Abstract
Background
Radiation-induced cognitive dysfunction is a significant side effect of cranial irradiation for brain tumors. Clinically, pediatric patients are more vulnerable than adults. However, the underlying mechanisms of dysfunction, including reasons for age dependence, are still largely unknown. Previous studies have focused on the loss of hippocampal neuronal precursor cells and deficits in memory. However, survivors may also experience deficits in attention, executive function, or other non-hippocampal–dependent cognitive domains. We hypothesized that brain irradiation induces age-dependent deficits in cortical synaptic plasticity.
Methods
In vivo recordings were used to test neuronal plasticity along the direct pathway from the cornu ammonis 1 (CA1)/subicular region to the prefrontal cortex (PFC). Specifically, long-term potentiation (LTP) in the CA1/subicular-PFC pathway was assessed after cranial irradiation of juvenile and adult Sprague Dawley rats. We further assessed a potential role for glutamate toxicity by evaluating the potential neuroprotective effects of memantine.
Results
LTP was greatly inhibited in both adult and juvenile animals at 3 days after radiation but returned to near-normal levels by 8 weeks—only in adult rats. Memantine given before, but not after, irradiation partially prevented LTP inhibition in juvenile and adult rats.
Conclusion
Cranial radiation impairs neuroplasticity along the hippocampal–PFC pathway; however, its effects vary by age. Pretreatment with memantine offered protection to both juvenile and adult animals. Deficits in cortical plasticity may contribute to radiation-induced cognitive dysfunction, including deficits in attention and age-dependent sensitivity of such pathways, which may underlie differences in clinical outcomes between juveniles and adults after cranial irradiation.
Keywords: cognitive impairment, cranial radiation, long-term potentiation, memantine, neuroplasticity
Importance of the study
Patients with brain tumors can experience significant cognitive dysfunction after cranial irradiation. Cognitive dysfunction is most pronounced in pediatric cancer survivors, and both adults and children experience deficits in attention and other higher cognitive domains. Investigations in previous decades have focused on deficits in learning and memory, with a focus on neurogenesis and the hippocampus. However, the mechanisms underlying attentional and other deficits remain unknown. This study describes age-dependent deficits in cortical synaptic plasticity that were prevented by the N-methyl-D-aspartic acid (NMDA) receptor antagonist memantine. These results provide evidence for direct, NMDA receptor-dependent, cortical neuronal injury after radiation and reveal pathways that may underlie age-dependent outcomes.
Radiation therapy (RT) is a standard treatment for primary brain tumors and brain metastases. However, successful treatment outcomes are often associated with long-term declines in cognitive function, with memory being the most often studied. However, deficits in other intellectual domains have also been recorded and have significant negative effects on survivors.1 Although cognitive impairments after cranial irradiation are seen in patients of all ages, children are more vulnerable than adults.1,2 Survivors of childhood brain tumors may suffer from failure to acquire new memories and from deficits in other cognitive areas, including processing speed and attention, negatively impacting overall academic achievement and quality of life.2,3 However, to date, the mechanisms by which radiation negatively influences brain function (including both the location and the specific type of the damage) as well as the reasons for the observed profound age dependency are unknown.
Historically, studies of radiation-induced cognitive deficits have focused on the loss of neural progenitor cells in the hippocampus. In recent years, interest has grown in how radiation affects neuronal structure and function, mainly in the hippocampus, as memories are thought to be encoded by modification of synaptic strength. In terms of neuronal architecture, radiation has been shown to affect the morphology of hippocampal dendritic cells.4 Functionally, we found in a previous ex vivo study that radiation suppresses long-term potentiation (LTP) in the hippocampal dentate gyrus.5 LTP represents a persistent strengthening of synapses and is the most promising cellular correlate of learning and memory.6 We later extended these studies to demonstrate that radiation causes rapid and dynamic changes in the synaptic structural plasticity of cultured hippocampus cells.7 Notably, we also implicated abnormal glutamate signaling as a mechanism underlying both structural and functional deficits in the hippocampus.7
To date, no studies have investigated changes in neuronal integrity or function after radiation in brain regions outside the hippocampus that control higher-order cognitive functions beyond memory. The prefrontal cortex (PFC), which is one the main cortical inputs to the hippocampus, is crucial not only for memory but also for diverse cognitive processes, including perception, decision making, and emotion.8 A direct monosynaptic pathway originates in the hippocampal cornu ammonis 1 (CA1)/subicular region and projects to the medial orbital and prelimbic areas of the PFC.9 The plasticity of synapses within this pathway has been investigated and highlighted in several theories of cognitive function.10–12 Given the known effects of radiation on attention and other cognitive domains controlled by extra-hippocampal brain regions,13 we hypothesized that radiation would adversely affect synaptic plasticity in the PFC. To test this hypothesis, we exposed both adult and juvenile rats to cranial irradiation and assessed LTP at set intervals thereafter using in vivo recordings along the CA1/subicular-PFC direct pathway. We further tested whether the N-methyl-D-aspartate (NMDA) antagonist memantine influenced the effects of radiation on LTP in this pathway.
Materials and Methods
The Institutional Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center approved all procedures in accordance with federal guidelines.
Animal Care and Preparation
Male 20-day-old (juvenile) or 8-week-old (adult) Sprague Dawley rats from Harlan Laboratory were used in all experiments. Animals were housed with food and water ad libitum in a temperature-controlled room (23 ± 0.5°C) with a 12-hour light/dark cycle. For drug treatment studies, memantine (5 mg/kg) or the same volume of water (vehicle control) were delivered by oral gavage at 25 hours and 1 hour before cranial irradiation or at 1 hour and 25 hours after cranial irradiation. On day 0, rats were anesthetized with isoflurane and either irradiated to a dose of 10 Gy with an XRAD 225Cx (Precision X-Ray) or given sham radiation. A single dose of 10 Gy was used in the present study, since previous studies have shown cognitive impairments and altered expression of the plasticity-related immediate early gene Arc in rodents at this dose.14–16
Electrophysiology Recording In Vivo
On days 3, 14, 28, and 56 after irradiation, rats were anesthetized with urethane (Sigma, 1.5 g/kg i.p.) and placed in stereotaxic frames with body temperature maintained at 37°C by a homeothermic warming blanket. Tungsten microelectrodes (WPI) were positioned in the PFC through a small burr hole in the skull by using coordinates based on the atlas of Paxinos and Watson17 (adult rats: 3.0 mm anterior to bregma, 0.8 mm lateral to the midline, 3.5–4.0 mm deep; juvenile rats: 1.2 mm anterior to bregma, 0.3 mm lateral to the midline, 1.7–2.2 mm deep). A bipolar concentric stainless steel stimulating electrode (150 µm outer diameter with a 300 µm tip separation; WPI) was placed in the CA1/subicular region of the hippocampus (coordinates for adult rats: 6.5 mm posterior to bregma, 5.5 mm lateral to the midline, 5.5–6.5 mm deep; and for juvenile rats: 3 mm posterior to bregma, 2.2 mm lateral to the midline, and 2.7–3.3 mm deep) ipsilateral to the recording site. (Electrode positioning is depicted in Supplementary Figure S1A–B.) Electrical stimulation of the CA1/subicular region evoked a characteristic monosynaptic excitatory postsynaptic field potential (PSP) in the PFC with a peak latency of 18–22 ms, as observed previously.12 Test pulses (100 µs) were delivered every 30 s at an intensity that evoked a response of 70% of its maximum. At this intensity, the field potential is most likely to reflect summated PSPs. Field potentials were amplified, filtered (bandpass 0.1 Hz to 3 kHz), and digitized at 20 kHz. No PSP was detected if either electrode was off target (Supplementary Figure S1C).
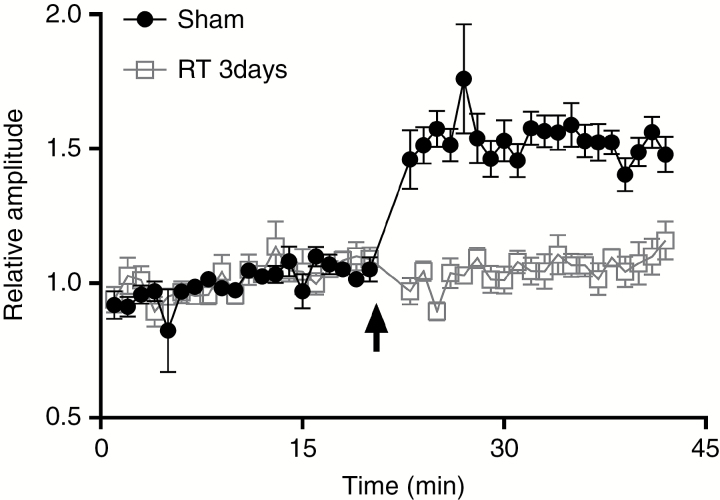
Once 20 minutes of stable baseline data were recorded, high-frequency stimulation composed of 2 series of 10 trains (250 Hz, 200 ms) at 0.1 Hz was delivered to the CA1/subicular at test intensity. This was followed by an additional 20 minutes of recording from the PFC with test stimulation. The PSP amplitudes induced by the test pulses before and after high-frequency stimulation were expressed as a percentage change of the mean response (Fig. 1).
Fig. 1.
Cranial irradiation inhibits long-term potentiation at the hippocampal–prefrontal cortex pathway. Cranial irradiation to a dose of 10 Gy inhibits long-term potentiation (LTP) at the hippocampal–PFC pathway. A baseline was obtained and confirmed to be stable for 20 minutes before high-frequency stimulation (HFS) was applied. The postsynaptic potential amplitudes indicate potentiation of synaptic transmission relative to the baseline. LTP amplitude was 153.5% ± 1.6% (relative to baseline) in sham radiation controls (n = 6), dropping to 104.9% ± 1.2% in irradiated rats (n = 6) at 3 days after irradiation. Two-way analysis of variance with repeated measures showed significant differences between treatment groups [F(3,76) = 103.8, P < 0.001].
Electrodes were marked with DiI dye on their surfaces to identify the tracks left by extracellular microelectrodes.18 At the end of each experiment, rats were perfused with 4% paraformaldehyde solution while still deeply anesthetized, and the brain was removed and fixed for 24 hours in 4% paraformaldehyde and then cryoprotected in 30% sucrose until it sank. The brain was then sectioned (50 µm), mounted onto glass slides, and stained with cresyl violet for histologic confirmation of the locations of both the recording and stimulating electrodes. Data are reported only for those rats in which the electrodes were confirmed to be in the PFC and CA1/subiculum regions.
Statistics
Statistical analyses were done with Microsoft Excel and SPSS. All data are expressed as means ± SEM. Potential differences between groups were calculated by analysis of variance with repeated measures, followed by a Tukey post hoc test for multiple comparisons. P-values of <0.05 were considered statistically significant.
Results
Radiation Inhibits LTP at the Hippocampal–PFC Pathway
High-frequency stimulation was used to trigger LTP along the hippocampal–PFC pathway. As shown in Fig. 1 and Supplementary Figure S1C, an immediate and prolonged increase in the amplitude of PFC potentials was observed immediately after the conditioning stimuli (153.5% ± 1.6% [n = 6], P < 0.01 vs baseline). In adult rats that received 10 Gy of whole-brain radiation, the postsynaptic field potential amplitude at 3 days afterward had decreased to 104.9% ± 1.2% after the conditioning stimuli, which was not statistically different from the baseline value (Fig. 1). This inhibition agrees with previous studies involving hippocampal slices, in which radiation inhibited synaptic function in the dentate gyrus region of the hippocampus within 30 minutes.5
LTP Is More Vulnerable to Radiation-Induced Inhibition in Juveniles than in Adults
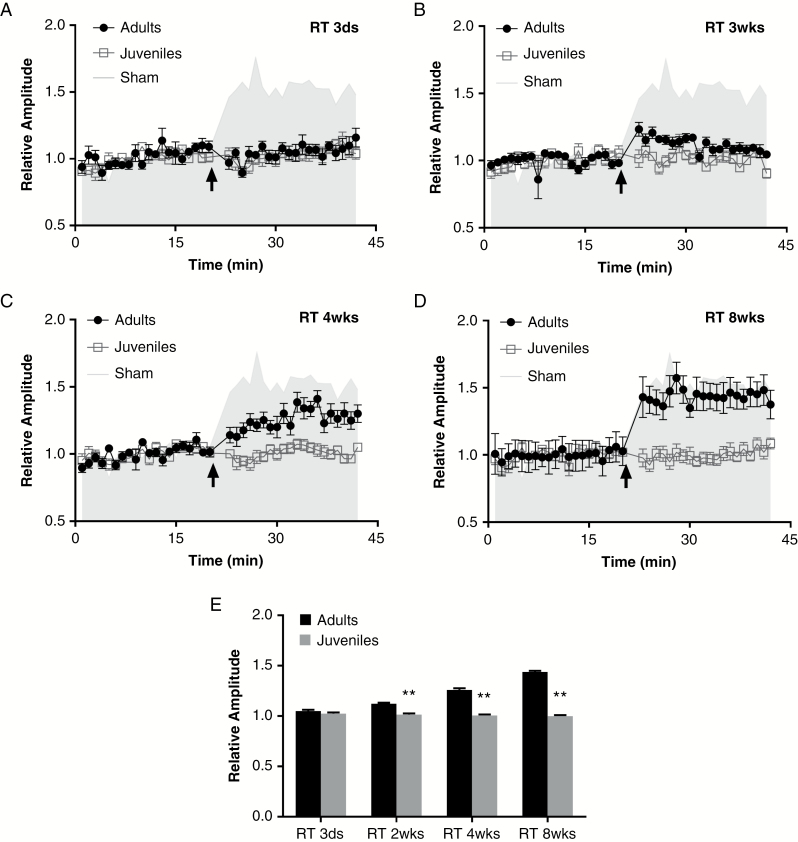
To investigate whether age affects the radiation-induced inhibition of LTP at hippocampal–PFC pathway synapses, we irradiated adult and juvenile rats with a single dose of 10 Gy and measured LTP 3 days, 14 days, 28 days, and 56 days later. LTP was significantly decreased in both adult (104.9% ± 1.2% [n = 6]) and juvenile rats (102.5% ± 1.2% [n = 6]) [F(2,57) = 442.2, P < 0.01 vs sham] at 3 days after irradiation (Fig. 2A). No significant difference was found in LTP amplitude between adults and juveniles (P = 0.398).
Fig. 2.
Long-term potentiation in juvenile rats is more vulnerable to sustained radiation-induced inhibition than in adults. Long-term potentiation (LTP) at the hippocampal–PFC pathway was measured in juvenile and adult rats at (A) 3 days, (B) 14 days, (C) 28 days, and (D) 56 days after irradiation (RT). The amplitudes of postsynaptic potentials in adult rats were 104.9% ± 1.2% (relative to baseline) at day 3, 112.1% ± 1.2% at day 14, 125.9% ± 1.7% at day 28, and 143.8% ± 1.1% at day 56 after irradiation (6 rats per group). Although these values were still lower than those in the control group (gray shading), significant effects between each time point were detected [F(3,76) = 165.4, P < 0.01]. Meanwhile, LTP in juvenile rats remained inhibited (relative to baseline) even at 8 weeks after irradiation (101.5% ± 1.1% at 14 days, 100.6% ± 1.0% at 28 days, and 100.0% ± 0.9% at 56 days after irradiation [6 rats per group]), and no changes were detected during this period [F(3,76) = 1.063, P = 0.370]. (E) Histogram of the relative amplitudes of post-stimuli pulses from adults and juveniles at each time point. **P < 0.001 vs adult rats.
The LTP recovered slowly after irradiation in adult rats (Fig. 2). Amplitudes of PSPs increased to 143.8% ± 1.1% at 8 weeks from being completely inhibited at 3 days after radiation. Strikingly, rats irradiated as juveniles experienced no such recovery; the LTP amplitudes of juvenile rats remained depressed relative to baseline levels at 5 months after irradiation (Fig. 2 and Supplementary Figure S2). No differences were detected at any time during the 8 weeks after irradiation [F(3,76) = 1.063, P = 0.370].
Giving Memantine Before but Not After Irradiation Prevents Radiation-Induced Inhibition of LTP
Memantine is a noncompetitive, low-affinity NMDA receptor (NMDAR) antagonist initially used to treat Alzheimer’s disease.19 Memantine has had moderate neuroprotective effects against radiation-induced brain damage in laboratory experiments5,20 and in clinical trials.21 Here, we tested whether memantine would protect against radiation-induced suppression of LTP in the hippocampal–PFC pathway.
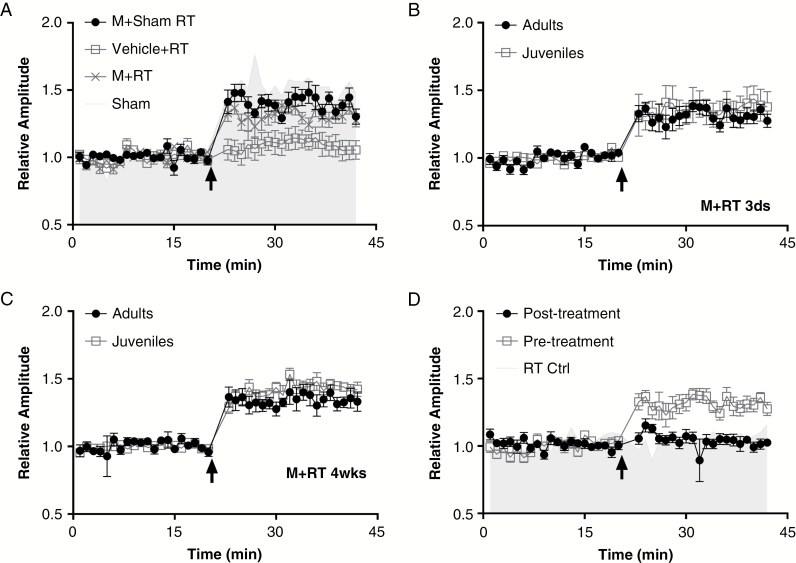
Memantine or vehicle was given by oral gavage at 25 hours and 1 hour before irradiation or sham treatment. In each group, 6 rats were used and identical stimuli applied to induce LTP at 3 days after treatment (radiation or sham). Memantine alone did not affect synaptic plasticity in the hippocampal–PFC pathway, as no differences were observed in LTP amplitudes between memantine-treated and vehicle-treated control groups without irradiation (Fig. 3A). Radiation-induced inhibition of LTP at the hippocampal–PFC connection was at least partially blocked by memantine pretreatment in both adult and juvenile rats. LTP amplitudes increased to 131.3% ± 1.0% of baseline in adults (Fig. 3A) and 135.9% ± 0.9% of baseline in juveniles, findings similar to, but still below, those of the sham controls. Similar results were obtained in adult and juvenile rats 4 weeks after irradiation. No changes in LTP amplitude were found between 3 days and 28 days after irradiation in either adults or juveniles pretreated with memantine (Fig. 3B–C).
Fig. 3.
Giving memantine before irradiation, but not afterward, preserved long-term potentiation at the hippocampal–PFC pathway. (A) Pretreatment with memantine (M) had significant protective effects on long-term potentiation (LTP) at 3 days after cranial irradiation [RT]/sham exposure [F(3,76) = 242.4, P < 0.001]. Memantine (at 0.5 mg/kg) or vehicle was given by oral gavage to adult rats at 25 hours and 1 hour before irradiation. This pretreatment protected the LTP (131.3% ± 1.0% [n = 6]) from RT-induced depression (Vehicle + RT, 109.1% ± 0.8% [n = 6] P < 0.001), although that protection was not complete (compare with Sham, 153.1% ± 1.6% [n = 6], P < 0.001). Memantine treatment alone did not affect LTP at the hippocampal–PFC pathway (Memantine + sham, 151.3% ± 1.6% [n = 6] vs Sham, P = 0.755). (B, C), LTP measured 3 days after memantine + RT (B) or 4 weeks after memantine + RT (C) in juvenile and adult rats. No difference was detected in LTP preservation between juveniles and adults either at day 3 (131.3% ± 1.0% [n = 6] vs 135.9% ± 0.9% [n = 6], P = 0.526) or at week 4 (141.4% ± 1.2% [n = 6] vs 141.2% ± 1.8% [n = 6], P = 1.000), or groups × time [F(3.76) = 2.087, P = 0.109]. (D). Memantine was given (0.5 mg/kg by oral gavage) to adult rats at 1 hour and 25 hours before or after cranial irradiation (RT), with distinct outcomes according to protocol [F(2, 57) = 184.1, P < 0.001]. Specifically, giving memantine after irradiation did not prevent RT-induced inhibition of LTP (104.2% ± 1.1% [n = 6], P = 0.873 vs RT control, which was significantly lower than LTP when memantine was given before irradiation [138.8% ± 1.8% [n = 6], P < 0.001]).
We also gave memantine at 1 hour and 25 hours after irradiation to adult rats and tested its effects on LTP. Unlike the positive effects noted when memantine was given before irradiation, no protective effects were seen when memantine was given after radiation. Giving memantine after irradiation did not affect the RT-induced decrease in LTP: LTP amplitudes were greatly depressed to 104.2% ± 1.1% of baseline and were significantly lower than in the memantine-pretreated rats (138.8% ± 1.8%, n = 6, P < 0.001), and equivalent to the baseline values [F(2,57) = 3.24, P > 0.05] (Fig. 3D).
Discussion
In the present study, we found that cranial irradiation inhibited hippocampal–PFC LTP in both juvenile and adult rats, but this inhibition persisted only in animals irradiated as juveniles. Memantine, a noncompetitive NMDAR antagonist that has shown promise for preventing radiation-induced cognitive deficits,21 blocked radiation-induced deficits in LTP in both juvenile and adult rats, but only if it was given before radiation. Our observations provide the first direct evidence of impaired connectivity between brain regions after radiation; specifically, they reveal that radiation has acute and early delayed effects on synaptic plasticity in a non-neurogenic brain region.
Previous explorations of radiation-induced cognitive impairment have focused largely on the loss of proliferative cells, damage to vascular endothelia, or microglial activation.22–25 However, patients receiving cranial RT can have significant cognitive impairments even without detectable anatomic abnormalities.26 A new line of study now focuses on radiation-induced alterations in neuronal function, particularly synaptic plasticity. Brain irradiation has been linked with changes in hippocampal synaptic strength,27 expression of the immediate-early gene activity-regulated cytoskeleton-associated protein (Arc),15 and expression of NMDAR subunits,28 but most of the evidence generated to date has been collected from hippocampal slice preparations.29,30
Beyond the hippocampus, the PFC participates in numerous cognitive functions, including decision making, learning, working memory, and goal-oriented behavior, all of which may be negatively affected by radiation.31 Functional imaging of patients with psychiatric disorders revealed marked aberrations in the structure, activation, and functional coupling in this hippocampal–PFC pathway,32 suggesting that hippocampal–PFC communications are critical in several aspects of cognition10,11 and are sensitive to insult.33 LTP is one of several phenomena underlying synaptic plasticity, which is the ability of synapses to change their strength. As memories are thought to be encoded by modification of synaptic strength, LTP is widely considered one of the major cellular mechanisms underlying learning and memory. Therefore, LTP at such an important neuronal pathway likely reflects a mechanism for increasing synaptic strength, leading to selective stabilization of neural connections.12
Radiation-induced brain injury is typically considered in terms of acute, early-delayed, and late phases. In patients acute and early-delayed injuries manifest within days to about 6 months after irradiation; many are reversible or can resolve spontaneously.13 Such injuries could arise from transient demyelination, subcortical microvascular damage, or changes in synaptic structure. Late injuries, by contrast, are typically thought to be irreversible and likely involve a reduction in the proliferative capacity of glial or vascular endothelial cells; however, late changes in synaptic structure and function may also contribute to persistent deficits in cognition. Our purpose in this study was to investigate synaptic plasticity in a rodent model at various intervals after irradiation, focusing primarily on acute and early-delayed, but also late, radiation effects. Our findings constitute direct evidence that cranial irradiation has acute effects on neuronal pathway plasticity that resulted in persistent late injuries in juvenile rats (Fig. 2 and Supplementary Figure S2). We identified a striking age dependence in sensitivity to radiation-induced inhibition of LTP in the hippocampal–PFC pathway: LTP was eliminated at day 3 after cranial radiation exposure in both adult and juvenile rats. Although LTP was largely restored during the following weeks in adult rats, LTP inhibition was still evident even 5 months after radiation exposure in juvenile rats (Supplementary Figure S2), consistent with significant late effects. Our results are in line with those previous findings showing that juveniles are more sensitive to cranial irradiation than adults.3,34,35 Tomé and colleagues summarized and systematically compared 48 recent studies of the behavioral effects of radiation-induced cognitive deficits in rodents, including 13 studies in which researchers treated 3- to 4-week-old or 8-week-old rodents with a single dose of 10 Gy, as we did here.16 A trend was evident that neonatal and juvenile subjects generally were more susceptible to cognitive deficits and more likely to have permanent dysfunction after radiation exposure, consistent with previous conclusions.13,36
NMDARs are important contributors in the regulation of neuronal plasticity.37 The phosphorylation-dependent recruitment of NMDARs to the postsynaptic membrane results in increased synaptic efficacy, as revealed in an increase in LTP.28,38 We previously reported irradiation led to removal of NMDARs from the cell surface and insertion of inhibitory gamma-aminobutyric acid receptors (GABARs) into the plasma membrane.5 One explanation for the variation in restoration capacity (and hence the response to neuronal plasticity to irradiation) between juvenile and adult rats may reflect the different responses of glutaminergic receptors to irradiation. A study recently reported irradiation of the juvenile brain results in long-term alterations in the response to high-frequency stimulation, provoking a shift from LTP to long-term depression in the dentate gyrus.39 Those authors attributed this long-term alteration in synaptic strength to the depletion of adult-born granule cells, which generally exhibit weaker and even silent glutamatergic synapses relative to mature granule cells.40,41
Other evidence supporting our explanation, that various responses of glutaminergic receptors may contribute to the age-dependent alteration in neuronal plasticity, comes from our experiments with the NMDA inhibitor memantine. Memantine is used to treat vascular and Alzheimer dementias. Given the clinical similarities between these conditions and radiation-induced cognitive impairment in adult patients, memantine has also been tested for its ability to prevent such deficits in adult patients being treated for brain cancer. In one study, memantine had protective effects against radiation-induced cognitive dysfunction in patients with brain metastases.21,42 Another pilot study, in which 14 patients underwent dynamic contrast-enhanced MRI to assess changes in vascular permeability after whole-brain RT, showed that memantine could protect the cerebral vasculature from radiation damage.43 However, memantine is rarely used in preclinical studies of radiation-induced brain injury, and clinically a recent survey indicated that few radiation oncologists in the United States use memantine for patients receiving whole-brain RT.44 We previously reported that memantine prevented or minimized radiation-induced changes in synaptic structures in hippocampus cultures7; we are currently investigating the behavioral consequences of this action. In the current study, we found that giving memantine before irradiation effectively reduced the RT-induced inhibition of LTP at the hippocampal–PFC connection in both adult and juvenile rats. This neuroprotective effect was observed as long as 4 weeks after the irradiation. However, giving memantine to rats that had already received cranial irradiation had no protective effect.
Another proposed explanation for age-dependent sensitivity to radiation is an increase in apoptotic cells with a corresponding decrease in proliferating cells in juvenile rats.45,46 Neuroinflammatory responses have also been implicated in radiation-induced brain injury and seem to differ in adult versus juvenile animals. Activated microglia, serving as brain macrophages, were increased at 2 months after a single 10-Gy dose of whole-brain irradiation in adult rodents,47,48 but in juvenile rodents, microglia increased only transiently at 6 hours after irradiation, followed by a decrease at 1 week and at subsequent time points.49,50 These findings suggest that juvenile rodents may be susceptible to different forms of radiation-induced neuroinflammation that damages the central nervous system. To the best of our knowledge, ours is the first study that indicates an age difference in terms of synaptic plasticity at hippocampal–PFC connections evoked by cranial irradiation.
In summary, we found that cranial irradiation inhibited hippocampal–PFC LTP in both juvenile and adult rats, but this inhibition persisted only in animals irradiated as juveniles. Our observations provide the first direct evidence of impaired connectivity between brain regions after radiation; specifically, they reveal that radiation has acute, early-delayed, and late effects on synaptic plasticity in a non-neurogenic brain region. Such effects could contribute to the cognitive dysfunction observed after RT in pediatric patients. Our finding that memantine had protective effects when administered before, but not after, irradiation implicates excitotoxicity in radiation-induced synaptic damage. It also has important clinical implications, in that patients may be expected to benefit only if the drug is given before RT. Finally, our discovery that memantine had a prolonged protective effect in juveniles is important, because to date memantine has demonstrated modest protective effects, but only in adult patients.19 However, if memantine is given to pediatric patients who are to receive cranial irradiation, its protective effects on cognitive function may be of even greater magnitude.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This work was supported by the Cancer Prevention and Research Institute of Texas grant RP140430; and National Cancer Institute, National Institutes of Health grants 1R01 CA208535-01 (to D.R.G.) and P30 CA016672 (to The University of Texas MD Anderson Cancer Center).
Conflict of interest statement. None declared.
Acknowledgments
We are grateful to Dr Yan Li for assistance with experiments and Christine Wogan for editorial review.
References
- 1. Robison LL, Armstrong GT, Boice JD, et al. . The Childhood Cancer Survivor Study: a National Cancer Institute–supported resource for outcome and intervention research. J Clin Oncol. 2009;27(14):2308–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brière ME, Scott JG, McNall-Knapp RY, Adams RL. Cognitive outcome in pediatric brain tumor survivors: delayed attention deficit at long-term follow-up. Pediatr Blood Cancer. 2008;50(2):337–340. [DOI] [PubMed] [Google Scholar]
- 3. Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5(7):399–408. [DOI] [PubMed] [Google Scholar]
- 4. Parihar VK, Limoli CL. Cranial irradiation compromises neuronal architecture in the hippocampus. Proc Natl Acad Sci U S A. 2013;110(31):12822–12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu PH, Coultrap S, Pinnix C, et al. . Radiation induces acute alterations in neuronal function. PLoS One. 2012;7(5):e37677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cooke SF, Bliss TV. Plasticity in the human central nervous system. Brain. 2006;129(Pt 7):1659–1673. [DOI] [PubMed] [Google Scholar]
- 7. Duman JG, Dinh J, Zhou W, et al. . Memantine prevents acute radiation-induced toxicities at hippocampal excitatory synapses. Neuro Oncol. 2017;doi: 10.1093/neuonc/nox203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang Y, Raine A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: a meta-analysis. Psychiatry Res. 2009;174(2):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thierry AM, Gioanni Y, Dégénétais E, Glowinski J. Hippocampo-prefrontal cortex pathway: anatomical and electrophysiological characteristics. Hippocampus. 2000;10(4):411–419. [DOI] [PubMed] [Google Scholar]
- 10. Damasio AR. Time-locked multiregional retroactivation: a systems-level proposal for the neural substrates of recall and recognition. Cognition. 1989;33(1–2):25–62. [DOI] [PubMed] [Google Scholar]
- 11. Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99(2):195–231. [DOI] [PubMed] [Google Scholar]
- 12. Laroche S, Jay TM, Thierry AM. Long-term potentiation in the prefrontal cortex following stimulation of the hippocampal CA1/subicular region. Neurosci Lett. 1990;114(2):184–190. [DOI] [PubMed] [Google Scholar]
- 13. Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD. Radiation-induced brain injury: a review. Front Oncol. 2012;2:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raber J, Rola R, LeFevour A, et al. . Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162(1):39–47. [DOI] [PubMed] [Google Scholar]
- 15. Rosi S, Andres-Mach M, Fishman KM, Levy W, Ferguson RA, Fike JR. Cranial irradiation alters the behaviorally induced immediate-early gene arc (activity-regulated cytoskeleton-associated protein). Cancer Res. 2008;68(23):9763–9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tomé WA, Gökhan Ş, Gulinello ME, et al. . Hippocampal-dependent neurocognitive impairment following cranial irradiation observed in pre-clinical models: current knowledge and possible future directions. Br J Radiol. 2016;89(1057):20150762. [DOI] [PubMed] [Google Scholar]
- 17. Paxinos G, Watson C.. The Rat Brain in Stereotaxic Coordinates. Cambridge, MA:Academic Press; 2007. [Google Scholar]
- 18. DiCarlo JJ, Lane JW, Hsiao SS, Johnson KO. Marking microelectrode penetrations with fluorescent dyes. J Neurosci Methods. 1996;64(1):75–81. [DOI] [PubMed] [Google Scholar]
- 19. Reisberg B, Doody R, Stöffler A, Schmitt F, Ferris S, Möbius HJ; Memantine Study Group Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med. 2003;348(14):1333–1341. [DOI] [PubMed] [Google Scholar]
- 20. Chen HS, Pellegrini JW, Aggarwal SK, et al. . Open-channel block of N-methyl-D-aspartate (NMDA) responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J Neurosci. 1992;12(11):4427–4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brown PD, Pugh S, Laack NN, et al. ; Radiation Therapy Oncology Group (RTOG) Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rola R, Raber J, Rizk A, et al. . Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188(2):316–330. [DOI] [PubMed] [Google Scholar]
- 23. Karlsson N, Kalm M, Nilsson MK, Mallard C, Björk-Eriksson T, Blomgren K. Learning and activity after irradiation of the young mouse brain analyzed in adulthood using unbiased monitoring in a home cage environment. Radiat Res. 2011;175(3):336–346. [DOI] [PubMed] [Google Scholar]
- 24. Roughton K, Kalm M, Blomgren K. Sex-dependent differences in behavior and hippocampal neurogenesis after irradiation to the young mouse brain. Eur J Neurosci. 2012;36(6):2763–2772. [DOI] [PubMed] [Google Scholar]
- 25. Tofilon PJ, Fike JR. The radioresponse of the central nervous system: a dynamic process. Radiat Res. 2000;153(4):357–370. [DOI] [PubMed] [Google Scholar]
- 26. Sundgren PC, Cao Y. Brain irradiation: effects on normal brain parenchyma and radiation injury. Neuroimaging Clin N Am. 2009;19(4):657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85(6):2423–2431. [DOI] [PubMed] [Google Scholar]
- 28. Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. LTP leads to rapid surface expression of NMDA but not AMPA receptors in adult rat CA1. Nat Neurosci. 2002;5(1):27–33. [DOI] [PubMed] [Google Scholar]
- 29. Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures?Neuron. 2010;65(1):7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O’Mara SM, Sanchez-Vives MV, Brotons-Mas JR, O’Hare E. Roles for the subiculum in spatial information processing, memory, motivation and the temporal control of behaviour. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(5):782–790. [DOI] [PubMed] [Google Scholar]
- 31. Kesner RP, Churchwell JC. An analysis of rat prefrontal cortex in mediating executive function. Neurobiol Learn Mem. 2011;96(3):417–431. [DOI] [PubMed] [Google Scholar]
- 32. Godsil BP, Kiss JP, Spedding M, Jay TM. The hippocampal-prefrontal pathway: the weak link in psychiatric disorders?Eur Neuropsychopharmacol. 2013;23(10):1165–1181. [DOI] [PubMed] [Google Scholar]
- 33. Paulus FM, Krach S, Bedenbender J, et al. . Partial support for ZNF804A genotype-dependent alterations in prefrontal connectivity. Hum Brain Mapp. 2013;34(2):304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 1994;12(3):627–642. [DOI] [PubMed] [Google Scholar]
- 35. Danoff BF, Cowchock FS, Marquette C, Mulgrew L, Kramer S. Assessment of the long-term effects of primary radiation therapy for brain tumors in children. Cancer. 1982;49(8):1580–1586. [DOI] [PubMed] [Google Scholar]
- 36. Lamproglou I, Chen QM, Boisserie G, et al. . Radiation-induced cognitive dysfunction: an experimental model in the old rat. Int J Radiat Oncol Biol Phys. 1995;31(1):65–70. [DOI] [PubMed] [Google Scholar]
- 37. MacDonald JF, Jackson MF, Beazely MA. Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors. Crit Rev Neurobiol. 2006;18(1–2):71–84. [DOI] [PubMed] [Google Scholar]
- 38. Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. Analysis of glutamate receptor surface expression in acute hippocampal slices. Sci STKE. 2002;2002(137):PL8. [DOI] [PubMed] [Google Scholar]
- 39. Zanni G, Zhou K, Riebe I, et al. . Irradiation of the juvenile brain provokes a shift from long-term potentiation to long-term depression. Dev Neurosci. 2015;37(3):263–272. [DOI] [PubMed] [Google Scholar]
- 40. Abrahamsson T, Gustafsson B, Hanse E. Synaptic fatigue at the naive perforant path-dentate granule cell synapse in the rat. J Physiol. 2005;569(Pt 3):737–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chancey JH, Adlaf EW, Sapp MC, Pugh PC, Wadiche JI, Overstreet-Wadiche LS. GABA depolarization is required for experience-dependent synapse unsilencing in adult-born neurons. J Neurosci. 2013;33(15):6614–6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Attia A, Page BR, Lesser GJ, Chan M. Treatment of radiation-induced cognitive decline. Curr Treat Options Oncol. 2014;15(4):539–550. [DOI] [PubMed] [Google Scholar]
- 43. Wong P, Leppert IR, Roberge D, et al. . A pilot study using dynamic contrast enhanced-MRI as a response biomarker of the radioprotective effect of memantine in patients receiving whole brain radiotherapy. Oncotarget. 2016;7(32):50986–50996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Slade AN, Stanic S. The impact of RTOG 0614 and RTOG 0933 trials in routine clinical practice: the US Survey of Utilization of Memantine and IMRT planning for hippocampus sparing in patients receiving whole brain radiotherapy for brain metastases. Contemp Clin Trials. 2016;47:74–77. [DOI] [PubMed] [Google Scholar]
- 45. Fukuda A, Fukuda H, Swanpalmer J, et al. . Age-dependent sensitivity of the developing brain to irradiation is correlated with the number and vulnerability of progenitor cells. J Neurochem. 2005;92(3):569–584. [DOI] [PubMed] [Google Scholar]
- 46. Schindler MK, Forbes ME, Robbins ME, Riddle DR. Aging-dependent changes in the radiation response of the adult rat brain. Int J Radiat Oncol Biol Phys. 2008;70(3):826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8(9):955–962. [DOI] [PubMed] [Google Scholar]
- 48. Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63(14):4021–4027. [PubMed] [Google Scholar]
- 49. Hellström NA, Björk-Eriksson T, Blomgren K, Kuhn HG. Differential recovery of neural stem cells in the subventricular zone and dentate gyrus after ionizing radiation. Stem Cells. 2009;27(3):634–641. [DOI] [PubMed] [Google Scholar]
- 50. Kalm M, Fukuda A, Fukuda H, et al. . Transient inflammation in neurogenic regions after irradiation of the developing brain. Radiat Res. 2009;171(1):66–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.