Abstract
Alveolar capillary dysplasia with misalignment of the pulmonary veins (ACD/MPV) is a rare and lethal disorder mainly involving the vascular development of the lungs. Since its first description, significant achievements in research have led to a better understanding of the underlying molecular mechanism of ACD/MPV and genetic studies have identified associations with genomic alterations in the locus of the transcription factor FOXF1. This in turn has increased the awareness among clinicians resulting in over 200 cases reported so far, including genotyping of patients in most recent reports. Collectively, this promoted a better stratification of the patient group, leading to new perspectives in research on the pathogenesis. Here, we provide an overview of the clinical aspects of ACD/MPV, including guidance for clinicians, and review the ongoing research into the complex molecular mechanism causing this severe lung disorder.
Keywords: pulmonary development, pulmonary vascular biology, neonatal lung disease, bronchopulmonary dysplasia, transcription factors
Clinical presentation
The clinical presentation of alveolar capillary dysplasia with misalignment of the pulmonary veins (ACD/MPV) is characterized by respiratory distress and cyanosis caused by severe pulmonary hypertension (PH) and insufficient oxygen uptake.1–3 Ninety-five percent of ACD/MPV patients are born at full term with normal birth weights and Apgar scores4,5 and most patients develop symptoms within the first 24 h of life.1,6,7 In up to 80% of the cases, associated malformations are found for which surgery is occasionally needed.3,8–14 These malformations predominantly affect the gastrointestinal tract, but also affect the cardiovascular and urogenital system.2–5,9,15,16 Irrespective of the co-morbidities, the mortality of ACD/MPV is almost 100%. The vast majority succumb to hypoxemic respiratory failure within days to weeks after presentation despite receiving supportive care including extracorporeal membrane oxygenation (ECMO).4,12,17–24 Atypical, milder cases of ACD/MPV patients that present after 24 h of life or survive beyond the neonatal period have sporadically been described and will be discussed in the histology section.
Incidence
Since ACD/MPV was first described in 1981, over 200 cases have been reported.3,25 In 2000, Al-Hathlol et al. reported two ACD/MPV patients among 226 infants autopsied over a 10-year period in the province of Manitoba, Canada.4 During these 10 years, the total number of deliveries was 170,000, with 340 infants dying within the neonatal period. It is unclear whether other ACD/MPV cases were among the remaining deceased neonates because an autopsy or biopsy is needed for confirmation. Based on this study, the incidence can be estimated at approximately 1/100,000. In The Netherlands, the incidence can be estimated at 1/200,000 if calculated from the total number of ACD/MPV patients (20) diagnosed in either the Radboudumc Amalia Children's Hospital Nijmegen or the Sophia Children’s Hospital Rotterdam since 1993 and the total number of living births during this period (4.3 million26). However, also in The Netherlands, it is likely that ACD/MPV is underdiagnosed and sometimes misdiagnosed as idiopathic PH due to the lack of autopsies.
Diagnosis
The current gold standard to unambiguously diagnose ACD/MPV is histological examination of the lungs. In the majority of studies, most cases were diagnosed postmortem by autopsy.4,5,27 In one study, 85% of cases were diagnosed premortem by open lung biopsy.1 In this study, the median time from admission to lung biopsy was 6.5 days. Of neonatal patients who underwent an open lung biopsy during ECMO therapy in the Sophia Children’s Hospital Rotterdam or the Radboudumc Amalia Children’s Hospital Nijmegen, 32% were diagnosed with ACD/MPV. In all these patients, treatment was withdrawn due to futility. In order to avoid unnecessary and expensive ECMO treatment, it is recommended to perform a lung biopsy before starting ECMO therapy or, if it has been initiated already, within the first week of ECMO.28 Considering the non-uniformly distribution of lung involvement observed in some patients, it is recommended to obtain more than one lung sample to avoid false-negatives.
Echocardiography is used to confirm PH and to exclude structural cardiovascular abnormalities.1,4,7,10,18,19,21,29 An X-ray of the lungs does not show features specific for ACD/MPV, but can show unspecific diffuse haziness or pneumothoraces, most likely caused by mechanical ventilation.4,7,10,18,19,29 A computed tomography (CT) scan may show bilateral widespread ground-glass infiltrates with septal line thickening,12,21 while a CT angiography does not result in the diagnosis either due to the limitations of imaging most peripheral capillaries of the lung. Photoacoustic imaging, however, is able to perform molecular imaging at a high resolution inside different tissues and therefore overcomes the limitations of a CT angiography. So far, photoacoustic imaging has only been used in biomedical research settings, but since studies in humans are very promising, it could provide an excellent tool to diagnose ACD/MPV in the future.30
During pregnancy, there are no clinical signs associated with ACD/MPV. However, if there is a high suspicion for ACD/MPV, for instance after an earlier familial case, fetal genetic testing for deletions and mutations in the FOXF1 locus could contribute to an early diagnosis.16,31 In addition, a decreased total fetal lung volume (FLV) measured by prenatal magnetic resonance imaging (MRI) and ultrasound together with a lowered MRI signal intensity can be used to detect fetal pulmonary hypoplasia associated with multiple diffuse lung developmental disorders among others, including ACD/MPV.32 An early detection of ACD/MPV allows adequate prenatal counseling regarding the dismal prognosis although the postnatal clinical course must be observed as it differs between patients. Histological examination will be necessary to confirm the diagnosis.
Histology
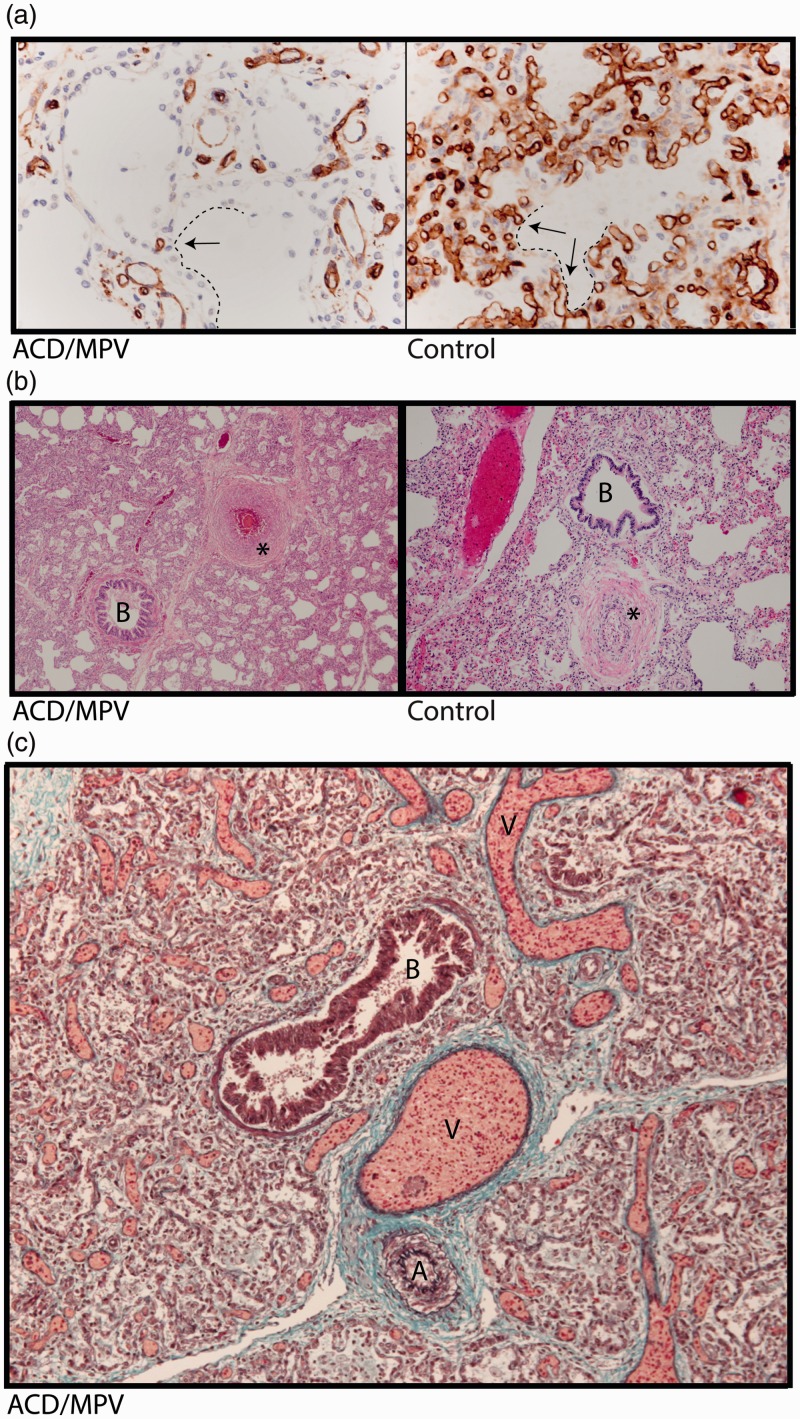
On gross examination, the lungs in cases of ACD/MPV appear bulky with up to a threefold increase in expected weight due to increased interstitial tissue.33–35 The most significant differences between ACD/MPV and healthy lungs are noted on a microscopic level. In normal lungs, the alveolar capillaries are in close contact with the alveolar epithelium lined by flattened type 1 pneumocytes to provide sufficient gas exchange. In cases of ACD/MPV, the lung tissue is characterized by diffuse thickening of interalveolar septa and marked reduction of pulmonary alveolar capillaries. In addition, these scanty alveolar capillaries are located away from the alveolar epithelium which is predominantly composed of hyperplastic cuboidal type 2 pneumocytes (Fig. 1a).3–5,33–35 Typically, in cases of ACD/MPV, the walls of small peripheral pulmonary arteries are thickened due to hypertrophic smooth muscle cells in the media (Fig. 1b). Further, the majority of ACD/MPV patients present with peripheral veins in the bronchovascular bundle adjacent to the pulmonary arteries outside the interlobular septa while normally they are located within the septa. This phenomenon is called “misalignment of the pulmonary veins” (Fig. 1c).
Fig. 1.
Lung histology of a 2-week-old infant with ACD/MPV. (a) Immunostaining for CD31 (brown color) highlighting a reduced number of alveolar capillary endothelial cells located away (arrows) from the inner side of the alveoli (dashed lines) in ACD/MPV compared to control lung. (b) Illustration of a hypertrophic arterial wall (*) in hematoxylin and eosin stained lung tissue from an ACD/MPV case compared to control. (c) Illustration of the misaligned pulmonary veins (V) adjacent to the bronchiole (B) and thickened pulmonary arteriole (A) in trichrome stained ACD/MPV lung tissue. Magnifications: (a) 200×; (b) 50×; (c) 100×.
It is postulated that the milder phenotype of atypical ACD/MPV patients, including late presenters and long-term survivors, is correlated with the extent of affected lung tissue.12,19–21,24,36 Although a few atypical patients with diffuse histological findings similar to the typical patients have been described,18,22 a very recent study showed a heterogeneous non-uniform distribution of histological findings in all atypical ACD/MPV patients.36 Nevertheless, correlating the histological features with phenotypical differences between patients remains difficult due to factors such as incomplete autopsy records, varying co-existing malformations, and variations in treatment, especially concerning treatment withdrawal.5 Either way, when a biopsy is obtained to confirm the diagnosis of ACD/MPV, consideration should be given to the possibility of uneven distribution of lesions. This is especially important if no other life-threatening co-malformations are found and the prognosis is based solely on the severity of lung lesions.
Treatment
Similar to persistent PH of the newborn (PPHN), the clinical approach to ACD/MPV consists of general supportive cardiorespiratory care and vasodilatory agents to reduce PH. In most cases, intubation and mechanical ventilation are immediately required at time of presentation due to the very low oxygen saturation levels. Rarely, only supplemental oxygen is temporarily enough to stabilize the patient before mechanical ventilation is needed.4,7,10,15,17,20,37 To reduce the pulmonary vascular resistance, inhaled nitric oxide and oral or intravenous vasodilatory agents such as milrinone, sildenafil, or epoprostenol are used. In the short term, this can improve the saturation levels, but literature shows that the dosage needs to be increased repeatedly and that eventually the severe hypoxemia will be fatal.4,5,7,10,18,20,22,23,37,38 Interestingly, one atypical ACD/MPV patient was still alive on oral vasodilators and supplemental oxygen at the age of 38 months when the case report was written.21
ECMO therapy can be used to stabilize infants in life-threatening conditions if the diagnosis is not clear. Yet, the vast majority of ACD/MPV patients cannot be weaned from ECMO, while ECMO itself is invasive, comes with risks of complications, and is expensive.1,4,7,8,19,23,28,29,37 Ideally, an open lung biopsy is performed before ECMO therapy has started or at least before a “second-run” ECMO therapy is considered.7,14,27,28 On the other hand, ECMO therapy can provide an excellent bridge to lung transplantation in ACD/MPV patients with a mild phenotype.12,36 To date, seven ACD/MPV patients receiving bilateral lung transplantation have been reported in the United States with a comparable five-year survival (56%) to infants transplanted for other indications at the St. Louis Children’s Hospital, Missouri.36,39 All of the transplanted ACD/MPV patients had an atypical milder phenotype than the classical ACD/MPV patients.12,36,40 Although the overall survival in this young age group is still disappointing, ECMO therapy followed by lung transplantation is the potential treatment of choice for selected ACD/MPV patients.36,41–43
Pathogenesis
Although the pathogenesis of ACD/MPV has not yet been fully defined, several hypotheses implicating various factors and events have been proposed. It is thought that ACD/MPV is caused by an early disturbance in embryonic lung development. Already at the end of the embryonic phase of lung development (five weeks of gestation), the two lung buds are surrounded by a vascular network that is connected to the systemic circulation. During the pseudoglandular phase (5–16 weeks of gestation), this network expands by angiogenesis when the two buds branch into the loose mesenchymal tissue. In the canalicular and saccular stage (16–26 and 26–38 weeks of gestation, respectively), expansion of the network continues, increasing the capillary density in the developing lung.44,45 The deficiency of capillaries in ACD/MPV lung tissue implies a disturbance already in the early embryonic and pseudoglandular phase. The presence of thickened septa and simplified acini with a reduced number of type 1 pneumocytes in ACD/MPV lungs indicate that also the saccular stage is affected, in which normally the alveolar saccules are formed with thin septa covered by type 1 pneumocytes.35,46,47
The cause of PH in ACD/MPV is still a topic of discussion. It was suggested that chronic hypoxemia in the newborn induces hypertrophic arterial changes which cause PH.35,38 However, this seems unlikely because the extent of arterial changes in ACD/MPV newborns would imply the presence of hypoxemia already during early fetal life when the oxygen is still provided by the placenta. Moreover, this theory is inconsistent with the poor outcome of ACD/MPV patients despite mechanical ventilation or ECMO therapy which diminishes the hypoxemia. Another, more acceptable, theory is that the PH results from a marked reduction in the alveolar capillary bed.18,33 However, abrupt deterioration in late presenters without any signs of PH during the time before deterioration questions this theory as well.4,18,23,33,34 Collectively, the progressive PH combined with structural histological changes in all ACD/MPV patients suggests a disturbance in normal pulmonary vascular development and is currently the subject of several studies in different laboratories.
The origin of the “misaligned pulmonary veins” found in peripheral ACD/MPV lung tissue, and whether they are indeed malpositioned, is not clear. Considering the presence of valves, it is more likely that the anomalous veins originate from bronchial veins that normally do not extend beyond the larger bronchopulmonary branches.33–35 This theory is supported by a study showing “misaligned pulmonary veins” originating from bronchial veins and act as shunt vessels between the bronchial and pulmonary veins.48
Role of FOXF1 in ACD/MPV
In 2009, Stanckiewizc and Shaw-Smith suggested an association between ACD/MPV and haploinsufficiency of the Forkhead Box F1 (FOXF1) gene.2 Since then, they have accumulated the largest ACD/MPV sample collection of 141 patients in which they identified 86 pathogenic variants containing copy number variations (CNVs), point mutations, and one complex rearrangement, all involving the FOXF1 gene or its regulatory region.49 This regulatory region was identified by defining the shortest region of overlapping genomic deletions in ACD/MPV patients.50,51 The remaining 55 cases were not genetically tested due to insufficient DNA quality.49 Additionally, a variety of heterozygous genomic variants in the FOXF1 locus of ACD/MPV patients have been reported by other research groups.8,16,20 Most of the variants are collected in the Leiden Open Variation Database (LOVD).52
FOXF1, first described by Pierrou et al. in 1994, is a member of the Forkhead box transcription factors (TFs) and plays a role during embryonic development, specifically in lung development.53 Studies in rodent embryos show that FOXF1 is already expressed at the primitive streak stage in the mesodermal lateral plate and continues to be expressed during development in mesenchymal lung tissue.54–58 Very little is known about FOXF1 expression during human lung development, but in newborns FOXF1 is expressed in mesenchymal stromal cells from tracheal aspirates.59 FOXF1 is regulated by Sonic hedgehog (SHH) signaling, one of the key regulators of embryonic development.60 Further research on the signaling pathways involving FOXF1 is ongoing. A recent study showed that the serotonin transporter (SERT) protein, important during pulmonary vascular remodeling and adaptation at birth, might be one of the downstream targets of FOXF1 as the expression of SERT protein was completely absent in ACD/MPV patients while expression levels in other disorders causing PH in the newborn were normal.61 Although rodent models enable us to study the function of FOXF1 during lung development, the phenotype of heterozygous FoxF1 mice does not completely correlate with ACD/MPV. Similar to ACD/MPV, heterozygous deletion of FoxF1 gives rise to multiple foregut and lung defects including lobular and alveolar underdevelopment, compact lung mesenchyme, and hemorrhagic lesions. However, the pulmonary branching defects dominate in these models while the main problem in ACD/MPV is the underdeveloped pulmonary vascularization.62 These differences illustrate the difficulties of studying the pathogenesis of ACD/MPV.
Imprinting of FOXF1
The question why a heterozygous genomic alteration in the FOXF1 gene or its regulatory region causes haploinsufficiency and results in ACD/MPV is still unanswered. The most suggested and studied explanation is parental imprinting where epigenetic marks determine if a particular gene is only expressed on the paternal or maternal allele.2,50,54,63 Assuming this hypothesis is true, all genomic alterations associated with ACD/MPV would be located on the same parental chromosome. Indeed, all but one of the deletions located in the regulatory region did arise on the maternal chromosome. However, mutations located in the FOXF1 transcription region are found on both paternal and maternal alleles. Still, this might implicate parental imprinting of the regulatory region instead of the transcription region itself.49 Unfortunately, detailed studies on imprinting of the FOXF1 locus in fetal, neonatal, and adult lung tissue have not been able to confirm this idea.50,64
Phenotypical differences of ACD/MPV
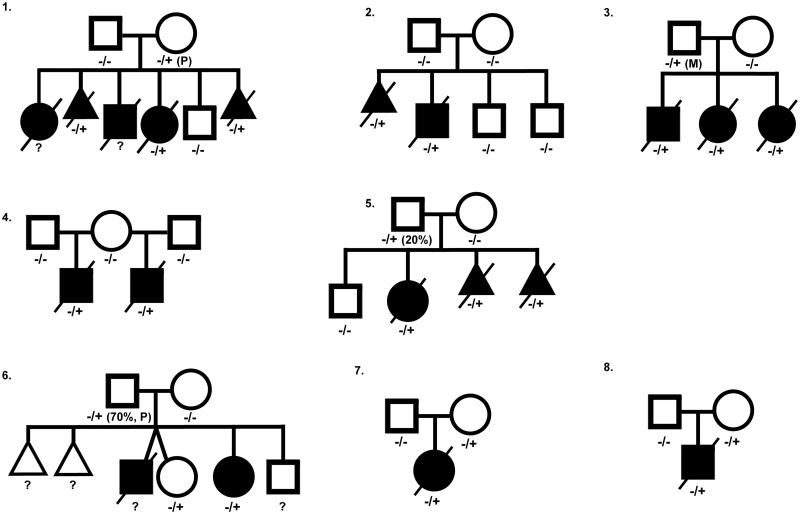
Although earlier studies suggested a high correlation between ACD/MPV and genomic alterations in the FOXF1 locus, familial cases show large phenotypical differences and, again, illustrate the complexity of the molecular mechanisms causing ACD/MPV. In total, eight ACD/MPV families with associated genomic variants in the FOXF1 transcription region have been reported so far (Fig. 2; Table 1).49,63,65,66 In six of these families, the pathogenic variant was also found in one of the parents including two fathers who were mosaic. None of those parents exhibited signs of ACD/MPV. The phenotypical variance is also illustrated by family 6 (Fig. 2) that was previously described by Reiter et al.66 In this family, two children were affected by ACD/MPV but only one of them showed classical features of ACD/MPV. The other child presented with an atypical form and survived. Interestingly, another sister carried the same mutation but was unaffected.49,66 The genotype–phenotype variation might be associated with altered FOXF1 expression levels, similar to FoxF1 heterozygote knock-out mice that show variation in the phenotype severity correlated to FoxF1 expression levels.58 Different expression levels can be the result of so-called “modifier genes” that modify the expression of the FOXF1 gene. In combination with mutations or deletions in the FOXF1 locus, alterations in these modifier genes might lead to ACD/MPV. This idea is supported by the finding that the survival of heterozygous FoxF1 knock-out mice depends on their genetic background.62
Fig. 2.
Pedigrees of published ACD/MPV families corresponding to Table 1, illustrating the complex inheritance pattern. Filled square or circle = affected male or female; open square or circle = unaffected male or female; crossed out = deceased; open triangle = spontaneous abortion; crossed-out filled triangle = terminated pregnancy. −/+, presence of heterozygous variant in the FOXF1 gene; −/−, no variant in FOXF1 gene present; P, variant located on paternal allele; M, variant located on maternal allele.
Table 1.
Overview of published ACD/MPV families with genomic alterations in the FOXF1 gene.
| Family number (Fig. 2) | Genomic alteration (all located in first FOXF1 exon) | Protein change | Inheritance | Parental inheritance | Reference |
|---|---|---|---|---|---|
| 1 | c.416G > T | Arg139Leu | Maternal | De novo paternal allele | Sen et al., 2013 |
| 2 | c.253T > A | Phe85Ile | Not found in parental DNA: germline mosaicism? | — | Sen et al., 2013 |
| 3 | Insertion 5 UTR side | Unknown | Paternal | De novo maternal allele | Szafranski et al., 2016 |
| 4 | c.849_850del | p.Ile285fs | Not found in parental DNA: maternal germline mosaicism? | — | Szafranski et al., 2016 |
| 5 | c90_96del | p.Ser31fs | Paternal; 20% mosaicism in blood nucleated cells | De novo, allele unknown | Szafranski et al., 2016 |
| 6 | c.231C > A | p.Phe77Leu | Paternal; 70% mosaicism in blood nucleated cells | De novo paternal allele | Reiter et al., 2016 |
| 7 | c.253T > C | p.Phe85Leu | Maternal | Unknown | Sen et al., 2013 |
| 8 | c.294C > A | p.His98Gln | Maternal | Unknown | Sen et al., 2013 |
Summary
ACD/MPV is a rare, almost uniformly lethal, developmental disorder of the lungs. Patients suffer from severe hypoxemia and PH that progresses over time. Although the awareness of ACD/MPV is growing among clinicians, it can be confused with idiopathic PH as the clinical picture is similar. This prolongs the time to confirm the diagnosis and contributes to unnecessary suffering of patients and a high cost of therapy. As soon as the response to medical treatment deviates from the expected, a histological examination should be performed. Moreover, an open lung biopsy is ideally performed before ECMO therapy or surgical interventions for co-occurring anomalies are initiated. For a very small selection of atypical ACD/MPV patients, a lung transplantation can be considered although survival rates are still disappointing. ACD/MPV is associated with haploinsufficiency of FOXF1, a TF regulated by SHH that plays a significant role during early lung development. Although phenotypical differences are present, prenatal or postnatal genetic testing could contribute to earlier detection and allows adequate consultation about the prognosis and the process of decision-making.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Eulmesekian P, Cutz E, Parvez B, et al. Alveolar capillary dysplasia: a six-year single center experience. J Perinat Med 2005; 33: 347–352. [DOI] [PubMed] [Google Scholar]
- 2.Stankiewicz P, Sen P, Bhatt SS, et al. Genomic and genic deletions of the FOX gene cluster on 16q24.1 and inactivating mutations of FOXF1 cause alveolar capillary dysplasia and other malformations. Am J Hum Genet 2009; 84: 780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishop NB, Stankiewicz P, Steinhorn RH. Alveolar capillary dysplasia. Am J Respir Crit Care Med 2011; 184: 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Hathlol K, Phillips S, Seshia MK, et al. Alveolar capillary dysplasia. Report of a case of prolonged life without extracorporeal membrane oxygenation (ECMO) and review of the literature. Early Hum Dev 2000; 57: 85–94. [DOI] [PubMed] [Google Scholar]
- 5.Sen P, Thakur N, Stockton DW, et al. Expanding the phenotype of alveolar capillary dysplasia (ACD). J Pediatr 2004; 145: 646–651. [DOI] [PubMed] [Google Scholar]
- 6.Al-Hathlol K, Idiong N, Hussain A, et al. A study of breathing pattern and ventilation in newborn infants and adult subjects. Acta Paediatr 2000; 89: 1420–1425. [DOI] [PubMed] [Google Scholar]
- 7.Michalsky MP, Arca MJ, Groenman F, et al. Alveolar capillary dysplasia: a logical approach to a fatal disease. J Pediatr Surg 2005; 40: 1100–1105. [DOI] [PubMed] [Google Scholar]
- 8.Ma Y, Jang MA, Yoo HS, et al. A novel de novo pathogenic variant in FOXF1 in a newborn with alveolar capillary dysplasia with misalignment of pulmonary veins. Yonsei Med J 2017; 58: 672–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arreo Del Val V, Avila-Alvarez A, Schteffer LR, et al. Alveolar capillary dysplasia with misalignment of the pulmonary veins associated with aortic coarctation and intestinal malrotation. J Perinatol 2014; 34: 795–797. [DOI] [PubMed] [Google Scholar]
- 10.Miranda J, Rocha G, Soares H, et al. Alveolar capillary dysplasia with misalignment of pulmonary veins (ACD/MPV): a case series. Case Rep Crit Care 2013; 2013: 327250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabah R, Poulik JM. Congenital alveolar capillary dysplasia with misalignment of pulmonary veins associated with hypoplastic left heart syndrome. Pediatr Dev Pathol 2001; 4: 167–174. [DOI] [PubMed] [Google Scholar]
- 12.Szafranski P, Dharmadhikari AV, Wambach JA, et al. Two deletions overlapping a distant FOXF1 enhancer unravel the role of lncRNA LINC01081 in etiology of alveolar capillary dysplasia with misalignment of pulmonary veins. Am J Med Genet A 2014; 164A: 2013–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garola RE, Thibeault DW. Alveolar capillary dysplasia, with and without misalignment of pulmonary veins: an association of congenital anomalies. Am J Perinatol 1998; 15: 103–107. [DOI] [PubMed] [Google Scholar]
- 14.Gerrits LC, De Mol AC, Bulten J, et al. Omphalocele and alveolar capillary dysplasia: a new association. Pediatr Crit Care Med 2010; 11: e36–37. [DOI] [PubMed] [Google Scholar]
- 15.Antao B, Samuel M, Kiely E, et al. Congenital alveolar capillary dysplasia and associated gastrointestinal anomalies. Fetal Pediatr Pathol 2006; 25: 137–145. [DOI] [PubMed] [Google Scholar]
- 16.Prothro SL, Plosa E, Markham M, et al. Prenatal diagnosis of alveolar capillary dysplasia with misalignment of pulmonary veins. J Pediatr 2016; 170: 317–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdallah HI, Karmazin N, Marks LA. Late presentation of misalignment of lung vessels with alveolar capillary dysplasia. Crit Care Med 1993; 21: 628–630. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed S, Ackerman V, Faught P, et al. Profound hypoxemia and pulmonary hypertension in a 7-month-old infant: late presentation of alveolar capillary dysplasia. Pediatr Crit Care Med 2008; 9: e43–46. [DOI] [PubMed] [Google Scholar]
- 19.Boggs S, Harris MC, Hoffman DJ, et al. Misalignment of pulmonary veins with alveolar capillary dysplasia: affected siblings and variable phenotypic expression. J Pediatr 1994; 124: 125–128. [DOI] [PubMed] [Google Scholar]
- 20.Goel D, Oei JL, Lui K, et al. Antenatal gastrointestinal anomalies in neonates subsequently found to have alveolar capillary dysplasia. Clin Case Rep 2017; 5: 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito Y, Akimoto T, Cho K, et al. A late presenter and long-term survivor of alveolar capillary dysplasia with misalignment of the pulmonary veins. Eur J Pediatr 2015; 174: 1123–1126. [DOI] [PubMed] [Google Scholar]
- 22.Kodama Y, Tao K, Ishida F, et al. Long survival of congenital alveolar capillary dysplasia patient with NO inhalation and epoprostenol: effect of sildenafil, beraprost and bosentan. Pediatr Int 2012; 54: 923–926. [DOI] [PubMed] [Google Scholar]
- 23.Shankar V, Haque A, Johnson J, Pietsch J. Late presentation of alveolar capillary dysplasia in an infant. Pediatr Crit Care Med 2006; 7: 177–179. [DOI] [PubMed] [Google Scholar]
- 24.Oldenburg J, Van Der Pal HJ, Schrevel LS, et al. Misalignment of lung vessels and alveolar capillary dysplasia. Histopathology 1995; 27: 192–194. [DOI] [PubMed] [Google Scholar]
- 25.Janney CG, Askin FB, Kuhn C., 3rd Congenital alveolar capillary dysplasia–an unusual cause of respiratory distress in the newborn. Am J Clin Pathol 1981; 76: 722–727. [DOI] [PubMed] [Google Scholar]
- 26.Central Bureau for Statistics. (2018, June 5). Statistics Netherlands: Birth; key figures. Retrieved from http://statline.cbs.nl/Statweb/publication/?DM=SLEN&PA=37422eng&D1=0,3-27,32-39,48-51,53-54&D2=0,5,10,15,20,25,30,35,43-66&LA=EN&HDR=T&STB=G1&VW=T.
- 27.Inwald D, Brown K, Gensini F, et al. Open lung biopsy in neonatal and paediatric patients referred for extracorporeal membrane oxygenation (ECMO). Thorax 2004; 59: 328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houmes RJ, Ten Kate CA, Wildschut ED, et al. Risk and relevance of open lung biopsy in pediatric ECMO patients: the Dutch experience. J Pediatr Surg 2017; 52: 405–409. [DOI] [PubMed] [Google Scholar]
- 29.Ng PC, Lewindon PJ, Siu YK, et al. Congenital misalignment of pulmonary vessels: an unusual syndrome associated with PPHN. Acta Paediatr 1995; 84: 349–353. [DOI] [PubMed] [Google Scholar]
- 30.Zackrisson S, van de Ven S, Gambhir SS. Light in and sound out: emerging translational strategies for photoacoustic imaging. Cancer Res 2014; 74: 979–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parris T, Nik AM, Kotecha S, et al. Inversion upstream of FOXF1 in a case of lethal alveolar capillary dysplasia with misalignment of pulmonary veins. Am J Med Genet A 2013; 161A: 764–770. [DOI] [PubMed] [Google Scholar]
- 32.Zirpoli S, Munari AM, Rustico M, et al. Fetal-MRI prenatal diagnosis of severe bilateral lung hypoplasia: alveolar capillary dysplasia case report. J Prenat Med 2016; 10: 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cullinane C, Cox PN, Silver MM. Persistent pulmonary hypertension of the newborn due to alveolar capillary dysplasia. Pediatr Pathol 1992; 12: 499–514. [DOI] [PubMed] [Google Scholar]
- 34.Sirkin W, O’Hare BP, Cox PN, et al. Alveolar capillary dysplasia: lung biopsy diagnosis, nitric oxide responsiveness, and bronchial generation count. Pediatr Pathol Lab Med 1997; 17: 125–132. [PubMed] [Google Scholar]
- 35.Haraida S, Lochbuhler H, Heger A, et al. Congenital alveolar capillary dysplasia: rare cause of persistent pulmonary hypertension. Pediatr Pathol Lab Med 1997; 17: 959–975. [PubMed] [Google Scholar]
- 36.Towe CT, White FV, Grady RM, et al. Infants with atypical presentations of alveolar capillary dysplasia with misalignment of the pulmonary veins who underwent bilateral lung transplantation. J Pediatr 2018; 194: 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castilla-Fernandez Y, Copons-Fernandez C, Jordan-Lucas R, et al. Alveolar capillary dysplasia with misalignment of pulmonary [corrected] veins: concordance between pathological and molecular diagnosis. J Perinatol 2013; 33: 401–403. [DOI] [PubMed] [Google Scholar]
- 38.Steinhorn RH, Cox PN, Fineman JR, et al. Inhaled nitric oxide enhances oxygenation but not survival in infants with alveolar capillary dysplasia. J Pediatr 1997; 130: 417–422. [DOI] [PubMed] [Google Scholar]
- 39.Eldridge WB, Zhang Q, Faro A, et al. Outcomes of lung transplantation for infants and children with genetic disorders of surfactant metabolism. J Pediatr 2017; 184: 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boston US, Fehr J, Gazit AZ, et al. Paracorporeal lung assist device: an innovative surgical strategy for bridging to lung transplant in an infant with severe pulmonary hypertension caused by alveolar capillary dysplasia. J Thorac Cardiovasc Surg 2013; 146: e42–43. [DOI] [PubMed] [Google Scholar]
- 41.Khan MS, Heinle JS, Samayoa AX, et al. Is lung transplantation survival better in infants? Analysis of over 80 infants. J Heart Lung Transplant 2013; 32: 44–49. [DOI] [PubMed] [Google Scholar]
- 42.Benden C, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Sixteenth Official Pediatric Lung and Heart-Lung Transplantation Report–2013; focus theme: age. J Heart Lung Transplant 2013; 32: 989–997. [DOI] [PubMed] [Google Scholar]
- 43.Hayes D, Jr., Naguib A, Kirkby S, et al. Comprehensive evaluation of lung allograft function in infants after lung and heart-lung transplantation. J Heart Lung Transplant 2014; 33: 507–513. [DOI] [PubMed] [Google Scholar]
- 44.Parera MC, van Dooren M, van Kempen M, et al. Distal angiogenesis: a new concept for lung vascular morphogenesis. Am J Physiol Lung Cell Mol Physiol 2005; 288: L141–149. [DOI] [PubMed] [Google Scholar]
- 45.Schachtner SK, Wang Y, Scott Baldwin H. Qualitative and quantitative analysis of embryonic pulmonary vessel formation. Am J Respir Cell Mol Biol 2000; 22: 157–165. [DOI] [PubMed] [Google Scholar]
- 46.Ameis D, Khoshgoo N, Keijzer R. Abnormal lung development in congenital diaphragmatic hernia. Semin Pediatr Surg 2017; 26: 123–128. [DOI] [PubMed] [Google Scholar]
- 47.Schittny JC. Development of the lung. Cell Tissue Res 2017; 367: 427–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galambos C, Sims-Lucas S, Ali N, et al. Intrapulmonary vascular shunt pathways in alveolar capillary dysplasia with misalignment of pulmonary veins. Thorax 2015; 70: 84–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szafranski P, Gambin T, Dharmadhikari AV, et al. Pathogenetics of alveolar capillary dysplasia with misalignment of pulmonary veins. Hum Genet 2016; 135: 569–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szafranski P, Dharmadhikari AV, Brosens E, et al. Small noncoding differentially methylated copy-number variants, including lncRNA genes, cause a lethal lung developmental disorder. Genome Res 2013; 23: 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szafranski P, Herrera C, Proe LA, et al. Narrowing the FOXF1 distant enhancer region on 16q24.1 critical for ACDMPV. Clin Epigenetics 2016; 8: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fokkema IF, Taschner PE, Schaafsma GC, et al. LOVD v.2.0: the next generation in gene variant databases. Hum Mutat 2011; 32: 557–563. [DOI] [PubMed] [Google Scholar]
- 53.Pierrou S, Hellqvist M, Samuelsson L, et al. Cloning and characterization of seven human forkhead proteins: binding site specificity and DNA bending. EMBO J 1994; 13: 5002–5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dharmadhikari AV, Szafranski P, Kalinichenko VV, et al. Genomic and epigenetic complexity of the FOXF1 locus in 16q24.1: implications for development and disease. Curr Genomics 2015; 16: 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahlapuu M, Pelto-Huikko M, Aitola M, et al. FREAC-1 contains a cell-type-specific transcriptional activation domain and is expressed in epithelial-mesenchymal interfaces. Dev Biol 1998; 202: 183–195. [DOI] [PubMed] [Google Scholar]
- 56.Peterson RS, Lim L, Ye H, et al. The winged helix transcriptional activator HFH-8 is expressed in the mesoderm of the primitive streak stage of mouse embryos and its cellular derivatives. Mech Dev 1997; 69: 53–69. [DOI] [PubMed] [Google Scholar]
- 57.Mahlapuu M, Ormestad M, Enerback S, et al. The forkhead transcription factor Foxf1 is required for differentiation of extra-embryonic and lateral plate mesoderm. Development 2001; 128: 155–166. [DOI] [PubMed] [Google Scholar]
- 58.Kalinichenko VV, Lim L, Stolz DB, et al. Defects in pulmonary vasculature and perinatal lung hemorrhage in mice heterozygous null for the Forkhead Box f1 transcription factor. Dev Biol 2001; 235: 489–506. [DOI] [PubMed] [Google Scholar]
- 59.Bozyk PD, Popova AP, Bentley JK, et al. Mesenchymal stromal cells from neonatal tracheal aspirates demonstrate a pattern of lung-specific gene expression. Stem Cells Dev 2011; 20: 1995–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ormestad M, Astorga J, Landgren H, et al. Foxf1 and Foxf2 control murine gut development by limiting mesenchymal Wnt signaling and promoting extracellular matrix production. Development 2006; 133: 833–843. [DOI] [PubMed] [Google Scholar]
- 61.Castro EC, Sen P, Parks WT, et al. The role of serotonin transporter in human lung development and in neonatal lung disorders. Can Respir J 2017; 2017: 9064046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mahlapuu M, Enerback S, Carlsson P. Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations. Development 2001; 128: 2397–2406. [DOI] [PubMed] [Google Scholar]
- 63.Sen P, Gerychova R, Janku P, et al. A familial case of alveolar capillary dysplasia with misalignment of pulmonary veins supports paternal imprinting of FOXF1 in human. Eur J Hum Genet 2013; 21: 474–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alsina Casanova M, Monteagudo-Sanchez A, Rodiguez Guerineau L, et al. Maternal mutations of FOXF1 cause alveolar capillary dysplasia despite not being imprinted. Hum Mutat 2017; 38: 615–620. [DOI] [PubMed] [Google Scholar]
- 65.Sen P, Yang Y, Navarro C, et al. Novel FOXF1 mutations in sporadic and familial cases of alveolar capillary dysplasia with misaligned pulmonary veins imply a role for its DNA binding domain. Hum Mutat 2013; 34: 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reiter J, Szafranski P, Breuer O, et al. Variable phenotypic presentation of a novel FOXF1 missense mutation in a single family. Pediatr Pulmonol 2016; 51: 921–927. [DOI] [PubMed] [Google Scholar]