Short abstract
Previously, we reported that orally administered Emu Oil (EO) increases mucosal thickness in the small intestine and colon in rodent models of chemotherapy-induced mucositis and colitis. However, it remains unclear whether mucosal thickening (crypt and villus lengthening) represents a process of normal or aberrant growth. We sought to determine if villus height (VH) and crypt depth (CD) measurements returned to normal in EO-treated rats following withdrawal of EO therapy. Dark agouti rats (n = 8/group) were gavaged daily for 10 days with water, olive oil (OO), or EO (0.5 mL or 1 mL). Groups of rats were euthanized on days 10 and 17. Intestinal weights, lengths, VH, and CD were quantified. P < 0.05 was considered significant. On day 10, jejuno–ileum weight was increased by OO (26%) and EO (0.5 mL: 15%; 1 mL: 29%) compared to water controls (P < 0.01), which was normalized by day 17. On days 10 and 17, jejuno-ileum length was greater in OO- (12%) and EO-treated rats (0.5 mL: 8%; 1 mL: 12%; P < 0.05), relative to water controls. On day 10, OO and EO increased ileal VH (OO: 32%; 0.5 EO: 22%; EO: 35%; P < 0.01) and CD (OO: 17%; 0.5 EO: 13%; EO: 22%) compared to water controls. Importantly, however, after withdrawal of all oils, VH and CD measurements returned to normal control values. Moreover, the VH:CD ratio (potential indicator of dysplasia) remained unchanged in all experimental groups on days 10 and 17. The restoration of normal intestinal architecture following cessation of Emu Oil therapy supports its safety for application in intestinal disorders.
Impact statement
Uncontrolled inflammation and intestinal proliferation can predispose to the development of colorectal cancer. In previous pre-clinical studies, we demonstrated that oral administration of Emu Oil promotes intestinal repair via stimulation of the mucosa in response to tissue injury and inflammation. Therefore, it was important to determine if Emu Oil administration did not promote the precocious development of colorectal cancer. The current study revealed that Emu Oil returned indicators of intestinal proliferation back to normal values after a period of seven days. These data strongly support the safety of Emu Oil for further studies in the context of bowel inflammation.
Keywords: Emu Oil, intestinal mucosa, dark agouti rats
Introduction
The emu (Dromaius novaehollandiae), belonging to the ratite family, is a large flightless bird endemic to Australia. The emu is farmed worldwide for its meat, leather, feathers, eggs and more recently, its oil. The oil extraction process entails rendering the macerated tissue (sourced from subcutaneous and retroperitoneal emu fat) then filtering the liquefied fat to obtain purified oil.1
Fatty acids predominate in Emu Oil (accounting for approximately 98%)1,2,3 with variable levels of skin permeation enhancing factors and natural antioxidants.2,4 Mashtoub et al.4 and Bennett et al.5 demonstrated that Emu Oil possesses both antioxidant properties in vitro and a protective role against oxidative damage in a biological membrane model system.
Indigenous Australians, and subsequently early European settlers, first used Emu Oil for healing of wounds and burns, alleviation of pain, and treatment of inflamed joints. At present, Emu Oil is available as a concentrated oil (both liquid and encapsulated) and in various skin, hair, and body care products. Indeed, a wealth of anecdotal evidence suggests applications for Emu Oil in relief from inflammatory arthritic pain, skin conditions, and general wellbeing. More recently, well-controlled experimental studies have highlighted the anti-inflammatory and reparative properties of orally administered Emu Oil in intestinal disorders characterized by inflammation, ulceration, malabsorption, and mucosal damage.6–10
In a rat model of dextran sulfate sodium-induced ulcerative colitis, a form of inflammatory bowel disease, orally administered Emu Oil reduced colonic tissue damage and facilitated mucosal repair.6 Improvements were indicated by reduced colonic damage and enhanced crypt elongation.6 Nonetheless, Emu Oil did not impact colonic mucosal architecture in normal animals.6 Lindsay et al.7 reported therapeutic efficacy of Emu Oil in the early phase of recovery from mucositis. Moreover, Mashtoub et al.8 investigated the effects of Emu Oil, in comparison with olive oil, during the later stages of recovery from 5-fluorouracil (5-FU)-induced mucositis. Olive oil was selected as a control, with oleic acid levels similar to Emu Oil, in order to determine any non-specific oil-related effects. Emu Oil reduced small intestinal myeloperoxidase activity, indicative of reduced inflammation, and maintained small intestinal villus height and crypt depth during the phase of maximal damage.8 Subsequently, Emu Oil enhanced compensatory mucosal thickening, suggesting an acceleration of the repair process.8 These results were not consistently reflected in the olive oil-treated animals, highlighting the specific efficacy of Emu Oil in this model. In a similar study of 5-FU-induced mucositis, Emu Oil in combination with the green-lipped mussel extract, Lyprinol, decreased myeloperoxidase activity, attenuated mid-small intestinal crypt depth blunting, and reduced histologically assessed intestinal damage severity.9 Furthermore, the anti-inflammatory properties of Emu Oil have been reaffirmed in a rat model of non-steroidal anti-inflammatory drug (NSAID)-induced enteropathy.10
Villus and crypt lengthening (features of intestinal growth) may represent a mode of Emu Oil efficacy in models of colitis6 and chemotherapy-induced mucositis.8,9 Moreover, Mashtoub et al., in normal rats, demonstrated that 8–11 days of orally administered Emu Oil and olive oil resulted in thickening of the small intestinal mucosa. Although mucosal thickening could be considered a desirable component of bowel repair, uncontrolled crypt lengthening could potentially represent a malignant neoplastic process. To date, the safety of orally-administered Emu Oil has not yet been fully elucidated.
It was hypothesized that the stimulus for villus and crypt lengthening would be eliminated following cessation of Emu Oil treatment in normal rats, restoring mucosal homeostasis. Therefore, the current study sought to determine if villus height and crypt depth measurements would return to normal values in rats following withdrawal of Emu Oil administration; ultimately supporting its safety for application in intestinal disorders.
Methods
Experimental procedures
Animal studies
Female dark agouti rats (120–140 g each) were acclimatized for two days prior to experimentation in individual metabolism cages (Tecniplast Inc., Exton, PA, USA) and were provided ad libitum access to food (standard 18% casein-based diet11) and water. During the experimental period, rats remained in these individual housing conditions at room temperature with a light:dark cycle of 12 h. The animal trial was conducted in compliance with the Australian Code of Practice for the Care and Use of Animals and was approved by the Animal Ethics Committees of the Women’s and Children’s Hospital and The University of Adelaide.
A total of 64 rats were assigned to four groups at random (n = 8/group/time point); Group 1: 1 mL water, Group 2: 1 mL olive oil, Group 3: 0.5 mL Emu Oil, Group 4: 1 mL Emu Oil. Water, olive oil, or Emu Oil was orally administered (via gavage) once per day from days 0 to 10 (treatment period; total of 10 daily gavages). Gavages ceased on day 10, whereby half the rats (32 animals) were then euthanized (n = 8/group at time point 1 (day 10)). The remaining 32 rats did not receive any further gavages (recovery period; total of 8 non-gavage days including day 10) and were euthanized on day 17 (n = 8/group at time point 2 (day 17)).
The volumes of water and oil utilized in the current study were directly derived from our previous studies,6–8,10 deemed to be optimal in these studies from both scientific and ethical perspectives; 1 mL is recognized as the maximum volume capable of being administered safely to adult rats by the oro-gastric route.
Oil preparation
Emu Oil was sourced from Emus farmed in North-E astern South Australia, prepared by Emu Tracks Pty Ltd (Marleston, SA, Australia). Emu adipose tissue was rendered and filtered (using methodologies specified by Technology Investment Corporation) with appropriate considerations for delivery of product consistency and quality assurance. Fatty acid analysis of Emu Oil and olive oil (Conga Foods, Spain) was carried out using gas chromatography, as described previously12 (Table 1). Both Emu Oil and olive oil were individually stored in 50 mL opaque containers at 4°C.
Table 1.
Major fatty acid composition (%) of Emu Oil and olive oil used in the current study.
| Fatty acid | Common name | Emu | Olive |
|---|---|---|---|
| Major FA composition of emu and olive oils | |||
| 16:0 | Palmitic acid | 24 | 10.4 |
| 16:1n-7 | Palmitoleic acid | 4.3 | 0.7 |
| 18:0 | Stearic acid | 8.5 | 3.1 |
| 18:1n-9 | Oleic acid | 49.1 | 73.9 |
| 18:2n-6 | Linoleic acid | 9.5 | 8.4 |
| 18:3n-3 | α-Linolenic acid | 1.1 | 0.7 |
| Saturated | 32.5 | 13.5 | |
| MUFA | 53.4 | 74.6 | |
| PUFA | 10.6 | 9.1 | |
| UFA:SFA ratio | 2 | 6.2 | |
MUFA: monounsaturated fatty acid; PUFA: polyunsaturated fatty acid; UFA: unsaturated fatty acid; SFA: saturated fatty acid.
Daily metabolic data and tissue collection
Body weight was recorded daily to determine 1. Daily body weight change in rats expressed as a % of starting body weight and 2. Overall difference in body weight, calculated during the treatment period (day 10 minus day 0) and recovery period (day 17 minus day 10). Additionally, food and water intake, and fecal and urine output were monitored and measured daily.
On days 10 or 17, rats were sacrificed by CO2 asphyxiation and cervical dislocation. The gastrointestinal tract was removed and measured, then emptied of contents and weighed. Segments of the small intestinal tract (jejunum and ileum; 2 cm) were placed in 10% buffered formalin for histological analysis. Visceral organs were weighed and discarded.
Histological analysis
Jejunal and ileal samples were processed for quantitative analysis, including villus height and crypt depth measurements, as described in Mashtoub et al.8
Statistical analyses
Using SigmaPlot version 12.3 (San Jose, CA, USA), sample size calculations indicated that a total of eight mice per group would enable the detection of a 40% difference in villus height between groups with 95% power (alpha = 0.05).
SPSS version 16.0 for Windows (IL, USA) was used to compare groups. All data sets were normally distributed (as per Shapiro–Wilk statistic) and thus analyzed using a one-way analysis of variance (ANOVA) and Tukey’s post hoc test and expressed as mean with their standard errors. P < 0.05 was considered significant.
Results
Metabolic data
Neither Emu Oil nor olive oil significantly impacted daily body weight change or overall difference in bodyweight during either the treatment or recovery periods, compared with water-treated animals (P > 0.05; Figures 1 and 2). Olive oil, unlike Emu Oil, increased total water intake by 23% during days 0–10, compared to water gavage controls (P < 0.05; Figure 3(a)), although urine output remained unchanged (P = 0.17; Figure 3(b)). Food intake significantly decreased in Emu Oil (low dose: 8%; high dose: 16%) and olive oil-treated animals (16%) compared to water gavage controls (P < 0.01; Figure 3(c)). However, fecal weight was not significantly affected by either oil (P > 0.05; Figure 3(d)).
Figure 1.
Daily body weight change of rats gavaged daily with 1 mL water, 1 mL olive oil, 0.5 mL Emu Oil, or 1 mL Emu Oil from days 0 to 10 (n = 16/group) and 10 to 17 (n = 8/group). Data are expressed as mean (body weight change as a % of starting body weight) ± SEM. (A color version of this figure is available in the online journal.)
Figure 2.
Overall difference in body weight during treatment period (days 0–10; n = 16/group) and recovery period (days 10–17; n = 8/group). Rats were gavaged daily with 1 mL Water, 1 mL olive oil, 0.5 mL Emu Oil, or 1 mL Emu Oil from days 0 to 10. Data are expressed as mean (g; body weight difference) ± SEM. (A color version of this figure is available in the online journal.)
Figure 3.
Metabolic parameters; (a) water intake (b) urine output (c) food intake and (d) fecal output of rats gavaged daily with 1 mL water, 1 mL olive oil, 0.5 mL Emu Oil, or 1 mL Emu Oil from days 0 to 10 (treatment period; n = 16/group). Data are expressed as mean (g or ml) ± SEM. ***P < 0.001, **P < 0.01, *P < 0.05 compared to 1 mL water; ^P < 0.05 compared to 1 mL olive oil. (A color version of this figure is available in the online journal.)
Organ data
On day 10, both doses of Emu Oil significantly increased jejuno-ileum weight; although this effect was more pronounced in 1 mL Emu Oil (high dose)-treated animals (29%) compared with 0.5 mL Emu Oil treatment (15%; P < 0.01). Furthermore, olive oil-treated rats exhibited significantly increased jejuno-ileum weight (26% increase; P < 0.001; Figure 4). Importantly, however, jejuno-ileum weight in all oil-treated animals returned to normal (water gavage) levels by day 17 (Figure 4). Similarly, jejuno-ileum length was increased by Emu Oil (low dose: 8%; high dose: 12%) and olive oil (12%) on day 10 (P < 0.01; Figure 5). Although not stimulated any further over the ensuing seven days, jejuno-ileum length remained greater than water gavage controls following withdrawal of the highest dose of Emu Oil and olive oil (P < 0.05; Figure 5). Nonetheless, when expressed as a % of body weight, average jejuno-ileal length did not significantly vary between groups on days 10 or 17 (data not shown). Neither oil significantly impacted duodenal or colonic weights or lengths (P > 0.05; Figures 4 and 5) nor did either oil significantly affect visceral organ weights on either days 10 or 17 (P > 0.05; Table 2).
Figure 4.
Gastrointestinal organ weights on day 10 and day 17 (n = 8/group). Rats were gavaged daily with 1 mL water, 1 mL olive oil, 0.5 mL Emu Oil, or 1 mL Emu Oil from days 0 to 10. Data are expressed as mean (g) ± SEM. ***P < 0.001, **P < 0.01 compared to 1 mL Water. JI: jejuno-ileum. (A color version of this figure is available in the online journal.)
Figure 5.
Gastrointestinal organ lengths on day 10 and day 17 (n = 8/group). Rats were gavaged daily with 1 mL water, 1 mL olive oil, 0.5 mL Emu Oil, or 1 mL Emu Oil from days 0 to 10. Data are expressed as mean (cm) ± SEM. ***P < 0.001, **P < 0.01, *P < 0.05 compared to 1 mL water. JI: jejuno-ileum. (A color version of this figure is available in the online journal.)
Table 2.
Visceral organ weights of rats on days 10 and 17.
|
Thymus |
Heart |
Lung |
Liver |
Spleen |
Kidneys |
Stomach |
Caecum |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | 10 | 17 | 10 | 17 | 10 | 17 | 10 | 17 | 10 | 17 | 10 | 17 | 10 | 17 | 10 | 17 |
| 1 mL water | 26 ± 2 | 27 ± 1 | 52 ± 1 | 54 ± 2 | 90 ± 3 | 90 ± 3 | 487 ± 11 | 521 ± 30 | 25 ± 1 | 28 ± 2 | 106 ± 30 | 110 ± 40 | 70 ± 2 | 82 ± 6 | 41 ± 3 | 44 ± 4 |
| 1 mL olive oil | 26 ± 1 | 26 ± 2 | 51 ± 2 | 55 ± 2 | 94 ± 3 | 104 ± 9 | 478 ± 20 | 501 ± 11 | 25 ± 1 | 27 ± 1 | 102 ± 30 | 112 ± 40 | 71 ± 4 | 74 ± 1 | 49 ± 3 | 45 ± 3 |
| 0.5 mL Emu Oil | 26 ± 2 | 27 ± 1 | 52 ± 1 | 50 ± 1 | 89 ± 7 | 86 ± 5 | 486 ± 14 | 505 ± 11 | 25 ± 1 | 26 ± 1 | 103 ± 30 | 110 ± 20 | 69 ± 2 | 76 ± 1 | 46 ± 2 | 39 ± 3 |
| 1 mL Emu Oil | 26 ± 2 | 28 ± 2 | 55 ± 3 | 54 ± 1 | 99 ± 6 | 96 ± 5 | 513 ± 11 | 521 ± 15 | 30 ± 1 | 29 ± 1 | 110 ± 40 | 111 ± 30 | 70 ± 1 | 76 ± 2 | 49 ± 2 | 44 ± 3 |
Note: Rats were gavaged daily with 1 mL water, 1 mL olive oil, 0.5 mL Emu Oil, or 1 mL Emu Oil from days 0 to 10. Data are expressed as mean (g) ± SEM. All values × 10−2.
Mucosal measurements
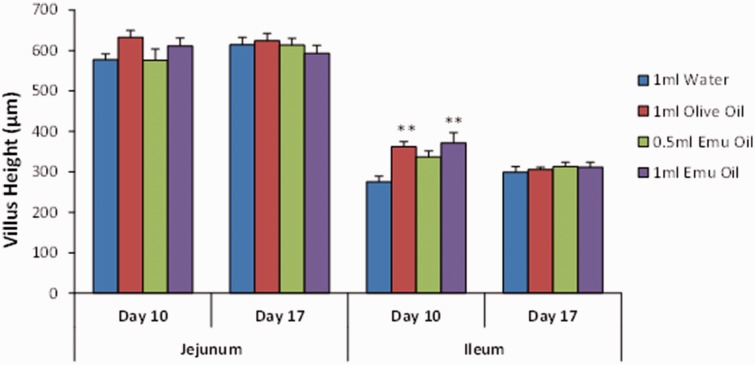
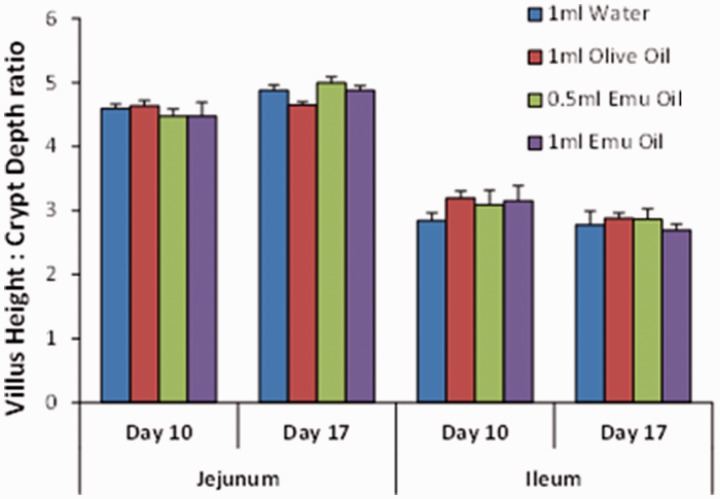
Administration of Emu Oil and olive oil resulted in significantly increased ileal villus height (Emu Oil low dose: 22%; high dose: 35%; olive oil: 32%) and crypt depth (Emu Oil low dose: 13%; high dose: 22%; olive oil: 17%) on day 10 – an effect not observed in the jejunum (P > 0.05; Figures 6 and 7). Importantly, however, on day 17 (following oil withdrawal), ileal villus height and crypt depth measurements returned to normal control levels (Figures 6 and 7). Moreover, jejunal and ileal villus height:crypt depth ratio, known to be altered in conditions such as neoplasia,13 remained unchanged in Emu Oil and olive oil-treated animals on days 10 and 17, compared to water gavage controls (P > 0.05; Figure 8).
Figure 6.
Villus height in the jejunum and ileum on days 10 and 17 (n = 8/group). Rats were gavaged daily with 1 mL water, 1 mL olive oil, 0.5 mL Emu Oil, or 1 mL Emu Oil from days 0 to 10. Data are expressed as mean (µm) ± SEM. (A color version of this figure is available in the online journal.)
Figure 7.
Crypt depth in the jejunum and ileum on days 10 and 17 (n = 8/group). Rats were gavaged daily with 1 mL water, 1 mL olive oil, 0.5 mL Emu Oil, or 1 mL Emu Oil from days 0 to 10. Data are expressed as mean (µm) ± SEM. ***P < 0.001, **P < 0.01 compared to 1 mL water. (A color version of this figure is available in the online journal.)
Figure 8.
Villus height to crypt depth ratio in the jejunum and ileum of rats gavaged daily with 1 mL water, 1 mL olive oil, 0.5 mL Emu Oil, or 1 mL Emu Oil from days 0 to 10 (n = 8/group). Data are expressed as mean (ratio) ± SEM. (A color version of this figure is available in the online journal.)
Discussion
In the current study, 10 days oral administration of Emu Oil or olive oil resulted in ileal mucosal thickening in healthy rats; however, importantly, there was no continued stimulation of intestinal growth once oil treatments were ceased. The restoration of normal villus and crypt dynamics following Emu Oil withdrawal provides added reassurance that the growth stimulatory properties of Emu Oil, observed in previous studies,6,8,9 represented processes of normal intestinal growth and repair in response to tissue injury. These outcomes were further supported by the unchanged villus height to crypt depth ratio, known to be altered in dysplasia. Collectively, the data implied that the intestinal growth process was controlled and not indicative of a precursor to the development of colonic neoplasia.
Orally administered Emu Oil has been identified as a potential therapeutic for intestinal inflammatory disorders,6–10 evidenced by improved clinical indicators of disease severity, anti-inflammatory properties (reduced myeloperoxidase activity, indicative of acute inflammation), restoration of normal mucosal architecture, and increased intestinal barrier function (stimulation of mucin-secreting goblet cell production). These findings highlight the potential efficacy of Emu Oil in other intestinal conditions characterized by inflammation, including colitis-associated colorectal cancer.14 The mode of Emu Oil action is yet to be fully elucidated; however, its growth stimulatory properties and therapeutic efficacy may be attributed to its fatty acid composition, their ratios and components within the non-triglyceride fraction, including skin permeation enhancing factors and antioxidants. Despite similar mucosal growth stimulatory effects of both Emu Oil and olive oil in the current study, the differing compositions of the two oils may have been responsible for the superior therapeutic efficacy of Emu Oil demonstrated in previous pre-clinical studies of different bowel disorders.8,10
Sebe et al.15 demonstrated that intestinal mucosal integrity was not pronounced in 5-FU-injected mice treated with a fish oil-, DHA-, or EPA-supplemented diet, despite reducing apoptosis. These findings highlight the importance of fatty acid ratios and synergism to confer intestinal protection. Mucosal stimulation following Emu Oil treatment in this study therefore potentially represented a process of increased crypt cell proliferation (hyperplasia), increased cell hypertrophy, reduced apoptosis, or a combination of these factors.8,13,16 Accordingly, in future studies, it will be important to define the impact of Emu Oil on other cellular processes including hypertrophy and hyperplasia incorporating studies of intestinal cell kinetics (proliferation and apoptosis).
During the period of daily oil treatment in normal dark agouti rats (days 0–10), total food intake significantly decreased in both Emu Oil and olive oil-treated animals compared to normal water gavage controls. A similar non-specific oil effect was also observed by Mashtoub et al.8,9 in dark agouti rats. Following daily oil gavage in these studies, a sizeable proportion of the total daily energy requirements may have been fulfilled, thus resulting in reduced casein-based diet intake. In the current study, overall caloric intake was maintained, evidenced by comparable bodyweights in oil- and water-treated controls. Interestingly, Abimosleh et al.2,10 did not report any food intake changes in Emu Oil and olive oil-treated Sprague Dawley rats, highlighting the potential metabolic or energy requirement differences between rat strains.
Consistent with previous studies by Mashtoub et al.8 and Abimosleh et al.,10 in the current study, daily oral administration of Emu Oil and olive oil resulted in increased small intestinal (jejuno-ileum) weights on day 10, relative to normal water controls, highlighting a generalized oil effect. Concomitant ileal mucosal thickening or adherence of oils to the luminal epithelial surface may have accounted for increased small intestinal weights on day 10. Importantly, weights normalized by day 17, consistent with restored ileal villus height and crypt depth. Small intestinal lengths were increased in low dose (0.5 mL) Emu Oil on day 10, which returned to normal levels following cessation of oil treatment (day 17). However, increased small intestinal length in olive oil (1 mL)-treated and high dose (1 mL) Emu Oil-treated animals remained elevated on day 17 compared to water controls. This unexpected effect could potentially represent an increased absorptive capacity of the small intestine, an as yet undefined mode of action for the two test oils. It remains to be determined whether this was a result of the oil composition or alternatively, a non-specific effect of high oil volume. Nonetheless, when expressed as a % of body weight, average jejuno-ileal length did not significantly vary between groups. Importantly, as observed by Abimosleh et al.10 and Mashtoub et al.,8 the high volume of daily oil gavage did not result in increased liver weight (indicative of liver toxicity or steatosis), reaffirming the safety of Emu Oil administration in healthy subjects.
Calviello et al.17 reported that supplementation of rats with low doses of the n-3 polyunsaturated fatty acids (PUFAs), eicosapentaenoic acid (EPA; 20:5 n-3) and docosahexaenoic acid (DHA; 22:6 n-3) reduced colonocyte proliferation and increased apoptosis in the normal rat colonic mucosa. Moreover, in colorectal cancer cell lines, the LCFAs palmitic acid, α-linoleic acid, conjugated linoleic acid, and DHA induced cell apoptosis.18–22 Furthermore, the protective effect of fish oil (a concentrated source of n-3 PUFAs) on colorectal carcinogenesis in rats has been associated with increased colonic crypt cell apoptosis.23,24 Interestingly, Sebe et al.15 demonstrated that a diet enriched with both EPA and DHA protected the small intestine against mucosal damage caused by 5-FU in mice. Intestinal mucosal protection was quantified by: 1. attenuation of mucosal ablation; 2. reduction of the pro-apoptotic genes BH3-interacting domain death agonist, Caspase-3, -8 and -9; 3. reduced TUNEL-stained apoptotic cells; and 4. greater expression of the anti-apoptotic genes BCL-2 and IAP-1. Torres et al.25 reported an increase in mid-small intestinal proliferating cells following administration of high dose Lyprinol (a rich source of n-3 P U F As) in a rat model of 5-FU-induced mucositis. The anti-apoptotic effects of EPA and DHA and proliferative effect of n-3-rich oils seemingly imply a consequent promotion of tumorigenesis; however, as indicated, it has been well documented that n-3 PUFAs are protective against colorectal carcinogenesis. Moreover, there have been no reports of any inhibitory effects of EPA and DHA on chemotherapeutic efficacy.26–28 Conflicting influences of fatty acids on cell kinetics, dependent upon the disease setting, potentially indicates differing expression or activity of genes in signal transduction pathways related to the control of cell growth and apoptosis.
Intestinal pathologies characterized by inflammation and mucosal ablation include chemotherapy-induced mucositis,29 NSAID-enteropathy,30 and the inflammatory bowel diseases.31 In this context, reduced inflammation and regeneration of the mucosa are essential to restore intestinal integrity, absorptive capacity, and barrier function. Efforts to ameliorate several chronic inflammatory disorders focus on enhanced consumption of n-3 and n-9 fatty acids (accounting for 1% and 49% of Emu Oil, respectively) due to their anti-inflammatory properties. Furthermore, saturated LCFAs, in particular palmitic acid (accounting for 24% of Emu Oil), have been implicated in the improvement of intestinal epithelial barrier function. Benoit et al.32 revealed that saturated LCFAs enhance MUC 2 (a high molecular weight glycoprotein secretion from intestinal epithelial goblet cells) synthesis and differentiation of goblet cells, leading to the establishment of a thick mucus gel. Conversely, monounsaturated or polyunsaturated LCFAs decreased MUC 2 secretion. Accordingly, Benoit et al. suggested the therapeutic application of palmitic acid in pathologies of altered intestinal permeability and goblet cells, such as the inflammatory bowel diseases.
Emu Oil, at the doses examined, did not result in any indications of sustained uncontrolled intestinal growth, supporting its safety for use in healthy subjects and its application in intestinal disorders. Relative doses of Emu Oil at or below those investigated in the current study are recommended for any subsequent clinical study since these doses have not revealed any indications of aberrant intestinal growth.
Authors’ contributions
SM is the recipient of a National Health and Medical Research Council Early Career (Peter Doherty) Biomedical Postdoctoral Fellowship. SM contributed to the intellectual development of the Emu Oil project, experimental design, analysis and interpretation of data and manuscript preparation. KYC and KAL contributed to the planning and undertaking of animal trials. GSH contributed to the study design, data interpretation, and manuscript preparation.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the Rural Industries Research and Development Corporation of Australia.
References
- 1.Beckerbauer LM, Thiel-Cooper R, Ahn DU, Sell JL, Parrish FC, Jr, Beitz DC. Influence of two dietary fats on the composition of Emu Oil and meat. Poult Sci 2001; 80:187–94 [DOI] [PubMed] [Google Scholar]
- 2.Abimosleh SM, Tran CD, Howarth GS. Emu Oil: a novel therapeutic for disorders of the gastrointestinal tract? J Gastroenterol Hepatol 2012; 27:857–61 [DOI] [PubMed] [Google Scholar]
- 3.Mashtoub S., Potential therapeutic applications for Emu Oil, Lipid. Technology 2017; 29(3-4): 28–31 [Google Scholar]
- 4.Mashtoub S, Bennett DC, Tran CD, Howarth GS. Processing and storage of ratite oils affects primary oxidation status and radical scavenging ability. Anim Product Sci 2014; 55:1332–7 [Google Scholar]
- 5.Bennett DC, Code WE, Godin DV, Cheng KM. Comparison of the antioxidant properties of Emu Oil with other avian oils. Aust J Exp Agricul 2008; 48:1345–50 [Google Scholar]
- 6.Abimosleh SM, Lindsay RJ, Butler RN, Cummins AG, Howarth GS. Emu Oil increases colonic crypt depth in a rat model of ulcerative colitis. Dig Dis Sci 2012; 57:887–96 [DOI] [PubMed] [Google Scholar]
- 7.Lindsay RJ, Geier MS, Yazbeck R, Butler RN, Howarth GS. Orally administered Emu Oil decreases acute inflammation and alters selected small intestinal parameters in a rat model of mucositis. Br J Nutr 2010; 104:513–9 [DOI] [PubMed] [Google Scholar]
- 8.Mashtoub S, Tran CD, Howarth GS. Emu Oil expedites small intestinal repair following 5-fluorouracil-induced mucositis in rats. Exp Biol Med 2013; 238:1305–17 [DOI] [PubMed] [Google Scholar]
- 9.Mashtoub S, Lampton LS, Eden GL, Cheah KY, Lymn KA, Bajic JE, Howarth GS. Emu Oil combined with Lyprinol reduces small intestinal damage in a rat model of chemotherapy-induced mucositis. Nutr Cancer 2016; 68:1171–80 [DOI] [PubMed] [Google Scholar]
- 10.Abimosleh SM, Tran CD, Howarth GS. Emu Oil reduces small intestinal inflammation in the absence of clinical improvement in a rat model of indomethacin-induced enteropathy. Evid Based Complement Alternat Med 2013; 2013:429706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomas FM, Knowles SE, Owens PC, Read LC, Chandler CS, Gargosky SE, Ballard FJ. Effects of full-length and truncated insulin-like growth factor-I on nitrogen balance and muscle protein metabolism in nitrogen-restricted rats. J Endocrinol 1991; 128:97–105 [DOI] [PubMed] [Google Scholar]
- 12.Portolesi R, Powell BC, Gibson RA. Competition between 24:5n-3 and ALA for Delta 6 desaturase may limit the accumulation of DHA in HepG2 cell membranes. J Lipid Res 2007; 48:1592–8 [DOI] [PubMed] [Google Scholar]
- 13.Shaw D, Gohil K, Basson MD. Intestinal mucosal atrophy and adaptation. World J Gastroenterol 2012; 18:6357–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chartier LC, Howarth GS, Lawrance IC, Trinder D, Barker SJ, Mashtoub S. Emu Oil improves clinical indicators of disease in a mouse model of colitis-associated colorectal cancer. Digest Dis Sci 2018; 63:135–45 [DOI] [PubMed] [Google Scholar]
- 15.Sebe M, Tsutsumi R, Yamaguchi S, Horikawa YT, Harada N, Oyama T, Kakuta N, Tanaka K, Tsutsumi YM, Nakaya Y, Sakaue H. The synergystic effects of omega-3 fatty acids against 5-fluorouracil-induced mucosal impairment in mice. BMC Nutr 2016; 2:2–10 [Google Scholar]
- 16.Drozdowski L, Thomson ABR. Intestinal mucosal adaptation. World J Gastroenterol 2006; 12:4614–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calviello G, Palozza P, Maggiano N, Piccioni E, Franceschelli P, Frattucci A, Di Nicuolo F, Bartoli GM. Cell proliferation, differentiation, and apoptosis are modified by n-3 polyunsaturated fatty acids in normal colonic mucosa. Lipids 1999; 34:599–604 [DOI] [PubMed] [Google Scholar]
- 18.Engelbrecht AM, Toit-Kohn JL, Ellis B, Thomas M, Nell T, Smith R. Differential induction of apoptosis and inhibition of the PI3-kinase pathway by saturated, monounsaturated and polyunsaturated fatty acids in a colon cancer cell model. Apoptosis 2008; 13:1368–77 [DOI] [PubMed] [Google Scholar]
- 19.Nano JL, Nobili C, Girard-Pipau F, Rampal P. Effects of fatty acids on the growth of Caco-2 cells. Prostaglandins Leukot Essent Fatty Acids 2003; 69:207–15 [DOI] [PubMed] [Google Scholar]
- 20.Kato T, Kolenic N, Pardini RS. Docosahexaenoic acid (DHA), a primary tumor suppressive omega-3 fatty acid, inhibits growth of colorectal cancer independent of p53 mutational status. Nutr Cancer 2007; 58:178–87 [DOI] [PubMed] [Google Scholar]
- 21.Lee SH, Yamaguchi K, Kim JS, Eling TE, Safe S, Park Y, Baek SJ. Conjugated linoleic acid stimulates an anti-tumorigenic protein NAG-1 in an isomer specific manner. Carcinogenesis 2006; 27:972–81 [DOI] [PubMed] [Google Scholar]
- 22.Toit-Kohn JL, Louw L, Engelbrecht AM. Docosahexaenoic acid induces apoptosis in colorectal carcinoma cells by modulating the PI3 kinase and p38 MAPK pathways. J Nutr Biochem 2009; 20:106–14 [DOI] [PubMed] [Google Scholar]
- 23.Chang WL, Chapkin RS, Lupton JR. Fish oil blocks azoxymethane-induced rat colon tumorigenesis by increasing cell differentiation and apoptosis rather than decreasing cell proliferation. J Nutr 1998; 128:491–7 [DOI] [PubMed] [Google Scholar]
- 24.Latham P, Lund EK, Johnson IT. Dietary n-3 PUFA increases the apoptotic response to 1,2-dimethylhydrazine, reduces mitosis and suppresses the induction of carcinogenesis in the rat colon. Carcinogenesis 1999; 20:645–50 [DOI] [PubMed] [Google Scholar]
- 25.Torres DM, Tooley KL, Butler RN, Smith CL, Geier MS, Howarth GS. Lyprinol only partially improves indicators of small intestinal integrity in a rat model of 5-fluorouracil-induced mucositis. Cancer Biol Ther 2008;7(2):295–302 [DOI] [PubMed] [Google Scholar]
- 26.Wynter MP, Russell ST, Tisdale MJ. Effect of n-3 fatty acids on the antitumour effects of cytotoxic drugs. In Vivo 2004; 18:543–7 [PubMed] [Google Scholar]
- 27.Schley PD, Jijon HB, Robinson LE, Field CJ. Mechanisms of omega-3 fatty acid-induced growth inhibition in MDA-MB-231 human breast cancer cells. Breast Cancer Res Treat 2005; 92:187–95 [DOI] [PubMed] [Google Scholar]
- 28.Yang T, Fang S, Zhang HX, Xu LX, Zhang ZQ, Yuan KT, Xue CL, Yu HL, Zhang S, Li YF, Shi HP, Zhang Y. N-3 PUFAs have antiproliferative and apoptotic effects on human colorectal cancer stem-like cells in vitro. J Nutr Biochem 2013; 24:744–53 [DOI] [PubMed] [Google Scholar]
- 29.Sonis ST. Pathobiology of mucositis. Semin Oncol Nurs 2004; 20:11–5 [DOI] [PubMed] [Google Scholar]
- 30.Boelsterli UA, Redinbo MR, Saitta KS. Multiple NSAID-induced hits injure the small intestine: underlying mechanisms and novel strategies. Toxicol Sci 2013; 131:654–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hendrickson BA, Gokhale R, Cho JH. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev 2002; 15:79–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benoit B, Bruno J, Kayal F, Estienne M, Debard C, Ducroc R, Plaisancie P. Saturated and unsaturated fatty acids differently modulate colonic goblet cells in vitro and in rat pups. J Nutr 2015; 145:1754–62 [DOI] [PubMed] [Google Scholar]