Abstract
Early-life adversity is associated with increased risk for substance abuse in later life, with women more likely to report past and current stress as a mediating factor in their substance use and relapse as compared to men. Preclinical models of neonatal and peri-adolescent (early through late adolescence) stress all support a direct relationship between experiences of early-life adversity and adult substance-related behaviors, and provide valuable information regarding the underlying neurobiology. This review will provide an overview of these animal models and how these paradigms alter drug and alcohol consumption and/or seeking in male and female adults. An introduction to the corticotropin-releasing factor (CRF) and serotonin systems, their development and their interactions at the level of the dorsal raphe will be provided, illustrating how this particular stress system is sexually dimorphic, and is well positioned to be affected by stressors early in development and throughout maturation. A model for CRF-serotonin interactions in the dorsal raphe and how these influence dopaminergic activity within the nucleus accumbens and subsequent reward-associated behaviors will be provided, and alterations to the activity of this system following early-life adversity will be identified. Overall, converging findings suggest that early-life adversity has long-term effects on the functioning of the CRF-serotonin system, highlighting a potentially important and targetable mediator linking stress to addiction. Future work should focus on identifying the exact mechanisms that promote long-term changes to the expression and activity of CRF receptors in the dorsal raphe. Moreover, it is important to clarify whether similar neurobiological mechanisms exist for males and females, given the sexual dimorphism both in CRF receptors and serotonin indices in the dorsal raphe and in the behavioral outcomes of early-life adversity.
Keywords: Early-life stress, Drug reward, Sex differences, Corticotropin-releasing factor, Dorsal raphe nucleus, Serotonin
Abbreviations: 5-HIAA, 5–Hydroxyindoleacetic Acid; BNST, Bed Nucleus of the Stria Terminalis; CeA, Central Nucleus of the Amygdala; CRF, Corticotropin-Releasing Factor; CRF-BP, Corticotropin-Releasing Factor Binding Protein; dRN, Dorsal Raphe Nucleus; LC, Locus Coeruleus; MDMA, 3,4-Methylenedioxymethamphetamine; NAc, Nucleus Accumbens; NMDA, N-methyl-d-aspartate; PND, Postnatal Day; TPH2, Tryptophan Hydroxylase 2; VTA, Ventral Tegmental Area
Highlights
-
•
Early life stress increases risk for substance abuse in adulthood.
-
•
Stress and drugs increase CRF which alters serotonin release in the brain.
-
•
CRF2 receptor expression in the dorsal raphe is altered by early life stress.
-
•
Resultant changes to serotonin output facilitates dopamine in the accumbens.
-
•
CRF2-sertotonin-dopamine interactions may link early life stress with substance abuse.
1. Introduction
Neglect as well as physical and sexual abuse of minors are highly prevalent in the United States, with approximately 676,000 child victims and 3.5 million child protective services investigations in 2016 (U.S. Department of Health and Human Services, 2018). Stressful experiences during childhood and adolescence, a time when brain regions important for executive function and reward processing are still developing, can have long lasting influences into adulthood. The goal of the current review is to provide a working neurobiological model describing how early-life adversity may influence substance use during adulthood, which will inform future directions in the field. To do so, we will introduce the problem of early-life adversity and the influence it has on substance use later in life, discuss corticotropin-releasing factor (CRF) and serotonin as major players in early-life stress outcomes in adulthood, and give an overview of the development of both CRF and serotonin systems and associated sex differences that may increase vulnerability to the effects of early-life adversity. Finally, we will describe how these systems interact and are influenced by early-life adversity to increase substance use susceptibility later in life.
2. The problem of early-life adversity and substance use
Childhood, peri-adolescence (early through late adolescence including puberty), and early adulthood mark a particularly vulnerable time to the effects of stress. Exposure to stress early in life may be particularly deleterious on behavioral outcomes in adulthood given the correspondence these time periods have to brain development (for review see Kolb and Gibb, 2011; Kolb et al., 2013). In humans, early-life adversity arising from physical, emotional, and sexual abuse correlates with an increased risk of substance use and other mental health disorders including anxiety disorders, major depressive disorder, and schizophrenia (Espejo et al., 2007; Heim et al., 2008; Copeland et al., 2013; Merrick et al., 2017; Seidenfaden et al., 2017). Children exposed to early-life adversity through abuse and/or neglect also have increased risk of drug use, relapse following drug abstinence, and overall increased prevalence of substance use disorders during adulthood (Messina et al., 2008; Van Dam et al., 2014; Teixeira et al., 2017). Adolescents from a family with a history of substance use disorders have an increased likelihood of experiencing more frequent and severe stressors, and initiation of substance use is indirectly influenced by stress exposure; indicative of a cycle of stress and substance use (Charles et al., 2015a; b). Adults that report childhood sexual abuse also report earlier initiation of and increased use of illicit drugs (Harrison et al., 1989; Ompad et al., 2005; Nelson et al., 2006). Furthermore, children exposed to two or more stressful life events have an increased risk for developing alcohol dependence compared to children that did not experience any stressful life events (Pilowsky et al., 2009), with stress likely being a greater mediating factor in women compared to in men (Simpson and Miller, 2002; Widom et al., 2006; Enoch, 2011). Similarly, in women, emotional abuse has a stronger association with age of first alcohol use and severity of substance abuse compared to men (Hyman et al., 2006), and childhood emotional abuse is associated with cocaine relapse in women but not in men (Hyman et al., 2008). Combined, these findings imply that trauma or stress early in development may play a more prominent role in promoting adult drug-taking behaviors in women. These data suggest that women have a higher likelihood for a stress-induced trajectory to drug taking, resulting in a more rapid transition from drug taking to dependence and greater propensity for relapse compared to men (Hudson and Stamp, 2011; Becker et al., 2012). Overall, it appears that exposure to adverse experiences early in life increases risk of substance use during adulthood, but it is important to note that not all children exposed to early-life adversity go on to develop a substance use disorder, arguing for the importance and contribution of both genetics, epigenetics, and the influence of other environmental factors that may contribute to these individual differences.
3. Effects of early life adversity on reward processes and drug responses in preclinical models
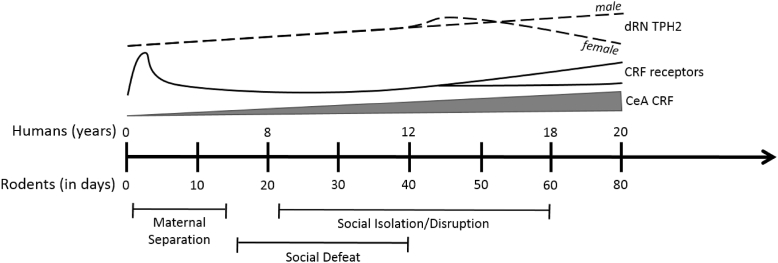
Three main types of paradigms have been used in rodents to model early-life adversity and directly test the impact of early-life stress on drug-related behaviors. These include social isolation/social disruption, social defeat stress, and maternal separation, which differ in the developmental period of stress exposure (Fig. 1, see figure legend for age ranges corresponding to weaning, peri-adolescence, adolescence and puberty). Social isolation/disruption from weaning to either mid-adolescence or up to adulthood (Fig. 1) interrupts the development of play behavior and results in aggression, anxiety, and hyperactivity during adulthood (Hall, 1998; Varlinskaya et al., 1999; Heidbreder et al., 2000; Lukkes et al., 2009c; Watt et al., 2017). Social isolation/disruption during periods of development corresponding to pre-pubescence and adolescence results in a sensitized response to amphetamine (McCormick et al., 2005; Mathews et al., 2008), enhanced acquisition of cocaine self-administration at low doses, and decreased acquisition at higher doses in adulthood (Schenk et al., 1987; Boyle et al., 1991; Phillips et al., 1994; Howes et al., 2000; Kosten et al., 2000, 2004; 2006; Yajie et al., 2005; Gipson et al., 2011; Baarendse et al., 2014). Further, social isolation/disruption during a more discreet period of development from postnatal day (PND) 21–42 (weaning to mid-adolescence) followed by weeks of re-socialization results in increased motivation for cocaine while not altering extinction responding, or cue- cocaine- or yohimbine-induced reinstatement of cocaine seeking during adulthood (Baarendse et al., 2014). Alterations in drug-taking behavior are not specific to stimulants, given that social isolation/disruption from weaning to early adulthood also increases ethanol consumption and self-administration during adulthood in both rats and mice (McCool and Chappell, 2009; Lopez et al., 2011). Increased sensitivity to opiates is often reported following post-weaning social isolation, but findings are not always consistent among the different paradigms (Neisewander et al., 2013). For example, social isolation increases morphine and sufentanil self-administration but decreases morphine or heroin preferences in conditioned place preference tasks (Alexander et al., 1978; Weinhold et al., 1993; Schenk et al., 1983, 1985; Kennedy et al., 2011). Together, these studies indicate that social isolation/disruption following weaning typically results in increased self-administration of a variety of drug classes and often enhances other indices of substance-associated behaviors during adulthood.
Fig. 1.
Timeline of neural development of the corticotropin-releasing factor (CRF) and dorsal raphe nucleus (dRN) serotonin systems in relation to the time periods in which different stress paradigms are employed. Note that peri-adolescence for the rat is considered to begin at weaning (PND 21), encompassing adolescence from PND 32 to PND 56–60 (although adolescence may begin later in males) (Sengupta, 2013). Rats are thought to attain puberty by PND 50 (Sengupta, 2013). The time period in which each early-life stress paradigm is indicated represents the age ranges used by a majority of studies described here, although a number of social isolation/disruption or social defeat studies use more specific age ranges to restrict stress to discrete developmental periods, as detailed in the text. CRF expression in the central nucleus of the amygdala (CeA; gray bar) is thought to increase over development, whereas CRF receptor expression (solid lines) in most brain regions peaks very early in postnatal development. As indicated by the bifurcating black solid line, CRF receptor expression shows a second developmental change over puberty, with either a decrease or increase in expression dependent on brain region studied, sex of individual and receptor (CRF1 or CRF2). The expression of tryptophan hydroxylase 2 (TPH2) in the dRN (dashed lines), as a measure of serotonin levels/neurons, increases over development in males, but peaks around PND42 in females and then declines to adulthood. See text for citations.
Social defeat involves an imbalance of power between a dominant and subordinate animal. Social defeat stress can be used as a model of peer victimization and bullying frequently experienced during childhood and adolescence (Fig. 1) (Bjorkqvist, 2001; Watt et al., 2009; Burke et al., 2017). This type of stress during the adolescent period effectively results in heightened novelty seeking and risk taking in adulthood (Watt et al., 2009) along with executive function deficits (Novick et al., 2013; Watt et al., 2017). Social defeat in pre-adolescence (e.g., PND 14–38) increases conditioned place preferences for cocaine and MDMA, and enhances alcohol consumption and motivation to administer alcohol in adulthood (Lo Iacono et al., 2016; Garcia-Pardo et al., 2015; Rodriguez-Arias et al., 2015). Similarly, social defeat restricted to mid-adolescence (PND 35–44) results in augmented locomotion following a low dose of amphetamine and increased amphetamine conditioned place preference during adulthood (Burke et al., 2011, 2013). Adolescent social defeat also increased the percentage of rats that voluntarily acquired the task of cocaine self-administration acquisition, the motivation to lever press for cocaine as measured using a progressive ratio schedule, and the amount of cocaine consumed when given unlimited access during a 24 h binge later in life (Burke and Miczek, 2015; Burke et al., 2016). Furthermore, a metric used to determine how stressful the social defeat was during adolescence in individual rats was positively correlated with the amount of cocaine consumed under progressive ratio and unlimited access schedules approximately 40 days later in adulthood (Burke and Miczek, 2015; Burke et al., 2016). Thus, similar to the social isolation model, social defeat stress applied either prior to or during adolescence effectively enhances drug and alcohol responding in adulthood, suggestive of long-term effects of stress on reward-associated neural systems.
Maternal separation is used to describe a variety of neonatal paradigms during which the pups are separated from the mother for various lengths of time (for review of methodologies see Kosten et al., 2012; Nylander and Roman, 2013; Tractenberg et al., 2016). Studies using this paradigm often have inconsistent timing and lengths of separation (i.e., methodologies), making comparisons difficult. Despite these shortfalls, there are remarkable consistencies regarding the influence that prolonged (three or more hrs) daily maternal separation has on later drug reactivity and responding for drugs in adulthood. Maternal separation from PND 1–14 increases the rewarding effects of acute amphetamine exposure during adulthood, as measured by a decrease in intracranial self-stimulation current threshold (Der-Avakian and Markou, 2010). Similar to findings following social isolation/social disruption, maternal separation results in greater methamphetamine self-administered and earlier acquisition while not altering extinction or cue-induced reinstatement (Moffett et al., 2006; Lewis et al., 2013, 2106). Exposure to maternal separation also has effects on alcohol and opiate intake and preferences during adulthood. To illustrate, male rats exposed to maternal separation exhibit increased self-administered alcohol binge drinking, corresponding to increased impulsivity and increased ethanol preference (Huot et al., 2001; Ploj et al., 2003; Jaworski et al., 2005; Gondre-Lewis et al., 2016), heightened morphine conditioned place preference and increased morphine self-administration (Michaels and Holtzman, 2008; Vazquez et al., 2005, 2006; 2007).
Overall, these findings indicate that the long-lasting effects of early-life adversity may be conserved across various drug classes. Collectively these studies also demonstrate that social isolation/social disruption, social defeat, and maternal separation have similar outcomes for drug-related behaviors in adulthood despite their different methodologies and the variety of developmental time periods in which these stressors are experienced (Fig. 1). Interestingly, similar outcomes are noted with prenatal stressors (Kippin et al., 2015; Reynaert et al., 2015). This suggests that there may not be a critical period in development by which stress has negative consequences for later substance use, and instead, any period of development early in life is a sensitive time for the effects of stress. However, due to the different stages of brain development affected by each specific early-life adversity model (Fig. 1), it is likely that the underlying neural substrates promoting enhanced drug taking or preferences differ among the developmental stressors (discussed further in Section 6).
Despite sex differences in the prevalence of mental health disorders including anxiety disorders, major depressive disorder, and substance use disorders (Sinha et al., 2007; Ter Horst et al., 2009; Hudson and Stamp, 2011; Becker et al., 2012; Valentino et al., 2013), little work in rodent studies has been done to characterize the influence of stress during development in both males and females on outcomes in adulthood. Furthermore, the differential trajectory of brain development (Becker et al., 2007; Ball et al., 2014) urges for further investigation in both males and females. Studies that have probed for potential sex differences in the effects of early-life adversity on later drug or alcohol measures in rodents are surprising. Contrary to the findings discussed above (Simpson and Miller, 2002; Widom et al., 2006; Enoch, 2011), where early-life adversity has a greater impact on later alcohol use in women, rodent studies either fail to find a sex difference or show that female rodents are not affected by the early-life stressor when alcohol preference or intake is measured in adulthood (e.g. Roman et al., 2004; Gustafsson et al., 2005; Lopez et al., 2011). It is possible that the effects of early-life adversity in female rodents are more apparent for responses to stimulants. For example, female rats briefly handled during week two of life show increased self-administration of cocaine during adulthood, whereas males do not (Flagel et al., 2003). Likewise, social instability stress from PND 33–48 results in increased nicotine-induced locomotion in adult female but not male rats (McCormick et al., 2005). However, there are just as many instances where stimulant responses in males and females during adulthood are similar following early-life adversity. For example, maternal separation for an hour a day from PND 2–12 increases responding for cocaine during extinction and cue-induced reinstatement similarly in both male and female rats (Lynch et al., 2005). Also, social instability of rats from PND 30–45 increases amphetamine-induced locomotion equally among both sexes (Mathews et al., 2008). Further work is needed to determine whether animal models sufficiently model human sex differences in the impact of early-life adversity on later substance use, and to determine whether any sex differences in stress and drug responsivity are due to the activational rather than organizational effects of gonadal hormones (for review see Ball et al., 2014).
The exact neurobiological mechanisms through which early-life adversity leads to increased substance use in adulthood remain to be elucidated. Numerous preclinical studies have shown that stress experienced in early-life results in long term functional alterations in the mesocorticolimbic system and corresponding changes in reward processing, particularly in enhancing responses to a range of abused substances (see Moffett et al., 2006; Miczek et al., 2008; Enoch, 2011; Bardo et al., 2013; Delavari et al., 2016; Watt et al., 2017 for reviews). Not surprisingly, many of the observed neurobiological effects involve direct changes to dopaminergic signaling in the nucleus accumbens (NAc), along with modifications to activity of other stress-responsive factors that impinge upon accumbal function either directly or indirectly, including CRF, GABAergic, glutamatergic, noradrenergic, neurotrophic, opioid, and glucocorticoid systems (see Fone and Porkess, 2008; Sinha, 2008; Bardo et al., 2013; Mantsch et al., 2014; Jawahar et al., 2015; Watt et al., 2017). Here, we will focus on a neural system characterized by CRF-serotonin interactions that is upstream of the dopaminergic mesocorticolimbic pathways, and we will examine the emerging body of converging evidence suggesting that early life stress has long-term effects on the functioning of the CRF-serotonin system.
4. Corticotropin-releasing factor (CRF) and serotonin interactions mediating stress and reward reponses
Topographically organized, functional subsets of serotonergic neurons of the dorsal raphe nucleus (dRN) are often associated with mediating the negative consequences of early life adversity (Lowry et al., 2008; Lukkes et al., 2009c; Burke et al., 2017) and also with drug-associated behaviors (Vuong et al., 2010; Valentino et al., 2010). Similarly, the actions of the stress-related neuropeptide CRF in the brain have been implicated in early life adversity and addiction (Sinha, 2008; Lukkes et al., 2009c; Koob et al., 2013). The dRN is a major site for CRF interactions with a distributed serotonergic system, and the neurons of the dRN receive CRF projections likely from specific subregions of the central nucleus of the amygdala (CeA) and bed nucleus of the stria terminalis (BNST) (Gray, 1993; Fox and Lowry, 2013). Both local GABAergic interneurons and serotonergic projection neurons of the dRN subregions are targeted by CRF terminals and both neuronal types possess CRF1 and CRF2 receptors (Day et al., 2004; Waselus et al., 2005; Lukkes et al., 2011, Fig. 2). Both serotonin and CRF are implicated in behavioral and emotional states that are risk factors for substance use and dependence, such as anxiety, dysphoria, and impulsivity (Lowry and Moore, 2006; Lukkes et al., 2009c; Valentino et al., 2010; Cunningham and Anastasio, 2014; Muller and Homberg, 2015; Ouzir and Errami, 2016; Roberto et al., 2017). Therefore it is not surprising that CRF-dRN interactions and resultant serotonergic output to the extended limbic system have gained attention as a potential important mediator linking stress to addiction (e.g. Vuong et al., 2010; Scholl et al., 2010; Valentino et al., 2010).
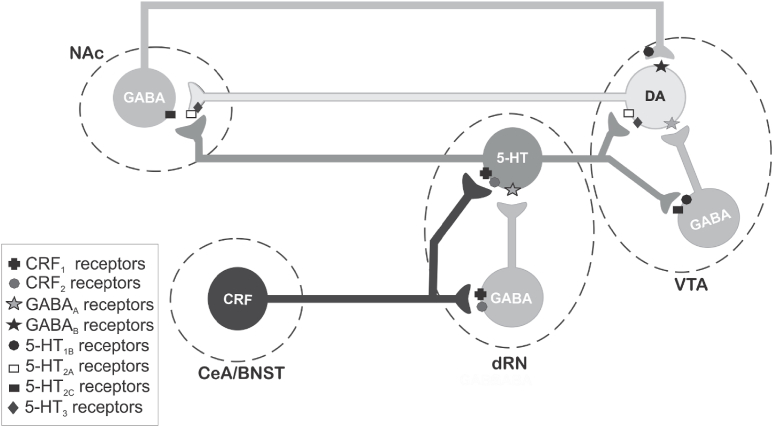
Fig. 2.
Simplified schematic diagram illustrating how corticotropin-releasing factor (CRF) receptor activity in the dorsal raphe nucleus (dRN) may increase dopamine release in the nucleus accumbens (NAc). Stressors and drugs of abuse increase release of CRF in the dRN (a presumptive functional CRF projection has been indicated from the central nucleus of the amygdala [CeA] and/or the bed nucleus of the stria terminalis [BNST]). CRF activation of CRF2 receptors in the dRN appear to increase serotoninergic neural activity and serotonin output likely via CRF2 Gs-coupled receptor excitation of serotonergic neurons. Resultant serotonin released in the ventral tegmental area (VTA) can increase dopamine cell firing and NAc dopamine release via activation of serotonin type 2A (5-HT2A) or type 3 (5-HT3) receptors on dopamine neurons, or by disinhibition via 5-HT1B inhibition of inhibitory GABAergic neurons. Furthermore, serotonin released in the NAc facilitates dopamine release in the NAc via presynaptic 5-HT2A and 5-HT3 receptors located on dopamine terminals. See text for citations. Note that 5-HT2C receptors located on GABAergic neurons do not appear to play a major role in increasing mesoaccumbal dopamine release as activation of these receptors result in hyperpolarization of dopaminergic neurons presumably via activation of GABAergic neurons, and thus reduce NAc dopamine release (Navailles et al., 2006).
Generally, findings from electrophysiological studies within the dRN and measures of serotonin release in limbic structures following global dRN manipulation align to conclude that CRF1 receptor activity decreases, whereas CRF2 receptor activity increases, dRN serotonergic neuronal activity and serotonin release (Kirby et al., 2000; Amat et al., 2004; Pernar et al., 2004; Forster et al., 2008; Lukkes et al., 2008, 2009a; Waselus et al., 2009). Although both receptor types are localized to serotonergic and GABAergic neurons in the dRN, the opposing effects of CRF1 vs CRF2 receptors on serotonergic output from this region may reflect predominant excitation of GABAergic neurons in the dRN by CRF1 receptors and activation of serotonergic neurons by local CRF2 receptors (Fig. 2). Of relevance, CRF2 receptors appear to be equally localized to serotonergic and GABAergic neurons in the caudal dRN but are almost exclusively localized to serotonergic neurons in the rostral dRN (Day et al., 2004), which may account for a predominant increase in serotonin output from the dRN when CRF2 receptors are globally activated by pharmacological manipulation of the dRN. While CRF1 receptors in other brain regions are more often associated with stress responses and fear or anxiety-like behaviors in preclinical models (Heinrichs et al., 1997; Skutella et al., 1998; Timpl et al., 1998; Muller et al., 2003; Gehlert et al., 2005), there appears to be a more important role for CRF2 receptors specifically in the dRN in mediating adaptive and maladaptive stress responses (Lukkes et al., 2009c; Waselus et al., 2009; Wood et al., 2013). For example, activation of CRF2, but not CRF1, receptors throughout the dRN results in increased serotonin release in various amygdala subregions, prefrontal cortex and NAc (Amat et al., 2004; Forster et al., 2008; Lukkes et al., 2008; Scholl et al., 2010), and pharmacological blockade of CRF2 receptors in the dRN reduces fear or anxiety-like behaviors in a variety of animal models (Takahashi et al., 2001; Hammack et al., 2003; Cooper and Huhman, 2007; Vuong et al., 2010; Lukkes et al., 2009b; Bledsoe et al., 2011). Interestingly, CRF1 and CRF2 receptors differentially respond to stress (Waselus et al., 2009; Wood et al., 2013) which may account for the prominent role of dRN CRF2 receptors in stress-related outcomes. Within the dRN, CRF2 receptors are trafficked to the neuronal membrane while CRF1 receptors are internalized in response to an acute swim stress or chronic social stress (Waselus et al., 2009; Wood et al., 2013). These molecular changes manifest in stress-induced alterations in dRN serotonergic neuronal firing patterns that shift from CRF1-mediated inhibition to CRF2-mediated excitation, accompanied by a change in cognitive performance that is dependent upon increased cortical serotonin transmission (Waselus et al., 2009; Wood et al., 2013; Snyder et al., 2015). Overall, the CRF2 receptor in the dRN plays an important role in facilitating serotonergic transmission in the limbic system, which appears to become more pronounced in response to both acute and chronic stress.
The activity of CRF2 receptors in the dRN represents a potential neurobiological mechanism underlying the link between stress and drug seeking. Increased serotonin release in the NAc is elicited by CRF2 receptor activation in the dRN and enhanced by stress (Lukkes et al., 2008, 2009a). Serotonin in the NAc can, in turn, increase NAc dopamine release (Fig. 2; Parsons and Justice, 1993; De Deurwaerdere et al., 1998) and dopaminergic activity in the NAc is strongly implicated in reward salience and drug seeking (Bardo, 1998; Tzschentke, 1998; Di Chiara, 2002; Grace et al., 2007; Phillips et al., 2008; Floresco, 2015). Specifically, activation of 5-HT2A and 5-HT3 receptors within the NAc increases accumbal dopamine release likely via direct actions on dopamine terminals (Fig. 2), and augments associated responses to drugs of abuse (Parsons and Justice, 1993; De Deurwaerdere et al., 1998; Parsons et al., 1999; Bowers et al., 2000; Yan and Yan, 2001; Neumaier et al., 2002; Alex and Pehek, 2007). While serotonin in the dopamine cell body region of the ventral tegmental area (VTA) can enhance NAc dopamine release either directly via 5-HT2A and 5-HT3 receptors on dopamine neurons or via 5-HT1B receptor disinhibition via GABAergic neurons (Fig. 2; Yan and Yan, 2001; Alex and Pehek, 2007), it is actions of serotonin in the NAc, but not the VTA, that are critical for serotonergic modulation of the rewarding effects of intracranial self-stimulation and psychostimulants (Katsidoni et al., 2011). Given that CRF2 receptor responses in the dRN are augmented after stress (Lukkes et al., 2009a; Waselus et al., 2009; Wood et al., 2013), these converging lines of evidence suggest that drug seeking enhanced by stress may result in part from dRN CRF2 receptor-driven serotonergic facilitation of NAc dopamine release.
In other monoaminergic cell body regions, the stimulatory effects of CRF2 receptors appear to be related to the presence or activity of the CRF binding protein (CRF-BP). While traditionally thought of as a ‘ligand-sink’ (Jahn et al., 2005), the CRF-BP is necessary for CRF2 receptor-mediated potentiation of NMDA-induced activation of dopamine neurons in the VTA (Ungless et al., 2003) and plays an important role in stress-induced reinstatement of cocaine self-administration that is dependent on CRF2 receptor activation in the VTA (Wang et al., 2007). Therefore, it has been suggested that the CRF-BP may play a permissive role in CRF's actions at the CRF2 receptor in the VTA (Ungless et al., 2003). Moreover, the CRF-BP promotes trafficking of CRF2α receptors (neural CRF2 receptors) to the cellular membrane in cultured mesencephalic neurons (Slater et al., 2016). Whether the CRF-BP plays similar roles in the dRN is an important future direction, as the CRF-BP may provide a novel pharmacological target to manipulate CRF2 receptor function and trafficking in the dRN following early-life adversity.
5. Ontogeny and sexual dimorphism of CRF-Serotonin systems
The CRF system, with its influence on monoaminergic function in the brain, is well positioned to be affected by stressors from early in life and throughout maturation (Fig. 1), with potential long-term consequences for later drug-related behaviors. This is because CRF expression peaks early in development (PND 8) within the paraventricular nucleus of the hypothalamus (PVN), and later in other brain regions such as the hippocampus (PND 18) (Chen et al., 2001; Korosi and Baram, 2008). An exception to this is CRF expression in the CeA, which appears to increase over the course of development alongside increasing expression of the CRF-BP in this region (Vazquez et al., 2006). Given that one target of CRF afferents from the CeA is likely the dRN (Gray, 1993), this latter finding provides the intriguing possibility that CRF innervation of, or CRF activity in the dRN similarly increases over maturation and could be more susceptible to perturbation over a longer developmental period than other neural CRF systems.
The expression of CRF receptors in the brain initially was also thought to peak very early in development, with rat whole brain homogenates showing increases from birth to peak at PND 8, declining thereafter through the rest of development (Insel et al., 1988). In a similar timeframe, CRF receptor mRNA expression peaks between PND 2–9 in the hippocampus, amygdala, and cortex (Avishai-Eliner et al., 1996). However, when studies have included later developmental time points and examined both sexes, clear pre- and post-pubertal sex differences emerge for CRF receptor ontogeny that are often receptor and brain region specific. For example, CRF1 receptor expression is similar among male and female pre-pubertal rats in the amygdala, but decreases in males and increases in females after puberty (measured in adulthood at PND 98; Weathington et al., 2012, 2014). Of the variety of corticolimbic brain regions studied, each with their own sex- and age-dependent changes in CRF1 receptor expression, a general pattern of greater CRF1 receptor expression in post-pubertal females emerges (Weathington et al., 2012, 2014). In contrast, amygdala CRF2 receptor expression is higher in adult males because of an increase in CRF2 receptor expression over adolescence that is absent in females (Weathington et al., 2012, 2014). Greater CRF2 receptor expression in males also holds true for many other limbic brain regions (Weathington et al., 2014). Overall, there appears to be a sex difference in CRF1/2 receptor expression in the post-pubertal brain as a result of differential maturation patterns. Males have greater CRF2 receptor expression as adults whereas females exhibit greater expression of anxiogenic CRF1 receptors throughout adolescence. These latter findings suggest a possible mechanism for higher incidence of anxiety disorders in females (Weathington et al., 2012).
Only one study to date (Lukkes et al., 2016) has explored the ontogeny and sexual dimorphism of CRF receptors within the dRN, and currently no information regarding sex-dependent expression or the developmental trajectory of the CRF-BP in the dRN is available. Overall, there is very little developmental change in either CRF1 or CRF2 mRNA in the dorsal and ventrolateral subregions of dRN from early adolescence (PND 25) to adulthood (PND 90) (Lukkes et al., 2016). Earlier ages were not examined, leaving the possibility that CRF receptor expression in the dRN, like other brain regions (e.g., Insel et al., 1988; Avishai-Eliner et al., 1996), peaks very early in development. However, like other brain regions, clear sex differences in CRF receptor expression are apparent in subregions of the dRN that project to the cortex and NAc (Lukkes et al., 2016). In contrast to other brain regions discussed earlier, CRF2 receptor mRNA expression in the dorsal ventrolateral dRN is higher in females compared to males (Lukkes et al., 2016). Whether this results in a change of the net activity of the dRN to affect serotonergic output to the limbic system in a sex-specific manner is not known, but if this is the case, it may help explain the increased risk for females in the development of stress psychopathology, including stress-induced drug relapse (Sinha et al., 2007; Ter Horst et al., 2009; Hudson and Stamp, 2011; Becker et al., 2012).
The exact mechanisms underlying sexual dimorphism of CRF receptor expression are not understood, but sex steroids may play an important role given the greater divergence in expression in post-pubertal animals (Weathington et al., 2012, 2014; Lukkes et al., 2016). For instance, non-aromatizable androgen administration to castrated male rats increases CRF2 mRNA in a variety of limbic structures (Weiser et al., 2008). This effect aligns with the natural pattern of higher CRF2 receptor expression in the same limbic regions of the male brain (Weathington et al., 2012, 2014), and suggests that such heightened CRF2 receptor expression in post-pubertal males is mediated by androgens or androgenic metabolites such as dihydrotestosterone and 3α-andronstanediol. However, it is unclear how such findings relate to the contrasting higher expression of CRF2 mRNA in the dorsal ventrolateral dRN of females (Lukkes et al., 2016), especially as only male rats and mice express androgen receptors in the dRN (Sheng et al., 2004). Alternatively, the pattern seen in the dRN of females may reflect actions of estrogenic steroids, receptors for which are also located in the rodent dRN (Sheng et al., 2004). Support for an estrogen-mediated effect comes from studies showing that estradiol upregulates CRF2 receptor mRNA expression in the dRN of nonhuman primates (Sanchez et al., 2010; Bethea et al., 2015), possibly via demethylation of the CRF2 receptor gene promoter that has been observed in myocardial cells (Cong and Ni, 2014). Further research that examines the effects of sex steroids on both CRF receptor types in a variety of limbic and monoamine cell body brain regions will clarify a potential and differential role for these hormones in the sexually dimorphic pattern observed over later development.
In addition to sex differences in total cell expression of CRF receptors, differences between males and females in CRF receptor cell surface expression, receptor trafficking and G-protein coupling have been documented (Bangasser et al., 2010, 2013; Valentino et al., 2012; Bangasser, 2013). For example, female rats show greater coupling of cortical CRF1 receptor-Gs protein coupling in control conditions, whereas stress increases this coupling in male rats to female stress-naïve levels, and has no effect in females (Bangasser et al., 2010). Furthermore, stress enhances the association between cortical CRF1 receptors and β-arrestin2 (necessary for receptor internalization) in males only (Bangasser et al., 2010). The sex difference in β-arrestin2 association with CRF1 receptors is in line with observations of CRF-induced or stress-induced internalization of CRF receptors in the locus coeruleus (LC) of males and recruitment of CRF receptors to the cellular membrane in the LC of females (Bangasser et al., 2010, 2013). Similarly, proestrus female rats exhibit higher membrane localization of CRF1/2 receptors in hippocampal dendrites compared to males (Williams et al., 2011), suggestive of differential CRF receptor trafficking in the hippocampus among the sexes. Interestingly, there appears to be little role of ovarian steroids since ovariectomy does not alter the coupling or trafficking of CRF receptors in the cortex or LC of females (Bangasser et al., 2010). Overall, it appears that increased sensitivity of neurons in the female brain to CRF (Bangasser et al., 2010, 2013; Bangasser, 2013) may result from increased coupling to Gs proteins, and that sex differences in stress responses and stress-related psychiatric illness could be explained by differential effects of stress on CRF receptor coupling and trafficking (Valentino et al., 2012, 2013; Bangasser, 2013). While stress-induced receptor trafficking is certainly an important phenomenon in the dRN (Waselus et al., 2009; Wood et al., 2013), it remains to be seen whether there are sex differences in CRF receptor coupling or trafficking in this serotonergic region.
Like CRF receptor expression, TPH2 mRNA expression is sexually dimorphic, with levels increasing throughout adolescence in males, but peaking at PND 42 in females before declining into adulthood (Lukkes et al., 2016). Since TPH2 mRNA expression reflects TPH2 levels in neurons (Bach et al., 2014), the latter finding strongly suggests differential serotonergic activity in the dorsal ventrolateral dRN in males and females over late adolescence that may help explain sexually dimorphic outcomes in response to stress exposure during this time (Burke et al., 2017). In contrast to the lack of change in CRF receptor expression across adolescence in the dRN discussed earlier, tryptophan hydroxylase 2 (TPH2; an isozyme of the rate-limiting enzyme of serotonin synthesis) mRNA expression increases in the dorsal ventrolateral dRN, suggesting increased serotonergic activity over development (Lukkes et al., 2016). This pattern mirrors the increase in serotonin and its metabolite 5–hydroxyindoleacetic acid (5-HIAA) that is observed in limbic and brainstem structures up until at least mid-adolescence (PND 45) (Chen et al., 1997).
Overall, the earlier developmental processes characterizing the CRF system, combined with later sex-specific maturation of CRF receptors and serotonergic activity, places CRF interactions with the serotonergic dRN in a prime position to be affected by stressors from very early postnatal development to at least mid-adolescence (Fig. 1). Therefore, dysfunction of CRF-serotonin interactions at the level of the dRN may represent an important neural mechanism by which early-life adversity increases the risk for drug-associated problems later in life.
6. Early-life adversity alters CRF-Serotonin interactions and drug-related behaviors
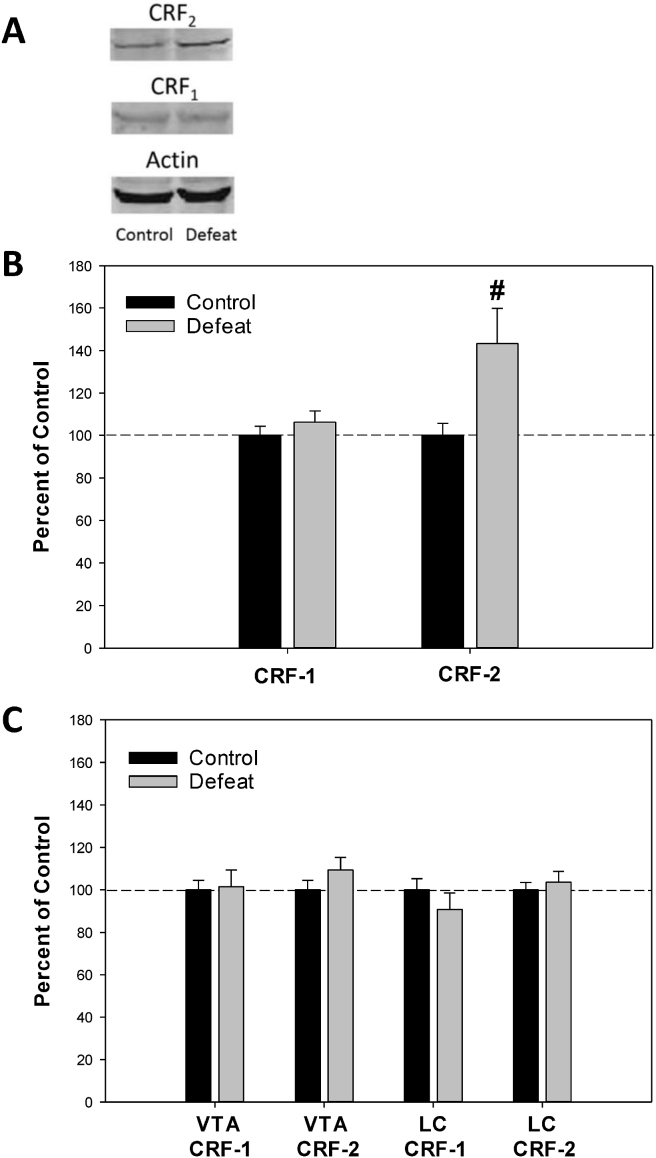
Early-life adversity exerts effects on CRF and serotonergic signaling in mesolimbic reward circuitry that can persist into adulthood (Plotsky et al., 2005; Miczek et al., 2008; Lukkes et al., 2009c; Burke and Miczek, 2014), with deleterious consequences for reward processing and goal-directed behavior. For example, peri-adolescent social stress (social isolation/disruption or social defeat) has been shown to upregulate CRF2 receptors in the lateral dRN of adult male rats (Lukkes et al., 2009a; Bledsoe et al., 2011, Fig. 3A and B). This effect is specific to CRF2 receptors, as expression and activity of CRF1 receptors in the adult dRN is not altered by either peri-adolescent stressor (Lukkes et al., 2009a; Bledsoe et al., 2011, Fig. 3A and B). Moreover, the upregulation of CRF2 receptors in rats defeated in adolescence is only evident in the dRN, and is not seen in either the VTA or the locus coeruleus (LC, Fig. 3C). Increased CRF2 receptors in the lateral dRN following peri-adolescent stress results in prolonged CRF2-induced serotonin release in the NAc core in adulthood (Lukkes et al., 2009a). As described earlier, increased serotonin in the NAc facilitates dopamine release in this region via direct actions on serotonin receptors (Fig. 2), and augments responses to drugs of abuse (Parsons and Justice, 1993; Parsons et al., 1999; Neumaier et al., 2002; Alex and Pehek, 2007). Drugs of abuse also activate extrahypothalamic CRF neurons and increase CRF levels in the brain (Sarnyai et al., 1993; Richter and Weiss, 1999; Zorrilla et al., 2014). Thus, drug- or stress-induced increases in CRF2 receptor activation in the dRN following peri-adolescent stress, and the influence of this on accumbal serotonin and dopamine, represents a neural substrate linking stress experience during adolescence with increased drug responsivity and drug seeking later in life. It should be noted that the studies to date have been performed exclusively in male rodents. As discussed earlier, females have greater CRF2 receptor mRNA expression and also higher TPH2 mRNA levels in the dorsal ventrolateral dRN (Lukkes et al., 2016) that could play an important role in sexually-dimorphic responses to early-life adversity. However, neither the effects of social isolation nor defeat on CRF receptors in the dRN and resultant accumbal monoaminergic activity have been determined for females.
Fig. 3.
Corticotropin-releasing factor (CRF) receptor expression in monoaminergic cell body regions was measured from adult male rats (PND 60) using methods described by Lukkes et al. (2009a). Rats were exposed to either adolescent social defeat or control handling conditions from PND 35–39, as detailed by Watt et al. (2009) – a paradigm known to increase cocaine and amphetamine responses in adulthood (Burke et al., 2011, 2013, 2016; Burke and Miczek, 2015). A) Representative Western blot images of CRF2 receptor, CRF1 receptor, and actin (loading control) expression in the dorsal raphe nucleus (dRN). B) CRF2, but not CRF1 receptor expression was increased in the dRN of adult male rats exposed to adolescent social defeat. C) No changes in either CRF1 or CRF2 receptor expression was observed in the ventral tegmental area (VTA) or locus coeruleus (LC) in adult male rats exposed to adolescent social defeat. #P < 0.05 compared to controls. All data represented as mean values + S.E.M. N = 20 per group.
Enhanced CRF2 receptor-serotonin interactions at the level of the dRN may not only increase drug-seeking behaviors, but also the negative affect commonly associated with drug withdrawal and relapse (Koob et al., 2013). Selective antagonism of CRF2, but not CRF1 receptors globally in the dRN, prevents anxiety-like behaviors of adult rats exposed to adolescent social isolation/disruption and those undergoing amphetamine withdrawal (Lukkes et al., 2009b; Vuong et al., 2010; Bledsoe et al., 2011). Therefore, increased expression of CRF2 receptors in the dRN following peri-adolescent social stress may have important consequences for negative affect-induced reinstatement of drug seeking in individuals exposed to early-life adversity.
In contrast to social isolation/disruption and defeat, maternal separation decreases CRF2 receptor expression while CRF1 receptor mRNA is increased in the adult dRN (Bravo et al., 2011). This difference in CRF receptor mRNA expression may be a function of the earlier developmental time period in which maternal separation is applied, corresponding to the greatest change in CRF receptor expression over development (Fig. 1). If these alterations to CRF receptor mRNA are translated to receptor expression and function, this would result in reduced accumbal serotonin and dopamine responses to stress and drugs of abuse (Fig. 2). On the other hand, dRN serotonergic neurons in adult rats are more responsive to stress (Pollano et al., 2017) and prolonged maternal separation increases the number of serotonin neurons and the capacity for serotonin synthesis (as reflected by TPH mRNA) in the ventrolateral subregion of the rat dRN in adulthood (Gardner et al., 2009; van der Doelen et al., 2017). This is important because it is the ventrolateral subregion of the dRN that innervates the NAc (Van Bockstaele et al., 1993), allowing the potential for greater serotonergic responses in the NAc following maternal separation. Maternal separation has been shown to alter serotonin signaling in the NAc, but results can be conflicting. Male rats exhibit either decreased (Oreland et al., 2011) or increased (Xue et al., 2013) basal NAc serotonin tissue content in adulthood, while acute stress-induced increases in the serotonergic metabolite 5-HIAA within the NAc of middle-aged (15 month) female rats is exacerbated by maternal separation (Arborelius and Eklund, 2007). In line with reduced CRF2 receptor expression (and presumably attenuated facilitation of dopamine in the NAc), adult rats that experienced maternal separation exhibit blunted NAc dopamine release in response to food rewards either before or following chronic mild stress (Minami et al., 2017; Romani-Perez et al., 2017). More aligned with enhanced sensitivity of dRN neurons and greater serotonergic innervation of the NAc, maternal separation augments the accumbal dopaminergic response to both acute amphetamine (Hall et al., 1999) and acute stress (Brake et al., 2004) in adulthood. These contrasting findings may be reconciled by a reward deficiency syndrome which is hypothesized to underlie greater drug seeking following maternal separation (Delavari et al., 2016). This syndrome may reflect both dampened tonic and heightened phasic serotonin release in the NAc, respectively resulting in deficient accumbal dopamine to cause a blunted hedonic state that encourages initial drug taking (Wand, 2008), but augmented stress-induced dopamine release that would promote drug seeking during abstinence.
Epigenetic processes have emerged as a critical mediator between genes and postnatal environment in conferring risk for later emergence of neuropsychiatric disorders, including addiction (see Cadet, 2016; Ajonijebu et al., 2017 for recent reviews). In brief, these processes broadly encompass changes to gene expression that are not secondary to alterations in DNA sequences (Jablonka, 2012). Among the most well studied epigenetic processes are chromatin histone acetylation, which typically promotes gene expression and methylation either of histones or of DNA cytosine-guanine dinucleotide (CpG) sites to result in repression of gene transcription (Smith and Meissner, 2013; Guintivano and Kaminsky, 2016). Of relevance, maternal separation in rats has been shown to cause both increased acetylation and hypomethylation of the Crf promoter in the adult hippocampus and hypothalamus, leading to increased expression of CRF mRNA and protein (Chen et al., 2012; Wang et al., 2014). Maternal separation has also been shown to increase CRF mRNA specifically in the dRN (Bravo et al., 2011). Therefore, neonatal stress-induced epigenetic modifications resulting in increased CRF in the dRN may explain downregulation of the CRF2 receptor in rats exposed to maternal separation (Bravo et al., 2011), given that ligand and receptor availability are often inversely related (as discussed by Weathington et al., 2014). Moreover, CRF-related epigenetic modifications induced by maternal separation are found in first and second generation male and female offspring (Franklin et al., 2010). While more studies in this area are necessary, these findings highlight the possibility that the negative consequences of early-life adversity may be transmitted to subsequent generations via epigenetic modifications of neural CRF systems.
To date, epigenetic effects of peri-adolescent stress on either CRF or CRF receptors do not appear to have received much attention, with most studies examining the influence of stress exposure earlier in postnatal life. Hypomethylation at multiple CpG sites of the Crf2 promoter, which results in greater promoter activity indicating disinhibition of gene expression, has been observed in otherwise healthy individuals who report higher generalized anxiety (Schartner et al., 2017). This phenotype is reminiscent of increased anxiety and increased CRF2 receptor expression in the dRN of adult rats exposed to social isolation/disruption during adolescence (Lukkes et al., 2009a; b; Bledsoe et al., 2011). As such, it is tempting to speculate that long-term upregulation of CRF2 receptor expression in the dRN as observed following early-life adversity (Lukkes et al., 2009a, Fig. 3) may result from reduced methylation of the Crf2 gene, but this is yet to be determined. If true, it may have implications for understanding sex differences in behavioral responses to abused substances following early-life (particularly adolescent) adversity, given that adolescent female rats show a 10-fold higher expression of CRF2 mRNA in the dRN compared to males (Lukkes et al., 2016). This sex specific ontogeny could conceivably increase susceptibility of females to stress-induced changes in epigenetic regulation of the CRF2 receptor, with detrimental consequences for serotonergic and dopaminergic signaling in reward pathways.
7. Conclusions
Both human and preclinical studies clearly demonstrate that early-life adversity is associated with a greater propensity for substance abuse in later life. Converging evidence suggests that this may be in part due to alterations of CRF2 receptors in the dRN following early-life adversity, which have the ability to promote long-lasting alterations to accumbal monoaminergic transmission and related behaviors. While heightened drug- and alcohol-related behaviors appear similar between pre-adolescent stress (e.g. maternal separation) and stressors later in peri-adolescent development (e.g. social isolation/disruption and social defeat), underlying mechanisms may differ with opposite effects on CFR2 receptor expression and activity within the mesolimbic system. Regardless, the CRF2 receptor may represent a pharmacotherapeutic target for reducing the negative consequences of early-life adversity, but translational studies are hampered by the lack of CRF2 receptor antagonists that effectively cross the blood-brain barrier (Forster et al., 2012; Vinzant et al., 2017). Recent progress in nanomaterials have been employed to facilitate CRF2 receptor ligands crossing the blood-brain barrier to have efficacy at the level of the dRN (Vinzant et al., 2017), increasing the promise of the CRF2 receptor as a novel target for reducing the impact of early-life adversity. However, advancements in preventing and treating substance dependence associated with early-life adversity will continue to be hampered by a paucity of neurobiological studies within preclinical female models. Future work should be directed at understanding sex differences in CRF-serotonin interactions within the dRN and how sexually dimorphic expression of CRF receptors in the dRN, and in dRN serotonin development, translate to greater vulnerability of women to be negatively affected by early-life adversity.
Acknowledgements
This work was supported by the National Institute on Drug Abuse (R15 DA035478 to MJW and RO1 DA019921 to GLF), the National Science Foundation (1257679 to MJW), and a South Dakota Governor's Research Center Grant – Center for Genetics and Behavioral Health.
References
- Ajonijebu D.C., Abboussi O., Russell V.A., Mabandla M.V., Daniels W.M.U. Epigenetics: a link between addiction and social environment. Cell. Mol. Life Sci. 2017;74:2735–2747. doi: 10.1007/s00018-017-2493-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alex K.D., Pehek E.A. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol. Ther. 2007;113:296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander B.K., Coambs R.B., Hadaway P.F. The effect of housing and gender on morphine self- administration in rats. Psychopharmacology (Berlin) 1978;58:175–179. doi: 10.1007/BF00426903. [DOI] [PubMed] [Google Scholar]
- Amat J., Tamblyn J.P., Paul E.D., Bland S.T., Amat P., Foster A.C., Watkins L.R., Maier S.F. Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neuroscience. 2004;129:509–519. doi: 10.1016/j.neuroscience.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Arborelius L., Eklund M.B. Both long and brief maternal separation produces persistent changes in tissue levels of brain monoamines in middle-aged female rats. Neuroscience. 2007;145:738–750. doi: 10.1016/j.neuroscience.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S., Yi S.J., Baram T.Z. Developmental profile of messenger RNA for the corticotropin-releasing hormone receptor in the rat limbic system. Brain Res. Dev. Brain Res. 1996;91:159–163. doi: 10.1016/0165-3806(95)00158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarendse P.J., Limpens J.H., Vanderschuren L.J. Disrupted social development enhances the motivation for cocaine in rats. Psychopharmacology (Berlin) 2014;231:1695–1704. doi: 10.1007/s00213-013-3362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach H., Arango V., Kassir S.A., Tsaava T., Dwork A.J., Mann J.J., Underwood M.D. Alcoholics have more tryptophan hydroxylase 2 mRNA and protein in the dorsal and median raphe nuclei. Alcohol Clin. Exp. Res. 2014;38:1894–1901. doi: 10.1111/acer.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball G.F., Balthazart J., McCarthy M.M. Is it useful to view the brain as a secondary sexual characteristic? Neurosci. Biobehav. Rev. 2014;46:628–638. doi: 10.1016/j.neubiorev.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Bardo M.T. Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Crit. Rev. Neurobiol. 1998;12:37–67. doi: 10.1615/critrevneurobiol.v12.i1-2.30. [DOI] [PubMed] [Google Scholar]
- Bardo M.T., Neisewander J.L., Kelly T.H. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol. Rev. 2013;65:255–290. doi: 10.1124/pr.111.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Curtis A., Reyes B.A., Bethea T.T., Parastatidis I., Ischiropoulos H. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol. Psychiatr. 2010;15:896–904. doi: 10.1038/mp.2010.66. mp201066 [pii] 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A. Sex differences in stress-related receptors: ''micro'' differences with ''macro'' implications for mood and anxiety disorders. Biol. Sex Differ. 2013;4:2. doi: 10.1186/2042-6410-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Reyes B.A., Piel D., Garachh V., Zhang X.Y., Plona Z.M., Van Bockstaele E.J., Beck S.G., Valentino R.J. Increased vulnerability of the brain norepinephrine system of females to corticotropin-releasing factor overexpression. Mol. Psychiatr. 2013;18:166–173. doi: 10.1038/mp.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J.B., Monteggia L.M., Perrot-Sinal T.S., Romeo R.D., Taylor J.R., Yehuda R., Bale T.L. Stress and disease: is being female a predisposing factor? J. Neurosci. 2007;27:11851–11855. doi: 10.1523/JNEUROSCI.3565-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J.B., Perry A.N., Westenbroek C. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol. Sex Differ. 2012;3:14. doi: 10.1186/2042-6410-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea C.L., Reddy A.P., Flowers M., Shapiro R.A., Colman R.J., Abbott D.H., Levine J.E. High fat diet decreases beneficial effects of estrogen on serotonin-related gene expression in marmosets. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2015;58:71–80. doi: 10.1016/j.pnpbp.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkqvist K. Social defeat as a stressor in humans. Physiol. Behav. 2001;73:435–442. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- Bledsoe A.C., Oliver K.M., Scholl J.L., Forster G.L. Anxiety states induced by post-weaning social isolation are mediated by CRF receptors in the dorsal raphe nucleus. Brain Res. Bull. 2011;85:117–122. doi: 10.1016/j.brainresbull.2011.03.003. S0361-9230(11)00080-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers B.J., Henry M.B., Thielen R.J., McBride W.J. Serotonin 5-HT(2) receptor stimulation of dopamine release in the posterior but not anterior nucleus accumbens of the rat. J. Neurochem. 2000;75:1625–1633. doi: 10.1046/j.1471-4159.2000.0751625.x. [DOI] [PubMed] [Google Scholar]
- Boyle A.E., Gill K., Smith B.R., Amit Z. Differential effects of an early housing manipulation on cocaine-induced activity and self-administration in laboratory rats. Pharmacol. Biochem. Behav. 1991;39:269–274. doi: 10.1016/0091-3057(91)90178-5. [DOI] [PubMed] [Google Scholar]
- Brake W.G., Zhang T.Y., Diorio J., Meaney M.J., Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur. J. Neurosci. 2004;19:1863–1874. doi: 10.1111/j.1460-9568.2004.03286.x. [DOI] [PubMed] [Google Scholar]
- Bravo J.A., Dinan T.G., Cryan J.F. Alterations in the central CRF system of two different rat models of comorbid depression and functional gastrointestinal disorders. Int. J. Neuropsychopharmacol. 2011;14:666–683. doi: 10.1017/S1461145710000994. [DOI] [PubMed] [Google Scholar]
- Burke A.R., DeBold J.F., Miczek K.A. CRF type 1 receptor antagonism in ventral tegmental area of adolescent rats during social defeat: prevention of escalated cocaine self-administration in adulthood and behavioral adaptations during adolescence. Psychopharmacology (Berlin) 2016;233:2727–2736. doi: 10.1007/s00213-016-4336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke A.R., Forster G.L., Novick A.M., Roberts C.L., Watt M.J. Effects of adolescent social defeat on adult amphetamine-induced locomotion and corticoaccumbal dopamine release in male rats. Neuropharmacology. 2013;67:359–369. doi: 10.1016/j.neuropharm.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke A.R., McCormick C.M., Pellis S.M., Lukkes J.L. Impact of adolescent social experiences on behavior and neural circuits implicated in mental illnesses. Neurosci. Biobehav. Rev. 2017;76:280–300. doi: 10.1016/j.neubiorev.2017.01.018. [DOI] [PubMed] [Google Scholar]
- Burke A.R., Miczek K.A. Escalation of cocaine self-administration in adulthood after social defeat of adolescent rats: role of social experience and adaptive coping behavior. Psychopharmacology (Berlin) 2015;232:3067–3079. doi: 10.1007/s00213-015-3947-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke A.R., Watt M.J., Forster G.L. Adolescent social defeat increases adult amphetamine conditioned place preference and alters D2 dopamine receptor expression. Neuroscience. 2011;197:269–279. doi: 10.1016/j.neuroscience.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke A.R., Miczek K.A. Stress in adolescence and drugs of abuse in rodent models: role of dopamine, CRF, and HPA axis. Psychopharmacology (Berlin) 2014;231:1557–1580. doi: 10.1007/s00213-013-3369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet J.L. Epigenetics of stress, addiction, and resilience: therapeutic implications. Mol. Neurobiol. 2016;53:545–560. doi: 10.1007/s12035-014-9040-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles N.E., Mathias C.W., Acheson A., Bray B.C., Ryan S.R., Lake S.L., Liang Y., Dougherty D.M. Increased pre- and early-adolescent stress in youth with a family history of substance use disorder and early substance use initiation. J. Youth Adolesc. 2015;44:1954–1967. doi: 10.1007/s10964-015-0271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles N.E., Ryan S.R., Acheson A., Mathias C.W., Liang Y., Dougherty D.M. Childhood stress exposure among preadolescents with and without family histories of substance use disorders. J. Soc. Psychol. Addict. Behav. 2015;29:192–200. doi: 10.1037/adb0000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.C., Turiak G., Galler J., Volicer L. Postnatal changes of brain monoamine levels in prenatally malnourished and control rats. Int. J. Dev. Neurosci. 1997;15:257–263. doi: 10.1016/s0736-5748(96)00121-9. [DOI] [PubMed] [Google Scholar]
- Chen J., Evans A.N., Liu Y., Honda M., Saavedra J.M., Aguilera G. Maternal deprivation in rats is associated with corticotrophin-releasing hormone (CRH) promoter hypomethylation and enhances CRH transcriptional responses to stress in adulthood. J. Neuroendocrinol. 2012;24:1055–1064. doi: 10.1111/j.1365-2826.2012.02306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Bender R.A., Frotscher M., Baram T.Z. Novel and transient populations of corticotropin-releasing hormone-expressing neurons in developing hippocampus suggest unique functional roles: a quantitative spatiotemporal analysis. J. Neurosci. 2001;21:7171–7181. doi: 10.1523/JNEUROSCI.21-18-07171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham K.A., Anastasio N.C. Serotonin at the nexus of impulsivity and cue reactivity in cocaine addiction. Neuropharmacology. 2014;76:460–478. doi: 10.1016/j.neuropharm.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong B., Ni X. Estrogen up-regulates CRHR2 expression via demethylation of CRHR2 gene promoter in cardiomyocytes. Int. J. Cardiol. 2014;172:496–497. doi: 10.1016/j.ijcard.2014.01.017. [DOI] [PubMed] [Google Scholar]
- Cooper M.A., Huhman K.L. Corticotropin-releasing factor receptors in the dorsal raphe nucleus modulate social behavior in Syrian hamsters. Psychopharmacology. 2007;194:297–307. doi: 10.1007/s00213-007-0849-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland W.E., Wolke D., Angold A., Costello E.J. Adult psychiatric outcomes of bullying and being bullied by peers in childhood and adolescence. JAMA Psych. 2013;70:419–426. doi: 10.1001/jamapsychiatry.2013.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day H.E., Greenwood B.N., Hammack S.E., Watkins L.R., Fleshner M., Maier S.F., Campeau S. Differential expression of 5HT-1A, alpha 1b adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. J. Comp. Neurol. 2004;474:364–378. doi: 10.1002/cne.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Deurwaerdere P., Stinus L., Spampinato U. Opposite change of in vivo dopamine release in the rat nucleus accumbens and striatum that follows electrical stimulation of dorsal raphe nucleus: role of 5-HT3 receptors. J. Neurosci. 1998;18:6528–6538. doi: 10.1523/JNEUROSCI.18-16-06528.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delavari F., Sheibani V., Esmaeili-Mahani S., Nakhaee N. Maternal separation and the risk of drug abuse in later life. Addict Health. 2016;8:107–114. [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A., Markou A. Neonatal maternal separation exacerbates the reward-enhancing effect of acute amphetamine administration and the anhedonic effect of repeated social defeat in adult rats. Neuroscience. 2010;170:1189–1198. doi: 10.1016/j.neuroscience.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav. Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Enoch M.A. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology (Berlin) 2011;214:17–31. doi: 10.1007/s00213-010-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo E.P., Hammen C.L., Connolly N.P., Brennan P.A., Najman J.M., Bor W. Stress sensitization and adolescent depressive severity as a function of childhood adversity: a link to anxiety disorders. J. Abnorm. Child Psychol. 2007;35:287–299. doi: 10.1007/s10802-006-9090-3. [DOI] [PubMed] [Google Scholar]
- Flagel S.B., Vázquez D.M., Robinson T.E. Manipulations during the second, but not the first, week of life increase susceptibility to cocaine self-administration in female rats. Neuropsychopharmacology. 2003;28:1741. doi: 10.1038/sj.npp.1300228. [DOI] [PubMed] [Google Scholar]
- Floresco S.B. The nucleus accumbens: an interface between cognition, emotion, and action. Annu. Rev. Psychol. 2015;66:25–52. doi: 10.1146/annurev-psych-010213-115159. [DOI] [PubMed] [Google Scholar]
- Fone K.C., Porkess M.V. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci. Biobehav. Rev. 2008;32:1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Forster G.L., Novick A.M., Scholl J.L., Watt M.J. The role of the amygdala in anxiety disorders. In: Ferry B., editor. Rejeka, Croatia: InTech. 2012. [Google Scholar]
- Forster G.L., Pringle R.B., Mouw N.J., Vuong S.M., Watt M.J., Burke A.R., Lowry C.A., Summers C.H., Renner K.J. Corticotropin-releasing factor in the dorsal raphe nucleus increases medial prefrontal cortical serotonin via type 2 receptors and median raphe nucleus activity. Eur. J. Neurosci. 2008;28:299–310. doi: 10.1111/j.1460-9568.2008.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J., Lowry C. Corticotropin-releasing factor-related peptides, serotonergic systems, and emotional behavior. Front. Neurosci. 2013;7:169. doi: 10.3389/fnins.2013.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T.B., Russig H., Weiss I.C., Gräff J., Linder N., Michalon A. Epigenetic transmission of the impact of early stress across generations. Biol.Psych. 2010;68:408–415. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- García-Pardo M.P., Blanco-Gandía M.C., Valiente-Lluch M., Rodríguez-Arias M., Miñarro J., Aguilar M.A. Long-term effects of repeated social stress on the conditioned place preference induced by MDMA in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2015;63:98–109. doi: 10.1016/j.pnpbp.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Gardner K.L., Hale M.W., Oldfield S., Lightman S.L., Plotsky P.M., Lowry C.A. Adverse experience during early life and adulthood interact to elevate tph2 mRNA expression in serotonergic neurons within the dorsal raphe nucleus. Neuroscience. 2009;163:991–1001. doi: 10.1016/j.neuroscience.2009.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert D.R., Shekhar A., Morin S.M., Hipskind P.A., Zink C., Gackenheimer S.L., Shaw J., Fitz S.D., Sajdyk T.J. Stress and central Urocortin increase anxiety-like behavior in the social interaction test via the CRF1 receptor. Eur. J. Pharmacol. 2005;509:145–153. doi: 10.1016/j.ejphar.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Gipson C.D., Beckmann J.S., El-Maraghi S., Marusich J.A., Bardo M.T. Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacology (Berlin) 2011;214:557–566. doi: 10.1007/s00213-010-2060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondre-Lewis M.C., Warnock K.T., Wang H., June H.L., Jr., Bell K.A., Rabe H., Tiruveedhula V.V., Cook J., Luddens H., Aurelian L., June H.L., Sr. Early life stress is a risk factor for excessive alcohol drinking and impulsivity in adults and is mediated via a CRF/GABA(A) mechanism. Stress. 2016;19:235–247. doi: 10.3109/10253890.2016.1160280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace A.A., Floresco S.B., Goto Y., Lodge D.J. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Gray T.S. Amygdaloid CRF pathways. Role in autonomic, neuroendocrine, and behavioral responses to stress. Ann. N. Y. Acad. Sci. 1993;697:53–60. doi: 10.1111/j.1749-6632.1993.tb49922.x. [DOI] [PubMed] [Google Scholar]
- Guintivano J., Kaminsky Z.A. Role of epigenetic factors in the development of mental illness throughout life. Neurosci. Res. 2016;102:56–66. doi: 10.1016/j.neures.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Gustafsson L., Ploj K., Nylander I. Effects of maternal separation on voluntary ethanol intake and brain peptide systems in female Wistar rats. Pharmacol. Biochem. Behav. 2005;81:506–516. doi: 10.1016/j.pbb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Hall F.S. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit. Rev. Neurobiol. 1998;12:129–162. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- Hall F.S., Wilkinson L.S., Humby T., Robbins T.W. Maternal deprivation of neonatal rats produces enduring changes in dopamine function. Synapse. 1999;32:37–43. doi: 10.1002/(SICI)1098-2396(199904)32:1<37::AID-SYN5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Hammack S.E., Schmid M.J., LoPresti M.L., Der-Avakian A., Pellymounter M.A., Foster A.C.…Maier S.F. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J. Neurosci. 2003;23:1019–1025. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P.A., Hoffmann N.G., Edwall G.E. Differential drug use patterns among sexually abused adolescent girls in treatment for chemical dependency. Int. J. Addict. 1989;24:499–514. doi: 10.3109/10826088909081832. [DOI] [PubMed] [Google Scholar]
- Heidbreder C.A., Weiss I.C., Domeney A.M., Pryce C., Homberg J., Hedou G., Feldon J., Moran M.C., Nelson P. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience. 2000;100:749–768. doi: 10.1016/s0306-4522(00)00336-5. [DOI] [PubMed] [Google Scholar]
- Heim C., Newport D.J., Mletzko T., Miller A.H., Nemeroff C.B. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Heinrichs S.C., Lapsansky J., Lovenberg T.W., De Souza E.B., Chalmers D.T. Corticotropin releasing factor CRF1, but not CRF2, receptors mediate anxiogenic-like behavior. Regul. Pept. 1997;71:15–21. doi: 10.1016/s0167-0115(97)01005-7. [DOI] [PubMed] [Google Scholar]
- Howes S.R., Dalley J.W., Morrison C.H., Robbins T.W., Everitt B.J. Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacology (Berlin) 2000;151:55–63. doi: 10.1007/s002130000451. [DOI] [PubMed] [Google Scholar]
- Hudson A., Stamp J.A. Ovarian hormones and propensity to drug relapse: a review. Neurosci. Biobehav. Rev. 2011;35:427–436. doi: 10.1016/j.neubiorev.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Huot R.L., Thrivikraman K.V., Meaney M.J., Plotsky P.M. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berlin) 2001;158:366–373. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- Hyman S.M., Garcia M., Sinha R. Gender specific associations between types of childhood maltreatment and the onset, escalation and severity of substance use in cocaine dependent adults. Am. J. Drug Alcohol Abuse. 2006;32:655–664. doi: 10.1080/10623320600919193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman S.M., Paliwal P., Chaplin T.M., Mazure C.M., Rounsaville B.J., Sinha R. Severity of childhood trauma is predictive of cocaine relapse outcomes in women but not men. Drug Alcohol Depend. 2008;92:208–216. doi: 10.1016/j.drugalcdep.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T.R., Battaglia G., Fairbanks D.W., De Souza E.B. The ontogeny of brain receptors for corticotropin-releasing factor and the development of their functional association with adenylate cyclase. J. Neurosci. 1988;8:4151–4158. doi: 10.1523/JNEUROSCI.08-11-04151.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonka E. Epigenetic variations in heredity and evolution. Clin. Pharmacol. Ther. 2012;92:683–688. doi: 10.1038/clpt.2012.158. [DOI] [PubMed] [Google Scholar]
- Jahn O., Radulovic J., Stiedl O., Tezval H., Eckart K., Spiess J. Corticotropin-releasing factor binding protein--a ligand trap? Mini Rev. Med. Chem. 2005;5:953–960. doi: 10.2174/138955705774329500. [DOI] [PubMed] [Google Scholar]
- Jawahar M.C., Murgatroyd C., Harrison E.L., Baune B.T. Epigenetic alterations following early postnatal stress: a review on novel aetiological mechanisms of common psychiatric disorders. Clin. Epigenet. 2015;14:122. doi: 10.1186/s13148-015-0156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski J.N., Francis D.D., Brommer C.L., Morgan E.T., Kuhar M.J. Effects of early maternal separation on ethanol intake, GABA receptors and metabolizing enzymes in adult rats. Psychopharmacology (Berlin) 2005;181:8–15. doi: 10.1007/s00213-005-2232-4. [DOI] [PubMed] [Google Scholar]
- Katsidoni V., Apazoglou K., Panagis G. Role of serotonin 5-HT2A and 5-HT2C receptors on brain stimulation reward and the reward-facilitating effect of cocaine. Psychopharmacology (Berlin) 2011;213:337–354. doi: 10.1007/s00213-010-1887-7. [DOI] [PubMed] [Google Scholar]
- Kennedy B.C., Panksepp J.B., Runckel P.A., Lahvis G.P. Social influences on morphine-conditioned place preference in adolescent BALB/cJ and C57BL/6J mice. Psychopharmacology (Berlin) 2011;219:923–932. doi: 10.1007/s00213-011-2421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin T.E., Campbell J.C., Ploense K., Knight C.P., Bagley J. Prenatal stress and adult drug-seeking behavior: interactions with genes and relation to nondrug-related behavior. Adv Neurobiol. 2015;10:75–100. doi: 10.1007/978-1-4939-1372-5_5. [DOI] [PubMed] [Google Scholar]
- Kirby L.G., Rice K.C., Valentino R.J. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Kolb B., Gibb R. Brain plasticity and behaviour in the developing brain. J. Can. Acad. Child Adol. Psych. 2011;20:265–276. [PMC free article] [PubMed] [Google Scholar]
- Kolb B., Mychasiuk R., Muhammad A., Gibb R. Brain plasticity in the developing brain. Prog. Brain Res. 2013;207:35–64. doi: 10.1016/B978-0-444-63327-9.00005-9. [DOI] [PubMed] [Google Scholar]
- Koob G.F., Buck C.L., Cohen A., Edwards S., Park P.E., Schlosburg J.E., Schmeichel B., Vendruscolo L.F., Wade C.L., Whitfield T.W., Jr., George O. Addiction as a stress surfeit disorder. Neuropharmacology. 2013;76:370–382. doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F. Addiction is a reward deficit and stress surfeit disorder. Front. Psychiatr. 2013;4:72. doi: 10.3389/fpsyt.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosi A., Baram T.Z. The central corticotropin releasing factor system during development and adulthood. Eur. J. Pharmacol. 2008;583:204–214. doi: 10.1016/j.ejphar.2007.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten T.A., Kim J.J., Lee H.J. Early life manipulations alter learning and memory in rats. Neurosci. Biobehav. Rev. 2012;36:1985–2006. doi: 10.1016/j.neubiorev.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten T.A., Miserendino M.J., Kehoe P. Enhanced acquisition of cocaine self-administration in adult rats with neonatal isolation stress experience. Brain Res. 2000;875:44–50. doi: 10.1016/s0006-8993(00)02595-6. [DOI] [PubMed] [Google Scholar]
- Kosten T.A., Sanchez H., Zhang X.Y., Kehoe P. Neonatal isolation enhances acquisition of cocaine self-administration and food responding in female rats. Behav. Brain Res. 2004;151:137–149. doi: 10.1016/j.bbr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Kosten T.A., Zhang X.Y., Kehoe P. Heightened cocaine and food self-administration in female rats with neonatal isolation experience. Neuropsychopharmacology. 2006;31:70–76. doi: 10.1038/sj.npp.1300779. [DOI] [PubMed] [Google Scholar]
- Lewis C.R., Bastle R.M., Manning T.B., Himes S.M., Fennig P., Conrad P.R., Colwell J., Pagni B.A., Hess L.A., Matekel C.G., Newbern J.M., Olive M.F. Interactions between early life stress, nucleus accumbens mecp2 expression, and methamphetamine self-administration in male rats. Neuropsychopharmacology. 2016;41:2851–2861. doi: 10.1038/npp.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C.R., Staudinger K., Scheck L., Olive M.F. The effects of maternal separation on adult methamphetamine self-administration, extinction, reinstatement, and MeCP2 immunoreactivity in the nucleus accumbens. Front. Psychiatr. 2013;4:55. doi: 10.3389/fpsyt.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Iacono L., Valzania A., Visco-Comandini F., Viscomi M.T., Felsani A., Puglisi-Allegra S., Carola V. Regulation of nucleus accumbens transcript levels in mice by early-life social stress and cocaine. Neuropharmacology. 2016;103:183–194. doi: 10.1016/j.neuropharm.2015.12.011. [DOI] [PubMed] [Google Scholar]
- Lopez M.F., Doremus-Fitzwater T.L., Becker H.C. Chronic social isolation and chronic variable stress during early development induce later elevated ethanol intake in adult C57BL/6J mice. Alcohol. 2011;45:355–364. doi: 10.1016/j.alcohol.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry C.A., Moore F.L. Regulation of behavioral responses by corticotropin-releasing factor. Gen. Comp. Endocrinol. 2006;146:19–27. doi: 10.1016/j.ygcen.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Lowry C.A., Hale M.W., Evans A.K., Heerkens J., Staub D.R., Gasser P.J., Shekhar A. Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Ann. N. Y. Acad. Sci. 2008;1148:86–94. doi: 10.1196/annals.1410.004. [DOI] [PubMed] [Google Scholar]
- Lukkes J.L., Forster G.L., Renner K.J., Summers C.H. Corticotropin-releasing factor 1 and 2 receptors in the dorsal raphé differentially affect serotonin release in the nucleus accumbens. Eur. J. Pharmacol. 2008;578:185–193. doi: 10.1016/j.ejphar.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes J.L., Summers C.H., Scholl J.L., Renner K.J., Forster G.L. Early life social isolation alters corticotropin-releasing factor responses in adult rats. Neuroscience. 2009;158:845–855. doi: 10.1016/j.neuroscience.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes J., Vuong S., Scholl J., Oliver H., Forster G. Corticotropin-releasing factor receptor antagonism within the dorsal raphe nucleus reduces social anxiety-like behavior after early-life social isolation. J. Neurosci. 2009;29:9955–9960. doi: 10.1523/JNEUROSCI.0854-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes J.L. Watt, Lowry M.J., Forster C.A., L G. Consequences of post-weaning social isolation on anxiety behavior and related neural circuits in rodents. Front. Behav. Neurosci. 2009;3:1–12. doi: 10.3389/neuro.08.018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes J.L., Staub D.R., Dietrich A., Truitt W., Neufeld-Cohen A., Chen A. Topographical distribution of corticotropin-releasing factor type2receptor-like immunoreactivity in the rat dorsal raphe nucleus: co-localization with tryptophan hydroxylase. Neuroscience. 2011;183:47–63. doi: 10.1016/j.neuroscience.2011.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes J.L., Norman K.J., Meda S., Andersen S.L. Sex differences in the ontogeny of CRF receptors during adolescent development in the dorsal raphe nucleus and ventral tegmental area. Synapse. 2016;70:125–132. doi: 10.1002/syn.21882. [DOI] [PubMed] [Google Scholar]
- Lynch W.J., Mangini L.D., Taylor J.R. Neonatal isolation stress potentiates cocaine seeking behavior in adult male and female rats. Neuropsychopharmacology. 2005;30:322–329. doi: 10.1038/sj.npp.1300594. [DOI] [PubMed] [Google Scholar]
- Mantsch J.R., Vranjkovic O., Twining R.C., Gasser P.J., McReynolds J.R., Blacktop J.M. Neurobiological mechanisms that contribute to stress-related cocaine use. Neuropharmacology. 2014;76:383–394. doi: 10.1016/j.neuropharm.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews I.Z., Mills R.G., McCormick C.M. Chronic social stress in adolescence influenced both amphetamine conditioned place preference and locomotor sensitization. Dev. Psychobiol. 2008;50:451–459. doi: 10.1002/dev.20299. [DOI] [PubMed] [Google Scholar]
- McCool B.A., Chappell A.M. Early social isolation in male Long-Evans rats alters both appetitive and consummatory behaviors expressed during operant ethanol self-administration. Alcohol Clin. Exp. Res. 2009;33:273–282. doi: 10.1111/j.1530-0277.2008.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick C.M., Robarts D., Kopeikina K., Kelsey J.E. Long-lasting, sex- and age-specific effects of social stressors on corticosterone responses to restraint and on locomotor responses to psychostimulants in rats. Horm. Behav. 2005;48:64–74. doi: 10.1016/j.yhbeh.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Merrick M.T., Ports K.A., Ford D.C., Afifi T.O., Gershoff E.T., Grogan-Kaylor A. Unpacking the impact of adverse childhood experiences on adult mental health. Child Abuse Negl. 2017;69:10–19. doi: 10.1016/j.chiabu.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina N., Marinelli-Casey P., Hillhouse M., Rawson R., Hunter J., Ang A. Childhood adverse events and methamphetamine use among men and women. J. Psychoact. Drugs Suppl. 2008;5:399–409. doi: 10.1080/02791072.2008.10400667. [DOI] [PubMed] [Google Scholar]
- Michaels C.C., Holtzman S.G. Early postnatal stress alters place conditioning to both mu- and kappa- opioid agonists. J. Pharmacol. Exp. Therapeut. 2008;325:313–318. doi: 10.1124/jpet.107.129908. [DOI] [PubMed] [Google Scholar]
- Miczek K.A., Yap J.J., Covington H.E., 3rd Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol. Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami S., Satoyoshi H., Ide S., Inoue T., Yoshioka M., Minami M. Suppression of reward-induced dopamine release in the nucleus accumbens in animal models of depression: differential responses to drug treatment. Neurosci. Lett. 2017;650:72–76. doi: 10.1016/j.neulet.2017.04.028. [DOI] [PubMed] [Google Scholar]
- Moffett M.C., Harley J., Francis D., Sanghani S.P., Davis W.I., Kuhar M.J. Maternal separation and handling affects cocaine self-administration in both the treated pups as adults and the dams. J. Pharmacol. Exp. Therapeut. 2006;317:1210–1218. doi: 10.1124/jpet.106.101139. [DOI] [PubMed] [Google Scholar]
- Muller C.P., Homberg J.R. The role of serotonin in drug use and addiction. Behav. Brain Res. 2015;277:146–192. doi: 10.1016/j.bbr.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Muller M.B., Zimmermann S., Sillaber I., Hagemeyer T.P., Deussing J.M., Timpl P., Kormann M.S., Droste S.K., Kuhn R., Reul J.M., Holsboer F., Wurst W. Limbic corticotropin releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat. Neurosci. 2003;6:1100–1107. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- Navailles S., Moison D., Ryczko D., Spampinato U. Region-dependent regulation of mesoaccumbens dopamine neurons in vivo by the constitutive activity of central serotonin2C receptors. J. Neurochem. 2006;99:1311–1319. doi: 10.1111/j.1471-4159.2006.04188.x. [DOI] [PubMed] [Google Scholar]
- Neisewander J.L., Peartree N.A., Pentkowski N.S. Emotional valence and context of social influences on drug abuse-related behavior in animal models of social stress and prosocial interaction. Psychopharmacol. (Berl) 2012;224(1):33–56. doi: 10.1007/s00213-012-2853-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E.C., Heath A.C., Lynskey M.T., Bucholz K.K., Madden P.A., Statham D.J., Martin N.G. Childhood sexual abuse and risks for licit and illicit drug-related outcomes: a twin study. Psychol. Med. 2006;36:1473–1483. doi: 10.1017/S0033291706008397. [DOI] [PubMed] [Google Scholar]
- Neumaier J.F., Vincow E.S., Arvanitogiannis A., Wise R.A., Carlezon W.A., Jr. Elevated expression of 5-HT1B receptors in nucleus accumbens efferents sensitizes animals to cocaine. J. Neurosci. 2002;22:10856–10863. doi: 10.1523/JNEUROSCI.22-24-10856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick A.M., Miiller L.C., Forster G.L., Watt M.J. Adolescent social defeat decreases spatial working memory performance in adulthood. Behav. Brain Funct. 2013;9:39. doi: 10.1186/1744-9081-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander I., Roman E. Is the rodent maternal separation model a valid and effective model for studies on the early-life impact on ethanol consumption? Psychopharmacology (Berlin) 2013;229:555–569. doi: 10.1007/s00213-013-3217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ompad D.C., Ikeda R.M., Shah N., Fuller C.M., Bailey S., Morse E., Kerndt P., Maslow C., Wu Y., Vlahov D., Garfein R., Strathdee S.A. Childhood sexual abuse and age at initiation of injection drug use. Am. J. Publ. Health. 2005;95:703–709. doi: 10.2105/AJPH.2003.019372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreland S., Raudkivi K., Oreland L., Harro J., Arborelius L., Nylander I. Ethanol-induced effects on the dopamine and serotonin systems in adult Wistar rats are dependent on early-life experiences. Brain Res. 2011;1405:57–68. doi: 10.1016/j.brainres.2011.06.031. [DOI] [PubMed] [Google Scholar]
- Ouzir M., Errami M. Etiological theories of addiction: a comprehensive update on neurobiological, genetic and behavioural vulnerability. Pharmacol. Biochem. Behav. 2016;148:59–68. doi: 10.1016/j.pbb.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Parsons L.H., Justice J.B., Jr. Perfusate serotonin increases extracellular dopamine in the nucleus accumbens as measured by in vivo microdialysis. Brain Res. 1993;606:195–199. doi: 10.1016/0006-8993(93)90984-u. [DOI] [PubMed] [Google Scholar]
- Parsons L.H., Koob G.F., Weiss F. RU 24969, a 5-HT1B/1A receptor agonist, potentiates cocaine-induced increases in nucleus accumbens dopamine. Synapse. 1999;32:132–135. doi: 10.1002/(SICI)1098-2396(199905)32:2<132::AID-SYN6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Pernar L., Curtis A.L., Vale W.W., Rivier J.E., Valentino R.J. Selective activation of corticotropin-releasing factor-2 receptors on neurochemically identified neurons in the rat dorsal raphe nucleus reveals dual actions. J. Neurosci. 2004;24:1305–1311. doi: 10.1523/JNEUROSCI.2885-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A.G., Vacca G., Ahn S. A top-down perspective on dopamine, motivation and memory. Pharmacol. Biochem. Behav. 2008;90:236–249. doi: 10.1016/j.pbb.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Phillips G.D., Howes S.R., Whitelaw R.B., Wilkinson L.S., Robbins T.W., Everitt B.J. Isolation rearing enhances the locomotor response to cocaine and a novel environment, but impairs the intravenous self-administration of cocaine. Psychopharmacology (Berlin) 1994;115:407–418. doi: 10.1007/BF02245084. [DOI] [PubMed] [Google Scholar]
- Pilowsky D.J., Keyes K.M., Hasin D.S. Adverse childhood events and lifetime alcohol dependence. Am. J. Publ. Health. 2009;99:258–263. doi: 10.2105/AJPH.2008.139006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploj K., Roman E., Nylander I. Long-term effects of maternal separation on ethanol intake and brain opioid and dopamine receptors in male Wistar rats. Neuroscience. 2003;121:787–799. doi: 10.1016/s0306-4522(03)00499-8. [DOI] [PubMed] [Google Scholar]
- Plotsky P.M., Thrivikraman K.V., Nemeroff C.B., Caldji C., Sharma S., Meaney M.J. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Pollano A., Trujillo V., Suarez M.M. How does early maternal separation and chronic stress in adult rats affect the immunoreactivity of serotonergic neurons within the dorsal raphe nucleus? Stress. 2017:1–10. doi: 10.1080/10253890.2017.1401062. [DOI] [PubMed] [Google Scholar]
- Reynaert M.L., Marrocco J.E., Gatta E., Mairesse J., Van Camp G., Fagioli F., Maccari S., Nicoletti F., Morley-Fletcher S. A self-medication hypothesis for increased vulnerability to drug abuse in prenatally restraint stressed rats. Adv Neurobiol. 2015;10:101–120. doi: 10.1007/978-1-4939-1372-5_6. [DOI] [PubMed] [Google Scholar]
- Richter R.M., Weiss F. In vivo CRF release in rat amygdala is increased during cocaine withdrawal in self-administering rats. Synapse. 1999;32:254–261. doi: 10.1002/(SICI)1098-2396(19990615)32:4<254::AID-SYN2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Roberto M., Spierling S.R., Kirson D., Zorrilla E.P. Corticotropin-releasing factor (CRF) and addictive behaviors. Int. Rev. Neurobiol. 2017;136:5–51. doi: 10.1016/bs.irn.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Arias M., Vaccaro S., Arenas M.C., Aguilar M.A., Miñarro J. The novelty-seeking phenotype modulates the long-lasting effects of adolescent MDMA exposure. Physiol. Behav. 2015;141:190–198. doi: 10.1016/j.physbeh.2015.01.023. [DOI] [PubMed] [Google Scholar]
- Roman E., Ploj K., Nylander I. Maternal separation has no effect on voluntary ethanol intake in female Wistar rats. Alcohol. 2004;33:31–39. doi: 10.1016/j.alcohol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Romani-Perez M., Lepinay A.L., Alonso L., Rincel M., Xia L., Fanet H.…Darnaudery M. Impact of perinatal exposure to high-fat diet and stress on responses to nutritional challenges, food-motivated behaviour and mesolimbic dopamine function. Int. J. Obes. 2017;41:502–509. doi: 10.1038/ijo.2016.236. [DOI] [PubMed] [Google Scholar]
- Sanchez R.L., Reddy A.P., Bethea C.L. Ovarian steroid regulation of the midbrain corticotropin releasing factor and urocortin systems in macaques. Neuroscience. 2010;171:893–909. doi: 10.1016/j.neuroscience.2010.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnyai Z., Biro E., Gardi J., Vecsernyes M., Julesz J., Telegdy G. Alterations of corticotropin-releasing factor-like immunoreactivity in different brain regions after acute cocaine administration in rats. Brain Res. 1993;616:315–319. doi: 10.1016/0006-8993(93)90224-b. [DOI] [PubMed] [Google Scholar]
- Schartner C., Ziegler C., Schiele M.A., Kollert L., Weber H., Zwanzger P.…Domschke K. CRHR1 promoter hypomethylation: an epigenetic readout of panic disorder? Eur. Neuropsychopharmacol. 2017;27:360–371. doi: 10.1016/j.euroneuro.2017.01.005. [DOI] [PubMed] [Google Scholar]
- Schenk S., Hunt T., Colle L., Amit Z. Isolation versus grouped housing in rats: differential effects of low doses of heroin in the place preference paradigm. Life Sci. 1983;32:1129–1134. doi: 10.1016/0024-3205(83)90118-2. [DOI] [PubMed] [Google Scholar]
- Schenk S., Ellison F., Hunt T., Amit Z. An examination of heroin conditioning in preferred and nonpreferred environments and in differentially housed mature and immature rats. Pharmacol. Biochem. Behav. 1985;22:215–220. doi: 10.1016/0091-3057(85)90380-6. [DOI] [PubMed] [Google Scholar]
- Schenk S., Lacelle G., Gorman K., Amit Z. Cocaine self-administration in rats influenced by environmental conditions: implications for the etiology of drug abuse. Neurosci. Lett. 1987;81:227–231. doi: 10.1016/0304-3940(87)91003-2. [DOI] [PubMed] [Google Scholar]
- Scholl J.L., Vuong S.M., Forster G.L. Chronic amphetamine treatment enhances corticotropin-releasing factor-induced serotonin release in the amygdala. Eur. J. Pharmacol. 2010;644:80–87. doi: 10.1016/j.ejphar.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenfaden D., Knorr U., Soendergaard M.G., Poulsen H.E., Fink-Jensen A., Jorgensen M.B., Jorgensen A. The relationship between self-reported childhood adversities, adulthood psychopathology and psychological stress markers in patients with schizophrenia. Compr. Psychol. 2017;72:48–55. doi: 10.1016/j.comppsych.2016.09.009. [DOI] [PubMed] [Google Scholar]
- Sengupta P. The laboratory rat: relating its age with human's. Int. J. Preventive Med. 2013;4:624–630. [PMC free article] [PubMed] [Google Scholar]
- Sheng Z., Kawano J., Yanai A., Fujinaga R., Tanaka M., Watanabe Y., Shinoda K. Expression of estrogen receptors (alpha, beta) and androgen receptor in serotonin neurons of the rat and mouse dorsal raphe nuclei; sex and species differences. Neurosci. Res. 2004;49:185–196. doi: 10.1016/j.neures.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Simpson T.L., Miller W.R. Concomitance between childhood sexual and physical abuse and substance use problems. A review. Clin. Psychol. Rev. 2002;22:27–77. doi: 10.1016/s0272-7358(00)00088-x. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann. N. Y. Acad. Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R., Fox H., Hong K.I., Sofuoglu M., Morgan P.T., Bergquist K.T. Sex steroid hormones, stress response, and drug craving in cocaine-dependent women: implications for relapse susceptibility. Exp. Clin. Psychopharmacol. 2007;15:445–452. doi: 10.1037/1064-1297.15.5.445. [DOI] [PubMed] [Google Scholar]
- Skutella T., Probst J.C., Renner U., Holsboer F., Behl C. Corticotropin-releasing hormone receptor (type I) antisense targeting reduces anxiety. Neuroscience. 1998;85:795–805. doi: 10.1016/s0306-4522(97)00682-9. [DOI] [PubMed] [Google Scholar]
- Slater P.G., Cerda C.A., Pereira L.A., Andres M.E., Gysling K. CRF binding protein facilitates the presence of CRF type 2alpha receptor on the cell surface. Proc. Natl. Acad. Sci. U.S.A. 2016;113:4075–4080. doi: 10.1073/pnas.1523745113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Z.D., Meissner A. DNA methylation: roles in mammalian development. Nat. Rev. Genet. 2013;14:204–220. doi: 10.1038/nrg3354. [DOI] [PubMed] [Google Scholar]
- Snyder K.P., Hill-Smith T.E., Lucki I., Valentino R.J. Corticotropin-releasing factor in the rat dorsal raphe nucleus promotes different forms of behavioral flexibility depending on social stress history. Neuropsychopharmacology. 2015;40:2517–2525. doi: 10.1038/npp.2015.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi L.K., Ho S.P., Livanov V., Graciani N., Arneric S.P. Antagonism of CRF(2) receptors produces anxiolytic behavior in animal models of anxiety. Brain Res. 2001;902:135–142. doi: 10.1016/s0006-8993(01)02405-2. [DOI] [PubMed] [Google Scholar]
- Teixeira C.A.B., Lasiuk G., Barton S., Fernandes M.N.F., Gherardi-Donato E. An exploration of addiction in adults experiencing early-life stress: a metasynthesis. Rev. Lat. Am. Enfermagem. 2017;25:e2939. doi: 10.1590/1518-8345.2026.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter Horst G.J., Wichmann R., Gerrits M., Westenbroek C., Lin Y. Sex differences in stress responses: focus on ovarian hormones. Physiol. Behav. 2009;97(2):239–249. doi: 10.1016/j.physbeh.2009.02.036. [DOI] [PubMed] [Google Scholar]
- Timpl P., Spanagel R., Sillaber I., Kresse A., Reul J.M., Stalla G.K., Blanquet V., Steckler T., Holsboer F., Wurst W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropinreleasing hormone receptor 1. Nat. Genet. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- Tractenberg S.G., Levandowski M.L., de Azeredo L.A., Orso R., Roithmann L.G., Hoffmann E.S., Brenhouse H., Grassi-Oliveira R. An overview of maternal separation effects on behavioural outcomes in mice: evidence from a four-stage methodological systematic review. Neurosci. Biobehav. Rev. 2016;68:489–503. doi: 10.1016/j.neubiorev.2016.06.021. [DOI] [PubMed] [Google Scholar]
- Tzschentke T.M. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog. Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Ungless M.A., Singh V., Crowder T.L., Yaka R., Ron D., Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health & Human Services . 2018. Administration for Children and Families, Administration on Children, Youth and Families, Children's Bureau.https://www.acf.hhs.gov/cb/research-data-technology/statistics-research/child-maltreatment Child maltreatment 2016. Available from: [Google Scholar]
- Valentino R.J., Lucki I., Van Bockstaele E. Corticotropin-releasing factor in the dorsal raphe nucleus: linking stress coping and addiction. Brain Res. 2010;1314:29–37. doi: 10.1016/j.brainres.2009.09.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino R.J., Reyes B., Van Bockstaele E., Bangasser D. Molecular and cellular sex differences at the intersection of stress and arousal. Neuropharmacology. 2012:13–20. doi: 10.1016/j.neuropharm.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino R.J., Bangasser D., Van Bockstaele E.J. Sex-biased stress signaling: the corticotropin-releasing factor receptor as a model. Mol. Pharmacol. 2013;83:737–745. doi: 10.1124/mol.112.083550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele E.J., Biswas A., Pickel V.M. Topography of serotonin neurons in the dorsal raphe nucleus that send axon collaterals to the rat prefrontal cortex and nucleus accumbens. Brain Res. 1993;624:188–198. doi: 10.1016/0006-8993(93)90077-z. [DOI] [PubMed] [Google Scholar]
- Van Dam N.T., Rando K., Potenza M.N., Tuit K., Sinha R. Childhood maltreatment, altered limbic neurobiology, and substance use relapse severity via trauma-specific reductions in limbic gray matter volume. JAMA Psychiatry. 2014;71:917–925. doi: 10.1001/jamapsychiatry.2014.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Doelen R.H.A., Robroch B., Arnoldussen I.A., Schulpen M., Homberg J.R., Kozicz T. Serotonin and urocortin 1 in the dorsal raphe and Edinger-Westphal nuclei after early life stress in serotonin transporter knockout rats. Neuroscience. 2017;340:345–358. doi: 10.1016/j.neuroscience.2016.10.072. [DOI] [PubMed] [Google Scholar]
- Varlinskaya E.I., Spear L.P., Spear N.E. Social behavior and social motivation in adolescent rats: role of housing conditions and partner's activity. Physiol. Behav. 1999;67:475–482. doi: 10.1016/s0031-9384(98)00285-6. [DOI] [PubMed] [Google Scholar]
- Vazquez V., Penit-Soria J., Durand C., Besson M.J., Giros B., Dauge V. Maternal deprivation increases vulnerability to morphine dependence and disturbs the enkephalinergic system in adulthood. J. Neurosci. 2005;25(18):4453–4462. doi: 10.1523/JNEUROSCI.4807-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez D.M., Bailey C., Dent G.W., Okimoto D.K., Steffek A., Lopez J.F., Levine S. Brain corticotropin-releasing hormone (CRH) circuits in the developing rat: effect of maternal deprivation. Brain Res. 2006;1121:83–94. doi: 10.1016/j.brainres.2006.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez V., Weiss S., Giros B., Martres M.P., Dauge V. Maternal deprivation and handling modify the effect of the dopamine D3 receptor agonist, BP 897 on morphine-conditioned place preference in rats. Psychopharmacol. (Berl) 2007;193(4):475–486. doi: 10.1007/s00213-007-0789-9. [DOI] [PubMed] [Google Scholar]
- Vinzant N., Scholl J.L., Wu C.-M., Kindle T., Koodali R., Forster G.L. Iron oxide nanoparticle delivery of peptides to the brain: reversal of anxiety during drug withdrawal. Front. Neurosci. 2017;11 doi: 10.3389/fnins.2017.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong S.M., Oliver H.A., Scholl J.L., Oliver K.M., Forster G.L. Increased anxiety-like behavior of rats during amphetamine withdrawal is reversed by CRF2 receptor antagonism. Behav. Brain Res. 2010;208:278–281. doi: 10.1016/j.bbr.2009.11.036. S0166-4328(09)00715-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand G. The influence of stress on the transition from drug use to addiction. Alcohol Res. Health. 2008;31:119–136. [PMC free article] [PubMed] [Google Scholar]
- Wang A., Nie W., Li H., Hou Y., Yu Z., Fan Q., Sun R. Epigenetic upregulation of corticotrophin-releasing hormone mediates postnatal maternal separation-induced memory deficiency. PLoS One. 2014;9 doi: 10.1371/journal.pone.0094394. e94394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., You Z.B., Rice K.C., Wise R.A. Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berlin) 2007;193:283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- Waselus M., Valentino R.J., Van Bockstaele E.J. Ultrastructural evidence for a role of gamma-aminobutyric acid in mediating the effects of corticotropin-releasing factor on the rat dorsal raphe serotonin system. J. Comp. Neurol. 2005;482:155–165. doi: 10.1002/cne.20360. [DOI] [PubMed] [Google Scholar]
- Waselus M., Nazzaro C., Valentino R.J., Van Bockstaele E.J. Stress-induced redistribution of corticotropin-releasing factor receptor subtypes in the dorsal raphe nucleus. Biol. Psychol. 2009;66:76–83. doi: 10.1016/j.biopsych.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt M.J., Burke A.R., Renner K.J., Forster G.L. Adolescent male rats exposed to social defeat exhibit altered anxiety behavior and limbic monoamines as adults. Behav. Neurosci. 2009;123:564–576. doi: 10.1037/a0015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt M.J., Weber M.A., Davies S.R., Forster G.L. Impact of juvenile chronic stress on adult cortico-accumbal function: implications for cognition and addiction. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2017;79:136–154. doi: 10.1016/j.pnpbp.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathington J.M., Cooke B.M. Corticotropin-releasing factor receptor binding in the amygdala changes across puberty in a sex-specific manner. Endocrinology. 2012;153:5701–5705. doi: 10.1210/en.2012-1815. [DOI] [PubMed] [Google Scholar]
- Weathington J.M., Hamki A., Cooke B.M. Sex- and region-specific pubertal maturation of the corticotropin-releasing factor receptor system in the rat. J. Comp. Neurol. 2014;522:1284–1298. doi: 10.1002/cne.23475. [DOI] [PubMed] [Google Scholar]
- Weinhold L.L., Sharpe L.G., Jaffe J.H. Housing conditions influence acquisition of sufentanil aerosol self-administration in rats. Pharmacol. Biochem. Behav. 1993;44:141–144. doi: 10.1016/0091-3057(93)90291-z. [DOI] [PubMed] [Google Scholar]
- Weiser M.J., Goel N., Sandau U.S., Bale T.L., Handa R.J. Androgen regulation of corticotropin-releasing hormone receptor 2 (CRHR2) mRNA expression and receptor binding in the rat brain. Exp. Neurol. 2008;214:62–68. doi: 10.1016/j.expneurol.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom C.S., Schuck A.M., White H.R. An examination of pathways from childhood victimization to violence: the role of early aggression and problematic alcohol use. Violence Vict. 2006;21:675–690. [PubMed] [Google Scholar]
- Williams T.J., Akama K.T., Knudsen M.G., McEwen B.S., Milner T.A. Ovarian hormones influence corticotropin releasing factor receptor colocalization with delta opioid receptors in CA1 pyramidal cell dendrites. Exp. Neurol. 2011;230:186–196. doi: 10.1016/j.expneurol.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S.K., Zhang X.Y., Reyes B.A., Lee C.S., Van Bockstaele E.J., Valentino R.J. Cellular adaptations of dorsal raphe serotonin neurons associated with the development of active coping in response to social stress. Biol. Psychiatr. 2013;73:1087–1094. doi: 10.1016/j.biopsych.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X., Shao S., Li M., Shao F., Wang W. Maternal separation induces alterations of serotonergic system in different aged rats. Brain Res. Bull. 2013;95:15–20. doi: 10.1016/j.brainresbull.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Yajie D., Lin K., Baoming L., Lan M. Enhanced cocaine self-administration in adult rats with adolescent isolation experience. Pharmacol. Biochem. Behav. 2005;82:673–677. doi: 10.1016/j.pbb.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Yan Q.S., Yan S.E. Activation of 5-HT(1B/1D) receptors in the mesolimbic dopamine system increases dopamine release from the nucleus accumbens: a microdialysis study. Eur. J. Pharmacol. 2001;418:55–64. doi: 10.1016/s0014-2999(01)00913-x. [DOI] [PubMed] [Google Scholar]
- Zorrilla E.P., Logrip M.L., Koob G.F. Corticotropin releasing factor: a key role in the neurobiology of addiction. Front. Neuroendocrinol. 2014;35:234–244. doi: 10.1016/j.yfrne.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]