Version Changes
Revised. Amendments from Version 2
Revisions, corrections and details addition have been done throughout the manuscript as a response to the last comments/questions of reviewers. Significant improvements made are including an explanation about the reasons why fluconazole 0.31 mg/ml did not show good antimycotic properties as expected. References supported this explanation were also provided. Some parts of this explanation also answers a question from reviewer about the reason for determining dose of fluconazole. Correction and enrichment in methods were also performed to provide better clarity and readability. The method used was a modification of those previously used by two different researchers in analyzing biofilm formation by bacteria. Further details, especially those related to the low respondent involved, could not be made because research presented was still in the preliminary stage.
Abstract
Background: Candida albicans is an opportunistic fungus that might infect the oral cavity. Increased colony numbers of C. albicans in the mouth can be caused by multiple factors, such as smoking, weakened immune system, antibiotics use and immune-compromised condition. Smoking can increase expression of virulence factors of C. albicans and make it stronger. One virulence factor of C. albicans is biofilm formation. The ability of creating biofilm makes C. albicans more tolerant to commercial antifungal agents. The objective of this preliminary study was to examine the ability of the seaweed G. verrucosa extracts to inhibit the formation of biofilm by C. albicans isolated from the saliva of a smoker.
Methods: The extract of G. verrucosa was prepared by maceration using 96% methanol and subjected for phytochemical analysis. C. albicans was isolated from the saliva of a smoker who voluntarily participated in the study after providing informed consent. In triplicate, the fungus was cultured in the growth medium containing increased concentrations of G. verrucosa (6.25, 12.5, 25, 50, 75 and 100% ).The same reaction using fluconazole 0.31 µg/ml C. albicans was prepared as positive control. Biofilm formation was accessed based on optical density of cell mixtures using an ELISA reader. The data obtained were subjected to Kruskal-Wallis test at a significance limit of 0.05.
Results: Methanol extract of seaweed G. verrucosa contained three bio-active compounds namely steroids, terpenoid, and tannins. Inhibitory activity of seaweed extracts on C. albicans biofilm formation increased as their concentration increased. The highest inhibitory effect was recorded at fungus culture treated with seaweed concentration of 25% at 24 hours of time exposure.
Conclusions: Seaweed G. verrucosa extract contained steroids, terpenoids and tannins that were able to effectively inhibit the formation of biofilm by C. albicans at the concentration of 25% after 24 hours of time exposure.
Keywords: Candida albicans, oral candidiasis, seaweed Gracilaria verrucosa
Introduction
Smoking is a common problem in both developed and developing countries, including in Indonesia. Based on a survey by the Tobacco Atlas in 2015, Indonesia has the highest number of smokers in Asia, with 66% of men in Indonesia being active smokers 1. Smoking can lead to addiction owing to the nicotine contents, and harm due to the presence of toxic compounds such as carbon monoxide, ammonia and tar contents in tobacco 1. Substances in cigarettes can also contribute for the occurrence of oral candidiasis, infection of in the mouth cavity caused by the fungus Candida albicans 2. This fungus is part of the normal flora of the human mouth, but it can become pathogenic in certain conditions, for example, due to nicotine exposure 3.
The C. albicans that infects human tissues generally form biofilm 3, an extracellular matrix consisting of C. albicans colonies 4. The size of the biofilm increases when the fungus was exposed to substances in cigarette smoke because cigarette contains chemicals that can initiate the growth of and nourish C. albicans 5, 6.
Currently, fluconazole and nystatin are the most effective drugs for treating oral candidiasis. Unfortunately, these drugs could result in undesired side effects. Prolonged use of fluconazole, for example, can leads to resistance 7 whereas high dosages of nystatin give gastrointestinal discomfort and increase plaque formation 8. Therefore, plant-derived antifungals may be a viable oral treatment option for candidiasis. One of these potential plants is Gracilaria verrucosa. This seaweed contains several bioactive compounds, including alkaloids, flavonoids, phenolics, saponins, steroids and terpenoids 9. Aceh Province, Indonesia, has large G. verrucosa resources, although this aquatic plant has not been commonly used for medicinal purposes. Hence, the objective of the present study was to examine the ability of seaweed extract to inhibit the biofilm formation of C. albicans isolated from the saliva of smoker.
Methods
Time and site
The study was conducted in August 2017 at the Laboratory of Microbiology, Veterinary Faculty, Syiah Kuala University. The G. verrucosa seaweeds were collected from a farmer in Pulo Aceh, Aceh Province.
Ethics
All research protocols used in this study were approved by the Research Ethics Committee of the Dentistry Faculty of Syiah Kuala University No. 1741/UN11.1.21/TU/2017.
Saliva collection
The saliva was collected from an active smoker who worked as administrative staff at the Faculty of Dentistry Medicine of Syiah Kuala University and voluntarily participated in this study after completing informed consent. Inclusion criterion of the volunteer was active smoker who smoked 20 cigarettes per day. Saliva was collected once by spitting into a glass jar (15 ml) right after the subject finished smoking, and added with 10 ml of PBS (0.01 M, pH 7.2). The jar was centrifuged at 10,000 rpm for 10 minutes. The precipitate was stored for the microbiological examination.
Candida albicans isolation and preparation
Precipitate was cultured in ChromAgar Candida medium and incubated at 37°C for 2 days. The C. albicans fungus grew as green colonies. One colony of C. albicans was mixed with 5 ml of peptone in a tube and incubated at 37°C for 24 hours. Turbidity of medium was compared to a 0.5 McFarland solution standard, which was equivalent to 1.5 × 10 8 CFU/ml.
Seaweed extraction
Seaweed extraction was performed based by maceration using 96% methanol 10. In brief, a total of 500 g of fresh seaweeds were washed with tap water then with distilled water. Seaweed samples were at 25°C for 24 hours, chopped into small pieces (2 mm), and soaked in 1.5 liters of 96% methanol. Macerate was filtered using Whatman filter paper No. 42. Filtrate collected was concentrated using a vacuum rotary evaporator (Laborta 4003 control, Heildolph) 60°C and at a speed of 80–90 rpm for 3 days. Concentrated extract was put in a sealed dark bottle and stored at 4°C.
Phytochemical tests
Flavonoid test. A 0.5 cm magnesium plate was rinsed in 5 ml of seaweed extract, mixed with two drops of HCl, and heated by passing it over a Bunsen flame. Red or purple coloration formed on the heating indicated the presence of flavonoids 11.
Alkaloid test. Seaweed extract (5 ml) was mixed with 8 ml of HCl and filtered. Filtrate was subjected to Mayer, Wagner and Dragendroff tests for alkaloids 11. This was done by mixing 2 ml of filtrate with 5 g potassium mercuric iodide (Mayer test), 2 ml of Wagner reagent, or 2 ml of bismuth potassium iodide solution (Dragendroff test). The formation of white or pale precipitates (Mayer test), brown or reddish-brown precipitates (Wagner test) and red precipitates (Dragendroff test) indicated the presence of alkaloids.
Tannin/phenolic test. Two drops of 1% FeCl 3 was added to 1 ml seaweed extract. The change in the color to a blackish green indicated the presence of tannin/phenolic 12.
Saponin test. Seaweed extract, 1 ml, was diluted in 20 ml of distilled water and shaken vertically for 15 seconds. Persistent foaming indicated the presence of saponin content.
Steroid test. Two milliliters of seaweed extract was added with 2 ml of CHCl 3, 2 drops of H 2S and 1 ml of CH 3COOH. The formation of green or blue precipitates indicated the presence of steroid 11.
Terpenoid test. Seaweed extracts (5 ml) was mixed with 2 ml of chloroform. Concentrated H 2SO 4, 3 ml, were carefully added. The formation of reddish brown layer at the interface of extract and chloroform solution indicated the presence of terpenoids 13.
Examination of biofilm formation
Casein-peptone lecithin polysorbate broths (Merck-1117230500), 100 µl, were poured in each well of a 96-well plate, incubated at room temperature for 5 minutes and discarded by blotting the plate on paper towels 2–5 times. Fifty microliter C. albicans culture had turbidity was equal to 0.5 McFarland standard were added to each well and incubated for 5 minutes to attachment of fungal cells on casein. Cell mixtures were washed by aspiration. In triplicate, 50 µl of decreased concentrations of seaweed extracts (100, 75, 50, 25, 12.5 and 6.25%) were added. The same reaction using fluconazole 0.31 µg/ml was prepared as positive control. The plates were then incubated at 37°C for 24, 48 or 72 hours. Each well was added with 200 µl of 0.1% violet crystal, incubated at 25°C for 15 min, and washed three times with 200 µl of 0.01 M PBS. The crystal violet in each well was then removed by adding 100 µl of 96% ethanol for 2 min. The biofilm formation was analyzed by reading optical density of mixture using an ELISA reader at 620 nm 14, 15.
Data analysis
The data obtained were subjected to Kruskal-Wallis test using SPSS software v20.0 for windows.
Results
The results of phytochemical tests in Table 1 show that methanol extract of seaweed G. verrucosa contained bioactive compounds belonged to steroids, terpenoids, and tannins/polyphenols.
Table 1. Phytochemical contents of seaweed Gracilaria verrucosa extract.
| Substance | Reagent | Result | Indication |
|---|---|---|---|
| Alkaloid | Mayer | - | White deposit |
| Wagner | - | Brown deposit | |
| Dragendroff | - | Red deposit | |
| Steroid | Uji Lieberman-Burchard | + | Green or blue colors |
| Terpenoid | Uji Lieberman-Burchard | + | Red or purple colors |
| Saponin | Shuffling method | - | Stable foams |
| Flavonoid | 0.5 Mg and HCl | - | Red or purple colors |
| Tannin/Phenolic | MgCl 3 | + | Dark green |
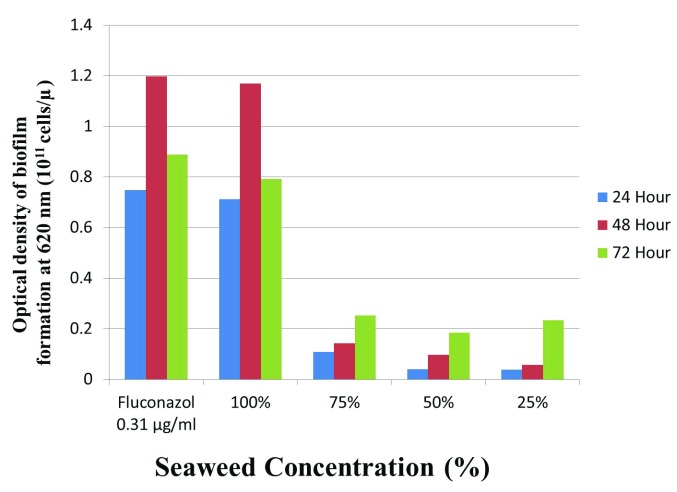
In general, the inhibitory effect was reduced as seaweed concentration increased ( Figure 1). The highest inhibition after 24 and 48 hours time exposures was recorded from C. albicans culture treated with 25% seaweed extract, followed by those with 50% and 75%. The best inhibition after 75 hours time exposure, however, was found in C. albicans culture treated with 25% seaweed extract. In all time exposures, the inhibitory effect caused by fluconazole (control) was the worst, followed by those caused by 100% seaweed extracts, but there was no difference between these two groups. Results of Kruskal-Wallis analysis showed that seaweed extract significantly ( p<0.05) inhibited the formation of biofilm by C. albicans, indicated potential of the extract to inhibit the growth of the fungus.
Figure 1. The formation of biofilm by Candida albicans exposed to seaweed Gracilaria verrucosa extract on different time exposures and concentrations.
Copyright: © 2018 Mubarak Z et al.
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Discussion
The study showed that 25–75% seaweed extracts are promising for inhibiting the growth of C. albicans as shown by much lower biofilm formation after 24, 48 and 72 hours exposure compared to those caused by positive control (fluconazole) and 100% seaweed extract. This indicated potential of seaweed extract as natural anti-fungus to treat oral candidiosis caused by against C. albicans in smokers.
C. albicans is a normal micro-organism in the human mouth. This fungus, however, can be pathogenic in certain circumstances 3 such as in the mouth of smokers 2. Smoking can stimulate synthesis of HWP1, EAP1 and SAP2 proteins in C. albicans, causing higher virulence of the fungus. This can lead to increased formation of biofilm and finally cause oral candidiasis 6. Smoking also cause a decreased immune function, making individuals more susceptible to oral infections, including candidiasis 4, 6.
The low effectiveness of fluconazole 0.31 μg/ml, the leading choice of therapy against C. albicans, against this fungus was probably caused by several factors such as low therapeutic dose and resistance of the fungus to the drug. Pfaller et al., who complement CLSI guidelines with the EUCAST guidelines about species-specific interpretative breakpoints for fluconazole susceptibility suggested that the breakpoints for C. albicans are <2 mg/ml, 4 mg/ml and >8 mg ml for susceptible, susceptible dose-dependent, and resistant isolates, respectively 16. Dosage criteria for analyzing candida species using MIC test were susceptible (MIC < 8 μg/ml; range, 0.25 to 4 μg/ml), susceptible dose-dependent (MIC 8 – 16 μg/ml; range, >4 to <16 μg/ml), and resistant (MIC > 16 mg/ml; range, 16 to >128 mg/ml) 17. Fluconazole dose of 0.31 μg/ml used in this study was slightly higher than minimum dose ideally used to test antifungal resistance of the antifungal. This also meant that C. albicans strain isolated from smoker respondent involved in this study already developed resistance to the dose used. Increasing resistance of C. albicans against this fluconazole, one of the most azole antimycotics have been reported 18, 19. This resistance, that is assumed related to prolonged or repeated exposure to low-dose of the antifungus 20, must be confirmed by further study.
The stronger ability of 25–75% seaweed extracts to inhibit the formation of C. albicans biofilm significantly compared to those of fluconazole (positive control) was probably caused by bioactive compound presence in the extracts. These included steroids, terpenoids, and tannins/polyphenols, all of which have been known their benefit for human health. According to Sampaio et al. 21, antifungal activity of a substance strongly depends on the composition of its bioactive compounds. Steroids can kill C. albicans through their lypophilic properties, interfering with the formation of fungal spores and mycelium 22. This activity weakens C. albicans, inhibiting the formation of the biofilm. To function optimally, steroids require oligosaccharides that are also present in the seaweed content 23
Terpenoids are derivatives of saponins that may act as an antifungals by damaging organelles of the fungus and by inhibiting secretion of enzymes, leading to growth the inhibition C. albicans cells. Terpenoids can also damage the morphology of C. albicans 24. Tannins may inhibit chitin synthesis in C. albicans cell walls, leading to lost of membrane cell protection and disrupted cellular metabolism. Tannins also can inhibit ergosteron activity of C. albicans 25.
The effectiveness of seaweed extracts in inhibiting fungal growth is influenced by at least three factors, namely concentration, exposure time, and contact surface media 26. The present study showed that diluted concentration (25–75%) of extracts showed better inhibitory effect on the growth of C. albicans than the more concentrated 100% extract. Concentrated extract usually has lesser effectiveness in vitro due to solubility and import problems. In more aqueous condition plant extracts generally show better medicinal properties with increased concentration as shown by a study testing antifungal activity of ethanol extracts of Syzygium jombolanum, Cassia siamea, Ordina wodier, Momodica charantia and Melia azedarach as well as two algal species Sargassum wightii and Saulerpa scalpellformis against 25 C. albicans isolates in vitro 27.
This study also showed the best inhibitory effect of seaweed extracts was recorded at 24 hour of exposure. This is probably because the farnesol, a quorum-sensing molecule that has the potency to inhibit C. albicans growth 28, works effectively after 48–72 hour of exposure.
This study, however, was still preliminary due to limited number of subject and unavailability of non-smoker, control. Virulence factor of C. albicans analyzed was also only biofilm formation. Since more virulence factors are possibly synthesized by the fungus under certain environmental condition, further studies are need to be conducted to investigate effect of individual bioactive compound contained in the seaweeds on the formation of biofilm and on the expression other virulence factors of C. albicans isolated form both smoker and non-smoker individuals.
Conclusion
Extract of Gracilaria verrucosa seaweed could inhibit the growth of C. albicans isolated from the saliva of a smoker. The inhibitory effect decrease with the increase of concentration, and reached the highest at concentration of 25% and time exposure of 24 hours.
Data availability
The data referenced by this article are under copyright with the following copyright statement: Copyright: © 2018 Mubarak Z et al.
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication). http://creativecommons.org/publicdomain/zero/1.0/
Dataset 1. The raw data of the Triplo anti-Biofilm seaweed to C. albicans for 24, 48 and 72 h at a wavelength 620 nm. DOI: 10.5256/f1000research.14879.d204270 29.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 3; referees: 3 approved]
References
- 1. Lian TY, Dorotheo U: The Tobacco Control Atlas.Seutawan Co.2016; 3. Reference Source [Google Scholar]
- 2. Mayer FL, Wilson D, Hube B: Candida albicans pathogenicity mechanisms. Virulence. 2013;4(2):119–128. 10.4161/viru.22913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adel E, Khadijed PS, Mohammad M: Oral Cavity Candidiasis as a Complication of Fungal Diseases in Diabetic Patients in South-East of Iran. IJIAS. 2016;14(4):1134–1138. Reference Source [Google Scholar]
- 4. Little JW, Falace D, Miller C: Dental Management of The Medically Compromised Patient.Mosby: Elsevier;2013. Reference Source [Google Scholar]
- 5. Keten HS, Keten D, Ucer H, et al. : Prevalence of oral Candida carriage and Candida species among cigarette and maras powder users. Int J Clin Exp Med. 2015;8(6):9847–54. [PMC free article] [PubMed] [Google Scholar]
- 6. Semlali A, Killer K, Alanazi H, et al. : Cigarette smoke condensate increases C. albicans adhesion, growth, biofilm formation, and EAP1, HWP1 and SAP2 gene expression. BMC Microbiol. 2014;14:61. 10.1186/1471-2180-14-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peron IH, Reichert-Lima F, Busso-Lopes AF, et al. : Resistance Surveillance in Candida albicans: A Five-Year Antifungal Susceptibility Evaluation in a Brazilian University Hospital. PLoS One. 2014;11(7): e0158126. 10.1371/journal.pone.0158126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lyu X, Zhao C, Yan ZM, et al. : Efficacy of nystatin for the treatment of oral candidiasis: a systematic review and meta-analysis. Drug Des Devel Ther. 2016;10:1161–1171. 10.2147/DDDT.S100795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sahat JH: Warta Ekspor. September ed. Jakarta: DGNED;2013; 3. [Google Scholar]
- 10. Prima MI: Uji Aktivitas Antibakteri Ekstrak Metanol Ganggang Merah Gracilaria verrucosa terhadap Beberapa Bakteri Patogen Gram Positif dan Gram Negatif. Published Only In Database.2012. Reference Source [Google Scholar]
- 11. Vimalkumar CS, Hosagaudar VB, Suja SR, et al. : Comparative Preliminary Phytochemical Analysis Of Ethanolic Extracts of Leaves of Olea dioica Roxb., Infected with The Rust Fungus Zaghouania oleae (E.J.Butler) Cummins and Non-infected Plants. J Pharmacogn Phytochem. 2014;3(4):69–72. Reference Source [Google Scholar]
- 12. Hariyanto Ih, Inarah F, Suci PR, et al. : Skrining Fitokimia dan Analisis Kromatografi Lapis Tipis dari Ekstrak Ethanol Herba Pacar Air ( Impatiens balsamina Linn.). Published Only In Database.2014. Reference Source [Google Scholar]
- 13. Khan MA, Qureshi AR, Ullah F, et al. : Phytochemical analysis of selected medicinal plants of Margalla Hills and surroundings. JMPR. 2011;5(25):6017–7. Reference Source [Google Scholar]
- 14. Metzler A: Developing a Crystal Violet Assay to Quantify Biofilm Production Capabilities of Staphylococcus aureus. Published Only In Database. 2016. Reference Source [Google Scholar]
- 15. Roberts SK, Wei GX, Wu CD: Evaluating biofilm growth of two oral pathogens. Lett Appl Microbiol. 2002;35(6):552–6. 10.1046/j.1472-765X.2002.01228.x [DOI] [PubMed] [Google Scholar]
- 16. Pfaller MA, Andes D, Diekema DJ, et al. : Wild-type MIC distributions, epidemiological cutoff values and species-specific clinical breakpoints for fluconazole and Candida: time for harmonization of CLSI and EUCAST broth microdilution methods. Drug Resist Updat. 2010a;13(6):180–195. 10.1016/j.drup.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 17. Graybill JR, Montalbo E, Kirkpatrick WR, et al. : Fluconazole versus Candida albicans: a complex relationship. Antimicrob Agents Chemother. 1998;42(11):2938–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gygax SE, Vermitsky JP, Chadwick SG, et al. : Antifungal resistance of Candida glabrata vaginal isolates and development of a quantitative reverse transcription-PCR-based azole susceptibility assay. Antimicrob Agents Chemother. 2008;52(9):3424–3426. 10.1128/AAC.00462-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pfaller MA, Diekema DJ, Gibbs DL, et al. : Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: a 10.5-year analysis of susceptibilities of Candida Species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J Clin Microbiol. 2010;48(4):1366–1377. 10.1128/JCM.02117-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Richardson DM: Changing patterns and trends in systemic fungal infections. J Antimicrob Chemother. 2005;56 Suppl 1:i5–i11. 10.1093/jac/dki218 [DOI] [PubMed] [Google Scholar]
- 21. Sampaio BL, Edrada-Ebel R, Da Costal FB: Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: a model for environmental metabolomics of plants. Sci Rep. 2016;6: 29265. 10.1038/srep29265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Subsiha S, Subramoniam A: Antifungal Activities of Steroid From Pallavicinia lyellii, a liverwort. Indian J Pharmacol. 2005;37(5):304–308. 10.4103/0253-7613.16854 [DOI] [Google Scholar]
- 23. Cammarata A, Upadhyay SK, Jursic BS, et al. : Antifungal activity of 2α,3β-functionalized steroids stereoselectively increases with the addition of oligosaccharides. Bioorg Med Chem Lett. 2011;21(24):7379–7386. 10.1016/j.bmcl.2011.10.015 [DOI] [PubMed] [Google Scholar]
- 24. Martínez A, Rojas N, García L, et al. : In vitro activity of terpenes against Candida albicans and ultrastructural alterations. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118(5):553–9. 10.1016/j.oooo.2014.07.009 [DOI] [PubMed] [Google Scholar]
- 25. Hastuti SU, Ummah PIY, Khasanah NH: Antifungal Activity of Piper aduncum and Peperomia pellucida Leaf Ethanol Extract Against Candida albicans. AIP Conf Proc. 2017;1844: 020006. 10.1063/1.4983417 [DOI] [Google Scholar]
- 26. Kenakin PT: A Pharmacology Primer: Theory, Application, and Methods. 2 ndedition. USA: Elvesier.2006;36–38. 10.1016/B978-0-12-370599-0.X5000-X [DOI] [Google Scholar]
- 27. Prabhakar K, Kumar LS, Rajendran S, et al. : Antifungal Activity of Plant Extracts against Candida Species from Oral Lesions. Indian J Pharm Sci. 2008;70(6):801–803. 10.4103/0250-474X.49128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alem AM, Oteef MD, Flowers TH, et al. : Production of tyrosol by Candida albicans biofilms and its role in quorum sensing and biofilm development. Eukaryot Cell. 2006;5(10):1770–1779. 10.1128/EC.00219-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mubarak Z, Humaira A, Gani BA, et al. : Dataset 1 in: Preliminary study on the inhibitory effect of seaweed Gracilaria verrucosa extract on biofilm formation of Candida albicans cultured from the saliva of a smoker. F1000Research. 2018. 10.5256/f1000research.14879.d204270 [DOI] [PMC free article] [PubMed] [Google Scholar]