuberculosis.London: Verso,2003. Reference Source [Google Scholar]
- 19. Krieger N: Epidemiology and the web of causation: has anyone seen the spider? Soc Sci Med. 1994;39(7):887–903. 10.1016/0277-9536(94)90202-X [DOI] [PubMed] [Google Scholar]
- 20. Pareek M, Greenaway C, Noori T, et al. : The impact of migration on tuberculosis epidemiology and control in high-income countries: a review. BMC Med. 2016;14:48. 10.1186/s12916-016-0595-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arshad S, Bavan L, Gajari K, et al. : Active screening at entry for tuberculosis among new immigrants: a systematic review and meta-analysis. Eur Respir J. 2010;35(6):1336–45. 10.1183/09031936.00054709 [DOI] [PubMed] [Google Scholar]
- 22. Klinkenberg E, Manissero D, Semenza JC, et al. : Migrant tuberculosis screening in the EU/EEA: yield, coverage and limitations. Eur Respir J. 2009;34(5):1180–9. 10.1183/09031936.00038009 [DOI] [PubMed] [Google Scholar]
- 23. PHE: TB screening for the UK. (Public Health England and UK Home Office).2013. Reference Source [Google Scholar]
- 24. Fok A, Numata Y, Schulzer M, et al. : Risk factors for clustering of tuberculosis cases: a systematic review of population-based molecular epidemiology studies. Int J Tuberc Lung Dis. 2008;12(5):480–92. [PubMed] [Google Scholar]
- 25. Choudhury IW, West CR, Ormerod LP: The outcome of a cohort of tuberculin-positive predominantly South Asian new entrants aged 16-34 to the UK: Blackburn 1989-2001. J Public Health (Oxf). 2014;36(3):390–5. 10.1093/pubmed/fdt110 [DOI] [PubMed] [Google Scholar]
- 26. MacPherson DW, Gushulak BD: Balancing prevention and screening among international migrants with tuberculosis: population mobility as the major epidemiological influence in low-incidence nations. Public Health. 2006;120(8):712–23. 10.1016/j.puhe.2006.05.002 [DOI] [PubMed] [Google Scholar]
- 27. Marks GB, Bai J, Stewart GJ, et al. : Effectiveness of postmigration screening in controlling tuberculosis among refugees: a historical cohort study, 1984–1998. Am J Public Health. 2001;91(11):1797–9. 10.2105/AJPH.91.11.1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dorsett R: Ethnic minorities in the inner city. Findings York: Joseph Rowntree Foundation.1998. Reference Source [Google Scholar]
- 29. Bakhshi S: Tuberculosis in the United Kingdom: A Tale of Two Nations.Leicester: Troubador Publishing Ltd. Reference Source [Google Scholar]
- 30. Murray M, Nardell E: Molecular epidemiology of tuberculosis: achievements and challenges to current knowledge. Bull World Health Organ. 2002;80(6):477–82. [PMC free article] [PubMed] [Google Scholar]
- 31. Glynn JR, Bauer J, de Boer AS, et al. : Interpreting DNA fingerprint clusters of Mycobacterium tuberculosis. European Concerted Action on Molecular Epidemiology and Control of Tuberculosis. Int J Tuberc Lung Dis. 1999;3(12):1055–60. [PubMed] [Google Scholar]
- 32. Walker TM, Lalor MK, Broda A, et al. : Assessment of Mycobacterium tuberculosis transmission in Oxfordshire, UK, 2007-12, with whole pathogen genome sequences: an observational study. Lancet Respir Med. 2014;2(4):285–92. 10.1016/S2213-2600(14)70027-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Asadi L, Heffernan C, Menzies D, et al. : Effectiveness of Canada's tuberculosis surveillance strategy in identifying immigrants at risk of developing and transmitting tuberculosis: a population-based retrospective cohort study. Lancet Public Health. 2017;2(10):e450–e7. 10.1016/S2468-2667(17)30161-5 [DOI] [PubMed] [Google Scholar]
- 34. Aldridge RW, Zenner D, White PJ, et al. : Tuberculosis in migrants moving from high-incidence to low-incidence countries: a population-based cohort study of 519 955 migrants screened before entry to England, Wales, and Northern Ireland. Lancet. 2016;388(10059):2510–8. 10.1016/S0140-6736(16)31008-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bhopal RS: Migration, Ethnicity, Race, and Health in Multicultural Societies.2nd ed. Oxford: Oxford University Press,2014. Reference Source [Google Scholar]
- 36. French CE, Antoine D, Gelb D, et al. : Tuberculosis in non-UK-born persons, England and Wales, 2001-2003. Int J Tuberc Lung Dis. 2007;11(5):577–84. [PubMed] [Google Scholar]
- 37. Basch L, Glick-Schiller N, Szanton Blanc C: Nations Unbound: transnational projects, postcolonial predicaments and deterritorialized nation-states.Langhorne: Gordon and Breach.1994. Reference Source [Google Scholar]
- 38. Çaglar AS: Constraining metaphors and the transnationalisation of spaces in Berlin. J Ethn Migr Stud. 2001;27(4):601–13. 10.1080/13691830120090403 [DOI] [Google Scholar]
- 39. Foreign travel associated illness: a focus on those visiting friends and relatives; 2008 report.London: Health Protection Agency,2008. Reference Source [Google Scholar]
- 40. Cobelens FG, van Deutekom H, Draayer-Jansen IW, et al. : Risk of infection with Mycobacterium tuberculosis in travellers to areas of high tuberculosis endemicity. Lancet. 2000;356(9228):461–5. 10.1016/S0140-6736(00)02554-X [DOI] [PubMed] [Google Scholar]
- 41. Ormerod LP, Green RM, Gray S: Are there still effects on Indian Subcontinent ethnic tuberculosis of return visits?: a longitudinal study 1978-97. J Infect. 2001;43(2):132–4. 10.1053/jinf.2001.0872 [DOI] [PubMed] [Google Scholar]
- 42. Tocque K, Bellis MA, Beeching NJ, et al. : A case-control study of lifestyle risk factors associated with tuberculosis in Liverpool, North-West England. Eur Respir J. 2001;18(6):959–64. 10.1183/09031936.01.00211701 [DOI] [PubMed] [Google Scholar]
- 43. Singh H, Joshi M, Ormerod LP: A case control study in the Indian subcontinent ethnic population on the effect of return visits and the subsequent development of tuberculosis. J Infect. 2006;52(6):440–2. 10.1016/j.jinf.2005.08.029 [DOI] [PubMed] [Google Scholar]
- 44. Denholm JT, Thevarajan I: Tuberculosis and the traveller: evaluating and reducing risk through travel consultation. J Travel Med. 2016;23(3). 10.1093/jtm/taw008 [DOI] [PubMed] [Google Scholar]
- 45. Marx FM, Fiebig L, Hauer B, et al. : Higher Rate of Tuberculosis in Second Generation Migrants Compared to Native Residents in a Metropolitan Setting in Western Europe. PLoS One. 2015;10(6):e0119693. 10.1371/journal.pone.0119693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reed MB, Pichler VK, McIntosh F, et al. : Major Mycobacterium tuberculosis lineages associate with patient country of origin. J Clin Microbiol. 2009;47(4):1119–28. 10.1128/JCM.02142-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Coscolla M, Gagneux S: Does M. tuberculosis genomic diversity explain disease diversity? Drug Discov Today Dis Mech. 2010;7(1):e43–e59. 10.1016/j.ddmec.2010.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Portevin D, Gagneux S, Comas I, et al. : Human macrophage responses to clinical isolates from the Mycobacterium tuberculosis complex discriminate between ancient and modern lineages. PLoS Pathog. 2011;7(3):e1001307. 10.1371/journal.ppat.1001307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang C, Peyron P, Mestre O, et al. : Innate immune response to Mycobacterium tuberculosis Beijing and other genotypes. PLoS One. 2010;5(10):e13594. 10.1371/journal.pone.0013594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wagner KS, Lawrence J, Anderson L, et al. : Migrant health and infectious diseases in the UK: findings from the last 10 years of surveillance. J Public Health (Oxf). 2014;36(1):28–35. 10.1093/pubmed/fdt021 [DOI] [PubMed] [Google Scholar]
- 51. Pareek M, Evans J, Innes J, et al. : Ethnicity and mycobacterial lineage as determinants of tuberculosis disease phenotype. Thorax. 2013;68(3):221–9. 10.1136/thoraxjnl-2012-201824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rodrigues LC, Diwan VK, Wheeler JG: Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol. 1993;22(6):1154–8. 10.1093/ije/22.6.1154 [DOI] [PubMed] [Google Scholar]
- 53. Trunz BB, Fine P, Dye C: Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367(9517):1173–80. 10.1016/S0140-6736(06)68507-3 [DOI] [PubMed] [Google Scholar]
- 54. Fine PE: Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346(8986):1339–45. 10.1016/S0140-6736(95)92348-9 [DOI] [PubMed] [Google Scholar]
- 55. Narayanan PR: Influence of sex, age & nontuberculous infection at intake on the efficacy of BCG: re-analysis of 15-year data from a double-blind randomized control trial in South India. Indian J Med Res. 2006;123(2):119–24. [PubMed] [Google Scholar]
- 56. Colditz GA, Brewer TF, Berkey CS, et al. : Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271(9):698–702. 10.1001/jama.1994.03510330076038 [DOI] [PubMed] [Google Scholar]
- 57. Mahomed H, Kibel M, Hawkridge T, et al. : The impact of a change in bacille Calmette-Guérin vaccine policy on tuberculosis incidence in children in Cape Town, South Africa. Pediatr Infect Dis J. 2006;25(12):1167–72. 10.1097/01.inf.0000243765.33880.54 [DOI] [PubMed] [Google Scholar]
- 58. Moyo S, Verver S, Mahomed H, et al. : Age-related tuberculosis incidence and severity in children under 5 years of age in Cape Town, South Africa. Int J Tuberc Lung Dis. 2010;14(2):149–54. [PubMed] [Google Scholar]
- 59. Palmer CE, Long MW: Effects of infection with atypical mycobacteria on BCG vaccination and tuberculosis. Am Rev Respir Dis. 1966;94(4):553–68. [DOI] [PubMed] [Google Scholar]
- 60. Weiszfeiler JG, Karasseva V: Mixed mycobacterial infections. Rev Infect Dis. 1981;3(5):1081–3. 10.1093/clinids/3.5.1081 [DOI] [PubMed] [Google Scholar]
- 61. Rook GA, Bahr GM, Stanford JL: The effect of two distinct forms of cell-mediated response to mycobacteria on the protective efficacy of BCG. Tubercle. 1981;62(1):63–8. 10.1016/0041-3879(81)90038-6 [DOI] [PubMed] [Google Scholar]
- 62. Stanford JL, Shield MJ, Rook GA: How environmental mycobacteria may predetermine the protective efficacy of BCG. Tubercle. 1981;62(1):55–62. 10.1016/0041-3879(81)90037-4 [DOI] [PubMed] [Google Scholar]
- 63. Fifteen year follow up of trial of BCG vaccines in south India for tuberculosis prevention. Tuberculosis Research Centre (ICMR), Chennai. Indian J Med Res. 1999;110:56–69. [PubMed] [Google Scholar]
- 64. Black GF, Fine PEM, Warndorff DK, et al. : Relationship between IFN-gamma and skin test responsiveness to Mycobacterium tuberculosis PPD in healthy, non-BCG-vaccinated young adults in Northern Malawi. Int J Tuberc Lung Dis. 2001;5(7):664–72. [PubMed] [Google Scholar]
- 65. Brandt L, Feino Cunha J, Weinreich Olsen A, et al. : Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect Immun. 2002;70(2):672–8. 10.1128/IAI.70.2.672-678.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hirsch A: Handbook of geographical and historical pathology. Vol. III. Diseases of organs and parts London: New Syndenham Society,1886. Reference Source [Google Scholar]
- 67. Abel L, Fellay J, Haas DW, et al. : Genetics of human susceptibility to active and latent tuberculosis: present knowledge and future perspectives. Lancet Infect Dis. 2018;18(3):e64–e75. 10.1016/S1473-3099(17)30623-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kallman FJ, Reisner D: Twin studies on the significance of genetic factors in tuberculosis. Am Rev Tuberc. 1942;47:549–74. [Google Scholar]
- 69. Comstock GW: Tuberculosis in twins: a re-analysis of the Prophit survey. Am Rev Respir Dis. 1978;117(4):621–4. [DOI] [PubMed] [Google Scholar]
- 70. Thye T, Browne EN, Chinbuah MA, et al. : IL10 haplotype associated with tuberculin skin test response but not with pulmonary TB. PLoS One. 2009;4(5):e5420. 10.1371/journal.pone.0005420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zembrzuski VM, Basta PC, Callegari-Jacques SM, et al. : Cytokine genes are associated with tuberculin skin test response in a native Brazilian population. Tuberculosis (Edinb). 2010;90(1):44–9. 10.1016/j.tube.2009.11.002 [DOI] [PubMed] [Google Scholar]
- 72. Sveinbjornsson G, Gudbjartsson DF, Halldorsson BV, et al. : HLA class II sequence variants influence tuberculosis risk in populations of European ancestry. Nat Genet. 2016;48(3):318–22. 10.1038/ng.3498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stein CM, Zalwango S, Malone LL, et al. : Genome scan of M. tuberculosis infection and disease in Ugandans. PLoS One. 2008;3(12):e4094. 10.1371/journal.pone.0004094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cobat A, Gallant CJ, Simkin L, et al. : Two loci control tuberculin skin test reactivity in an area hyperendemic for tuberculosis. J Exp Med. 2009;206(12):2583–91. 10.1084/jem.20090892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jabot-Hanin F, Cobat A, Feinberg J, et al. : Major Loci on Chromosomes 8q and 3q Control Interferon γ Production Triggered by Bacillus Calmette-Guerin and 6-kDa Early Secretory Antigen Target, Respectively, in Various Populations. J Infect Dis. 2016;213(7):1173–9. 10.1093/infdis/jiv757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Daya M, van der Merwe L, van Helden PD, et al. : The role of ancestry in TB susceptibility of an admixed South African population. Tuberculosis (Edinb). 2014;94(4):413–20. 10.1016/j.tube.2014.03.012 [DOI] [PubMed] [Google Scholar]
- 77. Delgado JC, Baena A, Thim S, et al. : Ethnic-specific genetic associations with pulmonary tuberculosis. J Infect Dis. 2002;186(10):1463–8. 10.1086/344891 [DOI] [PubMed] [Google Scholar]
- 78. Stead WW: Variation in vulnerability to tuberculosis in America today: random, or legacies of different ancestral epidemics? Int J Tuberc Lung Dis. 2001;5(9):807–14. [PubMed] [Google Scholar]
- 79. Coussens AK, Wilkinson RJ, Nikolayevskyy V, et al. : Ethnic variation in inflammatory profile in tuberculosis. PLoS Pathog. 2013;9(7):e1003468. 10.1371/journal.ppat.1003468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. McMillen C: Discovering Tuberculosis: A Global History 1900 to the Present. New Haven: Yale University Press;2015. Reference Source [Google Scholar]
- 81. Goodman A: The problematics of 'race' in contemporary biological anthropology. In: Biological Anthropology: The State of the Science.2nd ed. NT Boas and LD Wolfe (eds). Bend Oregon: International Institute for Human Evolutionary Research;1977. Reference Source [Google Scholar]
- 82. Gagneux S: Host-pathogen coevolution in human tuberculosis. Philos Trans R Soc Lond B Biol Sci. 2012;367(1590):850–9. 10.1098/rstb.2011.0316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Smith NH, Hewinson RG, Kremer K, et al. : Myths and misconceptions: the origin and evolution of Mycobacterium tuberculosis. Nat Rev Microbiol. 2009;7(7):537–44. 10.1038/nrmicro2165 [DOI] [PubMed] [Google Scholar]
- 84. Lux M: Perfect subjects: race, tuberculosis, and the Qu'Appelle BCG Vaccine Trial. Can Bull Med Hist. 1998;15(2):277–95. 10.3138/cbmh.15.2.277 [DOI] [PubMed] [Google Scholar]
- 85. MacDonald N, Hébert PC, Stanbrook MB: Tuberculosis in Nunavut: a century of failure. CMAJ. 2011;183(7):741–3. 10.1503/cmaj.110160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Barnes I, Duda A, Pybus OG, et al. : Ancient urbanization predicts genetic resistance to tuberculosis. Evolution. 2011;65(3):842–8. 10.1111/j.1558-5646.2010.01132.x [DOI] [PubMed] [Google Scholar]
- 87. Martineau AR: Old wine in new bottles: vitamin D in the treatment and prevention of tuberculosis. Proc Nutr Soc. 2012;71(1):84–9. 10.1017/S0029665111003326 [DOI] [PubMed] [Google Scholar]
- 88. Talat N, Perry S, Parsonnet J, et al. : Vitamin d deficiency and tuberculosis progression. Emerg Infect Dis. 2010;16(5):853–5. 10.3201/eid1605.091693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Huang SJ, Wang XH, Liu ZD, et al. : Vitamin D deficiency and the risk of tuberculosis: a meta-analysis. Drug Des Devel Ther. 2016;11:91–102. 10.2147/DDDT.S79870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gibney KB, MacGregor L, Leder K, et al. : Vitamin D deficiency is associated with tuberculosis and latent tuberculosis infection in immigrants from sub-Saharan Africa. Clin Infect Dis. 2008;46(3):443–6. 10.1086/525268 [DOI] [PubMed] [Google Scholar]
- 91. Ho-Pham LT, Nguyen ND, Nguyen TT, et al. : Association between vitamin D insufficiency and tuberculosis in a Vietnamese population. BMC Infect Dis. 2010;10(1):306. 10.1186/1471-2334-10-306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Nnoaham KE, Clarke A: Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol. 2008;37(1):113–9. 10.1093/ije/dym247 [DOI] [PubMed] [Google Scholar]
- 93. Chocano-Bedoya P, Ronnenberg AG: Vitamin D and tuberculosis. Nutr Rev. 2009;67(5):289–93. 10.1111/j.1753-4887.2009.00195.x [DOI] [PubMed] [Google Scholar]
- 94. Sita-Lumsden A, Lapthorn G, Swaminathan R, et al. : Reactivation of tuberculosis and vitamin D deficiency: the contribution of diet and exposure to sunlight. Thorax. 2007;62(11):1003–7. 10.1136/thx.2006.070060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Pareek M, Innes J, Sridhar S, et al. : Vitamin D deficiency and TB disease phenotype. Thorax. 2015;70(12):1171–80. 10.1136/thoraxjnl-2014-206617 [DOI] [PubMed] [Google Scholar]
- 96. Kruijshaar ME, Abubakar I: Increase in extrapulmonary tuberculosis in England and Wales 1999-2006. Thorax. 2009;64(12):1090–5. 10.1136/thx.2009.118133 [DOI] [PubMed] [Google Scholar]
- 97. Thieden E, Philipsen PA, Heydenreich J, et al. : Vitamin D level in summer and winter related to measured UVR exposure and behavior. Photochem Photobiol. 2009;85(6):1480–4. 10.1111/j.1751-1097.2009.00612.x [DOI] [PubMed] [Google Scholar]
- 98. Holick MF: Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. 10.1056/NEJMra070553 [DOI] [PubMed] [Google Scholar]
- 99. Eggemoen AR, Knutsen KV, Dalen I, et al. : Vitamin D status in recently arrived immigrants from Africa and Asia: a cross-sectional study from Norway of children, adolescents and adults. BMJ Open. 2013;3(10):e003293. 10.1136/bmjopen-2013-003293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Primary vitamin D deficiency in adults. Drug Ther Bull. 2006;44(4):25–9. 10.1136/dtb.2006.44425 [DOI] [PubMed] [Google Scholar]
- 101. Martin CA, Gowda U, Renzaho AM: The prevalence of vitamin D deficiency among dark-skinned populations according to their stage of migration and region of birth: A meta-analysis. Nutrition. 2016;32(1):21–32. 10.1016/j.nut.2015.07.007 [DOI] [PubMed] [Google Scholar]
- 102. Lawson M, Thomas M, Hardiman A: Dietary and lifestyle factors affecting plasma vitamin D levels in Asian children living in England. Eur J Clin Nutr. 1999;53(4):268–72. 10.1038/sj.ejcn.1600717 [DOI] [PubMed] [Google Scholar]
- 103. Zhang R, Naughton DP: Vitamin D in health and disease: current perspectives. Nutr J. 2010;9:65. 10.1186/1475-2891-9-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chan TY: Vitamin D deficiency and susceptibility to tuberculosis. Calcif Tissue Int. 2000;66(6):476–8. 10.1007/s002230010095 [DOI] [PubMed] [Google Scholar]
- 105. Finch PJ, Millard FJ, Maxwell JD: Risk of tuberculosis in immigrant Asians: culturally acquired immunodeficiency? Thorax. 1991;46(1):1–5. 10.1136/thx.46.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bonilla C, Ness AR, Wills AK, et al. : Skin pigmentation, sun exposure and vitamin D levels in children of the Avon Longitudinal Study of Parents and Children. BMC Public Health. 2014;14:597. 10.1186/1471-2458-14-597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hunt SP, O'Riordan JL, Windo J, et al. : Vitamin D status in different subgroups of British Asians. Br Med J. 1976;2(6048):1351–4. 10.1136/bmj.2.6048.1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wilkinson RJ, Llewelyn M, Toossi Z, et al. : Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet. 2000;355(9204):618–21. 10.1016/S0140-6736(99)02301-6 [DOI] [PubMed] [Google Scholar]
- 109. Farrow S: Allelic variation and the vitamin D receptor. Lancet. 1994;343(8908):1242. 10.1016/S0140-6736(94)92147-4 [DOI] [PubMed] [Google Scholar]
- 110. Chen C, Liu Q, Zhu L, et al. : Vitamin D receptor gene polymorphisms on the risk of tuberculosis, a meta-analysis of 29 case-control studies. PLoS One. 2013;8(12):e83843. 10.1371/journal.pone.0083843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sutaria N, Liu CT, Chen TC: Vitamin D Status, Receptor Gene Polymorphisms, and Supplementation on Tuberculosis: A Systematic Review of Case-Control Studies and Randomized Controlled Trials. J Clin Transl Endocrinol. 2014;1(4):151–60. 10.1016/j.jcte.2014.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zmuda JM, Cauley JA, Ferrell RE: Molecular epidemiology of vitamin D receptor gene variants. Epidemiol Rev. 2000;22(2):203–17. 10.1093/oxfordjournals.epirev.a018033 [DOI] [PubMed] [Google Scholar]
- 113. Andraos C, Koorsen G, Knight JC, et al. : Vitamin D receptor gene methylation is associated with ethnicity, tuberculosis, and TaqI polymorphism. Hum Immunol. 2011;72(3):262–8. 10.1016/j.humimm.2010.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Stevenson CR, Forouhi NG, Roglic G, et al. : Diabetes and tuberculosis: the impact of the diabetes epidemic on tuberculosis incidence. BMC Public Health. 2007;7:234. 10.1186/1471-2458-7-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Root HF: The association of diabetes and tuberculosis. N Engl J Med. 1934;210(8):127–47. 10.1056/NEJM193401182100304 [DOI] [Google Scholar]
- 116. Jeon CY, Murray MB: Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5(7):e152. 10.1371/journal.pmed.0050152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Martens GW, Arikan MC, Lee J, et al. : Tuberculosis susceptibility of diabetic mice. Am J Respir Cell Mol Biol. 2007;37(5):518–24. 10.1165/rcmb.2006-0478OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ogbera AO, Kapur A, Abdur-Razzaq H, et al. : Clinical profile of diabetes mellitus in tuberculosis. BMJ Open Diabetes Res Care. 2015;3(1):e000112. 10.1136/bmjdrc-2015-000112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pealing L, Wing K, Mathur R, et al. : Risk of tuberculosis in patients with diabetes: population based cohort study using the UK Clinical Practice Research Datalink. BMC Med. 2015;13:135. 10.1186/s12916-015-0381-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Stevenson CR, Critchley JA, Forouhi NG, et al. : Diabetes and the risk of tuberculosis: a neglected threat to public health? Chronic Illn. 2007;3(3):228–45. 10.1177/1742395307081502 [DOI] [PubMed] [Google Scholar]
- 121. Thomas C, Nightingale CM, Donin AS, et al. : Socio-economic position and type 2 diabetes risk factors: patterns in UK children of South Asian, black African-Caribbean and white European origin. PLoS One. 2012;7(3):e32619. 10.1371/journal.pone.0032619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Sproston KaM, J: Health Survey for England 2004: Volume 1: The health of ethnic minority groups.Leeds: The Information Centre.2006;127–47. [Google Scholar]
- 123. Holman N, Forouhi NG, Goyder E, et al. : The Association of Public Health Observatories (APHO) Diabetes Prevalence Model: estimates of total diabetes prevalence for England, 2010–2030. Diabet Med. 2011;28(5):575–82. 10.1111/j.1464-5491.2010.03216.x [DOI] [PubMed] [Google Scholar]
- 124. Bhopal R, Hayes L, White M, et al. : Ethnic and socio-economic inequalities in coronary heart disease, diabetes and risk factors in Europeans and South Asians. J Public Health Med. 2002;24(2):95–105. 10.1093/pubmed/24.2.95 [DOI] [PubMed] [Google Scholar]
- 125. Bhopal RS: A four-stage model explaining the higher risk of Type 2 diabetes mellitus in South Asians compared with European populations. Diabet Med. 2013;30(1):35–42. 10.1111/dme.12016 [DOI] [PubMed] [Google Scholar]
- 126. Zumla A, Malon P, Henderson J, et al. : Impact of HIV infection on tuberculosis. Postgrad Med J. 2000;76(895):259–68. 10.1136/pmj.76.895.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Chretien J: Tuberculosis and HIV. The cursed duet. Bull Int Union Tuberc Lung Dis. 1990;65(1):25–8. [PubMed] [Google Scholar]
- 128. Getahun H, Gunneberg C, Granich R, et al. : HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis. 2010;50 Suppl 3:S201–7. 10.1086/651492 [DOI] [PubMed] [Google Scholar]
- 129. Sonnenberg P, Glynn JR, Fielding K, et al. : How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J Infect Dis. 2005;191(2):150–8. 10.1086/426827 [DOI] [PubMed] [Google Scholar]
- 130. Corbett EL, Watt CJ, Walker N, et al. : The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163(9):1009–21. 10.1001/archinte.163.9.1009 [DOI] [PubMed] [Google Scholar]
- 131. McShane H: Co-infection with HIV and TB: double trouble. Int J STD AIDS. 2005;16(2):95–100; quiz 101. 10.1258/0956462053057576 [DOI] [PubMed] [Google Scholar]
- 132. Zhang M, Gong J, Iyer DV, et al. : T cell cytokine responses in persons with tuberculosis and human immunodeficiency virus infection. J Clin Invest. 1994;94(6):2435–42. 10.1172/JCI117611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Bell LCK, Noursadeghi M: Pathogenesis of HIV-1 and Mycobacterium tuberculosis co-infection. Nat Rev Microbiol. 2018;16(2):80–90. 10.1038/nrmicro.2017.128 [DOI] [PubMed] [Google Scholar]
- 134. Tavares AM, Fronteira I, Couto I, et al. : HIV and tuberculosis co-infection among migrants in Europe: A systematic review on the prevalence, incidence and mortality. PLoS One. 2017;12(9):e0185526. 10.1371/journal.pone.0185526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. WHO: World Health Organisation: Global Health Observatory Data Repository.2016. Reference Source [Google Scholar]
- 136. Pradhan RP, Katz LA, Nidus BD, et al. : Tuberculosis in dialyzed patients. JAMA. 1974;229(7):798–800. 10.1001/jama.1974.03230450032020 [DOI] [PubMed] [Google Scholar]
- 137. Hu HY, Wu CY, Huang N, et al. : Increased risk of tuberculosis in patients with end-stage renal disease: a population-based cohort study in Taiwan, a country of high incidence of end-stage renal disease. Epidemiol Infect. 2014;142(1):191–9. 10.1017/S0950268813000551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Dobler CC, McDonald SP, Marks GB: Risk of tuberculosis in dialysis patients: a nationwide cohort study. PLoS One. 2011;6(12):e29563. 10.1371/journal.pone.0029563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Al-Efraij K, Mota L, Lunny C, et al. : Risk of active tuberculosis in chronic kidney disease: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2015;19(12):1493–9. 10.5588/ijtld.15.0081 [DOI] [PubMed] [Google Scholar]
- 140. Romanowski K, Clark EG, Levin A, et al. : Tuberculosis and chronic kidney disease: an emerging global syndemic. Kidney Int. 2016;90(1):34–40. 10.1016/j.kint.2016.01.034 [DOI] [PubMed] [Google Scholar]
- 141. Kato S, Chmielewski M, Honda H, et al. : Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3(5):1526–33. 10.2215/CJN.00950208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. National Collaborating Centre for Chronic Conditions (UK), Centre for Clinical Practice at NICE (UK), National Institute for Health and Clinical Excellence: Guidance: Tuberculosis: Clinical Diagnosis and Management of Tuberculosis, and Measures for Its Prevention and Control.London: National Institute for Health and Clinical Excellence (UK). Royal College of Physicians of London. Updated text, Copyright (c) 2011, National Institute for Health and Clinical Excellence.2011. 22720337 [Google Scholar]
- 143. Lightstone L: Preventing renal disease: the ethnic challenge in the United Kingdom. Kidney Int Suppl. 2003;63(83):S135–8. 10.1046/j.1523-1755.63.s83.29.x [DOI] [PubMed] [Google Scholar]
- 144. Ball S, Lloyd J, Cairns T, et al. : Why is there so much end-stage renal failure of undetermined cause in UK Indo-Asians? QJM. 2001;94(4):187–93. 10.1093/qjmed/94.4.187 [DOI] [PubMed] [Google Scholar]
- 145. Ostermann M, Palchaudhuri P, Riding A, et al. : Incidence of tuberculosis is high in chronic kidney disease patients in South East England and drug resistance common. Ren Fail. 2016;38(2):256–61. 10.3109/0886022X.2015.1128290 [DOI] [PubMed] [Google Scholar]
- 146. New JP, Middleton RJ, Klebe B, et al. : Assessing the prevalence, monitoring and management of chronic kidney disease in patients with diabetes compared with those without diabetes in general practice. Diabet Med. 2007;24(4):364–9. 10.1111/j.1464-5491.2007.02075.x [DOI] [PubMed] [Google Scholar]
- 147. Khan S: Vitamin D deficiency and secondary hyperparathyroidism among patients with chronic kidney disease. Am J Med Sci. 2007;333(4):201–7. 10.1097/MAJ.0b013e31803bb129 [DOI] [PubMed] [Google Scholar]
- 148. Winston JA: HIV and CKD epidemiology. Adv Chronic Kidney Dis. 2010;17(1):19–25. 10.1053/j.ackd.2009.08.006 [DOI] [PubMed] [Google Scholar]
- 149. Hossain MP, Goyder EC, Rigby JE, et al. : CKD and poverty: a growing global challenge. Am J Kidney Dis. 2009;53(1):166–74. 10.1053/j.ajkd.2007.10.047 [DOI] [PubMed] [Google Scholar]
- 150. Dubos R, Dubos J: The White Plague: Tuberculosis, Man, and Society.New Brunswick, N.J.: Rutgers University Press,1996. Reference Source [Google Scholar]
- 151. Weiss KB, Addington WW: Tuberculosis: poverty's penalty. Am J Respir Crit Care Med. 1998;157(4 Pt 1):1011. 10.1164/ajrccm.157.4.ed02-98 [DOI] [PubMed] [Google Scholar]
- 152. Lönnroth K, Jaramillo E, Williams BG, et al. : Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med. 2009;68(12):2240–6. 10.1016/j.socscimed.2009.03.041 [DOI] [PubMed] [Google Scholar]
- 153. McKeown T: The Modern Rise of Population.London: Edward Arnold.1976. Reference Source [Google Scholar]
- 154. Szreter S: The Importance of Social Intervention in Britain's Mortality Decline c.1850–1914: a Re-interpretation of the Role of Public Health. Soc Hist Med. 1988;1(1):1–38. 10.1093/shm/1.1.1 [DOI] [Google Scholar]
- 155. Burki T: Tackling tuberculosis in London's homeless population. Lancet. 2010;376(9758):2055–6. 10.1016/S0140-6736(10)62282-9 [DOI] [PubMed] [Google Scholar]
- 156. Mangtani P, Jolley DJ, Watson JM, et al. : Socioeconomic deprivation and notification rates for tuberculosis in London during 1982-91. BMJ. 1995;310(6985):963–6. 10.1136/bmj.310.6985.963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Krieger N: Discrimination and Health. In: Berkman LF, Kawachi I, eds., Social Epidemiology Press OU editor. New York.2000. Reference Source [Google Scholar]
- 158. 2011 Census analysis: Ethnicity and the Labour Market, England and Wales.Office for National Statistics.2014. Reference Source [Google Scholar]
- 159. Tala E: Migration, ethnic minorities and tuberculosis. Eur Respir J. 1989;2(6):492–3. [PubMed] [Google Scholar]
- 160. Population of the UK by Country of Birth and Nationality: 2015.Office for National Statistics.2016. Reference Source [Google Scholar]
- 161. Statutory Homelessness: October to December Quarter 2015.Department for Communities and Local Government.2016. Reference Source [Google Scholar]
- 162. Overcrowding and Under-Occupation by Ethnic Group, 2011.Office for National Statistics.2014. Reference Source [Google Scholar]
- 163. Farmer P: Infections and Inequalities: The Modern Plagues.California: University of California Press,1999. Reference Source [Google Scholar]
- 164. Singer M: Farewell to adaptationism: unnatural selection and the politics of biology. Med Anthropol Q. 1996;10(4):496–515. 10.1525/maq.1996.10.4.02a00050 [DOI] [PubMed] [Google Scholar]
- 165. Mason PH, Roy A, Spillane J, et al. : SOCIAL, HISTORICAL AND CULTURAL DIMENSIONS OF TUBERCULOSIS. J Biosoc Sci. 2016;48(2):206–32. 10.1017/S0021932015000115 [DOI] [PubMed] [Google Scholar]
- 166. Crofts JP, Gelb D, Andrews N, et al. : Investigating tuberculosis trends in England. Public Health. 2008;122(12):1302–10. 10.1016/j.puhe.2008.04.011 [DOI] [PubMed] [Google Scholar]
- 167. Parslow R, El-Shimy NA, Cundall DB, et al. : Tuberculosis, deprivation, and ethnicity in Leeds, UK: 1982-1997. Arch Dis Child. 2001;84(2):109–13. 10.1136/adc.84.2.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Tocque K, Regan M, Remmington T, et al. : Social factors associated with increases in tuberculosis notifications. Eur Respir J. 1999;13(3):541–5. 10.1183/09031936.99.13354199 [DOI] [PubMed] [Google Scholar]
- 169. Offer C, Lee A, Humphreys C: Tuberculosis in South Asian communities in the UK: a systematic review of the literature. J Public Health (Oxf). 2016;38(2):250–7. 10.1093/pubmed/fdv034 [DOI] [PubMed] [Google Scholar]
- 170. Beckhurst C, Evans S, MacFarlane AF, et al. : Factors influencing the distribution of tuberculosis cases in an inner London borough. Commun Dis Public Health. 2000;3(1):28–31. [PubMed] [Google Scholar]
- 171. Ho MJ: Migratory journeys and tuberculosis risk. Med Anthropol Q. 2003;17(4):442–58. 10.1525/maq.2003.17.4.442 [DOI] [PubMed] [Google Scholar]
- 172. Abarca Tomás B, Pell C, Bueno Cavanillas A, et al. : Tuberculosis in migrant populations. A systematic review of the qualitative literature. PLoS One. 2013;8(12):e82440. 10.1371/journal.pone.0082440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Raphaely N: Understanding the health needs of migrants in the South East Region.London: Health Protection Agency and Department of Health,2010. Reference Source [Google Scholar]
- 174. Samers M: Migration.Oxford: Routledge,2010. Reference Source [Google Scholar]
- 175. Brugha T, Jenkins R, Bebbington P, et al. : Risk factors and the prevalence of neurosis and psychosis in ethnic groups in Great Britain. Soc Psychiatry Psychiatr Epidemiol. 2004;39(12):939–46. 10.1007/s00127-004-0830-9 [DOI] [PubMed] [Google Scholar]
- 176. The Mental and Emotional Wellbeing of Africans in the UK: A research and discussion paper.African Health Policy Network,2013. Reference Source [Google Scholar]
- 177. Prince M, Patel V, Saxena S, et al. : No health without mental health. Lancet. 2007;370(9590):859–77. 10.1016/S0140-6736(07)61238-0 [DOI] [PubMed] [Google Scholar]
- 178. Wieland ML, Weis JA, Yawn BP, et al. : Perceptions of tuberculosis among immigrants and refugees at an adult education center: a community-based participatory research approach. J Immigr Minor Health. 2012;14(1):14–22. 10.1007/s10903-010-9391-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Gerrish K, Naisby A, Ismail M: The meaning and consequences of tuberculosis among Somali people in the United Kingdom. J Adv Nurs. 2012;68(12):2654–63. 10.1111/j.1365-2648.2012.05964.x [DOI] [PubMed] [Google Scholar]
- 180. Johnson A: Beliefs and barriers related to understanding TB amongst vulnerable groups in South East London.2006. Reference Source [Google Scholar]
- 181. Nnoaham KE, Pool R, Bothamley G, et al. : Perceptions and experiences of tuberculosis among African patients attending a tuberculosis clinic in London. Int J Tuberc Lung Dis. 2006;10(9):1013–7. [PubMed] [Google Scholar]
- 182. Poss JE: The meanings of tuberculosis for Mexican migrant farmworkers in the United States. Soc Sci Med. 1998;47(2):195–202. 10.1016/S0277-9536(98)00062-8 [DOI] [PubMed] [Google Scholar]
- 183. Coreil J, Lauzardo M, Heurtelou M: Cultural feasibility assessment of tuberculosis prevention among persons of Haitian origin in South Florida. J Immigr Health. 2004;6(2):63–9. 10.1023/B:JOIH.0000019166.80968.70 [DOI] [PubMed] [Google Scholar]
- 184. Kulane A, Ahlberg BM, Berggren I: "It is more than the issue of taking tablets": the interplay between migration policies and TB control in Sweden. Health Policy. 2010;97(1):26–31. 10.1016/j.healthpol.2010.02.014 [DOI] [PubMed] [Google Scholar]
- 185. Festenstein F, Grange JM: Tuberculosis in Ethnic Minority Populations in Industrialised Countries. In: JDH Porter and JM Grange, eds., Tuberculosis: An Interdisciplinary Perspective London: Imperial College Press,2010. Reference Source [Google Scholar]
- 186. Kraut AM: Silent Travellers: Germs, Genes and the 'Immigrant Menace'.Baltimore: John Hopkins University Press,1994. Reference Source [Google Scholar]
- 187. Goffman E: Stigma: Notes on the Management of Spoiled Identity. London: Penguin Books. Reference Source [Google Scholar]
- 188. Kelly P: Isolation and stigma: the experience of patients with active tuberculosis. J Community Health Nurs. 1999;16(4):233–41. 10.1207/S15327655JCHN1604_3 [DOI] [PubMed] [Google Scholar]
- 189. Baral SC, Karki DK, Newell JN: Causes of stigma and discrimination associated with tuberculosis in Nepal: a qualitative study. BMC Public Health. 2007;7:211. 10.1186/1471-2458-7-211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Courtwright A, Turner AN: Tuberculosis and stigmatization: pathways and interventions. Public Health Rep. 2010;125 Suppl 4:34–42. 10.1177/00333549101250S407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Sterne JA, Rodrigues LC, Guedes IN: Does the efficacy of BCG decline with time since vaccination? Int J Tuberc Lung Dis. 1998;2(3):200–7. [PubMed] [Google Scholar]
- 192. McBride D: From Tuberculosis to AIDS: Epidemics Among Urban Blacks Since 1900. Albany: State University of New York.1991. Reference Source [Google Scholar]
- 193. Bollini P, Siem H: No real progress towards equity: health of migrants and ethnic minorities on the eve of the year 2000. Soc Sci Med. 1995;41(6):819–28. 10.1016/0277-9536(94)00386-8 [DOI] [PubMed] [Google Scholar]
- 194. Stagg HR, Jones J, Bickler G, et al. : Poor uptake of primary healthcare registration among recent entrants to the UK: a retrospective cohort study. BMJ Open. 2012;2(4): pii: e001453. 10.1136/bmjopen-2012-001453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Hart JT: The inverse care law. Lancet. 1971;1(7696):405–12. 10.1016/S0140-6736(71)92410-X [DOI] [PubMed] [Google Scholar]
- 196. Bender A, Andrews G, Peter E: Displacement and tuberculosis: recognition in nursing care. Health Place. 2010;16(6):1069–76. 10.1016/j.healthplace.2010.06.007 [DOI] [PubMed] [Google Scholar]
- 197. Ito KL: Health culture and the clinical encounter: Vietnamese refugees' responses to preventive drug treatment of inactive tuberculosis. Med Anthropol Q. 1999;13(3):338–64. 10.1525/maq.1999.13.3.338 [DOI] [PubMed] [Google Scholar]
- 198. Sumartojo E: When tuberculosis treatment fails. A social behavioral account of patient adherence. Am Rev Respir Dis. 1993;147(5):1311–20. 10.1164/ajrccm/147.5.1311 [DOI] [PubMed] [Google Scholar]
- 199. Salway SAP, Tod A: Reducing Health Inequalities Implementation Theme. Briefing Paper 2: How to incorporate attention to inequality in CLAHRC (SY) activity. Discussion Paper. South Yorkshire.2009. Reference Source [Google Scholar]
- 200. Kline R: The 'snowy white peaks' of the NHS: a survey of discrimination in governance and leadership and the potential impact on patient care in London and England. Middlesex University Research Repository.2014. Reference Source [Google Scholar]
- 201. MacPherson SW: The Stephen Lawrence Inquiry.1999. Reference Source [Google Scholar]
- 202. Joseph HA, Waldman K, Rawls C, et al. : TB perspectives among a sample of Mexicans in the United States: results from an ethnographic study. J Immigr Minor Health. 2008;10(2):177–85. 10.1007/s10903-007-9067-5 [DOI] [PubMed] [Google Scholar]
- 203. Ho MJ: Sociocultural aspects of tuberculosis: a literature review and a case study of immigrant tuberculosis. Soc Sci Med. 2004;59(4):753–62. 10.1016/j.socscimed.2003.11.033 [DOI] [PubMed] [Google Scholar]
- 204. Taylor K: Asylum seekers, refugees, and the politics of access to health care: a UK perspective. Br J Gen Pract. 2009;59(567):765–72. 10.3399/bjgp09X472539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Reeves M, de Wildt G, Murshali H, et al. : Access to health care for people seeking asylum in the UK. Br J Gen Pract. 2006;56(525):306–8. [PMC free article] [PubMed] [Google Scholar]
- 206. Story A, Murad S, Roberts W, et al. : Tuberculosis in London: the importance of homelessness, problem drug use and prison. Thorax. 2007;62(8):667–71. 10.1136/thx.2006.065409 [DOI] [PMC free article] [PubMed] [Google Scholar]