Short abstract
Hyperhomocysteinemia (HHcy) is associated with suppressed lipolytic response in adipocytes/adipose tissue, however, the underlying mechanism remains to be extensively studied. Nuclear factor erythroid 2-related factor 2 (Nrf2), a master transcriptional factor regulating antioxidant generation, has been recently reported to mediate lipid metabolism. Employing both fully differentiated 3T3-L1 adipocytes and male C57BL/6 mice, in the present study, we investigated the potential involvement of Nrf2 activation in HHcy-mediated lipolytic suppression. Our results showed that homocysteine (Hcy) treatment resulted in suppressed lipolysis, evidenced by increased intracellular triglyceride (TG) accumulation, decreased glycerol and free fatty acid (FFA) in fully differentiated 3T3-L1 adipocytes. Interestingly, Hcy exposure was associated with Nrf2 activation in adipocytes. Further studies showed that Nrf2 knockdown via siRNA transfection ameliorated Hcy-induced glycerol release in adipocytes. On the contrary, Nrf2 activators, epigallocatechin gallate (EGCG) and tert-butylhydroquinone (t-BHQ), increased intracellular TG content and decreased glycerol release in adipocytes. Importantly, our in vitro observations were corroborated by our in vivo findings, in which Hcy feeding (0.1% wt/vol) for four weeks induced Nrf2 expression in adipose tissue and lowered circulating FFA and glycerol levels in mice. Furthermore, EGCG injection (5 mg/kg/d) decreased circulating glycerol levels in comparison to the control group in mice. In conclusion, these results indicated that Nrf2 activation in response to HHcy plays an important role in mediating Hcy-suppressed lipolysis in adipocytes.
Keywords: Adipose, homocysteine, 3T3-L1 adipocytes, nuclear factor erythroid 2-related factor 2, lipolysis, hyperhomocysteinemia
Impact statement
The hyperhomocysteinemia (HHcy) was associated with many metabolic abnormalities, including atherosclerosis and alcoholic liver disease. We previously reported that homocysteine (Hcy) suppressed lipolytic response in adipocytes/adipose tissue. However, the underlying mechanism was still elusive. In the current study, we uncovered that Hcy was a potent activator of nuclear factor erythroid 2-related factor 2 (Nrf2), an antioxidant transcriptional factor, and Hcy-triggered Nrf2 activation played a critical role in mediating Hcy-inhibited lipolytic suppression in adipocyte. The specific role of Nrf2 in hyperhomocysteinemia-related dysfunction of lipid metabolism provided a new molecular candidate for the application of the diagnosis and the therapy to these diseases mentioned above.
Introduction
Hyperhomocysteinemia (HHcy) was associated with many metabolic disorders, including atherosclerosis, hypertension, diabetes, alcoholic liver disease and neurodegenerative disease and had been considered as an independent risk factor for cardiovascular disease.1–3 Available evidence indicated that the metabolic imbalance of homocysteine (Hcy) was associated with adipose tissue dysfunction. A higher plasma Hcy level was detected in type 2 diabetics than that in healthy subjects, and among type 2 diabetes patients, the morbidity of HHcy in the obese patients were higher than those of the non-obese.4 Meanwhile, Hcy inhibited the uptake of glucose into adipocytes by provoking endoplasmic reticulum (ER) stress and inflammation.5 The presence of HHcy in Japanese patients with type 2 diabetes mellitus-associated insulin resistance was correlative with increased visceral adiposity.6 Although the detrimental impact of HHcy on adipose tissue function had been well-documented, the exact cellular/molecular mechanism behind remained elusive. Adipose tissue functioned as the main body of energy reserve in mammals. During fed-state, adipose tissue stored excess energy as free fatty acid (FFA) incorporates into triglycerides (TG) in a process known as lipogenesis.7 In contrast, during fasting state, adipose tissue released fatty acids to provide energy resource to maintain whole-body energy homeostasis via lipolysis, a process defined as the enzymatic cleavage of fatty acids from TG.8 Changes in lipolysis was a contributing factor to several metabolic disease including type 2 diabetes.9–11 Our previous study provided initial evidence supporting that Hcy conferred an inhibitory effect on lipolysis, potentially via activating AMP-activated protein kinase (AMPK) and subsequent hormone-sensitive lipase (HSL) Ser565 phosphorylation, leading to its inhibition.12
Oxidative stress was closely related to energy metabolism and a fundamental factor affecting the incidence of obesity.13–16 CDDO-Im, a potent activator of nuclear factor erythroid 2-related factor 2 (Nrf2) signaling both in vitro and in vivo, inhibited body weight, adipose mass, and hepatic lipid accumulation.17 Nrf2 was an obligatory transcription factor activated in response to oxidative stress and therefore was focused by many scientific groups in the research realm of metabolic diseases.18,19 It regulated the expression of antioxidant enzymes, such as NAD(P)H quinone oxidoreductase 1 (NQO1) and γ-glutamatecysteine ligase catalytic subunit (Gclc).20 Under normal conditions, Nrf2 was kept in the cytoplasm by kelch-like ECH-associated protein 1 (Keap1). Perplexingly, both Nrf2 knockout mice and Keap1 knockout (constitutive Nrf2 activation) mice led to weight loss.21–24 Oxidative stress was one of HHcy-related pathological alterations in metabolic diseases.25,26 We previously reported that Hcy promoted the expression and activation of Nrf2 in liver.27 However, the direct effect of Hcy on Nrf2 activation in adipocytes and its potential contribution to Hcy-triggered adipose tissue dysfunction received very little investigative attention. Therefore, the aim of this study was to study the effect of antioxidant transcriptional factor Nrf2 on Hcy-inhibited lipolysis and the underlying mechanism in fully differentiated 3T3-L1 adipocytes and adipose tissue.
Materials and methods
Cell culture and differentiation of 3T3-L1 preadipocytes
Mouse 3T3-L1 preadipocytes were purchased from American Type Culture Collection (Manassas, VA, USA) and grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) at 37°C in a 5% CO2 atmosphere until confluence and induced to differentiate. For differentiation, 3T3-L1 preadipocytes were exposed to differentiation medium containing 0.5 mmol/L isobutylmethylxanthine, 1 μmol/L dexamethasone, 10 μg/mL insulin (Beyotime Biotechnology, Shanghai, China), and 10% FBS for three days. Cells were then transferred to DMEM with 10 μg/mL insulin and 10% FBS and refed every two days. Maturation of adipocytes was confirmed by Oil Red O staining of lipid droplets on day 11.
Oil Red O staining and quantification of lipid droplet in fully differentiated 3T3-L1 adipocytes
Lipid droplets in fully differentiated 3T3-L1 adipocytes were stained with Oil Red O. Cells were fixed with 4% paraformaldehyde and incubated with 60% filtered Oil Red O (Sigma, St. Louis, MO, USA) for 20 min. Cells were then washed twice with 60% isopropanol to remove excess dye and photographed under microscopy. To quantify intracellular lipid accumulation, stained lipid droplets were dissolved with a solution of 4% NP40 in isopropanol. Optical density was measured at 515 nm by spectrophotometer.
Glycerol and FFA assay
The content of FFA and glycerol in the plasma of mice or in the culture medium of adipocytes served as an index of lipolysis. We used the commercial enzyme-linked immunosorbent assay kit (ELISA) (Shang Hai Mei Lian, Shanghai, China) to measure the level of FFA or glycerol. The plasma or medium was incubated with relative working liquid, and the reaction was developed at 37°C. The absorbance was detected by SpectraMAX 190 instrument at the wavelength of 450 nm.
Western blotting analysis
The fully differentiated 3T3-L1 adipocytes or adipose tissue were lyzed in RIPA buffer (Beyotime Biotechnology) and supplemented with Protease and phosphatase inhibitor cocktail (Beyotime Biotechnology) for 10 min. Protein concentration was quantified using Enhanced BCA Protein Assay Kit (Beyotime Biotechnology). And isolated protein samples were separated by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose transfer membrane. And the membranes were respectively incubated with anti-Nrf2 antibody (Abcam, Cambridge, UK), anti-HSL antibody or anti-phospho-HSL antibody (Cell Signaling Technology, Danvers, MA) overnight at 4°C followed by Horseradish peroxidase-conjugated secondary antibodies (ZSGB-BIO, Beijing, China) and enhanced chemiluminescence substrate kit (HaiGene, Harbin, Heilongjiang, China) were used in the detection of specific proteins.
Quantitative real-time RT-PCR
Total RNA was extracted from either the fully differentiated 3T3-L1 adipocytes or the adipose tissue by using TRIzol Reagent (HaiGene). A 2 μg RNA was converted to cDNAs using Golden 1st cDNA Synthesis Kit (HaiGene). The cDNA was amplified with a SYBR Green PCR Master Mix (Roche Diagnostics, Mannheim, Germany) on an ABI 7300 Sequence Detection System. Relative gene expression was calculated using the 2−ΔΔCt method according to 18S as the internal control.
Animals and treatments
Male C57BL/6 mice (8 wk old) weighing 22 ± 0.5 g were obtained from the Harbin Medical University Experimental Animal Center. All studies were approved by the animal Ethical Committee of Harbin Medical University, Daqing, China. Ten mice were divided into two groups: control diet (Con), and control diet supplemented with Hcy (Hcy). Hcy was supplemented in the drinking water for four weeks at a concentration of 0.1% (wt/vol) (Sigma). Another 10 mice were also divided into two groups: control group (Con) and EGCG injection group (EGCG). Mice were treated with either EGCG (5 mg/kg/d) (Sigma) or saline for two weeks via intraperitoneal injection. At the end of the experiment, the mice were euthanized, and the plasma and adipose tissue were harvested for assays.
Statistical analysis
All data were expressed as means ± SEM. Statistical analysis was performed by GraphPad Prism Software version 5 (GraphPad Software, La Jolla, CA) and statistical differences between experimental groups were assessed by using unpaired t-test followed by one-way ANOVA. P < 0.05 was considered significant.
Result
Hcy increased intracellular TG content and inhibited lipolysis in fully differentiated 3T3-L1 adipocytes
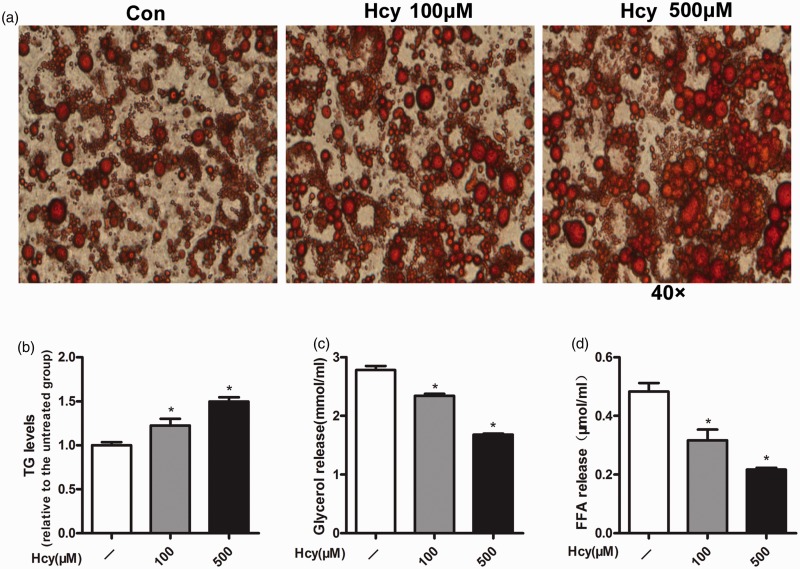
To examine the anti-lipolytic effect of Hcy, intracellular TG content in 3T3-L1 adipocytes were measured by histological examination (Oil Red O staining) (Figure 1(a)) and biochemical assay. Compared with the control group, inclusion of Hcy (100 or 500 μmol/L) in the culture medium for eight days promoted intracellular TG accumulation in a dose-dependent manner (Figure 1(b)). To determine the effect of Hcy on the lipolysis, the fully differentiated 3T3-L1 adipocytes were treated with Hcy (100 or 500 μmol/L) for 24 h, and the levels of glycerol and FFA in the culture medium were measured. As shown in Figure 1(c) and (d), Hcy treatment decreased the release of glycerol and FFA in a dose-dependent manner in fully differentiated 3T3-L1 adipocytes.
Figure 1.
Homocysteine (Hcy) increases fat accumulation and inhibits lipolysis in fully differentiated 3T3-L1 adipocytes. (a) After incubation with Hcy (100 or 500 μmol/L) for eight days (from days 3 to 11), differentiated 3T3-L1 adipocytes were fixed for Oil Red O staining. (b) Total lipid was isolated for intracellular triglyceride (TG) determination. (c and d) Fully differentiated 3T3-L1 adipocytes were treated with Hcy (100 or 500 μmol/L) for 24 h. (c) Measurement of glycerol. (d) FFA assay. Data are means ± SEM (n ≥ 3). *P < 0.05 compared with control. Con: control; Hcy: homocysteine. (A color version of this figure is available in the online journal.)
Hcy promoted the activation of Nrf2 in fully differentiated 3T3-L1 adipocytes
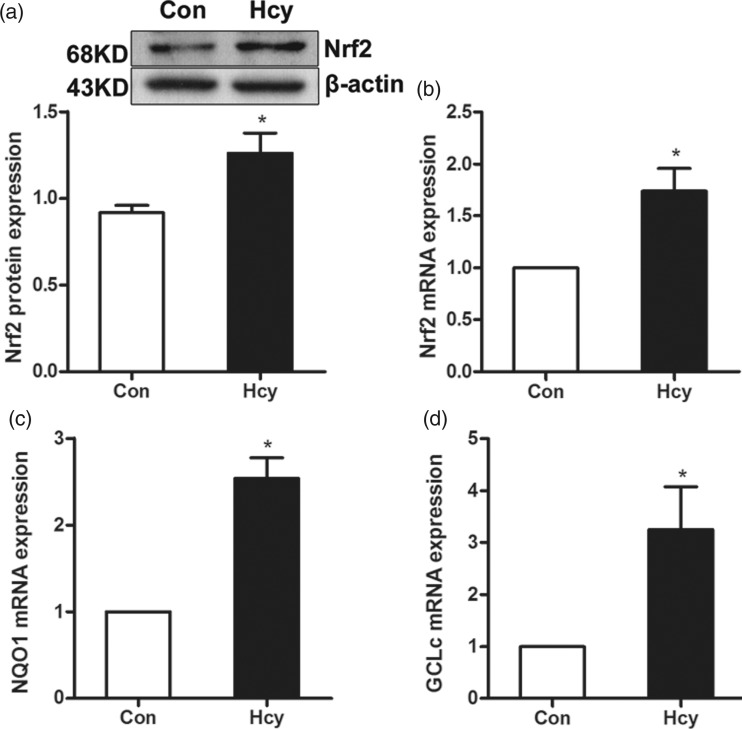
The fully differentiated 3T3-L1 adipocytes were treated with Hcy (100 μmol/L) for 24 h and the expression of Nrf2 was determined by both real time RT-PCR and Western blotting analysis. In comparison to the control group, Hcy exposure increased the gene expression and protein abundance of Nrf2 in fully differentiated 3T3-L1 adipocytes (Figure 2(a) and (b)). To determine the role of Hcy exposure in Nrf2 activation, the gene expressions of NQO1 and Gclc, the downstream target genes of Nrf2 activation, were examined by real time RT-PCR. As shown in Figure 2(c) and (d), the gene expression of both NQO1 and Gclc were significantly elevated by Hcy exposure in fully differentiated 3T3-L1 adipocytes.
Figure 2.
Hcy elevates the Nrf2 activity in fully differentiated 3T3-L1 adipocytes. Fully differentiated 3T3-L1 adipocytes were treated with Hcy (100 μmol/L) for 24 h. (a) The protein expression of Nrf2 was increased. (b) The mRNA expression of Nrf2 was increased. (c and d) The mRNA level of NQO1 and Gclc was also increased. Data are means ± SEM (n ≥ 3). *P < 0.05 compared with control. Con: control; Hcy: homocysteine.
Nrf2 activation contributed to Hcy-mediated inhibition of glycerol release in fully differentiated 3T3-L1 adipocytes
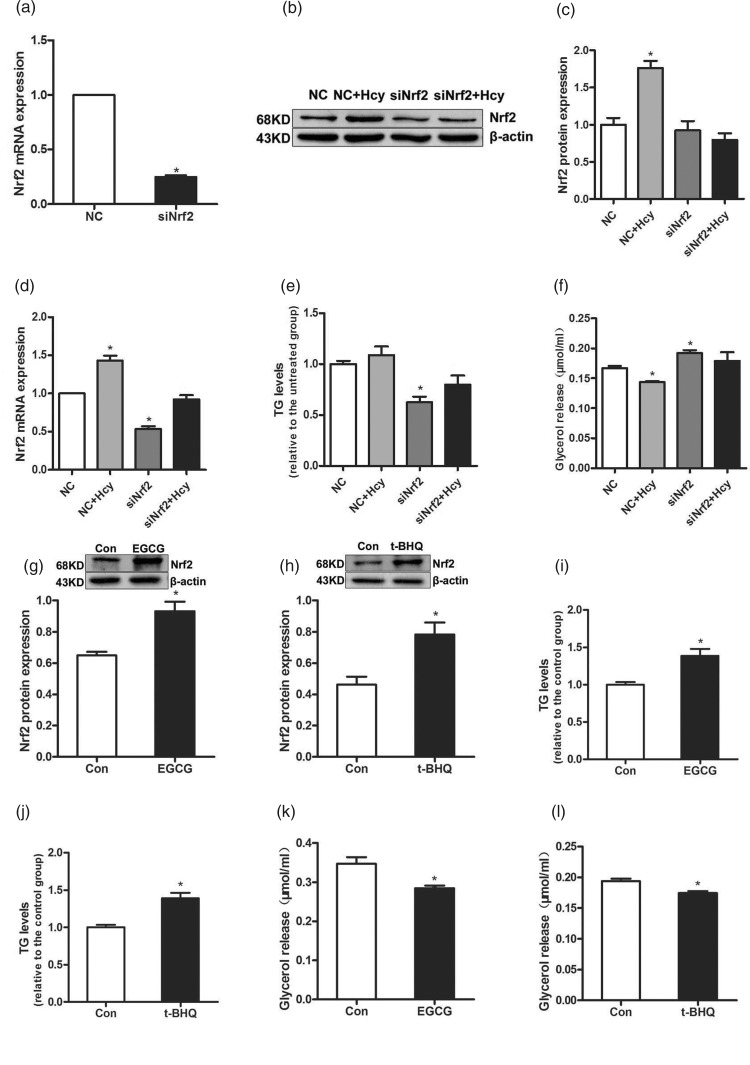
To verify the relevance between Nrf2 activation and Hcy-inhibited lipolytic reaction, the fully differentiated 3T3-L1 adipocytes were transfected with either NC siRNA or Nrf2 siRNA for 4 h, followed by treatment with Hcy (100 μmol/L) for 4 h. Firstly, in comparison to the NC group, Nrf2 siRNA treatment remarkably decreased the gene expression of Nrf2 (Figure 3(a)). And Hcy treatment elevated the gene expression and protein abundance of Nrf2 compared with the NC group in fully differentiated 3T3-L1 adipocytes (Figure 3(b), (c) and (d)). In comparison to the NC group, siRNA knockdown of Nrf2 lowered intracellular TG level (Figure 3(e)). Importantly, Hcy treatment decreased glycerol release from the adipocytes in comparison to the NC group. However, after Nrf2 siRNA transfection, the glycerol release of adipocytes altered little between Nrf2 siRNA+Hcy group and Nrf2 siRNA group, and Nrf2 knockdown ameliorated Hcy-suppressed glycerol release in adipocytes (Figure 3(f)). To further explore whether Nrf2 activation is involved in Hcy-mediated lipolytic suppression, the fully differentiated 3T3-L1 adipocytes were treated with Nrf2 activator EGCG (100 μmol/L) or t-BHQ (50 μmol/L) for 24 h. And the protein levels of Nrf2 were increased by the two Nrf2 activators in adipocytes (Figure 3(g) and (h)). As shown in Figure 3(i)–(l), Nrf2 activation is associated with increased intracellular TG accumulation and decreased glycerol release in adipocytes in both EGCG and t-BHQ group compared with the control group.
Figure 3.
Nrf2 mediates Hcy-inhibited glycerol release in fully differentiated 3T3-L1 adipocytes. (a) Fully differentiated 3T3-L1 adipocytes were transfected with Nrf2 siRNA. Fully differentiated 3T3-L1 adipocytes were transfected with siRNA for Nrf2 and treated with 100 μmol/L Hcy. (b, c and d) Transfection of siRNA for Nrf2 into 3T3-L1 adipocytes decreased the protein and mRNA expression levels of Nrf2. (e and f) The cells and medium were harvested for TG and glycerol assays. (g and h) Cells were incubated with EGCG (100 μmol/L) or t-BHQ (50 μmol/L) for 24 h, the expression level of Nrf2 protein was increased. (i and j) The levels of TG. (k and l) Measurement of glycerol assay. Data are means ± SEM (n ≥ 3). *P < 0.05 compared with NC or control. NC: non-targeted control siRNA; Hcy: homocysteine; EGCG: epigallocatechin gallate; t-BHQ: tert-butylhydroquinone.
Nrf2 mediated Hcy-inhibited glycerol release in adipose tissue in mice
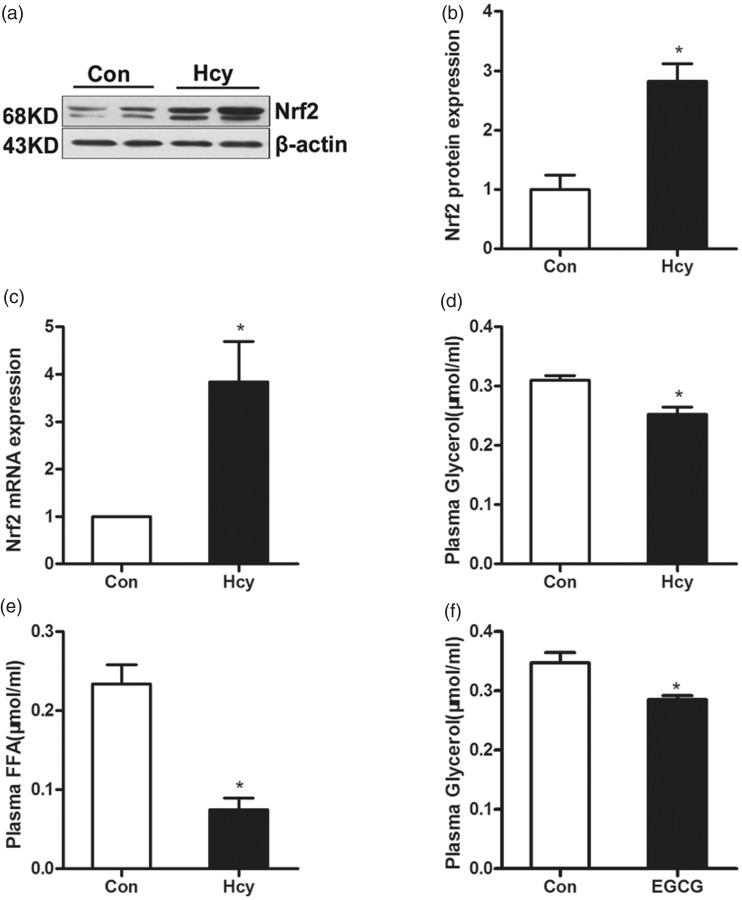
Male C57BL/6 mice were fed with Hcy (0.1%, wt/vol) for four weeks. The gene expression and protein abundance of Nrf2 in epididymal fat pads was determined by Western blotting and real time RT-PCR. As shown in Figure 4(a)–(c), chronic Hcy feeding elevated the gene expression and protein abundance of Nrf2 in adipose tissue in mice. Furthermore, the levels of glycerol and FFA in plasma were examined by biochemical assay kit and the result showed that Hcy supplementation significantly lowered the levels of glycerol and FFA in plasma compared with the control group (Figure 4(d) and (e)). In another experiment, male C57BL/6 mice were intraperitoneally injected with saline (control group) or EGCG (5 mg/kg/d) for two weeks. Nrf2 activator EGCG injection led to a decrease in the level of glycerol in plasma compared with the control group (Figure 4(f)).
Figure 4.
Nrf2 activation decreases plasma glycerol in mice. Male C57BL/6 mice were fed with Hcy (0.1%, wt/vol) for four weeks. (a, b and c) Hcy supplementation increased the level of Nrf2 protein and mRNA expression in mice adipose tissue. (d and e) The level of plasma glycerol and FFA. (f) EGCG (5 mg/kg/d) injection decreased plasma glycerol levels. Data are means ± SEM (n ≥ 3). *P < 0.05 compared with control. Con: control; Hcy: homocysteine; EGCG: epigallocatechin gallate.
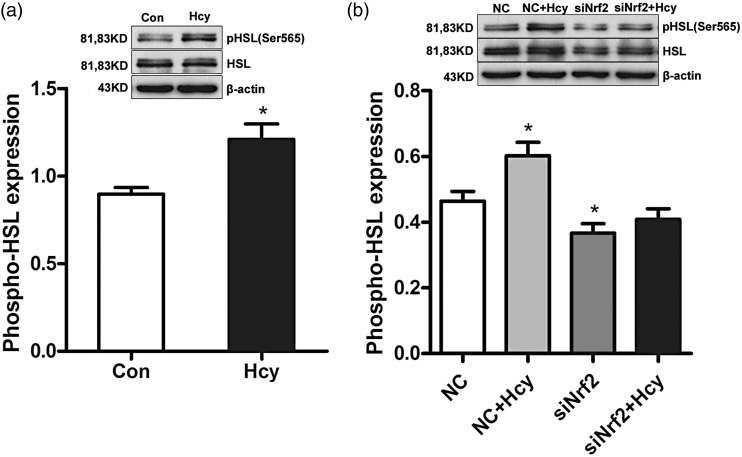
Nrf2 mediated Hcy-induced HSL Ser565 phosphorylation in fully differentiated 3T3-L1 adipocytes
HSL, the rate-limiting enzyme in lipolysis, catalyzes the lipolytic reaction of TG in adipocyte. The phosphorylation level of HSL Ser565 is an inhibitive indication of HSL activity. The anti-phospho-HSL S565 antibody was applied to demonstrate the specific role of Nrf2 in Hcy-inhibited lipolysis in fully differentiated 3T3-L1 adipocytes. The quantification of the phosphorylation level of HSL Ser565 was normalized by the protein level of HSL. As shown in Figure 5(a), Hcy significantly increased HSL phosphorylation at Ser565 in adipocytes. Nrf2 siRNA treatment decreased the phosphorylation level of HSL at Ser565 compared with that in the NC group in adipocytes. Importantly, Nrf2 siRNA+Hcy treatment caused little alteration in the phosphorylation level of HSL Ser565 in comparison to that in the Nrf2 siRNA group, and Nrf2 knockdown significantly relieved Hcy-elevated phosphorylation level of HSL Ser565 in fully differentiated 3T3-L1 adipocytes (Figure 5(b)).
Figure 5.
Nrf2 mediates Hcy-induced HSL Ser565 phosphorylation in fully differentiated 3T3-L1 adipocytes. The quantification of the phosphorylation level of HSL Ser565 was normalized by HSL protein expression. (a) Fully differentiated 3T3-L1 adipocytes were incubated with Hcy (100 μmol/L) for 24 h. Total protein was isolated and subjected to Western blotting for evaluation of activation of HSL. (b) Fully differentiated 3T3-L1 adipocytes were transfected with Nrf2 siRNA and treated with 100 μmol/L Hcy. Transfection of siRNA for Nrf2 into 3T3-L1 adipocytes decreased the phosphorylation of HSL at Ser565. Data are means ± SEM (n ≥ 3). *P < 0.05 compared with control or NC. Con: control; Hcy: homocysteine; NC: non-targeted control siRNA.
Discussion
The present study was conducted to examine the effect of Hcy on the lipolytic response in adipocytes and to explore the mechanistic pathways involved. Here, we provided initial evidence supporting that Hcy-inhibited lipolytic effect was regulated with Nrf2 activation in adipocytes. Mechanistic investigation revealed that Nrf2 activation contributed to Hcy-inhibited lipolysis via promoting HSL Ser565 phosphorylation, an inhibitive indicator for lipolysis. Those in vitro observations were corroborated by our in vivo findings that Hcy supplementation augmented Nrf2 activation in adipose tissue, concomitant with lowered levels of glycerol and FFA in plasma in mice. Furthermore, Nrf2 activator EGCG administration decreased circulating glycerol levels in mice in comparison to the control group. These findings altogether indicated that Nrf2 is a critical mediator in HHcy-induced lipolytic suppression.
HHcy has been implicated as an independent risk factor of atherosclerosis, potentially via inducing oxidative stress on the endothelium of blood vessels,28,29,30,31 which is counteracted by a series of antioxidants and detoxification enzymes, especially glutathione S-transferase (GSTs).14 Nrf2 is an important transcription factor that regulates cellular anti-oxidative stress.32 It binds to antioxidant-response elements (AREs) and initiates gene regulation of detoxification enzymes and antioxidant enzymes.33 Our previous experiments showed that Nrf2 mediated Hcy-induced GSH expression and cellular protection in HepG2 cells.24 Based on our previous study, we examined the effects of Hcy on Nrf2 expression in fully differentiated 3T3-L1 adipocytes. Our data revealed that Hcy treatment increased Nrf2 expression at the levels of mRNA and protein. Nrf2 activation was further confirmed by increased expression of NQO1 and Gclc, two direct downstream targets of Nrf2. Those in vitro observations were corroborated by our in vivo findings that Hcy supplementation increased the gene expression and protein abundance of Nrf2 in adipose tissue in mice. These data altogether suggested that, in consistent with our previous observation in hepatocytes,27 Hcy is an activator of Nrf2 in adipocytes.
The oxidative stress in adipose tissue plays a pathological role in obesity-related diseases. Reactive oxygen species (ROS) play a role in the control of body weight by exerting different effects on hypothalamic neurons, which control satiety and hunger behavior.34 Chronic hypoxia in adipose tissue causes oxidative stress in human and animal adipocytes and reduces the production of beneficial adipokines.35 Oxidative stress also leads to disruption of lipid metabolism and reduces lipoprotein clearance by decreasing lipoprotein lipase activity in mice and diminished subcutaneous adipose tissue lipolysis by decreased efficiency of beta-adrenergic, growth hormone and parathyroid hormone lipolytic signaling in humans.36 Nrf2 plays a major role in both maintaining redox balance and mediating a cytoprotective response against stressors.37 These previous studies provide rational for us to posit that Nrf2 may potentially implicate in Hcy-mediated inhibition of lipolysis. The data obtained in the current study indeed support our hypothesis. Using both genetic and pharmacological approaches, our data suggest that Nrf2 activation plays an important role in Hcy-induced anti-lipolytic effects.
HSL plays a critical role in regulating fatty acids release from adipose tissue. In our previous publication, we reported that Hcy suppressed lipolytic process via activating the AMPK pathway, which led to HSL inhibition via inducing its Ser565 phosphorylation.12 In the current study, we reported for the first time that, similar to the AMPK pathway, Nrf2 activation-mediated anti-lipolytic reaction in response to Hcy led to an increased HSL Ser565 phosphorylation as well. However, whether Nrf2 activation in response to Hcy exposure is a cause or consequence of AMPK activation remains unknown and warrant further investigation in the future. And, there is differentiated alteration between AMPK and Nrf2 pathway. AMPK, a key cellular energy sensor, potently inhibited the lipolytic reaction including the release of both glycerol and FFA in adipocytes. But Nrf2 only mediated Hcy-inhibited glycerol release in full differentiated 3T3-L1 adipocytes and had little effect on Hcy-elevated intracellular TG levels and Hcy-inhibited FFA release in the same cell line. However, compared with released FFA that was reabsorbed by cultured 3T3-L1 adipocytes rapidly, glycerol release was a more convincing indicator for the lipolytic reaction in adipocyte. Furtherly, in our investigation, we provided the direct molecular evidence that Nrf2 mediated Hcy-elevated HSL Ser565 phosphorylation, a key indicator for lipolytic reaction in adipocytes. Therefore, all the data suggested that Hcy inhibits the lipolytic process, at least in part, via activating the Nrf2 pathway.
In summary, our results demonstrated that Nrf2 activation mediated Hcy-inhibited lipolysis in fully differentiated 3T3-L1 adipocytes. And Nrf2 activation might be a critical mediator in HHcy-induced lipolytic suppression.
Supplemental Material
Supplemental material, Supplemental Figure for Nuclear factor erythroid 2-related factor 2 activation mediates hyperhomocysteinemia-associated lipolysis suppression in adipocytes by Xin Li, Yuhong Cheng, Xiuli Zhong, Bing Zhang, Zhiwei Bao, Yi Zhang and Zhigang Wang in Experimental Biology and Medicine
Authors’ contributions
ZW designed the experiments; XL, XZ, BZ, ZB and YZ carried out the experiments, ZW and XL analyzed the data; ZW, XL and CH wrote the paper.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Natural Science Foundation (81370523), Postdoctoral Science Foundation Special Project (201104420), China Postdoctoral Science Foundation General Project (20100471022), Heilongjiang Young Key Academic Staff support program (1251G039), Science and Technology Bureau of Daqing (zdy-2016-038), Postgraduate Innovative Program (YJSCX2016-40HYD) and Health and Family Planning committee of Heilongjiang Province (2016-489).
References
- 1.Keshteli AH, Baracos VE, Madsen KL. Hyperhomocysteinemia as a potential contributor of colorectal cancer development in inflammatory bowel diseases: a review. Wjg 2015; 21:1081–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhanji RA, Ma M, Bain VG, Montano-Loza AJ. Hyperhomocysteinemia is associated with severity of cirrhosis and negative impact after liver transplantation. Liver Int 2016; 36:696–704 [DOI] [PubMed] [Google Scholar]
- 3.Baggott JE, Tamura T. Homocysteine, iron and cardiovascular disease: a hypothesis. Nutrients 2015; 7:1108–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonseca VA, Fink LM, Kern PA. Insulin sensitivity and plasma homocysteine concentrations in non-diabetic obese and normal weight subjects. Atherosclerosis 2003; 167:105–9 [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Zhang H, Jiang C, Xu M, Pang Y, Feng J, Xiang X, Kong W, Xu G, Li Y, Wang X. Hyperhomocysteinemia promotes insulin resistance by inducing endoplasmic reticulum stress in adipose tissue. J Biol Chem 2013; 288:9583–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masaki T, Anan F, Anai M, Higuchi K, Tsubone T, Gotoh K, Chiba S, Katsuragi I, Tanaka K, Kakuma T, Yoshimatsu H. Hyperhomocysteinemia is associated with visceral adiposity in Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 2007; 77:168–73 [DOI] [PubMed] [Google Scholar]
- 7.Contreras GA, Strieder-Barboza C, Raphael W. Adipose tissue lipolysis and remodeling during the transition period of dairy cows. J Anim Sci Biotechnol 2017; 8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schweiger M, Romauch M, Schreiber R, Grabner GF, Hutter S, Kotzbeck P, Benedikt P, Eichmann TO, Yamada S, Knittelfelder O, Diwoky C, Doler C. Mayer N, Cecco WD, Breinbauer R, Zimmermann R, Zechner R. Pharmacological inhibition of adipose triglyceride lipase corrects high-fat diet-induced insulin resistance and hepatosteatosis in mice. Nat Commun 2017; 8:14859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fruhbeck G, Mendez GL, Fernandez-Formoso JA, Fernandez S, Rodriguez A. Regulation of adipocyte lipolysis. Nutr Res Rev 2014; 27:63–93 [DOI] [PubMed] [Google Scholar]
- 10.Jacome-Sosa MM, Parks EJ. Fatty acid sources and their fluxes as they contribute to plasma triglyceride concentrations and fatty liver in humans. Curr Opin Lipidol 2014; 25:213–20 [DOI] [PubMed] [Google Scholar]
- 11.Jou J, Choi SS, Diehl AM. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis 2008; 28:370–9 [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Pini M, Yao T, Zhou Z, Sun C, Fantuzzi G, et al. Homocysteine suppresses lipolysis in adipocytes by activating the AMPK pathway. Am J Physiol Endocrinol Metab 2011; 301:E703–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yokota T, Kinugawa S, Yamato M, Hirabayashi K, Suga T, Takada S, Harada K, Morita N, Oyama-Manabe N, Kikuchi Y, Okita K, Tsutsui H. Systemic oxidative stress is associated with lower aerobic capacity and impaired skeletal muscle energy metabolism in patients with metabolic syndrome. Diabetes Care 2013; 36:1341–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meher AK, Sharma PR, Lira VA, Yamamoto M, Kensler TW, Yan Z, Leitinger N. Nrf2 deficiency in myeloid cells is not sufficient to protect mice from high-fat diet-induced adipose tissue inflammation and insulin resistance. Free Radic Biol Med 2012; 52:1708–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lunder M, Janic M, Ziberna L, Drevensek G, Sabovic M. A low-dose atorvastatin and losartan combination directly improves aortic ring relaxation and diminishes ischaemic-reperfusion injury in isolated rat hearts. Med Sci Monit 2012; 18:BR366–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004; 114:1752–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin S, Wakabayashi J, Yates MS, Wakabayashi N, Dolan PM, Aja S, Liby KT, Sporn MB, Yamamoto M, Kensler TW. Role of Nrf2 in prevention of high-fat diet-induced obesity by synthetic triterpenoid CDDO-imidazolide. Eur J Pharmacol 2009; 620:138–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho HK, White CC, Fernandez C, Fausto N, Kavanagh TJ, Nelson SD, Bruschi SA. Nrf2 activation involves an oxidative-stress independent pathway in tetrafluoroethylcysteine-induced cytotoxicity. Toxicol Sci 2005; 86:354–64 [DOI] [PubMed] [Google Scholar]
- 19.Klaassen CD, Reisman SA. Nrf2 the rescue: effects of the antioxidative/electrophilic response on the liver. Toxicol Appl Pharmacol 2010; 244:57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu KC, Cui JY, Klaassen CD. Beneficial role of Nrf2 in regulating NADPH generation and consumption. Toxicol Sci 2011; 123:590–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seo HA, Lee IK. The role of Nrf2: adipocyte differentiation, obesity, and insulin resistance. Oxid Med Cell Longev 2013; 2013:184598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pi J, Leung L, Xue P, Wang W, Hou Y, Liu D, Yehuda-Shnaidman E, Lee C, Lau J, Kurtz TW, Chan JY. Deficiency in the nuclear factor E2-related factor-2 transcription factor results in impaired adipogenesis and protects against diet-induced obesity. J Biol Chem 2010; 285:9292–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chartoumpekis DV, Ziros PG, Psyrogiannis AI, Papavassiliou AG, Kyriazopoulou VE, Sykiotis GP, Habeos IG. Nrf2 represses FGF21 during long-term high-fat diet-induced obesity in mice. Diabetes 2011; 60:2465–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu J, Kulkarni SR, Donepudi AC, More VR, Slitt AL. Enhanced Nrf2 activity worsens insulin resistance, impairs lipid accumulation in adipose tissue, and increases hepatic steatosis in leptin-deficient mice. Diabetes 2012; 61:3208–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang SY, Siow YL, Au-Yeung KK, House JOK. Folic acid supplementation inhibits NADPH oxidase-mediated superoxide anion production in the kidney. Am J Physiol Renal Physiol 2011; 300:F1891–98 [DOI] [PubMed] [Google Scholar]
- 26.da Cunha AA, Ferreira AG, da Cunha MJ, Pederzolli CD, Becker DL, Coelho JG, Dutra-Filho CS, Wyse AT. Chronic hyperhomocysteinemia induces oxidative damage in the rat lung. Mol Cell Biochem 2011; 358:153–60 [DOI] [PubMed] [Google Scholar]
- 27.Zhang B, Dong JL, Chen YL, Liu Y, Huang SS, Zhong XL, Cheng YH, Wang ZG. Nrf2 mediates the protective effects of homocysteine by increasing the levels of GSH content in HepG2 cells. Mol Med Rep 2017; 16:597–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barai I, Gadhvi K, Nair P, Prasad S. The importance of laboratory medicine in the medical student curriculum. Med Educ Online 2015; 20:30309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikolic T, Zivkovic V, Srejovic I, Stojic I, Jeremic N, Jeremic J, Radonjic K, Stankovic S, Obrenovic R, Djuric D, Jakovljevic V. Effects of atorvastatin and simvastatin on oxidative stress in diet-induced hyperhomocysteinemia in Wistar albino rats: a comparative study. Mol Cell Biochem 2018; 437:109–18 [DOI] [PubMed] [Google Scholar]
- 30.Beltowski J, Jamroz-Wisniewska A. Hydrogen sulfide in the adipose tissue-physiology, pathology and a target for pharmacotherapy. Molecules 2016; 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park J, Kwon OS, Cho SY, Paick JS, Kim SW. Chronic administration of atorvastatin could partially ameliorate erectile function in streptozotocin-induced diabetic rats. PLoS One 2017; 12:e0172751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao H, Eguchi S, Alam A, Ma D. The role of nuclear factor-erythroid 2 related factor 2 (Nrf-2) in the protection against lung injury. Am J Physiol Lung Cell Mol Physiol 2017; 312:L155–62 [DOI] [PubMed] [Google Scholar]
- 33.Su ZY, Shu L, Khor TO, Lee JH, Fuentes F, Kong AN. A perspective on dietary phytochemicals and cancer chemoprevention: oxidative stress, nrf2, and epigenomics. Top Curr Chem 2013; 329:133–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manna P, Jain SK. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metab Syndr Relat Disord 2015; 13:423–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang B, Wood IS, Trayhurn P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch 2007; 455:479–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Netzer N, Gatterer H, Faulhaber M, Burtscher M, Pramsohler S, Pesta D. Hypoxia, oxidative stress and fat. Biomolecules 2015; 5:1143–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hochmuth CE, Biteau B, Bohmann D, Jasper H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell 2011; 8:188–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental Figure for Nuclear factor erythroid 2-related factor 2 activation mediates hyperhomocysteinemia-associated lipolysis suppression in adipocytes by Xin Li, Yuhong Cheng, Xiuli Zhong, Bing Zhang, Zhiwei Bao, Yi Zhang and Zhigang Wang in Experimental Biology and Medicine