Highlights
-
•
There is no established diagnostic and treatment approach for laryngeal chondrosarcoma due to its low frequency.
-
•
90% of laryngeal chondrosarcoma are low-grade tumors and therefore conservative surgery is recommended as initial treatment.
-
•
Percutaneous biopsy could save patients from a of total laryngectomy.
Keywords: Laryngeal sarcoma, Sarcoma, Chondrosarcoma, Laryngeal chondrosarcoma, Laryngeal tumor, Case report
Abstract
Introduction
Chondrosarcoma constitutes 0.2% of all malignant tumors of the larynx. Many surgeons treat it with total laryngectomy due to the limited experience with this neoplasm because its rarity, and although several conservative approaches have been proposed, the data of these techniques are limited and based on retrospective series.
Presentation of case
A 52-year-old male with a transglottic submucosal tumor and glottic stenosis in fiberoptic examination showed by tomography a laryngeal tumor that infiltrates vocal cords, glottis, cricoid and thyroid cartilage of 3 × 2.7 × 4 cm. Patient was submitted to total laryngectomy with selective bilateral neck dissection because obstructive tumor. Pathology reported a cricoid cartilage tumor consistent with grade 2 chondrosarcoma.
Discussion
Biopsy by laryngoscopy is considered the standard procedure for the diagnosis of laryngeal tumors, however the need for general anesthesia and the difficulty in intubation in some patients with large tumors make difficult to obtain an adequate biopsy in some cases with submucosal tumor. Conservative surgeries should be individualized based on the size and location of the tumor as well as on the patient’s general conditions. Radical treatment is recommended for high-grade and large tumors in which conservative surgery would destabilize the cricoid ring.
Conclusion
There is no diagnostic and treatment approach established for laryngeal chondrosarcoma, we believe that percutaneous biopsy would be the diagnostic test of choice because it is less invasive and has a high sensitivity and specificity; it could also identify patients who are candidates for conservative surgeries.
1. Introduction
This work has been reported in line with the SCARE criteria [21]. Chondrosarcoma constitutes 0.2% of all malignant tumors of the larynx [1] being located more frequently in the cricoid cartilage [2], although there have been reported between 400 and 600 cases in the literature, some have been reported more than once in different series [3,4]. Presentation symptoms include hoarseness, dysphonia, dyspnea, cough, stridor, hemoptysis and a palpable tumor in the neck [1]; lymph node metastases has an incidence of 2.0% [5] and distant metastases occur in 8.5% of the cases, the most frequent sites are lung and bone [6]. Laryngoscopy biopsy is the standard procedure for diagnosis [7], although the need for general anesthesia and the difficulty in intubation in some patients make it difficult to obtain an adequate biopsy [8], this is the reason which some authors recommend percutaneous guided biopsy.
In tomography (CT), chondrosarcoma appears as a well-defined hypodense lesion with calcifications in its interior, distortion of structures and cartilaginous destruction [6] although it is considered that MRI has greater sensitivity to delineate the relationships of the tumor with peripheral soft tissues [9]. The endoscopic approach allows macroscopic resection of the lesion with favorable functional results and minimal morbidity [2], however recurrences have been reported in up to 50% of cases [9,10]; many surgeons perform total laryngectomy due to the limited experience with these neoplasms due to their rarity [11], and although several conservative approaches have been proposed, due to their low frequency, the related data of these techniques are limited and based on retrospective series [2]. Radical treatment is recommended for high-grade [1] and large-sized invasive tumors [5,12]. The prognosis is favorable with a specific disease survival at 1, 5, 10 and 20 years of 97.7%, 91.4%, 81.8% and 68%, however local recurrence reaches rates up to 40% [1].
2. Presentation of case
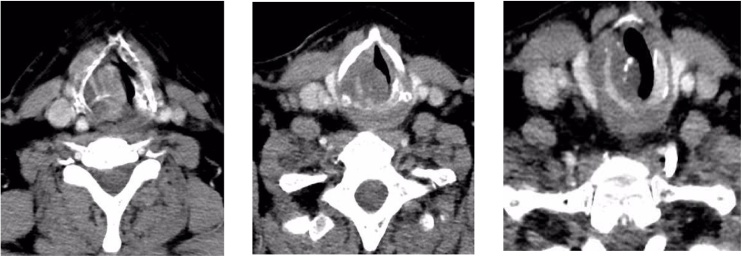
A 52-year-old man with a smoking history of 30 years of evolution acceded to consult with dyspnea, dysphonia and progressive stridor of 1 year of evolution. Physical examination did not showed cervical adenopathy and fiberoptic examination disclosed a submucosal transglottic tumor with glottic stenosis. CT revealed a laryngeal tumor that compromised vocal cords, glottis, cricoid and thyroid cartilage of 3 × 2.7 cm in diameter and 4 cm in length (Fig. 1).
Fig. 1.
CT scan: Neoplasm in larynx that infiltrates vocal cords and part of the glottis as well as cricoid cartilage and right lateral part of the thyroid with dimensions of 3 x 2.7 cm on its axial axis and a craniocaudal extension of 4 cm.
Due to the intralaringeal tumor extension and the secondary obstruction of the airway, we decided to perform an emergent total laryngectomy and a selective bilateral neck dissection (levels II – Vb) because the high risk of occult nodal metastases since taking into account the history of smoking and the tumor´s behavior we decided to treat it as a locally advanced epidermoid carcinoma (T3 Nx M0 EC III), and given the functional status of the larynx, the patient was not considered a candidate for a preservative laryngectomy. We decided to perform the bilateral selective neck dissection first in order to have a better surgical field for the larigenctomy, and because of the tumor characteristic we did not consider a frozen section, and also we did not have a pathologist in this moment. We did not perform a tracheostomy because as an emergent surgery this procedure associates with a lower decanulization rate and also it is a risk factor for a stoma recurrence and a surgical site infection. Likewise we did not perform a guided biopsy due to patient conditions.
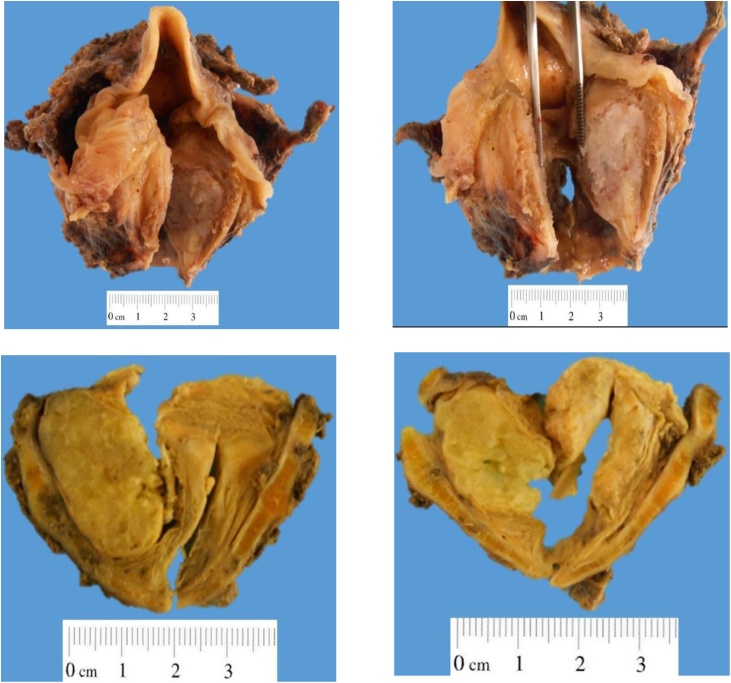
A neoplasm originated in the cartilaginous skeleton of the larynx was revealed. The histopathological study reported a grade 2 chondrosarcoma of cricoid cartilage without extralaryngeal extension of 3.5 × 3 × 2.3 cm with negative margins (Fig. 2), without lymphovascular permeability or neoplastic cells in the rest of laryngeal structures. The product of selective neck dissections did not showed any evidence of metastatic disease. He was discharged from the hospital 5 days after surgery with adequate tolerance to food intake and without complications of the surgical wound or the tracheostomy; currently the patient is in surveillance without evidence of recurrence after two years of surgery.
Fig. 2.
Glottic stenosis due to lesion covered by normal appearance mucosa with subglottic extension. The tumor apparently originates from the cricoid cartilage, has a solid surface, bright, white and measures 3.5 × 2 × 2 cm. The tumor expands the cartilage and infiltrates the mucosa without ulcerating it and infiltrates both paraglottic spaces with the pre-epiglottic space free of tumor.
3. Discussion
Chondrosarcoma occupies the third place in presentation frequency, after squamous cell carcinoma and laryngeal adenocarcinoma [1]. Although it predominates in the cricoid cartilage (75%) [2], especially in its posterior lamina [1], it can emerge from the thyroid (20%), arytenoids (3%) involving the vocal cords [3], or multiple sites [1], and in some cases it is not possible to define the site of origin [1]; in the epiglottis it is rare due to its elastic composition [12].
Its etiology is unknown; due to the tendency of hyaline cartilage to ossification, it is believed that it arises from disorganized ossification [1]. The injection of polytetrafluoroethylene [6], smoking [3], exposure to radiation and the antecedent of concurrent malignancies [1] have been reported as risk factors; some argue that it could have to chondroma as a precursor [1]; in Thompson and Gannon´s review, 60.4% of the chondrosarcomas arose from a preexisting chondroma [14].
Laryngeal chondrosarcoma predominates in males with an H:M ratio of 3:1 [1], the average age of diagnosis is 62.5 years [1], and has slow growth, even in high-grade lesions [5]. The average size of low grade chondrosarcoma is 3.7 cm [8]; in the face of rapid accelerated growth of the lesion, high-grade chondrosarcoma may be suspected [8].
Laryngoscopy biopsy is considered the standard procedure for the diagnosis of laryngeal lesions [7], however the need of general anesthesia, the difficulty in intubation in some patients with large tumors [7], and the submucosal location of chondrosarcoma make it difficult to obtain an adequate biopsy [8]. Many authors have reported the difficulty in differentiating low-grade chondrosarcoma from the chondroma. Given this scenario, size may be useful for differentiation [6] since, as a general rule, chondroma diagnosis is reserved for lesions smaller than 1 cm without cellular atypia [10].
Based on the mitotic index, the cellularity and the nuclear size, the degree of differentiation according to the classification of Jaffe and Lichtenstein for bone chondrosarcomas is determined [3] and two histological variants have been described, the undifferentiated and the clear cell, being the undifferentiated variant associated with the worse prognosis. Although the expression of vimentin and S100 protein may be useful for the diagnosis of high grade chondrosarcoma, in the low and intermediate grade their value is limited [10].
In a prospective study of 66 patients with laryngeal tumors undergoing ultrasound-guided percutaneous biopsy, sensitivity, specificity, positive predictive value and negative predictive value were reported of 91.9%, 100%, 100% and 54.5% respectively [7]. This diagnostic approach could be indicated in patients with some contraindication to general anesthesia, as those with multiple concomitant tumors that limit orotracheal intubation, and/or in patients in whom ruling out a malignant lesion would make them candidates for conservative surgery [15].
Laser endoscopic surgery allows macroscopic resection of the lesion with favorable functional results and minimal morbidity [2], however it does not allow an adequate evaluation of surgical margins [2] and recurrences have been reported in up to 50% of cases [9,10]. Despite this, there are reports of 5-year disease-free survival of 85.7% and overall survival of 100% [2]; in addition, this technique also provides a histological diagnosis before opting for a more radical treatment [9].
Total laryngectomy continues to be the treatment of choice in up to 29.4% of patients [2]. Voice-sparing surgeries provide disease-free survival of up to 30 years; in the Thompson and Gannon’s review, only 33% of the patients underwent total laryngectomy, the rest of patients were treated with preservative techniques reporting a cure rate greater than 90%; in the follow-up, local and/or distant recurrence only occurred in 18% of the patients (20/111), of which 10 patients underwent salvage laryngectomy and the rest were newly submitted to conservative treatment [14]. Based on these findings and because most chondrosarcomas are low or intermediate grade (>95%) [2] conservative surgery is recommended as initial treatment [14].
The conservative surgeries should be individualized based on the size and location of the lesion as well as the general conditions and age of the patient, in addition to these embroideries, the site of tumor origin is the most important factor to consider since unlike other cartilages, resection of a cricoid lamina lesion can cause a glottal collapse and laryngeal stenosis [12,16]; all preserving surgery must conserve at least one cricoarytenoid unit that give anatomic and functional support [2], the corresponding recurrent nerve [10], and a rigid support structure for the airway at the level of the crico-tracheal junction [2]. Some techniques that can be used are the supratracheal partial laryngectomy which has reported 5-year global disease-free survival rates of 78.7% and 69.1% in squamous cell laryngeal carcinomas [17] and the total cricoidectomy, in which due to the arytenoids remain supported on the tracheal rings and the preservation of the recurrent laryngeal nerves, the cricoarytenoid muscles retain the ability to tighten the vocal cords and narrow the cleft glottal preserving swallowing and phonation [16].
Radical treatment is recommended for high-grade [1] and large invasive tumors [12] in which conservative surgery would destabilize the cricoid ring [5]. Traditionally, all tumors with an extension in this cartilage greater than 50% have been treated with total laryngectomy [5,9]; the survival rate reported with total laryngectomy as primary treatment is 86% and 77% for recurrent lesions [10]. The treatment for recurrences ranges from endoscopic resection to total laryngectomy depending on the extent and location of the lesion and the functional status of the larynx [9]. Due to its low radiosensitivity, radiotherapy is reserved for extensive lesions, inoperable cases and recurrences [6,13], and there are reports of the use of radiofrequency as a palliative treatment in single tumors and that do not exceed more than 14 mm in diameter [13].
The management of airway obstruction in patients with laryngeal cancer who have not received definitive treatment is controversial [18] and there are three options to achieve an adequate airway; emergency tracheostomy, laser debulking, and emergency laryngectomy [19].
Although an emergency tracheostomy allows a biopsy and could contribute to improve the patient nutritional status before the definitive surgery, it has been reported that it constitutes a major risk factor for peristomal recurrence which presents an incidence of 5%–15% after total laryngectomy because the tumor cells may exfoliate and survive in the peristomal granulation tissue [18,19], and performing the laryngectomy through a previously contaminated field may increase the risk of wound infection and make difficult the construction of a definitive tracheostomy [19]. In addition, in comparison with an elective tracheostomy, the decannulation rate is lower [20].
Debulking with CO2 laser is the first option especially when the obstruction is in the supraglottic or glottis regions, however, although this technique does not increase the rate of infection or peristomal recurrence, it is associated with postoperative bleeding, laryngeal edema and aspiration pneumonia [19].
Emergency laryngectomy is defined as that performed in the first 24 h of admission in a previously untreated patient without a diagnosis of malignancy. This technique minimizes the risk of tumoral implantation in the peristomal area and reduces the risk of infection, and is recommended in glottic obstructions with tracheal extension [19].
4. Conclusion
Due to its low frequency, there is no established diagnostic and treatment approach for laryngeal chondrosarcoma. Strategies for its management are based on retrospective studies and case reports; this tumor could be suspected in a patient with a slow-growing laryngeal tumor and no lymph node or distant disease because more than 90% are low-grade tumors. Although there are tomographic characteristic findings of this tumor, we believe that percutaneous biopsy would be the diagnostic test of choice because it is a less invasive technique and has a high sensitivity and specificity; in addition, patients who could be candidates for endoscopic resections or partial laryngectomies could be selected accurately. By keeping in mind the diagnostic suspicion of this neoplasm, a more conservative treatment could be provided in earlies tumors.
Conflict of interest
None of the authors have conflict of interest.
Funding
None of the authors have sources of funding.
Ethical approval
Ethical approval was given by Instituto Nacional de Cancerología, Mexico City.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Dr. Carlos Hernández-Brito: Design, data collection, data analysis, writing the paper.
Dra. María Alejandra Salazar-Álvarez: Data collection and writing the paper.
Dr. Mario Enrique Álvarez-Bojorquez: Data collection and writing the paper.
Dr. Francisco Carlos Cisneros-Juvera: Data collection and writing the paper.
Dr. Javier López-Gómez: Data collection and writing the paper.
Dr. Ángel Elizalde-Méndez: Data collection and writing the paper.
Dr. Martín Granados-García: Data analysis, data interpretation and writing the paper.
Registration of research studies
Nothing to declare. This is a case report.
Guarantor
Carlos Hernández-Brito.
References
- 1.Chin O.Y., Dubal P.M., Sheikh A.B., Unsal A.A., Woo Park R.C., Baredes S., Eloy J.A. Laryngeal chondrosarcoma: a systematic review of 592 cases. Laryngoscope. 2017;127:430–439. doi: 10.1002/lary.26068. [DOI] [PubMed] [Google Scholar]
- 2.Piazza C., Paderno A., Nicolai P. Conservative surgery for laryngeal chondrosarcoma: a review of the most recently proposed approaches. Curr. Opin. Otolaryngol. Head Neck Surg. 2017;25:93–100. doi: 10.1097/MOO.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 3.Sauter A., Bersch C., Lambert K.L., Hörmann K., Naim R. Chondrosarcoma of the larynx and review of the literature. Anticancer Res. 2007;27:2925–2929. [PubMed] [Google Scholar]
- 4.Buda I., Hod R., Feinmesser R., Shvero J. Chondrosarcoma of the larynx. IMAJ. 2012;14:681–684. [PubMed] [Google Scholar]
- 5.Dubal P.M., Svider P.F., Kanumuri V.V., Patel A.A., Baredes S., Eloy J.A. Laryngeal chondrosarcoma: a population-based analysis. Laryngoscope. 2014;124:1877–1881. doi: 10.1002/lary.24618. [DOI] [PubMed] [Google Scholar]
- 6.Oliveira J.F., Branquinho F.A., Monteiro A.R., Portugal M.E., Guimarães A.M. Laryngeal chondrosarcoma—ten years of experience. Braz. J. Otorhinolaryngol. 2014;80:354–358. doi: 10.1016/j.bjorl.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Fioria E., Conteb G., Ansarinc M., De Benedettoc L., Bonellob L., Alteriod D., Maffinie F., Bellomia M., Preda L. The role of ultrasound-guided transcutaneous tru-cut biopsy in diagnosing untreated and recurrent laryngo-hypopharyngeal masses. Eur. J. Radiol. 2016;85:158–163. doi: 10.1016/j.ejrad.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Maglioccaa K.R., Edgara M.A., Coreyb A., Villaric C.R. Dedifferentiated chondrosarcoma of the larynx: radiological, gross, microscopic and clinical features. Ann. Diagn. Pathol. 2017;30:42–46. doi: 10.1016/j.anndiagpath.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Merrot O., Gleizal A., Poupart M., Pignat J.C. Cartilaginous tumors of the larynx: endoscopic laser management using YAG/KTP. Head Neck. 2009;31:145–152. doi: 10.1002/hed.20932. [DOI] [PubMed] [Google Scholar]
- 10.Damiani V., Crosetti E., Rizzotto G., Camaioni A., Succo G. Well and intermediate differentiated laryngeal chondrosarcoma: toward conservative surgery? Eur. Arch. Otorhinolaryngol. 2014;271:337–344. doi: 10.1007/s00405-013-2656-0. [DOI] [PubMed] [Google Scholar]
- 11.Zeitels S.M., Bums J.A., Wain J.C., Wright C.D., Rosenberg A.E. Function preservation surgery in patients with chondrosarcoma of the cricoid cartilage. Ann. Otol. Rhinol. Laryngol. 2011;120:603–607. doi: 10.1177/000348941112000908. [DOI] [PubMed] [Google Scholar]
- 12.Piazza C., Del Bon F., Grazioli P., Mangili S., Barbieri D., Nicolai P., Peretti G. Organ preservation surgery for low- and intermediate-grade laryngeal chondrosarcomas: analysis of 16 cases. Laryngoscope. 2014;124:907–912. doi: 10.1002/lary.24416. [DOI] [PubMed] [Google Scholar]
- 13.Perrot C., Cortese S., Eluecque H., Mastronicola R., Sergeant C., Marchal F., Demet S., Dolivet G. Laryngeal chondrosarcoma: repeated laser and radiofrequency ablation in the palliative setting. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2013;130:91–93. doi: 10.1016/j.anorl.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Thompson L.D.R., Gannon F.H. Chondrosarcoma of the larynx: a clinicopathologic study of 111 cases with a review of the literature. Am. J. Surg. Pathol. 2002;26:836–851. doi: 10.1097/00000478-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Preda L., De Fiori E., Rampinelli C., Ansarin M., Petralia G., Maffini F., Alterio D., Bonello L., Chiesa F., Bellomi M. US-guided transcutaneous tru-cut biopsy of laryngo-hypopharyngeal lesions. Eur. Radiol. 2010;20:1450–1455. doi: 10.1007/s00330-009-1682-1. [DOI] [PubMed] [Google Scholar]
- 16.de Vincentiis M., Greco A., Fusconi M., Pagliuca G., Martellucci S., Gallo A. Total cricoidectomy in the treatment of laryngeal chondrosarcomas. Laryngoscope. 2011;121:2375–2380. doi: 10.1002/lary.22337. [DOI] [PubMed] [Google Scholar]
- 17.Succoa G., Fantini M., Rizzotto G. Supratracheal partial laryngectomy: indications, oncologic and functional results. Curr. Opin. Otolaryngol. Head Neck Surg. 2017;25:127–132. doi: 10.1097/MOO.0000000000000344. [DOI] [PubMed] [Google Scholar]
- 18.Pittman A., Lindau R., Andersen P., Wax M.K. Stomal recurrence: salvage surgery and reconstruction utilizing microvascular free tissue transfer. Head Neck. 2014;36:1431–1434. doi: 10.1002/hed.23468. [DOI] [PubMed] [Google Scholar]
- 19.Chu P.Y., Lee T.L., Chang S.Y. Impact and management of airway obstruction in patients with squamous cell carcinoma of the larynx. Head Neck. 2011;31:98–102. doi: 10.1002/hed.21401. [DOI] [PubMed] [Google Scholar]
- 20.Fang C.H., Friedman R., White P.E., Mady L.J., Kalyoussef E. Emergent awake tracheostomy—the five-year experience at an urban tertiary care center. Laryngoscope. 2015;125:2476–2479. doi: 10.1002/lary.25348. [DOI] [PubMed] [Google Scholar]
- 21.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P. The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;30:A6148. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]